Abstract
The RAS signaling pathway is constitutively activated in psoriatic keratinocytes. We expressed activated H-RASV12G in suprabasal keratinocytes of adult mice and observed rapid development of a psoriasis-like skin phenotype characterized by basal keratinocyte hyperproliferation, acanthosis, hyperkeratosis, intraepidermal neutrophil microabscesses and increased Th1/Th17 and Tc1/Tc17 skin infiltration. The majority of skin infiltrating CD8+ T cells co-expressed IFN-γ and IL-17A. When RAS was expressed on a Rag1−/− background, microabscess formation, iNOS expression and keratinocyte hyperproliferation were suppressed. Depletion of CD8+ but not CD4+ T cells reduced cutaneous and systemic inflammation, the RAS-induced increase in cutaneous Th17 and IL-17+ γΔ T cells, and epidermal hyperproliferation to levels similar to a Rag1−/− background. Reconstitution of Rag1−/− inducible RAS mice with purified CD8+ T cells restored microabscess formation and epidermal hyperproliferation. Neutralization of IFN-γ but not IL-17A in CD8+ T cell reconstituted Rag1−/− mice expressing RAS blocked CD8-mediated skin inflammation, iNOS expression and keratinocyte hyperproliferation. These results show for that CD8+ T cells can orchestrate skin inflammation with psoriasis-like pathology in response to constitutive RAS activation in keratinocytes, and this is primarily mediated through IFN-γ.
Keywords: CD8+ T cells, RAS, psoriasis model, inflammation
INTRODUCTION
Psoriasis is a chronic inflammatory disease of the skin characterized by keratinocyte hyperproliferation with parakeratosis, increased angiogenesis and dermal and epidermal infiltration of inflammatory cells, including CD4+ and CD8+ T-cells, neutrophils, macrophages and dendritic cells (Monteleone et al., 2011). RAS is a small GTPase regulated by growth factor receptors such as EGFR, and signals through multiple pathways to activate gene expression and cell proliferation (Wennerberg et al., 2005). Abnormalities in the RAS-RAF-MAPK pathway in keratinocytes have been linked to the pathophysiology of psoriasis. RAS activity, but not expression, is elevated in psoriatic lesions independent of an inflammatory microenvironment (Lin et al., 1999), possibly due to reduced levels of the neurofibromatosis type 1 (NF1) protein (Karvonen et al., 2004). RAS effectors, including the MAPKs, p38, ERK1/2 and MSK1, are also elevated in psoriatic lesions, associated with activation of transcription factors regulating proinflammatory cytokine gene expression (Funding et al., 2006; Yu et al., 2007). Transgenic mice overexpressing components of the RAS-RAF-MAPK pathway in basal or suprabasal keratinocytes phenocopy certain aspects of the hyperproliferative and inflammatory phenotype of psoriasis (Hobbs et al., 2004; Tarutani et al., 2010) including neutrophil and T cell infiltration. Similarly basal or suprabasal expression of amphiregulin, an EGFR ligand likely to activate the RAS pathway generates a psoriasiform skin phenotype (Cook et al 1997; Cook et al 2004).
Many studies have documented the importance of both Th1 and Th17 polarized CD4+ T cells and more recently CD8+ T cells in the pathogenesis of psoriasis (Monteleone et al., 2011). There are increased numbers of circulating and skin-infiltrating IFN-γ-producing T cells and activation of many IFN-γ-induced immune response genes in psoriatic plaques (Austin et al., 1999) as well as increased numbers of Th17 cells that enhance and maintain the pro-inflammatory phenotype in T cells and keratinocytes and contribute to keratinocyte hyperproliferation (Kolls and Linden, 2004; Caruso et al., 2009; Zheng et al., 2007; Di Cesare et al., 2009; Monteleone et al., 2011). Psoriatic lesions also contain increased numbers of both IFN-γ and IL-17 producing CD8+ T cells (Ortega et al., 2009; Kryczek et al., 2008; Res et al., 2010). Recent studies suggest there is more plasticity between Tc1 and Tc17 polarized states than for CD4+ T cells, as Tc17 cells from psoriatic lesions co-expressed both IFN-γ and IL-17 as well as other Th1 and Th17 lineage cytokines and possessed cytotoxic activity (Ortega et al., 2009). However, in vitro Tc17 polarization is linked to a loss of cytotoxic functionality (Yen et al., 2009; Huber et al., 2009). The importance of these CD8+ T cells in the disease process has not been determined.
While mouse models of RAS pathway activation recapitulate many of the key histopathological features of psoriasis, less is known about effects on polarization of skin infiltrating CD4+ and CD8+ T cells. To assess this we have expressed an activated human Ha-RASV12G gene in the suprabasal layer of the epidermis of adult mice using an Involucrin (Inv) promoter-driven tetracycline transactivator (tTA) (Jaubert et al., 2004). Our results show that suprabasal expression of RAS causes a CD8+ T cell dependent psoriasis-like skin phenotype requiring IFN-γ.
RESULTS
Suprabasal RAS expression causes basal keratinocyte hyperproliferation and cutaneous inflammation associated with intraepidermal neutrophil microabscesses
Seven days after doxycycline (dox) removal, InvtTA x tetORASV12G double transgenic (DT) mice had overt phenotypic changes including scruffy hair coat, scaling, and inflamed ears and tails with no change in single transgenic (ST) littermates (Figure 1a) in parallel with transgene induction (Figure S1a). The skin of DT mice was hyperkeratotic, acanthotic and parakeratotic (Figure 1c) correlating with increased proliferation in the basal layer (Figure 1d). Extensive enlargement of dermal vasculature was also evident on H&E stained sections (see Figure 4a). In DT skin there was increased dermal CD45+ infiltrates (Figure 1e), coupled with increased expression of proinflammatory cytokines and chemokines such as TNF-α, IL-6, IL-23, IL-1β, IL-1α, CXCL1/2, S100A8/9, G-CSF, and GM-CSF (Figure S1b), as well as DefB2, Camp and S100A7, genes associated with human psoriasis (Figure S1c). DT skin had multiple intra-epidermal microabscesses filled with myeloperoxidase+ (MPO) neutrophils (Figure 1c, f), and the majority of resident leukocytes were Ly6G+/CD11b+ neutrophils (Figure 1k) but there was also an increase in mast cells (Figures 1g, h) and F4/80+ cells (Figures 1i, j). When DT mice were placed back on dox, reversal of the inflamed skin phenotype, hair regrowth and resolution of systemic neutrophilia was observed within 3 weeks (Figure S2). Neutrophils isolated from the spleens of DT mice had increased reactive oxygen species (ROS) relative to control mice (Figure 1l), and were cytotoxic in vitro to the papilloma tumor cell line, SP-1 (Figure 1m) and primary mouse keratinocytes (not shown). Antibody depletion of Gr-1+ cells prior to and during RAS-expression prevented microabscess formation (Figure S3a), indicating neutrophils were the main driver of epidermal cytotoxicity, but there was little effect on epidermal proliferation (Figure S3b and c).
Figure 1. Suprabasal expression of H-RASV12G in adult epidermis causes psoriasis-like phenotype with intraepidermal microabscesses.

(a) Photographs of representative shaved DT mice on dox (right) or off dox (left) for 7 days. Representative skin sections from ST mice (b,g,i) or DT mice off dox for 7 days (c–f,h,j). (b) H&E stained ST skin. (c) H&E stained DT section (arrow indicates intraepidermal microabscesses). IHC detection of (d) proliferation (anti-BrdU), (e) leukocytes (anti-CD45), (f) neutrophils (anti-myeloperoxidase), (g,h) mast cells (toluidine blue) and (i,j) macrophages (anti-F4/80) (20x). Arrows indicate representative stained cell. (k) FACS profile (n=5 mice) of Ly6G+/CD11b+ cells from ST or DT skin gated on viable CD45+ cells. (l) Flow histogram of H2DCFDA fluorescence in neutrophils from ST and DT mice. (m) In vitro cytotoxicity assay of sorted splenic Ly6G+/CD11b+ cells using SP1 papilloma cells as target. Data is from 3 independent experiments performed in triplicate, error bars = +/− SEM * significantly different from ST Ly6G+ cells. ST:single transgenic, DT: double transgenic – dox. Scale bars = 20 μm (b–f), 100μm (g–j)
Figure 4. CD8+ T cells are necessary and sufficient to cause neutrophil inflammation, microabscess formation and enhance keratinocyte proliferation.

(a–c) Histology from DT mice injected with (a) control IgG, (b) α-CD4 or (c) α-CD8 depleting antibodies before RAS induction. (d) FACS quantitation of skin Ly6G+ cells gated on the viable CD45+/CD11b+ population, n = 7, repeated twice. (e) BrdU+ basal keratinocytes from DT mice treated with indicated antibodies. (f, g) Histology of DTRag1−/− mice reconstituted with (f) saline or (g) CD8+ T cells. (h) FACS quantitation of skin Ly6G+ neutrophils gated on viable CD45+/CD11b+ cells from DTRag1 −/− reconstituted with saline (6 mice) or CD8+ T cells (7 mice), 3 experiments. (i) BrdU+ basal keratinocytes in saline or CD8+ T cell repleted DTRag1−/− mice. All mice were analyzed 7 days post-dox removal. Error bars = +/− SEM, * significantly different from control. ST:single transgenic, DT: double transgenic – dox. Scale bars = 50 μm.
Suprabasal RAS expression increases Th17, γδ-17 and Tc1/Tc17 skin residency
Suprabasal RAS expression also caused a significant increase in absolute numbers of skin infiltrating CD4+ and CD8+ T cells determined by semi-quantitative FACS, with all of the CD8+ T cells in the epidermis and the majority but not all of CD4+ T cells residing in the dermis (Figure 2a). Approximately 68% of skin-infiltrating CD3+ T cells were CD4+ and 5% were CD8+ T cells, as measured by FACS (not shown). Additionally, there was an increase in activated and IFN-γ expressing CD4+ and CD8+ T cells and Tregs in skin draining lymph node (SDLN) (Figure S4a-c). IL-17A+ T cells were undetectable in SDLN (data not shown), and Th2 cells were undetectable in SDLN and skin of RAS expressing mice (Figure S4d). In normal skin 14% of CD4+ T cells and 25% of γΔ T cells expressed IFN-γ and 0.9% and 2.7% percent were IL-17A+ respectively (Figure 2b and c). Following induction of RAS the frequency of Th1 and IFN-γ+ γΔ T cells did not change, but there was a ~5 fold increase in the frequency of Th17 cells, and a 2-fold increase in the percent of IL-17A expressing γΔ T cells (Figure 2b and c). There was also a substantial increase in FoxP3+ cells in DT skin (Figure 2d). In contrast, 86% of skin CD8+ T cells in DT mice were IFN-γ+ (Figure 2e). Nearly 75% of the skin infiltrating CD8+ T cells co-expressed both IL-17A and IFN-γ, and ~50% of these double positive CD8 cells also expressed the cytolytic effectors granzyme B and perforin (Figure 2f). A small percentage (~ 1%) of CD8+ T cells were detected in ST skin but IFN-γ and IL-17A cytokine expression was undetectable (not shown).
Figure 2. RAS triggers skin infiltration of CD4+ and CD8+ T cells expressing IFN-γ and IL-17A.
(a) Top: CD4+ and TCRβ+/CD8+ T cell counts in skin of ST and DT mice. N= 5 ST and 6 DT mice from two experiments. Bottom: IHC localization of CD4+ T cell infiltration (left) in the dermis and CD8+ T cell infiltration (right) in the epidermis of DT mice. (b, c, e) Intracellular FACS analysis for IL-17A and IFN-γ obtained from stimulated skin single cell suspensions gated on viable/CD45+ cells and CD4+ (b), γΔ TCR+ (c), and CD8+ (e) cells. (d) FoxP3 IHC in ST and DT skin. (f) Perforin/granzyme B FACS on IFN-γ+/IL-17+/CD8+ T cells. CD8+ T cells from ST mice were too scarce for analysis. Means +/-SEM from at least 6 mice per group. * p < 0.05 relative to control ST mice. ST:single transgenic, DT: double transgenic – dox. Arrows point to representative positively stained cells. Scale bars = 50 μm
CD8+ T Lymphocytes drive epidermal microabscesses formation, Th17 infiltration and maximal keratinocyte proliferation
To test the importance of lymphocytes in the inflammatory response, the inducible transgenes were placed on a Rag1−/− background (DTRag1−/−). In absence of lymphocytes RAS-induced MPO+ epidermal microabscesses (Figure 3a-d) and neutrophil and mast cell skin infiltration was suppressed (Figure 3d,e,g) but macrophage infiltration was unaffected (Figure 3f). Inducible nitric oxide (iNOS), elevated in keratinocytes, neutrophils and CD11c+ cells of psoriatic patients (Bruch-Gerharz et al., 1996)(Lowes et al., 2005) was also increased in DT skin and suppressed in DTRag1−/− skin (Figure 3h). Suprabasal RAS-induced proliferation decreased from 42% to 30% BrdU+ basal keratinocytes/FOV (Figure 3i) with a corresponding reduction in epidermal thickness in DTRag1−/− skin (Figure 3j).
Figure 3. Lymphocyte ablation blocks RAS-induced neutrophil infiltration and suppresses epidermal proliferation.
(a, b) Skin histology from (a) DTRag1+/+ and (b) DTRag1−/− mice. anti-MPO IHC in DTRag1+/+ (c) and DTRag1−/− mice (d), (40X). (e) toluidine blue stain and (f) anti-F4/80 IHC in DTRag1−/− mice (20x). (g) top: FACS of neutrophils from skin of DTRag1+/+ or −/− mice gated on live CD45+/CD11b+ cells; n =10; bottom: mast cells in ST or DTRag1+/+ and −/− mice off dox 7 days, n=5 mice per group. (h) Relative levels of iNOS mRNA expression from skin of DTRag1+/+ and −/− mice. (i) Quantitation of BrdU+ basal keratinocytes in DTRag1 +/+ and −/− mice from 5 mice per group. (j) Epidermal hyperplasia in skin of ST and DTRag1+/+ and −/− mice. Means +/− SEM from 3 ST and 8 DT mice of each genotype. Error bars = +/− SEM, * significantly different from control or indicated group. ST:single transgenic, DT: double transgenic – dox. Scale bars = 50 μm (a,b,e,f) 100 μm (c,d)
Initial studies showed that adoptive transfer of CD3+ T cells into DTRag1−/− mice restored neutrophil microabscesses (data not shown). To determine the importance of CD4+ or CD8+ T cells in this pathology we depleted these lymphocyte subsets from DT mice with antibodies. Depletion efficiency was ≥ 99% as measured by FACS from blood, spleen and lymph nodes (not shown). Depletion of CD4+ T cells had no effect on RAS-induced epidermal microabscesses (Figure 4b) or the severity of neutrophilia (Figure S5) and slightly enhanced the percentage of CD11b+/Ly6G+ skin infiltrates (Figure 4d). However depletion of CD8+ T cells suppressed microabscesses (Figure 4c), reduced circulating neutrophil numbers (Figure S5) and caused a 2-fold reduction in cutaneous CD11b+/Ly6G+ cells (Figure 4d). CD8+ but not CD4+ T cell depletion suppressed RAS-activated keratinocyte proliferation from 42% to 28% BrdU+ basal cells similar to that found in the DTRag1 −/− mice (Figure 4e).
CD8+ T cells are sufficient to cause epidermal microabscess formation and enhance keratinocyte proliferation
To determine if CD8 + T cells could restore RAS-induced cutaneous inflammation and enhance keratinocyte proliferation, we reconstituted DTRag1−/− mice with purified lymph node and splenic CD8+ T cells, and induced RAS expression for 7 days. Similar to CD3-repleted mice (not shown), transferred CD8+ T cells restored microabscesses (Figure 4g), neutrophil skin infiltration (Figure 4h) and keratinocyte proliferation (Figure 4i) in the absence of other lymphocytes. Reconstituting naïve ST littermates with CD8+ T cells had no effect on inflammation and epidermal proliferation. FACS analysis of lymph nodes demonstrated successful reconstitution in all CD8-repleted mice (data not shown).
CD8+ T cells aid in CD4+ T cell activation and skin residency of Th17 and γδ-17 cells
Since CD8+, but not CD4+ T lymphocytes were critical for the neutrophil driven pathology we examined their importance in mediating other RAS-induced inflammatory effects. In DT mice, depletion of CD8+ T cells significantly reduced the increase of effector CD4+ T cells (CD44hi/CD62lo) in inguinal LN (Figure 5a), and dampened CD4+ T cell infiltration into the skin (Figure 5b). CD8 depletion also blocked the increase in skin Th17 and IL-17+ γΔ T cells (Figure 5c). In contrast, CD4 depletion increased CD8+ T cell effector differentiation in LN (Figure 5d). These results suggest that CD8+ T cells are instrumental in orchestrating global inflammatory changes associated with suprabasal activation of RAS.
Figure 5. CD8+ T cells are necessary for LN CD4 activation and increase in skin IL-17+ T cells.
(a) Percent CD44hi/CD62Llo of CD4+ T cells in SDLN and (b) CD4+ T cells in skin from ST or DT mice injected with IgG isotype control or α-CD8. Means and SEM were calculated from 5 mice in each group. (c) IL-17A expression in ex vivo stimulated skin CD4+ or γΔ T cells isolated from ST or DT mice injected with IgG or α-CD8 depleting antibody. (d) Percent CD44hi/CD62Llo of CD8+ cells in SDLN of IgG or α-CD4 treated ST and DT mice. Error bars = +/− SEM, * significantly different from indicated group. All mice analyzed 7 days post-dox removal. ST:single transgenic, DT: double transgenic - dox
IFN-γ and not IL-17 mediates CD8+ T cell driven inflammation
Since IFN-γ and IL-17A expressing CD8+ T cells were present in inflamed skin of DT mice, we tested if either cytokine was responsible for the CD8-driven inflammatory responses. CD8+ T cell transferred DTRag1−/− mice were injected with neutralizing antibodies to IL-17A or IFN-γ and parameters of skin inflammation were measured after RAS induction. Surprisingly, neutralization of IL-17A had no effect on neutrophil infiltration (Figure 6a). In contrast, anti-IFN-γ reduced Ly6G+ skin infiltration (Figure 6b), epidermal hyperplasia (Figure 6c) and keratinocyte proliferation (Figure 6d). Finally, iNOS, a direct target of IFN-γ, was reduced in CD8-repleted, IFN-γ neutralized skin relative to isotype control groups (Figure 6e) suggesting that CD8+ T cells alone could cause iNOS upregulation through the paracrine activities of IFN-γ from CD8+ T cells or other cellular sources responding to transferred CD8+ T cells.
Figure 6. IFN-γ neutralization suppresses CD8-driven skin inflammation and epidermal proliferation.
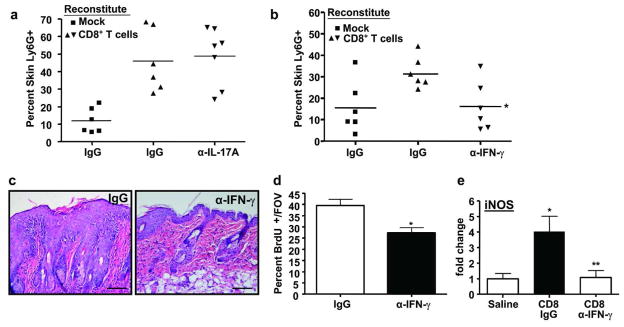
Ly6G+/CD11b+ percentages from skin of DTRag1−/− mice reconstituted with saline or CD8+ T cells followed by injections with α-IL-17A (a), α-IFN-γ (b) or relevant control IgG prior to and during dox removal. Each symbol represents an individual mouse. * significantly different from CD8 reconstituted + IgG. (c) Histology of CD8+ T cell-repleted DTRag1−/− mice administered IgG or α-IFN-γ. (d) Quantitation of BrdU+ basal keratinocytes in CD8+ T cell reconstituted DTRag1−/− mice given α-IFN-γ. (e) qRT-PCR analysis of iNOS expression from skin RNA of CD8+ T cell or saline reconstituted DTRag1 −/− mice administered IgG or IFN-γ neutralizing antibody. Error bars = +/− SEM, * significantly different from saline, ** significantly different from CD8 reconstituted + IgG, p<.05. Scale bars = 50 μm.
DISCUSSION
Here we have documented a mouse model of suprabasal RAS expression that recapitulates many features of a psoriasis-like skin phenotype including basal keratinocyte hyperproliferation, acanthosis, hyperkeratosis, T cell driven inflammation dominated by neutrophil infiltration and formation of intraepidermal neutrophil microabscesses. Similar pathologies (Munro’s microabscesses) have been observed in severe pustular psoriasis as well as psoriatic-like mouse models including PKC-α overexpression targeted to basal layer epithelia (Cataisson et al., 2003; Cataisson et al., 2006) and topical imiquimod application (van der Fits et al., 2009). Our results suggest that this cytotoxic damage is caused by hyperactivated neutrophils dependent on CD8+ T cells.
With minor differences such as the extent of microabscess formation, the skin phenotype is similar to other mouse models that express individual components of the RAS-RAF-MAPK pathway or EGFR ligands that likely activate this pathway (Tarutani et al., 2010; Cook et al., 2004; Hobbs et al., 2004). Suprabasal RAS causes a CD4+ predominant T cell skin infiltrate with a significant increase in intraepidermal CD8+ T cells similar to suprabasal MEK expression (Hobbs et al., 2004) and psoriatic skin. Consistent with the biology of psoriasis (Monteleone et al., 2011; Cai et al., 2011; Di Cesare et al., 2009) we observed a significant increase in the percentage of Th17 and IL-17+ γΔ T cells in RAS-expressing skin. Since we did not detect IL-17+ T cells in SDLN it is likely that this represents polarization of these cells within the skin microenvironment due to elevated IL-6, IL-23 (Figure S1b) and TGFβ1 (Glick et al., 1991). Despite their abundance in lesional skin, depletion of CD4+ T cells had no effect on keratinocyte proliferation, neutrophil skin infiltration or microabscess formation, but instead slightly increased percentage of skin Ly6G+ cells and activated CD8+ T cells in the SDLN. This suggests that a significant fraction of CD4+ T cells are primarily functioning as Tregs, and this is supported by increased Treg numbers in the skin and SDLN following RAS expression (Figure 2d and S1d). Similar results were obtained in a K14-VEGF model of psoriasis-like inflammation, where CD4 depletion enhanced ear thickness and increased proinflammatory cytokine expression (Teige et al., 2009). Additionally, CD4 depletion in another psoriasis-like model (Involucrin-α2β1 integrin transgenic) had little effect on disease phenotype, but the role of CD8+ T cells was not examined (Teige et al., 2010).
Although CD8+ T cells infiltrating the skin in response to RAS represent a minor component of total T cells, our data suggest they are critical for initiating the psoriasis-like phenotype. While epidermal microabscesses were caused by neutrophils, the neutrophil inflammatory phenotype and maximal keratinocyte hyperproliferation was dependent on CD8+ T cells. Thus the psoriasiform pathology was ameliorated on a Rag1−/− background and by depletion of CD8+ T cells while naïve CD8+ T cell reconstitution into DTRag1−/− mice restored these phenotypes. Conversely, infiltration of F4/80+ cells was independent of lymphocytes and may be contributing to the observed residual epidermal hyperplasia in DTRag1−/− mice. Strikingly, depletion of CD8+ T cells reduced the percentage of activated CD4+ T cells in SDLN and Th17 and IL-17+ γΔ T cells in the skin. It is possible that CD8+ T cells are required for initiating or propagating an inflammatory signal that indirectly activates SDLN CD4+ T cells and an inflammatory microenvironment that causes Th17 polarization of skin infiltrating CD4 T cells, but more studies are needed to determine the underlying mechanism.
The overwhelming majority (~75%) of skin infiltrating CD8+ T cells co-expressed both IFN-γ+ and IL-17+ and the majority of these also expressed the cytotoxic effectors granzyme B and perforin. These results are similar to those of Ortega and colleagues who found cytotoxic activity in ex vivo isolated IFN-γ+/IL-17+ CD8+ T cells from psoriatic plaques (Ortega et al., 2009), and suggest greater plasticity in CD8 polarization. In contrast, in vitro conditioned Tc17 differentiation is associated with reduced cytotoxicity and downregulation of perforin, granzyme B and the transcription factor Eomes (Hinrichs et al., 2009; Yen et al., 2009). Thus, further study of the cytotoxic and molecular profile of these RAS-activated CD8+ T cells is needed to understand their unique properties and relationship to psoriatic CD8+ T cells.
Both IFN-γ and IL-17 play significant roles in psoriasis (Di Cesare et al., 2009; Monteleone et al., 2011), but the importance CD8+ T cell as a source for these cytokines is not clear. Within the context of this suprabasal RAS model, IFN-γ plays a primary role during disease onset, as neutralization of IFN-γ but not IL-17A in CD8-reconstituted, RAS expressing Rag1−/− mice suppressed neutrophil skin infiltration and keratinocyte proliferation. While it remains to be directly demonstrated that IFN-γ from CD8+ T cells is responsible, the observations that CD8+ T cells infiltrate the epidermis in response to RAS coupled with multiple studies showing that IFN-γ can induce epidermal hyperproliferation (Barker et al., 1993; Sarra et al., 2011) suggest this may be a direct effect. Since IFN-γ can program APCs to induce IL-17+ T cells (Kryczek et al., 2008) it is plausible that CD8-derived IFN-γ can provide the same signal. Given the strong evidence linking the IL-17/23 axis to the pathogenesis of psoriasis (Di Cesare et al., 2009), it is not clear if these results represent a peculiarity to this model, or reflect an initial requirement for CD8+ T cells for the response that is sustained and amplified by IL-17 expressing CD4 or γΔ T cells. It is also possible that IL-17F rather than IL17A is critical for the inflammatory changes we observe here (Iwakura et al., 2011). Adoptive transfer studies using Ifnγ −/− and Il-17−/− CD4+ and CD8+ T cells are needed to elucidate the relative contributions of each of these cytokines.
It is surprising that the more abundant IFN-γ + CD4+ and γδ T cells in the RAS-expressing skin do not fulfill the same function as IFN-γ+/CD8+ T cells. Likely, an additional CD8-specific cytokine such as TNF-α is required along with IFN-γ to drive the cytotoxic neutrophil phenotype, as IFN-γ/IL-17 co-expressing CD8+ T cells also upregulate TNF-α in psoriasis and murine skin tumors (Ortega et al., 2009; Roberts et al., 2007). While we have observed increased TNF-α and IL-1α in RAS expressing skin the importance of these clinically relevant cytokine pathways remains to be determined. However, epidermal localization of CD8+ T cells may also be critical for initiating inflammation that is further amplified and maintained by Th1 and Th17 cells in the dermis. In support of this, epidermal localization of VLA-1 (α1β1)+ IFN-γ+ CD3+ cells correlated with disease progression of psoriatic lesions, and inhibition of epidermal T cell infiltration reduced the psoriasiform phenotype (Conrad et al., 2007). Moreover, a CXCL16/CXCR6 signaling axis appears to be critical for epidermal infiltration of CD8+ but not CD4+ T cells in psoriasis (Gunther et al., 2012). Whether the intra-epidermal localization of CD8+ T cells is necessary in the suprabasal RAS model and driven by a similar CXCL16/CXCR6 chemotactic pathway remains to be tested.
Taken together, our model of suprabasal RAS expression parallels the CD8+ T cell phenotype from psoriatic patients, and highlights the ability of these cells to initiate skin inflammation, keratinocyte proliferation and regulate the abundance of skin Th17 cells. This model could represent a unique tool to understand the role of CD8+ T cells in the pathogenesis of skin inflammatory diseases such as psoriasis.
MATERIALS and METHODS
Animal Studies
InvtTA mice (Jaubert et al., 2004) were crossed with a homozygous tetOHRASV12G line (Chin et al., 1999) to give tetORAS (ST) and double transgenic (DT) InvtTA/tetOHRASV12G offspring, and genotypes were determined by PCR. Transgene induction was suppressed by doxycycline (10 μg/ml) in drinking water of breeding pairs and ST and DT weaned mice, and 6–8 week old mice were placed on water without dox to induce RAS expression. No difference in histology or immune parameters was seen between ST–dox or DT+dox mice. Leukocytes were depleted with 500 μg of RB6-8C5 (α-Gr-1), GK1.5 (α-CD4), YTS169.4 (α-CD8β), and HB94 (IgG) monoclonal antibodies administered intraperitoneally every other day (α-Gr-1) or once (α-CD4, α-CD8β) coincident with dox removal, and > 95% depletion was validated by flow cytometry. InvtTA and tetORAS transgenes were bred onto a Rag1−/− background to assess lymphocyte function. Rag1−/− mice were reconstituted by retro-orbital transfer of 5 million negatively selected FACS and/or MACS® (Miltenyi Biotec) purified T cells isolated from inguinal lymph nodes and spleen of non-transgenic FVB/n mice (1:4 ratio) 2 days prior to transgene induction. Purities of > 95% were routinely achieved for adoptive transfer and repletion was validated by flow cytometry. Differential leukocyte counts were determined on a Mascot™ Hemavet 950FS blood analyzer (Drew Scientific Inc.). 500 μg of neutralizing α-IFN-γ, XMG1.2 (BioXcell) and α-IL-17A, TC11-18H10.1 (Biolegend) antibodies were administered IP every 3 days beginning on day 0 through one day before animal sacrifice. To measure proliferation, mice were injected intraperitoneally with 6 mg of 5-Bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) 1 hour before sacrifice. All mice were on FVB/n background and animal studies were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals following protocols approved by The Pennsylvania State University IACUC.
Cytotoxicity Assay
Detection of neutrophil mediated cytotoxicity was done as described (Corey et al., 1997) with modifications, using the KDalert™ GAPDH Assay Kit (Applied Biosystems) to quantitate GAPDH. Percent cytotoxicity was calculated using the following equation: % killing = [GAPDHCo-culture – (GAPDHeffectors + GAPDHtarget)]/GAPDHtotal effector lysates * 100.
FACS analysis
FACS analysis of single cell suspensions isolated from dorsal skin was performed as described (Mohammed et al., 2010). Intracellular cytokines were detected by treating single cell suspensions with PMA/ionomycin and Brefeldin A (eBioscience) for 4 ½ hours at 37° C. Cells were stained for extracellular surface antigens, fixed with 4% paraformaldehyde and permeabilized with 0.2% saponin/1% BSA/1x PBS. ROS was measured in CD11b+/Ly6G+ cells using the dye carboxy-H2DCFDA (Molecular Probes) according to manufacturer’s protocol. Fluorescently stained single cell suspensions were analyzed on an FC500 (Beckman Coulter) or an LSRFortessa (BD Biosciences) cytometer. Cell sorting was performed on a Cytopeia Influx Sorter (BD Biosciences).
Statistical Analysis
Student’s t test was used to calculate p values between experimental groups of two only. For groups of 3 or more, one-way ANOVA was used along with Tukey’s post-analysis t tests. GraphPad Prism 4.0 was employed to format all figures and calculate significance. * indicates p < 0.05.
Flow antibodies, Tissue Analysis and Biochemical and Molecular Analysis are described in Supplemental Material
Supplementary Material
Acknowledgments
Grant Support: National Cancer Institute CA117597, and National Psoriasis Foundation to ABG. AJG was supported through fellowship awards from Bristol Myers Squibb and The College of Agricultural Sciences, The Pennsylvania State University.
The authors thank staff of the Microscopy and Cytometry Facility of the Huck Institutes of the Life Sciences in assisting with flow cytometry and tissue histology, and Shailaja Hegde for her assistance with adoptive lymphocyte transfer studies. Support from the National Cancer Institute and National Psoriasis Foundation to ABG, and Bristol Myers Squibb to AJG.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Barker JN, Goodlad JR, Ross EL, Yu CC, Groves RW, MacDonald DM. Increased epidermal cell proliferation in normal human skin in vivo following local administration of interferon-gamma. Am J Pathol. 1993;142:1091–1097. [PMC free article] [PubMed] [Google Scholar]
- Bruch-Gerharz D, Fehsel K, Suschek C, Michel G, Ruzicka T, Kolb-Bachofen V. A proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. J Exp Med. 1996;184:2007–2012. doi: 10.1084/jem.184.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15:1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S, et al. Activation of cutaneous protein kinase Calpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol. 2003;171:2703–2713. doi: 10.4049/jimmunol.171.5.2703. [DOI] [PubMed] [Google Scholar]
- Cataisson C, Pearson AJ, Tsien MZ, Mascia F, Gao JL, Pastore S, et al. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J Clin Invest. 2006;116:2757–2766. doi: 10.1172/JCI27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de FA, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- Cook PW, Brown JR, Cornell KA, Pittelkow MR. Suprabasal expression of human amphiregulin in the epidermis of transgenic mice induces a severe, early-onset, psoriasis-like skin pathology: expression of amphiregulin in the basal epidermis is also associated with synovitis. Exp Dermatol. 2004;13:347–356. doi: 10.1111/j.0906-6705.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Corey MJ, Kinders RJ, Brown LG, Vessella RL. A very sensitive coupled luminescent assay for cytotoxicity and complement-mediated lysis. J Immunol Methods. 1997;207:43–51. doi: 10.1016/s0022-1759(97)00098-7. [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Funding AT, Johansen C, Kragballe K, Otkjaer K, Jensen UB, Madsen MW, et al. Mitogen- and stress-activated protein kinase 1 is activated in lesional psoriatic epidermis and regulates the expression of pro-inflammatory cytokines. J Invest Dermatol. 2006;126:1784–1791. doi: 10.1038/sj.jid.5700252. [DOI] [PubMed] [Google Scholar]
- Glick AB, Sporn MB, Yuspa SH. Altered regulation of TGF-β 1 and TGF-α in primary keratinocytes and papillomas expressing v-Ha-ras. Mol Carcinog. 1991;4:210–219. doi: 10.1002/mc.2940040308. [DOI] [PubMed] [Google Scholar]
- Gunther C, Carballido-Perrig N, Kaesler S, Carballido JM, Biedermann T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J Invest Dermatol. 2012;132:626–634. doi: 10.1038/jid.2011.371. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Silva-Vargas V, Groves R, Watt FM. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J Invest Dermatol. 2004;123:503–515. doi: 10.1111/j.0022-202X.2004.23225.x. [DOI] [PubMed] [Google Scholar]
- Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Jaubert J, Patel S, Cheng J, Segre JA. Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J Invest Dermatol. 2004;123:313–318. doi: 10.1111/j.0022-202X.2004.23203.x. [DOI] [PubMed] [Google Scholar]
- Karvonen SL, Koivunen J, Nissinen M, Yla-Outinen H, Bjorkstrand AS, Peltonen J. Neurofibromatosis type 1 tumour suppressor gene expression is deficient in psoriatic skin in vivo and in vitro: a potential link to increased Ras activity. Br J Dermatol. 2004;150:211–219. doi: 10.1111/j.1365-2133.2004.05767.x. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Baldassare JJ, Voorhees JJ, Fisher GJ. Increased activation of Ras in psoriatic lesions. Skin Pharmacol Appl Skin Physiol. 1999;12:90–97. doi: 10.1159/000029850. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed J, Ryscavage A, Perez-Lorenzo R, Gunderson AJ, Blazanin N, Glick AB. TGFbeta1-induced inflammation in premalignant epidermal squamous lesions requires IL-17. J Invest Dermatol. 2010;130:2295–2303. doi: 10.1038/jid.2010.92. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Pallone F, MacDonald TT, Chimenti S, Costanzo A. Psoriasis: from pathogenesis to novel therapeutic approaches. Clin Sci (Lond) 2011;120:1–11. doi: 10.1042/CS20100163. [DOI] [PubMed] [Google Scholar]
- Ortega C, Fernandez A, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarra M, Caruso R, Cupi ML, Monteleone I, Stolfi C, Campione E, et al. IL-21 promotes skin recruitment of CD4(+) cells and drives IFN-gamma-dependent epidermal hyperplasia. J Immunol. 2011;186:5435–5442. doi: 10.4049/jimmunol.1003326. [DOI] [PubMed] [Google Scholar]
- Tarutani M, Imai Y, Yasuda K, Tsutsui H, Nakanishi K, Yamanishi K. Neutrophil-dominant psoriasis-like skin inflammation induced by epidermal-specific expression of Raf in mice. J Dermatol Sci. 2010;58:28–35. doi: 10.1016/j.jdermsci.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Teige I, Backlund A, Svensson L, Kvist PH, Petersen TK, Kemp K. Induced keratinocyte hyper-proliferation in alpha2beta1 integrin transgenic mice results in systemic immune cell activation. Int Immunopharmacol. 2010;10:107–114. doi: 10.1016/j.intimp.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Teige I, Hvid H, Svensson L, Kvist PH, Kemp K. Regulatory T cells control VEGF-dependent skin inflammation. J Invest Dermatol. 2009;129:1437–1445. doi: 10.1038/jid.2008.375. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Li CY, Dai HY, Cai DX, Wang KY, Xu YH, et al. Expression and localization of the activated mitogen-activated protein kinase in lesional psoriatic skin. Exp Mol Pathol. 2007;83:413–418. doi: 10.1016/j.yexmp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





