Abstract
The effect of the fast skeletal muscle troponin activator, CK-2066260, on calcium-induced force development was studied in skinned fast skeletal muscle fibers from wildtype (WT) and nebulin deficient (NEB KO) mice. Nebulin is a sarcomeric protein that when absent (NEB KO mouse) or present at low levels (nemaline myopathy (NM) patients with NEB mutations) causes muscle weakness. We studied the effect of fast skeletal troponin activation on WT muscle and tested whether it might be a therapeutic mechanism to increase muscle strength in nebulin deficient muscle. We measured tension–pCa relations with and without added CK-2066260. Maximal active tension in NEB KO tibialis cranialis fibers in the absence of CK-2066260 was ∼60% less than in WT fibers, consistent with earlier work. CK-2066260 shifted the tension-calcium relationship leftwards, with the largest relative increase (up to 8-fold) at low to intermediate calcium levels. This was a general effect that was present in both WT and NEB KO fiber bundles. At pCa levels above ∼6.0 (i.e., calcium concentrations <1 µM), CK-2066260 increased tension of NEB KO fibers to beyond that of WT fibers. Crossbridge cycling kinetics were studied by measuring ktr (rate constant of force redevelopment following a rapid shortening/restretch). CK-2066260 greatly increased ktr at submaximal activation levels in both WT and NEB KO fiber bundles. We also studied the sarcomere length (SL) dependence of the CK-2066260 effect (SL 2.1 µm and 2.6 µm) and found that in the NEB KO fibers, CK-2066260 had a larger effect on calcium sensitivity at the long SL. We conclude that fast skeletal muscle troponin activation increases force at submaximal activation in both wildtype and NEB KO fiber bundles and, importantly, that this troponin activation is a potential therapeutic mechanism for increasing force in NM and other skeletal muscle diseases with loss of muscle strength.
Introduction
The skeletal muscle sarcomere is a highly organized and intricate structure that consists of a regular array of actin-based thin filaments that interdigitate with myosin-based thick filaments; these filaments slide past each other as muscles contract, a process driven by the mechano-chemical cycling of the thick-filament based myosin crossbridges [1]. The force level that is generated by the sarcomere depends on the degree to which the thin and thick filaments overlap, the degree of thin filament activation, and on the kinetics of the crossbridge cycle that determine the fraction of the crossbridges that develops force. Recent studies on mouse models deficient in nebulin[2]–[4] indicate that the large filamentous protein nebulin plays an important role in each of these force determinants and, importantly, that when nebulin levels are reduced in nemaline myopathy (NM) patients, severe muscle weakness ensues[5]–[7].
The C-terminus of nebulin is anchored at the Z-disk, the majority of the nebulin filament is co-extensive with the thin filament, and the N-terminus of nebulin is near the pointed end of the thin filament (Fig. 1A) [8]–[11]. The human NEB (nebulin) gene is large, containing 183 exons in human that encode a ∼800 kDa protein [12], [13]. The bulk of the molecule is comprised of small modules that are organized into seven-module super-repeats that match the 38.5 nm repeat of the actin filament [12]–[15]. This arrangement allows each nebulin module to interact with a single actin monomer, and each nebulin super-repeat to associate with a single tropomyosin/troponin complex [14], [15]. Recent work indicates that nebulin’s C-terminus interacts with the actin nucleating protein N-WASP, that this interaction plays an important role in skeletal muscle hypertrophy, and that this process is controlled by phosphorylation of nebulin’s serine rich domain [16].
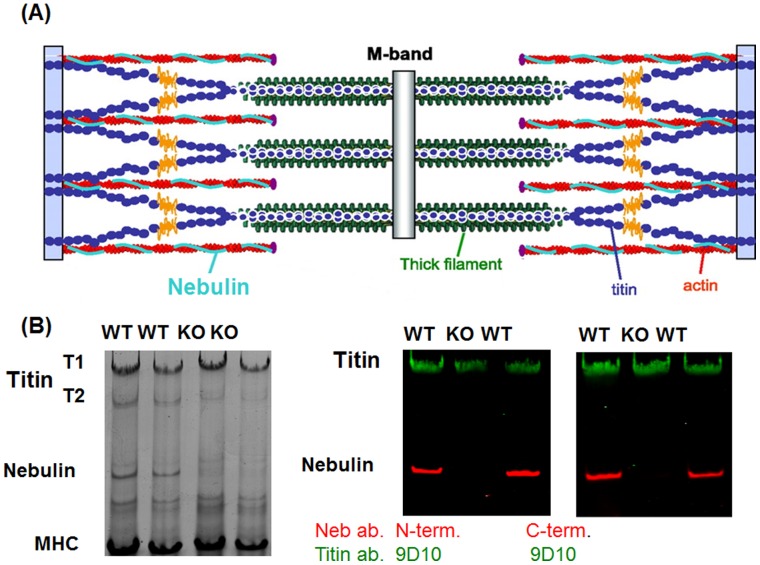
Figure 1. Location of nebulin in the sarcomere and protein expression in WT and NEB KO mouse TC muscle.
A) Nebulin is a large sarcomeric protein that is coextensive with the actin filament. B) Protein expression in WT and NEB KO mouse TC muscle. (1% agarose gel). Left coomassie blue stained gel. Right: Westernblot with anti-titin antibody 9D10 (green) and anti-nebulin antibody raised to nebulin’s N-terminus (left) and anti-nebulin antibody raised to nebulin’s C-terminus. Nebulin is a ∼800 kDa protein that is only present in WT mice.
Studies using nebulin KO mouse models have shown that thin filaments are shorter in nebulin deficient muscle, indicating that nebulin contributes to thin filament length specification [3], [4]. Furthermore, on the descending limb of the force-sarcomere length relationship, the shorter thin filaments of nebulin deficient muscle give rise to reduced filament overlap and reduced force [3], [4], [17]. Finally, nebulin plays a role in increasing calcium sensitivity and, importantly, in increasing the fraction of the crossbridges that generates force, by altering crossbridge cycling kinetics [2], [18]. Subsequent studies on biopsies from NM patients support that nebulin’s compromised role in augmenting force generation contributes to the muscle weakness of nebulin-based NM patients [2], [6]. Therapeutic approaches to lessen force impairment in NM patients are currently unavailable.
A class of fast skeletal troponin activators was recently discovered that can augment force development of skeletal muscle by slowing the dissociation rate of calcium from the troponin complex and enhancing crossbridge formation at a given calcium concentration [19]. Given that deficits in force production described above in NM might be improved by this mechanism of action, the purpose of this study was to establish the effect of the fast skeletal troponin activator, CK-2066260, on force generation in 1) skinned fibers from WT mice, and 2) skinned fibers from NEB-KO mice.
Methods
Ethics Statement
Experiments were approved by the University of Arizona Institutional Animal Care and Use Committee (protocol 07–090) and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
Animal Model
We used wildtype (WT) and nebulin-deficient (NEB KO) mice in which the nebulin gene is inactivated by targeting its promoter region [3]. Inactivation of the nebulin gene and genotyping of mice was as described previously [3]. NEB KO mice die at early age [3] and we were restricted to comparing ∼1 week old WT and NEB KO mice. We focused our studies on the tibialis cranialis (TC) muscle (this muscle is also known as tibialis anterior) which is a fast fiber muscle type that is convenient to use for skinned fiber studies. Mice were anesthetized using isoflurane and the TC muscles were dissected. For the mechanical studies, the muscle was chemically skinned using 1% Triton X-100 in relaxing solution. For protein expression studies, muscles were quick-frozen in liquid nitrogen and stored at −80°C. In addition to PCR genotyping, absence of nebulin expression was also confirmed by 1% agarose protein gels (Fig. 1B shows examples).
Muscle Preparations
Skinned TC muscles were dissected into muscle fiber bundles (cross-sectional area (CSA) ∼0.02 mm2; length ∼1.5 mm) and small aluminum clips were glued at each end of the muscle strip. Note that fiber bundles were used for ease of experimentation (single fibers are only ∼10 µm in diameter). Because the bundles contain different fiber types that express neonatal myosin, MHC type IIA and IIB, and small amounts of MHC type I [2], to know the effect of CK-2066260 on the individual fiber types requires future follow-up studies at the single fiber level. The muscles were attached to a force transducer (model 405A, Aurora Scientific) and a length controller (model 322C, Aurora Scientific) [20]. We used a custom-designed experimental setup (model 802D, Aurora Scientific) with an experimental stage that was mounted on top of an inverted microscope with 8 isolated wells that were pre-loaded with activating solutions that contain a range of calcium concentrations. The fiber bundles were visualized via a CCD camera, and from the video images the sarcomere length was measured on-line using a spatial autocorrelation function (model 901, Aurora Scientific). The experimental stage was temperature controlled at 15°C. The thickness and width of the preparation were measured and the CSA was calculated by assuming an elliptical cross-section. The CSA was used to convert measured forces into tension (in mN/mm2).
Skinned Muscle Solutions
We used relaxing solution (RS), pre-activating solution (Pre-A), and maximal activating solution (AS, pCa 4.5). Sub-maximal activating solutions were obtained by mixing RS and AS according to Fabiato and Fabiato [21]. All solutions contained the following: BES, 40 mM; DTT, 1 mM; creatine phosphate (PCr), 33 mM; creatine phosphokinase (CPK), 200 U/ml; the ionic strength was adjusted to 180 mM with K-proprionate; pH 7.0 at 15°C. Relaxing solution, pre-activating solution and activating solution contains 6.9, 6.7, 6.6 mM MgCL2, respectively. For Na-ATP the values were 6.0, 6.0 and 6.2 mM, for EGTA 10.0, 1.0, and 0.0 mM, for Ca-EGTA 0, 0, and 10 mM and for K-proprionate 3.3, 30.4, and 2.1 mM, respectively.
Fast Skeletal Troponin Activator
CK-2066260 belongs to a class of fast skeletal troponin activators discovered by Cytokinetics [19] and was provided by the Research and Early Development department at Cytokinetics (South San Francisco, CA). CK-2066260 selectively binds to and activates the fast skeletal troponin complex by increasing its sensitivity to calcium. CK-2066260 was added to all experimental solutions (relaxing, pre-activating and activating solutions) from a DMSO stock (final DMSO concentration 1%). Control solutions contained 1% DMSO with no CK-2066260 (vehicle control).
Tension-pCa Measurements
Fiber bundles while in relaxing solution were stretched to either sarcomere length (SL) 2.1 µm or 2.6 µm, were held at the extended length for 7 min, and were then released. During the hold phase the preparation was first incubated in a pre-activating solution and was then activated in the following sequence pCa 6.4, 6.25, 6.1, 5.95, 5.75 and 4.5 (see Fig. 2A). The muscle was then relaxed again and released. To study the length-dependence of activation (LDA), we first obtained a tension-pCa curve at SL of 2.1 µm, then a relation at 2.6 µm both without CK-2066260 and after that the same sequence was repeated in the presence of 10 µM CK-2066260. Experiments were also performed in which the tension-pCa curves were constructed by activating the fiber bundles at a certain pCa solution (randomly selected) followed by relaxation and a repeat with activation at a different pCa.
Figure 2. Experimental Protocol and effect of CK-2066260 on active tension at submaximal activation.
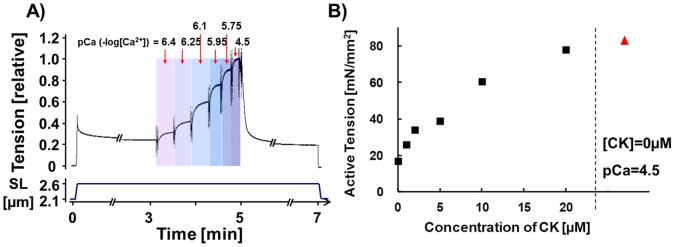
A) Skinned skeletal muscle fibers (TC muscle) were stretched, held for 7 min, and were then released. During the hold phase, the muscle was exposed to various pCa activating solutions, and was then relaxed again. B) Effect of CK-2066260 on active tension at submaximal activation level (pCa = 6.25). Active tensions at a range of CK-2066260 concentrations (black symbols). The maximal active tension (pCa 4.5) at 0 µM CK-2066260 is shown to the right in red.
Active tension was measured at the plateau of each activation (Fig. 2A). Active tension was normalized by the maximal active tension at pCa 4.5 (Tmax), and plotted against the pCa to determine the tension-pCa curve. The tension-pCa curves were fit to the Hill equation: T/Tmax (relative tension) = [Ca2+]n H/(K+[Ca2+]n H), where n H is the Hill coefficient, and pCa50 (pCa value that results in 50% of the maximal activate tension measured at pCa 4.5) was calculated as (-logK)/n H. In the LDA experiments, we determined the differences between pCa50 of the tension-pCa curves measured at SL 2.1 and 2.6 µm and used this as an index of length-dependent activation (i.e. ΔpCa50). Rundown was determined from a maximal activation (pCa 4.5; SL 2.1 µm) prior to measurement of the first tension-pCa curve and once more after the last curve (typically we measured 4 curves). Experiments in which rundown was less than 10% were accepted in this study (on average rundown was absent).
K tr Measurement
The rate constant of tension redevelopment (ktr) was measured at pCa 5.95, 5.75 and 4.5 by using the large release, rapid shortening, rapid re-stretch approach [22]. First, the muscle preparation was activated and once the steady-state was reached, the preparation was rapidly shortened by 20% of the initial length, which reduced tension to zero. This was followed by unloaded shortening lasting 30 msec. The remaining bound crossbridges were mechanically detached by rapidly (1 msec) re-stretching the muscle fiber bundles to its original length, after which tension redevelops. Measurements were performed by controlling fiber length as the quality of the striation patterns did not permit control of sarcomere length. ktr was determined by fitting the rise of tension redevelopment following re-stretch by a mono-exponential equation: T = Tss(1-e-Ktr.t), where T is tension at time t, Tss is steady-state tension, and ktr is the rate constant of tension redevelopment.
Protein Gels and Westernblots
The anti-titin antibody (9D10 [23], [24]) was obtained from the Developmental Studies Hybridoma Bank, University of Iowa. For details on nebulin antibodies, see acknowledgments. Nebulin antibodies were obtained from Myomedix, Mannheim Germany (www.myomedix.com). For additional technical details, please see[25]–[27].
Statistical Analysis
Data are shown as mean ± SEM. Paired or unpaired t-tests or ANOVA was used, as appropriate, to test for statistical significance with p<0.05.
Results
We performed tension-pCa studies on skinned muscle fiber bundles from ∼1 week old WT and NEB KO mice (for protocol see Fig. 2A) and studied the effect of the fast skeletal muscle activator CK-2066260 on tension. The initial studies were performed at a sarcomere length (SL) of 2.1 µm because at this sarcomere length WT and NEB KO fibers have a similar degree of filament overlap [2], which simplifies interpretation of results. We first established the CK-2066260 dose-response curve by measuring active tension in skinned WT fiber bundles at pCa 6.25 and adding CK-2066260 at concentrations that ranged from 0–20 µM. Tension in the absence of CK-2066260 was ∼25% of the maximal tension at pCa 4.5 and the addition of CK-2066260 increased tension in a dose-dependent manner (Fig. 2B) with 20 µM CK-2066260 resulting in a tension that was near the maximal tension, Tmax (pCa 4.5). Thus, in the µM range CK-2066260 is highly effective in increasing submaximal active tensions of wildtype mouse skeletal muscle.
Effect of CK-2066260 on Calcium Sensitivity of Active Tension
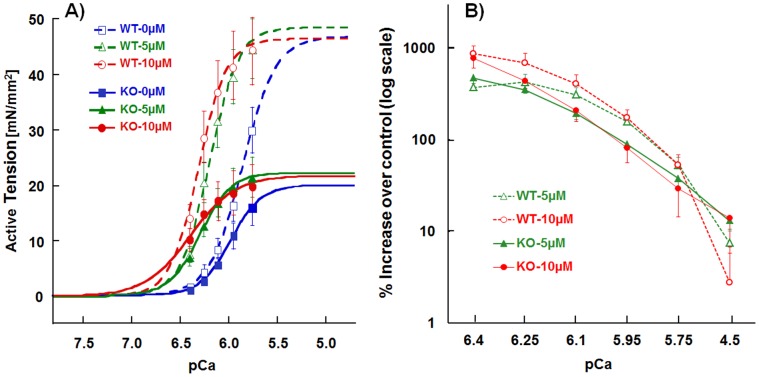
We measured the full tension – pCa relations in WT and NEB KO fiber bundles, normalized tensions to Tmax and used the normalized curves to derive the pCa50 (pCa that results in a tension that is 50% of Tmax). Tmax in KO fiber bundles was only 42% of WT fibers (Table 1, 0 µM CK-2066260). This finding is consistent with previous work on nebulin deficient mouse muscle that reported the tension in KO fibers to be ∼45% of WT fibers [2]. In the present study, calcium sensitivity was found to be increased in the NEB KO (Table 1), whereas previously a reduction was found [2]. Increased calcium sensitivity might be explained by the upregulation of a low level (∼10%) of slow skeletal muscle TnT (sTnT) and slow TnI (sTnI) in nebulin KO TC muscle [2], or a change in their post-translational modification. This notion is supported by experiments in which in both NEB KO and WT fibers the native troponin complex was replaced by recombinant troponin and calcium sensitivity was reduced [2]. Next we studied the effect of CK-2066260 on the tension-pCa curve and selected, based on the measured dose-response curve, 5 and 10 µM CK-2066260. In both genotypes CK-2066260 substantially shifted the tension-calcium relationship to the left as compared to vehicle alone (0 µM CK-2066260), with the most prominent tension increase observed at lower activation levels, while peak tension (Tmax) was not affected (Fig. 3A). To highlight the effect of CK-2066260 on submaximal active tension, we plotted the relative increase in active tension in the presence of 10 µM CK-2066260 over that measured in absence of CK-2066260, at various pCa levels (Fig. 3B). The increase in tension was ∼800% at pCa 6.4 and decreased as calcium concentration increased, with only a small ∼5% increase in WT and a ∼10% increase in KO at pCa 4.5 (p>0.05 compared to the absence of CK-2066260). There were no differences between the genotypes in the relative increase in tension in CK-2066260. In summary, CK-2066260 was highly effective in increasing active tension levels with very large effects at low to intermediate levels of activation.
Table 1. Tmax (tension at pCa 4.5); calcium sensitivity (pCa50) and Hill coefficient (nH) of WT and KO in 0, 5, and 10 µM CK-2066260.
| Genotype/parameter | CK-2066260 concentration | ||
| 0 µM | 5 µM | 10 µM | |
| WT- Tmax (mN/mm2) | 46.6±5.5 | 49.7±5.1 | 47.8±6.0 |
| KO- Tmax (mN/mm2) | 19.9±4.0** | 22.1±4.1** | 22.2±4.5** |
| WT- pCa50 | 5.85±0.04 | 6.18±0.04# # | 6.30±0.04# #√√ |
| Δ from 0 CK (pCa unit) | 0.34±0.02 | 0.45±0.02√√ | |
| KO- pCa50 | 5.98±0.03* | 6.29±0.01* # # | 6.43±0.05# #√√ |
| Δ from 0 CK(pCa unit) | 0.31±0.02 | 0.45±0.04√ | |
| WT- nH | 2.64±0.07 | 3.21±0.20 | 3.29±0.34 |
| KO- nH | 2.83±0.15 | 3.14±0.38 | 2.16±0.58 |
| WT- Tmax (SL 2.6 µm) | 58.7±6.0 | NA | 66.7±6.4# |
| KO- Tmax (SL 2.6 µm) | 34.0±5.7** | NA | 44.5±8.0**# # |
| WT- nH (SL 2.6 µm) | 2.7±0.28 | NA | 2.31±0.24 |
| KO- nH (SL 2.6 µm) | 3.09±0.11 | NA | 1.47±0.24*# # |
Results are mean ± SEM from 5 WT and 5 KO mice, except for the last 4 rows that are from 6 WT and 6 KO mice.
comparison WT vs. KO;
comparison versus 0 µM CK-2066260;
comparison versus 5 µM CK-2066260; single symbol p<0.05, double symbol p<0.01. All measurements were at a sarcomere length (SL) of 2.1 µm, except the bottom 4 rows that were at 2.6 µm.
Figure 3. Tension–pCa relations in WT and NEB KO skinned TC muscle fibers.
A) Average active tension-pCa curves in WT (open symbols, dashed) and KO (closed symbols, solid) mice with 0 (blue), 5 µM (green) and 10 µM (red) CK-2066260 at SL = 2.1 µm. Curves in CK were shifted to the left in both WT and KO, i.e. calcium sensitivity is increased. Note that at pCa>6.0, CK-2066260 increased active tension of NEB-KO muscle beyond that of WT. B) Relative active tension increase (plotted on a log scale) in 5 and 10 µM of CK over 0 µM of CK at various pCa levels. Regardless of genotype, active tensions were significantly increased from ∼800% at the pCa 6.4 to ∼10% at pCa 4.5. Results from five WT and five KO mice.
In WT muscle fibers, pCa50 was significantly increased by 0.34 and 0.45 pCa units in 5 µM and 10 µM CK-2066260, respectively. In nebulin deficient fibers, a similar increase occurred with values of 0.31 and 0.45 pCa units (Table 1). We also determined the cooperativity of activation (Hill coefficient, nH) and found this to be unchanged (Table 1). Thus, CK-2066260 alters the activation characteristics of mouse skinned fibers by greatly increasing calcium sensitivity.
Because all studies were performed in the presence of 1% DMSO (CK-2066260 solvent) we studied whether DMSO itself affects tension. We compared the tension-pCa relation in solutions without and with 1% DMSO (but no CK-2066260) and measured for each muscle preparation multiple tension-pCa relations (as in our CK-2066260 experiments). DMSO did cause a small increase in active tension, but the same relative increase occurred at all pCa levels. Consequently 1% DMSO did not cause a change in pCa50 nor in nH (measurement of cooperativity) and is therefore a safe concentration to use in our experiments. Finally, we also evaluated whether the activation protocol with progressively increasing calcium levels (explained in Fig. 2A) gave the same results as when performing experiments in which the tension-pCa curves were constructed by activating the fiber bundles by a single pCa solution (randomly selected) followed by relaxation, and repeating this sequence at different pCa values. Figure S1 shows that both protocols gave the same results.
In summary, CK-2066260 increases tension in both WT and nebulin deficient fibers, with large effects at submaximal activation levels. Thus, CK-2066260 is a highly effective calcium sensitizer.
Effect of CK-2066260 on Rate Constant of Tension Development (ktr)
To gain insights in the effect of CK-2066260 on crossbridge cycling kinetics we measured ktr, the rate constant of force redevelopment following a release/shortening/restretch protocol. This rate constant reflects the sum of the apparent rate constant of conversion of non-force generating to force generating crossbridges, fapp, and the apparent rate constant of conversion of force generating to non-force generating crossbridges, gapp [22]. ktr was measured at three pCa levels (5.95, 5.75, and 4.5) and the effect of 10 µM CK-2066260 was determined. Fig. 4A shows example experiments and Fig. 4B and 4C summarized results of WT (B) and NEB KO (C) fiber bundles. Under all experimental conditions was ktr less in NEB KO than WT fiber bundles. ktr increased with activation level and, importantly, was significantly increased by CK-2066260. The largest effects were present at submaximal activation level where ktr was increased by more than 150% (Fig. 4D). These results indicate that in WT and NEB KO fibers CK-2066260 alters crossbridge cycling kinetics.
Figure 4. Effect of CK 2066260 on the rate constant of force redevelopment (ktr).
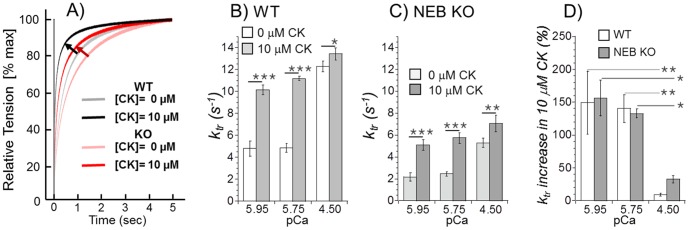
A) Examples of ktr experiments on WT and NEB KO fiber bundles activated at pCa 5.95 before (control) and after the addition of 10 µM CK-2066260. Shown is the tension recovery phase following the re-stretch (see Methods for details). In the presence of 10 µM CK-2066260 (darker traces) tension recovery is faster than in absence of CK-2066260. B) and C) ktr results of WT (B) and NEB KO fibers (C). Addition of 10 µM CK-2066260 increases ktr in both WT and NEB-KO fibers. A two-way ANOVA reveals in WT bundles significant effects of pCa and CK-2066260 on ktr with a significant interaction between pCa and CK (p<0.001). The same is the case for the KO data, except that the p-value of the interaction term is p = 0.02. D) Change in ktr in CK 2066260 (as percentage of control values). Results from six WT and six KO mice.
Length Dependent Effects of CK-2066260
Because muscle operates at a range of sarcomere lengths, a separate set of studies was performed in which tension-pCa curves were measured at SL 2.1 µm and 2.6 µm. To keep rundown to a minimum during the experiment we tested only 10 µM CK-2066260, which required a manageable four force-pCa curves per muscle preparation. An increase in SL caused a leftward shift in the tension-pCa relation (see the small shifts between the pair of broken lines and between the pair of solid lines in Fig. 5), which reflects the well-known phenomenon of length dependence of activation in striated muscle [28]. This SL-induced shift was characterized by determining the ΔpCa50 (pCa50 at SL 2.6 µm minus pCa50 at SL 2.1 µm) and the result is shown in Fig. 6A. The data did not reveal a significant difference between the genotypes. However, within genotype a significant difference was found in the KO fibers as the SL effect was larger in fibers that were exposed to CK-2066260 (Fig. 6A).
Figure 5. Effect of 10 µM CK-2066260 on tension-pCa curves at short (2.1 µm) and long SL (2.6 µm).
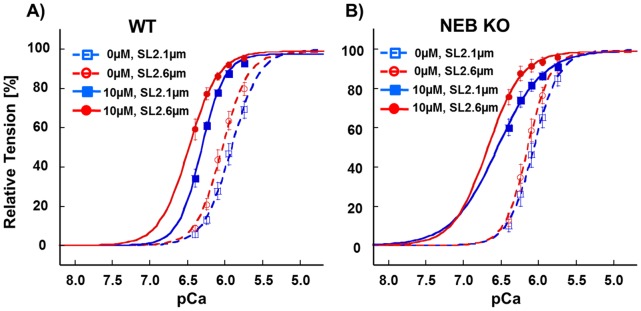
Result in WT in A and in NEB KO fibers in B. Increasing SL slightly shifts the curves left-ward (i.e., calcium sensitivity is increased) and CK results in an additional large shift. Results from six WT and six KO mice.
Figure 6. SL-induced ΔpCa50 at 0 and 10 µM CK-2066260 and CK 2066260-induced ΔpCa50 at SL 2.1 µm and SL 2.6 µm.
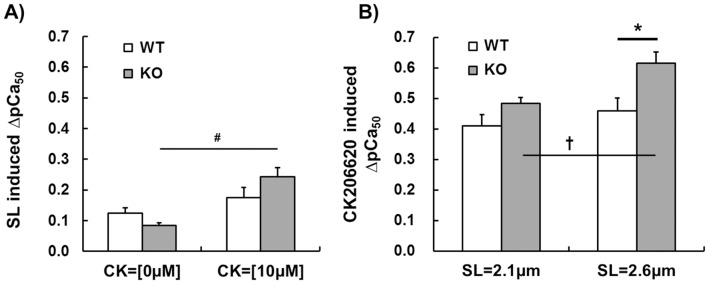
A) Effect of increasing SL from 2.1 to 2.6 µm on calcium sensitivity (SL-induced ΔpCa50) without and with 10 µM CK 2066260. In KO fibers CK-2066260 increased the SL-induced ΔpCa50 (#, p<0.05). B) CK-2066260 (10 µM) induced ΔpCa50 at SL 2.1 µm and 2.6 µm. Only in KO fibers CK 2066260-induced ΔpCa50 was significantly greater at SL 2.6 µm compared to 2.1 µm (†, p<0.05). Furthermore, at SL 2.6 µm CK 2066260-induced ΔpCa50 in KO fibers was significantly greater than in WT (*, p<0.05). Results from six WT and six KO mice.
We also determined the length-dependence of the CK-2066260 effect on calcium sensitivity by determining the ΔpCa50 (pCa50 in 10 µM CK-2066260 minus pCa50 in 0 µM CK-2066260, see shift between same color broken and solid curves in Figure 5). The results (Fig. 6B) reveal ΔpCa50 values of 0.4–0.6 pCa units, which is several times larger than the values obtained by increasing SL (compare Figure 6B with Figure 6A). Thus compared to SL increase, CK-2066260 is a much more efficacious calcium sensitizer. Furthermore, CK-2066260 at a SL of 2.6 µm increases calcium sensitivity to a significantly higher degree in KO fibers than in WT fibers and the CK-2066260 effect on KO fibers is significantly larger at long SL than at short SL (Fig. 6B).
Tmax was significantly increased by CK-2066260 at the long SL in both genotypes (WT: 58.7±6.0 to 66.7±6.4 mN/mm2; KO: 34.0±5.7 to 44.5±8.0 mN/mm2 (see Table 1, near bottom). Finally, we also determined the cooperatively of activation (nH) at SL 2.6 µm and found that CK-2066260 did not affect nH of WT fibers but that nH was reduced in KO fibers (Table 1, bottom two rows).
Discussion
Compromised neural input and excitation-contraction coupling is well-known to underlie muscle weakness in many skeletal muscle diseases. However, reduced force production can also result from alterations in sarcomeric proteins [29]. A prime example is nemaline myopathy (NM), the most common non-dystrophic congenital skeletal muscle myopathy [30] that is caused by mutations in six genes that all encode thin filament proteins (TMP2 [31], TMP3 [32], NEB [33], ACTA1 [34], TNNT1 [35], CFL2 [36]). Of these NM genes NEB is most important as it accounts for ∼50 percent of NM cases [33]. Treatment options for NM currently do not exist and gene therapy is still far away. Considering that a hallmark feature of NM is muscle weakness [37] we studied whether the fast skeletal muscle troponin activator CK-2066260 can restore muscle strength in skinned fast skeletal muscle fiber bundles from wildtype and nebulin deficient mice. In both genotypes CK-2066260 causes a large increase in calcium sensitivity of force development with a ∼8-fold increase in force at low activating calcium levels.
CK-2066260 is structurally and functionally closely related to the fast skeletal muscle troponin activator CK-2017357 that is currently in phase II clinical trials for Amyotrophic Lateral Sclerosis [19]. CK-2017357 slows the dissociation rate of calcium from troponin thereby stabilizing the open-conformation of the troponin/tropomyosin complex and enhancing crossbridge formation at a given calcium concentration [19]; the analogue CK-2066260 acts similarly (data not shown). In the present study, we observed that CK-2066260 greatly increases the calcium-sensitivity of force generation with an increase in pCa50 of 0.4–0.6 pCa units (Fig. 6B). A large effect is already seen at low doses with a 2 µM dose nearly doubling force at a pCa of 6.25 (Fig. 2B). This effect of CK-2066260 is similar to that of CK-2017357 in human and rabbit fast skeletal muscle fibers [19], indicating that this class of troponin activators is effective in a wide range of species.
Nebulin deficient muscle fibers produced active tensions that were much less than in WT muscle; for example the deficit in Tmax (maximal active tension at pCa 4.5) was ∼60% at a SL of 2.1 µm (Table 1), which is consistent with earlier findings [2]. Nebulin deficiency results in thin filaments that are on average shorter than in WT muscle [3], [4]; however, it is unlikely that this explains the large tension deficit because the effect of the shorter thin filament length on active tension is minimal at the SL of 2.1 µm where KO fibers reach their optimal sarcomere length and WT fibers are on the ascending limb of the force-sarcomere length relation [2]. An additional known effect of nebulin deficiency that impacts Tmax is an increase in gapp (apparent rate constant with which force generating crossbridges detach) and a decrease in fapp (apparent rate constant with which crossbridges enter the force generating state) with the net effect being a reduction in the fraction of crossbridges that generate force [2], [7], [18]. These earlier conclusions were based on an increased tension cost (which reflects gapp) and a reduction in ktr in NEB KO fibers (ktr represents the sum of fapp and gapp [22]) and a similar ktr reduction was found in the present study (Fig. 4). We consider it likely therefore that the tension deficit of nebulin KO fibers in the present study has a large contribution from altered crossbridge cycling kinetics.
We found that ktr increases with activation level (Fig. 4B and C), consistent with the work of others [38]–[40]. This Ca2+-dependence of ktr is likely due to complex kinetic interactions between crossbridge cycling and Ca2+-dependent thin-filament dynamics that increase fapp as sub-maximal calcium levels are increased [22], [40]. TnC likely plays an important role in force kinetics since previous studies have provided evidence that TnC mutants with a slower Ca2+ dissociation increase ktr at submaximal activation levels [40], [41]. Similarly, we found that CK-2066260 causes a pronounced increase in ktr (Fig. 4), consistent with the slowing of Ca2+ dissociation from TnC caused by skeletal muscle troponin activators [19]. We propose that the greater calcium sensitivity of the thin filament induced by CK-2066260 increases ktr because of an increase in fapp. Considering that force is proportional to fapp/(fapp+gapp) (the fraction of cycling crossbridges that develops force [22]), the effect of CK-2066260 will not only be a more rapid rise in force but also an increase in peak force at submaximal activation levels. Thus, both effects are consequences of the increase in calcium sensitivity of the sarcomere caused by CK-2066260.
Our finding that CK-2066260 increased Tmax at SL 2.6 µm (13% in the WT muscle fibers and 30% in the NEB KO muscle fibers) was unexpected considering that the effect of the activator is thought to be an increase in calcium sensitivity only. The increase in Tmax suggests that a saturating level of calcium does not cause a maximal number of crossbridges to attach and develop force. It has been reported that even a maximal tetanic contraction in normal healthy muscle has only ∼40% of the available crossbridges attached [34]. If underlying the CK-2066260 induced increase in ktr at saturating calcium levels (Fig. 4B–D) is solely an increase in fapp, then an increase in Tmax would be expected. A reduction in the number of force generating crossbridges was previously reported in nebulin-deficient muscle both in the mouse KO model and in NM patients [2]; this finding might underlie the larger effect of CK-2066260 at maximal activation on both ktr (increase 9% in WT and 34% in KO) and Tmax (increase 13% in WT and 30% in KO). Finally, the finding that the coefficient of cooperativity (nH) is reduced (at 10 µM CK-2066260) in only the NEB KO fibers (Table 1) suggests that the absence of nebulin alters the CK-2066260-thin filament interactions and its downstream effects.
The conclusion that absence of nebulin alters the effect of CK-2066260 on the thin filament is also supported by the length dependency of the CK-2066260 effect on calcium sensitivity. It is well known that increasing SL causes an increase in calcium sensitivity [42], a phenomenon that is especially prominent in cardiac muscle (where it underlies the Frank-Starling mechanism of the heart) but it also occurs in skeletal muscle [43]. In the present study, an increase in SL caused a left-shift in the force-pCa relation of ∼0.1 pCa units, with no difference between the genotypes (Fig. 6A). Although calcium sensitivity is already increased at the long SL, CK-2066260 causes a further increase and this increase exceeds the one at short length (Fig. 6B). At the long SL (2.6 µm) CK-2066260 had a larger effect in NEB KO fibers than in WT fibers (Fig. 6B) which suggests that nebulin deficiency sensitizes troponin to CK-2066260. Although additional studies are required to fully understand the mechanistic basis of these findings, our results establish that CK-2066260 is a potent activator of fast skeletal muscle, especially of nebulin deficient muscle.
In summary, we investigated the effect of the fast skeletal muscle troponin activator CK-2066260 on force development in fast skeletal muscle of wildtype and NEB KO mice. CK-2066260 was found to greatly increase force development at submaximal activation levels. Considering that most muscles during normal activity operate in the submaximal activation regime [44] the beneficial effect of CK-2066260 on in vivo muscle force may be substantial. It is also noteworthy that the class of troponin activators to which CK-206620 belongs is specific to fast skeletal troponin and does not affect cardiac muscle [19], which is an important consideration in terms of therapeutic index. The beneficial effect of CK-2066260 on fast skeletal muscle can be well visualized by our finding that at pCa levels above ∼6.0 (i.e., calcium levels <1 µM) CK-2066260 increases the tension of NEB KO fibers to beyond that of WT fibers (compare red and green solid curves in Fig. 3A with blue broken line). We conclude that fast skeletal troponin activators may ameliorate muscle weakness in NM and other patients with compromised muscle strength.
Supporting Information
Effect of 10 µM CK-2066260 on tension-pCa curves in WT (dashed) and NEB KO fibers (solid) with a randomized activation protocol (see text for details). Regardless of genotype, tension-pCa curves were shifted to left with addition of CK-2066260 (blue-0 µM of CK, red-10 µM of CK). Results from 6 WT and 6 KO mice. The change in pCa50 between 0 and 10 mM CK-2066260 (ΔpCa50) obtained with the ‘randomize’d protocol is 0.46 pCa units, which is the same as that obtained with the protocol in which calcium in progressively increased (as in Fig. 2), see inset.
(TIF)
Funding Statement
Generous support by ‘A Foundation for Building Strength in Nemaline Myopathy’ is acknowledged. Additional support from NIH AR05387 (HG), SarcoSI and NEMMYOP(SL and CACO) and a VIDI grant (016.126.319) to CACO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Huxley HE (2004) Fifty years of muscle and the sliding filament hypothesis. Eur J Biochem 271: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 2. Chandra M, Mamidi R, Ford S, Hidalgo C, Witt C, et al. (2009) Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J Biol Chem 284: 30889–30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, et al. (2006) Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J 25: 3843–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bang ML, Li X, Littlefield R, Bremner S, Thor A, et al. (2006) Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol 173: 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ottenheijm CA, Witt CC, Stienen GJ, Labeit S, Beggs AH, et al. (2009) Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet 18: 2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ottenheijm CA, Hooijman P, DeChene ET, Stienen GJ, Beggs AH, et al. (2010) Altered myofilament function depresses force generation in patients with nebulin-based nemaline myopathy (NEM2). J Struct Biol 170: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawlor MW, Ottenheijm CA, Lehtokari VL, Cho K, Pelin K, et al. (2011) Novel mutations in NEB cause abnormal nebulin expression and markedly impaired muscle force generation in severe nemaline myopathy. Skelet Muscle 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kruger M, Wright J, Wang K (1991) Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: correlation of thin filament length, nebulin size, and epitope profile. J Cell Biol 115: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright J, Huang QQ, Wang K (1993) Nebulin is a full-length template of actin filaments in the skeletal muscle sarcomere: an immunoelectron microscopic study of its orientation and span with site-specific monoclonal antibodies. J Muscle Res Cell Motil 14: 476–483. [DOI] [PubMed] [Google Scholar]
- 10. Pappas CT, Krieg PA, Gregorio CC (2010) Nebulin regulates actin filament lengths by a stabilization mechanism. J Cell Biol 189: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castillo A, Nowak R, Littlefield KP, Fowler VM, Littlefield RS (2009) A nebulin ruler does not dictate thin filament lengths. Biophys J 96: 1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Labeit S, Kolmerer B (1995) The complete primary structure of human nebulin and its correlation to muscle structure. J Mol Biol 248: 308–315. [DOI] [PubMed] [Google Scholar]
- 13. Kazmierski ST, Antin PB, Witt CC, Huebner N, McElhinny AS, et al. (2003) The complete mouse nebulin gene sequence and the identification of cardiac nebulin. J Mol Biol 328: 835–846. [DOI] [PubMed] [Google Scholar]
- 14. Jin JP, Wang K (1991) Cloning, expression, and protein interaction of human nebulin fragments composed of varying numbers of sequence modules. J Biol Chem 266: 21215–21223. [PubMed] [Google Scholar]
- 15. Jin JP, Wang K (1991) Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett 281: 93–96. [DOI] [PubMed] [Google Scholar]
- 16. Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, et al. (2010) Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science 330: 1536–1540. [DOI] [PubMed] [Google Scholar]
- 17. Granzier HL, Akster HA, Ter Keurs HE (1991) Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol 260: C1060–1070. [DOI] [PubMed] [Google Scholar]
- 18. Bang ML, Caremani M, Brunello E, Littlefield R, Lieber RL, et al. (2009) Nebulin plays a direct role in promoting strong actin-myosin interactions. FASEB J 23: 4117–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, et al. (2012) Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med 18: 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muhle-Goll C, Habeck M, Cazorla O, Nilges M, Labeit S, et al. (2001) Structural and functional studies of titin’s fn3 modules reveal conserved surface patterns and binding to myosin S1–a possible role in the Frank-Starling mechanism of the heart. J Mol Biol 313: 431–447. [DOI] [PubMed] [Google Scholar]
- 21. Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505. [PubMed] [Google Scholar]
- 22. Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A 85: 3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang SM, Greaser ML (1985) Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J Muscle Res Cell Motil 6: 293–312. [DOI] [PubMed] [Google Scholar]
- 24. Wang SM, Greaser ML, Schultz E, Bulinski JC, Lin JJ, et al. (1988) Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol 107: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, et al. (2006) Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warren CM, Krzesinski PR, Greaser ML (2003) Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 27. Lahmers S, Wu Y, Call DR, Labeit S, Granzier H (2004) Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res 94: 505–513. [DOI] [PubMed] [Google Scholar]
- 28. Konhilas JP, Irving TC, de Tombe PP (2002) Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch 445: 305–310. [DOI] [PubMed] [Google Scholar]
- 29. Laing NG, Nowak KJ (2005) When contractile proteins go bad: the sarcomere and skeletal muscle disease. Bioessays 27: 809–822. [DOI] [PubMed] [Google Scholar]
- 30. Sanoudou D, Beggs AH (2001) Clinical and genetic heterogeneity in nemaline myopathy–a disease of skeletal muscle thin filaments. Trends Mol Med 7: 362–368. [DOI] [PubMed] [Google Scholar]
- 31. Donner K, Ollikainen M, Ridanpaa M, Christen HJ, Goebel HH, et al. (2002) Mutations in the beta-tropomyosin (TPM2) gene–a rare cause of nemaline myopathy. Neuromuscul Disord 12: 151–158. [DOI] [PubMed] [Google Scholar]
- 32. Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, et al. (1995) A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy NEM1. Nat Genet 10: 249. [DOI] [PubMed] [Google Scholar]
- 33. Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, et al. (1999) Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A 96: 2305–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nowak KJ, Wattanasirichaigoon D, Goebel HH, Wilce M, Pelin K, et al. (1999) Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 23: 208–212. [DOI] [PubMed] [Google Scholar]
- 35. Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, et al. (2000) A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 67: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agrawal PB, Greenleaf RS, Tomczak KK, Lehtokari VL, Wallgren-Pettersson C, et al. (2007) Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am J Hum Genet 80: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. North KN, Laing NG, Wallgren-Pettersson C (1997) Nemaline myopathy: current concepts. The ENMC International Consortium and Nemaline Myopathy. J Med Genet 34: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Metzger JM, Greaser ML, Moss RL (1989) Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol 93: 855–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Metzger JM, Moss RL (1990) Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 247: 1088–1090. [DOI] [PubMed] [Google Scholar]
- 40. Moreno-Gonzalez A, Gillis TE, Rivera AJ, Chase PB, Martyn DA, et al. (2007) Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres. J Physiol 579: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Regnier M, Martyn DA, Chase PB (1996) Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophys J 71: 2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI (1986) Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res 58: 755–768. [DOI] [PubMed] [Google Scholar]
- 43. Konhilas JP, Irving TC, de Tombe PP (2002) Length-dependent activation in three striated muscle types of the rat. J Physiol 544: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jasmin BJ, Gardiner PF (1987) Patterns of EMG activity of rat plantaris muscle during swimming and other locomotor activities. J Appl Physiol 63: 713–718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of 10 µM CK-2066260 on tension-pCa curves in WT (dashed) and NEB KO fibers (solid) with a randomized activation protocol (see text for details). Regardless of genotype, tension-pCa curves were shifted to left with addition of CK-2066260 (blue-0 µM of CK, red-10 µM of CK). Results from 6 WT and 6 KO mice. The change in pCa50 between 0 and 10 mM CK-2066260 (ΔpCa50) obtained with the ‘randomize’d protocol is 0.46 pCa units, which is the same as that obtained with the protocol in which calcium in progressively increased (as in Fig. 2), see inset.
(TIF)