Abstract
Ischaemia–reperfusion injury (IRI) in the liver, a major complication of haemorrhagic shock, resection and transplantation, is a dynamic process that involves the two interrelated phases of local ischaemic insult and inflammation-mediated reperfusion injury. This Review highlights the latest mechanistic insights into innate–adaptive immune crosstalk and cell activation cascades that lead to inflammation-mediated injury in livers stressed by ischaemia–reperfusion, discusses progress in large animal experiments and examines efforts to minimize liver IRI in patients who have received a liver transplant. The interlinked signalling pathways in multiple hepatic cell types, the IRI kinetics and positive versus negative regulatory loops at the innate–adaptive immune interface are discussed. The current gaps in our knowledge and the pathophysiology aspects of IRI in which basic and translational research is still required are stressed. An improved appreciation of cellular immune events that trigger and sustain local inflammatory responses, which are ultimately responsible for organ injury, is fundamental to developing innovative strategies for treating patients who have received a liver transplant and developed ischaemia–reperfusion inflammation and organ dysfunction.
Introduction
Liver transplantation is the standard of care in patients with end-stage liver disease and those with tumours of hepatic origin.1 In 2011, 16,107 patients in the USA were waiting for a liver transplant, but only 6,341 procedures were performed, indicating a shortage of about 10,000 donor livers per year.2 During the same time, 1,589 patients died whilst waiting for a liver transplant and an additional 1,349 patients were removed from the waiting list because they became too sick to undergo surgery.2 Organ shortage has prompted the use of extended criteria donor organs from older, steatotic, or non-heart-beating donors, as well as organs that have been subjected to prolonged periods of warm and cold storage. However, these ‘marginal’ organs are particularly susceptible to ischaemia–reperfusion injury (IRI) as a result of damage during procurement, preservation and surgery. Indeed, IRI not only contributes to the donor organ shortage (as organs might be harvested and then deemed too damaged for transplant) but might also lead to poor early graft function and primary nonfunction. Moreover, the cellular damage surrounding organ removal and storage affects transplantation outcomes as it is a major risk factor for both acute and chronic rejection. Despite its obvious clinical importance, the mechanisms that account for organ IRI are only partially understood and remain one of the most understudied areas in clinical and experimental transplantation.3,4
In this Review, we first focus on our current understanding of cellular and molecular mechanisms that trigger local immune activation and inflammatory cascades in procured livers subjected to revascularization. We then summarize research progress in large animal models, and finally address the current status of clinical trials focusing on liver IRI in patients who receive a liver transplant. By presenting new insights into tissue inflammation responses driven by complex innate–adaptive immunity, we had to limit our discussion of other essential IRI pathogenic mechanisms, such as parenchymal cell death programmes, the complement system and the role of mitochondria in generating reactive oxygen species (ROS) and nitrogen species. Progress pertaining to the aforementioned factors might lead to innovative therapies in liver IRI (including preconditioning and postconditioning approaches). These issues have been discussed elsewhere.5–11
Types and stages of liver IRI
Two major types of liver injury that are attributable to ischaemia–reperfusion can be distinguished.4,12 The ‘warm’ IRI, which is initiated by hepatocellular damage, develops in situ during liver transplantation surgery or during various forms of shock or trauma, and might lead to liver or even multiorgan failure. The ‘cold’ IRI, which is initiated by damage to hepatic sinusoidal endothelial cells and disruption of the microcirculation, occurs during ex vivo preservation and is usually coupled with warm IRI during liver transplantation surgery. Although initial cellular targets of the two IRI types might be different, they do share a common mechanism in the disease aetiology; that is, local inflammatory innate immune activation. Mechanistic appreciation of different IRI types is of current interest because, as discussed later, cold static hepatic preservation and warm ex vivo liver perfusion are being compared in large animal models of IRI for future clinical use.
The activation of liver Kupffer cells and neutrophils, the production of cytokines and chemokines, the generation of ROS, increased expression of adhesion molecules and infiltration by circulating lymphocytes and/or monocytes are immunological cascades present in both types of IRI.3,48 Distinct from alloreactive responses against liver grafts, tissue inflammation triggered by ischaemia–reperfusion occurs immediately after reperfusion. This inflammation is predominantly an innate-immune-dominated response that is mediated by the sentinel pattern recognition receptor (PRR) system. Endogenous ligands generated as a result of hepatocellular damage—danger-associated molecular patterns, rather than exogenous pathogen-associated molecular patterns—have a key role in tissue inflammation. However, ischaemia–reperfusion elicits an equally robust adaptive immune response in the liver that is dependent on CD4+ T cells. Our own studies suggest that although innate activation might induce ischaemia–reperfusion damage both in situ and in liver transplants, the ‘cold’ preservation injury favours an early and massive T-cell influx into ischaemic liver grafts.13
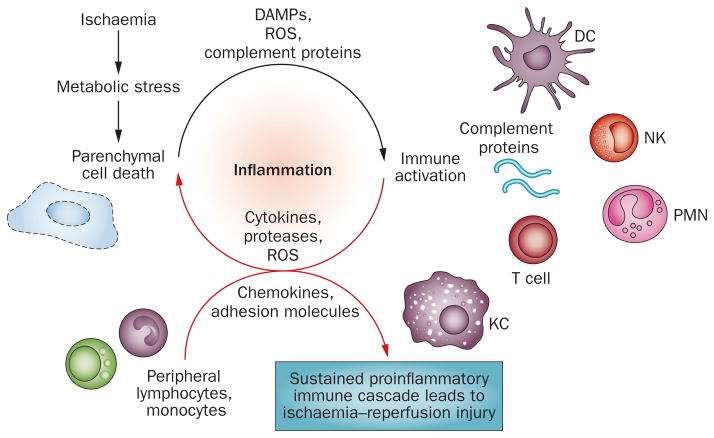
Two distinct stages of liver IRI, with unique mechanisms of hepatic damage, have been identified (Figure 1). Ischaemic injury, a localized process of cellular metabolic disturbances, results from glycogen consumption, lack of oxygen supply and ATP depletion, which lead to the initial parenchymal cell death. Reperfusion injury, which follows the ischaemic injury, results not only from metabolic disturbances but also from a profound inflammatory immune response that involves both direct and indirect cytotoxic mechanisms. Indeed, the liver’s response to inflammation is critical, as prevention of local immune activation uniformly ameliorates IRI. Therefore, dissection of innate immune activation is important for identifying novel therapeutic targets to alleviate proinflammatory mechanisms whilst sparing or augmenting anti-inflammatory mechanisms. Furthermore, crosstalk between the innate and adaptive immune responses triggered by ischaemia–reperfusion readily converts an immunologically quiescent liver into an inflammatory organ, even in a sterile environment.4 Most of the discussed studies were performed in an in situ model of segmental hepatic warm ischaemia–reperfusion in mice. Although only partially reflecting the real-life transplant setting, the model takes advantage of the genetically targeted mouse strains. A clinically more relevant mouse model, combining cold and warm IRI components followed by orthotopic liver transplantation, has only been established in the past decade.14
Figure 1.
The distinct stages of liver ischaemia–reperfusion injury. Ischaemic injury, a localized process of hepatic metabolic disturbances, results from glycogen consumption, lack of oxygen supply and ATP depletion. The cell-death-released DAMPs, activation of complement (a group of proteins that are involved in tissue injury and/or repair) induced by tissue injury and mitochondrial ROS production triggered by oxygenation all contribute to liver immune activation after reperfusion, which involves multiple liver nonparenchymal cell types, including Kupffer cells, dendritic cells, T cells, NK cells and neutrophils (PMNs). The ischaemia–reperfusion-activated proinflammatory immune cascade sustains itself by recruiting peripheral immune cells from the circulation, and is responsible for the ultimate liver reperfusion injury. Abbreviations: DAMPs, danger-associated molecular patterns; DC, dendritic cells; KC, Kupffer cells; NK, natural killer cell; PMN, polymorphonuclear cells; ROS, reactive oxygen species.
Ischaemia–reperfusion activates TLRs
On the basis of seminal observations in the mid 1990s from patients who received a renal transplant,15 Professor Walter Land’s ‘injury hypothesis’ states that IRI activates a cascade of innate-dominated proinflammatory immune responses, which then trigger the adaptive immune response that culminates in allograft rejection.16 Indeed, ample evidence indicates that all vertebrates use the same sentinel innate immune receptor systems—PRRs—in response to tissue damage in the absence of infection.17–22 PRRs are divided into four classes: Toll-like receptors (TLRs); C-type lectin receptors; retinoic acid-inducible gene I-like receptors and NOD-like receptors (NLRs). The first two are transmembrane proteins and the last two are cytoplasmic proteins. These PRRs are expressed predominantly in macrophages and dendritic cells, and their activation triggers upregulation of the transcription of genes involved in the inflammatory response.23 TLRs are the most thoroughly studied PRRs in the liver IRI immune cascade (Figure 2). The TLR system consists of at least 13 members, which function as homodimers or heterodimers of type I transmembrane glycoproteins. Most TLRs are expressed on the cell surface, except TLR3, TLR7, TLR8 and TLR9, which are located in the endoplasmic reticulum membrane. Co-receptors are required for some TLRs to recognize their ligands, for example, lymphocyte antigen 96 and CD14 are required for TLR4 to bind lipopolysaccharides. TLR ligation triggers multiple intracellular signalling pathways and ultimately results in activation of transcriptional factors, such as nuclear factor κB (NF-κB), activator protein 1 and interferonregulatory factors (IRFs), which act in concert to initiate expression of genes encoding cytokines, chemokines and co-stimulatory molecules.24–26
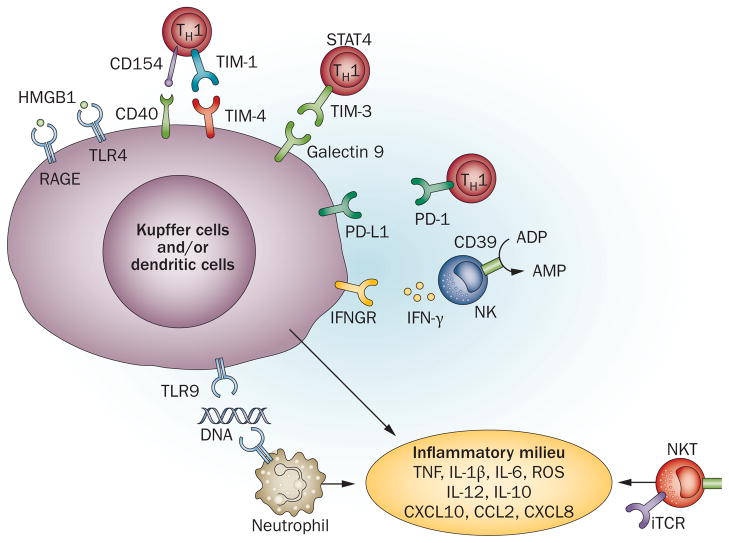
Figure 2.
A scheme of liver immune activation against ischaemia–reperfusion injury. The ischaemic insult induces initial cell death, which results in diverse ‘danger’ molecules, such as HMGB1, DNA fragments and histones activating TLR4, RAGE and TLR9 signalling on Kupffer cells and/or dendritic cells and neutrophils. T cells, particularly CD4+ TH1 effectors, might also facilitate and regulate local innate immune activation via CD154–CD40, TIM-1–TIM-3, TIM-4–galectin 9 and PD-L1 pathways. In addition, CD1d-mediated NKT and CD39-mediated NK cell activation contribute to hepatic immune activation against ischaemia–reperfusion. IFN-γ produced by activated NK cells promotes Kupffer cell and/or dendritic cell activation. The proinflammatory milieu, composed of TNF, IL-1β, IL-6, IL-12, CXCL10, CCL2, CXCL8 and ROS, further activates local immune cells and recruits circulating immune cells, culminating in inflammatory reperfusion injury. Abbreviations: HMGB1, high-mobility group protein B1; IFNGR, IFN-γ receptor; iTCR, invariant T cell receptor; NK, natural killer cell; NKT, natural killer T cell; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; PMN, polymorphonuclear cell; RAGE, receptor for advanced glycation end products; STAT4, signal of transducer and activator of transcription 4; TH1, T-helper type 1 cell; TIM, T cell, immunoglobulin, mucin-containing molecules; TLR, Toll-like receptor.
The liver receives blood that drains from the gastrointestinal system (where commensal bacteria reside) via the hepatic portal vein; therefore, gut-derived endotoxin might be translocated directly into the liver circulation. This phenomenon certainly occurs during liver ischaemia–reperfusion when hepatic portal vein occlusion results in blockage of the intestinal wall, leading to its increased permeability during reperfusion.4 TLR4 was the first innate immune receptor tested in liver IRI. Indeed, using mouse models of partial warm ischaemia, data from three separate laboratories demonstrated that livers in TLR4-deficient mice were protected against IRI and that local hepatic inflammation against ischaemia–reperfusion insult was suppressed in the absence of TLR4.27–29 Of note, TLR2 was not necessary for the development of liver IRI, which is unlike its essential role in other organs, such as the kidney or heart.30,31 The critical role of TLR4-specific activation in triggering liver IRI pathology was also confirmed in orthotopic liver transplantation, which consists of both warm and cold IRI components32 and in a steatotic liver IRI mouse model.33 Interestingly, the donor being deficient in TLR4 was sufficient to protect the liver from IRI in the transplant model, and TLR4 signalling on liver nonparenchymal cells seems to be more relevant than TLR4 signalling on parenchymal cells for liver IRI;18 however, a study has demonstrated that TLR4 has a unique role in liver parenchymal cells at the late stage of the disease process.34 These data indicate that TLR4 on both parenchymal and nonparenchymal cells in livers are involved in the process of hepatic IRI.34
The activation of TLR4 in liver IRI is dependent on a downstream signalling pathway mediated by IRF3 but independent of myeloid differentiation primary response gene MyD88.29 MyD88-deficient mice not only developed hepatocellular damage, but their liver IRI ‘signature’ proinflammatory cytokine (TNF and IL-1β) and chemokine (CXCL10) programmes were largely unaffected. As the MyD88-independent, IRF3-dependent signalling pathway of TLR4 just delays NF-κB activation, it seems that the MyD88-mediated activation of NF-κB in the early phase of IRI is not necessary for the proinflammatory immune response against ischaemia–reperfusion in the liver. This situation is very different from renal and heart IRI models in which either MyD88 and IRF3 or only MyD88 are required.35–38 The fact that the severity of liver IRI peaks at 6 h of reperfusion and that of kidney and heart injury last for days after reperfusion might partially explain this discrepancy. The other potential reason is that TLR4 activation in the liver is unique, as gut-derived endotoxin might have already tolerized the innate immune system in the liver, which has been shown to target the MyD88-dependent pathway.39,40 Both proinflammatory and anti-inflammatory gene programmes are induced by TLR4 activation in macrophages in vitro and livers in vivo.4 Glycogen synthase kinase 3β, a serine/threonine kinase, was found to differentially regulate these two programmes.41 We have shown that glycogen synthase kinase 3β inhibitor can be used as an effective therapeutic agent in liver IRI by selectively inhibiting the proinflammatory programme while sparing the immune-regulatory IL-10 gene programme.42
Although endotoxin has been implicated in IRI, the question of whether it provides the triggering signal for the liver inflammatory immune response against ischaemia–reperfusion remains controversial. Increased levels of lipopolysaccharide were detected in portal and systemic circulation after ischaemia–reperfusion in both animal models and patients who received a liver transplant.43 A protective effect of antiendotoxin monoclonal antibodies was detected in a steatotic hepatic IRI model.44 However, our own study using endotoxin-neutralizing peptides failed to show any organ protection in the early phase of liver ischaemia–reperfusion.45 One key point that might differentiate the latter result from the former is the time after reperfusion at which the measurements were taken (6 h versus 24 h). Perhaps endotoxin is not responsible for triggering the activation of the innate immune response of the liver during ischaemia–reperfusion, but instead it might participate in sustaining the inflammation response.
More than 20 distinct endogenous TLR2 and/or TLR4 ligands, representing intracellular proteins, extracellular matrix proteins, oxidatively modified lipids and other soluble mediators, have been identified.46 High mobility group protein 1 (HMGB1), originally discovered as a nuclear protein, has been identified as the key endogenous TLR4 ligand responsible for immune activation in the liver during ischaemia–reperfusion.47 HMGB1 can be released from damaged hepatocytes to subsequently stimulate liver nonparenchymal cells, including Kupffer cells, via TLR4 signalling (Figure 2). Hypoxic hepatocytes release HMGB1 through an active process facilitated by TLR4-dependent production of ROS. In turn, ROS induces the release of HMGB1 through a CaMK-dependent mechanism, and such a positive loop of HMGB1–TLR4 signalling might encourage a sustained inflammatory response in the liver during ischaemia–reperfusion.48
Of note, HMGB1 biology has become quite complex, and how this ubiquitously expressed protein might trigger the inflammation response is a matter of controversy. First, the relative importance of hypoxia and cellular necrosis in HMGB1-mediated stimulation of target cells remains to be determined. Second, questions concerning the molecular nature of TLR4 binding partners and putative roles of other receptors in immune activation, such as receptor for advanced glycation end products (RAGE) need to be addressed.49,50 Third, additional functions of HMGB1 have been discovered via its binding to nonorthotropic receptors.51,52 Indeed, RAGE has been shown to be essential in liver IRI by regulating expression of CXCL2 (also known as macrophage inflammatory protein 2) via an epidermal growth factor receptor (EGFR)-dependent mechanism, as well as influencing cell death and production of TNF in an EGFR-independent manner.51,52 TLR4-mediated upregulation of IRF1 in hepatocytes has also been identified as a necessary step for release of HMGB1 in response to hypoxia,53 consistent with the essential function of parenchymal rather than nonparenchymal IRF1 in the mechanism of IRI as a result of liver transplant.54 HMGB1 also promotes recruitment of inflammatory cells to damaged tissue by forming a complex with the chemokine CXCL12 and signalling via CXCR4, independent of RAGE and TLR4.55 Although mechanisms that govern inflammatory cell sequestration in the damaged organ versus cytokine release might be different, both biological processes do involve HMGB1.
In addition to HMGB1, other DAMPs released from damaged and/or necrotic cells might stimulate innate immune cells via various receptors, such as, heat-shock proteins, S100 proteins via TLR4, RNA via TLR3 and DNA via TLR9. TLR9 was found to function in cells derived from bone marrow, particularly neutrophils, during liver ischaemia–reperfusion, which boosted production of proinflammatory cytokines and chemokines.56 Inhibition of TLR9 had additive protective effects when HMGB1 was neutralized in livers subjected to ischaemia–reperfusion. 56 Nuclear histone proteins were identified as the potential endogenous ligand of TLR9 in the liver.57 Indeed, liver IRI resulted in increased levels of circulating histones and neutralization of histones was cytoprotective. Extracellular histones enhanced TLR9 activation mediated by DNA, whereas histone infusion exacerbated liver IRI pathology via TLR9 signalling. TLR3, which recognizes necrotic cell-derived RNA products, has also been shown to sustain inflammation in a mouse gastrointestinal ischaemia model.58
The role of other PRRs in liver IRI has only become unravelled in the past 5 years. Necrotic cells can be ‘sensed’ by inflammasomes as they release panels of proinflammatory mediators. In a model of sterile inflammation, one member of the NLR family, NLRP3 protein, was found to be involved in the mechanism of polymorphonuclear cell recruitment to sites of focal hepatic necrosis. 59 Gene silencing of NLRP3 attenuated liver damage, in association with reduced levels of IL-1β, IL-18, TNF and IL-6, diminished levels of HMGB1 and decreased local inflammatory cell infiltration.60 Interestingly, haemorrhagic shock-induced liver damage might develop independently of NLRP3, and caspase 1 was found to be cytoprotective during trauma, as it mitigated liver injury and inflammation.61
Although an array of PRR-targeting studies have shown promise in different animal models, the caveat is that most of these studies focus on the ‘correlation’ between genetic deletion and cytoprotection rather than establishing the actual cause of the reduced tissue damage. With limited mechanistic understanding of a successful anti-IRI therapy, exploring multiple PRR pathways with small molecules acting preferably in a synergistic manner might be required.
T cells in liver innate immune response
Despite the fact that IRI can develop in syngeneic grafts, ex vivo, or under sterile conditions, T cells, particularly of the CD4+ phenotype, are indispensable for the activation and regulation of the tissue proinflammatory immune response to ischaemia–reperfusion (Figure 2). The observation that systemic immunosuppression (with either ciclosporin or tacrolimus) attenuated hepatocellular damage provided initial indirect evidence for involvement of T cells in the development of IRI.62 Studies in T-cell deficient and CD4-deficient mouse systems have proven the pivotal function of CD4+ T cells in the mechanism of liver IRI.63–66 The obvious question that arises is how do T cells function in this predominantly innate response and in the absence of exogenous antigen stimulation?
The role of T cell co-stimulatory pathways in promoting IRI in the absence of alloantigen was initially shown in a study in which CD28 blockade with cytotoxic T-lymphocyte antigen 4 immunoglobulin (CTLA4Ig) protected rat kidneys from IRI by reducing infiltration of T cells and/or macrophages.67 Both CD28 and CD154 are important for the activation of liver inflammation pathways that lead to hepatocellular damage. Indeed, livers in CD154-knockout or CD28-knockout mice or in wild-type mice treated with anti-CD154 or CTLA4Ig were all protected from IRI.65 Furthermore, T-helper (TH)1-type cells have a major role in the pathogenesis of liver IRI, as Stat4-knockout mice (deficient in TH1 development), but not Stat6-knockout mice, were protected from the injury, whereas reconstitution of ‘nude’ mice with T cells from Stat6-knockout, but not Stat4-knockout, mice restored cardinal features of liver IRI.68 TH17 cells have also been implicated in autoimmune inflammatory diseases, and their putative role in IRI has started to be revealed. Although cellular sources of IL-17 in the liver remain to be determined, Il-17a knockout mice suffer less severe liver IRI concomitant with reduced neutrophil infiltration than wild-type mice. The effect of Il-17a deficiency was associated with fairly late stages of the disease and did not affect acute ischaemia–reperfusion liver damage.69 In agreement with the Lentsch group,70 our research group has detected CD4+ T-cell sequestration into postischaemic livers before any appreciable local neutrophil accumulation (H. Ji, personal communication). This sequestration occurs via released Il-17, which might then act upon hepatocytes and macrophages to produce CXCL2, a known neutrophil chemotactic factor.
By contrast, IL-22, which is produced by the same subset of CD4+ T cells that express IL-17, exerts protective functions in mouse models of acute liver failure.71–73 Unlike other cytokines, IL-22 is not involved in communication between immune cells, but instead it signals directly to hepatocytes via IL-22R1.74 Having documented that administration of recombinant IL-22 had a hepatoprotective effect via STAT3 activation,75 we favour the concept that IL-22 is well-positioned to orchestrate innate–adaptive networks by activating cell survival genes, preventing apoptosis and promoting hepatic regeneration in livers stressed by ischaemia–reperfusion.
Although the role of CD154 in liver IRI has been attributed to its co-stimulatory T-cell function, CD40 ligation on dendritic cells or macrophages by T-cell-derived CD154 is the critical activating signal to innate immune cells.4 Endogenous ligands that trigger liver IRI might be insufficient to fully activate and sustain a proinflammatory phenotype in an ischaemia–reperfusion-stressed liver. Thus, hepatic Kupffer cells might be less sensitive to TLR4 stimulation than peripheral macrophages because of their exposure to endotoxin from the gut that drained through the hepatic portal vein. Indeed, Kupffer cells might synthesize immune regulatory IL-10 when exposed to lipopolysaccharide in vitro.76 Liver dendritic cells have lower expression levels of TLR4 and are less susceptible to stimulation with lipopolysaccharide than dendritic cells in the spleen.77 In fact, conventional liver dendritic cells have been shown to exert immuneregulatory functions during ischaemia–reperfusion by producing IL-10 via a TLR9-mediated mechanism.78
The T cell, immunoglobulin, mucin-containing molecules (TIM) family of cell surface proteins has attracted much attention as novel regulators of host immunity.79 Stimulation of T cells amplifies expression of TIM-1, a phosphatidylserine receptor, that is expressed primarily on CD4+ TH2 and TH1 cells, whereas TIM-4, one of the major TIM-1 ligands, is expressed mostly by macrophages and antigen-presenting cells. Therefore, interactions between TIM-1 and TIM-4 constitute a novel molecular mechanism of T cell–macrophage regulation at the innate–adaptive immune interface, and might be a therapeutic target. Indeed, treatment with anti-TIM-1 monoclonal antibodies ameliorated the hepatocellular damage, accompanied by decreased local polymorphonuclear cell infiltration and/or activation, inhibition of T cell and/or macrophage sequestration and diminished homing of TIM-4+ cells expressing the TIM-1 ligand in ischaemic livers.80 The induction of proinflammatory cytokine and/or chemokine programmes was also blunted,80 this data is supported by findings from a renal IRI model.81 By contrast, the TIM-3–galectin pathway constitutes a ‘negative’ co-stimulation signalling pathway between TH1 cells and macrophages, and has been shown to promote immunotolerance in transplant recipients.79 Interestingly, TIM-3 blockade worsened the hepatocellular damage, and caused increased IFN-γ but decreased IL-10 expression in ischaemia–reperfusion-stressed livers.82 The programmed death 1 programmed death ligand 1 (also known as B7–H1) ‘negative’ T cell pathway has also been shown to promote hepatoprotection in warm and cold liver IRI models.83,84 Thus, multiple co-stimulatory signalling pathways, both positive and negative, might function in a two-way traffic approach to promote versus inhibit liver inflammatory immune responses against ischaemia–reperfusion insult (Figure 2).
In addition to ‘traditional’ T cells, natural killer (NK) and natural killer T (NKT) cells might also have distinct roles in the mechanism of IRI. Although general depletion of cells expressing the antigen NK1.1 (both NK and NKT cells) fails to affect the severity of IRI at its early stages,85 this depletion does reduce the hepatocellular damage in the later phases.86 Activation of NKT cells (which make up almost 50% of liver T cells) that is triggered by ischaemia–reperfusion is mediated by CD1d, which is expressed by most liver cells. This activation also presents glycolipid antigens (that are possibly released by necrotic cells) to NKT cell invariant T cell receptors. Furthermore, subsets of NKT cells have distinct roles in vivo. Indeed, type II NKT cells were shown to prevent liver IRI when activated by a specific glycolipid ligand sulphatide (self-glycolipid 3-sulphated β-galactosyl ceramide.87 NK cell activation triggered by ischaemia–reperfusion is dependent on CD39 to hydrolyse ADP to AMP. Indeed, CD39-deficient livers were resistant to IRI, and NK-derived IFN-γ production was diminished, possibly because of activation of the type 2 purinergic receptor.88 Thus, T cells, NKT cells and NK cells are all involved, although possibly at different stages, in the activation of the innate immune system by ischaemia–reperfusion, by providing co-stimulatory signalling via direct cell–cell interactions or cytokine stimulation to Kupffer cells and/or dendritic cells. This, in turn, promotes the proinflammatory phenotype of innate immune activation.
Liver IRI in large animal models
Although indispensable for dissecting basic molecular mechanisms of liver IRI, the rodent studies discussed earlier fall short of addressing critical clinical problems, such as using grafts procured from extended criteria donors and donation after cardiac death (DCD) as no models exist that would reproducibly mimic these situations. Approximately 60% of the potential DCD livers are discarded because of irreversible ischaemia–reperfusion damage.1 In those DCD livers that are used, long-term survival outcomes are inferior compared with those from donation after brain death. With no established therapies for IRI available, limiting the duration of cold preservation and re-warming during surgery remain primary strategies to reduce the detrimental effects of liver IRI. Major efforts are being directed towards developing innovative techniques to circumvent warm IRI in clinically relevant pig liver DCD models. Emerging therapeutic protocols can be categorized on the basis of the timing of organ resuscitative therapy during the different phases of liver preservation. Organ preservation and resuscitation can be instituted immediately after cardiac death, when the organ is still in the donor, using extracorporeal membrane oxygenation.89,90 They can also be used after removal of the organ from the donor until its implantation via an ex vivo machine perfusion, or during revascularization of the liver after ischaemia, which is termed regulated hepatic reperfusion.91
Initiating preservation and resuscitation immediately after cardiac death and before organ removal provides a theoretical advantage of shortening the ischaemic period of the liver. Normothermic recirculation with extracorporeal membrane oxygenation restores warm oxygenated blood flow to the intra-abdominal organs and provides a beneficial preconditioning effect that reverses warm ischaemia-mediated cellular injury.92,93 Accruing clinical data suggest that normothermic recirculation might improve graft function, and ultimately salvage DCD livers that would have been otherwise discarded.89,90
Current research in organ preservation is shifting from a static hypothermic preservation to dynamic ex vivo machine perfusion of the liver. Unlike static hypothermic preservation, machine perfusion provides continuous circulation and therefore improved preservation of the microcirculation, effective delivery of nutrients and oxygen for cellular metabolism, removal of metabolic waste products and administration of cytoprotective and/or immunomodulating agents. Machine perfusion also enables the surgical team to assess organ viability and potentially extend the period of organ storage.94 Liver perfusion protocols, which are based on the temperature of the perfusate, can be categorized as normothermic (37 °C), subnormothermic (20 °C) or hypothermic (0–4 °C).
The rationale for normothermic machine perfusion is to maintain physiological temperature and avoid hypoxia to enable maintenance of cellular metabolic function during organ storage, avoiding depletion of cellular energy stores, accumulation of metabolic waste products and the direct adverse events of hypothermia. By using this approach, stable metabolic and haemodynamic function in a pig liver was reported for up to 72 h in a nontransplant setting.95 Normothermic extracorporeal perfusion for 4 h at 37 °C in a pig DCD liver model enabled successful transplantation of livers exposed to up to 60 min of warm ischaemia.96 Superior outcomes of DCD liver transplantation were reported after prolonged periods of normothermic perfusion compared with cold static storage in a pig model.97
Hypothermic organ perfusion is based on reducing cellular metabolism by lowering the temperature of the organ. Indeed, hypothermic oxygenated perfusion of pig DCD liver grafts can confer a survival benefit that is associated with a reduction of necrosis and platelet adhesion, improvement of bile flow and the return to usual levels of ATP and glutathione.98 The first prospective clinical study has confirmed machine perfusion as a safe and reliable preservation strategy.99 However, cold perfusion limits the duration of organ storage because it might increase vascular resistance and damage to sinusoidal endothelium and endoplasmic reticulum.100
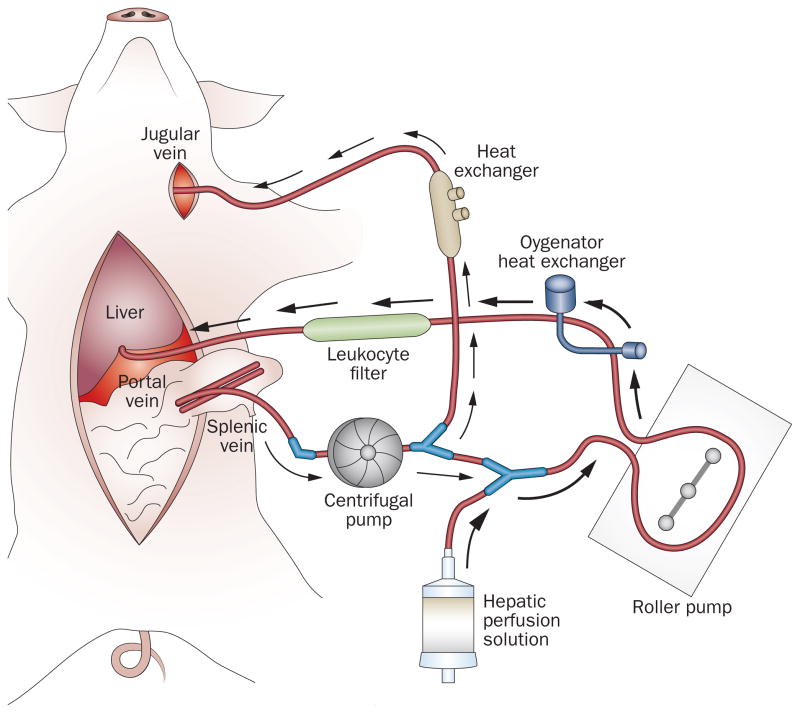
A critical factor of reperfusion injury is microcirculatory failure, which is associated with insufficient energy supply, impaired ATP regeneration and incomplete recovery of hepatocellular excretory function.101,102 Resuscitation of mitochondria (in which the mitochondria are protected from damage and orderly energy production is sustained) offers a potential avenue to mitigate IRI because of its central role in energy production, the generation of ROS and the initiation of apoptosis and necrosis. A group at UCLA, USA, has proposed a novel concept of regulated hepatic reperfusion to modulate IRI during the critical period of organ revascularization.91 The strategy aims to nurture ischaemic hepatocytes by providing a substrate-enriched, leukocyte-depleted, oxygen-saturated perfusate in a pressure, temperature and flow-controlled milieu to enable compromised cells to withstand reperfusion insult of warm blood during allograft revascularization (Figure 3). In a pig model, regulated hepatic reperfusion mitigated IRI, provided intraoperative postreperfusion haemodynamic stability, preserved hepatocellular function and improved survival after prolonged periods of warm ischaemia.91 This novel strategy might have direct applicability to clinical hepatic surgery and transplantation of marginal grafts. In human liver surgery, regulated hepatic reperfusion can be administered after a prolonged hepatic warm ischaemia from portal trial occlusion (the Pringle manoeuvre). In liver transplantation, regulated hepatic reperfusion can be applied during organ procurement after cardiac death and/or before organ revascularization during liver implantation.
Figure 3.
Regulated hepatic reperfusion and splenojugular venovenous bypass circuits. An extracorporeal centrifugal pump recirculates the animal’s splanchnic venous blood to the heart through a splenojugular venovenous bypass (the direction of the blood flow is indicated by white arrows) to avoid congestion of the splanchnic circulation during total portal occlusion. During regulated hepatic reperfusion, an amount of the animal’s splanchnic venous blood is diverted through a Y-connector from the centrifugal pump and mixed with hepatic perfusion solution in a 4:1 dilution ratio (perfusate). Another extracorporeal roller pump recirculates the perfusate through a paediatric oxygenator heat exchanger and leukoreduction filter before perfusion of the liver through the portal vein (the direction of the perfusate flow is indicated by black arrows). The roller pump regulates the reperfusion pressure (8–12 mmHg) and the oxygenator heat exchanger maintains the perfusate oxygen saturation (to 100%) and temperature (30–32 °C). Permission obtained from Elsevier Ltd © Hong, J. C. et al. Am. J. Coll. Surg. 214, 505–515 (2012).
Liver IRI transplant clinical trials
Little progress has been made since the seminal study by a group of researchers from Munich, Germany, on the beneficial effects of human recombinant superoxide dismutase in mitigating endothelial cell damage mediated by free radicals and induced by ischaemia–reperfusion or to ameliorate acute and chronic rejection episodes in recipients of deceased donor renal transplants.15 Targeting hepatic IRI at the bedside can occur in preconditioning or postconditioning transplant settings. Preconditioning applies to pharmacological interventions in the donor or in the graft before implantation or reperfusion, whereas postconditioning refers to the time of reperfusion in transplant recipients. Although an array of agents have shown protective effects against liver IRI in animal models, only a few have been tested in clinical randomized controlled trials (RCTs) in liver transplantation (Table 1).
Table 1.
Randomized clinical trials on pharmacological strategies to minimize hepatic IRI in deceased donor liver transplantation
| Study | Pharmacological intervention | Mechanism | Patients (placebo/study drug) | Findings for intervention group |
|---|---|---|---|---|
| Klein et al. (1999)105 | Epoprostenol (iv bolus of 500 μg) before cross clamp | Improvement of sinusoidal perfusion | 53/53 | Decreased levels of AST and ALT after surgery |
| Bogetti et al. (2005)110 | Thymoglobulin (1.5 mg/kg) during anhepatic phase and 2 doses after surgery | Suppression of inflammatory immune response | 11/11 | Decreased levels of AST and bilirubin after surgery, improved initial allograft function |
| Khan et al. (2005)124 | NAC iv and portal flush of donor liver | Antioxidant hepatoprotection | 9/9 | No protective effects on liver IRI or on acute cellular rejection |
| HEGPOL trial126 | HEGPOL (glycin) in multiple iv doses in liver transplant recipients | Decreased Kupffer cell activation | 65/65 | Trial completed but not yet published |
| Baskin-Bey et al. (2007)111 | IDN-6556 in organ storage solution and recipient | Inhibition of pan-caspase (apoptosis) | 23 (placebo)/23/27/26* | Decreased apoptosis and decreased liver injury for the group with study drug in preservation and flush solution |
| Lang et al. (2007)118 | Inhaled NO (80 ppm) during liver transplantation | Downregulation of endogenous NO production | 10/10 | Decreased levels of AST after surgery, decreased hepatocyte apoptosis, improved rate of liver function |
| Kotsch et al. (2008)106 | Donor treatment with iv methylprednisolone before recovery | Suppression of inflammatory immune response | 50/50 | Decreased levels of AST, decreased serum levels of cytokine, improved levels of biomarkers after surgery and decreased incidence of acute rejection |
| Hilmi et al. (2010)125 | NAC (12 doses) in liver transplant patients | Antioxidant/GSH-mediated hepatoprotection | 50/50 | No effects on liver/renal injury, no increase in GSH levels in some patients (possibly because of inadequate dose/duration of NAC) |
| Kristo et al. (2011)123 | Intraoperative intraportal organ perfusion with tacrolimus | Suppression of inflammatory immune response | 13/13 | No effects on early graft function despite decreased immune response and inflammation on a genome-wide basis |
| Busuttil et al. (2011)113 | Preimplantation allograft and recipient treatment with rPSGL-Ig | Blockade of leukocyte adhesion cascade | 24/23 | Decreased levels of AST after surgery in recipients with high DRI, and improved biomarkers (IL-10, CXCL10) |
The first group received placebo through the process, the second group received IDN-6556 during cold storage and flush and placebo was given to the recipient, the third group received a solution of 5 μg/ml IDN-6556 during cold storage and flush and then 0.5 mg/kg of IDN-6556 every 6 h for 24 h after the surgery, the fourth group received a solution of 15 μg/ml IDN-6556 during cold storage and flush and then 0.5 mg/kg of IDN-6556 every 6 h for 48 h after the surgery.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DRI, donor risk index; GSH, glutathione; IP-10, inducible protein 10; IRI, ischaemia–reperfusion injury; iv, intravenous; NAC, N-acetyl cysteine; NO, nitric oxide; rPSGL-Ig, recombinant P-selectin glycoprotein ligand IgG.
In agreement with the ability of prostaglandins to exert cytoprotection against liver IRI in rodents,103,104 systemic bolus of prostacyclin (also known as the drug epoprostenol) in the donor before the graft retrieval improved early sinusoidal perfusion and diminished the sharp increase in levels of hepatocellular enzymes as a result of local IRI after liver transplantation.105 Confirming experimental findings on the key pathogenic function of adaptive–innate immunity crossregulation,4 administration of immunosuppressive drugs that target T cells seems to be an attractive approach to suppress the inflammation response that results from brain death and ischaemia–reperfusion.106,107 For instance, continuous treatment of brain dead donors with methylprednisolone resulted in a considerably reduced incidence of hepatic IRI and lowered the rate of acute rejection.106 The rationale behind this study relates to the enhanced immune activation in brain dead donors, which in turn might trigger inflammation and damage mediated by ischaemia–reperfusion in liver transplants. Donor pretreatment with methylprednisolone not only reduced serum levels of IL-2, IL-6, CCL2, TNF and CXCL10 but also downregulated macrophage (CD68) local sequestration along with expression of major histocompatibility complex class II, tumour necrosis factor ligand superfamily member 6, TNF, CXCL10 and intercellular adhesion molecule 1 at the graft site.107
Following encouraging data from patients who received a renal transplant,108,109 the effects of thymoglobulin (an anti-thymocyte globulin) on IRI were studied in deceased donor liver transplantation.110 Indeed, treatment with thymoglobulin during the anhepatic phase (when the patient’s liver has been removed and the donor liver has not been transplanted) and shortly after surgery enabled compromised livers to be transplanted, as this strategy improved the initial graft function and ameliorated the hepatocellular damage. This effect might have resulted from blockade of the adhesion cascade, decreased β2 integrin expression and depletion of activated CD4+ T cells, which are all known to be instrumental in the liver IRI immune cascade.4 These findings imply that combined rather than individual donor and recipient immunosuppressive treatment is more efficacious against IRI in patients who receive a liver transplant.
Activation of caspase is involved in the initiation and execution of hepatocyte apoptosis, the key effector mechanism of ischaemia–reperfusion liver damage.4 Therefore, the pan-caspase inhibitor, IDN-6556, was used in a limited number of patients at centres where the incidence of primary liver transplant nonfunction was minimal.111 Although IDN-6556 administered in cold storage and flush solutions during liver transplantation offered local protection against ischaemia–reperfusion-mediated apoptosis and early hepatocellular damage, adjunctive intravenous administration of IDN-6556 abrogated the beneficial effect of organ-alone exposure to the inhibitor.111 Indeed, preventing caspase activation might hinder neutrophil apoptosis and, in doing so, might prolong and/or worsen the local inflammatory response.112
Busuttil et al.113 have pioneered the concept of targeting the cell adhesion cascade as a new means to mitigate IRI in patients who receive a liver transplant. Instead of using immunosuppressive agents, the selectin antagonist known as recombinant P-selectin glycoprotein ligand IgG (rPSGL-Ig), blocks the initial tethering of leukocytes to activated platelets and endothelium.114 Indeed, treatment with rPSGL-Ig has repeatedly shown its efficacy against organ IRI in experimental settings.115–117 Furthermore, in an RCT of recipients of a deceased-donor liver, rPSGL-Ig improved early hepatocellular function when given as an intravenous bolus combined with pretransplant allograft ex vivo treatment.113 Interestingly, rPSGL-Ig therapy was particularly effective in recipients with a donor risk index above the study average, in whom improved normalization of levels of aspartate aminotransferase correlated with decreased CXCL10 yet augmented immune regulatory IL-10 levels. These early clinical findings document the efficacy of a refined cell adhesion rather than broadly immunosuppressive targeted therapy to mitigate hepatocellular damage mediated by ischaemia–reperfusion in procedures that use marginal liver in association with modulation of CXCL10 and IL-10, the signature biomarkers in experimental liver IRI.
Another therapeutic concept of potential importance is the administration of nitric oxide, as demonstrated by the reduction of hepatocyte apoptosis and restoration of allograft liver function, without affecting the hepatic inflammatory markers, in patients who received inhaled nitric oxide before and after reperfusion. 118 Currently, three RCTs (NCT01172691, NCT00948194 and NCT00582010)119–121 are underway in the US to evaluate the protective effects of inhaled NO in liver transplantation.122
Some RCTs have ultimately failed to ameliorate liver IRI in patients who have received a liver transplant (Table 1). Indeed, although treatment with calcineurin inhibitors has been reported to reduce experimental organ IRI in small animals, intraoperative intraportal donor organ perfusion with tacrolimus did not affect early liver graft function in patients who received a transplant, despite decreased immune responses and local inflammation on a genome-wide basis.123 Similarly, at least two antioxidant RCTs that have used N-acetyl cysteine either as a donor organ flush124 or as a post-transplant regimen125 did not produce beneficial effects, perhaps because of an inadequate increase of glutathione levels in some of the patients undergoing therapy based on N-acetyl cysteine.125 Further studies with appropriate dose ranging and in larger cohorts of patients are required to establish whether or not calcineurin inhibitors and antioxidant regimens are effective treatment modalities for organ IRI in deceased donor liver transplantation. Some major challenges remain, as we still need to establish how relevant currently used animal experimental models are for patients who receive liver transplants. In addition, our appreciation of the human pathophysiology of liver warm and cold IRI is still fairly poor.
Conclusion
One of the major problems facing organ transplantation is the widening disparity between the numbers of potential recipients who vie for the donor supply.1 Although the number of liver transplants in the USA has increased 3.7-fold between 1988 and 2011, the number of patients on waiting lists has increased 15-fold, and many of them die while waiting for the life-saving organ.2 The leading factor in the decreasing number of donor livers available for transplantation is IRI, that is, local tissue damage that occurs during organ harvesting and the subsequent peritransplant period. Cellular immune activation, cytoprotective functions and immune regulation are all critically involved in the pathogenesis of hepatic IRI. Despite their distinctive roles in different liver cell types, these pathways might function in series and in a synergistic or counteractive fashion. The intricate network of functional interactions among molecular targets can be amplified, highly regulated and in many cases flexible to be either cell or tissue-type specific. Therefore, dissecting innate–adaptive immune cross-regulation in clinically relevant animal models of liver IRI is warranted to provide the rationale for much needed novel agents to fill the current therapeutic gaps. However, a great deal of work is yet to be done to translate our current experience from bench to bedside in the continuing effort to improve donor organ quality, save lives, benefit patient outcomes and ultimately enhance the overall success of clinical liver transplantation.
Key points.
The cellular damage incurred by organ procurement and preservation affects transplantation outcomes, contributes to donor organ shortage and represents a major risk factor for acute and chronic liver graft rejection
Liver ischaemia–reperfusion injury (IRI) is a local proinflammatory response that is mediated by the innate immune system
Several pattern recognition receptors, including Toll-like receptor (TLR)4, TLR9 and the inflammasome, are involved in liver immune activation against ischaemia–reperfusion in distinct cell types at different stages of IRI development
Different subsets of T cells participate in innate immune responses triggered by ischaemia–reperfusion via positive and negative co-stimulatory pathways
Studies of regulated hepatic reperfusion in large animals might lead to successful clinical use of liver grafts procured from extended criteria donors and donation after cardiac death
Review criteria.
Relevant literature on the pathophysiology of liver ischaemia–reperfusion injury (IRI) in experimental settings and in liver transplant patients were identified by searching the PubMed database for articles published up to June 2012. The search terms used in combination with “liver IRI” were “transplantation”, “inflammation”, “T cells”, “innate immunity”, “Toll-like receptors” and “macrophages”. All papers identified were English-language, full-text original research articles published in peer-reviewed journals.
Footnotes
Author contributions
All authors contributed equally to researching data for the article, discussions of content and reviewing and editing the manuscript. Y. Zhai, H. Petrowsky, J. C. Hong and J. W. Kupiec-Weglinski wrote the article.
Competing interests
The authors declare no competing interests.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Organ Procurement and Transplantation Network. 2012 [online], http://optn.transplant.hrsa.gov/data/
- 3.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 4.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltzchig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeschke H, Woolbright BL. Current strategies to minimize ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev. 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Amara M, et al. Liver ischemia/reperfusion injury: Processes in inflammatory networks—a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 9.Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15:1172–1182. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 10.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 11.Richards JA, Wigmore SJ, Devey LR. Heme oxygenase system in hepatic ischemia-reperfusion injury. World J Gastroenterol. 2010;16:6068–6078. doi: 10.3748/wjg.v16.i48.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda T, et al. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992;16:454–461. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- 13.Shen XD, et al. Disruption of type-I IFN pathway ameliorates preservation damage in mouse orthotopic liver transplantation via HO-1 dependent mechanism. Am J Transplant. 2012;12:1730–1739. doi: 10.1111/j.1600-6143.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen XD, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 15.Land W, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 16.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79:505–514. doi: 10.1097/01.tp.0000153160.82975.86. [DOI] [PubMed] [Google Scholar]
- 17.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 18.Fox-Marsh A, Harrison LC. Emerging evidence that molecules expressed by mammalian tissue grafts are recognized by the innate immune system. J Leukoc Biol. 2002;71:401–409. [PubMed] [Google Scholar]
- 19.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 20.Lotze MT, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 21.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann NY Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 26.Baccala R, et al. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 27.Tsung A, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 28.Wu HS, et al. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250–253. [PubMed] [Google Scholar]
- 29.Zhai Y, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 30.Leemans JC, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arslan F, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic Toll-like receptor-2 and reduced by systemic administration of a novel anti-Toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 32.Shen XD, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 33.Ellett JD, et al. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009;15:1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui W, Jinxiang Z, Heshui W, Zhuoya L, Qichang Z. Bone marrow and non-bone marrow TLR4 regulates hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun. 2009;389:328–332. doi: 10.1016/j.bbrc.2009.08.149. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulskens WP, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaczorowski DJ, et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;87:1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shigeoka AA, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 39.Broad A, Kirby JA, Jones DE. Toll-like receptor interactions: tolerance of MyD88-dependent cytokines but enhancement of MyD88-independent interferon-beta production. Immunology. 2007;120:103–111. doi: 10.1111/j.1365-2567.2006.02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren F, et al. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268–1270. [PubMed] [Google Scholar]
- 44.Fiorini RN, et al. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–1573. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhai Y, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–1022. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 46.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 47.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsung A, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hreggvidsdottir HS, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 50.Qin YH, et al. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol. 2009;183:6244–6250. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]
- 51.Zeng S, et al. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J Hepatol. 2009;50:929–936. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Zeng S, et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39:422–432. doi: 10.1002/hep.20045. [DOI] [PubMed] [Google Scholar]
- 53.Dhupar R, et al. Interferon regulatory factor 1 mediates acetylation and release of high mobility group Box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 54.Ueki S, et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51:1692–1701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bamboat ZM, et al. Toll-like receptor 9 inhibition confers protection from liver ischemiareperfusion injury. Hepatology. 2009;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 60.Zhu P, et al. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum Gene Ther. 2011;22:853–864. doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- 61.Menzel CL, et al. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med. 2011;17:1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Zwacka RM, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabb H, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 65.Shen XD, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 66.Burne MJ, et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL. The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest. 1997;100:1199–1203. doi: 10.1172/JCI119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen XD, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 69.Kono H, et al. Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. J Immunol. 2011;187:4818–4825. doi: 10.4049/jimmunol.1100490. [DOI] [PubMed] [Google Scholar]
- 70.Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007;82:457–464. doi: 10.1189/jlb.0107062. [DOI] [PubMed] [Google Scholar]
- 71.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 72.Kreymborg K, et al. IL-22 is expressed by TH17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 73.Ki SH, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role off signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 75.Chestovich PJ, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–492. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knolle P, et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 77.De Creus A, et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 78.Bamboat ZM, et al. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 80.Uchida Y, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rong S, et al. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:484–495. doi: 10.1681/ASN.2010030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uchida Y, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji H, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueki S, et al. Hepatic B7 Homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54:216–228. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen X, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beldi G, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magliocca JF, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58:1095–1101. doi: 10.1097/01.ta.0000169949.82778.df. [DOI] [PubMed] [Google Scholar]
- 90.Fondevila C, et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849–1855. doi: 10.1111/j.1600-6143.2007.01846.x. [DOI] [PubMed] [Google Scholar]
- 91.Hong JC, et al. Regulated hepatic reperfusion mitigates ischemia and reperfusion injury and improves survival after prolonged liver warm ischemia: a pilot study on a novel concept of organ resuscitation in a large animal model. J Am Coll Surg. 2012;214:505–515. doi: 10.1016/j.jamcollsurg.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Valdecasas JC, et al. Liver conditioning after cardiac arrest: the use of normothermic recirculation in an experimental animal model. Transpl Int. 1998;11:424–432. doi: 10.1007/s001470050169. [DOI] [PubMed] [Google Scholar]
- 93.Fondevila C, et al. Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg. 2011;254:1000–1007. doi: 10.1097/SLA.0b013e31822b8b2f. [DOI] [PubMed] [Google Scholar]
- 94.Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160–166. doi: 10.1097/MOT.0b013e328337342b. [DOI] [PubMed] [Google Scholar]
- 95.Butler AJ, et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73:1212–1218. doi: 10.1097/00007890-200204270-00005. [DOI] [PubMed] [Google Scholar]
- 96.Schon MR, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114–123. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brockmann J, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 98.de Rougemont O, et al. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250:674–683. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 99.Guarrera JV, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372–381. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 100.Minor T, Manekeller S, Sioutis M, Dombrowski F. Endoplasmic and vascular surface activation during organ preservation: refining upon the benefits of machine perfusion. Am J Transplant. 2006;6:1355–1366. doi: 10.1111/j.1600-6143.2006.01338.x. [DOI] [PubMed] [Google Scholar]
- 101.Goto M, et al. Hepatic tissue oxygenation as a predictive indicator of ischemia-reperfusion liver injury. Hepatology. 1992;15:432–437. doi: 10.1002/hep.1840150313. [DOI] [PubMed] [Google Scholar]
- 102.Kamiike W, et al. Correlation between cellular ATP level and bile excretion in the rat liver. Transplantation. 1985;39:50–55. doi: 10.1097/00007890-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 103.Monden M, Fortner JG. Twenty-four- and 48-hour canine liver preservation by simple hypothermia with prostacyclin. Ann Surg. 1982;196:38–42. doi: 10.1097/00000658-198207000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghonem N, et al. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. Am J Transplant. 2011;11:2508–2516. doi: 10.1111/j.1600-6143.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 105.Klein M, et al. Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation. 1999;67:1128–1132. doi: 10.1097/00007890-199904270-00007. [DOI] [PubMed] [Google Scholar]
- 106.Kotsch K, et al. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation: a prospective randomized controlled trial. Ann Surg. 2008;248:1042–1050. doi: 10.1097/SLA.0b013e318190e70c. [DOI] [PubMed] [Google Scholar]
- 107.Weiss S, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 108.Mehrabi A, et al. Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant. 2007;22(Suppl 8):viii54–viii60. doi: 10.1093/ndt/gfm651. [DOI] [PubMed] [Google Scholar]
- 109.Goggins WC, et al. A prospective randomized clinical trial of intraoperative versus postoperative thymoglobulin in adult cadaveric renal transplant recipients. Transplantation. 2003;76:798–802. doi: 10.1097/01.TP.0000081042.67285.91. [DOI] [PubMed] [Google Scholar]
- 110.Bogetti D, et al. Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury. Clin Transplant. 2005;19:507–511. doi: 10.1111/j.1399-0012.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 111.Baskin-Bey ES, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 112.Alvarado-Kristensson M, et al. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med. 2004;199:449–458. doi: 10.1084/jem.20031771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Busuttil RW, et al. rPSGL-Ig for improvement of early liver allograft function: a double-blind, placebo-controlled, single-center phase II study. Am J Transplant. 2011;11:786–797. doi: 10.1111/j.1600-6143.2011.03441.x. [DOI] [PubMed] [Google Scholar]
- 114.Yang J, Galipeau J, Kozak CA, Furie BC, Furie B. Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood. 1996;87:4176–4186. [PubMed] [Google Scholar]
- 115.Dulkanchainun TS, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amersi F, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. Am J Transplant. 2002;2:600–608. doi: 10.1034/j.1600-6143.2002.20704.x. [DOI] [PubMed] [Google Scholar]
- 117.Tsuchihashi S, et al. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616–624. doi: 10.4049/jimmunol.176.1.616. [DOI] [PubMed] [Google Scholar]
- 118.Lang JD, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.US National Library of Medicine. ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/results?term=NCT01172691.
- 120.US National Library of Medicine. ClinicalTrials.gov. 2009 [online], http://clinicaltrials.gov/ct2/results?term=NCT00948194.
- 121.US National Library of Medicine. ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/results?term=NCT00582010.
- 122.US National Library of Medicine. ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov.
- 123.Kristo I, et al. Effect of intraportal infusion of tacrolimus on ischaemic reperfusion injury in orthotopic liver transplantation: a randomized controlled trial. Transpl Int. 2011;24:912–919. doi: 10.1111/j.1432-2277.2011.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khan AW, et al. A prospective randomized trial of N-acetyl cysteine administration during cold preservation of the donor liver for transplantation. Ann Hepatol. 2005;4:121–126. [PubMed] [Google Scholar]
- 125.Hilmi IA, et al. N-acetylcysteine does not prevent hepatorenal ischaemia-reperfusion injury in patients undergoing orthotopic liver transplantation. Nephrol Dial Transplant. 2010;25:2328–2333. doi: 10.1093/ndt/gfq077. [DOI] [PubMed] [Google Scholar]
- 126.ISRCTN Register. HEGPOL: Randomised, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation. 2012 doi: 10.1186/1471-2482-5-18. [online], http://www.controlled-trials.com/ISRCTN69350312/ [DOI] [PMC free article] [PubMed]