Abstract
Leishmaniasis is one of the world's most neglected diseases, largely affecting the poorest of the poor, mainly in developing countries. Over 350 million people are considered at risk of contracting leishmaniasis, and approximately 2 million new cases occur yearly1. Leishmania donovani is the causative agent for visceral leishmaniasis (VL), the most fatal form of the disease. The choice of drugs available to treat leishmaniasis is limited 2;current treatments provide limited efficacy and many are toxic at therapeutic doses. In addition, most of the first line treatment drugs have already lost their utility due to increasing multiple drug resistance 3. The current pipeline of anti-leishmanial drugs is also severely depleted. Sustained efforts are needed to enrich a new anti-leishmanial drug discovery pipeline, and this endeavor relies on the availability of suitable in vitro screening models.
In vitro promastigotes 4 and axenic amastigotes assays5 are primarily used for anti-leishmanial drug screening however, may not be appropriate due to significant cellular, physiological, biochemical and molecular differences in comparison to intracellular amastigotes. Assays with macrophage-amastigotes models are considered closest to the pathophysiological conditions of leishmaniasis, and are therefore the most appropriate for in vitro screening. Differentiated, non-dividing human acute monocytic leukemia cells (THP1) (make an attractive) alternative to isolated primary macrophages and can be used for assaying anti-leishmanial activity of different compounds against intracellular amastigotes.
Here, we present a parasite-rescue and transformation assay with differentiated THP1 cells infected in vitro with Leishmania donovani for screening pure compounds and natural products extracts and determining the efficacy against the intracellular Leishmania amastigotes. The assay involves the following steps: (1) differentiation of THP1 cells to non-dividing macrophages, (2) infection of macrophages with L. donovani metacyclic promastigotes, (3) treatment of infected cells with test drugs, (4) controlled lysis of infected macrophages, (5) release/rescue of amastigotes and (6) transformation of live amastigotes to promastigotes. The assay was optimized using detergent treatment for controlled lysis of Leishmania-infected THP1 cells to achieve almost complete rescue of viable intracellular amastigotes with minimal effect on their ability to transform to promastigotes. Different macrophage:promastigotes ratios were tested to achieve maximum infection. Quantification of the infection was performed through transformation of live, rescued Leishmania amastigotes to promastigotes and evaluation of their growth by an alamarBlue fluorometric assay in 96-well microplates. This assay is comparable to the currently-used microscopic, transgenic reporter gene and digital-image analysis assays. This assay is robust and measures only the live intracellular amastigotes compared to reporter gene and image analysis assays, which may not differentiate between live and dead amastigotes. Also, the assay has been validated with a current panel of anti-leishmanial drugs and has been successfully applied to large-scale screening of pure compounds and a library of natural products fractions (Tekwani et al. unpublished).
Keywords: Infection, Issue 70, Immunology, Infectious Diseases, Molecular Biology, Cellular Biology, Pharmacology, Leishmania donovani, Visceral Leishmaniasis, THP1 cells, Drug Screening, Amastigotes, Antileishmanial drug assay
Protocol
1. Maintain and Subculture THP1 Cell Culture
Maintain THP1 cells in RPMI-1640 medium (with 10% FBS and pH 7.4) at 37 °C in a 5% CO2 incubator.
Subculture cells twice a week to prevent cell count from exceeding 1x106 cells/ml. This is important to maintain their transformation ability.
2. Maintain and Subculture Leishmania donovani Promastigotes Culture
Maintain the L. donovani promastigotes (S1, sudan strain) in RPMI-1640 medium (without Sodium Bicarbonate and Sodium Pyruvate) with 10% FBS at 26 °C.
Subculture L. donovani promastigotes twice a week, with highest cells concentration in the range of 20-25x106 promastigotes/ml.Caution: All the media and solutions should be brought to the room temperature before use.
3. Seeding and Differentiation of the THP1 cells in a 96-well Microplate and 16-chamber Glass Culture Slide.
Prepare a diluted THP1 culture with cell count of 2.5x105 cells/ml from a four-day-old cell culture (cell count should not exceed 106 cells/ml) in RPMI-1640 with 10% heat-inactivated FBS. Prepared 20 ml of culture for each 96-well plate and 4 ml of culture for each 16-well chamber slide.
Add phorbol 12-myristate 13-acetate (PMA) (for differentiation of THP1) to diluted cell culture suspension (10 μl/20 ml culture from the stock of 50 μg/ml in DMSO) (final PMA concentration in diluted cells culture should be 25 ng/ml).
To compare the Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation- Assay, set up the assays simultaneously in clear, flat-bottom, 96-well plate and 16-chamber, glass, microscopic culture slide.
Dispense 200 μl of THP1-PMA-treated cells to each well or chamber.
Incubate the 96-well plates and 16-well chamber slides in a 37 °C, 5% CO2 incubator overnight to allow almost complete differentiation of the cells.
Note: The THP1 cells, which normally grow in suspension, are differentiated into adherent macrophages.
4. Infection of the Transformed THP1 Cells with Leishmania donovani Promastigotes
For infection of differentiated THP1 cell culture with Leishmania donovani promastigotes, the majority of parasites should be in the infective metacyclic stage (long cylindrical forms, ~ 5-6 day old culture).
A 1:10 THP1 cell to parasite ratio is optimal for the infection in both the Digital-Image-Analysis-Direct-Counting-Assay and the Promastigote-Rescue-Transformation- Assay.
Prepare a diluted culture of L. donovani promastigotes with a parasite count of 2.5x106 parasite/ml (for THP1 cells:parasites ratio = 1:10) from a 5 to 6-day-old culture in RPMI-1640 medium with 2% FBS.
From the step 3.5 (after overnight differentiation of THP1 cell culture) take out the plates and chamber slides, remove the medium and wash the cell cultures once with serum-free RPMI-1640 medium.
After careful washing of PMA-treated THP1 cells with serum-free, warm RPMI-1640 (~37 °C) medium, replace the serum-free medium with 200 μl of the diluted culture of L. donovani promastigotes (2.5x106 parasites/ml) from step 4.3. Set up the control wells of THP1 cells without the parasite and the parasites without THP1 cells in each plate and 16-well chamber slides.
After adding parasite to the THP1 cell culture , incubate the plate and slides at 37 °C, 5% CO2 for 24 hr to allow the parasites to infect the differentiated THP1 cells.
5. Treatment of Infected Macrophages with Test Drugs/Compounds
Test Amphotericin B, Pentamidine and Miltefosine as standard anti-leishmanial drugs for screening. Prepare stock solutions of the drugs/test compounds in water or DMSO as mentioned in reagent table. Test each drug compound at 6 concentrations.
Serially dilute (1:5) the standard drugs and test compounds in a fresh 96-well plate or 2 ml tubes (for chamber slides) with RPMI-1640 medium with 2% FBS. The drugs/test compound concentrations in this plate are 2X of final concentrations.
Wash the culture plates and chamber slides infected with L. donovani promastigotes (from step 4.5) at least 5 times with serum-free, RPMI-1640 medium. Add 100 μl culture medium (RPMI-1640 with 2% FBS) into each well/chamber. The multiple washings are necessary to ensure complete removal of non-internalized promastigotes.
Add 100 μl medium from serially-diluted standard anti-leishmanial drugs or the test compounds to each well or chamber. Set up the infected THP1 cells controls without drugs simultaneously in each plate/chamber slide.
Incubate the plates and chamber slides at 37 °C, 5% CO2 for 48 hr.
6. Chamber Slide Staining, Fluorescent Microscope Imaging and Image Analysis for Quantification of Infection and Effect of Drug Treatment
After a 48 hr incubation, wash the chamber slide 3 times with serum-free RPMI-1640 medium. Peel off the plastic chambers from the slides and fix the cells by immersing the slides in methanol for 30 sec. Leave the slides in bio-hood under airflow for drying.
Prepare a SYBR Green I staining solution (5X) by diluting (1:2,000) the stock (10,000X) with water.
Stain the slides under dark conditions with the diluted SYBR Green I stain solution for 15 min at room temperature, wash once with water and leave the slides under the air flow for drying.
Place a full glass coverslip over the stained slide with the help of mounting medium.
Capture the digital images of THP1 cells: without infection (blank), with infection (control), infected cells treated with different standard drugs or test compounds in different dilutions (Figure 3, 5 and 6)) using a Nikon Eclipse 90i fluorescent microscope accompanied by NIS element AR 3.2 software.
Count the macrophage nuclei (big) and the parasite nuclei (small with kinetoplast) using ImageJ (Figure 7), which is a publically-available, Java-based image processing program developed at the National Institutes of Health (http://rsb.info.nih.gov/ij/download.html). Express the data as the number of amastigotes per 100 transformed THP1 cells.
7. The Parasite-Rescue-Transformation-Assay: Controlled lysis of L. donovani Amastigotes-infected Macrophages
Wash the 96-well microplate from step 5.5 three times with serum-free RPMI-1640 culture medium.
Among different detergents tested at different concentrations, 0.05% SDS treatment for 30 sec is optimal for controlled lysis (maximum cell lysis with minimum loss of rescued parasites' viability).
Remove the serum-free medium from each well after the last washing and add 20 μl of RPMI-1640 (with 0.05% SDS) to each well. Shake the plate for 30 sec and add 180 μl complete RPMI-1640 (with 10% FBS) to each well.
Incubate the plates at 26 °C for 48 hr for transformation of rescued amastigotes to promastigotes.
8. Quantitative Analysis of Transformed Promastigote (alamarBlue assay)
After a 48 hr incubation at 26 °C, all the rescued live amastigotes are transformed into promastigotes (Figure 1D). Add 10 μl of alamarBlue into each well of the 96-well plates.
Incubate the plates at 26 °C overnight.
After overnight incubation, read the plates for standard fluorescence on a Fluostar Galaxy fluorimeter (BMG Lab Technologies) at 544 nm excitation, 590nm emission.
Prepare the dose response curves (percent growth vs. concentration of the drug or test compound) with ExcelFit (Figures 7-9) and compute IC50/IC90 values from these curves (Table 1).
9. Standardization of THP1 Cells to Parasites Ratio
Standardize the THP1 cells to parasites ratio to determine sensitivity of the assay. Note: Low/high parasites numbers/infection may compromise with sensitivity and selectivity of the screening.
Standardize the THP1 cells to parasites ratio by following the protocol described above for THP1 cells seeding and Leishmania promastigote infection (section 3 & 4) except use different ratios of THP1 cells to parasites as 1:1.25, 1:2.5, 1:5 and 1:10.
Set up the experiment both in 16-chamber slide (for image analysis) and 96-well plate (for parasite-rescue assay) formats to compare the parasite rescue and image analysis assays.
10. Standardization of different detergents for controlled cell lysis
The primary objective of this experiment is to optimize the protocol for controlled lysis of the THP1 cells to achieve maximum/complete THP1 cells lysis without significantly affecting the viability of the rescued amastigote parasites.
Test different detergents such as Tween 20, Tween 80, Triton X-100, NP-40 and SDS at different concentrations and different durations of treatment (Figure 2).
THP1 cells to parasites ratio is 1:10 and other conditions are similar as above sections 1-8.
Test the 0.05% SDS treatment further for different treatment times to further optimize the controlled cell lysis.
Representative Results
A quantitative analysis was done for both Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for 24, 48, 72 and 96 hr post-drug treatment
In the direct counting method, infection of L. donovani-infected macrophages (THP1 cells) was calculated by the following equation:
![]()
The amastigotes (determined by counting amastigotes nuclei)/100 transformed THP1 cells (determined by counting counted at THP1 cell nuclei) (Figure 7) is a more accurate measure to analyze the effect of different standard or test compounds than the percentage of infected THP1 cells, as reported in some previous papers, because this number is directly related to overall effect of compounds, either through a decrease in parasites in macrophage cells or total removal of parasite from the macrophage cells.Infection was calculated from digital images of infected THP1 cells treated with different standard drugs at different dilutions for various time intervals (Figure 10 and Table 1). The read-out for the Digital-Image-Analysis-Direct-Counting-Assay was amastigotes infection/100 transformed THP1 cells, while the read-out for the Parasite-Rescue-Transformation-Assay was relative fluorescence units (RFU), which is directly proportional to the number of live Leishmania amastigotes rescued from the infected macrophages and transform into promastigotes. The alamarBlue assay is routinely used for Leishmania promastigotes anti-leishmanial drug screening.
The assay was initially standardized and optimized for controlled lysis of Leishmania-infected THP1 cells. The objective was to optimize the conditions for detergent treatment, which yield almost complete lysis of THP1 cells with minimal effect on the viability of the rescued amastigotes. Figure 1 depicts a microscopic view of the complete assay protocol. Intact THP1 cells infected with Leishmania amastigotes can be seen in Figure 1A. Figure 1B shows lysis of the THP1 cells after detergent treatment. Figure 1C shows rescued Leishmania amastigotes, which have been partially transformed into promastigotes and Figure 1D shows almost complete transformation of amastigotes into promastigotes and their subsequent proliferation. The growth of these transformed promastigotes can be quantitatively monitored with addition of alamarBlue and measurement of fluorescence on a microplate reader. Treatment with NP-40 (Figure 2A) and Triton X-100 (Figure 2B) lysed the infected THP1 cells; however, it also influenced the viability and transformation of the rescued amastigotes. Treatment with Tween 20 (Figure 2C) and Tween 80 (Figure 2D) did not cause optimal lysis of THP1 cells resulting into an incomplete rescue of amastigotes as indicated by low numbers of transformed promastigotes. Treatment with 0.05% SDS for 30 sec (Figure 2E) yielded almost complete lysis of Leishmania-infected THP1 cells and did not affect viability and transformation of rescued amastigotes. Further optimization showed that treatment of cells with 0.05% SDS for 20-30 sec yielded highest rescue of viable Leishmania amastigotes (Figure 2F). In subsequent experiments, treatment with 0.05% SDS for 30 sec was used. Procedure for SDS treatment is the same for single or multiple plates. In multiple plates, SDS treatment was implemented column by column with a multichannel pipette. Serum-free medium was removed from all 8 wells of one column of the plate and 20 μl of 0.05% SDS was added in 8 wells of the same column and diluted after 30 sec with RPMI-1640 with 10% FBS. During initial standardization of the assay, the plates were checked under the microscope for non-internalized promastigotes. A minimum of five washings were necessary for removal of parasites before step 5 of treatment of infected macrophage cells with standard compounds and three washings were necessary before step 7 of SDS treatment. Thus, the cells were washed 8 times and no visible non-internalized promastigotes remained before control lysis of infected THP1 cells.
Digital Image Analysis and Direct Counting
The digital images of Leishmania-infected THP1 cells were captured on Nikon Eclipse 90i fluorescent microscope after staining with SYBR Green I. Both macrophage nuclei and intracellular Leishmania nuclei with characteristic kinetoplast DNA were observed under the fluorescent filters (Figure 3). Further, the images of infected THP1 cells were also captured under DIC. When both the images were merged, the outlines of THP1 cells with intracellular amastigotes were seen more clearly (Figure 4). The ImageJ software was used to analyze these images. ImageJ is a public domain, Java-based, image-processing program developed at the National Institutes of Health (http://rsb.info.nih.gov/ij/download.html). ImageJ has been designed with an open architecture that provides extensibility via Java plugins and recordable macros. Custom acquisition, analysis and processing plugins can be developed using ImageJ's built-in editor and a Java compiler. For differential counting of THP1 cells nuclei and parasite nuclei by ImageJ, the image was opened in ImageJ. Cell counter was found in Analyze option in plugin of the Software. The image was initialized and cell counter type 1 was selected for THP1 cell nuclei and cell counter type 2 was selected for parasite nuclei (Figure 3). Differential counting was done for at least 200 THP1 cell nuclei and the intracellular amastigotes present in these THP1 cell nuclei. A comparison of parasite-rescue assay and image analysis method was made for evaluating the infectivity of THP1 cells with different macrophage:promastigotes ratios (Figure 4). Figure 5 represents the differential infectivity in THP1 cells at different macrophage:promastigote ratios. Both the methods showed comparable results and the macrophage:promastigote ratio of 1:10 yielded optimum and reproducible infectivity.
Once the conditions for parasite-rescue/transformation assay and the digital image analysis were optimized, the utility of these assays was evaluated for anti-leishmanial drug screening. The Leishmania-infected THP1 cells were treated with different concentrations of standard anti-leishmanial drugs namely Amphotericin B, Pentamidine and Miltefosine for different time intervals ranging from 24 to 96 hr. The experiment for parasite rescued/transformation assay was done in triplicate and the experiment for direct cells counting method was done in duplicate. Figure 6 shows microscopic images of the control uninfected, control infected untreated and Leishmania-infected, treated THP1 cells. The dose response curves were prepared from the parasite-rescue and transformation assay (concentration of the drug vs. transformed parasites) and the image analysis assay (number of amastigotes/100 THP1 cells) (Figures 7-9). The IC50 of the drugs were computed by ExcelFit and are presented in Table 1. Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay showed comparable results. Digital-Image-Analysis-Direct-Counting-Assay was less optimal during the early time points of 24 and 48 hr drug treatments, while the Parasite-Rescue-Transformation-Assay showed results more consistent with the reported values at all the time points during the 24-96 hr after drug treatments. This difference in results with Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay may be due to presence of non-viable amastigotes during early periods of drug treatment in Digital-Image-Analysis-Direct-Counting-Assay.
 Figure 1. A microscopic view of the Leishmania donovani amastigotes rescue and transformation to promastigotes. A - Adherent THP1 cells infected with Leishmania amastigotes; B - Adherent, infected THP1 cells after controlled lysis C - Transformed Leishmania donovani promastigotes from the amastigotes rescued from infected THP1 macrophage cells D - Growth and proliferation of transformed Leishmania donovani promastigotes.
Figure 1. A microscopic view of the Leishmania donovani amastigotes rescue and transformation to promastigotes. A - Adherent THP1 cells infected with Leishmania amastigotes; B - Adherent, infected THP1 cells after controlled lysis C - Transformed Leishmania donovani promastigotes from the amastigotes rescued from infected THP1 macrophage cells D - Growth and proliferation of transformed Leishmania donovani promastigotes.
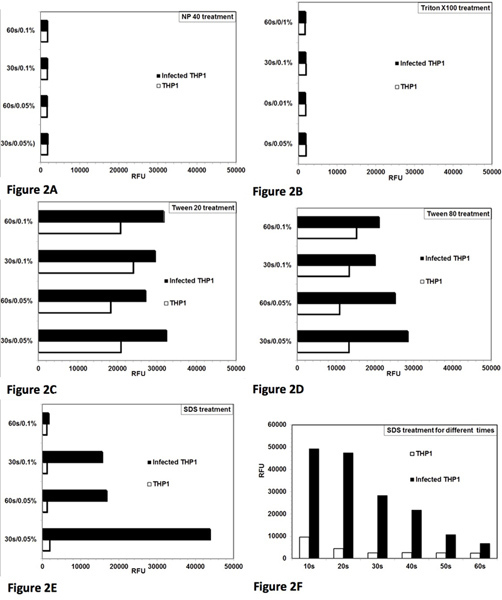 Figure 2. Optimization of controlled lysis of infected THP1 cells to achieve maximum rescue of live Leishmania donovani amastigotes and their transformation to promastigotes. Analysis of lysis of THP1 cells and rescue of amastigotes from the Leishmania-infected THP1 cells with different detergents. Two concentrations (0.05% and 0.1%) of detergent and two time periods (30 sec and 60 sec) for treatment were tested. RFU = relative fluorescence units. Each bar represents the mean of duplicate observations. [A] NP-40 treatment caused lysis of THP1 cells and also affected the viability of the rescued amastigote parasites. [B] Triton X-100 treatment caused lysis of THP1 cells and also affected the viability of the rescued amastigote parasites [C] Tween 80 caused partial lysis of THP1 cells to rescue the amastigotes.[D] Tween 80 caused partial lysis of THP1 cells to rescue the amastigotes. [E] SDS treatment caused almost complete lysis of THP1 cells and did not affect viability of the rescued amastigotes at 0.05%/30 sec. [F] Treatment with 0.05% SDS for 20-30 sec caused almost complete lysis of THP1 cells and rescued viable parasite amastigotes to transform into promastigotes. Click here to view larger figure.
Figure 2. Optimization of controlled lysis of infected THP1 cells to achieve maximum rescue of live Leishmania donovani amastigotes and their transformation to promastigotes. Analysis of lysis of THP1 cells and rescue of amastigotes from the Leishmania-infected THP1 cells with different detergents. Two concentrations (0.05% and 0.1%) of detergent and two time periods (30 sec and 60 sec) for treatment were tested. RFU = relative fluorescence units. Each bar represents the mean of duplicate observations. [A] NP-40 treatment caused lysis of THP1 cells and also affected the viability of the rescued amastigote parasites. [B] Triton X-100 treatment caused lysis of THP1 cells and also affected the viability of the rescued amastigote parasites [C] Tween 80 caused partial lysis of THP1 cells to rescue the amastigotes.[D] Tween 80 caused partial lysis of THP1 cells to rescue the amastigotes. [E] SDS treatment caused almost complete lysis of THP1 cells and did not affect viability of the rescued amastigotes at 0.05%/30 sec. [F] Treatment with 0.05% SDS for 20-30 sec caused almost complete lysis of THP1 cells and rescued viable parasite amastigotes to transform into promastigotes. Click here to view larger figure.
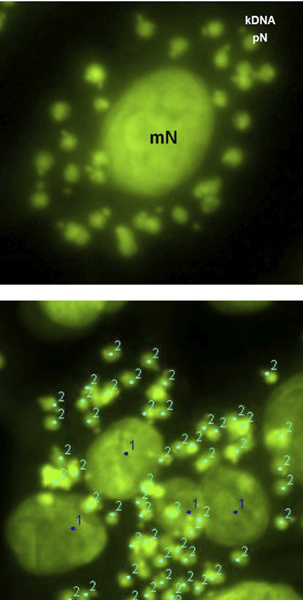 Figure 3. Fluorescent digital image of a differentiated THP1 cell infected in vitro with Leishmania donovani amastigotes. The characteristic kDNA can also be seen with each parasite nucleus. The macrophage nucleus (mN) (1) and the parasite nuclei (pN) (2) can be differentially marked and differentially counted by ImageJ analysis software for quantitative evaluation of the infection. The quantification was done as number of amastigotes/100 THP1 cells.
Figure 3. Fluorescent digital image of a differentiated THP1 cell infected in vitro with Leishmania donovani amastigotes. The characteristic kDNA can also be seen with each parasite nucleus. The macrophage nucleus (mN) (1) and the parasite nuclei (pN) (2) can be differentially marked and differentially counted by ImageJ analysis software for quantitative evaluation of the infection. The quantification was done as number of amastigotes/100 THP1 cells.
 Figure 4. Comparison between Digital-Image-Analysis-Direct-Counting-Assay (lower panel) and Parasite-Rescue-Transformation-Assay (upper panel). The macrophage:promastigote ratio of 1:10 yielded optimal infection. Both showed comparable results. The parasite-rescue assay showed some background values. Each bar shows mean of duplicate values.
Figure 4. Comparison between Digital-Image-Analysis-Direct-Counting-Assay (lower panel) and Parasite-Rescue-Transformation-Assay (upper panel). The macrophage:promastigote ratio of 1:10 yielded optimal infection. Both showed comparable results. The parasite-rescue assay showed some background values. Each bar shows mean of duplicate values.
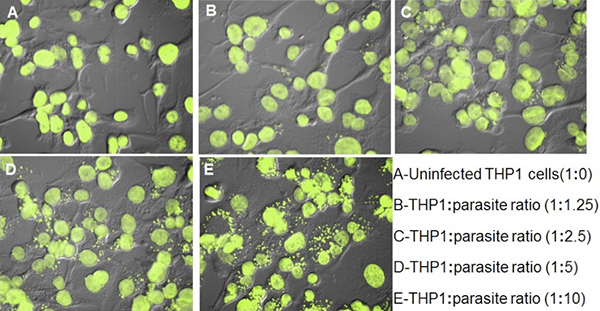 Figure 5. THP1 cells infected with Leishmania promastigotes by different THP1:promastigote ratio. The quantitative results as number of amastigotes/100 THP1 cells are presented in Figure 4.
Figure 5. THP1 cells infected with Leishmania promastigotes by different THP1:promastigote ratio. The quantitative results as number of amastigotes/100 THP1 cells are presented in Figure 4.
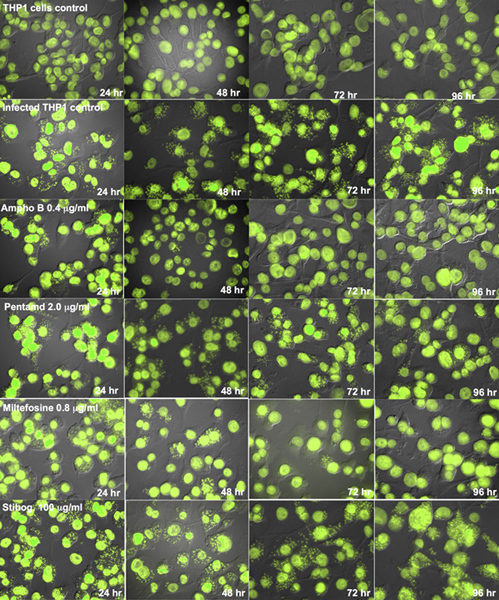 Figure 6. Digital images (Fluorescent + DIC) of THP1 cells infected with Leishmania
donovani amastigotes after treatment with standard anti-leishmanial drugs for different time periods.Results were quantified as number of amastigotes/100 THP1 cells and used to compute the percent growth compared to untreated controls and determine the IC50 values.
Figure 6. Digital images (Fluorescent + DIC) of THP1 cells infected with Leishmania
donovani amastigotes after treatment with standard anti-leishmanial drugs for different time periods.Results were quantified as number of amastigotes/100 THP1 cells and used to compute the percent growth compared to untreated controls and determine the IC50 values.
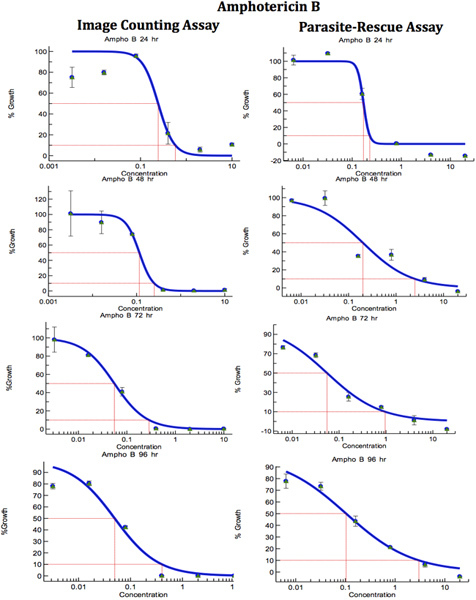 Figure 7. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Amophotericin B). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different periods. IC50 (μg/ml) values were computed from the dose response curve by Excelfit. Click here to view larger figure.
Figure 7. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Amophotericin B). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different periods. IC50 (μg/ml) values were computed from the dose response curve by Excelfit. Click here to view larger figure.
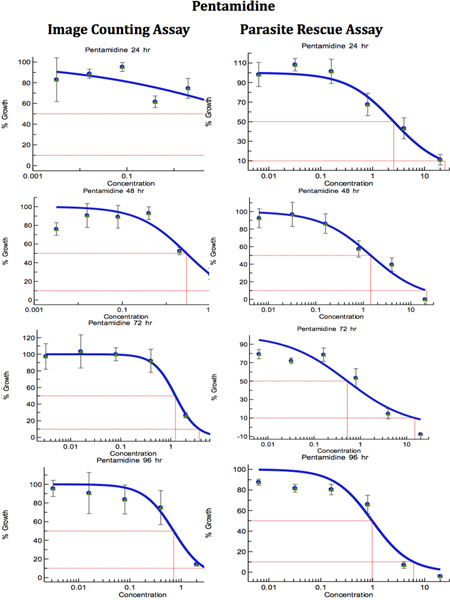 Figure 8. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Pentamidine). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different periods. IC50 (μg/ml) values were computed from the dose response curves by Excelfit. Click here to view larger figure.
Figure 8. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Pentamidine). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different periods. IC50 (μg/ml) values were computed from the dose response curves by Excelfit. Click here to view larger figure.
 Figure 9. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Mitefosine). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different time periods. IC50 (μg/ml) values were computed from the dose response curves by Excelfit. Click here to view larger figure.
Figure 9. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening (Mitefosine). The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different time periods. IC50 (μg/ml) values were computed from the dose response curves by Excelfit. Click here to view larger figure.
| Test Drug | 24 hra | 48 hra | 72 hra | 96 hra | ||||
| IACAb | PRTAc | IACAb | PRTAc | IACAb | PRTAc | IACAb | PRTAc | |
| Amphotericin B | 0.24± 0.03 | 0.17± 0.01* | 0.12± 0.04 | 0.20± 0.07 | 0.06± 0.01 | 0.06± 0.01 | 0.11± 0.03 | 0.10± 0.03 |
| Pentamidine | >10 | 2.55± 1.16* | 2.88± 0.58 | 1.43± 0.91 | 1.24± 0.35 | 1.52± 0.16 | 0.71± 0.63 | 0.98± 0.33 |
| Miltefosine | 0.38± 0.02 | 0.19± 0.08* | 0.24± 0.06 | 0.30± 0.08 | 0.36±0.02 | 0.16± 0.06 | 0.21± 0.15 | 0.17± 0.10 |
Table 1. Comparison of Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay for anti-leishmanial drug screening. The infected macrophages were treated with different concentrations of standard anti-leishmanial drug for different periods. IC50 (μg/ml) values were computed from the dose response curves by Excelfit (Figures 7-9). aHours post drug treatment; bIACA = Image Analysis and Direct Counting Assay; cPRTA = Parasite-Rescue and Transformation Assay. Values given are IC50 (concentration of the drug causing 50% inhibition in parasite growth) as μg/ml and are the mean ± S.D. of at least three experiments. * Statistically different (<0.05) compared to IC50 values with IACA.
Discussion
There are several methods available for anti-leishmanial drug screening based on macrophage-amastigote models. Assays can be done with the macrophages collected from host animals namely peritoneal exudate cells (PEC), peripheral blood monocyte cells (PBMC) 6 or bone marrow-derived macrophages (BMM) or in monocytic cell lines such as mouse (J774 and RAW264.7)7 and human (THP1, U937, HL-60)8 monocytic cells. The assays, which use dividing host cells, must ensure that the confounding effects of drug activity on both parasite and host cells number are considered. The differentiated primary macrophages collected from various sources such as mice and rats are non-dividing in nature, but these cell preparations may not have homogeneous cell populations. Monocytic cells-derived cell lines are homogenous in nature and are a better model for the macrophage-amastigote-based screening. Out of different monocytic cell lines, differentiated THP1 cells (human acute monocytic leukemia cell line) can form a non-dividing monolayer and offer an attractive alternative to primary isolated macrophages.
The macrophage-amastigote-based screening can be done in several ways. Classical microscopic evaluation based on direct cell and parasite counting9 is labor intensive. The absence of automation limits the utility of this assay. Counting of cells is time consuming and may give inaccurate determination of IC50 values since determination of parasite viability through a staining procedure is difficult. Many fluorescent dyes and monoclonal antibodies may be employed for flow cytometric assays10, 11, but these assays are also limited due to less sensitivity and limitation of time interval of drug-treatment to only one day. There are several reporter gene assays available for quantifying the growth of intracellular amastigotes12,13,14. An automated screening may be possible using reporter genes, but these assays also have certain drawbacks. First, majority of these assays require drug selection for maintaining the episomal expression of the reporter genes, which may not be ideal for a drug screening experiment. The way by which the reporter gene is introduced could also influence the physiological properties of the parasite and have an impact on the screening. If the reporter gene is the part of an episomal plasmid, the relative output of reporter may depend on the copy number of the transfected plasmid (which varies from cell to cell) rather than on the activity of the drug 14. Some reporter parasites which are transformed parasites do not need selective pressure to maintain the reporter gene; however, there could be biological consequences either by disrupting the genomic architecture or just by the presence of the foreign reporter proteins 15. In some reporter gene based assays, there are issues of sensitivity and background activity 16. Most importantly, many of the reporter gene expression assays, specially the one with GFP reporter gene15, may not differentiate between the live and dead intracellular amastigotes. Assays based on luciferase reporter gene may discern between living and dead intracellular amastigotes, but substrate and cell lysis buffer for these assays are expensive for large-scale screening17. To overcome these demerits and limitations of previous macrophage-amastigote-based screening assays, we have developed and optimized this parasite-rescue and transformation assay. This assay is based on THP1 cells, which have good homogeneity and are non-dividing in nature, as host cells.
The Parasite-Rescue-Transformation-Assay assay described here is comparable to the assay based on Digital-Image-Analysis-Direct-Counting of the intracellular amastigotes. Fluorescent and DIC microscopy, digital image analyses by ImageJ for differential counting of the macrophage nuclei and the parasite nuclei have further refined the microscopic counting assay. Capturing the images under fluorescent light filters and differential interference contrast (DIC) filters have improved the quality of the digital image for more accurate counting of the intracellular parasites. Both fluorescent and DIC images can be merged to obtain the digital images with clear macrophage cell outlines and fluorescent intracellular nuclei. The macrophage nuclei and the parasite nuclei can be differentially recognized with ImageJ. Therefore, both Digital-Image-Analysis-Direct-Counting-Assay and Parasite-Rescue-Transformation-Assay have the potential for automation and application to large-scale screening. The critical steps in the Parasite-Rescue-Transformation-Assay are: (a) repeated washings of THP1 cell cultures after exposure to Leishmania promastigotes, to ensure almost complete removal of the non-internalized promastigotes and (b) controlled lysis of the infected THP1 cells with SDS. Both the steps may also be controlled with automation and should not compromise with throughput of the assay. The second step of washings, after exposure of the Leishmania infected THP1 cells to the test drugs/compounds removes the remaining non-internalized parasites, if any. The Parasite-Rescue-Transformation-Assay offers significant advantages over existing microscopic, reporter gene and image analysis assays. The assay is simple, robust, and reproducible, can be automated for large-scale screening and therefore should have important application in screening of large compounds libraries for new anti-leishmanial drug discovery. Further, the assay can also be applied for evaluating infectivity of clinical, as well as, laboratory isolates of Leishmania in vitro.
Disclosures
No conflicts of interest declared.
Acknowledgments
The NCNPR-USDA-ARS Scientific agreement No. 58-6408-2-0009; CDMRP grant Award # W81XWH-09-2-0093 by U.S. Army Medical research and Materiel Command.
References
- WHO. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniasis. Xii. Geneva: 2010. pp. 22–26. [Google Scholar]
- Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 2006;123(3):399–410. [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug Resistance in Leishmaniasis. Clinical Microbiology reviews. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye AlamarBlue. Parasitology International. 2000;48(3):265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- Callahan HL, Portal AC, Devereaux R, Grogl M. An Axenic Amastigote System for Drug Screening. Antimicrob. Agents Chemother. 1997;41(4):818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K, Escobar P, Croft SL. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 2010;65(3):508–511. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- Kolodziej H, Kiderlen AF. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitized RAW 264.7 cells. Phytochemistry. 2005;66(17):2056–2071. doi: 10.1016/j.phytochem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Maia C, et al. Infectivity of five different types of macrophages by Leishmania infantum. Acta Tropica. 2007;103(2):150–155. doi: 10.1016/j.actatropica.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Neal RA, Croft SL. An in vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J. Antimicrob. Chemother. 1984;14:463–475. doi: 10.1093/jac/14.5.463. [DOI] [PubMed] [Google Scholar]
- Abdullah SM, Flath B, Presber HW. Comparison of different staining procedures for the flow cytometric analysis of U-937 cells infected with different Leishmania-species. J. Microbiol Methods. 1999;37(2):123–138. doi: 10.1016/s0167-7012(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Giorgio CD, et al. Flow Cytometric Detection of Leishmania Parasites in Human Monocyte-Derived Macrophages: Application to Antileishmanial-Drug Testing. Antimicrob Agents Chemother. 2000;44(11):3074–3078. doi: 10.1128/aac.44.11.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, et al. High throughput screening of Leishmania donovani clinical isolates against drug using a colorimetric β Lactamase assay. Indian J. Exp. Biol. 2009;47(6):475–479. [PMC free article] [PubMed] [Google Scholar]
- Chan MMY, Bulinski JC, Chang KP, Fong DA. Microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol. Res. 2003;89(4):266–271. doi: 10.1007/s00436-002-0706-4. [DOI] [PubMed] [Google Scholar]
- Buckner FS, Wilson AJ. Colorimetric assay for screening compounds against Leishmania Amastigote Grown in macrophages. Am. J. Trop. Med. 2005;72(5):600–605. [PubMed] [Google Scholar]
- Sereno D, et al. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol. Int. 2007;56(1):3–7. doi: 10.1016/j.parint.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Gupta S, Nishi Visceral leishmaniasis: experimental models for drug discovery. Indian J. Med. Res. 2011;133:27–39. [PMC free article] [PubMed] [Google Scholar]
- Roy G, et al. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. Infections in macrophages and in animal models. Mol. Biochem. Parasitol. 2000;110(2):195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]


