Abstract
Natural killer T (NKT) cells are a unique subset of T cells that display markers characteristic of both natural killer (NK) cells and T cells1. Unlike classical T cells, NKT cells recognize lipid antigen in the context of CD1 molecules2. NKT cells express an invariant TCRα chain rearrangement: Vα14Jα18 in mice and Vα24Jα18 in humans, which is associated with Vβ chains of limited diversity3-6, and are referred to as canonical or invariant NKT (iNKT) cells. Similar to conventional T cells, NKT cells develop from CD4-CD8- thymic precursor T cells following the appropriate signaling by CD1d 7. The potential to utilize NKT cells for therapeutic purposes has significantly increased with the ability to stimulate and expand human NKT cells with α-Galactosylceramide (α-GalCer) and a variety of cytokines8. Importantly, these cells retained their original phenotype, secreted cytokines, and displayed cytotoxic function against tumor cell lines. Thus, ex vivo expanded NKT cells remain functional and can be used for adoptive immunotherapy. However, NKT cell based-immunotherapy has been limited by the use of autologous antigen presenting cells and the quantity and quality of these stimulator cells can vary substantially. Monocyte-derived DC from cancer patients have been reported to express reduced levels of costimulatory molecules and produce less inflammatory cytokines9,10. In fact, murine DC rather than autologous APC have been used to test the function of NKT cells from CML patients11. However, this system can only be used for in vitro testing since NKT cells cannot be expanded by murine DC and then used for adoptive immunotherapy. Thus, a standardized system that relies on artificial Antigen Presenting Cells (aAPC) could produce the stimulating effects of DC without the pitfalls of allo- or xenogeneic cells12, 13. Herein, we describe a method for generating CD1d-based aAPC. Since the engagement of the T cell receptor (TCR) by CD1d-antigen complexes is a fundamental requirement of NKT cell activation, antigen: CD1d-Ig complexes provide a reliable method to isolate, activate, and expand effector NKT cell populations.
Keywords: Immunology, Issue 70, Medicine, Molecular Biology, Cellular Biology, Microbiology, Cancer Biology, Natural killer T cells, in vitro expansion, cancer immunology, artificial antigen presenting cells, adoptive transfer
Protocol
1. Generation of aAPC
Before adding proteins to beads, prepare all reagents and buffers: 0.1M Borate buffer; 1X D-PBS (no Ca2+ and Mg2+); Bead Wash Buffer (1X PBS+5% Human AB serum + 0.02% sodium azide); Complete medium (RPMI medium +100 mM sodium pyruvate,10 mM non-essential vitamin solution, 100 mM MEM Vitamin solution, 1% 2-mercaptoethanol, 10 μM ciprofloxacin, 5% Human AB serum); MACS buffer (1 L PBS free of Ca2+ and Mg2+, 5 g BSA, and 2 mmol EDTA).
Rinse 1 ml Dynabeads M-450 Epoxy beads with 3 ml sterile 0.1 M Borate buffer (boric acid and water, pH 7.0-7.4) in a 5 ml clear borosilicate glass threaded vial.
In a separate 1.5 ml microcentrifuge tube, add 100 μg hCD1d-Ig dimer and 20 μg costimulatory molecules (example: anti-CD28mAb) to 1 ml PBS w/o Ca2+ or Mg2+.
Place bead containing glass vial on magnet and aspirate borate buffer from beads. Add protein mixture from step 1.2 to glass vial and replace cap. Mix immediately by inverting the vial, cover the cap with parafilm, and place on a rotator and incubate overnight at 4 °C.
The next day, place glass vial on magnet and remove protein mixture, while carefully avoiding beads. Wash the beads by adding 3 ml bead wash buffer (PBS with 5% AB serum +0.02% sodium azide), and incubating at 4 °C on a rotator for 5 min. Repeat twice.
Beads can be stored in this bead wash mixture. To make functional aAPC, remove a small aliquot and count the beads using a hemacytometer. Check that the proteins are stably loaded onto the beads by staining with antibodies (ex. PE- conjugated anti-mouse IgG1) and performing flow cytometric analyses.
To load beads with antigen, remove 5 x 107 beads, and add to a small 1.5 ml glass vial, rinse beads with 1 ml sterile PBS. Resuspend washed beads with 1 ml sterile PBS and add antigen; example: Load with α-GalCer/KRN7000 (5mg/ml). * Note: While, we have not detected any issues with lipid solubility or micelle formation, lipids should be handled according to manufacturer's recommendations. Specifically, KRN7000 was reconstituted in DMSO (1mg/ml) for these studies. KRN7000 and other glycolipid antigens can also be dissolved in 0.2 mg/ml PBS containing 0.5% Tween-20 (sonicate 2 hr. at 37 °C). IMPORTANT- The aAPC need to be loaded for at least 48-72 hr prior to use.
2. Isolation of CD161+CD3+ Cells
Collect peripheral blood mononuclear cells (PBMC). For Ficoll density gradient centrifugation separation of lymphocytes from a buffy coat or leukopheresis pack, first dilute heparinized blood with an equal volume of 1X PBS at room temperature.
Add 15 ml of Ficoll (warmed to room temperature) to 50 ml conical tubes. Slowly overlay 25 ml of the diluted blood mixture on top of the Ficoll. Centrifuge at 2,000 rpm for 30 min at room temperature with the brake off.
Carefully remove the lymphocyte interface (white ring between the media and Ficoll) with a Pasteur pipette and transfer to a new 50 ml conical tube.
Wash the cells by filling up the tube to 50 ml with PBS and centrifuging at 1,500 rpm for 5 min. Discard the supernatant and combine the tubes from a single individual to a single tube and wash the peripheral blood mononuclear cells (PBMC) again with 20 ml PBS. Then count the PBMC and resuspend at a concentration of 5 x 107 cells/ml in MACS buffer (1 L PBS free of Ca2+ and Mg2+, 5 g BSA, and 2 mmol EDTA).
In order to isolate the T cell fraction, start with 2 ml of PBMC (108 cells) and add 100 μl of Pan T cell enrichment solution from the EasySep Human T Cell Enrichment Kit. Incubate at room temperature for 10 min.
Add 100 μl of magnetic particles to the solution and incubate at room temperature for another 10 min. Bring the final volume of solvent to 2.5 ml and place the tube in the purple magnet for 5 min. Quickly pour off the CD3+ fraction into a 15 ml conical tube.
Wash the cells by adding 5 ml cold MACS buffer, count the number of viable cells, and remove an aliquot for FACS staining.
To select the CD161+ cells, first resuspend enriched T cells in 980 μl ice cold MACS buffer, add 10 μg anti CD161 mAb, and incubate in refrigerator for 10 min.
Centrifuge the cells at 1,500 rpm at 4 °C for 5 min. Then reconstitute the cell pellet in 800 μl of MACS buffer. Add 200 μl of anti-mouse IgG1 microbeads and incubate the solution for 10 min at 4 °C.
During this incubation step, equilibrate a LS column by adding 3 ml MACS buffer.
Next, wash the cells by centrifuging 1,500 rpm at 4 °C for 5 min. Resuspend the cells in 3 ml MACS buffer. Then pipette the cells into the LS MACS separating column. Make sure to avoid generating bubbles by pipetting slowly. Rinse the column by adding 3 ml of MACS buffer. Repeat twice.
Add 3 ml fresh MACS buffer and remove column from magnet. Place column into a 15 ml conical tube. Insert plunger and push out contents to obtain purified CD161+CD3+ cells. Count NKT cell enriched fraction. You should have 2-4 million cells.
3. aAPC-mediated NKT Cell Expansion
Set up co-culture by adding 106 enriched CD161+CD3+ T cells and 106 aAPC in 16 ml complete medium (complete medium + IL-2, 100 U/ml). Plate this mixture by adding 160 μl/well final volume to a 96 well tissue-culture treated polystyrene, U-bottom plate with low-evaporation lid. Perform medium exchange every 7th day by adding 80 μl of fresh medium.
Harvest cells, count, and perform FACS staining on day 12-14.
The expanded NKT cells can be replated as described above in step 3.1, specifically resuspend 106 expanded T cells and 106 aAPC in 16 ml complete medium. Plate this mixture by adding 160 μl/well to a 96 well U-bottom plate. Continue to refresh coculture medium every 7th day by adding 80 μl of fresh medium.
It is best to freeze extra NKT cells following the second round of expansion (1 x106/cryovial in 1 ml-5% DMSO/ 95% FBS.)
4. Functional Test: aAPC-mediated Stimulation of NKT Cells
Set up 5x104 NKT cells/ well with 5x105 aAPC in 200 μl final volume (complete medium) in 96 well U-bottom plate.
Harvest cell culture supernatant for ELISA after 24-48 hr.
Representative Results
Herein we describe a method for generating CD1d-Ig based aAPC, made by covalent coupling of CD1d-Ig and anti-CD28 mAb to magnetic beads to stimulate NKT cells as a standardized method for the propagation of NKT cells (Figure 1).First, one must demonstrate that the CD1d-Ig fusion proteins are stably immobilized onto the surface of the magnetic beads. As shown in Figure 2A, CD1d-Ig and anti-CD28 antibodies were both expressed on the surface of the magnetic beads. To examine the stimulatory capacity of the aAPC, we co-cultured NKT cell hybridomas with aAPC overnight, harvested the culture supernatants and measured IL-2 production by ELISA. We found that CD1d-Ig based aAPC were able to stimulate the NKT cell hybridomas at levels equal to or higher than their cellular counterparts (Figure 3, data not shown). Interestingly, we found that our mouse NKT cell hybridomas are stimulated by human CD1d-based aAPC (Figure 2B), which provides simple method for testing each batch of aAPC.
Next, we sought to demonstrate the propagation potential of aAPC, thus human T cells were isolated from the peripheral blood. First the CD161+CD3+ T cell fraction was enriched by magnetic bead separation. Then, the T cells were stimulated biweekly with α-GalCer-loaded aAPC. Importantly, we found that even with a relatively low initial NKT cell population (0.03%), we were able to expand the cells to ~67% Vα24+Vβ11+ (Figure 3). We have expanded NKT cells from the PBMC of many healthy volunteers and cancer patients and have found that α-GalCer loaded aAPC were able to expand the NKT cells population in both groups. Notably, the expansion rate was highly donor dependent. As expected the higher the initial population of Vα24+ cells, the greater the percentage of expansion. In addition, when using a starting population of 2 million cells CD161+CD3+T cells, one can obtain >107 cells after two rounds of expansion (Table 1). Approximately 80-90% of the expanded NKT cells are CD4+, ~5% CD8+, and the remaining are presumably CD4-CD8- double negative NKT cells. These expanded NKT cells can be used for functional studies as shown in Figure 4A-C. We have found that our ex vivo expanded NKT cells remain responsive to α-GalCer stimulation and are potent producers of IL-17A, TNF-α, and IFN-γ. It should be noted that if the initial T cell enrichment population is low and one is unable to perform the second CD161 enrichment step, the aAPC-mediated expansion may not yield the expected results (see Figure 4D, Donor 1). However, if the percentage of circulating NKT cells is higher than 0.1%, one should still be able to obtain a significant expansion of iNKT cells. Collectively, these data demonstrate that CD1d based-aAPC can be used to effectively expand and stimulate primary human NKT cells.
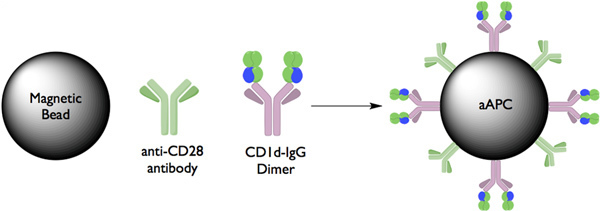 Figure 1. Schematic diagram of CD1d:Ig-based aAPCs Extracellular portions of the CD1d molecule are fused to the constant region of an immunoglobulin heavy chain protein separated by a short amino acid linker. These molecules can be easily loaded with lipid antigens, such as α-GalCer, simply by incubating them with an excess of the lipid of interest. aAPC were made by coupling CD1d-Ig and anti-CD Abs to magnetic beads. In this system, CD1d-Ig is used to provide the cognate antigen-specific signal through the TCR and anti-CD28 mAb provides the costimulatory signal.
Figure 1. Schematic diagram of CD1d:Ig-based aAPCs Extracellular portions of the CD1d molecule are fused to the constant region of an immunoglobulin heavy chain protein separated by a short amino acid linker. These molecules can be easily loaded with lipid antigens, such as α-GalCer, simply by incubating them with an excess of the lipid of interest. aAPC were made by coupling CD1d-Ig and anti-CD Abs to magnetic beads. In this system, CD1d-Ig is used to provide the cognate antigen-specific signal through the TCR and anti-CD28 mAb provides the costimulatory signal.
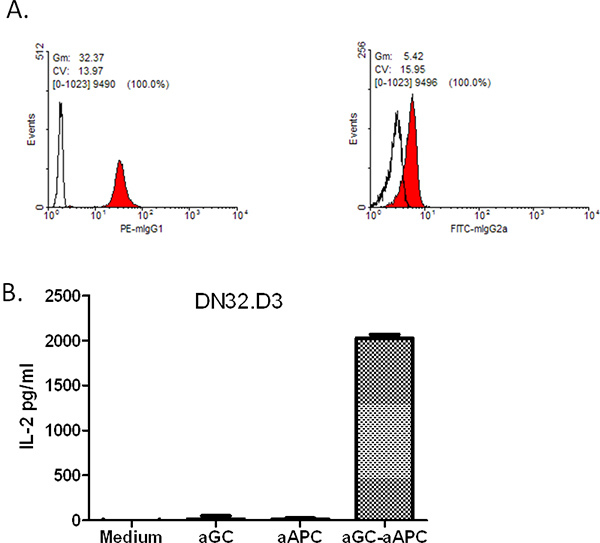 Figure 2. FACS staining of surface proteins on aAPCs. A) aAPCs were tested for the presence of CD1d:IgG dimer (via staining with PE-conjugated anti-mouse IgG1) as well as anti-CD28 antibody (using FITC-conjugated anti-mouse IgG2a).Open histograms indicate isotype control; filled histograms represent the indicated antibodies. CD1d-Ig Expressing aAPC can Stimulate IL-2 Production by NKT cells. B) The Vα14+ mouse NKT cell hybridoma, DN32.D3, was cocultured with either medium, soluble antigen (α-GalCer), unloaded aAPC or α-GalCer-loaded aAPC. Culture supernatants were harvested and standard sandwich ELISA was used to measure IL-2 production.
Figure 2. FACS staining of surface proteins on aAPCs. A) aAPCs were tested for the presence of CD1d:IgG dimer (via staining with PE-conjugated anti-mouse IgG1) as well as anti-CD28 antibody (using FITC-conjugated anti-mouse IgG2a).Open histograms indicate isotype control; filled histograms represent the indicated antibodies. CD1d-Ig Expressing aAPC can Stimulate IL-2 Production by NKT cells. B) The Vα14+ mouse NKT cell hybridoma, DN32.D3, was cocultured with either medium, soluble antigen (α-GalCer), unloaded aAPC or α-GalCer-loaded aAPC. Culture supernatants were harvested and standard sandwich ELISA was used to measure IL-2 production.
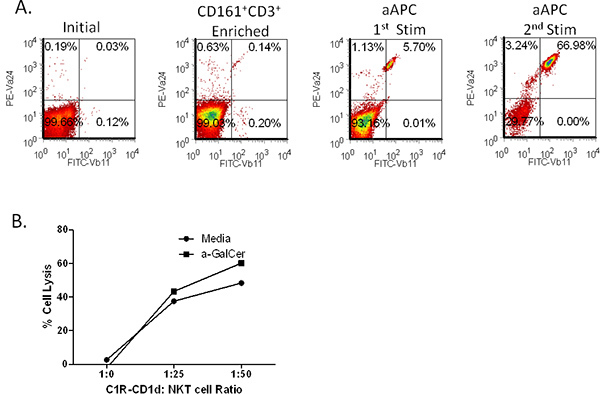 Figure 3. Expansion of NKT cells by CD1d-Ig coated artificial antigen presenting cells. (A) Primary CD3+CD161+ double positive cells were isolated from PBMCs using magnetic separation. The sorted cells were stimulated with α-GalCer loaded,CD1d-Ig coated aAPC for 14 days. The cells were stained for Vα24 and Vβ11 following aAPC stimulation. (B) Primary human NKT cell mediated lysis of a B cell lymphoma line. C1R-CD1d cells incubated with NKT cells at the indicated ratios in the presence or absence of antigen, a-GalCer (100 ng/ml) in 96-well U- bottom plates for 20-24 hr. NKT cell mediated cell lysis was assessed by by standard 51Cr-release assay.
Figure 3. Expansion of NKT cells by CD1d-Ig coated artificial antigen presenting cells. (A) Primary CD3+CD161+ double positive cells were isolated from PBMCs using magnetic separation. The sorted cells were stimulated with α-GalCer loaded,CD1d-Ig coated aAPC for 14 days. The cells were stained for Vα24 and Vβ11 following aAPC stimulation. (B) Primary human NKT cell mediated lysis of a B cell lymphoma line. C1R-CD1d cells incubated with NKT cells at the indicated ratios in the presence or absence of antigen, a-GalCer (100 ng/ml) in 96-well U- bottom plates for 20-24 hr. NKT cell mediated cell lysis was assessed by by standard 51Cr-release assay.
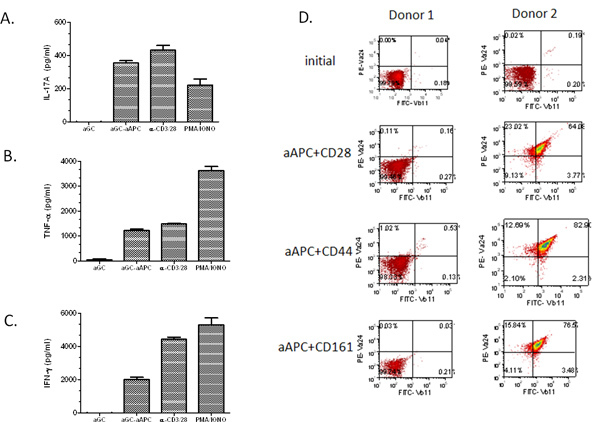 Figure 4. Cytokine profiles of aAPC-expanded NKT cells. After stimulation with α GalCer loaded aAPC for two weeks, the expanded NKT cells(1×105/well) were cocultured with soluble α-GalCer, PMA/Ionomycin, anti-CD3/28 microbeads, or α-GalCer loaded aAPC (2×105/well) for 48 hr. (A) IL-17A, (B) TNF-α, and (C) IFN-γ production was measured by standard cytokine ELISA. Data shown are net cytokine production after subtracting the negative controls (media and empty beads). (D) Primary T cells were isolated from PBMC using magnetic bead separation. The sorted cells were stimulated for two weeks with the indication a-GalCer loaded-aAPC. The cells were stained using Abs specific for Vα24+ and Vβ11+ and analyzed by flow cytometry.
Figure 4. Cytokine profiles of aAPC-expanded NKT cells. After stimulation with α GalCer loaded aAPC for two weeks, the expanded NKT cells(1×105/well) were cocultured with soluble α-GalCer, PMA/Ionomycin, anti-CD3/28 microbeads, or α-GalCer loaded aAPC (2×105/well) for 48 hr. (A) IL-17A, (B) TNF-α, and (C) IFN-γ production was measured by standard cytokine ELISA. Data shown are net cytokine production after subtracting the negative controls (media and empty beads). (D) Primary T cells were isolated from PBMC using magnetic bead separation. The sorted cells were stimulated for two weeks with the indication a-GalCer loaded-aAPC. The cells were stained using Abs specific for Vα24+ and Vβ11+ and analyzed by flow cytometry.
Discussion
aAPC can be used to study the basic requirements for NKT cell activation and it has potential clinical value for ex vivo expansion of NK T cells for adoptive immunotherapy. Mescher et al. described one of the first bead based systems, where biotinylated murine MHC class I-peptide-single chain constructs were combined with biotinylated costimulatory molecules B7.1 and B7.2 via streptavidin to the surface of latex microspheres 14, 15. This approach has successfully been used to stimulate antigen-specific T cells from transgenic mice. In addition, since this approach uses a single chain MHC-peptide complex to ensure homogenous loading of the MHC molecules, each target peptide antigen would require a new transfection for expression of the desired single chain MHC-peptide complex, thus limiting the generality of the approach. Importantly, Dr. Schneck's group pioneered the bead based-aAPC, by developing another non-cellular bead based aAPC, made by coupling HLA-Ig, signal 1, and anti-CD28, signal 2, onto magnetic beads. HLA-Ig, a unique multimeric form of HLA fused to an immunoglobulin molecular scaffold 16, 17 was developed by his group. Subsequently, they developed MHC-Ig based aAPC, which have been shown to effectively expand CMV and MART-1 specific CTL 18. Here, we have demonstrated that CD1d-Ig based aAPC can be used to expand functional NKT cells. One study has used a similar system to examine the physical interaction of NK cells with CD1d 19.
Notably, we have designed an artificial antigen presenting cell which is adaptable to any requirements we find necessary for optimal NKT cell proliferation. The aAPC expansion method provides a simple and reliable method for expanding and enriching human NKT cells. Our aAPC can be modified to systematically evaluate the role of a panel of potential costimulatory molecules and assess their role on NKT cell proliferation and function. Thus, aAPC represent a robust versatile technology useful for inducing and expanding NKT cells. The generation of aAPCs takes less than one week and is suitable for the production of large quantities of beads. However, a critical step in generating the aAPC is to confirm that CD1d-Ig is stably immobilized on the surface of the beads and to assess their functionality to ensure consistency from batch to batch. A potential limitation of the system is that there is not a mechanism in place to turn off stimulation, other than mechanical removal of the beads. Specifically, the engagement of the T cell receptor (TCR) with the antigen: CD1d/MHC complex typically generate the immunological synapse in concert with accessory/adhesion molecules, which can result in the induction of inhibitory or suppressive factors on both the T cell and antigen presenting cell. In the aAPC system, these factors may be upregulated by the T cell, but the bead will not express the cognate ligands for these receptors.
In addition, CD4+ NKT cells have been shown to suppress antitumor responses in mice and humans, therefore it is possible that nonselective activation of all NKT cells (i.e. global stimulation with α-GalCer) or activation of the wrong subset could result in unwanted immunological outcomes. Consequently, one must phenotypically and functionally characterize the aAPC-expanded NKT cell population. As shown in Figure 4, we have found that stimulation with α-GalCer-loaded aAPC expressing anti-CD28 can result NKT cells producing Th1, Th2, and Th17 type cytokines. Murine studies have reported that challenge with IL-33, a recently identified cytokine, resulted in increased levels of circulating inflammatory cytokines such as IL-5 and IL-13. Treatment of NKT cells with IL-33 enhanced their cytokine production 20. IL-33 is a specific ligand for ST2 and it has been shown that soluble ST2 can block IL-33 signaling. Thus, as an example of a future application, aAPC expressing ST2 could be generated and used to determine if one could selectively inhibit the production of Th2 cytokines while inducing Th1 cytokine secretion by NKT cells. It has also been reported that i.v. injection of Kb-expressing aAPC into C57BL/6 mice resulted in decreased lung metastasis of tumor 21. Importantly, these data demonstrate that aAPC traffic to the lung and are able to activate effector T cell subsets. Therefore, one could generate multiple types of aAPC and examine the interplay between antigen specific T cell subsets. To summarize, these studies demonstrate that CD1d-Ig based aAPC can be used to replace normal cellular APC, and have to the potential to enhance current clinical approaches for NKT cell based- adoptive immunotherapy.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Priyanka Subrahmanyam for helpful discussions. The authors have no competing financial interest. This work was supported by grants from the American Cancer Society, NIH/NCI K01 CA131487, R21 CA162273, R21 CA162277, and P30 Tumor Immunology and Immunotherapy Program to T.J. Webb. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Fowlkes BJ. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Prigozy TI. Glycolipid antigen presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- Davodeau F. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J. Immunol. 1997;158:5603–5611. [PubMed] [Google Scholar]
- Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4-CD8- T cells. J. Exp. Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H. Dominant expression of a distinctive V14+ T-cell antigen receptor α chain in mice. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7518–7522. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J. Exp. Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli SA, Modlin RL. The CD1 System: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- Harada Y, et al. Expansion of alpha-galactosylceramide-stimulated Valpha24+ NKT cells cultured in the absence of animal materials. J. Immunother. 2005;28:314–321. doi: 10.1097/01.cji.0000163593.66910.ad. [DOI] [PubMed] [Google Scholar]
- Bella SD, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br. J. Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H, et al. Dysfunctional and Short-Lived Subsets in Monocyte-Derived Dendritic Cells from Patients with Advanced Cancer. Clinical Immunology. 2002;105:286–295. doi: 10.1006/clim.2002.5293. [DOI] [PubMed] [Google Scholar]
- Shimizu K, et al. Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J. Immunol. 2006;177:3484–3492. doi: 10.4049/jimmunol.177.5.3484. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi T, Schneck J, Kawamura A, Tsuji M. Human CD1 dimeric proteins as indispensable tools for research on CD1-binding lipids and CD1-restricted T cells. Journal of immunological. 2009;345:49–59. doi: 10.1016/j.jim.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J. Immunol. Methods. 2009;346:38–44. doi: 10.1016/j.jim.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham EL, Jensen PL, Mescher MF. Activation of antigen-specific T cells by artificial cell constructs having immobilized multimeric peptide-class I complexes and recombinant B7-Fc proteins. J. Immunol. Methods. 2001;249:111–119. doi: 10.1016/s0022-1759(00)00335-5. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Shrikant P, Mescher MF. In vivo augmentation of tumor-specific CTL responses by class I/peptide antigen complexes on microspheres (large multivalent immunogen) J. Immunol. 2003;170:228–235. doi: 10.4049/jimmunol.170.1.228. [DOI] [PubMed] [Google Scholar]
- Dal Porto J. A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6671–6675. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. PNAS. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelke M, et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- Huang MMS, Borszcz P, Sidobre S, Kronenberg M, Kane KP. CD1d1 Displayed on Cell Size Beads Identifies and Enriches an NK Cell Population Negatively Regulated by CD1d1. J. Immunol. 2004;172:5304–5312. doi: 10.4049/jimmunol.172.9.5304. [DOI] [PubMed] [Google Scholar]
- Bourgeois E. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- Ugel S, et al. In vivo administration of artificial antigen-presenting cells activates low-avidity T cells for treatment of cancer. Cancer Res. 2009;69:9376–9384. doi: 10.1158/0008-5472.CAN-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]


