Abstract
Adult stem cells are well known for their self-renewal and regenerative capacity. The mechanisms protecting these cells from inflammatory damage have not been well elucidated. This study investigated the immunoprotective properties of corneal epithelial stem cells from inflammation by producing glial cell-derived neurotrophic factor (GDNF). Primary human limbal epithelial cells (HLECs) cultured from limbal explants were treated with interleukin (IL)-17A, tumor necrosis factor (TNF)-α, or hyperosmotic media, with or without GDNF or nuclear factor kappa B (NF-κB) inhibitor (NF-κB-I) for 4–48 hours. Inflammatory mediators and Th17-inducing cytokines were determined by real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and immunobead assays. NF-κB activation was detected by p65 phosphorylation, immunostaining and Western blotting. GDNF and its receptor, GDNF family receptor α-1, were exclusively immunolocalized in the basal layer of limbal epithelium, whereas IL-17 receptor was negative in these cells. Exogenous IL-17A stimulated the expression and production of inflammatory cytokines (TNF-α, IL-6, and IL-1β) and chemokine IL-8 by HLECs. Th17-inducing cytokines, transforming growth factor (TGF)-β1, IL-6, IL-23, and IL-1β, were significantly increased at mRNA and protein levels by HLECs exposed to TNF-α or hyperosmotic media. IL-17 activated NF-κB by p65 phosphorylation at serine 536 and nuclear translocation. GDNF or NF-κB-I blocked IL-17-induced NF-κB p65 activation and production of inflammatory mediators. Furthermore, GDNF suppressed the production of Th17-inducing cytokines through inhibiting NF-κB activation. These findings demonstrate that limbal progenitor cell-produced neurotrophic factor GDNF suppresses IL-17-mediated inflammation via NF-κB signaling pathway. This may represent a unique immunoprotective property of limbal stem cells against inflammatory challenges on the ocular surface.
Keywords: Glial cell-derived neurotrophic factor, Adult stem cell, Immunoprotection, Th17, Corneal epithelium
Introduction
The concept that the corneal epithelium is maintained by stem cells residing in the limbal region of the eye was originally proposed by Davanger and Evensen [1]. Since then, substantial evidence supporting the limbal location of corneal epithelial stem cells has emerged, although a definitive marker identifying the limbal stem cells remains elusive. The major evidence includes the following: limbal epithelial basal cells retain tritiated thymidine for long periods thus indicating that they are slow cycling [2]; limbal basal cells have a greater proliferative capacity in vitro than central and paracentral corneal epithelial cells [3]; wounding or surgical removal of the limbus results in delayed healing with noncorneal epithelium [4]. Evidence of limbal stem cell deficiency is often seen in patients with severe or chronic ocular surface inflammation. The ability of these cells to resist damaging effects of inflammation is essential for their survival.
Glial cell line-derived neurotrophic factor (GDNF) is a potent neurotrophic factor (NTF) that enhances the survival of cells in the peripheral and central nervous system, such as dopaminergic neurons, motor neurons, and auditory neurons. GDNF binds to its high-affinity membrane-bound GDNF family receptor (GFR) α-1 and initiates a signaling cascade [5]. Although NTFs are defined as molecules that maintain neuronal cells, they possess a range of functions outside the nervous system [6, 7]. For example, GDNF was identified as an essential growth factor supporting self-renewal of spermatogonial stem cells. GDNF receptor GFRα-1 is strongly expressed by a subset of spermatogonia, including the stem cells for spermatogenesis [8, 9]. Interestingly, we have found that GDNF and GFRα-1 are components of the human corneal epithelial precursor cell phenotype [10] and that GDNF gene delivery enhances survival of human corneal epithelial cells [11].
Interleukin (IL)-17-producing T lymphocytes have been recently shown to comprise a lineage of T-helper cells known as T-helper-17 (Th17) cells, which are distinct from Th1 and Th2 cells. The Th17 pathway has been shown to be involved in the pathogenesis of many autoimmune and inflammatory diseases, particularly those occurring in mucosal tissues such as the ocular surface [12–15]. The initiation of Th17 cell differentiation from naive CD4+ T-cells requires transforming growth factor (TGF)-β and IL-6; and the survival, expansion, and efficient production of IL-17 by these Th17 cells require additional cytokines including IL-23, IL-21, and IL-1β [16–18]. IL-17 initiates proinflammatory effects by binding to the IL-17 receptor (IL-17R), which is expressed by a variety of cell types including epithelial, endothelial, and fibroblastic stromal cells [19, 20]. We have observed that mucosal epithelium are enriched for Th17-inducing cytokines, Th17 inflammation developed on ocular surface, and the inducing cytokines produced by ocular surface epithelium promote Th17 differentiation of naïve CD4+ T-cells [21–23].
In this study, we show that GDNF and its receptor GFRα-1 are exclusively localized to the basal layer of limbal epithelium where stem cells reside, whereas IL-17R are not expressed by this cell layer. We further investigated the potential role of GDNF in suppressing IL-17-mediated inflammation in corneal epithelial stem cells. Our findings indicate that the immunoprotective property of adult stem cells by producing a novel neurotrophic factor GDNF, which may have therapeutic implication for treating Th17-mediated ocular surface diseases.
Materials and Methods
Material and Reagents
Cell culture dishes, plates, centrifuge tubes, and other plastic ware were purchased from Becton Dickinson (Lincoln Park, NJ, http://www.bd.com). Dulbecco-modified Eagle medium (DMEM), Ham F-12, amphotericin B, gentamicin, 0·25% trypsin/0·03% EDTA solution, and Fluor 488 (green)-conjugated goat anti-rabbit IgG were from Invitrogen (Carlsbad, CA, http://www.invitrogen.com). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Optimal cutting temperature compound and cryomolds were from Sakura Finetek (Torrance, CA, http://www.sakuraeu.com). Affinity-purified rabbit antibody against GDNF was purchased from Lab Vision (Fremont, CA, http://www.labvision.com). Rabbit antibodies against human GFRα-1, IL-17R, nuclear factor kappa B (NF-κB) p65, and antibody-specific blocking peptides were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, http://www.scbt.com). RNeasy Mini Kit was from Qiagen (Valencia, CA, http://www.qiagen.com). Ready-To-Go-Primer First-Strand Beads and Enhanced Chemiluminescence (ECL) Plus reagents were from GE Health Care, Inc. Tagman gene expression assays and real-time polymerase chain reaction (PCR) Master Mix were from Applied BioSystems (Foster City, CA, http://www.appliedbiosystems.com). Fast activated cell-based ELISA (FACE) NF-κB p65 (ser536) Profiler and Nuclear Extract kits were from Active Motif (Carlsbad, CA, http://www.activemotif.com); Ready Gel for protein electrophoresis (4%–15% Tris-HCl), prestained SDS-polyacrylamide gel (PAGE) low-range standards, precision plus protein standards, precision protein strep tactinhorseradish peroxidase (HRP) conjugate were from Bio-Rad (Hercules, CA, http://www.bio-rad.com). TGF-β1 and IL-23 ELISA kits were from R&D Systems (Minneapolis, MN, http://www.rndsystems.com). Immobilon-P polyvinylidendifluoride (PVDF) membrane and multiplex immunobead assays for tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-8 were from Millipore (Billerica, MA; http://www.millipore.com). Propidium iodide (PI) and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO; http://www.sigmaaldrich.com).
Human Corneal Limbal Tissues and Primary Limbal Epithelial Cultures
Human corneoscleral tissues, which did not meet the criteria for clinical use, from donors aged 17–64 years were obtained from the Lions Eye Bank of Texas (Houston, TX). These tissues were preserved in Optisole-GS (Bausch and Lomb Inc, Rochester, NY) at 4°C before use. Fresh human corneoscleral tissues (less than 72 hours postmortem) were cut through the central cornea and limbus, frozen, and sectioned for immunostaining or prepared for explant culture. The corneal and limbal specimens were prepared using a previously described method [24]. The limbal area was defined by the end of the Bowman’s layer, where vascularized soft connective tissue was noted. The peripheral cornea was defined as the region located 2 mm from this limbal landmark toward the cornea, where the underlying stroma was compact and avascular. The central cornea was defined as the portion of the cornea inside a ring, 4 mm inside the limbal landmark.
Primary human limbal epithelial cells (HLECs) were established from limbal explants without 3T3 fibroblasts using a previously described method [25] with modification. In brief, each limbal rim was cut into 12 equal pieces (about 2 × 2 mm2 size each). Two pieces with their epithelium side up were directly placed into a well of six-well culture plates or one pierce into a well of eight-chamber slides, and they were covered with a drop of FBS overnight. The explants were then cultured in a supplemented hormonal epidermal medium containing 5% FBS (SHEM) at 37°C under 5% CO2 and 95% humidity. The media was renewed every 2–3 days.
Cell Treatment for IL-17 Inflammatory Effects and Th17-Inducing Cytokines
To investigate the inflammatory effects of IL-17, confluent primary HLECs (5 × 105 cells/well) were switched to a serum-free medium (SHEM without FBS) for 24 hours, then treated with a recombinant human IL-17A at concentrations of 1–10 ng/ml for 4–48 hours with or without NF-κB activation inhibitor quinazoline (NF-κB inhibitor [NF-κB-I], 5 µM) or GDNF (10 ng/ml), which was preloaded to the medium 1 hour before adding the IL-17A.
To evaluate Th17-inducing cytokines produced by primary HLECs in response to TNF-α and hyperosmotic stress, the cells were treated with proinflammatory cytokine TNF-α (1–10 ng/ml) or hyperosmotic media (400–500 mOsM) that were achieved by adding 50–90 mM sodium chloride as described previously [26], for 4–48 hours in the absence or presence of 5 µM NF-κB-I or 10 ng/ml GDNF, which was preloaded in medium for 1 hour.
The cells treated for 4–8 hours were subjected to RNA extraction, reverse transcription (RT), and real-time PCR; and the cells treated for 24–48 hours were used for protein assays by enzyme-linked immunosorbent assay (ELISA), Luminex immunobead assays, and Western blot analysis. To detect phospho-p65, the cultured HLECs were seeded at 50,000 cells/well in 96-well plates and treated with above-mentioned conditions for a shorter time of 15, 30, or 60 minutes. All experiments were repeated at least three times using cultures initiated from different donor corneas.
Immunofluorescent Staining
Immunofluorescent staining was performed as a method previously reported [24]. Briefly, corneal- and limbal-frozen sections were fixed with cold methanol (for GDNF, p65) or 2% paraformaldehyde (for GFR-α1 and IL-17R) at 4°C for 10 minutes. The sections were incubated with primary antibodies against GDNF (1:100, 2 µg/ml), GFRα-1 (1:50, 4 µg/ml), IL-17R (1:100, 2 µg/ml), or NF-κB p65 (1:50, 4 µg/ml) for 1 hour, followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody (1:300) applied for 1 hour, then PI (2 µg/ml) for 5 minutes for nuclear counterstaining. Sections were examined and photographed with an epifluorescent microscope (Eclipse 400, Nikon, Japan) with a digital camera (model DMX 1200, Nikon).
RNA Extraction, RT, and Quantitative Real-Time PCR
Total RNA was isolated from cells using a Qiagen RNeasy Mini kit according to manufacturer’s protocol and quantified by a NanoDrop ND-1000 Spectrophotometer and stored at −80°C. The first strand cDNA was synthesized by RT from 1 µg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described [27, 28]. The real-time PCR was performed in a Mx3005P system (Stratagene) with 20 µl reaction volume containing 5 µl of cDNA, 1 µl of TaqMan Gene Expression Assay, and 10 µl Master Mix. The thermocycler parameters were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 second, and 60°C for 1 minute. A nontemplate control was included to evaluate DNA contamination. The results were analyzed by the comparative threshold cycle method and normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [28, 29]. TaqMan Gene Expression Assays include human GAPDH (Assay ID Hs99999905_m1), TNF-α (Hs00174128_m1), IL-1β (Hs01555413_m1), IL-6 (Hs00174131_m1), IL-8 (Hs00174103_m1), TGF-β1 (Hs00171257_m1), and IL-23 (Hs00372324_m1).
ELISA and Luminex Immunobead Assay
Double-sandwich ELISA for human TGF-β1 and IL-23 was performed to determine their concentration in the supernatants of the media from cultures after different treatment, using commercial kits from R&D Systems and according to the manufacturer’s protocols. Absorbance was read at 450 nm with a reference wavelength of 570 nm by a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA). Supernatant concentrations of TNF-α, IL-1β, IL-6, and IL-8 were measured using Luminex multiplex immunobead assays from Millipore according to the manufacturer’s protocols. The reactions were detected with streptavidinphycoerythrin with a Luminex 100 IS 2.3 system (Luminex, Austin, TX).
FACE for NF-κB p65 Activation
NF-κB p65 activation was quantitatively measured by a FACE NF-κB p65 Profiler Kit (Active Motif) that specifically quantifies phosphorylated NF-κB p65 and total NF-κB p65 according to the manufacturer’s protocol. Briefly, HLECs were cultured in 96-well plates and stimulated with IL-17, TNF-α, or 400–500 mOsM hyperosmotic media with or without NF-κB-I or GDNF as described earlier. Following treatment, the cells were rapidly fixed to preserve activation-specific protein modifications. Each well is then incubated with a primary antibody that recognizes NF-κB p65 phosphorylated at Serine 536 or total NF-κB p65. After incubation with HRP-conjugated secondary antibody and colorimetric developing solution, the absorbance was read at 450 nm with a reference wavelength of 655 nm by a VERSAmax microplate reader.
Western Blot Analysis
Western blot analysis was performed using a previously reported method [26]. Cytoplasmic and nuclear extracts were prepared using a Nuclear Extract kit (Active Motif) according to manufacturer’s instructions. Total protein concentration in the supernatants of HLECs was measured by a Micro BCA protein assay kit. Cytoplasmic and nuclear extracts (50 µg per lane) were loaded to SDS-PAGE for electrophoresis and blotted onto PVDF membranes. NF-κB subunit p65 was detected with a rabbit anti-human p65 antibody (1:200, 1 µg/ml) followed by a HRP-conjugated goat anti-rabbit IgG (1:2,000 dilution). The signal bands were detected with an ECL chemiluminescence reagent using a Kodak image station 2000R (Eastman Kodak, New Haven, CT).
Statistical Analysis
Student’s t test was used to compare difference between two groups. One-way analysis of variance (ANOVA) test was used to make comparisons among three or more groups, and the Dunnett’s test was further used to compare each treated group with the control group. p values <.05 were considered statistically significant.
Results
An Exclusive Pattern of GDNF, GFRα-1, and IL-17R Localization in Human Corneal Limbus
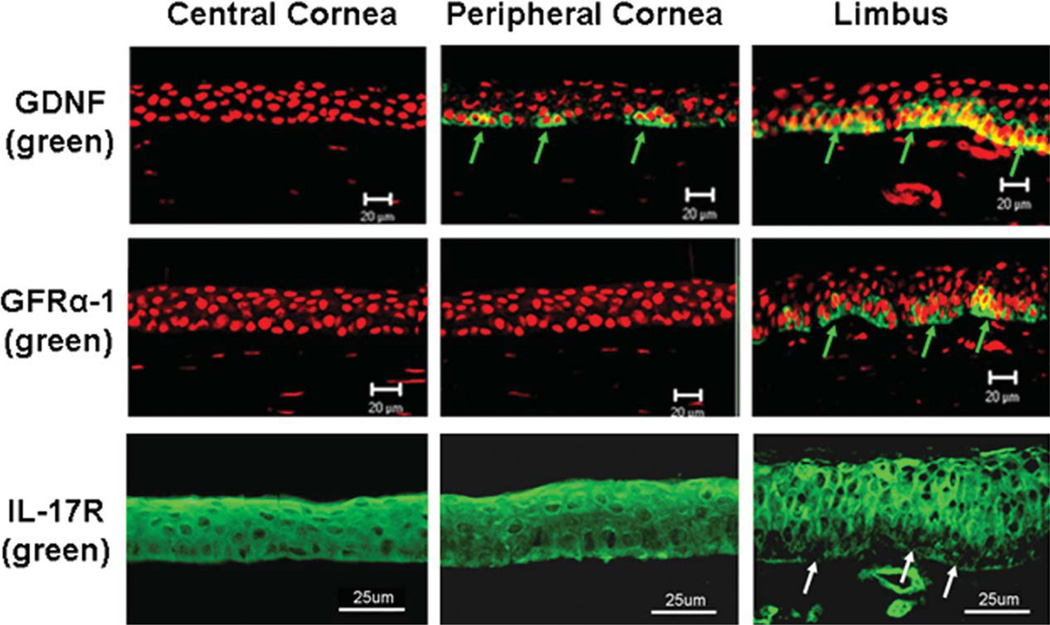
Immunofluorescent staining was performed on cryosections of human corneoscleral tissues. The meridional sections cut from the limbus through the central cornea displayed a traditional view of the limbus with about 8–10 layers of epithelium and the central cornea with about five layers of epithelium [24]. As shown in Figure 1, GDNF immunoreactivity was found to be exclusively localized to subsets of the basal cells of human limbal epithelium, whereas the suprabasal layers of the limbal epithelium and the entire central corneal epithelium were totally negative. Clusters of GDNF-positive cells were interspersed between negatively stained basal cells in the limbal basal layer. Viewing from the limbus toward the peripheral cornea, the positive cell clusters were observed to be looser and smaller and were separated with more negatively stained cells. GFRα-1 shared the same expression pattern with its ligand with staining restricted to the limbal basal layer. The specific immunoreactivity to GDNF and GFRα-1 was abolished in the negative control sections that were treated with antibody that was preneutralized by its respective blocking peptide for 2 hours.
Figure 1.
Representative images showing the immunofluorescent staining of GDNF, GFRα-1, and IL-17R (green color) with propidium iodide (red) nuclear counterstaining on frozen sections of human corneoscleral tissues. Green arrows: positive signals for GDNF or GFRα-1; White arrows: negative staining for IL-17R. Scale bar = 20 or 25 µm. Abbreviations: GDNF, glial cell-derived neurotrophic factor; GFRα-1, GDNF family receptor α-1; IL, interleukin.
IL-17R has been recently cloned and characterized from a human T-cell library, and it exhibits a broad tissue distribution including cornea [19, 20] in response to IL-17 inflammatory stimulation. We observed broad IL-17R immunoreactivity of the membrane and cytoplasm of cells on the human ocular surface, including epithelial and stromal cells. Interestingly, IL-17R was immunolocalized at most corneal and limbal epithelial layers except the basal cells of the limbal epithelium, where corneal epithelial stem cells reside and they did not express IL-17R (Fig. 1, bottom panel).
GDNF Suppresses IL-17-Induced Inflammatory Cytokine Production by HLECs
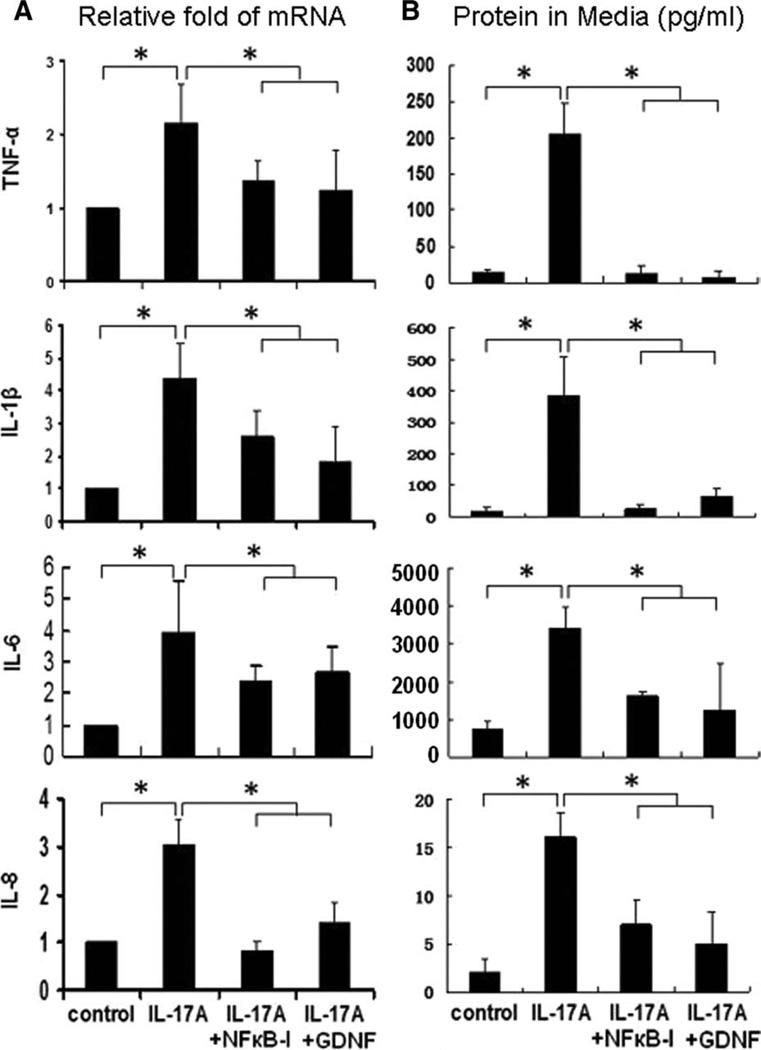
Among IL-17 cytokine family, IL-17A and IL-17F are the signature cytokines secreted by Th17 cells. Both IL-17A and IL-17F are inflammatory cytokines that stimulate a variety of tissues, including epithelium, to produce proinflammatory mediators; and IL-17A, also named IL-17, has been found to be more potent than IL-17F [30–32]. To investigate the inflammatory effect of exogenous IL-17 on HLECs, we measured the mRNA expression and protein production of cytokines (TNF-α, IL-1β, and IL-6) and chemokine IL-8 by quantitative real time PCR and Luminex immunobead assay. All real-time PCR results were standardized to control samples. As shown in Figure 2A, addition of recombinant human IL-17A (10 ng/ml) significantly increased mRNA levels (twofold to fourfold) of these inflammatory cytokines and chemokines expressed by HLECs. These stimulatory responses to IL-17A were confirmed by fourfold to sevenfold increase of TNF-α, IL-1β, IL-6, and IL-8 protein concentrations in HLECs (Fig. 2B). Interestingly, stimulated production of these inflammatory cytokines and chemokine were significantly suppressed at both mRNA (all p < .05, n = 4) and protein levels (p < .05 or .01) by a neurotrophic factor GDNF or a transcription factor NF-κB activation inhibitor quinazoline (NF-κB-I), which was added to cultures 1 hour before IL-17A.
Figure 2.
The inflammatory effects of IL-17A on human limbal epithelial cells (HLECs). Primary HLECs (5 × 105 cells/well) were treated with IL-17A at 10 ng/ml for 4–48 hours with or without NF-κB activation inhibitor quinazoline (NF-κB-I, 5 µM) or GDNF (10 ng/ml). The proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokine IL-8 were measured by reverse transcription and quantitative real-time polymerase chain reaction for mRNA (A) and by enzyme-linked immunosorbent assay or Luminex immunobead assays in culture supernatants (B). Results shown are mean ± SD of four independent experiments. *, p < .05; **, p < .01; by t test. Abbreviations: GDNF, glial cell-derived neurotrophic factor; IL, interleukin; NF-κB-I, nuclear factor kappa B inhibitor; TNF-α, tumor necrosis factor-α.
GDNF Suppresses the Production of Th17-Inducing Cytokines Stimulated by TNF-α and Hyperosmotic Stress in HLECs
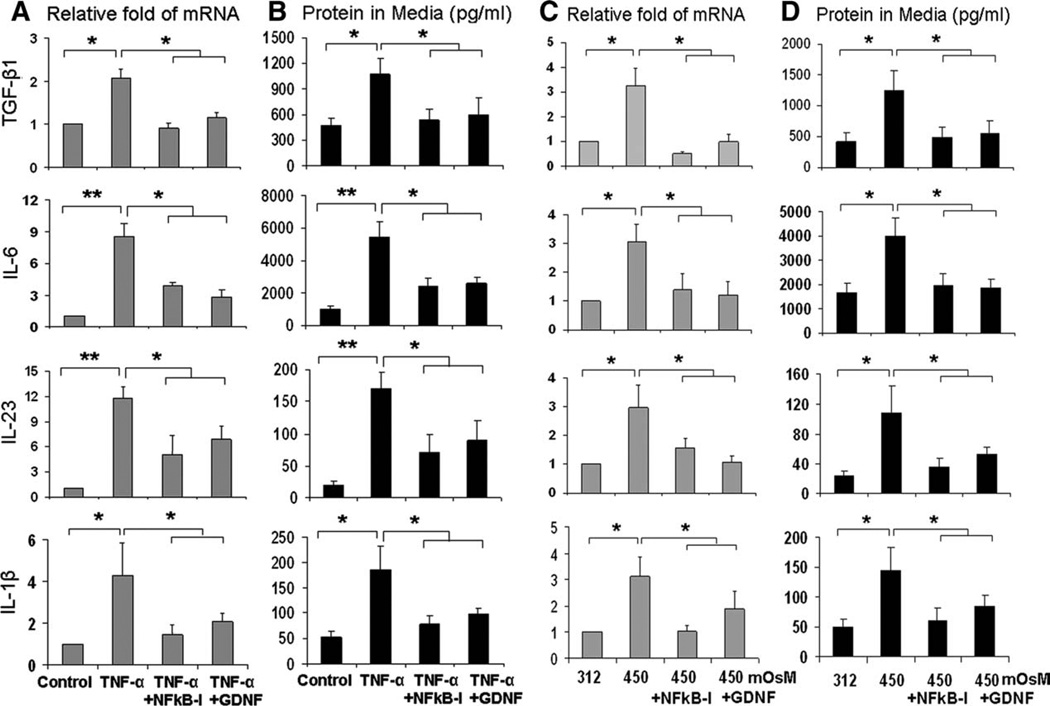
As one type of mucosal epithelia, the corneal epithelium is not only a target of Th17-cell-produced inflammatory cytokines, but it is also a producer of the Th17-inducing cytokines that participate in the induction of Th17 differentiation. In a recent in vitro study using mouse CD4+ T-cells, we have observed that TGF-β and IL-6, cytokines produced by the corneal epithelium, are essential for initiating Th17-cell differentiation, and IL-23 and IL-1β are major inducers that strongly promote the Th17 cell expansion [21]. Here, we evaluated whether physiological inflammatory stressors, including inflammatory cytokines and hyperosmotic media, stimulate production of these Th17-inducing cytokines in HLECs. To investigate factors regulating the expression and production of these Th17-inducing cytokine in response to an inflammatory stimulus, HLECs were treated with TNF-α (10 ng/ml) alone or in combination with GDNF (10 ng/ml) or NF-κB activation inhibitor quinazoline (NF-κB-I, 5 µM) for 4–48 hours. As shown in Figure 3A, TNF-α significantly stimulated mRNA levels of TGF-β1, IL-6, IL-23, and IL-1β by 2.1-to 11.8-fold (p < .05 or .01, respectively n = 4) in 4 hours; mRNA levels of IL-6 and IL-23 were observed to be much higher (8.5- to 11.8-fold, both p < .01, n = 4) than TGF-β1 and IL-1β (2.1- to 4.3-fold, p < .05) in response to TNF-α stimulation. Luminex immunobead assays or ELISA confirmed the stimulation of these inducing cytokine proteins in culture supernatants of HLECs exposed to TNF-α for 48 hours (Fig. 3B; p < .05 for TGF-β1 and IL-1β and p < .01 for IL-6 and IL-23, respectively, all n = 4). Interestingly, these stimulated responses of Th17-inducing cytokines were significantly suppressed at both mRNA and protein levels (Fig. 3A, 3B; all p < .05, n = 4) by neurotrophic factor GDNF or NF-κB-I, when they were added to the culture media 1 hour before TNF-α.
Figure 3.
Expression and production of Th17-inducing cytokines by human limbal epithelial cells (HLECs) exposed to TNF-α ([A] and [B]) and hyperosmotic media ([C] and [D]). The HLECs were treated with TNF-α (10 ng/ml) or hyperosmotic media (450 mOsM) for 4–48 hours in the absence or presence of 5 µM NF-κB-I or 10 ng/ml GDNF, which was preloaded to the medium for 1 hour. The Th17-inducing cytokines (TGF-β1, IL-6, IL-23, and IL-1β) were measured by reverse transcription and quantitative real-time polymerase chain reaction for mRNA ([A], [C]) and by enzyme-linked immunosorbent assay and Luminex immunobead assays in culture supernatants ([B], [D]). Results shown are mean ± SD of four independent experiments. *, p < .05; **, p < .01; by t test. Abbreviations: GDNF, glial cell-derived neurotrophic factor; IL, interleukin; NF-κB-I, NF-κB inhibitor; TGF, transforming growth factor; TNF-α, tumor necrosis factor-α.
The production of Th17-inducing cytokines were further evaluated in an in vitro hyperosmotic stress model. As shown in Figure 3C, the mRNA levels of TGF-β1, IL-6, IL-23, and IL-1β were significantly increased by 2.9- to 3.3-fold in primary-cultured HLECs that were switched from medium with normal osmolarity of 312 mOsM to a 450 mOsM hyperosmotic medium (created by adding 90 mM NaCl2) for 4 hours (all p < .05, n = 4). The production of TGF-β1, IL-6, IL-23, and IL-1β proteins also increased significantly to 2.9-to 4.5-fold (Fig. 3D, all p < .05, n = 4) in culture supernatants of HLECs exposed to hyperosmotic media for 48 hours. Interestingly, stimulated production of these Th17-inducing cytokines was significantly suppressed at both mRNA and protein levels (all p < .05, n = 4) by GDNF or NF-κB-I, which were added to the culture media 1 hour before 90 mM NaCl to increase the medium osmolarity to 450 mOsM.
GDNF Suppresses NF-κB Signaling Activation in IL-17 Stimulated Limbal Epithelial Cells
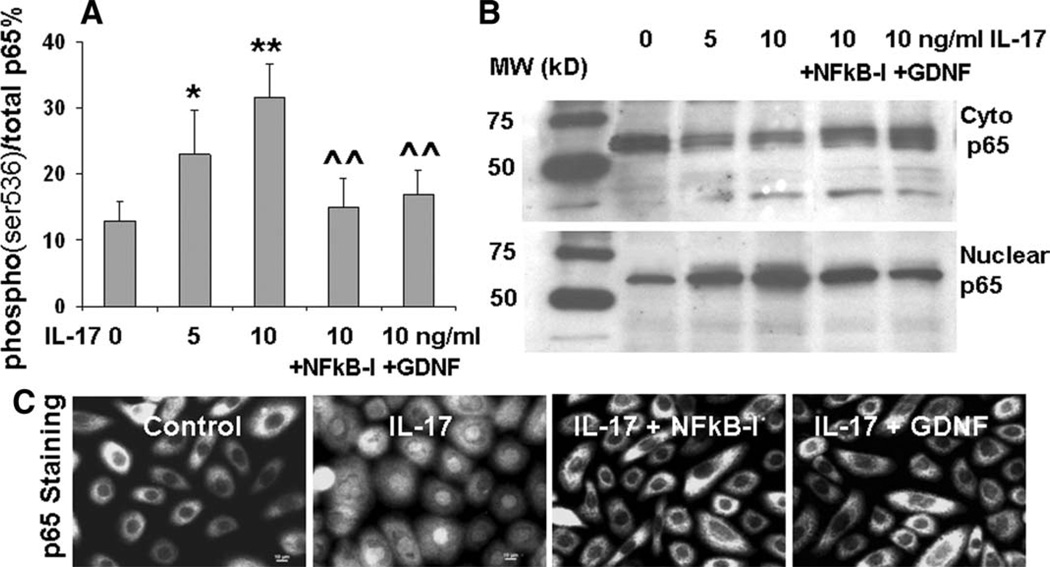
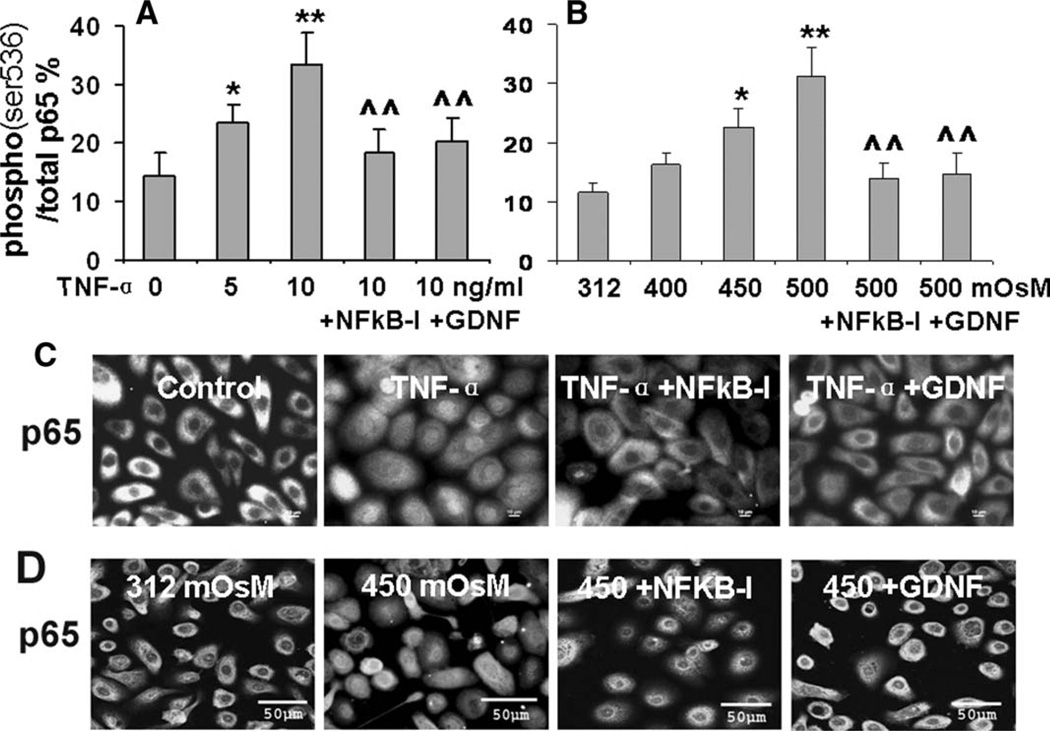
NF-κB is a ubiquitously expressed transcription factor with particular importance in immune and inflammatory responses. To gain an insight whether the IL-17-mediated inflammatory response by HLECs involves NF-κB activation, cells were treated with the NF-κB-I quinazoline 1 hour before the stimulation. As shown in Figure 2A and 2B, NF-κB-I significantly suppressed IL-17-stimulated production of inflammatory cytokines (TNF-α, IL-1β, IL-6) and chemokine IL-8 by HLECs at mRNA expression and protein levels. Using a fast-activated cell-based ELISA (FACE) In-Cell Western kit, we measured the NF-κB p65-signaling activation directly in the cells by applying a phospho-specific antibody against phospho-p65 (ser536) that recognizes only the phosphorylated (activated) state of signaling components. The cells were incubated for 30 minutes with IL-17 in the presence or absence of NF-κB-I that was added to culture media 1 hour earlier. As shown in Figure 4A, the activity of NF-κB p65 protein phosphorylated at ser536 over total p65 levels enhanced from 12.9% in controls to 23% and 31.5% in HLECs exposed to 5 and 10 ng/ml of IL-17A (p < .05 and < .01, respectively, n = 3). These were confirmed by evaluating p65 protein translocation from cytoplasm to nuclei by Western blot (Fig. 4B) and immunofluorescent staining (Fig. 4C). Interestingly, IL-17-stimulated p65 activation (ser536 phosphorylation and nuclear translocation) was markedly blocked by NF-κB-I and GDNF (Fig. 4A–4C). These results support the notion that GDNF suppresses the IL-17-induced inflammatory responses in HLECs by inhibiting the NF-κB signaling pathway.
Figure 4.
NF-κB activation in IL-17 stimulated inflammatory responses by human limbal epithelial cells (HLECs). The HLECs were preincubated with 5 µM quinazoline NF-κB-I or 10 ng/ml GDNF for 1 hour before adding 5–10 ng/ml IL-17A. (A): The cells in 96-well plates treated for 30 minutes were used for cell-based enzyme-linked immunosorbent assay quantification of p65 (ser536) phosphorylation (% Phospho-/total p65). Results shown are mean ± SD of three independent experiments, *, p < .05. **, p < .01 compared with controls; ^, p < .05. ^^, p < .01 compared with IL-17 stimulated levels, by analysis of variance (ANOVA) test. (B): The cells in six-well plates treated for 4 hours were subjected to cytoplasm and nuclear protein extraction to evaluate NF-κB p65 nuclear translocation by Western blot analysis. (C): The cells seeded in eight-chamber slides were fixed by methanol for immunofluorescent staining with rabbit anti-human p65 antibody and Alexa-Fluor 488-conjugated second antibodies. The images were representative of results obtained in three independent experiments. Abbreviations: C-p65, cytoplasm p65; GDNF, glial cell-derived neurotrophic factor; IL, interleukin; NF-κB-I, NF-κB inhibitor.
GDNF Suppresses the Stimulated Production of Th17-Inducing Cytokines by TNF-α and Hyperosmotic Stress via Inhibiting NF-κB Signaling
The NF-κB-signaling pathway also appeared to mediate the production of Th17-inducing cytokines in stress-challenged HLECs. As shown in Figure 3A–3D, NF-κB-I significantly suppressed production of Th17-inducing cytokines, TGF-β1, Il-6, IL-23, and IL-1β, by HLECs stimulated with 10 ng/ml TNF-α or 450 mOsM hyperosmolarity at both mRNA and protein levels. Measured with an ELISA-based signaling activation kit, NF-κB p65-signaling activity, presented as the percentage of phosphorylated NF-κB p65 protein at ser536 over total p65 levels, increased significantly to 1.64-(p < .05, n = 3) and 2.32-fold (p < .01, n = 3) in HLECs exposed to 5 and 10 ng/ml of TNF-α, respectively (Fig. 5A). When challenged by 450 and 500 mOsM hyperosmotic media (Fig. 5B), activated NF-κB p65 increased 1.94- and 2.68-fold, respectively (p < .05 and 0.01, n = 3, respectively). The NF-κB activation by p65 ser536 phosphorylation was further confirmed by directly observing translocation of p65 protein from cytoplasm to nuclei of cells exposed to TNF-α or 450 mOsM hyperosmolarity, by immunofluorescent staining as shown in Figure 5C and 5D. Interestingly, the stimulated p65 activation was markedly blocked by NF-κB-I and GDNF (Fig. 5A–5D). These results suggest that GDNF suppresses production of Th17-inducing cytokines in HLECs stimulated by TNF-α and hyperosmolarity stress via inhibition of the NF-κB-signaling pathway.
Figure 5.
Suppressive effect of GDNF on NF-κB activation in human limbal epithelial cells (HLECs) stressed with TNF-α or hyperosmotic media. The HLECs were preincubated with 5 µM quinazoline NF-κB-I or 10 ng/ml GDNF for 1 hour before treatment with TNF-α (5–10 ng/ml; [A], [C]) or hyperosmotic media (400–500 mOsM; [B], [D]). The cells in 96-well plates treated for 30 minutes were used for cell-based enzymelinked immunosorbent assay quantification of p65 (ser536) phosphorylation (% Phospho-/total p65; [A], [B]). Results shown are mean ± SD of three independent experiments, *, p < .05; **, p < .01, compared with controls; ^, p < .05; ^^, p < .01, compared with the stimulated levels, by ANOVA test. The cells seeded in eight-chamber slides were fixed with methanol for immunofluorescent staining with rabbit anti-human p65 antibody and Alexa-Fluor 488-conjugated secondary antibodies ([C], [D]). The images were representative of those observed in the three independent experiments. Abbreviations: GDNF, glial cell-derived neurotrophic factor; NF-κB-I, NF-κB inhibitor; TNF-α, tumor necrosis factor-α.
Discussion
In addition to self-renewal and tissue regeneration, adult stem cells are also known to be resistant to various immune and inflammatory challenges. Adult mesenchymal stem cells (MSCs) initially attracted interest for their ability to undergo differentiation toward cells of different lineages. Recently, increasing evidence reveals that MSCs have a unique antiinflammatory immunomodulatory properties that directly suppress immune responses through production of toleragenic cytokines, inhibition of lymphocyte proliferation, and delivery of reparative and protective signals after injury, suggesting that MSCs could be used to dampen immune-mediated diseases and transplant rejection [33–35]. Hematopoietic stem cells were found to reduce cerebral postischemic inflammation, attenuate peripheral immune activation, and mediate neuroprotection after ischemic stroke [36]. Mesoangioblast stem cells were reported to be capable of responding to different cell stresses such as heat, heavy metals, and osmotic stress by constitutive expression of a heat shock protein 70 [37]. The hair bulge region has been recognized to represent an area of relative immune privilege that protects the hair follicle epithelial stem cell reservoir from autoaggressive immune attack, whereas loss of bulge immune privilege may play a central role in the pathogenesis of cicatricial alopecias [38]. Human amniotic fluid stem cells have been reported to modulate the kidney immune milieu in renal failure caused by acute tubular necrosis [39]. The immune privilege of these adult stem cells may be driven by secreted factors, including TGF-β, IL-10, IL-6, cyclooxygenases, and matrix metalloproteinase-2 and -9 (see review article [35, 40]). In this study, we revealed that production of the NTF by human corneal epithelial progenitor cells protects them from Th17-mediated inflammatory stress. Our data clearly demonstrated that GDNF and its receptor GFRα-1 were exclusively localized in the basal layer of limbal epithelium where stem cells reside, whereas IL-17 receptor was barely detected. Furthermore, GDNF not only suppressed IL-17-stimulated inflammatory response but also inhibited the production of Th17-inducing cytokines, by blocking NF-κB-signaling activation.
Recently, IL-17-producing T-helper cells has been identified as a third category of T-helper cells, named as Th17 [12]. Th17 cells play a critical role in immunoinflammatory responses seen in autoimmune and inflammatory diseases, such as rheumatoid arthritis and experimental autoimmune encephalitis [12–15]. The role of Th17 has been recently recognized in ocular surface diseases, such as dry eye syndrome and ocular cicatricial pemphigoid [41, 42], including in our observation that desiccating stress promoted Th17 differentiation that was involved in corneal epithelial barrier disruption in dry eye conditions [22, 23]. IL-17A is a 30- to 35-kDa homodimeric polypeptide cytokine cloned in 1993 and originally named cytotoxic T lymphocyte-associated antigen-8. The receptor for IL-17A (IL-17RA) is a single-pass transmembrane protein of approximately 130 kDa. In contrast to the restricted expression of IL-17A by Th17 cells, the IL-17RA is ubiquitously expressed, and thus most cells are potential physiological targets of IL-17A [43]. Interestingly, IL-17RA was strongly expressed by corneal and limbal epithelial cells except in the basal layer of limbal epithelium (Fig. 1) where stem cells reside. This finding lead us to hypothesize that those limbal stem cells may resistant to inflammatory effect of IL-17 produced by Th17 cells.
The immune-protective role of GDNF has long been recognized to reduce immunoreactivity of transplanted substantia nigra allografts in rat [44], promote survival of dopaminergic neurons [45], and prevent the degeneration of adult facial motoneurons [46]. Recently, a critical role of phosphatidylinositol 3-kinase/Akt/Forkhead (PI-3K/Akt/FOXO) signaling has been observed in enteric neuroblast precursor survival and neurite extension stimulated by GDNF [47], and GDNF expression was upregulated by an immunomodulator AS101 [48] and identified to be induced by lipopolysaccharides in immune cell lines and primary-cultured rat macrophages [49]. Furthermore, we have observed that GDNF gene delivery enhances survival of human corneal epithelium in culture and in bioengineered corneal constructs [11]. More interestingly, GDNF and its high-affinity receptor GFR-α1 were found to be exclusively expressed by subsets of limbal basal epithelial cells containing stem cells. The finding further extended our hypothesis that production of the neurotrophic factor GDNF by human corneal epithelial progenitor cells may protect them from Th17 inflammatory stress.
Further investigations in this study have confirmed our hypothesis. We observed that IL-17A significantly stimulated production of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokine IL-8 by HLECs at both mRNA and protein levels (Fig. 2). These stimulated responses by HLECs were significantly suppressed by GDNF or NF-κB activation inhibitor quinazoline (NF-κB-I). GDNF was also found to suppress the production of Th17-inducing cytokines, TGF-β and IL-6, which initiate the Th17-cell differentiation, as well as IL-23 and IL-1β, the major inducers that strongly expand Th17 cell [21], in HLECs stimulated by TNF-α and hyperosmotic stress. These findings suggest that GDNF protects HLECs from Th17-induced inflammatory responses through two levels of inhibition: directly suppressing the inflammatory effects of Th17 cell produced IL-17 and indirectly blocking production of Th17 inducers by HLECs in response to inflammation and hyperosmotic stress. The potential role of epithelium-derived inducers in Th17 differentiation has been observed in our previous reports [21, 22].
Finally, we investigated the potential signaling pathway by which GDNF exert its immune suppressive role in Th17 inflammatory responses. NF-κB has been recognized to be a common mediator in IL-17-induced inflammatory effects. NF-κB activation mediated IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells [50], and it is involved in IL-17 upregulated expression of cytokines and β-defensin-2 in human airway epithelium [51, 52]. We have observed that NF-κB activation inhibitor quinazoline has similar suppressive effects to GDNF on IL-17 stimulated production of proinflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokine IL-8 (Fig. 2), as well as on production of Th17-inducing cytokines (TGF-β, IL-6, IL-23 and IL-1β) stimulated by TNF-α and hyperosmotic stress (Fig. 3). IL-17-stimulated p65 activation (ser536 phosphorylation and nuclear translocation) was markedly blocked by GDNF and NF-κB-I (Fig. 4A–4C). These results support the notion that GDNF suppresses IL-17-induced inflammatory responses in HLECs though an NF-κB-signaling pathway. As shown in Figure 5A–5D, GDNF also suppressed production of Th17-inducing cytokines in HLECs exposed to TNF-α and hyperosmolarity stress through an NF-κB mechanism.
Conclusion
In conclusion, our findings demonstrated a unique immunoprotective property of human corneal epithelial stem cells against Th17 inflammatory stress via production of a novel neurotrophic factor GDNF and downregulation of the IL-17 receptor. Through blocking NF-κB signaling, limbal basal epithelial cell-derived GDNF not only directly suppressed IL-17 stimulated inflammation but also inhibited the production of Th17-inducing cytokines on ocular surface. This suggests that GDNF may have therapeutic implication for treating Th17-mediated inflammatory diseases.
Acknowledgments
We thank Dr. Marshall Bowes Hamill for his great support and the Lions Eye Bank of Texas for providing human corneoscleral tissues. This work was supported by Department of Defense CDMRP PRMRP Grant FY06 PR064719 (D.-Q.L.), National Institutes of Health Grant EY11915 (S.C.P.), National Natural Science Foundation of China 30872813 (H.Q.), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Footnotes
Author contributions: F.B.: conception and design, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing; H.Q.: conception and design, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing; P.M.: provision of study material, collection and/or assembly of data; L.Z.: provision of study material or patients, collection and/or assembly of data; K.-C.Y.: provision of study material or patients, collection and/or assembly of data; S.C.P.: conception and design, financial support, manuscript writing; D.-Q.L.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure Of Potential Conflicts Of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavker RM, Dong G, Cheng SZ, et al. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32:1864–1875. [PubMed] [Google Scholar]
- 5.Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 6.You L, Ebner S, Kruse FE. Glial cell-derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:2496–2504. [PubMed] [Google Scholar]
- 7.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 9.Oatley JM, Avarbock MR, Telaranta AI, et al. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci USA. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi H, Li DQ, Bian F, et al. Expression of glial cell-derived neurotrophic factor and its receptor in the stem-cell-containing human limbal epithelium. Br J Ophthalmol. 2008;92:1269–1274. doi: 10.1136/bjo.2007.132431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi H, Shine HD, Li DQ, et al. Glial cell-derived neurotrophic factor gene delivery enhances survival of human corneal epithelium in culture and the overexpression of GDNF in bioengineered constructs. Exp Eye Res. 2008;87:580–586. doi: 10.1016/j.exer.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C. Diversi.cation of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 13.Paradowska A, Maslinski W, Grzybowska-Kowalczyk A, et al. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2007;55:329–334. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oboki K, Ohno T, Saito H, et al. Th17 and allergy. Allergol Int. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 16.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 19.Moseley TA, Haudenschild DR, Rose L, et al. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 20.Molesworth-Kenyon SJ, Yin R, Oakes JE, et al. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Bian F, Ma P, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2010;222:95–102. doi: 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, de Paiva CS, Li D-Q, et al. Desiccating stress promotes Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51:3083–3091. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Jun Song X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D-Q, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo L, Li D-Q, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 28.Yoon KC, de Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: Effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 29.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, Mapk activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 30.McAllister F, Henry A, Kreindler JL, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic .brosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 32.Liang SC, Long AJ, Bennett F, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 33.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Bartholomew A, Polchert D, Szilagyi E, et al. Mesenchymal stem cells in the induction of transplantation tolerance. Transplantation. 2009;87:S55–S57. doi: 10.1097/TP.0b013e3181a287e6. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Xu D, Feng G, et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9. Diabetes. 2009;58:1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarting S, Litwak S, Hao W, et al. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke. 2008;39:2867–2875. doi: 10.1161/STROKEAHA.108.513978. [DOI] [PubMed] [Google Scholar]
- 37.Geraci F, Turturici G, Galli D, et al. Stress response in mesoangioblast stem cells. Cell Death Differ. 2006;13:1057–1063. doi: 10.1038/sj.cdd.4401794. [DOI] [PubMed] [Google Scholar]
- 38.Meyer KC, Klatte JE, Dinh HV, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 39.Perin L, Sedrakyan S, Giuliani S, et al. Protective effect of human amniotic .uid stem cells in an immunode.cient mouse model of acute tubular necrosis. Plos One. 2010;5:e9357. doi: 10.1371/journal.pone.0009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrie Aronin CE, Tuan RS. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:67–74. doi: 10.1002/bdrc.20174. [DOI] [PubMed] [Google Scholar]
- 41.Chauhan SK, El AJ, Ecoif.er T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambiase A, Micera A, Mantelli F, et al. T-helper 17 lymphocytes in ocular cicatricial pemphigoid. Mol Vis. 2009;15:1449–1455. [PMC free article] [PubMed] [Google Scholar]
- 43.Gaffen SL, Kramer JM, Yu JJ, et al. The IL-17 cytokine family. Vitam Horm. 2006;74:255–282. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 44.Shinoda M, Hoffer BJ, Olson L. Minor immunoreactivity in GDNF-, BDNF-, or NT-3-treated substantia Nigra allografts. J Neural Transplant Plast. 1997;6:83–96. doi: 10.1155/NP.1997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen ZY, Chen XQ, Lu CL, et al. The human recombinant GDNF and study of its biological activity. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 1999;31:203–206. [PubMed] [Google Scholar]
- 46.Sakamoto T, Watabe K, Ohashi T, et al. Adenoviral vector-mediated GDNF gene transfer prevents death of adult facial motoneurons. NeuroReport. 2000;11:1857–1860. doi: 10.1097/00001756-200006260-00011. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan S, Anitha M, Mwangi S, et al. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNFstimulated survival. Mol Cell Neurosci. 2005;29:107–119. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Okun E, Saida H, Albeck M, et al. Upregulation of carp GDNF mRNA by the immunomodulator AS101. Dev Comp Immunol. 2006;30:441–446. doi: 10.1016/j.dci.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Oh-Hashi K, Ito M, et al. Identi.cation of a novel GDNF mRNA induced by LPS in immune cell lines. Neurosci Res. 2008;61:11–17. doi: 10.1016/j.neures.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Awane M, Andres PG, Li DJ, et al. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, And IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. [PubMed] [Google Scholar]
- 51.Kao CY, Chen Y, Thai P, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 52.Huang F, Kao CY, Wachi S, et al. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]