Abstract
A major class of disease resistance (R) genes which encode nucleotide binding and leucine rich repeat (NB-LRR) proteins have been used in traditional breeding programs for crop protection. However, it has been difficult to functionally transfer NB-LRR-type R genes in taxonomically distinct families. Here we demonstrate that a pair of Arabidopsis (Brassicaceae) NB-LRR-type R genes, RPS4 and RRS1, properly function in two other Brassicaceae, Brassica rapa and Brassica napus, but also in two Solanaceae, Nicotiana benthamiana and tomato (Solanum lycopersicum). The solanaceous plants transformed with RPS4/RRS1 confer bacterial effector-specific immunity responses. Furthermore, RPS4 and RRS1, which confer resistance to a fungal pathogen Colletotrichum higginsianum in Brassicaceae, also protect against Colletotrichum orbiculare in cucumber (Cucurbitaceae). Importantly, RPS4/RRS1 transgenic plants show no autoimmune phenotypes, indicating that the NB-LRR proteins are tightly regulated. The successful transfer of two R genes at the family level implies that the downstream components of R genes are highly conserved. The functional interfamily transfer of R genes can be a powerful strategy for providing resistance to a broad range of pathogens.
Introduction
Plants trigger innate immune responses to pathogens via a two-layer surveillance system composed of pattern recognition receptors (PRRs) and nucleotide binding-leucine rich repeat (NB-LRR) proteins that are encoded by resistance (R) genes [1]. PRRs recognize microbe-associated molecular patterns (MAMPs) at an plasma membrane, and NB-LRR proteins subsequently detect pathogen-derived effectors inside the cell. The two layers are often called MAMPs-triggered Immunity (MTI) and effector-triggered immunity (ETI) [2]. Because MAMPs are conserved across a wide range of microbes, transfer of PRRs could confer resistance to a broad range of plant pathogens [3]. Likewise, some effectors are conserved within groups, so the transfer of NB-LRR proteins could confer resistance to phytopathogens carrying the common effectors. Furthermore, NB-LRR-based ETI is generally much stronger than PRR-mediated MTI, and thus NB-LRR transfer could be a powerful tool for disease control. R genes that confer NB-LRR-based ETI have been introgressed from wild relatives into susceptible varieties of the same species in traditional breeding programs. However, it has been difficult to transfer NB-LRR-type R genes to confer disease resistance in a different family [4]. Heterologous expression of NB-LRR-type R genes in a taxonomically distinct family often triggers either no response or inappropriate autoimmunity responses, suggesting that the regulatory or signaling components associated with NB-LRR protein-based resistance are potentially family specific [5]. There are, however, examples in which transient expression of particular R genes triggers correspoonding effetor dependent responses [6]. To date, stable transformaion with NB-LRR-type R genes in different families to confer actual resistance to pathogens has not been reported.
In a previous study we demonstrated that Arabidopsis thaliana (A. thaliana) (Brassicaceae) NB-LRR-type R genes RPS4 and RRS1 function together to confer resistance against multiple pathogen isolates [7], i.e., the fungal pathogen Colletotrichum higginsianum (C. higginsianum), and two taxonomically distinct bacteria, Pseudomonas syringae pv. tomato DC3000 carrying the effector AvrRps4 (Pst-avrRps4) [8] and Ralstonia solanacearum (R. solanacearum) strains, which express the PopP2 effector [9]. RPS4 and RRS1 are encoded in a head-to-head configuration within the Arabidopsis genome (Figure 1). As the two open reading frames are only 264 bp apart, the promoter regions of the gene pairs likely overlap, leading to the co-regulation [7], [10]. RPS4 encodes an NB-LRR with a TIR domain at the N terminus (TIR-NB-LRR), whereas RRS1 encodes a TIR-NB-LRR with a WRKY domain at the C-terminus. The precise mechanism of how RPS4 functions with RRS1 is not clear.
Figure 1. Schematic diagram of RPS4 and RRS1.
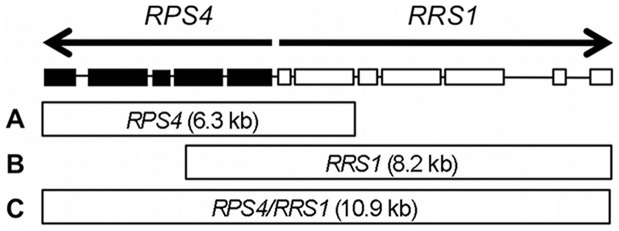
RPS4 and RRS1 genes are arranged in a head-to-head configuration in A. thaliana ecotype Wassilewskija chromosome V. Because the two open reading frames are only 264 bp apart, the promoter regions are likely to be overlapping, leading to co-regulation of the genes. Exons of RPS4 and RRS1 are indicated by black and white boxes, respectively. Intron positions are indicated as lines. (A) The 6.3 kbp genomic RPS4 fragment, including approximately 2.1 kbp upstream and 109 bp downstream regions, (B) The 8.2 kbp genomic RRS1 fragment, including approximately 1.8 kbp upstream and 176 bp downstream regions, (C) The 10.9 kbp genomic fragment containing both RPS4 and RRS1.
The goal of this study is to determine whether the dual R genes, RPS4 and RRS1, function as a cassette and therefore could confer disease resistance to different plant species. We show that a genomic fragment containing RPS4 and RRS1 under the control of their native promoters confers resistance to fungal and bacterial pathogens in the Brassicaceae, Solanaceae and Cucurbitaceae plants. We also demonstrate that the dual R gene transgenic plants do not trigger inappropriate auto-immunity responses. These results indicate that the dual R gene pair can confer multiple pathogen resistance in taxonomically distinct families.
Results
RPS4 and RRS1 Confer Resistance to C. higginsianum in Transgenic Brassica Rapa and Brassica Napus
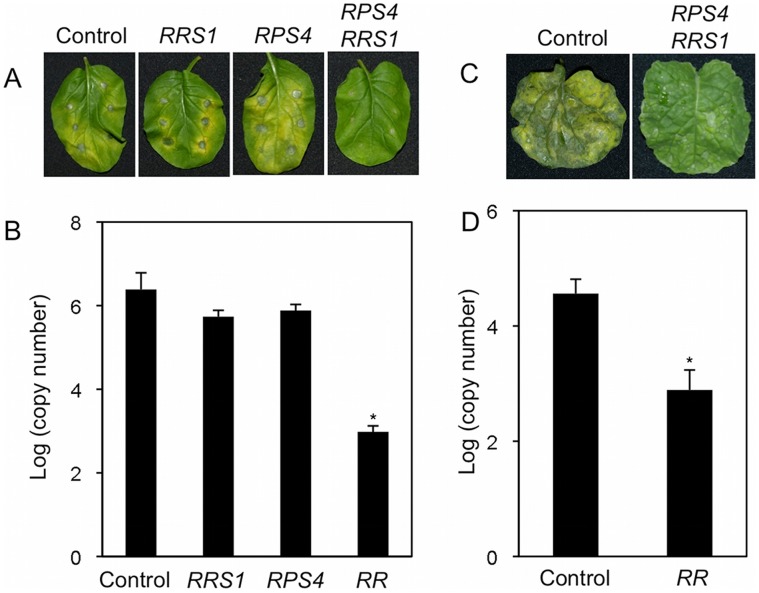
Colletotrichum sp. are ascomycete fungi causing anthracnose diseases on a large number of agronomically important crops and vegetables. C. higginsianum causes anthracnose disease in Brassicaceae plants. To investigate whether the RPS4 and RRS1 pair functions in other Brassicaceae plants, we generated transgenic Brassica rapa (B. rapa) L. Perviridis Group (Japanese Mustard Spinach, Komatsuna) and Brassica napus (B. napus) L. plants expressing either RPS4 or RRS1, and RPS4 and RRS1 together under the control of the native promoter (Figure 1). Agrobacterium-mediated transformation produced four B. rapa primary transformants (T1) carrying both RPS4 (6.3 kbp) and RRS1 (8.2 kbp) genomic fragments under control of the native Arabidopsis promoters. Seventeen B. rapa T1 plants carrying only RRS1 genomic fragment, but only one for RPS4 were obtained. We also obtained four B. napus T1 plants carrying a 10.9 kbp genomic fragment containing RPS4 and RRS1. Secondary transformants (T2) derived from bud pollination of B. rapa and selfing B. napus T1 plants were assessed for the presence of the transgenes by PCR (Figure S1) (data not shown). Expression of the transgenes in T2 plants was confirmed by qRT-PCR (Figure S2). When inoculated with C. higginsianum, wild-type (WT) B. rapa and B. napus plants developed the brown necrotic lesions typical of anthracnose. B. rapa and B. napus plants transformed with the RPS4 and RRS1 pair were highly resistant to the pathogen, developing only small necrotic flecks at the inoculated sites (Figure 2A–D). However, transgenic B. rapa plants expressing either RPS4 or RRS1 alone were as susceptible to C. higginsianum as WT (Figure 2A, B). These results indicate that RPS4 and RRS1 are both needed to confer resistance to the pathogen in Brassicaceae. Transgenic plants expressing RPS4 and RRS1 grew normally and did not constitutively express inducible defense gene PR1, suggesting that no autoimmunity response was induced by introducing the R genes (Figure S3A, B & S4).
Figure 2. Colletotrichum higginsianum resistance analysis in transgenic Brassica plants expressing RPS4 and RRS1.
(A) Infection phenotypes of B. rapa plants leaves inoculated with C. higginsianum. Four, three, and one independent T2 transgenic B. rapa lines carrying both RPS4 and RRS1 (RR), either RRS1 or RPS4 alone, respectively, were tested. Mature leaves of 2.5 true leaf stage seedlings were inoculated with spotting 5 µl of a conidial suspension of C. higginsianum (5×105 spores ml−1) on the leaf. Photographs were taken at 6 dpi. Each picture shows a representative of three independent experiments. (B) Quantification of C. higginsianum in planta by qRT-PCR. Mature leaves of 2.5 true leaf stage seedlings were spray-inoculated with a conidial suspension of C. higginsianum (5×105 spores ml−1). Inoculated B. rapa leaves were harvested at 4 dpi, and total RNA was isolated. QRT-PCR was performed with C. higginsianum actin (Chin-ACT) primers for each sample. (C) Infection phenotypes of B. napus plants leaves inoculated with C. higginsianum. Four independent T2 transgenic lines carrying both RPS4 and RRS1 (RR) were tested. Mature leaves of 2.5 true leaf stage seedlings were spray-inoculated with a conidial suspension of C. higginsianum (5×105 spores ml−1). Photographs were taken at 6 dpi. Each picture shows a representative of three independent experiments. (D) Quantification of C. higginsianum in planta by qRT-PCR. Inoculated B. napus leaves were harvested at 4 dpi, and total RNA was isolated. QRT-PCR was performed with Chin-ACT primers for each sample. Bars indicate SE. The asterisks indicate statistical significance from the WT controls (Dunnett’s method, P<0.05). This experiment was repeated three times with similar results.
Nicotiana benthamiana Plants Expressing RPS4 and RRS1 Recognize Bacterial Effectors AvrRps4 and PopP2
Because RPS4 and RRS1 function properly together in three different Brassicaceae plant species, we thought it is possible that this R gene pair would be able to break the restricted taxonomic functionality boundary when expressed in non-Brassicaceae plants. We generated transgenic Nicotiana benthamiana (N. benthamiana) (Solanaceae) plants expressing RPS4 and RRS1 under the control of their cognate promoters. We obtained thirteen N. benthamiana T1 plants carrying a 10.9 kbp genomic fragment containing RPS4 and RRS1 and two with only RPS4 and one with only RRS1. T2 and T3 progenies derived from selfing T1 and T2 plants, respectively, were assessed for the presence of the transgenes by PCR (Figure S1) and T3 homozygous lines were used in all assays described here. Expression of the transgenes in T3 plants was confirmed by qRT-PCR (Figure S2). To test functionality of the R genes, we used AvrRps4 or PopP2 effectors which are specifically recognized by the RPS4 and RRS1 pair in Arabidopsis. We found that AvrRps4 or PopP2 produced by Agrobacterium-mediated transient expression under control of a cauliflower mosaic virus (CaMV) 35S constitutive promoter induced cell death in N. benthamiana transformed with RPS4 and RRS1, but not in plants expressing only RPS4 or RRS1, indicating that the R gene pair is able to recognizes the AvrRps4 or PopP2 effectors in a non-Brassicaceae plant (Figure 3). We verified by qRT-PCR quantification that mRNAs for avrRps4 and popP2 accumulated to similar levels in transgenic plants. (Figure S5). No inappropriate autoimmune responses were induced by the introduced R gene pair in transgenic N. benthamiana plants (Figure S3C & S4).
Figure 3. Transient expression assay in Nicotiana benthamiana transformed with RPS4 and/or RRS1.
Transient expression assay of avrRps4 or popP2 was performed by Agrobacterium infiltration in four-week-old T3 homozygous transgenic N. benthamiana leaves expressing RPS4 and/or RRS1. Two, one, and two independent T2 transgenic lines carrying both RPS4 and RRS1, either RRS1 or RPS4 alone, respectively, were tested. Photographs were taken at 10 dpi.
Transgenic Tomato Plants Expressing RPS4 and RRS1 Confer Resistance to Two Taxonomically Distinct Bacteria
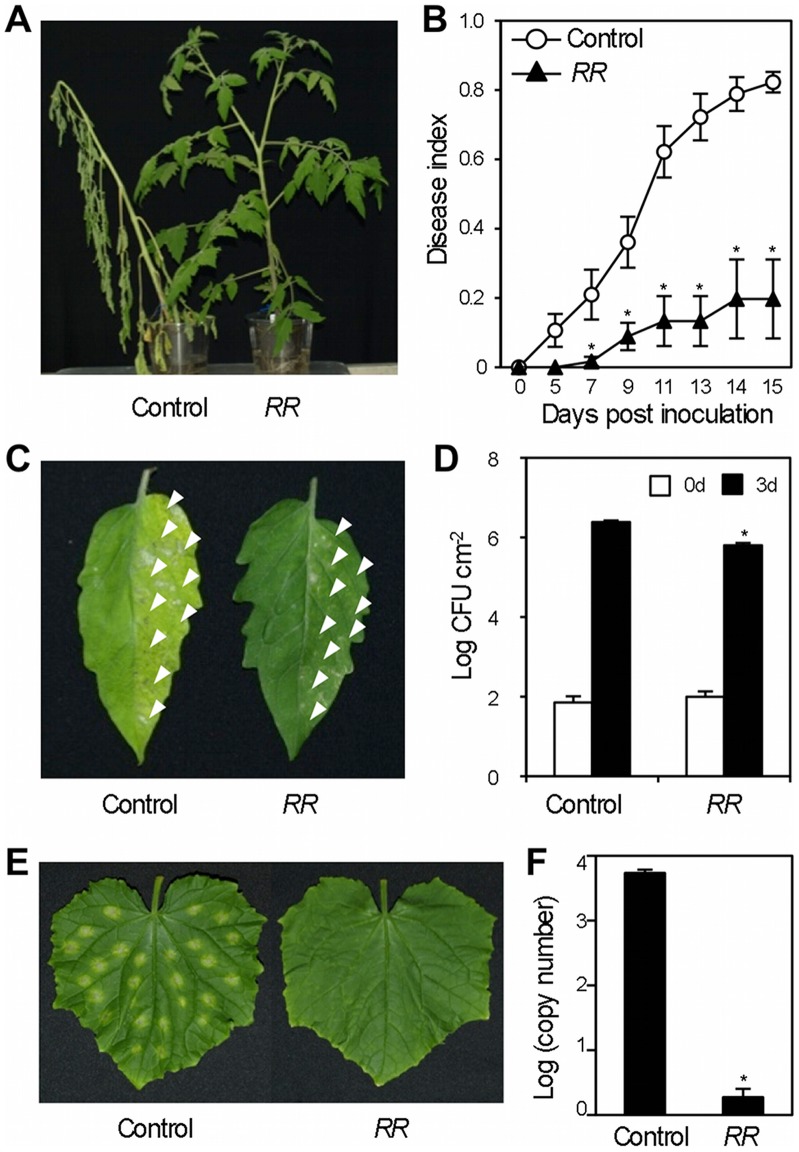
To test whether the RPS4/RRS1 pair confer immunity in another solanaceous plant, we generated transgenic tomato plants. Seven transgenic tomato T1 plants carrying a 10.9 kbp genomic fragment containing RPS4 and RRS1 were obtained and shown to contain these transgenes by PCR. T2 progenies derived from selfing T1 plants were assessed for the presence of the transgenes by PCR (Figure S1). Expression of the transgenes in T2 plants was confirmed by qRT-PCR (Figure S2). The bacterial wilt phytopathogen R. solanacearum is a serious soilborne disease that attacks almost 200 plant species in 33 plant families, including Solanaceae. Tomato plants (Solanum lycopersicum) transformed with RPS4 and RRS1 were resistant to R. solanacearum expressing popP2 (Figure 4A, B), but susceptible to R. solanacearum without popP2, indicating that the conferred resistance is specific for the PopP2 effector (Figure S6A). Pseudomonas syringae pv. tomato DC3000 (Pst) causes bacterial speck on tomato, a disease characterized by defoliation, blossom blight, and lesions on developing fruit. Transgenic tomato plants were resistant to Pst-avrRps4 (Figure 4C, D) but not to Pst containing a vector control (Figure S6B), indicating that conferred resistance is specific for the AvrRps4 effector. In addition, two independent T2 progenies segregated 3∶1 for resistance versus susceptibility to R. solanacearum expressing popP2 and Pst-avrRps4. These transgenic plants grew normally and showed no significant constitutive expression of inducible defense-related gene PR1 (Figure S3D & S4).
Figure 4. Transformation with RPS4/RRS1 breaks restricted taxonomic functionality.
(A,B) R. solanacearum resistance analysis in RPS4/RRS1 dual R gene-transformed tomato (RR) and control plants. Two independent T2 transgenic lines carrying both RPS4 and RRS1 have been tested. Six-week-old tomato plants were inoculated with R. solanacearum expressing popP2. (A) Disease symptoms on tomato plants inoculated with R. solanacearum expressing popP2 at 15 dpi. (B) Plants were rated every other day on a 0 to 5 disease index scale from 0 (no visible wilt) to 5 (the whole plant is dead). Each point represents the mean disease index (± SE) for three independent experiments, each containing 5 to 10 plants per treatment. The asterisks indicate statistical significance from the controls (Dunnett’s method, P<0.05). The control plants (vector control) wilted after inoculation with R. solanacearum expressing popP2, while RPS4/RRS1 transformed plants (RR) were resistant. (C,D) Infection assays with Pseudomonas syringae pv. tomato DC3000 carrying the effector AvrRps4 (Pst-avrRps4) in RPS4/RRS1 dual R gene-transformed tomato (RR) and control plants. Two independent T2 transgenic lines carrying both RPS4 and RRS1 were tested. The right sides of leaves of six-week-old tomato plants were infiltrated with bacterial suspensions (5×104 cfu ml−1). There were 10–12 inoculation sites per leaf. Inoculation sites indicated by arrowheads. (C) Disease symptoms on tomato leaves inoculated with Pst-avrRps4 at 7 dpi. (D) Leaves were harvested at 0 and 3 dpi. Leaves infected with Pst-avrRps4 developed chlorotic lesions at 3 dpi. Bars indicate SE (n = 6). The asterisk indicates statistical significance from the control (3d) (Dunnett’s method, P<0.05). The experiment was repeated at least two times with similar results. (E,F) Colletotrichum orbiculare resistance in RPS4/RRS1 dual R gene-transgenic cucumber (RR) and control plants. Four independent T2 transgenic lines carrying both RPS4 and RRS1 were tested. (E) Mature leaves of 2.5 true leaf stage seedlings were inoculated with spotting 5 µl of a conidial suspension of C. orbiculare (5×105 spores ml−1) on the leaf. Photographs were taken at 6 dpi. (F) Mature leaves of 2.5 true leaf stage seedlings were spray-inoculated with a conidial suspension of C. orbiculare (5×105 spores ml−1). Pathogen growth was determined by measuring C. orbiculare-actin mRNA by qRT-PCR. Bars indicate SE. The asterisk indicates statistical significance from the control (Dunnett’s method, P<0.05). The experiment was repeated at least two times with similar results.
Transgenic Cucumber Plants Expressing RPS4 and RRS1 are Resistant to Colletotrichum orbiculare
To investigate if the RPS4/RRS1 pair confers broad-range resistance, we tested Colletotrichum orbiculare (C. orbiculare) on cucumber (Cucumis sativus; Cucurbitaceae) (C. sativus). Six transgenic cucumber T1 plants carrying a 10.9 kbp genomic fragment containing RPS4 and RRS1 were obtained. T2 progenies derived from selfing T1 plants were assessed for the presence of the transgenes by PCR (Figure S1). Expression of the transgenes in T2 plants was confirmed by qRT-PCR (Figure S2). Wild type cucumber plants developed brown necrotic lesions surrounded by a yellow halo, a typical symptom of anthracnose disease (Figure 4E). In contrast, RPS4/RRS1 cucumber plants were highly resistant, developing only small necrotic flecks at the inoculated sites, indicative of an active defense reaction (Figure 4E, F). In addition, four independent T2 cucumber progenies segregated 3∶1 for resistance versus susceptibility to C. orbiculare. As in N. benthamiana and tomato, introduction of the RPS4/RRS1 pair did not induce autoimmunity, indicating that RPS4 and RRS1 are tightly regulated in cucumber (Figure S3E & S4). RPS4/RRS1 thus confers resistance to Colletotrichum sp. in taxonomically distinct families.
Discussion
We demonstrate here that the RPS4/RRS1 R gene pair from Arabidopsis functions in other Brassicaceae plants, B. rapa and B. napus, as well as in N. benthamiana, tomato and cucumber. This provides that transfer of NB-LRR-type R genes confers resistance to multiple pathogens in taxonomically distinct families. The transfer of either RPS4 or RRS1 failed to provide resistance in transgenic plants (Figure 2A, B & 3), thus the success of interfamily transfer is likely due to the “dual R” system.
The number of known pairs of R genes is increasing [11]. For example, the R gene pairs, RPP2A/RPP2 [12], N/NRG1 [13], RPM1/TAO1 [14], Lr10/RGA2 [15], Pi5-1/Pi5-2 [16], Pikm1-TS/Pikm2-TS [17], are only functional when both genes are present and we postulate that some of those pairs may also function in taxonomically distinct families when expressed together. It is also possible that some apparently singular R genes may require a complement to function. The mechanism of how these “dual R” proteins work together is unknown. However, one of the potential functions of the pair is as a negative regulator, as expression of single R genes often leads to inappropriate autoimmune responses in a taxonomically distinct family where no partner is available [18]. In this sense, expression of R proteins along with the corresponding proteins that are the target of pathogen effectors (often called ‘guardee’) may also be able to overcome restricted taxonomic functionality, since loss of the guardees often triggers R protein activation [1], [6].
Because RPS4 and RRS1 specifically react to bacterial effectors AvrRps4 and PopP2 in both Brassicaceae and Solanaceae plants, the recognition mechanism should be highly conserved across species, or built in the R protein pair itself. Recent reports indicate that AvrRps4 directly targets Enhanced Disease Susceptibility 1 (EDS1), but does not interact with RPS4 [19], [20]. EDS1 is a highly conserved key factor in TIR-NB-LRR-mediated ETI and interacts with several TIR-NB-LRR protiens, including RPS4 [21]. However, more recent report by Sohn et al. (2012) showed that AvrRps4 and EDS1 do not directly interacted [22]. Thus, EDS1 can be the direct or indirect target of AvrRps4 in both Brassicaceae and Solanaceae plants. If this is the case, RPS4 and RRS1 could confer broad resistance, as many pathogens would target EDS1 as the key immunity protein. Unlike the AvrRps4-RPS4/RRS1 example, PopP2, a YopJ-like family of cysteine proteases, directly binds to RRS1 [23]. As PopP2-dependent immune response requires RPS4 (Figure 3), it is possible that RPS4 can recognize the interaction between PopP2 and RRS1. In any case, PopP2 produced by Ralstonia could be detected in many plants by introgressing the RPS4/RRS1 cassette.
Perhaps the biggest surprise to us is that the RPS4/RRS1 pair confers immunity against both C. higginsianum and C. orbiculare in taxonomically distinct plant families. This implies that the target (i.e. host immunity-related proteins) of the unidentified Colletotrichum effectors is conserved in Brassicaceae and Cucurbitaceae, and/or that the effectors recognized by RPS4/RRS1 are highly conserved in Colletotrichum. As is the case for AvrRps4 and PopP2, Colletotrichum effectors may directly target EDS1 or RRS1. The sequenced Colletotrichum genomes do not contain apparent AvrRps4 and PopP2 homologs, suggesting that the targeting mechanism should be distinct from the bacterial targeting mechanism [24]. There are a number of potential highly conserved effectors in Colletotrichum spp., and some of them could be detected by the RPS4/RRS1 pair by an unknown mechanism. Isolation of the Colletotrichum effectors would resolve the problem. In any case, RPS4/RRS1 could provide resistance to Colletotrichum species among a number of agronomically important crops and vegetables.
Our study demonstrates that NB-LRR-type R gene-based immunity can be transferred to distantly related species once the right gene pair is identified. Each plant genome contains about 150 to 500 NB-LRR-type R genes [25], [26], some of which are likely to form pairs. As in the case of RPS4/RRS1, some gene pairs might provide a wide range resistance to multiple pathogens. Thus our finding provides a new strategy for creating pathogen-resistant vegetables and crops by a transgenic approach using previously unexploited resource of genetic resistance.
Materials and Methods
Plant Materials
Brassica rapa L. Perviridis Group (Japanese Mustard Spinach, Komatsuna cv. Osome, Takii & Co., Ltd., Kyoto, Japan), B. napus L. cv. Westar plants, tobacco (N. benthamiana), tomato (Solanum lycopersicum L. cultivar Moneymaker) and cucumber (C. sativus L. cultivar Shinhokusei No. 1) plants were grown in Soil-mix (Sakata Seed Corp., Yokohama, Japan) and expanded vermiculite (1.5–2 mm granules) at a ratio of 1∶1 in a growth chamber at 22°C with 12-h light for B. rapa, B. napus and cucumber, at 25°C with 16-h light for N. benthamiana, and at 25°C during the daylight hours for tomato.
Construction of the R Gene Plasmid
A 10.9 kbp genomic fragment containing RPS4 and RRS1 was amplified from A. thaliana ecotype Wassilewskija (Ws-0) genomic DNA by PCR using RR1 primers listed in Table S1, and cloned into pCR8GW-TOPO (Life Technologies, CA, USA). The resultant plasmid, pCR8GW-RR-Ws, was subcloned into the destination vector pBI-GW-NOS (Inplanta Innovations Inc., Yokohama, Japan) using the LR cloning reaction. The 6.3 kbp genomic RPS4 fragment, including approximately 2.1 kbp upstream and 109 bp downstream regions, was cloned into pBI101-SK+ [7]. The 8.2 kbp genomic RRS1 fragment, including approximately 1.8 kbp upstream and 176 bp downstream regions, was subcloned into the destination vector pGWB1 using the LR cloning reaction [7].
Transformation
B. rapa and B. napus were transformed by inoculating hypocotyl sections with Agrobacterium tumefaciens (A. tumefaciens) (Rhizobium radiobactor) strain EHA101 harboring the binary vector containing the fragments described above [27], [28]. N. benthamiana transformation was by the leaf disk method using A. tumefaciens strain LBA4404 harboring binary vector containing the fragments described above [29], [30]. Transformation of tomato was performed according to the cotyledon explant method using A. tumefaciens strain C58C1RifR (pGV2260) harboring the binary vector and genomic RPS4/RRS1 fragment described above [31]. Transformation of cucumber was by the cotyledon explant method using A. tumefaciens strain EHA105 harboring the binary vector with the genomic RPS4/RRS1 fragment described above [32], [33].
C. higginsianum Strain and Inoculations
C. higginsianum Saccardo isolates (MAFF305635) were obtained from the MAFF Genebank project, Japan. Mature leaves of 2.5 true leaf stage seedlings (12 h light cycle) were inoculated as described previously [7].
Quantification of C. higginsianum Actin mRNA
Plants inoculated with C. higginsianum were harvested at 4 d post inoculation (dpi) for qRT-PCR analysis. The primers for qRT-PCR are listed in Table S1. Quantification of C. higginsianum was performed as described previously [34]. QRT-PCR data for C. higginsianum actin (Chin-ACT) and Br-CBP20 expression from B. rapa, or Chin-ACT and B. napus actin (Bn-ACT) expression from B. napus were obtained from a standard curve of cycle times as a function of copy number. The abundance of Chin-ACT was normalized with Br-CBP20 or Bn-ACT in infected samples.
Transient Expression Assay in N. benthamiana
The avrRps4 and popP2 DNA fragments were amplified from Pst-avrRps4 and Ralstonia solanacearum strain RS1002 genomic DNA, respectively, by PCR using avrRps4 and popP2 primers listed in Table S1, and cloned into pCR8GW-TOPO. The pSfinx vector [35] was converted into a Gateway destination vector using the Gateway Vector Conversion System (Life Technologies). A Gateway Reading Frame Cassette B was introduced into the original cloning cassette of the pSfinx vector using the SmaI site. The avrRps4 and popP2 DNA fragments were cloned into the modified pSfinx vector using the LR cloning reaction (pSfinx-avrRps4 and pSfinx-popP2, respectively). N. benthamiana plants were grown in a growth chamber at 25°C with 16-h light. Plants at the age of four-week-old were used in the experiment. Overnight bacterial cultures of A. tumefaciens strain GV3101 (pMP90, pSoup) containing pSfinx-avrRps4 or pSfinx-popP2 were harvested by centrifugation. Cells were washed three times in induction buffer (10 mM MES, pH 5.6, 10 mM MgCl2 and 150 µM acetosyringone), re-suspended in induction buffer to an OD600 of 0.5, and incubated for 2 h at room temperature. Agrobacterium cells were hand-infiltrated into N. benthamiana leaves with a 1 ml syringe. Cell death responses began to appear on infiltrated leaves 5 d after infiltration.
Infection of Tomato with R. solanacearum
R. solanacearum strains RS1002 and RS1002-ΔpopP2 were described previously [7], [36]. R. solanacearum was streaked on tetrazolium medium (TTC) [37] and incubated at 28°C for 2 d. A single colony was grown in CPG liquid medium [38] at 28°C for 24 h. Bacterial cells were harvested and suspended with distilled water to an OD600 of 0.1 (about 1×108 cfu ml−1). Tomato cultivar Moneymaker (susceptible to R. solanacearum RS1002) plants were grown on 9 cm-diameter Jiffy peat pots containing Soil-mix and expanded vermiculite at the ratio of 1∶1 in a greenhouse at 24 to 25°C. Six-week-old seedlings at approximately the eight- to nine-leaf stage were inoculated by drenching the plant saucer near the root of each plant with 30 ml of the bacterial suspension. Plant roots were not wounded before inoculation. Observations for bacterial wilt symptoms were carried out at 5, 7, 9, 11, 13, 14 and 15 dpi according to the following scale: 0, no visible wilt; 1, one or two leaves wilted; 2, three or four leaves wilted; 3, five or six leaves wilted; 4, seven to nine leaves wilted; and 5, more than ten leaves wilted. The disease index (DI) was calculated as DI = Σ(grade × number of wilting plants)/(5× number of total plants) [39].
Infection of Tomato with Pst and Pst-avrRps4
Pst and Pst-avrRps4 [8], [40] were grown in liquid King’s B medium containing kanamycin (25 µg ml−1) and rifampicin (25 µg ml−1). Bacteria were harvested by centrifugation, cell pellets were washed with 10 mM MgSO4, and resuspended in 10 mM MgSO4 to a concentration of 5×104 cfu ml−1 for in planta growth assays. Tomato plants (susceptible to both Pst and Pst-avrRps4) were grown as above. Six-week-old seedlings at approximately the eight- to nine-leaf stage were used for virulence assays. Plants were transferred to a growth chamber with 95–100% humidity at 25°C with 12 h light 1 d prior to inoculation by infiltration. Abaxial leaf surfaces of tomato plants were infiltrated with the bacterial suspension with a 1 ml syringe. Bacterial growth was determined 0 and 3 d after infiltration using five leaf disks, as previously described [40].
C. orbiculare Inoculations
C. orbiculare strain 104-T [41] was maintained on potato dextrose agar (Difco, MI, USA) at 24°C in the dark. Conidia were obtained by gently scraping 7 d cultures as described [42]. Mature leaves of 2.5 true leaf stage seedlings were used for virulence assays. Quantification of C. orbiculare was performed by spotting 5 µl of a conidial suspension (5×105 conidia ml−1 in distilled water) on the surface of detached cucumber leaves, and then five leaf-disks were cut using a cork borer (No. 3) at 6 dpi. Leave disks were frozen in liquid nitrogen and ground using an SH-48 grinding apparatus (Kurabo, Osaka, Japan). Preparation of total RNA and first-strand cDNA, qRT-PCR were performed according to a method described previously [34]. Nucleotide sequences of C. orbiculare actin (Cor-ACT) primers are listed in Table S1. QRT-PCR data for Cor-ACT and C. sativus EF1α (Cs-EF1α) expression from cucumber were collected as copy number obtained from a standard curve of cycle times. The abundance of Cor-ACT was normalized against Cs-EF1α in infected samples.
Expression Analysis of Defense-related Gene in Transgenic Plants Expressing RPS4 and RRS1
Expression of the pathogenesis-related 1 (PR1) gene involved in the plant defense responses was determined by qRT-PCR of RNA from five leaf-disks cut from leaves of the 2.5 true leaf stage T2 transgenic B. rapa, B. napus and cucumber, four-week-old T3 transgenic N. benthamiana, and three-week-old T2 transgenic tomato plants carrying both RPS4 and RRS1 using a cork borer (No. 3). Total RNA was isolated and treated with RNase-free DNase (Promega, WI, USA). 500 ng of total RNA was synthesized with oligo dT primer using a PrimeScript RT reagent kit (Takara, Otsu, Japan). QRT-PCR was performed with SYBR Green PCR Master Mix (BIO-Rad Laboratories, CA, USA) using the first-strand cDNA as a template on an MJ Opticon (Bio-Rad Laboratories). QRT-PCR mixtures consisted of 1xSYBR Green I PCR Master Mix and 200 nM (each) sense and antisense primers. Following a preliminary denaturation step at 95°C for 30 s, the reaction mixtures were cycled 40X at 95°C for 5 s and at 65°C for 20 s. The target sample copy number was averaged for two reactions, and the experiment was repeated twice. Expression of the Br-CBP20 for B. rapa, Bn-ACT for B. napus, EF1α for N. benthamiana and cucumber, or Tip41 for tomato was used for normalization. PR1 gene expression is shown as relative values set at a value of 1 in the control plants. Nucleotide sequences of gene-specific primers for each gene are listed in Table S1. Bars indicate SE. This experiment was repeated twice with similar results.
Supporting Information
Diagram of RPS4 and RRS1 genes inserted into a binary vector. (A) The 6.3 kbp genomic RPS4 fragment, including approximately 2.1 kbp upstream and 109 bp downstream regions, (B) the 8.2 kbp genomic RRS1 fragment, including approximately 1.8 kbp upstream and 176 bp downstream regions, and (C) the 10.9 kbp genomic fragment containing both RPS4 and RRS1 were cloned into binary vector pBI101-SK+ [7], pGWB1 [7], and pBI-GW-NOS, respectively. Arrowboxes indicate RPS4 and/or RRS1 genome fragments. Lines indicate the polylinker regions of binary vector. Transgenic plants were assessed for the presence of the transgene by PCR. Locations of primers used for PCR are indicated by arrows. Specific PCR primers: KY and N3 for the polylinker of binary vector, RPS4-SF5 and NRS2-S4 for RPS4 gene, RRS1-S10-2 and RPS4-SF2 for RRS1 gene. The primers for qRT-PCR are listed in Table S1.
(TIF)
Expression of the transgene RPS4 and RRS1 in transgenic plants. Five leaf disks were cut from leaves of the 2.5 true leaf stage control and transgenic B. rapa (T2), B. napus (T2), N. benthamiana (T3), tomato (T2) and cucumber (T2) plants carrying both RPS4 and RRS1 (RR), either RRS1 or RPS4 alone, and total RNA was isolated for qRT-PCR analysis. Expression of the transgene RPS4 and RRS1 in transgenic plants was quantified by qRT-PCR. Bars indicate SE. The experiment was repeated twice with similar results. The primers used here are specific to the transgenes (Arabidopsis RPS4 and RRS1). As expression of RPS4 was slightly detected in B. napus wild-type plants, we assume B. napus has the homologue of Arabidopsis RPS4 or non-specific binding of the primers occurred for transcripts of B. napus.
(TIF)
Growth of transgenic plants expressing RPS4 and RRS1 . Each picture shows four-week-old T2 transgenic B. rapa (A), three-week-old T2 transgenic B. napus (B), six-week-old T3 transgenic N. benthamiana (C), four-week-old T2 transgenic tomato (D), four-week-old T2 transgenic cucumber (E) carrying both RPS4 and RRS1 (RR) and control plants. The experiment was repeated more than three times with similar results.
(TIF)
Expression of defense-related gene PR1 in transgenic plants under normal growth conditions. Five leaf disks were cut from leaves of the 2.5 true leaf stage T2 transgenic B. rapa, B. napus and cucumber, four-week-old T3 transgenic N. benthamiana, three-week-old T2 transgenic tomato carrying both RPS4 and RRS1 (RR) and control plants using a cork borer (No. 3). Total RNA was isolated for qRT-PCR analysis. PR1 gene expression is shown as relative values set at 1 in the control plants. Bars indicate SE. The experiment was repeated twice with similar results.
(TIF)
Quantification of avrRps4 and popP2 mRNA transiently expressed in transgenic N. benthamiana plants. The fully expanded leaves of four-week-old T3 homozygous transgenic N. benthamiana expressing RPS4 and/or RRS1, and control plants were infiltrated with A. tumefaciens strain GV3101 (pMP90, pSoup) containing pSfinx-avrRps4 or pSfinx-popP2. Total RNA was isolated 24 h post inoculation. Expression of avrRps4 and popP2 in transgenic plants was quantified by qRT-PCR. Non: non infiltrated leaves. Bars indicate SE. The experiment was repeated twice with similar results.
(TIF)
Growth of bacterial pathogens in RPS4 and RRS1 dual R gene-transformed tomato and control plants. (A) R. solanacearum resistance analysis in RPS4 and RRS1 dual R gene-transformed tomato (RR). Six-week-old tomato plants were inoculated with R. solanacearum strain RS1002-ΔpopP2. Plants were rated every other day on a 0 to 5 disease index scale from 0 (no visible wilt) to 5 (the whole plant is dead). Each point represents the mean disease index (± SE) for three independent experiments, each containing 5 to 10 plants per treatment. Both control plants and transformants wilted after inoculation with R. solanacearum strain RS1002-ΔpopP2. (B) Infection assays with Pseudomonas syringae pv. tomato DC3000 (Pst) in RPS4 and RRS1 dual R gene-transformed tomato (RR). Leaves of six-week-old tomato plants were infiltrated with bacterial suspensions (5×104 cfu ml−1). Leaves were harvested at 3 dpi. Growth of Pst in vector controls and dual R gene-transformed tomato had increased greatly by 3 dpi. Bars indicate SE. The experiment was repeated three times with similar results.
(TIF)
PCR primers used in this study.
(TIF)
Acknowledgments
We thank Masaki Iwabuchi (RIBS) for the valuable comments. We also thank Mariko Miyashita, Yoko Miyashita and Yasuyo Katayama for their excellent technical assistance, and Tsuyoshi Nakagawa (Shimane Univ.) for kindly providing pGWB1. The tomato resources used in this research were provided by the National BioResource Project (NBRP), Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The production of transgenic B. napus and tomato plants was supported by the RIKEN Plant Transformation Network.
Funding Statement
This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry to K.S., Y. Takano, Y. Narusaka and by JSPS Grant-in-Aid for Scientific Research (KAKENHI) (21580060 to Y. Narusaka, 21780038 to M.N. and 24228008 to K.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 2. Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814. [DOI] [PubMed] [Google Scholar]
- 3. Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, et al. (2010) Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol 28: 365–369. [DOI] [PubMed] [Google Scholar]
- 4. Tai TH, Dahlbeck D, Clark ET, Gajiwala P, Pasion R, et al. (1999) Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc Natl Acad Sci USA 96: 14153–14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joshi RK, Nayak S (2011) Functional characterization and signal transduction ability of nucleotide-binding site-leucine-rich repeat resistance genes in plants. Genet Mol Res 10: 2637–2652. [DOI] [PubMed] [Google Scholar]
- 6. Wulff BB, Horvath DM, Ward ER (2011) Improving immunity in crops: new tactics in an old game. Curr Opin Plant Biol 14: 468–476. [DOI] [PubMed] [Google Scholar]
- 7. Narusaka M, Shirasu K, Noutoshi Y, Kubo Y, Shiraishi T, et al. (2009) RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J 60: 218–226. [DOI] [PubMed] [Google Scholar]
- 8. Hinsch M, Staskawicz BJ (1996) Identification of a new Arabidopsis disease resistance locus, RPS4, and cloning of the corresponding avirulence gene, avrRps4, from Pseudomonas syringae pv. pisi . Mol Plant-Microbe Interact 9: 55–61. [DOI] [PubMed] [Google Scholar]
- 9. Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, et al. (2003) Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci USA 100: 8024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birker D, Heidrich K, Takahara H, Narusaka M, Deslandes L, et al. (2009) A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. Plant J 60: 602–613. [DOI] [PubMed] [Google Scholar]
- 11. Eitas TK, Dangl JL (2010) NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr Opin Plant Biol 13: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinapidou E, Williams K, Nott L, Bahkt S, Tör M, et al. (2004) Two TIR:NB:LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis . Plant J 38: 898–909. [DOI] [PubMed] [Google Scholar]
- 13. Peart JR, Mestre P, Lu R, Malcuit I, Baulcombe DC (2005) NRG1, a CCNB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr Biol 15: 968–973. [DOI] [PubMed] [Google Scholar]
- 14. Eitas TK, Nimchuk ZL, Dangl JL (2008) Arabidopsis TAO1 is a TIR-NBLRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc Natl Acad Sci USA 105: 6475–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loutre C, Wicker T, Travella S, Galli P, Scofield S, et al. (2009) Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J 60: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 16. Lee SK, Song MY, Seo YS, Kim HK, Ko S, et al. (2009) Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two CC-NBLRR genes. Genetics 181: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, et al. (2008) Two adjacent nucleotide-binding site–leucine-rich repeat class genes are required to confer pikm-specific rice blast resistance. Genetics 180: 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Dorey S, Swiderski M, Jones JD (2004) Expression of RPS4 in tobacco induces an AvrRps4-independent HR that requires EDS1, SGT1 and HSP90. Plant J 40: 213–224. [DOI] [PubMed] [Google Scholar]
- 19. Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, et al. (2011) Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334: 1401–1404. [DOI] [PubMed] [Google Scholar]
- 20. Bhattacharjee S, Halane MK, Kim SH, Gassmann W (2011) Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 334: 1405–1408. [DOI] [PubMed] [Google Scholar]
- 21. McDowell JM (2011) Plant science. Beleaguered immunity. Science 334: 1354–1355. [DOI] [PubMed] [Google Scholar]
- 22. Sohn KH, Hughes RK, Piquerez SJ, Jones JD, Banfield MJ (2012) Distinct regions of the Pseudomonas syringae coiled-coil effector AvrRps4 are required for activation of immunity. Proc Natl Acad Sci USA 109: 16371–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasset C, Bernoux M, Jauneau A, Pouzet C, Brière C, et al. (2010) Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1-R-mediated immunity in Arabidopsis. PLoS Pathog 6: e1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, et al. (2012) Life-style transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Gen 44: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15: 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itoh T, Tanaka T, Barrero RA, Yamasaki C, Fujii Y, et al. (2007) Curated genome annotation of Oryza sativa ssp. japonica and comparative genome analysis with Arabidopsis thaliana . Genome Res 17: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takasaki T, Hatakeyama K, Ojima K, Watanabe M, Toriyama K, et al. (1997) Factors influencing Agrobacterium-mediated transformation of Brassica rapa L. Breeding Sci. 47: 127–134. [Google Scholar]
- 28. Kohno-Murase J, Murase M, Ichikawa H, Imamura J (1994) Effects of an antisense napin gene on seed storage compounds in transgenic Brassica napus seeds. Plant Mol Biol 26: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 29. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, et al. (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 30. Clemente T (2006) Nicotiana (Nicotiana tobaccum, Nicotiana benthamiana). Meth Mol Biol 343: 143–154. [DOI] [PubMed] [Google Scholar]
- 31. Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47: 426–431. [DOI] [PubMed] [Google Scholar]
- 32. Tabei Y, Kitade S, Nishizawa Y, Kikuchi N, Kayano T, et al. (1998) Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botrytis cinerea). Plant Cell Rep 17: 159–164. [DOI] [PubMed] [Google Scholar]
- 33.Nanasato Y, Konagaya K, Okuzaki A, Tsuda M, Tabei Y (2012) Improvement of Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) by combination of vacuum infiltration and co-cultivation on filter paper wicks. Plant Biotechnol Rep DOI 10.1007/s11816-012-0260-1. [DOI] [PMC free article] [PubMed]
- 34. Narusaka M, Shiraishi T, Iwabuchi M, Narusaka Y (2010) Monitoring fungal viability and development in plants infected with Colletotrichum higginsianum by quantitative reverse transcription-polymerase chain reaction. J Gen Plant Pathol 76: 1–6. [Google Scholar]
- 35. Takken FL, Luderer R, Gabriëls SH, Westerink N, Lu R, et al. (2000) A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J 24: 275–283. [DOI] [PubMed] [Google Scholar]
- 36. Mukaihara T, Tamura N, Murata Y, Iwabuchi M (2004) Genetic screening of Hrp type III-related pathogenicity genes controlled by the HrpB transcriptional activator in Ralstonia solanacearum . Mol Microbiol 54: 863–875. [DOI] [PubMed] [Google Scholar]
- 37. Kelman A (1954) The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathology 44: 693–695. [Google Scholar]
- 38. Hendrick CA, Sequeira L (1984) Lipopolysaccharide-defective mutants of the wilt pathogen Pseudomonas solanacearum . Appl Environ Microbiol 48: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang HE, Liu CA, Lee MJ, Kuo CG, Chen HM, et al. (2007) Resistance Enhancement of Transgenic Tomato to Bacterial Pathogens by the Heterologous Expression of Sweet Pepper Ferredoxin-I Protein. Phytopathology 97: 900–906. [DOI] [PubMed] [Google Scholar]
- 40. Wei CF, Kvitko BH, Shimizu R, Crabill E, Alfano JR, et al. (2007) A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1–1 is able to cause disease in the model plant Nicotiana benthamiana . Plant J 51: 32–46. [DOI] [PubMed] [Google Scholar]
- 41. Akai S, Ishida N (1968) An electron microscopic observation on the germination of conidia of Colletotrichum lagenarium . Mycopathol Mycol Appl 34: 337–345. [DOI] [PubMed] [Google Scholar]
- 42. Kubo Y, Suzuki K, Furusawa I, Ishida N, Yamamoto M (1982) Relation of appressorium pigmentation and penetration of nitrocellulose membranes by Colletotrichum lagenarium . Phytopathology 72: 498–501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagram of RPS4 and RRS1 genes inserted into a binary vector. (A) The 6.3 kbp genomic RPS4 fragment, including approximately 2.1 kbp upstream and 109 bp downstream regions, (B) the 8.2 kbp genomic RRS1 fragment, including approximately 1.8 kbp upstream and 176 bp downstream regions, and (C) the 10.9 kbp genomic fragment containing both RPS4 and RRS1 were cloned into binary vector pBI101-SK+ [7], pGWB1 [7], and pBI-GW-NOS, respectively. Arrowboxes indicate RPS4 and/or RRS1 genome fragments. Lines indicate the polylinker regions of binary vector. Transgenic plants were assessed for the presence of the transgene by PCR. Locations of primers used for PCR are indicated by arrows. Specific PCR primers: KY and N3 for the polylinker of binary vector, RPS4-SF5 and NRS2-S4 for RPS4 gene, RRS1-S10-2 and RPS4-SF2 for RRS1 gene. The primers for qRT-PCR are listed in Table S1.
(TIF)
Expression of the transgene RPS4 and RRS1 in transgenic plants. Five leaf disks were cut from leaves of the 2.5 true leaf stage control and transgenic B. rapa (T2), B. napus (T2), N. benthamiana (T3), tomato (T2) and cucumber (T2) plants carrying both RPS4 and RRS1 (RR), either RRS1 or RPS4 alone, and total RNA was isolated for qRT-PCR analysis. Expression of the transgene RPS4 and RRS1 in transgenic plants was quantified by qRT-PCR. Bars indicate SE. The experiment was repeated twice with similar results. The primers used here are specific to the transgenes (Arabidopsis RPS4 and RRS1). As expression of RPS4 was slightly detected in B. napus wild-type plants, we assume B. napus has the homologue of Arabidopsis RPS4 or non-specific binding of the primers occurred for transcripts of B. napus.
(TIF)
Growth of transgenic plants expressing RPS4 and RRS1 . Each picture shows four-week-old T2 transgenic B. rapa (A), three-week-old T2 transgenic B. napus (B), six-week-old T3 transgenic N. benthamiana (C), four-week-old T2 transgenic tomato (D), four-week-old T2 transgenic cucumber (E) carrying both RPS4 and RRS1 (RR) and control plants. The experiment was repeated more than three times with similar results.
(TIF)
Expression of defense-related gene PR1 in transgenic plants under normal growth conditions. Five leaf disks were cut from leaves of the 2.5 true leaf stage T2 transgenic B. rapa, B. napus and cucumber, four-week-old T3 transgenic N. benthamiana, three-week-old T2 transgenic tomato carrying both RPS4 and RRS1 (RR) and control plants using a cork borer (No. 3). Total RNA was isolated for qRT-PCR analysis. PR1 gene expression is shown as relative values set at 1 in the control plants. Bars indicate SE. The experiment was repeated twice with similar results.
(TIF)
Quantification of avrRps4 and popP2 mRNA transiently expressed in transgenic N. benthamiana plants. The fully expanded leaves of four-week-old T3 homozygous transgenic N. benthamiana expressing RPS4 and/or RRS1, and control plants were infiltrated with A. tumefaciens strain GV3101 (pMP90, pSoup) containing pSfinx-avrRps4 or pSfinx-popP2. Total RNA was isolated 24 h post inoculation. Expression of avrRps4 and popP2 in transgenic plants was quantified by qRT-PCR. Non: non infiltrated leaves. Bars indicate SE. The experiment was repeated twice with similar results.
(TIF)
Growth of bacterial pathogens in RPS4 and RRS1 dual R gene-transformed tomato and control plants. (A) R. solanacearum resistance analysis in RPS4 and RRS1 dual R gene-transformed tomato (RR). Six-week-old tomato plants were inoculated with R. solanacearum strain RS1002-ΔpopP2. Plants were rated every other day on a 0 to 5 disease index scale from 0 (no visible wilt) to 5 (the whole plant is dead). Each point represents the mean disease index (± SE) for three independent experiments, each containing 5 to 10 plants per treatment. Both control plants and transformants wilted after inoculation with R. solanacearum strain RS1002-ΔpopP2. (B) Infection assays with Pseudomonas syringae pv. tomato DC3000 (Pst) in RPS4 and RRS1 dual R gene-transformed tomato (RR). Leaves of six-week-old tomato plants were infiltrated with bacterial suspensions (5×104 cfu ml−1). Leaves were harvested at 3 dpi. Growth of Pst in vector controls and dual R gene-transformed tomato had increased greatly by 3 dpi. Bars indicate SE. The experiment was repeated three times with similar results.
(TIF)
PCR primers used in this study.
(TIF)