Abstract
Phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is important in cell proliferation and survival, and it is frequently and aberrantly activated in pancreatic adenocarcinoma. Potential anti-tumor effect(s) of ZSTK474, a PI3K/Akt inhibitor, together with a key clinically relevant anti-tumor agent, gemcitabine (GEM), have been reported in a human pancreatic cancer xenograft mouse model (Yaguchi et al., Anti-tumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst 98:546-556, 2006). However, the precise molecular mechanism causing anti-tumor effects has not been well elucidated. In this study, we investigated the molecular mechanism of GEM plus ZSTK474 in reducing tumor cell survival in human pancreatic cancer cell lines. Our study showed that ZSTK474 inhibited cell growth by arresting cells at the G1 phase and by inducing apoptosis. ZSTK474 also inhibited the phosphorylation of Akt, GSK3β and BAD. The combination of GEM and ZSTK474 demonstrated synergistic anti-tumor effects on pancreatic cancer cells in both transient (3 days) and long-term (14 days) clonogenic assays. Thus, we elucidated the potential molecular mechanism leading to the enhanced anti-tumor effect when GEM and ZSTK474 are combined in treatment.
Keywords: PI3K/Akt pathway, ZSTK474, Gemcitabine, Apoptosis, Human pancreatic adenocarcinoma
Introduction
Pancreatic adenocarcinoma is one of the most common causes for cancer-related deaths in developed countries (1, 2). It is one of the most aggressive human solid tumors with an extremely poor prognosis because of its aggressive invasion, early metastasis, and resistance to existing chemotherapy and radiotherapy (3-5). Since gemcitabine (GEM) was introduced as an anti-tumor agent in 1996, it has been used as the standard first-line chemotherapeutic agent for the treatment of advanced and metastatic pancreatic cancer (6). However, its therapeutic efficacy seems marginal and pancreatic cancer easily acquires resistance to GEM after a few cycles of administration (7).
There are multiple signaling pathways that could potentially enhance growth and proliferation of pancreatic cancer, including nuclear factor kappa-B (NF-kB), phosphatidylinositol-3-kinase (PI3K)/Akt, and mitogen-activated protein kinase (MAPK). Moreover, the PI3K/Akt pathway has been reported as an important factor in confering chemoresistance to GEM in pancreatic cancer (8, 9). The pathway for PI3K is activated by various extracellular signals and leads to the phosphorylation of Akt and its downstream effectors (10). When Akt is phosphorylated, it, in turn, phosphorylates a variety of proteins leading to cell survival and proliferation (10). In addition, Akt is reported to be constitutively over-expressed in various pancreatic cancer cell lines (11).
Several previous studies have indicated that LY294002 and wortmannin, classical inhibitors of the PI3K/Akt pathway, can increase drug sensitivity in pancreatic adenocarcinoma cells in vitro and in vivo (12). Inhibition of the PI3K/Akt pathway by LY294002 or wortmannin enhances GEM-induced apoptosis in human pancreatic cancer cells (8). Therefore, the PI3K/Akt pathway may play a significant role in mediating drug resistance and is a promising target for therapeutic intervention in human pancreatic cancer (8, 12). Although these classic PI3K/Akt inhibitors have therapeutic potential when used either by themselves or in combination with GEM in the treatment pancreatic cancer, the severe cytotoxicity observed in preclinical animal studies limit their use for clinical trials (13). Accordingly, screening and identification of significantly effective novel PI3K/Akt inhibitor(s) to enhance clinical efficacy are important.
ZSTK474 is a pan-PI3K inhibitor, synthesized by Zenyaku Kogyo Co., Ltd (Tokyo, Japan) and can be given orally (14). It has shown potent anti-tumor activity against human cancer xenografts without toxic effects in critical organs (15). ZSTK474 has been reported to inhibit 39 human cancer cell lines including lung, stomach, ovarian, renal, colon, breast, brain, prostate cancer and melanoma in vitro (16, 17). Most notably, ZSTK474 competed with ATP to inhibit all four p110 isoforms of PI3K subunits with IC50 values of 16, 44, 5 and 49 nmol for p110α, -β, -δ, -γ, respectively (18). Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis, but G0/G1 arrest in prostate, lung, glioblastoma and colorectal cancer lines (19). In addition, ZSTK474 inhibits very specifically PI3K without targeting other types of protein kinases such as PI3K-related kinases such as mammalian target of rapamycin (mTOR) and DNA-activated protein kinase (DNA-PK) (15, 18).
In this study, we examined the anti-tumor efficacy of ZSTK474 in several human pancreatic cancer cell lines and also investigated the combination effects of ZSTK474 with various chemotherapeutic agents such as GEM and 5-FU. One aim of our study was to investigate the molecular mechanism of ZSTK474 alone or with GEM in suppressing growth.
Materials and Methods
Cell culture and reagents
MIA PaCa-2 and BxPC-3 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA), and AsPC-1 and Colo-357 cells were obtained from Tissue Culture Shared Resource of Georgetown University Medical School (GUMC; Washington, DC). AsPC-1, BxPC-3 and Colo-357 cells were cultured in RPMI 1640 media supplemented with fetal bovine serum (FBS; 20% for AsPC-1 and 10% for Colo-357 and BxPC-3 cells), 100 units/ml penicillin, 100μg/ml streptomycin and 1% sodium pyruvate. MIA PaCa-2 cells were cultured in Dulbecco's Modified Eagles’ Medium (DMEM) containing 10% FBS, 2.5% horse serum (HS), 100 units/ml penicillin, and 100 μg/ml streptomycin. All cell culture reagents were purchased from BioWhittaker (Walkersville, MD). ZSTK474 was purchased from LC Laboratories (Woburn, MA), and GEM was obtained from Sigma (St. Louis, MO).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and determination of the combination index (CI)
A total of 2500 human pancreatic cancer cells were plated in 96-well flat bottom plates and then exposed to test agents in various concentrations. At the indicated times, 10 μl of 1 mg/ml MTT (Sigma, St. Louis, MO) in PBS was added to each well for 4 h. After centrifugation and removal of the medium, 150 μl of DMSO (Sigma, St. Louis, MO) was added to each well to dissolve the formazan crystals. The absorbance was measured at 540 nm using an ELx808 Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT). Absorbance of untreated cells was designated as 100% and cell survival was expressed as a percentage of this value. Triplicate wells were assayed for each condition and standard deviation (SD) was determined. The drug interaction was evaluated by using the combination index (CI) according to the method of Chou and Talalay (20). For each combination experiment, the CI number was calculated by using CompuSyn software (ComboSyn, Inc., Paramus, NJ) and values of CI<1, CI=1, CI>1 indicated synergism, additive effect, and antagonism, respectively.
Western blot (WB) analysis
Cells were grown to ~70% confluence and reagents were added at the indicated concentrations. After exposure to ZSTK474 alone or in combination with GEM, cells were lysed in cell lysis buffer containing 20 mM Tris-HCl, 0.5 M NaCl, 0.25% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycophosphate, 10 mM NaF, 300 μM Na3VO4, 1 mM benzamidine, 2 μM PMSF, and 1 mM DTT. Protein concentrations were determined by a BCA protein assay kit (Thermo Scientific, Rockford, IL). Proteins were separated by SDS-PAGE, transferred on to PVDF membranes, blocked in 1 X blocking buffer (Sigma, St. Louis, MO) and probed with the following primary antibodies: phospho-GSK3β (S9), GSK3β, phospho-Akt (S473), Akt, phospho-BAD (S112), BAD (Cell Signaling Technology, Inc.,, Boston, MA), PARP (BD Biosciences, Franklin, NJ), and α-tubulin (Sigma, St. Louis, MO). Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma, St. Louis, MO) and visualized with a chemiluminescence kit (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer's instructions and exposed to X-ray film (American X-ray, Louisville, TN).
Clonogenic assay
Human pancreatic cancer cells (4 × 105 cells) were seeded in 60cm dishes. 24 h after plating, varying concentrations of the drugs, either as a single agent or in combination, were added to the dishes. After treatment, cells (2000 cells) were re-seeded in 60cm dishes (triplicate). Each culture dish was incubated for 14 days and photographed after staining with 0.5% crystal violet in PBS including 25% methanol. Colonies were examined under a light microscope and counted after capturing images by scanner. Colony numbers were calculated according to the percentage of the untreated cells.
Flow Cytometry
Human pancreatic cancer cell lines were collected after treatment of ZSTK474 with or without GEM by trypsinization, washed with PBS and fixed overnight in 70% ethanol at -20°C. Cells were then incubated with 20μg/ml propidium iodide and 40μg/ml RNase A in 1X PBS. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, CA). The acquired data were analyzed by Cell Quest Pro Analysis software (Becton Dickinson, San Jose, CA).
Caspase-3 activity assay
The Caspase-3 activity assay was carried out by using a caspase-3/CPP32 colorimetric assay kit (BioVision, Mountain View, CA) according to the manufacturer's instructions. Cells were treated with ZSTK474 or in combination with GEM with indicated concentrations for 48 h. Approximately 100 μg of protein was incubated with 200 μM Asp-Glu-Val-Asp-p-nitroanilide (DEVD-pNA) and 10 mM DTT at 37°C for 2 h. Absorbance was measured at 405 nm using an ELx808 Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT). Increasing percentage in CPP32 activity was determined by calculating these results in comparison with the percentage of un-induced control samples.
Statistical methods
Statistical comparisons were made using the two-tailed student's t-test where appropriate. Results were considered significant in all experiments at *P< 0.05, **P< 0.01 and ***P< 0.005. Data were expressed as the mean ± SD.
Results
ZSTK474 inhibits proliferation in human pancreatic adenocarcinoma cells
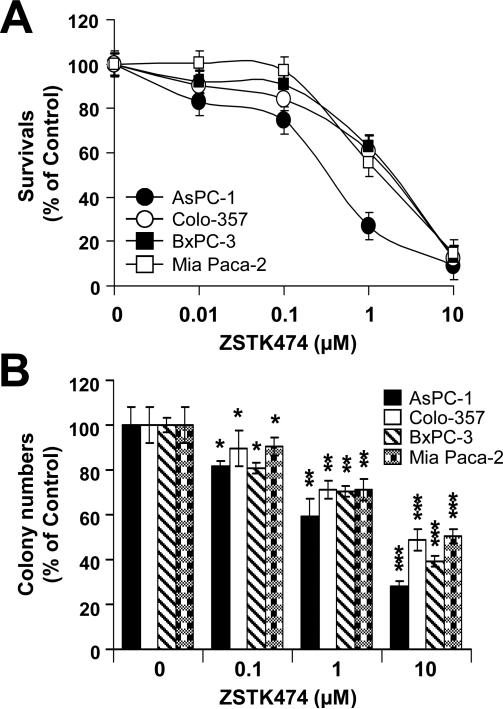
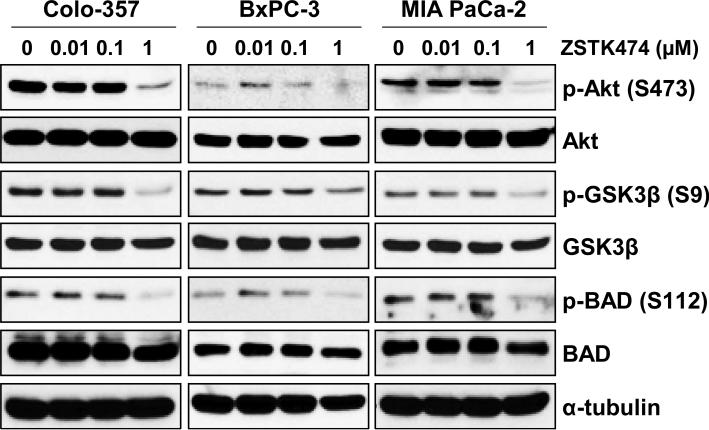
To evaluate the anti-tumor efficacy of ZSTK474 in human pancreatic adenocarcinoma cells, we measured cell viability of human pancreatic cancer cells lines (Colo-357, BxPC-3, MIA PaCa-2 and AsPC-1 cells) following ZSTK474 using MTT and clonogenic assays. The MTT assay showed that ZSTK474 inhibits cell proliferation in a dose-dependent manner (0, 0.01, 0.1, 1 or 10 μM) at 72 h (Fig. 1A). The IC50 of ZSTK474 was determined as 0.86, 1.16, 1.8 and 0.23 μM in Colo-357, BxPC-3, MIA PaCa-2 and AsPC-1 cells, respectively (Table 1). In addition, ZSTK474 suppressed the colony-forming ability of human pancreatic cancer cells, indicating the potential long-term anti-tumor efficacy of ZSTK474 (Fig. 1B). In order to determine the mechanisms responsible for the sensitivity of ZSTK474, we selected three human pancreatic cancer cell lines (Colo-357, BxPC-3 and MIA PaCa-2 cells). First, we determined the phosphorylated and total protein levels of Akt, BAD and GSK3β after 24 h of treatment with ZSTK474 in various concentrations. ZSTK474 substantially reduced the level of p-Akt (S473), p-BAD (S112) and p-GSK3β (S9) without significantly altering their total protein levels (Fig. 2). Especially, almost complete reduction of p-Akt (S473), p-BAD (S112) and p-GSK3β (S9) was observed by 1 μM ZSTK474 in all three human pancreatic cancer cell lines (Fig. 2). Taken together (Figs. 1 and 2), these data indicate that ZSTK474 suppresses proliferation by inhibiting the PI3K/Akt pathway and its downstream signaling.
Figure 1. Effects of ZSTK474 on cell survival in various human pancreatic cancer cell lines.
(A) AsPC-1, Colo-357, BxPC-3 and MIA PaCa-2 cells were treated with a various doses (0, 0.01, 0.1, 1 or 10 μM) of ZSTK474 for 72 h. Cell viability was measured by the MTT assay as described in Material and Methods. (B) AsPC-1, Colo-357, BxPC-3 and MIA PaCa-2 cells treated with ZSTK474 (0, 0.1, 1 or 10 μM) for 48 h were subjected to clonogenic assay as described in Material and Methods. Colony numbers were counted and calculated as a relative percentage (%) of the untreated control cells. Experiments were repeated three times and similar results were obtained. Error bars represent the standard deviation. *p < 0.05, significantly different from controls and 0.1 μM ZSTK474; **p < 0.01, significantly different from controls and 1 μM ZSTK474; and ***p < 0.005, significantly different from controls and 10 μM ZSTK474.
Table 1.
In vitro anti-tumor effect (s) of ZSTK474 in human pancreatic adenocarcinoma cells
| IC50 (μM) | ||||
|---|---|---|---|---|
| Human pancreatic cancer cells |
Colo-357 | BxPC-3 | MIA PaCa-2 | AsPC-1 |
| ZSTK474 | 0.86 | 1.16 | 1.8 | 0.23 |
Figure 2. Effects of ZSTK474 on the phosphorylation status of Akt and its downstream substrates.
Colo-357, BxPC-3 and MIA PaCa-2 cells were treated with increasing concentrations (0, 0.01, 0.1 or 1 μM) of ZSTK474 for 24 h. Cells were harvested, lysed and prepared for WB analysis. Antibodies to detect the total and phosphorylated forms of Akt (S473), GSK3β (S9), and BAD (S112) were used. The specific phosphorylation site(s) of each kinase is indicated in parentheses. Anti-α-tubulin antibody was used for a loading and transfer control.
ZSTK474 induces G1 arrest and apoptotic cell death
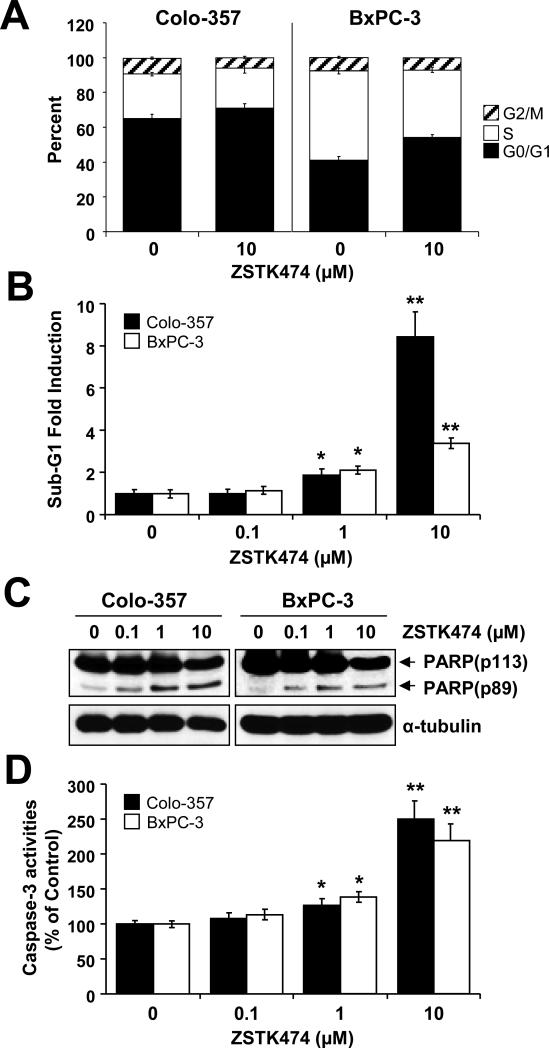
Since inhibition of PI3K/Akt is known to inhibit cell proliferation and survival, we determined to analyze cell cycle alterations and cell death after ZSTK474 treatment. Cells treated with ZSTK474 (10μM) for 48 h were harvested and subjected to FACS analysis. ZSTK474 increased the G1 population, compared with control cells (from 65% to 70.7% in Colo-357 and from 41.1% to 54.3% in BxPC-3; Fig. 3A), with a corresponding decrease in S-phase cells (from 25.6% to 23.2% in Colo-357 and from 51.14% to 38.48% in BxPC-3; Fig. 3A) and decreased the G2-phase cells (from 9% to 6% in Colo-357 and from 7.75% to 7.22% in BxPC-3; Fig. 3A). To further investigate whether ZSTK474 induces apoptosis, we analyzed the sub-G1 populations. Our results show that ZSTK474 increased the sub-G1 population in BxPC-3 and more significantly in Colo-357 at a higher dose (10μM) (Fig. 3B). The sub-G1 population significantly increased at 10μM ZSTK474 compared with control cells at 9-fold induction in Colo-357 cells and at 4-fold induction in BxPC-3 cells, indicating that ZSTK474 induced apoptotic cell death in these cells. Moreover, Western blot analysis revealed a pattern of molecular signaling events consistent with inhibition of cell proliferation and initiation of cell death. 48 h treatment with ZSTK474 significantly increased cleavage forms of PARP (Fig. 3C) and activation of caspase-3 activity (Fig. 3D) in a dose-dependent manner. Taken together, the results suggest that ZSTK474 suppresses tumor growth by inducing G1-phase arrest and apoptosis in human pancreatic cancer cells.
Figure 3. ZSTK474 induces G1 phase arrest and apoptotic cell death.
(A) Colo-357 and BxPC-3 cells were treated with 10 μM ZSTK474 for 48 h, harvested and subjected to FACS analysis. (B-D) Colo-357 and BxPC-3 cells were treated with ZSTK474 with variable doses (0, 0.1, 1 or 10 μM) for 48 h, harvested and subjected to FACS, WB analysis and caspase-3 activity assay. (B) The sub-G1 population from FACS analysis was analyzed and its fold induction was plotted in a bar graph. (C) Cells treated with ZSTK474 were subjected to WB analysis. An anti-PARP antibody was used to detect its cleavage form (an apoptosis marker). (D) Cells treated with ZSTK474 were harvested and subjected to caspase-3 activity assay kit as described in Material and Methods. Caspase-3 activities are calculated according to percentage of the untreated cells. *p < 0.05, significantly different from controls and 0.1 μM ZSTK474; **p < 0.01, significantly different from controls and 1 μM ZSTK474; and ***p < 0.005, significantly different from controls and 10 μM ZSTK474.
Synergistic cytotoxic effects of GEM plus ZSTK474
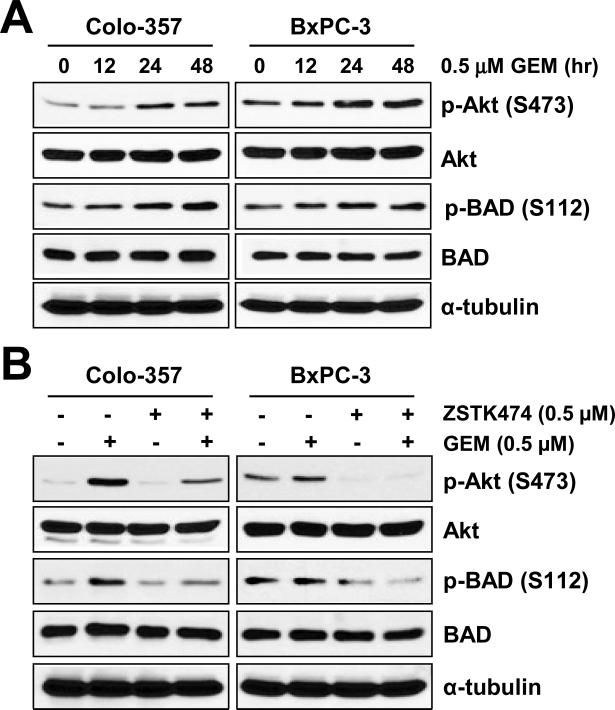
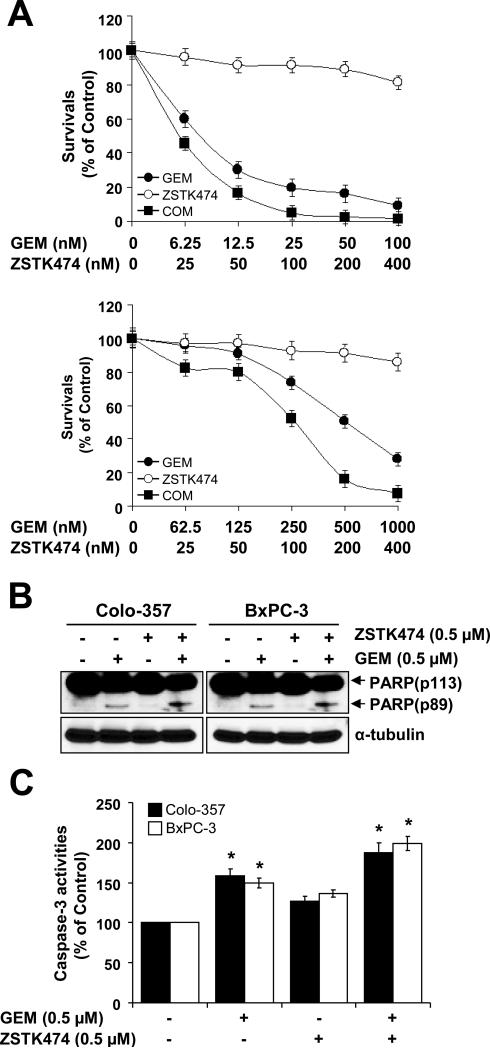
Previous studies reported that GEM induces Akt activation. Accordingly, we observed that GEM treatment substantially increased phosphorylation of Akt and BAD in both Colo-357 and BxPC-3 cells (Fig. 4A). Similar results were obtained in AsPC-1 and MIA PaCa-2 cells (data not shown). In order to study the effects of ZSTK474 on p-AKT (S473) and p-BAD (S112), cells were pre-treated with 0.5 μM of GEM for 24 h and cultured in the presence of ZSTK474 for an additional 24 h. As expected, ZSTK474 significantly suppressed GEM-induced phosphorylation of Akt and BAD (Fig. 4B). Since GEM induces Akt activity, we treated with GEM first for 24 h and then ZSTK474 for additional 48 h in fixed molar concentration ratios of 0.25:1 and 2.5:1 in Colo-357 and BxPC-3 cells, respectively. In our results, GEM plus ZSTK474 synergistically suppress cell survival of Colo-357 and BxPC-3 cell lines (Fig. 5A). Table 2 shows the CI values at ED50, ED75, and ED90 of Colo-357 and BxPC-3 cells. Our data show that pre-treatment with GEM gives more successful synergistic effects than the following sequential treatments: 1) treatment with the two drugs (GEM and ZSTK474) simultaneously or 2) treatment with ZSTK474 first and then GEM (data not shown). We also obtained similar synergistic effects by the combination effect of 5-FU and ZSTK474 measured by MTT assay (data not shown). To further address the synergism of GEM and ZSTK474, we analyzed the changes of apoptotic protein markers and caspase-3 activity. An apoptotic marker, cleaved PARP, was synergistically increased in the cells treated with GEM plus ZSTK474 (Fig. 5B). Measurement of caspase-3 activity also demonstrated that GEM plus ZSTK474 synergistically increased apoptosis in both Colo-357 and BxPC-3 cells (Fig. 5C). These results suggest that a specific blockade of PI3K/Akt by ZSTK474 induces apoptotic cell death via activation of caspase-3 and PARP cleavage in the cells pre-treated with GEM.
Figure 4. ZSTK474 supresses GEM-induced Akt activity.
(A) Colo-357 and BxPC-3 cells were treated with 0.5 μM GEM in time-dependent manner (0, 12, 24 or 48 h). Cells were harvested, lysed and WB analysis was carried out for total Akt, BAD, and their phosphorylated forms: p-Akt (S473), p-BAD (S112). The phosphorylation sites are indicated in parentheses. (B) Colo-357 and BxPC-3 cells were pre-treated with 0.5 μM GEM for 24 h and then treated with 0.5 μM ZSTK474 for 24 h. Cells were harvested, lysed and WB analysis was performed for total Akt, BAD, and their phosphorylated forms: p-Akt (S473) and p-BAD (S112).
Figure 5. Short-term effects of GEM plus ZSTK474.
(A) Colo-357 and BxPC-3 cells pre-treated with various doses of GEM for 24 h and further treated with various doses of ZSTK474 for 48 h with fixed molar concentration ratios of 0.25:1 and 2.5:1, respectively. Cell viability was determined by MTT assay. (B) Cells were pre-treated with 0.5 μM GEM for 24 h and treated with 0.5 μM ZSTK474 for an additional 24 h. These cells were then harvested, lysed and used for WB analysis. Anti-PARP antibody was used to detect apoptosis and α-tubulin antibody was used a loading and transfer control. (C) Cells treated with GEM plus ZSTK474 as described in (B) were lysed and caspase-3 activities were measured by using the caspase-3 colorimetric assay kit. Caspase-3 activities are calculated as a relative percentage of the untreated control sample. Experiments were repeated three times with similar results. Error bars represent the standard deviation. *p < 0.05, significantly different from controls and 0.1 μM ZSTK474; **p < 0.01, significantly different from controls and 1 μM ZSTK474; and ***p < 0.005, significantly different from controls and 10 μM ZSTK474.
Table 2. Synergistic inhibition of cell proliferation by combination effect of GEM plus ZZSTK474 in human pancreatic cancer cells.
Table 2 shows Cl-values obtained from experiments using the Colo-357 and BxPC-3 cells. These CI values were calculated by the Chou and Talalay method for drug interactions using Compusyn software for the different fractions affected (the CI values at ED50, ED75 and ED90). Values of CI<1, =1 and >1 indicate synergism, additive effects and antagonism, respectively.
| Combination index (CI) | |||
|---|---|---|---|
| ED50 | ED75 | ED90 | |
| GEM followed by ZSTK474 | |||
| Colo-357 | 0.63 | 0.4 | 0.26 |
| BxPC-3 | 0.43 | 0.41 | 0.38 |
Synergistic inhibition of colony formation by GEM plus ZSTK474
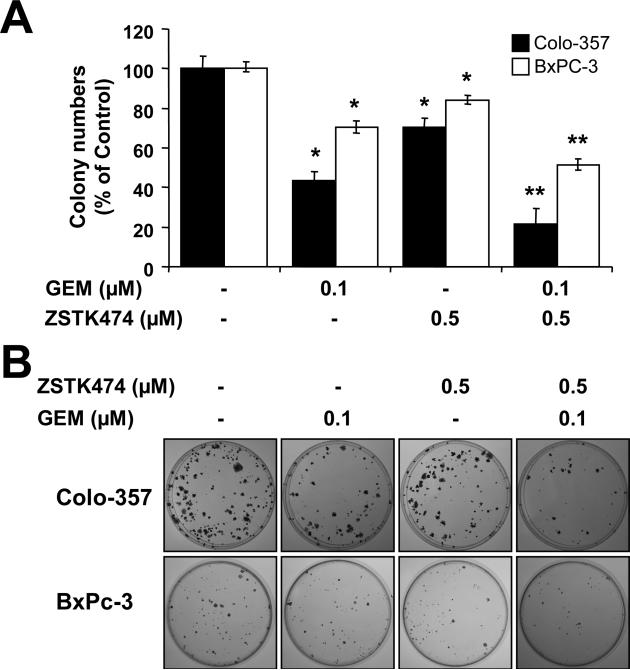
In order to confirm the long-term effects of GEM plus ZSTK474, cells pre-treated with GEM for 24 h were further treated with ZSTK474. 24 h later, both drugs in culturing media were removed and cells were continually cultured in fresh media for 14 days. Survived colonies were stained with crystal violet, and the numbers of colonies were counted and plotted as percentages of drug treatment versus non-drug treatment. Results showed that the clonogenicity was reduced to 43.4% and 70.5% of baseline after being exposed to GEM alone in Colo-357 and BxPC-3 cells, respectively (Fig. 6A). Similarly, ZSTK474 alone decreased the clonogenicity to 70.3% and 83.8% of baseline in Colo-357 and BxPC-3 cells, respectively (Fig. 6A). However, clonogenicity was significantly reduced to 21.4% and 51.4% of baseline in Colo-357 and BxPC-3 cells, respectively, when these cells were treated with GEM plus ZSTK474 (Fig. 6A). A photograph of a representative clonogenic assay is shown in Fig. 6B. Taken together, the results suggest that the combination of GEM and ZSTK474 results in a synergistic decrease in the clonogenic forming potential of Colo-357 and BxPC-3 cells.
Figure 6. Long-term effects of GEM plus ZSTK474.
(A) Colo-357 and BxPC-3 cells pre-treated with 0.5 μM GEM for 24 h were incubated with 0.5 μM ZSTK474 for additional 24 h. Then, cells were trypsinized, reseeded and cultured without GEM or ZSTK474. After 14 days colony numbers were counted and calculated as a relative percentage (%) of the untreated control cells. (B) Representative photograph of colony formation assay results are shown. Each experiment was performed in triplicate and similar results were obtained. Error bars represent the standard deviation. *p < 0.05, significantly different from controls and 0.1 μM ZSTK474; **p < 0.01, significantly different from controls and 1 μM ZSTK474; and ***p < 0.005, significantly different from controls and 10 μM ZSTK474.
Discussion
Our current study demonstrates that ZSTK474 treatment suppresses pancreatic cancer cell proliferation. This seems to be associated with ZSTK474's activity in inducing both cell cycle delay at G1 and apoptosis. A previous study shows that ZSTK474 with 10μM for 48 h only induced G1 arrest without inducing apoptosis in human lung, prostate and colorectal cancer cell lines (15). Raynaud et al., showed that LY294002, a PI3K/Akt inhibitor with low specificity, also affects most of its downstream protein kinases and stimulates the production of reactive oxygen species (21), which is an important apoptosis inducer. Although we do not have a clear explanation of these differential cellular responses against the two different PI3K/Akt inhibitors, it may be due to different chemical properties of these inhibitors and/or differential intrinsic capacity of apoptosis of cell types used for ours versus other's studies. Various cellular stress agents such as heat shock, UV irradiation, matrix detachment, cell cycle discordance, DNA damage and anti-tumor drugs are known to activate the PI3K/Akt pathway (22). GEM or cisplatin induced a transient increase of the phosphorylation levels of Akt in AsPC-1 cells in a dose-dependent manner (23, 24). Our data also demonstrates that GEM significantly increases the phosphorylation levels of Akt and its downstream signaling effectors like BAD and GSK3β in several human pancreatic cancer cell lines. Moreover, wortmanin and LY294002 block the phosphorylation of Akt in PK1 and PK8 pancreatic cancer cell lines and correlates with the enhancement of GEM-induced apoptosis (8). The identification of optimal dosing regimens and sequential schedules are important for the successful clinical evaluation of cancer therapeutics, especially when therapies are combined (25). In our experiments, the sequential treatment with GEM followed by ZSTK474 caused the most synergistic cell growth inhibition. In contrast, treatment with ZSTK474 followed by GEM demonstrated a mild additive or antagonistic effect, or simultaneous treatment of ZSTK474 and GEM resulted in reduced synergistic or additive effects as determined by MTT assay (data not shown). Although we do not exactly understand how the sequential treatment with GEM followed by ZSTK474 gives the better synergistic effects than other sequential treatment options, additional studies on this enhanced synergism may provide essential clues for choosing the best combination scheme for combination therapy of PKIs and anti-tumor agents such as GEM. Similarly, clonogenic assays from sequential treatment with GEM followed by ZSTK474 showed synergistic cell growth inhibition. Further studies are needed to confirm our MTT assay results using clonogenic assays and preclinical animal models.
Furthermore, it is known that orally administered ZSTK474 displays potent anti-tumor activity against human cancer xenografts in mice without evidence of critical toxicity, and it also reduced the phosphorylation of Akt after administration to mice (15). Thus, previous and current data support ZSTK474 as a novel anticancer drug candidate for the treatment of pancreatic cancer. In addition, ZSTK474 may be used in combination with chemotherapeutic agents to enhance treatment. In conclusion, our results show that ZSTK474 has strong anti-tumor efficacy and also enhances anti-tumor effects when combined with GEM in human pancreatic cancer cells. Our findings provide a rationale for the preclinical and clinical application of these combinations in pancreatic cancer.
Acknowledgments and Funding
Dr. Insoo Bae has been supported by National Institute of Health (1R03CA152530), by Susan G Komen for the Cure (FAS07038580) and by the Ministry of Education, Science and Technology through the National Research Foundation of Korea (R31-10069; World Class Uinversity (WCU) program).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Keighley MR. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther. 2003;18:7–30. doi: 10.1046/j.0953-0673.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 3.Bardeesy N, Depinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 4.Chua YJ, Zalcberg JR. Pancreatic cancer-is the wall crumbling? Ann Oncol. 2008;19:1224–1230. doi: 10.1093/annonc/mdn063. [DOI] [PubMed] [Google Scholar]
- 5.Pierantoni C, Pagliacci A, Scartozzi M, Berardi R, Berardi R, Bianconi M, Cascinu S. Pancreatic cancer: progress in cancer therapy. Crit Rev Oncol Hematol. 2008;67:27–38. doi: 10.1016/j.critrevonc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Ducreux M, Boige V, Malka D. Treatment of advanced pancreatic cancer. Semin Oncol. 2007;34:S25–30. doi: 10.1053/j.seminoncol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- 9.Yokoi K, Fidler U. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299–2309. doi: 10.1158/1078-0432.ccr-03-0488. [DOI] [PubMed] [Google Scholar]
- 10.Martelli AM, Faenza I, Billi AM, Manzoli L, Evangelisti C, Fala F, Cocco L. Intranuclear 3’-phosphoinositide metabolism and Akt signaling: new mechanisms for tumorigenesis and protection against apoptosis? Cell Signal. 2006;18:1101–1107. doi: 10.1016/j.cellsig.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient depravation: possible new biochemical target for cancer therapy. Cancer Res. 2000;60:6201–6207. [PubMed] [Google Scholar]
- 12.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3’-kinase-Akt pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–997. [PubMed] [Google Scholar]
- 13.Workan P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70:2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaguchi S, Izumisawa Y, Sato M, Nakagane T, Koshimizu I, Sakita K, Kato M, Yoshioka K, Sakato M, Kawashima S. In vitro cytotoxicity of imidazolyl-1,3,5-triazine derivatives. Biol Pharm Bull. 1997;20:698–700. doi: 10.1248/bpb.20.698. [DOI] [PubMed] [Google Scholar]
- 15.Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H, Hirono S, Yamazaki K, Yamori T. Anti-tumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:546–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 16.Kong D, Dan S, Yamazaki K, Yamori T. Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR39. Eur J Cancer. 2010;46:1111–1121. doi: 10.1016/j.ejca.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Dong DX, Yamori T. ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor identified using the JFCR39 drug discovery system. Acta Pharmacol Sin. 2010;31:1189–1197. doi: 10.1038/aps.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong D, Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3-kinase isoforms. Cancer Sci. 2007;98:1638–1642. doi: 10.1111/j.1349-7006.2007.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan S, Yoshimi H, Okamura M, Mukai Y, Yamori T. Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis but G0/G1 arrest. Biochem Biophys Res Commun. 2009;379:104–109. doi: 10.1016/j.bbrc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs and enzyme inhibitors. Adv Enz Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bierke LM, Kelland L, Valenti M, Patterson L, Gowan D, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saqhir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3 kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 22.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumad or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 23.Yotsumoto F, Fukami T, agi H, Funakoshi A, Yoshizato T, Kunoki M, Miyamoto S. Amphiregulin regulates the activation of ERK and Akt through epidermal growth factor receptor and HER3 signals involved in the progression of pancreatic cancer. Cancer Sci. 2010;101:2351–2360. doi: 10.1111/j.1349-7006.2010.01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara M, Izuishi K, Sano T, Hossain MA, Kimura S, Masaki T, Suzuki Y. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J Exp Clin Cancer Res. 2008;27:76. doi: 10.1186/1756-9966-27-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taquchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances anti-tumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]