Abstract
Effective removal of apoptotic cells, particularly apoptotic neutrophils, is essential for the successful resolution of acute inflammatory conditions. In the present experiments, we found that whereas interaction between vitronectin and integrins diminished the ability of macrophages to ingest apoptotic cells, interaction between vitronectin with urokinase type Plasminogen Activator receptor (uPAR) on the surface of apoptotic cells also had equally important inhibitory effects on efferocytosis. Pre-incubation of vitronectin with Plasminogen Activator inhibitor-1 (PAI-1) eliminated its ability to inhibit phagocytosis of apoptotic cells. Similar, incubation of apoptotic cells with soluble uPAR or antibodies to uPAR significantly diminished efferocytosis. In the setting of LPS-induced ALI, enhanced efferocytosis and decreased numbers of neutrophils was found in bronchoalveolar lavage obtained from vitronectin deficient (vtn−/−) mice compared to wild type (vtn+/+) mice. Furthermore, there was increased clearance of apoptotic vtn−/− as compared to vtn+/+ neutrophils after introduction into the lungs of vtn−/− mice. Incubation of apoptotic vtn−/− neutrophils with purified vitronectin prior to intratracheal instillation decreased efferocytosis in vivo. These findings demonstrate that the inhibitory effects of vitronectin on efferocytosis involve interactions with both the engulfing phagocyte as well as the apoptotic target cell.
INTRODUCTION
The removal of apoptotic cells, a process known as efferocytosis, plays a crucial role in the maintenance of tissue homeostasis and resolution of inflammatory and immune responses (1-3). Failure to effectively remove apoptotic cells, and particularly apoptotic neutrophils that accumulate in inflammatory foci, results in necrosis and cytolysis of dying cells with the concomitant release of tissue damaging intracellular contents. Recent studies have shown that the ability of host to effectively remove apoptotic cells has important effects on outcome in experimental models for sepsis, hemorrhage, burns or endotoxin induced acute lung injury, conditions that are clinically relevant particularly in the setting of critical illness (4-6).
Recognition of apoptotic cells by phagocytes is mediated by ‘eat-me’ signaling components that appear on the surface of the apoptotic cell (1, 2, 7-11). Phosphatidylserine (PtdSer), calreticulin, CD14, and oxidized low-density lipoprotein (LDL)-like moiety are well characterized apoptotic cell surface markers that are involved in the engulfment of apoptotic cells by phagocytes (12-15). Recent studies suggest that factors released by apoptotic cells, including lysophosphatidylcholine (LPC) or endothelial monocyte-activating polypeptide II, as well as the nucleotide extracellular gradient, participate in ‘find-me’ signaling, resulting in the accumulation of phagocytes around apoptotic cells (16-18). Some receptors are also capable of preventing the recognition of dying cells. For example, the appearance of complexes of CD31-CD31 or CD47-SIRPα (signal regulatory protein α) on the surface of apoptotic cells allows them to escape phagocytosis (19, 20). In addition to ligands appearing on the cell surface, soluble factors, including Gas6 and protein S, that bridge PtdSer and phagocytic receptors of the TAM family (Tyro3, Axl, Mer) enhance the uptake and ingestion of apoptotic cells by macrophages and other phagocytic cells (21). Finally, cytoskeletal rearrangement that allows for engulfment of the targeted cell and formation of phagosomes is required for effective clearance of apoptotic cells by phagocytes (22-24).
Vitronectin is a multifunctional glycoprotein found in large quantities in serum, the extracellular matrix, and platelets. Vitronectin consists of three distinct domains; a somatomedin B domain (SMB) that binds to the urokinase type plasminogen activator receptor (uPAR); a short RGD motif that interacts with integrins; and a hemopexin domain that forms complexes with heparin/complement (25-30). The ability of vitronectin to interact with these regulatory components affects cell adhesion, coagulation, fibrinolysis, complement activation, and apoptosis (31, 32). Recent studies suggest that interactions between vitronectin and integrin αvβ3, PAI-1, or uPAR can also modulate the clearance of apoptotic cells (33-35). The ability of vitronectin to affect biological processes associated with inflammation is likely to have pathophysiologic significance because tissue levels of vitronectin in the lungs and other anatomic sites are markedly increased in settings, such as acute lung injury, burns, and sepsis that are associated with neutrophil activation and tissue injury (36, 37).
In the present studies, we investigated the ability of vitronectin to modulate clearance of apoptotic cells under in vitro and in vivo conditions. Our results indicate that vitronectin can diminish efferocytosis by independently affecting the participation of both macrophages and apoptotic cells.
MATERIALS AND METHODS
Mice
Vitronectin-deficient mice (B6.129S2(D2)-Vtntm1Dgi/J), as well as control mice (C57BL/6J), were purchased from The Jackson Laboratory (Bar Harbor, ME). Vitronectin knockout male mice were crossed to B6D2F1/J female mice, and then backcrossed to C57BL/6J for 12 generations before being interbred. Male mice, 8 to 12 weeks of age, were used for experiments. All experiments were conducted in accordance with institutional review board approved protocols (UAB Institutional Animal Care and Use Committee).
Materials
Purified mouse vitronectin was purchased from Abcam (Cambridge, MA). Vitronectin lacking the SMB domain, delta SMB mutant (ΔSMB), or isolated SMB domain were expressed in Drosophila S2 cells using methods reported by Schar et al (38), and proteins purified as described by Thompson et al (39). Cyclo(Arg-Gly-Asp-D-Phe-Val) RGDfv and cyclo(Arg-Ala-Asp-D-Phe-Val) RADfv were purchased from Enzo Life Science (Plymouth Meeting, PA), whereas RGD-FITC was from AnaSpec (Fremont, CA). Recombinant mouse PAI-1 was a gift from Dr. Victoria Ploplis (Notre Dame, IN). suPAR were obtained from R&D Systems (Minneapolis, MN). Neutralizing antibody to integrin αvβ3 was from Millipore (Billerica, MA), and specific isotype control IgG was purchased from BD Biosciences (San Diego, CA). Mouse specific anti-integrin αvβ5 blocking antibody and isotype control IgG was a gift from Dr. Dean Sheppard (University of California, San Francisco). Anti-uPAR blocking antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). For immunocytochemistry, anti-vitronectin and anti-uPAR antibodies were from R&D Systems (Minneapolis, MN), whereas Alexa Fluor 488- and Alexa Fluor 555 secondary antibodies were purchased from Invitrogen (Carlsbad, CA, USA). Mouse specific anti-αvβ3 and αvβ5 antibody for immunocytochemistry were purchased from Novus Biologicals Litteton, CO) wheras anti-6His antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Propidium iodide and antibodies to annexin V were obtained from EMD Chemicals (Gibbstown, NJ). PKH26 was from Sigma-Aldrich (St. Louis, MO), whereas FITC-conjugated anti-CD11b and allophycocyanin-conjugated anti-CD 90.2 antibodies were from BD Biosciences (San Diego, CA). Custom antibody mixtures and negative selection columns for neutrophil isolation were purchased from Stem Cell Technologies (Vancouver, British Columbia, Canada). ELISA kits for measuring vitronectin were obtained from Molecular Innovations (Novi, MI).
Neutrophil and thymocyte isolation and culture
Bone marrow neutrophils were purified using a negative selection column (40, 41). Briefly, bone marrow cell suspensions were isolated from the femur and tibia of mice by flushing with RPMI 1640 medium with 5% FBS. The cell suspension was passed through a glass wool column and collected by washing with PBS containing 5% FBS. Negative selection to purify neutrophils was performed by incubation of the cell suspension with biotinylated primary antibodies specific for the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 (StemCell Technologies, Vancouver, BC, Canada, www.stemcell.com/technical/13309-PIS.pdf) for 15 min at 4°C followed by subsequent incubation with anti-biotin tetrameric antibodies (100 μl, StemCell Technologies) for 15 min. The complex of anti-tetrameric antibodies and cells was then incubated with colloidal magnetic dextran iron particles (60 μl, StemCell Technologies) for an additional 15 min at 4°C. The T cells, B cells, RBC, monocytes, and macrophages were captured in a column surrounded by a magnet, allowing the neutrophils to pass through. Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 98%. Thymocytes were isolated as previously described (42).
Purification and culture of peritoneal macrophages
Peritoneal macrophages were elicited in 8–10 week old mice using Brewer thioglycollate. Cells were collected 5 days after intraperitoneal injection of Brewer thioglycollate and were plated on coverslips (Fisherbrand, 12-545-82 12CIR-1D) in 24-well plates (2.5 × 105 cells/well) in serum free RPMI 1640 medium. After 1 hour, the plates were washed with culture medium to remove non-adherent cells. Macrophages were cultured in RPMI 1640 medium and used for phagocytosis assays on the day of isolation.
Induction of apoptosis in neutrophils and thymocytes
Apoptosis in neutrophils and thymocytes was induced as previously described . In brief, apoptosis in vtn+/+ or vtn−/− neutrophils was induced by incubation of the cells for 45 minutes at 43°C, followed by culture for an additional 2.5 hours at 37°C. Murine thymocytes (vtn+/+ or vtn−/−, 6 × 106 cells/ml) in RPMI 1640 medium were incubated with dexamethasone (1 μM) for 16 hours. Cells were then washed 3 times with RPMI 1640 medium to remove dexamethasone. Annexin/PI staining and flow cytometry showed that 90% or more of the thymocytes and greater than 70% if the neutrophils were apoptotic.
In vitro efferocytosis assay
In vitro efferocytosis assays were performed as previously described (43). Briefly, 2.5 × 106 apoptotic neutrophils or 106 apoptotic thymocytes suspended in RPMI medium were co-cultured with 2.5 × 105 macrophages on glass coverslips. Cells were incubated in media containing 5% mouse serum obtained from vtn+/+ or vtn−/− mice for 2 or 1.5 hours, as indicated in the Figure legends. Next, coverslips were washed three times with ice-cold PBS and cells stained with HEMA 3. Phagocytosis was evaluated by a blinded observer by counting for five-six randomly selected fields per slide. The phagocytosis index was calculated as the percentage of macrophages containing at least one engulfed neutrophil or thymocyte.
In vivo efferocytosis assay
In vivo efferocytosis was determined as previously described (6, 34). In brief, the effect of vitronectin on phagocytosis was determined using intratracheal instillation of apoptotic neutrophils into vtn+/+ or vtn−/− mice anesthetized with isofluorane. Mice were injected i.t with 5 ×106 vtn+/+ or vtn−/− viable or apoptotic neutrophils, or vtn−/− apoptotic neutrophils that were pre-incubated with purified vitronectin (100 nM) for 1 hour. Two hours later, the mice were sacrificed and bronchoalveolar lavage (BAL) performed using 1 ml sterile PBS containing 5 mM EDTA. Cells were then collected on cytospin slides, fixed, stained with HEMA 3, and phagocytosis index was determined by a blinded observer.
In selected experiments, apoptosis was induced in thymocytes that were labeled with PKH-26 red fluorescent dye. Mice were anesthetized with isoflurane, and 1 × 107 apoptotic thymocytes/PKH-26 in 50 or 100 μl of PBS were injected intratracheally or intraperitoneally respectively, as described in the figure legends. Two hours later, mice were sacrificed and bronchoalveolar lavage (BAL) or peritoneal lavage performed using 1 or 5 ml sterile PBS containing EDTA (5 μM), respectively. Isolated cells were washed with culture media and then incubated in PBS containing 1% albumin, FITC-conjugated anti-CD11b, and APC-conjugated anti-CD90.2 (thymocyte marker) antibodies followed by flow cytometry analysis. The phagocytic index was calculated as the ratio of FITC+PKH26+APC− cells to all cells gated. Engulfed thymocytes are not accessible to the APC-conjugated anti-CD90.2 antibody. Therefore, FITC+PKH26+APC− cells are identified as macrophages that have engulfed PKH-labeled thymocytes, whereas the FITC+PKH26+APC+cells are macrophages containing adherent thymocytes (e.g. not engulfed).
Imaging thymocytes, neutrophils and macrophages
Viable or apoptotic thymocytes were incubated with 4% paraformaldehyde in PBS for 20 minutes at room temperature. Cells were washed with PBS, pre-incubated with 1% BSA in PBS for 45 minutes, and then incubated with anti-uPAR antibodies (1:50 dilution) overnight at 4°C followed by fluorescent anti-rabbit antibody (Alexa-488, 1:1000 dilution) for 90 minutes at room temperature. Cells were washed with PBS and mounted with an emulsion oil solution containing DAPI to visualize nuclei. Microscopy was performed using a confocal laser scanning microscope (model LSM 710 confocal microscope, Carl Zeiss MicroImaging) provided by the High Resolution Imaging Facility at the University of Alabama at Birmingham. To determine co-localization between uPAR and vitronectin, viable and apoptotic neutrophils were incubated with 4% paraformaldehyde, washed with PBS, blocked with 3% BSA, and then cultured with antibodies to uPAR (1:20) and vitronectin (1:25) for 2 hours followed by incubation with fluorescent anti-rat Alexa-488 and anti-rabbit Alexa-555 antibodies at 1:1000 dilution for an additional 90 minutes at room temperature. In selected experiment, cells treated with RGD-FITC or vitronectin-Δ-SMB-6His for 60 minutes were stained with anti-αvβ3, anti-αvβ5 or anti-6His antibody (90 minutes at room temperature) and specific fluorescent secondary antibodies (for additional 60 minutes).
ELISA vitronectin
Amount of vitronectin in culture medium or BALs was determined using mouse specific vitronectin kit (Molecular Innovations. Novi, MI), and accordingly with instruction and vitronectin protein standard provided by manufacturer.
Model for LPS induced acute lung injury (ALI)
Acute lung injury was induced by intratracheal administration of 1 mg/kg LPS in 50 μl of PBS as previously described (40, 41, 44, 45). Briefly, mice were anesthetized with isoflurane, the tongue was gently extended, and LPS in PBS or PBS alone (control) was deposited into the pharynx. Mice were sacrificed 48 hours after LPS administration and BAL was obtained by lavaging the lungs three times with 1 ml of PBS.
Statistical analysis
Statistical significance was determined by the Wilcoxon rank sum test (independent 2-group Mann-Whitney U Test) as well as Student’s t test for comparisons between two groups. Multigroup comparisons were performed using one-way ANOVA with the Turkey’s post hoc test. A value of p < 0.05 was considered significant. Analyses were performed on SPSS version 16.0 for Windows.
RESULTS
Vitronectin diminishes engulfment of apoptotic cells by macrophages
The ability of vitronectin to affect efferocytosis was determined using peritoneal macrophages and apoptotic thymocytes or neutrophils. Cells were obtained from wild type mice (vtn+/+) or mice deficient in vitronectin (vtn−/−). Consistent with previous studies (33), complete deficiency of vitronectin in the cultures (e.g. vtn−/− cells and medium containing serum from vtn−/− mice ) was associated with markedly increased phagocytosis of apoptotic thymocytes as compared to that found when vtn+/+ macrophages and apoptotic cells were included in the cultures (Figures 1A and B). The effects of vitronectin deficiency were reversible upon co-incubation of vtn−/− cells with serum obtained from wild type mice (vtn+/+). Of note, the inhibitory effects of vitronectin were dependent on cell viability; in particular, exposure to vitronectin diminished the engulfment of apoptotic cells (Figures 1B), but had no effect on the low rate of uptake of viable cells by macrophages. As was found with apoptotic thymocytes, vitronectin deficiency also increased uptake of apoptotic neutrophils by peritoneal macrophages (Figure 1C).
Figure 1.
Vitronectin deficiency increases phagocytosis of apoptotic cells. Macrophages were co-cultured with apoptotic or viable thymocytes (vtn+/+ or vtn−/−) or neutrophils (vtn+/+ or vtn−/−) for 90 or 120 minutes respectively. Cells were incubated in serum obtained from wild type (vtn+/+) or vitronectin deficient (vtn−/−) mice as indicated. Representative images (A) and phagocytic indices (B and C) show that vitronectin deficiency (vtn−/−) increased phagocytosis of apoptotic thymocytes or apoptotic neutrophils. Means ± SD (n = 3), ***P < 0.001 compared to vtn+/+ apoptotic cells or to vtn−/− apoptotic cells cultured in vtn+/+ serum; #P < 0.05 compared to viable cells. Arrows in (A) indicate ingested apoptotic thymocytes.
Vitronectin diminishes efferocytosis through interactions with macrophages as well as with apoptotic cells
To determine if the inhibition of efferocytosis by vitronectin was mediated by binding of vitronectin to receptors on the surface of macrophages or apoptotic cells, or perhaps by affecting both cell types, vtn−/− macrophages cultured in vitronectin deficient medium were dose dependently treated with purified vitronectin or vtn+/+ serum obtained from wild type mice, then washed and co-cultured with vitronectin deficient apoptotic thymocytes in vitronectin deficient medium. As shown in Figure 2A and supplemental Figure s1A, inclusion of either purified vitronectin or serum containing vitronectin dose-dependently decreased the ability of macrophages to ingest apoptotic thymocytes. Decreases in efferocytosis were also found when apoptotic vtn−/− thymocytes were pre-incubated with purified vitronectin or vitronectin containing medium followed by co-culture with vtn−/− macrophages (Figure 2B or supplemental Figure s1B). The lowest levels of phagocytosis were found when both vtn−/− macrophages and apoptotic cells were treated with vitronectin (Figure 2C). As expected, large amounts of vitronectin were detected in vtn+/+ serum, whereas no vitronectin was found in vtn−/− serum (supplemental Figure s1G). Western blot analysis, confocal microscopy and ELISA confirmed the ability of purified vitronectin or vitronectin in serum to bind to viable and apoptotic vtn−/− cells (supplemental Figure s1E, F and H).
Figure 2.
Vitronectin inhibits efferocytosis through interactions with both apoptotic cells and macrophages. (A) Vtn−/− macrophages or (B) vtn−/− apoptotic thymocytes were pre-treated with purified vitronectin (0, 30, 100 or 300 nM) in medium without serum for 1 hour. The cells were then washed and co-cultured in medium supplemented with 5% serum (vtn−/−) for 90 minutes. The percentage of ingested thymocytes is shown. Means ± SD (n = 3), *P < 0.05 or **P < 0.01 compared to untreated cells. (C) Vtn−/− macrophages or vtn−/− apoptotic thymocytes were separately pre-treated with purified vitronectin (0 or 200 nM) for 60 minutes, then washed and co-cultured in medium containing vitronectin deficient serum (5%) for an additional 90 minutes. Means ± SD (n = 3), **P < 0.01 or ***P < 0.001 compared to untreated cells, whereas ##P < 0.01 compared to macrophages co-cultured with apoptotic thymocytes, both pre-treated with purified vitronectin.
Vitronectin domains differentially affect the interactions between macrophages and apoptotic thymocytes during efferocytosis
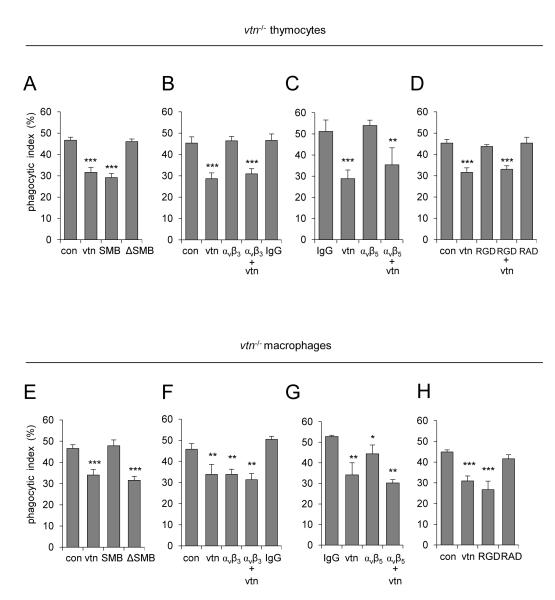
Pre-incubation of apoptotic thymocytes with full length vitronectin or with the vitronectin SMB domain alone produced similar inhibition of efferocytosis, but exposure of apoptotic thymocytes to vitronectin lacking the SMB domain (vitronectin-ΔSMB) had no effect of efferocytosis (Figure 3A). In contrast to apoptotic thymocytes, exposure of vtn−/− macrophages to full length vitronectin or vitronectin-ΔSMB, but not to the SMB domain alone, resulted in inhibition of efferocytosis (Figure 3E). Of note, confocal microscopy confirmed that vitronectin-ΔSMB was co-localized with αvβ3 and αvβ5 integrins on the cell surface of peritoneal macrophages (supplemental Figures s2A and B). These results suggest that whereas the SMB domain of vitronectin is involved in diminishing efferocytosis through interaction with receptors on apoptotic cells, other domains of vitronectin are responsible for its inhibitory effect on ingestion of apoptotic cells by macrophages.
Figure 3.
Effects of vitronectin, vitronectin SMB domain, vitronectin deficient in SMB domain (ΔSMB), RGD peptide, or antibodies to αvβ3 on efferocytosis. (A-D) Apoptotic vtn−/− thymocytes or (E-H) vtn−/− macrophages were pre-treated with vitronectin (vtn, 0 or 200 nM), SMB domain (SMB, 0 or 200 nM), ΔSMB (0 or 200 nM), anti-αvβ3 or anti-αvβ5 antibody (1 μg/ml), isotype specific IgG (1 μg/ml), RGDfv (1 μM), or RADfv (1 μM) for 1 hour and then washed. The percentage of engulfed thymocytes was determined after co-culture of macrophages with apoptotic thymocytes for 90 minutes. In selected experiments, cells were treated with anti-αvβ3 or αvβ5 antibody or RGDfv for 60 minutes, washed, and then cultured with purified vitronectin for an additional 60 minutes. Means ± SD (n = 3) are shown. (A) ***P < 0.001 compared to control (untreated) or cells pre-treated with ΔSMB only; (B) ***P < 0.001 compared to control, anti-αvβ3 or IgG pre-treated; (C) **P < 0.01 and ***P < 0.001 compared to IgG or anti-αvβ5; (D) ***P < 0.001 compared to control, RGD or RAD treated. In panel (E), ***P < 0.001 compared to control or SMB treated; (F) **P < 0.01 as compared to control and IgG; (G) *P < 0.05 (P = 0.036) and **P < 0.01 as compared to IgG, and (H) ***P < 0.001 compared to control and macrophages pre-treated with RAD.
Vitronectin is known to interact with the αvβ3 integrin and appears able to inhibit the stimulatory effects of αvβ3 on efferocytosis (33, 46, 47). Consistent with such previously reported findings, incubation of macrophages with specific antibodies that block the αvβ3 integrin resulted in decreased uptake of apoptotic thymocytes (Figure 3F). However, a similar approach to selectively block the αvβ3 integrin on apoptotic thymocytes had no effect on efferocytosis (Figure 3B). Similar results were obtained with specific antibodies that block the αvβ5 integrin (Figures 3C and G). These results demonstrate that in addition to its interactions with αvβ3, vitronectin can potentially modulate efferocytosis through binding between its RGD domain and the αvβ5 integrin (33, 48, 49). Previous studies (33), and as well as results shown in Figure 3H, demonstrate that pre-incubation of macrophages with the RGD peptide RGDfv or with full length vitronectin effectively diminished the ability of macrophages to engulf apoptotic cells. In contrast, exposure of apoptotic thymocytes to RGDfv did not affect efferocytosis and also did not affect vitronectin-dependent inhibition of efferocytosis (Figures 3D and H). These results suggest that the inhibitory effects of vitronectin on efferocytosis are mediated by binding to integrins on the surface of macrophages, but were integrin independent in case of apoptotic cells. Of note, confocal microscopy revealed co-localization between FITC-RGD peptide and αvβ3 or αvβ5 integrins in both, macrophages and apoptotic neutrophils (Supplemental Figures s2C and s3A).
Interactions between vitronectin and uPAR on the surface of apoptotic cells inhibit efferocytosis
As shown in Figures 4A and B, full length vitronectin or purified vitronectin SMB domain diminished efferocytosis of apoptotic vtn−/− thymocytes. Because the vitronectin SMB domain is known to bind to the uPA receptor (uPAR) (50, 51), we hypothesized that such interactions might be a relevant mechanism for the ability of vitronectin to affect the uptake of apoptotic cells by macrophages. Indeed, exposure of vtn−/− apoptotic thymocytes to uPAR blocking antibodies or deficiency of uPAR (uPAR−/− thymocytes) resulted in inhibition of efferocytosis (Figures 4A and 4D). Although a previous study (52) and the current experiments found decreased uPAR staining on the surface of apoptotic cells, uPAR was still readily detected on apoptotic thymocytes (Figure 4E and supplemental Figure s3B). As shown in Figure 4E, confocal microscopy revealed that uPAR also interacts with vitronectin.
Figure 4.
uPAR on the surface of apoptotic cells contributes to the ability of vitronectin to inhibit efferocytosis. (A) Apoptotic thymocytes (vtn−/) were pre-treated with blocking antibody to uPAR (0 or 1 μg/ml) or isotype specific IgG (0 or 1 μg/ml) for 30 minutes. Cells were washed and then treated with purified vitronectin (0 or 200 nM) for an additional 60 minutes. Phagocytic indices were determined after thymocytes were washed to remove unbound vitronectin followed by co-culture for 90 minutes with macrophages in medium containing vitronectin deficient serum (5%). Means ± SD (n = 3), ***P < 0.001 compared to control (untreated) or macrophages pre-treated with IgG alone. (B) Apoptotic thymocytes were pre-incubated with PAI-1 (0 or 100 nM), SMB (0 or 100 nM), or pre-formed PAI-1/SMB complexes for 60 minutes. PAI-1/SMB complexes were obtained after incubation of PAI-1 with SMB for 60 minutes at room temperature. Phagocytic indices are shown. Means ± SD (n = 3), **P < 0.01 comparing thymocytes incubated with SMB to control or treated with PAI-1 or PAI-1/SMB. In (C), phagocytic indices were obtained after apoptotic thymocytes were pre-treated with vitronectin (0 or 200 nM), soluble uPAR (suPAR, 0 or 200 nM), vitronectin/suPAR complexes, or vitronectin incubated with BSA. Means ± SD (n = 3), **P < 0.01 compared to control or thymocytes treated with suPAR alone or vitronectin/suPAR. Panel (D) shows phagocytic indices obtained after apoptotic wild type (uPAR+/+) or uPAR deficient (uPAR−/−) thymocytes were co-cultured with wild type macrophages. Means ± SD (n = 3), **P < 0.01. (E) Representative confocal images show the amount and co-localization (yellow) of vitronectin and uPAR on the surface of viable or apoptotic neutrophils (arrows). uPAR-red, vitronectin–green, nuclei-blue.
Interactions between vitronectin with PAI-1 and uPAR are mutually exclusive, although PAI-1 was demonstrated to have higher affinity for vitronectin then does uPAR (28, 53). Because SMB diminished efferocytosis, we anticipated that formation of PAI-1-SMB complexes will diminish binding between SMB and uPAR on the surface of apoptotic cells and preserve efficient clearance of such cells. As shown in Figure 4B, pre-incubation of vtn−/− apoptotic thymocytes with preformed complexes of PAI-1-SMB diminished the ability of the vitronectin SMB domain to inhibit efferocytosis. Of note, there was no evidence that the effects of PAI-1 were due to potential contamination with LPS (Figure s1C).
Previous studies have shown that soluble uPAR (suPAR) can bind to αv, β1, β2, or β3 integrins (25, 35, 54) as well as to SMB domain of vitronectin (30). Therefore, we examined whether interactions between soluble uPAR and vitronectin could affect efferocytosis. Inclusion of suPAR pre-incubated with vitronectin into cultures of vtn−/− apoptotic thymocytes increased the phagocytic index as compared to treatment with vitronectin alone (Figure 4C). However, unlike the situation with apoptotic cells, macrophages pre-incubated with suPAR still demonstrated diminished engulfment of apoptotic thymocytes after exposure to vitronectin (Figure s1D).
Vitronectin decreases efferocytosis in vivo
Although our experiments demonstrated that vitronectin can inhibit efferocytosis in vitro, we wished to confirm that such effects of vitronectin were also present under in vivo conditions. To examine this issue, acute lung injury (ALI) was induced by pulmonary exposure to LPS, a situation known to result in the appearance of significant amounts of vitronectin in interstitial lung tissue as well as in the alveolar space (Figure 5E) (36, 37). As shown in Figure 5, engulfment of apoptotic neutrophils was significantly increased in the lungs of LPS treated vtn−/− as compared to vtn+/+ mice. Decreased numbers of neutrophils were recovered from BAL fluid of vtn−/− as compared to vtn+/+ mice.
Figure 5.
Vitronectin deficiency enhances clearance of apoptotic neutrophils in the lungs of mice with LPS-induced acute lung injury. The percentage of macrophages with ingested neutrophils was determined in BAL fluid obtained from mice that received LPS (i.t.) 48 hours previously. Panel (A) shows representative images of macrophages engulfing apoptotic neutrophils and panel (B) shows phagocytic indices. (C) and (D) show total white cells and neutrophil numbers in BAL, whereas (E) demonstrates the amount of vitronectin detected in BAL obtained from control mice and mice subjected to i.t. LPS administration (Means ± SD; n = 3, *P < 0.05 compared to vtn or **P < 0.01 compared control to LPS treated mice).
Although these results suggest that deficiency of vitronectin can enhance the clearance of apoptotic neutrophils, another possibility is that there were increased numbers of neutrophils undergoing efferocytosis as a result of greater levels of apoptosis. To address this issue, vtn+/+ or vtn−/− mice were subjected to intratracheal administration of purified apoptotic vtn+/+ or vtn−/− neutrophils, and then phagocytic indices determined. As shown in Figure 6A, clearance of apoptotic neutrophils was significantly increased in vtn−/− mice injected with vtn−/− neutrophils as compared to vtn+/+ mice that were injected with vtn+/+ neutrophils. Of note, significantly less vitronectin was found in the lungs of control mice as compared to mice exposed to LPS (Figure 5E). Intratracheal administration of purified vitronectin into the lungs of control (vtn+/+) mice also decreased efferocytosis after instillation of vtn−/− apoptotic neutrophils (Figure 6B).
Figure 6.
Effects of vitronectin on clearance of apoptotic cells in vivo. (A) Phagocytic index was determined by measuring ingestion of apoptotic neutrophils by alveolar macrophages using BAL from mice (vtn+/+ or vtn−/−) obtained 2 hours after intratracheal administration of apoptotic neutrophils (vtn+/+ or vtn−/−). Means ± SD with three mice per group, *P < 0.05. (B) Wild type (vtn+/+) mice received purified vitronectin intratracheally (0 or 1 μg) 30 minutes before instillation of vtn+/+ apoptotic neutrophil. BAL was obtained 2 hours later. The percentages of alveolar macrophages with ingested neutrophils are shown. Means ± SD from three mice per group. *P < 0.05. In (C), apoptotic neutrophils (vtn−/−) were incubated with purified vitronectin (0 or 200 nM) for 1 hour, washed, and then administered intratracheally to vitronectin deficient (vtn−/−) mice. Phagocytic indices were determined in BAL obtained 2 hours after neutrophil administration. Means ± SD (4 mice per group) are shown. *P < 0.05 compared to mice that received neutrophils that were incubated without vitronectin. (D) Mice were treated as described in (C) using PKH26-labeled apoptotic thymocytes (vtn−/−) that were pre-incubated with or without vitronectin. Phagocytic indices were determined using flow cytometry. Means ± SD (4 mice/group) are shown. * P < 0.05 compared to the mice given untreated thymocytes. (E) Mice were subjected to intraperitoneal injection (i.p.) of PKH26-labeled apoptotic thymocytes (vtn−/−) treated with or without vitronectin. Peritoneal cells were isolated 2 hours later and phagocytic indices determined using flow cytometry. Means ± SEM using 3 mice per group.
To determine if vitronectin can affect efferocytosis by specifically binding to apoptotic cells, apoptotic vtn−/− neutrophils or thymocytes were incubated with or without vitronectin, then washed and injected intratracheally into vtn−/− mice. As shown in Figure 6C, there was decreased phagocytosis by alveolar macrophages of apoptotic cells that had been treated with purified vitronectin. Similar results were obtained from experiments in which fluorescently labeled apoptotic vtn−/− thymocytes were pre-incubated with or without purified vitronectin and then injected into the lungs of vtn−/− mice (Figure 6D). Confirmatory results were obtained using fluorescently labeled vtn−/− apoptotic thymocytes pre-incubated with or without vitronectin that were then injected into the intraperitoneal cavity of vtn−/− mice (Figure 6E). These data indicate that vitronectin plays a regulatory role in efferocytosis in vivo through interactions with receptors on the surface of apoptotic cells.
DISCUSSION
In the present experiments, we found that vitronectin effectively inhibits uptake of apoptotic cells by macrophages in vitro and in the lungs of mice with LPS-induced ALI. Our results showed that interactions between vitronectin and apoptotic cells as well as between vitronectin and macrophages prevent efferocytosis. However, the mechanisms for such inhibition of efferocytosis by vitronectin were dependent on interaction with differing receptors on macrophages and apoptotic cells. Although we found that vitronectin decreased phagocytosis by binding to the αvβ3 and αvβ5 integrin on the surface of macrophages, this did not appear to be the case for apoptotic cells. Using cells isolated from vitronectin deficient mice, antibodies to selective cell surface receptors, and specific vitronectin domains, we found that the inhibitory effects of vitronectin on the uptake of apoptotic cells by macrophages were mediated by interactions with the uPA receptor (uPAR) on the apoptotic cell.
Previous studies demonstrated that vitronectin receptors, including αvβ3 and the combination of PAI-1 and uPAR, can modulate the phagocytosis of apoptotic cells (33-35, 55). However, the direct role of vitronectin on efferocytosis has not been fully explored. Proteins able to bridge receptors on apoptotic cells and phagocytes, including vitronectin, milk fat globule EGF factor 8 (MFG-E8) or thrombospodin-1 (TSP-1), have been reported to participate in efferocytosis through binding to αvβ3 on macrophages (33, 46, 47, 56). Vitronectin can diminish interactions between αvβ3 and bridging molecules, such as MFG-E8 or TSP-1, thereby preventing recognition of phosphatidylserine on apoptotic cells (46, 47). In the present studies, incubation of macrophages with antibodies to αvβ3 or RGDfv decreased their ability to ingest apoptotic cells. Of note, interactions between αvβ3 and vitronectin or the Arg-Gly-Asp (RGD) peptide on macrophages, but not on apoptotic cells, have previously been shown to inhibit association of macrophages with apoptotic cells (33, 46, 57).
Previous studies have shown that the SMB domain of vitronectin is required for binding to uPAR (25, 28, 30). In the present experiments, culture of apoptotic cells with purified vitronectin SMB domain, but not with vitronectin lacking the SMB domain, diminished efferocytosis. These results indicate that uPAR on the surface of apoptotic cells is likely to be a target for the SMB domain of vitronectin. Indeed, similar to the inhibitory effects on efferocytosis found after incubation of the SMB domain with apoptotic cells, inactivation of uPAR on apoptotic cells by incubation with blocking antibodies also diminished phagocytosis. These data suggest that the ability of vitronectin to bind uPAR on apoptotic cells inhibits an important “eat me” signal that normally would enhance the uptake of apoptotic cells by macrophages. Of note, αvβ3 on apoptotic cells appeared to be dispensable in regulating efferocytosis, as evidenced by the lack of effect when apoptotic cells were pre-incubated with antibodies that block αvβ3 or with RGDfv, unlike the inhibition of efferocytosis found when macrophages were exposed to such agents.
uPAR affects many cellular functions, including the adhesion of cells to vitronectin and other matrix proteins (25, 58, 59). Soluble uPAR (suPAR) is released by cleavage of the uPAR GPI anchor in the plasma membrane (25, 50). As is the case for uPAR, suPAR is known to associate with αv, β1, β2, and β3 integrins (35, 54, 60, 61). In addition, association between suPAR and β3 integrins can be effectively blocked by RGDfv (54). These previous findings raise the possibility that suPAR can interfere with binding on the surface of macrophages between integrins, such as αvβ3, and extracellular ligands including MFG-E8 or TSP-1. Of note, our experiments found that exposure of apoptotic cells to suPAR had minimal or no effect on efferocytosis (supplemental Figure s1). However, because suPAR can bind to vitronectin, such interactions between vitronectin and suPAR are likely to prevent binding of vitronectin to uPAR on the apoptotic cell, thereby blocking the ability of vitronectin to inhibit efferocytosis, a finding confirmed in our experiments.
The specific mechanisms by which interactions between vitronectin, uPAR, and integrins affect efferocytosis have not been completely elucidated. The SMB domain of vitronectin has been shown to bind to the D1 domain and D1-D2 linker region of uPAR and such interactions may affect the association of uPAR with integrins through the uPAR D2 and/or D3 domains (30, 62-64).
Our findings reveal a novel mechanism by which vitronectin can inhibit efferocytosis and potentially affect the resolution of neutrophil associated inflammatory processes, such as those that occur in acute lung injury (Figure 7). Severe sepsis, hemorrhage, burns, and ventilator induced lung injury are associated with increased tissue levels of vitronectin, including in the pulmonary interstitium and alveolar space (36, 37). Of note, previous studies demonstrated that the severity of LPS-induced acute lung injury is diminished in vitronectin deficient (vtn−/−) mice (37). Our present results show that deficiency of vitronectin also is associated with diminished clearance of apoptotic neutrophils in the lungs of mice with LPS induced lung injury. In addition, pre-incubation of apoptotic cells with vitronectin diminished their uptake by alveolar macrophages after instillation into the lungs. These findings suggest that therapeutic approaches that inhibit binding between vitronectin with uPAR on apoptotic neutrophils should increase efferocytosis and therefore may diminish the severity of acute lung injury and other inflammatory processes in which neutrophils play a major role.
Figure 7.
The SMB domain and RGD motif of vitronectin inhibit efferocytosis through selective binding to integrins on macrophages and by binding to uPAR on apoptotic cells.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL76206 to Edward Abraham and GM87748, HL107585 to Jaroslaw Zmijewski.
References
- 1.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 2.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 3.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, Berdyshev EV, Birukov KG, Lee CH, Tuder RM, Twigg H. L. r., Vandivier RW, Petrache I. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem. 2010;285:40322–40332. doi: 10.1074/jbc.M110.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Friggeri A, Banerjee S, Bdeir K, Cines DB, Liu G, Abraham E. Urokinase-type plasminogen activator inhibits efferocytosis of neutrophils. Am J Respir Crit Care Med. 2010;182:1516–1523. doi: 10.1164/rccm.201003-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 8.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol. 1999;162:6800–6810. [PubMed] [Google Scholar]
- 10.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel RA, Krahling S, Callahan MK, Williamson P. CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes. Cell Death Differ. 1999;6:583–592. doi: 10.1038/sj.cdd.4400529. [DOI] [PubMed] [Google Scholar]
- 14.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 15.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, Clauss M. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci U S A. 1998;95:12322–12327. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauber K, Bohn E, Kröber SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 19.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman SL, Bucher C, Rhodes J, Bullock WE. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990;85:223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulahian TH, Imrich A, Deloid G, Winkler AR, Kobzik L. Signaling pathways required for macrophage scavenger receptor-mediated phagocytosis: analysis by scanning cytometry. Respir Res. 2008;9:59. doi: 10.1186/1465-9921-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khandani A, Eng E, Jongstra-Bilen J, Schreiber AD, Douda D, Samavarchi-Tehrani P, Harrison RE. Microtubules regulate PI-3K activity and recruitment to the phagocytic cup during Fcgamma receptor-mediated phagocytosis in nonelicited macrophages. J Leukoc Biol. 2007;82:417–428. doi: 10.1189/jlb.0706469. [DOI] [PubMed] [Google Scholar]
- 25.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 26.Preissner KT. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- 27.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10:541–544. doi: 10.1038/nsb943. [DOI] [PubMed] [Google Scholar]
- 30.Huai Q, Zhou A, Lin L, Mazar AP, Parry GC, Callahan J, Shaw DE, Furie B, Furie BC, Huang M. Crystal structures of two human vitronectin, urokinase and urokinase receptor complexes. Nat Struct Mol Biol. 2008;15:422–423. doi: 10.1038/nsmb.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preissner KT, Jenne D. Vitronectin: a new molecular connection in haemostasis. Thromb Haemost. 1991;66:189–194. [PubMed] [Google Scholar]
- 32.Preissner KT, Jenne D. Structure of vitronectin and its biological role in haemostasis. Thromb Haemost. 1991;66:123–132. [PubMed] [Google Scholar]
- 33.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 34.Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci U S A. 2008;105:11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park YJ, Liu G, Tsuruta Y, Lorne E, Abraham E. Participation of the urokinase receptor in neutrophil efferocytosis. Blood. 2009;114:860–870. doi: 10.1182/blood-2008-12-193524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh B, Janardhan KS, Kanthan R. Expression of angiostatin, integrin alphavbeta3, and vitronectin in human lungs in sepsis. Exp Lung Res. 2005;31:771–782. doi: 10.1080/01902140500324901. [DOI] [PubMed] [Google Scholar]
- 37.Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol. 2007;179:7079–7086. doi: 10.4049/jimmunol.179.10.7079. [DOI] [PubMed] [Google Scholar]
- 38.Schar CR, Blouse GE, Minor KH, Peterson CB. A deletion mutant of vitronectin lacking the somatomedin B domain exhibits residual plasminogen activator inhibitor-1-binding activity. J Biol Chem. 2008;283:10297–10309. doi: 10.1074/jbc.M708017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson LC, Goswami S, Ginsberg DS, Day DE, Verhamme IM, Peterson CB. Metals affect the structure and activity of human plasminogen activator inhibitor-1. I. Modulation of stability and protease inhibition. Protein Sci. 20:353–365. doi: 10.1002/pro.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med. 2008;178:168–179. doi: 10.1164/rccm.200710-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med. 2009;179:694–704. doi: 10.1164/rccm.200806-851OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 2003;171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 43.Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, Abraham E. Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol. 2011;186:6191–6198. doi: 10.4049/jimmunol.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol. 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- 45.Brass DM, Hollingsworth JW, McElvania-Tekippe E, Garantziotis S, Hossain I, Schwartz DA. CD14 is an essential mediator of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L77–83. doi: 10.1152/ajplung.00282.2006. [DOI] [PubMed] [Google Scholar]
- 46.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 47.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 49.Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 51.Reuning U, Magdolen V, Hapke S, Schmitt M. Molecular and functional interdependence of the urokinase-type plasminogen activator system with integrins. Biol Chem. 2003;384:1119–1131. doi: 10.1515/BC.2003.125. [DOI] [PubMed] [Google Scholar]
- 52.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterization of the surface of apoptotic neutrophils: implications for functional downregulation and recognition by phagocytes. Cell Death Differ. 2000;7:493–503. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 53.Lademann UA, Rømer MU. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1) Thromb Haemost. 2008;100:1041–1046. [PubMed] [Google Scholar]
- 54.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’mello V, Singh S, Wu Y, Birge RB. The urokinase plasminogen activator receptor promotes efferocytosis of apoptotic cells. J Biol Chem. 2009;284:17030–17038. doi: 10.1074/jbc.M109.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krispin A, Bledi Y, Atallah M, Trahtemberg U, Verbovetski I, Nahari E, Zelig O, Linial M, Mevorach D. Apoptotic cell thrombospondin-1 and heparin-binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood. 2006;108:3580–3589. doi: 10.1182/blood-2006-03-013334. [DOI] [PubMed] [Google Scholar]
- 57.Ren Y, Stuart L, Lindberg FP, Rosenkranz AR, Chen Y, Mayadas TN, Savill J. Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. J Immunol. 2001;166:4743–4750. doi: 10.4049/jimmunol.166.7.4743. [DOI] [PubMed] [Google Scholar]
- 58.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillig T, Engelholm LH, Ingvarsen S, Madsen DH, Gårdsvoll H, Larsen JK, Ploug M, Danø K, Kjøller L, Behrendt N. A composite role of vitronectin and urokinase in the modulation of cell morphology upon expression of the urokinase receptor. J Biol Chem. 2008;283:15217–15223. doi: 10.1074/jbc.C700214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.May AE, Kanse SM, Lund LR, Gisler RH, Imhof BA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 62.Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280:24792–24803. doi: 10.1074/jbc.M413954200. [DOI] [PubMed] [Google Scholar]
- 63.Chaurasia P, Aguirre-Ghiso JA, Liang OD, Gardsvoll H, Ploug M, Ossowski L. A region in urokinase plasminogen receptor domain III controlling a functional association with alpha5beta1 integrin and tumor growth. J Biol Chem. 2006;281:14852–14863. doi: 10.1074/jbc.M512311200. [DOI] [PubMed] [Google Scholar]
- 64.Gårdsvoll H, Ploug M. Mapping of the vitronectin-binding site on the urokinase receptor: involvement of a coherent receptor interface consisting of residues from both domain I and the flanking interdomain linker region. J Biol Chem. 2007;282:13561–13572. doi: 10.1074/jbc.M610184200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.