Abstract
Since 1978, we have witnessed a successful evolution of assisted reproductive technology (ART), with improvement of the pregnancy rates and a growing demand. However, in recent years, there has been increasing concern regarding its safety due to the potential health impact on its infants. The raise of the developmental origins of adult disease has positioned low birth weight (LBW) as a significant health issue. Despite multiple studies have associated ART with LBW, the etiology of this association remains largely unknown. This paper reviews the potential association between different components of ART and infertility with LBW, while acknowledging the limitations to interpretation of the existing literature.
Keywords: IVF, perinatal outcomes, low birth weight, pregnancy
INTRODUCTION
Since the late 1970’s, the management of infertility has undergone a radical transformation that has a profound impact on couples trying to become parents. In 1978, Steptoe and Edwards announced the birth of a healthy baby girl named Louise Brown. Baby Louise was quickly labeled the first “test-tube” baby and her birth ushered the modern reproductive interventions of assisted reproductive technology (ART). Since 1978, the combination of improving success rates and increasing demand for treatment has led to a dramatic rise in the number of infants born utilizing ART. Today, well over 5 million babies worldwide have been born by medical interventions and the number of ART babies is steadily growing with approximately 1–4% of the current births in developed countries conceived by in vitro fertilization (IVF) (1).
In recent years, there has been increasing concern regarding the safety of ART, and specifically IVF, due to the potential health impact on these infants. At present, multiple studies have suggested that IVF pregnancies may be at increased risk for preterm birth, low birth weight (LBW), congenital anomalies, perinatal mortality and several other pregnancy-related complications compared to unassisted pregnancies (2–7). The etiology of these poor perinatal outcomes is yet unknown. With the first children conceived through IVF now reaching adulthood and reproductive age, attention has increasingly focused on the health of children beyond the immediate neonatal period. Ultimately, patients and providers are most concerned about the long-term consequences of low birth weight and preterm delivery, which are still unclear. There is some suggestion that LBW may predispose infants to early onset of adult disease such as type 2 diabetes (8), hypertension (9), and cardiovascular disease (10, 11), although the association is not well defined. This is, in part, due to the scarcity of methodologically sound studies evaluating long-term outcomes in children conceived with IVF. Furthermore, if such a risk of adverse long-term behavioral and metabolic outcomes does exist, the precise etiological component of ART and the extent to which it may be modifiable remains uncertain.
While the specific etiologies of these poor perinatal outcomes is yet unknown, the increased risk of LBW has largely been attributed to higher rate of multiple gestation associated with ART. Recent meta-analyses have revealed a higher rate of LBW in IVF singletons and twins, respectively, compared to natural conceptions (12, 13). Although some data suggests that singleton infants are also at increased risk of LBW, even at term (3, 4, 14–19), other investigators have not observed such differences (20–23). For instance, Isaksson et al. reported that the incidence of LBW in IVF singletons was comparable to unassisted conceptions (24). In addition to matching for important confounders such as age at delivery, parity, and number of children at birth and area of residence, careful consideration was given to the selection of control group in this study. These authors state the inclusion of appropriate control groups with proper matching may virtually eliminate the increased rate of adverse outcomes found by other investigators. Furthermore, Dhont et al. found the incidence of LBW was significantly higher in IVF infants compared to spontaneously conceived pregnancies (8.1% vs. 4.7%). When adjusting for duration of gestation, this difference was no longer apparent (25). Research in this domain can be challenging given the need to separate the effects of ART from the multiple confounding factors that may influence the outcomes of interest.
POTENTIAL ETIOLOGIES
Multiple mechanisms could be responsible for the adverse outcomes associated with ART (Fig. 1). These include:
The effect of ovarian stimulation on egg quality or endometrial receptivity;
The effect of culturing the embryos in vitro during a very sensitive stage of development; and
The contributions of infertile parents, who represent a different population compared to fertile parents and therefore could be prone to more diseases.
Figure 1. Possible etiologies of LBW in children conceived after ART.
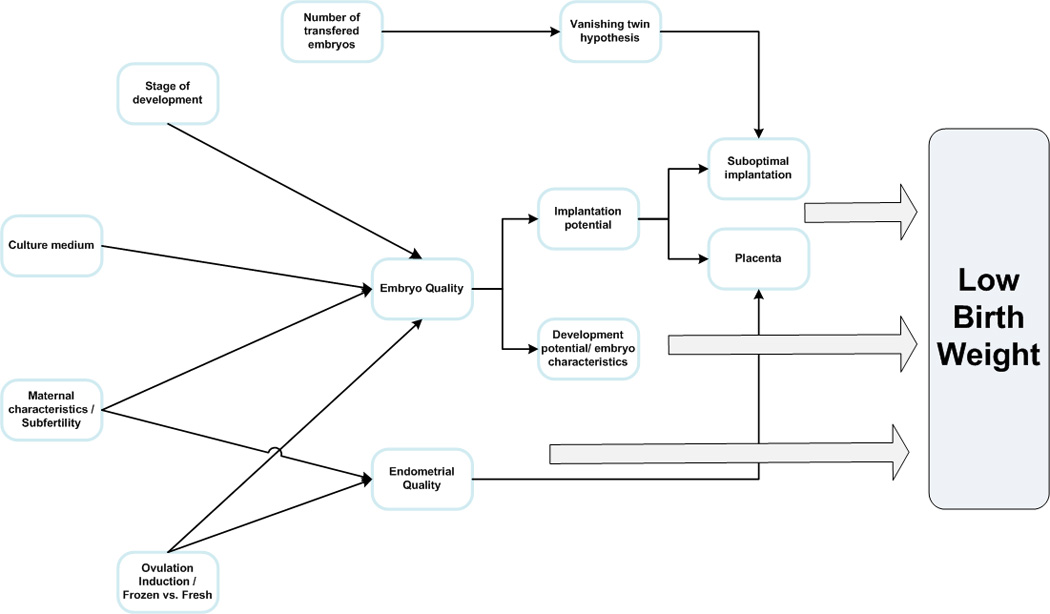
Multiple mechanisms have been proposed as potential etiologies for LBW in ART. Ovarian stimulation, maternal characteristics and subfertility may act through an impairment of the embryo or endometrial quality. The impairment in the endometrial quality may result in placental associated defects. The culture medium and the stage of embryo development at transfer may act via the embryo quality. The number of embryos trasfered may act through the vanishing twin hypothesis causing suboptimal implantation. The impairment of the embryo quality can result in either an insult to its implantation potential or its development potential.
Ovarian Stimulation
Ovarian stimulation has been associated with LBW when compared with spontaneous conceptions in conceptions with (26–28) and without IVF (4, 29). Several factors may account for this observation including an alteration in oocyte quality (30, 31), diminished endometrial receptivity and creation of a poor implantation environment (32, 33).
Clinical and animal studies suggest that supraphysiological estradiol levels have a direct toxic effect on the embryo, impairing its implantation potential (30, 34–36), in part by altering embryonic adhesion properties (30). Elevated estrogen levels can decrease the duration of the window of endometrial receptivity for implantation and impair uterine gene expression of implantation-related genes in mice (32). Delayed ovulation, which can occur in ovarian stimulation, has also been associated to a decrease in embryonic weight and fewer developing embryos per cycle in mice (31). Furthermore, endometrium exposed to controlled ovarian hyperstimulation exhibit derangements in morphologic (37), biochemical (35, 38) and gene expression features. However, these findings have not been replicated in human studies (39, 40).
Pregnancy associated plasma protein-A (PAPP-A), a macromolecular glycoprotein produced by the endometrial-placental interface, has been shown to be lower in the first trimester of pregnancies achieved using ovarian stimulation and its decrease may reflect impairment of early implantation (33, 41). Additionally, PAPP-A levels have been associated to a higher risk of small for gestational age (SGA, <10th percentile of weight) (42, 43) and are directly correlated with insulin-like growth factor (IGF) bioavailability. The IGF system plays an important role in the communication between the embryo and the endometrium, with IGF-2 likely stimulating implantation and invasion (28). The use of human menopausal gonadotropins during ovarian stimulation has been associated with increases IGF binding protein 1- a protein linked to intrauterine growth restriction (44).
Clinical studies revealed women undergoing standard ovarian stimulation had significantly lower mean birth weight infants those exposed to modified natural cycle with minimal stimulation (45). A recent retrospective cohort revealed that in addition, IVF patients with high peak estradiol levels had higher rates of SGA than those who had lower levels (OR 9.40; 95%CI 3.22–27.46) (28) and endometrial thickness may have a protective effect on LBW, supporting the role of endometrium in fetal growth and perinatal outcome after IVF (27). Additional studies are needed to identify the specific molecular mechanisms that underlying LBW.
Embryo – Endometrium Interface
Embryo Culture Media & Epigenetics
In vitro culture conditions have been investigated as a possible contributor to poor perinatal outcome, including abnormal birthweight. Emerging data from experimental studies suggests that blastocyst quality and expression of genes critical for the establishment and maintenance of pregnancy are greatly influenced by the post-fertilization conditions, specifically embryo culture environment (46). Several animal models have demonstrated that maturation media can influence levels of oocyte transcripts and DNA methylation (47, 48). Embryo culture has been associated with impaired methylation of H19/IGF-2 imprinting control region (ICR). Consequently, IGF2 can be silenced (49) and heterozygous mutations in mice result in 60% reduction in body weight compared to wild type (50). In humans, differential methylation and gene expression has been demonstrated in placentas and cord blood collected from infants conceived by IVF compared to natural conceptions. Of note, some of the differently expressed genes were related to adipocyte development, insulin signaling and obesity, suggesting a potential way to affect LBW (51). But mean human H19/IGF2 ICR methylation levels are similar in placentas and cord blood derived from ART and natural conceptions (49, 52) and IGF2 transcript levels were not correlated with birthweight (53).
In human IVF, a number of questions still remain about the contribution of media type on birthweight. Several groups report that the type of embryo culture medium has a significant effect on early embryonic development, subsequent fetal development and birthweight of IVF infants (54–56). However, a recent retrospective cohort study found no significant association between culture medium and birthweight, although these authors did not specifically report the frequency of LBW or SGA (57).
Fresh versus Frozen Embryo Transfer
The first successful live birth after cryopreservation of human embryos was reported in 1984 (58). In initial studies, embryo cryopreservation appeared reassuring and did not seem to adversely influence fetal-perinatal development when compared to fresh embryo transfer (59). Frozen embryo transfer (FET) can provide several benefits such as increasing the cumulative pregnancy rates, decreasing the risk of ovarian hyperstimulation syndrome by avoiding fresh transfer, reducing multiple gestation rates (60, 61), and may be more cost effective than fresh embryo transfer (62). The use of fresh embryos has been linked to a higher incidence of LBW compared to frozen embryos (5, 16, 63–66). In contrast, frozen embryo transfer (FET) seems to be associated with a better perinatal outcome than fresh embryo transfer, partly due to the embryo selection process, in which supernumerary embryos are required for freezing. In fact, a number of studies have suggested a “protective effect’ of frozen embryo transfer (FET) with lower rates of LBW and SGA compared to fresh transfers (64, 67, 68). A population-based sibling study found a higher mean birthweight in siblings born after frozen embryo transfer compared with fresh embryo transfer (17), further supporting the benefit of frozen embryo transfer.
Shih et al. evaluated the factors affecting LBW after ART, comparing data of fresh and frozen embryos (5). They eliminated the “patient effect”, in which good prognosis patients who produce more and higher quality embryos are less likely to have LBW babies, and identified a difference between the mean birthweight of singletons obtained from fresh and frozen embryos from the same patients. The authors speculate that FET outcomes are comparable to unassisted conceptions, and propose that LBW associated with fresh transfer may be related to ART procedures that differ from FET. Specifically, in fresh transfers, patients undergo controlled ovarian stimulation, anesthesia and needle aspiration of the follicles probably affect endometrial receptivity, implantation and early pregnancy (5). However, these findings are refuted by two retrospective cohort studies demonstrating a higher rate of LBW among infants resulting from FET compared to fresh transfers and natural conception (15, 62). Furthermore, Aflatoonian et al. concluded that preterm birth and LBW in singletons and multiple pregnancies were comparable between FET and fresh ET groups (69).
Although different hypotheses have been proposed, one prominent difference is the effect of ovarian stimulation and supraphysiologic estradiol levels on the endometrium that occurs with fresh embryo transfers, but is absent in FET. A large retrospective cohort study over 56,000 IVF singleton births in the US Society of Assisted Reproductive Technology registry investigated the association between the supraphysiologic hormonal environment and adverse perinatal outcomes by comparing infants born after fresh versus frozen and thawed embryo transfer (63). The results demonstrated that the odds of low birth weight, but not preterm delivery, was significantly higher after fresh transfer (aOR 1.35; 95%CI: 1.20–1.51), supporting an underlying role for maternal hormonal milieu in pregnancy outcomes.
Day of Transfer and Number of Embryos Transferred
There is conflicting data on the impact of cleavage versus blastocyst stage embryos on neonatal birthweight with various investigators identifying opposite results. While Kallen et al. found a higher rate of LBW in infants resulting from cleavage stage embryos (70), results from other studies, summarized in a Cochrane review, reported no differences in LBW rate comparing live births from cleavage versus blastocyst stage embryos (OR 1.13, 0.84–1.54) (2, 71, 72).
De Sutter et al. suggested that the number of transferred embryos is an important factor to consider when assessing risk of adverse perinatal outcomes. In a retrospective cohort study of patients undergoing single embryo transfer (SET) versus double embryo transfer (DET), mean birthweight was significantly higher in SET singletons compared with DET singletons. In addition, there was a three-fold increase odds of LBW (OR 3.38, 95% CI 1.86–6.12) in infants resulting from the transfer of two embryos as compared with singletons born after single embryo transfer. This finding is likely due to the relatively high frequency of vanishing twins after the transfer of two embryos (73).
Vanishing Twin Syndrome
Embryonic loss and absorption of one twin is commonly described as “vanishing twin syndrome”. Although the true prevalence is unknown, it is estimated that 10–30% of IVF singleton live births originated from a twin gestation in early pregnancy (74–77). A number of studies suggest poorer outcome in IVF survivors of a vanishing co-twin compared with singletons from single gestations. For example, in a case-control study of survivors of vanishing twin syndrome and matched singletons, both conceived by IVF-ICSI, Shebl et al. reported a significantly higher frequency of LBW in survivors (26.1 vs. 12.0%, p=0.04) (78). Pinborg et al. described a 1.7-fold increased risk of LBW for live-born survivors of a vanished co-twin compared with singletons originating from a single gestation (75). It is hypothesized that the presence of greater than one embryo at the time of implantation may impair the implantation potential of each embryo (73). Retrospective cohort studies have found a higher risk of LBW among singleton and twin survivors of a vanishing twin (75, 79, 80), the risk of which increased with gestational age at the time of vanishing (81). However, other groups have challenged these findings (74, 82, 83). Finally, selective reduction of multiple gestations conceived by IVF may predispose the surviving gestation to LBW (84, 85).
Placenta
Differences in birthweight seen in ART pregnancies may be related to placental-mediated mechanisms of growth restriction. For instance, pathologic studies of ART placentas have shown greater placental thickness and higher prevalence of anomalous insertion of the umbilical cord in ART-singletons than in controls (86) while ART twin placentae were thinner, weighed less and had more infarctions compared to non-ART-conceived twin pregnancies (87). Furthermore, higher frequency of pre-eclampsia, placenta previa and other placental-associated defects (peripartum hemorrhage, placental abruption and placenta accreta) have also been reported in pregnancies after ART when compared with the general population (21, 88, 89).
Underlying Infertility and Parental Characteristics
Some researchers have argued that the increased risk of adverse perinatal outcomes may be due to the underlying infertility of the couples seeking ART, independent of its treatment (18, 90, 91). Several studies have reported that women with untreated subfertility who became pregnant had a greater frequency of adverse outcomes than the general population (92–95), suggesting that a history of infertility may mediate some of the adverse risks attributed to fertility treatments. The frequency of perinatal complications was similar to that in subfertile women who conceived through ART (96). Several studies demonstrate that infants conceived after >12 months of attempting conception have a higher risk of LBW, SGA and preterm birth compared to infants conceived within 12 months (97–99), further supporting the role of underlying maternal factors relating to subfertility with adverse pregnancy outcomes.
A recent meta-analysis indicates a significant association between subfertility and LBW (aOR 1.34; 95%CI 1.21–1.48) and to SGA (aOR 1.07; 95%CI 1.03–1.33) and concludes that infertile couples who conceive without medical treatment are at higher risk of preterm birth and LBW with a modest elevated risk of SGA infant (6). In retrospective study, Romundstad et al. compared singletons from mothers who conceived both spontaneously and by means of ART. They used mothers as their own controls and controlled by the order of the mode of conception. They found no difference in LBW in the sibling-relationship comparison, suggesting that ART has no influence on LBW, but is rather an effect of the underlying infertility (22). Although, the latter was not directly supported by their data, they highlight the fact that the control group used by the traditional studies may not be appropriate.
Although IVF singletons have shown an increased risk of SGA and preterm birth compared to population- based frequencies (100–105), patients undergoing IVF are, on average, older and more often primiparous than the general obstetric population. Consequently, these patients carry additional age- and parity-related risks that are known associations with perinatal complications (25, 106–108). It is plausible that the underlying etiologies that contribute to infertility may be involved with the causal pathway of poor adverse perinatal outcomes. Several authors suggest that infertility due to a cervical disorder or anovulation (109), or prenatal exposure to stress and environmental pollutants may contribute to both infertility and perinatal outcomes (6, 110).
The evidence of an effect of female infertility has been reinforced by a lower rate of LBW in subgroups of male-factor infertility in retrospective cohort studies (14, 16, 109). However, a case-control study found no difference in a composite of LBW and preterm birth (27). Within male factor infertility, no differences have been found between LBW in patients with obstructive versus non obstructive azoospermia (111), or when comparing ICSI outcomes with either testicular, epididymal or ejaculated sperm (112).
LIMITATIONS OF INTERPRETATION
Although the majority of singleton IVF pregnancies are uncomplicated, emerging data suggests higher rates of adverse perinatal outcomes in singletons conceived by IVF compared to unassisted conceptions. To date, it has been difficult to isolate the contribution of ART-procedure related factors from underlying infertility characteristics to the overall risk. Additionally, these findings must be considered within the context of their methodological limitations. Principally, much of the current evidence relies on observational studies, including cohort and case-control designs, based on retrospective data. While some investigators have used large clinical registries with sufficient numbers, many studies consist of data from single center with modest sample sizes. Additionally, prospective studies and randomized controlled trials may not be feasible or appropriate when exploring ART outcomes, given the financial and ethical considerations.
The term “infertility” has been used to describe a heterogeneous group of patients and varies between studies. In addition, maternal characteristics associated with infertility may contribute to adverse perinatal outcomes. Women who utilize assisted reproduction differ from fertile women in a number of important traits that influence pregnancy outcomes such as age, parity, and socioeconomic status. Although some authors have attempted to account for known confounders, few studies acknowledge the influence of other potential cofactors such as parity, smoking status, alcohol consumption, duration of infertility and pre-existing maternal disease. New data suggests that duration and etiology of infertility are important predictors of perinatal outcomes (97, 98, 113–115) and further studies should account for these factors. Inherent differences in ART techniques pose a challenge to the interpretation of data. Maternal body mass index and weight gain during pregnancy, which have been associated with fetal growth, are often overlooked. ART pregnancies may be managed differently exposing patients to iatrogenic causes of preterm delivery and low birth weight. Patients and obstetricians may treat ART pregnancies as ‘‘precious’’, resulting in more intense monitoring and frequent intervention. In fact, a number of studies report higher rates of labor induction and elective caesarean section (20, 116, 117). In addition, we should acknowledge secular trends resulting from technological changes in ART procedures and advances in obstetrical and neonatal care that has occurred over time (118).
The choice of comparison group is critical to evaluation of the true risk associated with ART. While many studies have compared IVF infants to unassisted pregnancies conceived by fertile couples, questions still remain. Recent groups have incorporated infertile couples who conceive using superovulation, not IVF, and subfertile couples who conceive without assistance. This may better delineate the risk associated with ART procedures from underlying infertility. Alternative comparison groups include siblings conceived with and without ART, recipients using donor oocytes and gestational carriers. The lack of proper matching and comparison groups in studies to date have compromised the interpretation of results and promoted ambiguity in this area.
Another limitation is the use of surrogate outcomes and the inconsistencies in definition of these outcomes. For example, many studies only consider LBW, not SGA or IUGR, which may be more informative endpoints. LBW is not adjusted for gestational age, thus the higher rate of prematurity among ART-conceived babies may exaggerate the effect in this group. Further studies should evaluate SGA and IUGR in order to determine more accurately the effect of ART and to help clarify its etiology. Also, publication bias should be acknowledged as several studies use the same cohort of infants, either in part or entirety, resulting in duplication of data with overestimation of the results.
Finally, we acknowledge that LBW and prematurity are often evaluated as feasible endpoints to assess the effect of ART on long term morbidity and mortality. However, intrauterine growth restriction or other biologic factor that impacts fetal development may be the culprit for long term adverse effects of ART, not merely prematurity itself. Further, However, the underlying insult that induces preterm birth or LBW may also directly affect the adverse outcome of interest, suggesting collider effect (119, 120). Whitcomb and colleagues described the “birthweight paradox” as an observation related to birthweight, neonatal mortality and additional factors associated with both birthweight and mortality, such as maternal smoking, parity and race. Contrary to expectation, infants seemingly are the highest risk (e.g. those of low birthweight and with smoking mothers) appear to do better than those at lower risk (e.g. low birthweight and non-smoking mothers). While some theories suggest that maternal smoking may modify the risk of low birthweight, these findings may reflect artificial interpretation resulting from statistical modeling. As such, birthweight can be consider a collider - a variable in a causal system that is a shared effect of more than one factor (121). Overadjustment of intermediate factors may result in estimates that are biased toward the null and affect conclusions (122).
CONCLUSIONS
As ART utilization increases, there is growing concern about its safety, and the potential impact on long term health. However, the study of perinatal outcomes after assisted reproductive technologies remains challenging. Difficulties to overcome in the study design include: inability to randomize to treatment, the immense cost and extended period of time it takes to conduct a long term prospective cohort, the large loss to follow up rate (exacerbated by the years it may take for an outcome to become apparent), the use of surrogate markers for developmental outcomes, and the lack of specificity regarding exposure. Further epidemiologic and basic science research is needed to help determine the specific etiology and extent of the increased risks to childhood and long-term growth and development associated with ART. For example, examination of natural IVF or minimal controlled ovarian stimulation cycles may contribute to better understanding of the effects of embryo quality and endometrium. Animal models can be used to better assess the impact of embryo culture conditions, including the individual components of culture media, on perinatal outcomes. Further investigation into the mechanisms responsible for these effects may identify factors that may be modified to improve outcomes. More translational studies are needed as alterations in gene expression, particularly imprinted genes, and subtle epigenetic alterations may account for the phenotypic differences, such as birthweight, reported in ART infants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR’S ROLES
L.A.K and A.P.P prepared the manuscript. All authors have seen and approved the final version of the manuscript.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Nygren KGSE, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, de Mouzon J. International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report: assisted reproductive technology 2003. Fertility and Sterility. 2011;95:2209–2222. doi: 10.1016/j.fertnstert.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 2.Fernando D, Halliday JL, Breheny S, Healy DL. Outcomes of singleton births after blastocyst versus nonblastocyst transfer in assisted reproductive technology. Fertil Steril. 2012;97:579–584. doi: 10.1016/j.fertnstert.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi M, Nakai A, Satoh S, Matsuda Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil Steril. 2012;98:922–928. doi: 10.1016/j.fertnstert.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 4.D'Angelo DV, Whitehead N, Helms K, Barfield W, Ahluwalia IB. Birth outcomes of intended pregnancies among women who used assisted reproductive technology, ovulation stimulation, or no treatment. Fertil Steril. 2011;96:314–320. e2. doi: 10.1016/j.fertnstert.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 5.Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- 6.Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod. 2012 doi: 10.1093/humrep/des347. [DOI] [PubMed] [Google Scholar]
- 7.Kalra SK. Adverse perinatal outcome and in vitro fertilization singleton pregnancies: what lies beneath? Further evidence to support an underlying role of the modifiable hormonal milieu in in vitro fertilization stimulation. Fertil Steril. 2012;97:1295–1296. doi: 10.1016/j.fertnstert.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulindependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 11.Skilton MR, Viikari JS, Juonala M, Laitinen T, Lehtimaki T, Taittonen L, et al. Fetal growth and preterm birth influence cardiovascular risk factors and arterial health in young adults: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol. 2011;31:2975–2981. doi: 10.1161/ATVBAHA.111.234757. [DOI] [PubMed] [Google Scholar]
- 12.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 13.McDonald SD, Han Z, Mulla S, Ohlsson A, Beyene J, Murphy KE. Preterm birth and low birth weight among in vitro fertilization twins: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2010;148:105–113. doi: 10.1016/j.ejogrb.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 15.Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod. 2011;26:442–450. doi: 10.1093/humrep/deq325. [DOI] [PubMed] [Google Scholar]
- 16.Wang YA, Sullivan EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83:1650–1658. doi: 10.1016/j.fertnstert.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95:959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 19.Camarano L, Alkon A, Nachtigall RD, Schembri M, Weiss S, Croughan MS. Preterm delivery and low birth weight in singleton pregnancies conceived by women with and without a history of infertility. Fertil Steril. :2012. doi: 10.1016/j.fertnstert.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reubinoff BE, Samueloff A, Ben-Haim M, Friedler S, Schenker JG, Lewin A. Is the obstetric outcome of in vitro fertilized singleton gestations different from natural ones? A controlled study. Fertil Steril. 1997;67:1077–1083. doi: 10.1016/s0015-0282(97)81442-2. [DOI] [PubMed] [Google Scholar]
- 21.Fujii M, Matsuoka R, Bergel E, van der Poel S, Okai T. Perinatal risk in singleton pregnancies after in vitro fertilization. Fertil Steril. 2010;94:2113–2117. doi: 10.1016/j.fertnstert.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Gunnell D, et al. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372:737–743. doi: 10.1016/S0140-6736(08)61041-7. [DOI] [PubMed] [Google Scholar]
- 23.Wisborg K, Ingerslev HJ, Henriksen TB. In vitro fertilization and preterm delivery, low birth weight, and admission to the neonatal intensive care unit: a prospective follow-up study. Fertil Steril. 2010;94:2102–2106. doi: 10.1016/j.fertnstert.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Isaksson R, Gissler M, Tiitinen A. Obstetric outcome among women with unexplained infertility after IVF: a matched case-control study. Hum Reprod. 2002;17:1755–1761. doi: 10.1093/humrep/17.7.1755. [DOI] [PubMed] [Google Scholar]
- 25.Dhont M, De Sutter P, Ruyssinck G, Martens G, Bekaert A. Perinatal outcome of pregnancies after assisted reproduction: a case-control study. Am J Obstet Gynecol. 1999;181:688–695. doi: 10.1016/s0002-9378(99)70514-4. [DOI] [PubMed] [Google Scholar]
- 26.Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–596. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 27.Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, et al. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril. 2006;86:1634–1641. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 29.van der Spuy ZM, Steer PJ, McCusker M, Steele SJ, Jacobs HS. Outcome of pregnancy in underweight women after spontaneous and induced ovulation. Br Med J (Clin Res Ed) 1988;296:962–965. doi: 10.1136/bmj.296.6627.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–968. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 31.Bittner AK, Horsthemke B, Winterhager E, Grummer R. Hormone-induced delayed ovulation affects early embryonic development. Fertil Steril. 2011;95:2390–2394. doi: 10.1016/j.fertnstert.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amor DJ, Xu JX, Halliday JL, Francis I, Healy DL, Breheny S, et al. Pregnancies conceived using assisted reproductive technologies (ART) have low levels of pregnancyassociated plasma protein-A (PAPP-A) leading to a high rate of false-positive results in first trimester screening for Down syndrome. Hum Reprod. 2009;24:1330–1338. doi: 10.1093/humrep/dep046. [DOI] [PubMed] [Google Scholar]
- 34.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16:221–225. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 36.Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14 Suppl 2:107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 37.Kolb BA, Paulson RJ. The luteal phase of cycles utilizing controlled ovarian hyperstimulation and the possible impact of this hyperstimulation on embryo implantation. Am J Obstet Gynecol. 1997;176:1262–1267. doi: 10.1016/s0002-9378(97)70344-2. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 38.Develioglu OH, Hsiu JG, Nikas G, Toner JP, Oehninger S, Jones HW., Jr Endometrial estrogen and progesterone receptor and pinopode expression in stimulated cycles of oocyte donors. Fertil Steril. 1999;71:1040–1047. doi: 10.1016/s0015-0282(99)00137-5. [DOI] [PubMed] [Google Scholar]
- 39.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–4510. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 40.Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 41.Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. First-trimester screening markers are altered in pregnancies conceived after IVF/ICSI. Ultrasound Obstet Gynecol. 2009;33:8–17. doi: 10.1002/uog.6254. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Y, Bradshaw R, Stanley AP, Odibo AO. The impact of assisted reproductive technology on the association between first-trimester pregnancy-associated plasma protein a and human chorionic gonadotropin and adverse pregnancy outcomes. Am J Perinatol. 2011;28:347–354. doi: 10.1055/s-0030-1268707. [DOI] [PubMed] [Google Scholar]
- 43.Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. The efficiency of first-trimester serum analytes and maternal characteristics in predicting fetal growth disorders. Am J Obstet Gynecol. 2009;201:412, e1–e6. doi: 10.1016/j.ajog.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MR, Irvine R, Hills F, Bolton VN, Abbas AA, Brooks AA, et al. Superovulation, IGFBP-1 and birth weight. Eur J Obstet Gynecol Reprod Biol. 1995;59:193–195. doi: 10.1016/0028-2243(95)02040-y. [DOI] [PubMed] [Google Scholar]
- 45.Pelinck MJ, Keizer MH, Hoek A, Simons AH, Schelling K, Middelburg K, et al. Perinatal outcome in singletons after modified natural cycle IVF and standard IVF with ovarian stimulation. Eur J Obstet Gynecol Reprod Biol. 2010;148:56–61. doi: 10.1016/j.ejogrb.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Duranthon V, Watson AJ, Lonergan P. Preimplantation embryo programming: transcription, epigenetics, and culture environment. Reproduction. 2008;135:141–150. doi: 10.1530/REP-07-0324. [DOI] [PubMed] [Google Scholar]
- 47.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki J, Jr, Therrien J, Filion F, Lefebvre R, Goff AK, Smith LC. In vitro culture and somatic cell nuclear transfer affect imprinting of SNRPN gene in pre- and post-implantation stages of development in cattle. BMC Dev Biol. 2009;9:9. doi: 10.1186/1471-213X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi X, Ni Y, Zheng H, Chen S, Zhong M, Wu F, et al. Abnormal methylation patterns at the IGF2/H19 imprinting control region in phenotypically normal babies conceived by assisted reproductive technologies. Eur J Obstet Gynecol Reprod Biol. 2011;158:52–55. doi: 10.1016/j.ejogrb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 50.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 51.Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong EC, Hatakeyama C, Robinson WP, Ma S. DNA methylation at H19/IGF2 ICR1 in the placenta of pregnancies conceived by in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2011;95:2524–2526. e1–e3. doi: 10.1016/j.fertnstert.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 53.Turan N, Katari S, Gerson LF, Chalian R, Foster MW, Gaughan JP, et al. Inter- and intraindividual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet. 2010;6:e1001033. doi: 10.1371/journal.pgen.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25:605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 55.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27:1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 56.Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. 2012;27:2619–2626. doi: 10.1093/humrep/des252. [DOI] [PubMed] [Google Scholar]
- 57.Eaton JL, Lieberman ES, Stearns C, Chinchilla M, Racowsky C. Embryo culture media and neonatal birthweight following IVF. Hum Reprod. 2012;27:375–379. doi: 10.1093/humrep/der381. [DOI] [PubMed] [Google Scholar]
- 58.Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984;42:293–296. doi: 10.1016/s0015-0282(16)48029-5. [DOI] [PubMed] [Google Scholar]
- 59.Wennerholm UB, Hamberger L, Nilsson L, Wennergren M, Wikland M, Bergh C. Obstetric and perinatal outcome of children conceived from cryopreserved embryos. Hum Reprod. 1997;12:1819–1825. doi: 10.1093/humrep/12.8.1819. [DOI] [PubMed] [Google Scholar]
- 60.Bergh C, Werner C, Nilsson L, Hamberger L. Cumulative birth rates following cryopreservation of all embryos in stimulated in vitro fertilization (IVF) cycles. J Assist Reprod Genet. 1995;12:191–194. doi: 10.1007/BF02211797. [DOI] [PubMed] [Google Scholar]
- 61.Hyden-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod. 2005;20:2935–2938. doi: 10.1093/humrep/dei133. [DOI] [PubMed] [Google Scholar]
- 62.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010;25:914–923. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 63.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84:611–617. doi: 10.1016/j.fertnstert.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 65.Nakashima A, Araki R, Tani H, Ishihara O, Kuwahara A, Irahara M, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril. 2012 doi: 10.1016/j.fertnstert.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 66.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and metaanalysis. Fertil Steril. 2012 doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 67.Aytoz A, Van den Abbeel E, Bonduelle M, Camus M, Joris H, Van Steirteghem A, et al. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1999;14:2619–2624. doi: 10.1093/humrep/14.10.2619. [DOI] [PubMed] [Google Scholar]
- 68.Wikland M, Hardarson T, Hillensjo T, Westin C, Westlander G, Wood M, et al. Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod. 2010;25:1699–1707. doi: 10.1093/humrep/deq117. [DOI] [PubMed] [Google Scholar]
- 69.Aflatoonian A, Mansoori Moghaddam F, Mashayekhy M, Mohamadian F. Comparison of early pregnancy and neonatal outcomes after frozen and fresh embryo transfer in ART cycles. J Assist Reprod Genet. 2010;27:695–700. doi: 10.1007/s10815-010-9470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010;94:1680–1683. doi: 10.1016/j.fertnstert.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 71.Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007:CD002118. doi: 10.1002/14651858.CD002118.pub3. [DOI] [PubMed] [Google Scholar]
- 72.Schwarzler P, Zech H, Auer M, Pfau K, Gobel G, Vanderzwalmen P, et al. Pregnancy outcome after blastocyst transfer as compared to early cleavage stage embryo transfer. Hum Reprod. 2004;19:2097–2102. doi: 10.1093/humrep/deh398. [DOI] [PubMed] [Google Scholar]
- 73.De Sutter P, Delbaere I, Gerris J, Verstraelen H, Goetgeluk S, Van der Elst J, et al. Birthweight of singletons after assisted reproduction is higher after single- than after doubleembryo transfer. Hum Reprod. 2006;21:2633–2637. doi: 10.1093/humrep/del247. [DOI] [PubMed] [Google Scholar]
- 74.La Sala GB, Villani MT, Nicoli A, Gallinelli A, Nucera G, Blickstein I. Effect of the mode of assisted reproductive technology conception on obstetric outcomes for survivors of the vanishing twin syndrome. Fertil Steril. 2006;86:247–249. doi: 10.1016/j.fertnstert.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 75.Pinborg A, Lidegaard O, la Cour Freiesleben N, Andersen AN. Consequences of vanishing twins in IVF/ICSI pregnancies. Hum Reprod. 2005;20:2821–2829. doi: 10.1093/humrep/dei142. [DOI] [PubMed] [Google Scholar]
- 76.Landy HJ, Keith LG. The vanishing twin: a review. Hum Reprod Update. 1998;4:177–183. doi: 10.1093/humupd/4.2.177. [DOI] [PubMed] [Google Scholar]
- 77.Tummers P, De Sutter P, Dhont M. Risk of spontaneous abortion in singleton and twin pregnancies after IVF/ICSI. Hum Reprod. 2003;18:1720–1723. doi: 10.1093/humrep/deg308. [DOI] [PubMed] [Google Scholar]
- 78.Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Birth weight is lower for survivors of the vanishing twin syndrome: a case-control study. Fertil Steril. 2008;90:310–314. doi: 10.1016/j.fertnstert.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 79.Luke B, Brown MB, Grainger DA, Stern JE, Klein N, Cedars MI. The effect of early fetal losses on singleton assisted-conception pregnancy outcomes. Fertil Steril. 2009;91:2578–2585. doi: 10.1016/j.fertnstert.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 80.Luke B, Brown MB, Grainger DA, Stern JE, Klein N, Cedars MI. The effect of early fetal losses on twin assisted-conception pregnancy outcomes. Fertil Steril. 2009;91:2586–2592. doi: 10.1016/j.fertnstert.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 81.Pinborg A, Lidegaard O, Freiesleben NC, Andersen AN. Vanishing twins: a predictor of small-for-gestational age in IVF singletons. Hum Reprod. 2007;22:2707–2714. doi: 10.1093/humrep/dem225. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez-Gonzalez M, Serra V, Garcia-Velasco JA, Pellicer A, Remohi J. The "vanishing embryo" phenomenon in an oocyte donation programme. Hum Reprod. 2002;17:798–802. doi: 10.1093/humrep/17.3.798. [DOI] [PubMed] [Google Scholar]
- 83.La Sala GB, Nucera G, Gallinelli A, Nicoli A, Villani MT, Blickstein I. Spontaneous embryonic loss following in vitro fertilization: incidence and effect on outcomes. Am J Obstet Gynecol. 2004;191:741–746. doi: 10.1016/j.ajog.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 84.Cheang CU, Huang LS, Lee TH, Liu CH, Shih YT, Lee MS. A comparison of the outcomes between twin and reduced twin pregnancies produced through assisted reproduction. Fertil Steril. 2007;88:47–52. doi: 10.1016/j.fertnstert.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 85.Luke B, Brown MB, Nugent C, Gonzalez-Quintero VH, Witter FR, Newman RB. Risk factors for adverse outcomes in spontaneous versus assisted conception twin pregnancies. Fertil Steril. 2004;81:315–319. doi: 10.1016/j.fertnstert.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Daniel Y, Schreiber L, Geva E, Amit A, Pausner D, Kupferminc MJ, et al. Do placentae of term singleton pregnancies obtained by assisted reproductive technologies differ from those of spontaneously conceived pregnancies? Hum Reprod. 1999;14:1107–1110. doi: 10.1093/humrep/14.4.1107. [DOI] [PubMed] [Google Scholar]
- 87.Daniel Y, Schreiber L, Geva E, Lessing JB, Bar-Am A, Amit A. Morphologic and histopathologic characteristics of placentas from twin pregnancies spontaneously conceived and from reduced and nonreduced assisted reproductive technologies. J Reprod Med. 2001;46:735–742. [PubMed] [Google Scholar]
- 88.Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. 2010;25:265–274. doi: 10.1093/humrep/dep376. [DOI] [PubMed] [Google Scholar]
- 89.Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- 90.Pandian Z, Bhattacharya S, Templeton A. Review of unexplained infertility and obstetric outcome: a 10 year review. Hum Reprod. 2001;16:2593–2597. doi: 10.1093/humrep/16.12.2593. [DOI] [PubMed] [Google Scholar]
- 91.Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McElrath TF, Wise PH. Fertility therapy and the risk of very low birth weight. Obstet Gynecol. 1997;90:600–605. doi: 10.1016/s0029-7844(97)00362-1. [DOI] [PubMed] [Google Scholar]
- 93.Wang JX, Norman RJ, Kristiansson P. The effect of various infertility treatments on the risk of preterm birth. Hum Reprod. 2002;17:945–949. doi: 10.1093/humrep/17.4.945. [DOI] [PubMed] [Google Scholar]
- 94.Zhu JL, Basso O, Obel C, Hvidtjorn D, Olsen J. Infertility, infertility treatment and psychomotor development: the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2009;23:98–106. doi: 10.1111/j.1365-3016.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooper AR, O'Neill KE, Allsworth JE, Jungheim ES, Odibo AO, Gray DL, et al. Smaller fetal size in singletons after infertility therapies: the influence of technology and the underlying infertility. Fertil Steril. 2011;96:1100–1106. doi: 10.1016/j.fertnstert.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raatikainen K, Kuivasaari-Pirinen P, Hippelainen M, Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum Reprod. 2012;27:1162–1169. doi: 10.1093/humrep/des015. [DOI] [PubMed] [Google Scholar]
- 97.Henriksen TB, Baird DD, Olsen J, Hedegaard M, Secher NJ, Wilcox AJ. Time to pregnancy and preterm delivery. Obstet Gynecol. 1997;89:594–599. doi: 10.1016/s0029-7844(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 98.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18:2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 99.Thomson F, Shanbhag S, Templeton A, Bhattacharya S. Obstetric outcome in women with subfertility. BJOG. 2005;112:632–637. doi: 10.1111/j.1471-0528.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 100.Doyle P, Beral V, Maconochie N. Preterm delivery, low birthweight and small-forgestational- age in liveborn singleton babies resulting from in-vitro fertilization. Hum Reprod. 1992;7:425–428. doi: 10.1093/oxfordjournals.humrep.a137663. [DOI] [PubMed] [Google Scholar]
- 101.Olivennes F, Rufat P, Andre B, Pourade A, Quiros MC, Frydman R. The increased risk of complication observed in singleton pregnancies resulting from in-vitro fertilization (IVF) does not seem to be related to the IVF method itself. Hum Reprod. 1993;8:1297–1300. doi: 10.1093/oxfordjournals.humrep.a138245. [DOI] [PubMed] [Google Scholar]
- 102.Rufat P, Olivennes F, de Mouzon J, Dehan M, Frydman R. Task force report on the outcome of pregnancies and children conceived by in vitro fertilization (France: 1987 to 1989) Fertil Steril. 1994;61:324–330. [PubMed] [Google Scholar]
- 103.Seoud MA, Toner JP, Kruithoff C, Muasher SJ. Outcome of twin, triplet, and quadruplet in vitro fertilization pregnancies: the Norfolk experience. Fertil Steril. 1992;57:825–834. [PubMed] [Google Scholar]
- 104.Tan SL, Doyle P, Campbell S, Beral V, Rizk B, Brinsden P, et al. Obstetric outcome of in vitro fertilization pregnancies compared with normally conceived pregnancies. Am J Obstet Gynecol. 1992;167:778–784. doi: 10.1016/s0002-9378(11)91589-0. [DOI] [PubMed] [Google Scholar]
- 105.Wang JX, Clark AM, Kirby CA, Philipson G, Petrucco O, Anderson G, et al. The obstetric outcome of singleton pregnancies following in-vitro fertilization/gamete intra-fallopian transfer. Hum Reprod. 1994;9:141–146. doi: 10.1093/oxfordjournals.humrep.a138304. [DOI] [PubMed] [Google Scholar]
- 106.Kessler I, Lancet M, Borenstein R, Steinmetz A. The problem of the older primipara. Obstet Gynecol. 1980;56:165–169. [PubMed] [Google Scholar]
- 107.Prysak M, Lorenz RP, Kisly A. Pregnancy outcome in nulliparous women 35 years and older. Obstet Gynecol. 1995;85:65–70. doi: 10.1016/0029-7844(94)00330-g. [DOI] [PubMed] [Google Scholar]
- 108.Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54:6–10. doi: 10.1159/000064689. [DOI] [PubMed] [Google Scholar]
- 109.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8:e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baird DD, Wilcox AJ, Kramer MS. Why might infertile couples have problem pregnancies? Lancet. 1999;353:1724–1725. doi: 10.1016/S0140-6736(99)90050-8. [DOI] [PubMed] [Google Scholar]
- 111.Vernaeve V, Bonduelle M, Tournaye H, Camus M, Van Steirteghem A, Devroey P. Pregnancy outcome and neonatal data of children born after ICSI using testicular sperm in obstructive and non-obstructive azoospermia. Hum Reprod. 2003;18:2093–2097. doi: 10.1093/humrep/deg403. [DOI] [PubMed] [Google Scholar]
- 112.Belva F, De Schrijver F, Tournaye H, Liebaers I, Devroey P, Haentjens P, et al. Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26:1752–1758. doi: 10.1093/humrep/der121. [DOI] [PubMed] [Google Scholar]
- 113.Draper ES, Kurinczuk JJ, Abrams KR, Clarke M. Assessment of separate contributions to perinatal mortality of infertility history and treatment: a case-control analysis. Lancet. 1999;353:1746–1749. doi: 10.1016/S0140-6736(98)08500-6. [DOI] [PubMed] [Google Scholar]
- 114.Lambert RD. Safety issues in assisted reproductive technology: aetiology of health problems in singleton ART babies. Hum Reprod. 2003;18:1987–1991. doi: 10.1093/humrep/deg361. [DOI] [PubMed] [Google Scholar]
- 115.Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–359. doi: 10.1016/S0140-6736(07)60456-5. [DOI] [PubMed] [Google Scholar]
- 116.Verlaenen H, Cammu H, Derde MP, Amy JJ. Singleton pregnancy after in vitro fertilization: expectations and outcome. Obstet Gynecol. 1995;86:906–910. doi: 10.1016/0029-7844(95)00322-I. [DOI] [PubMed] [Google Scholar]
- 117.Koivurova S, Hartikainen AL, Gissler M, Hemminki E, Sovio U, Jarvelin MR. Neonatal outcome and congenital malformations in children born after in-vitro fertilization. Hum Reprod. 2002;17:1391–1398. doi: 10.1093/humrep/17.5.1391. [DOI] [PubMed] [Google Scholar]
- 118.Wilson CL, Fisher JR, Hammarberg K, Amor DJ, Halliday JL. Looking downstream: a review of the literature on physical and psychosocial health outcomes in adolescents and young adults who were conceived by ART. Hum Reprod. 2011;26:1209–1219. doi: 10.1093/humrep/der041. [DOI] [PubMed] [Google Scholar]
- 119.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–1068. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.VanderWeele TJ, Lantos JD, Lauderdale DS. Rising preterm birth rates, 1989-2004: changing demographics or changing obstetric practice? Soc Sci Med. 2012;74:196–201. doi: 10.1016/j.socscimed.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of colliderstratification bias and the birthweight paradox. Paediatr Perinat Epidemiol. 2009;23(5):394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Delbaere I, Vansteelandt S, De Bacquer D, Verstraelen H, Gerris J, De Sutter P, Temmerman M. Should we adjust for gestational age when analysing birth weights? The use of z-scores revisited. Hum Reprod. 2007;22:2080–2083. doi: 10.1093/humrep/dem151. [DOI] [PubMed] [Google Scholar]


