Abstract
OBJECTIVE
We determined the clinical impact and developmental changes of auditory-language-related augmentation of gamma activity at 50–120 Hz recorded on electrocorticography (ECoG).
METHODS
We analyzed data from 77 epileptic patients ranging 4 – 56 years in age. We determined the effects of seizure-onset zone, electrode location, and patient-age upon gamma-augmentation elicited by an auditory-naming task.
RESULTS
Gamma-augmentation was less frequently elicited within seizure-onset sites compared to other sites. Regardless of age, gamma-augmentation most often involved the 80–100 Hz frequency band. Gamma-augmentation initially involved bilateral superior-temporal regions, followed by left-side dominant involvement in the middle-temporal, medial-temporal, inferior-frontal, dorsolateral-premotor, and medial-frontal regions and concluded with bilateral inferior-Rolandic involvement. Compared to younger patients, those older than 10 years had a larger proportion of left dorsolateral-premotor and right inferior-frontal sites showing gamma-augmentation. The incidence of a post-operative language deficit requiring speech therapy was predicted by the number of resected sites with gamma-augmentation in the superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions of the left hemisphere assumed to contain essential language function (r2=0.59; p=0.001; odds ratio=6.04 [95% confidence-interval: 2.26 to 16.15]).
CONCLUSIONS
Auditory-language-related gamma-augmentation can provide additional information useful to localize the primary language areas.
SIGNIFICANCE
These results derived from a large sample of patients support the utility of auditory-language-related gamma-augmentation in presurgical evaluation.
Keywords: Epilepsy surgery, Intracranial EEG recording, Event-related synchronization, Ripples, High-frequency oscillations (HFOs), Oncogenicity, Outcome
1. INTRODUCTION
Primary or essential language areas are ultimately defined as brain regions of which resection results in impairment in language function (Geschwind, 1972). Identification of such areas is required in the presurgical evaluation of epileptic patients who undergo epilepsy surgery. Substantial evidence derived from lesion and electrical stimulation studies of humans suggests that the left superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions should generally contain sites essential for acoustic analysis followed by language comprehension, production and articulation with some degree of inter-subject spatial variability (Broca, 1861; Wernicke, 1911; Geschwind, 1972; Penfield and Boldrey, 1937; Lesser et al., 1986; Ojemann et al., 1989; Dronkers et al., 2004; Hickok and Poeppel, 2007). Neuroimaging studies of healthy individuals using functional MRI (fMRI) also showed that the aforementioned four regions of interest are activated during auditory-language tasks and that such activations are generally more extensive and intensive in the left hemisphere, irrespective of handedness (Pujol et al., 1999). Thus, neocortical resection involving the left hemisphere poses a greater risk of postoperative language deficits compared to surgery involving the right, unless essential language function has been reorganized to the right hemisphere. It has been widely considered that left-handed patients with left-sided seizure foci and early-onset left-sided neocortical lesions are most likely to have right-hemispheric language dominance (Rasmussen and Milner, 1977; Akanuma et al., 2003; Möddel et al., 2009).
Electrical stimulation currently serves as the clinical gold standard to estimate the location of primary language areas (Ojemann et al., 1989), but carries several limitations. Cortical stimulation via implanted subdural electrodes is a time-consuming procedure accompanied by a risk of after-discharges and electrically-induced seizures (Lee et al., 2010; Kojima et al., 2012). Trains of electrical stimuli could be simultaneously propagated to remote sites even without creating after-discharges and the testing of a given site may result in false positive detection of primary language cortex. For example, stimulation of left basal temporal sites simultaneously induced remote discharges involving the superior temporal gyrus along with transient language impairment; subsequent resection of the left basal temporal sites resulted in no language deficits (Ishitobi et al., 2000). Furthermore, electrical stimulation could fail to reliably elicit transient language impairment in some individuals, especially uncooperative or young patients (Schevon et al., 2007; Kojima et al., 2012).
Since the spatio-temporal characteristics of event-related gamma-augmentation recorded on intracranial ECoG were first reported at the end of the 20th century (Crone et al., 1998), a number of studies have demonstrated that language-related sites detected by gamma-augmentation on ECoG were co-localized with those defined by stimulation with high statistical significance (Sinai et al., 2005; Tanji et al., 2005; Towle et al., 2008; Wu et al., 2010; Miller et al., 2011; Kojima et al., 2012). Our recent study of 13 epileptic patients with left-hemispheric language dominance suggested by the Wada test showed that language-related gamma sites identified those suggested by stimulation, with sensitivity of 0.83–0.91 and specificity of 0.62–0.64 (Kojima et al., 2012). Surgically-inflicted cortical damage involving sites of significant gamma-augmentation in the superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions predicted the incidence of postoperative language deficits requiring speech therapy (Kojima et al., 2012). Thus, in the present study, we defined the summation of these four regions of interest as ‘canonical language regions’. Since populations in previous ECoG studies have typically not exceeded 15 patients, the utility of gamma-augmentation as a biomarker of underlying language function needs validation with a larger sample size.
In this study, we specifically addressed the following hypotheses: (i) A postoperative language deficit requiring speech therapy would be predicted by a logistic regression model incorporating gamma-augmentation measures in the ‘canonical language regions’ of the left hemisphere assumed to contain essential language function. Speech therapy is an expensive procedure, which inherently delays the return to home, school, or work. (ii) Auditory-language-related gamma-augmentation would be less frequently elicited in the seizure onset compared to non-seizure onset sites. (iii) Patient age would be correlated with the spectral frequency peak and the spatial pattern of gamma-augmentation. (iv) In addition to the ‘canonical language regions’, the middle-temporal, medial-temporal, and medial-frontal regions in both left and right hemispheres would show gamma-augmentation. Previous studies have suggested that these three regions might be involved in, to some extent, semantic/lexical processing (Mandonnet et al., 2007), verbal memory processing (Hamberger et al., 2010), and voluntary control over the initiation and suppression of articulation (Jürgens, 2002), respectively. Although, surgical excision of any of these three areas was not reported to be associated with acute/major postoperative dysphasia.
2. METHODS
2.1. Patients
This study has been approved by the Institutional Review Board at Wayne State University. The inclusion criteria consisted of: (i) patients with focal epilepsy who underwent extraoperative subdural ECoG recording as part of presurgical evaluation in Children’s Hospital of Michigan or Harper University Hospital in Detroit between January 2007 and May 2012; (ii) language mapping using measurement of gamma-augmentation elicited by an auditory-naming task (Brown et al., 2008; Kojima et al., 2012); and (iii) written informed consent obtained by patients or their guardians. The exclusion criterion was limited vocabulary of nouns (less than 60 nouns). This cohort study included a consecutive series of 77 English-speaking patients who satisfied these criteria (age range: 4–56 years; median age: 14 years) (Table 1).
Table 1.
| Demographic and Clinical Characteristics of the Patients | |
|---|---|
| Age - year | |
| Median | 14 |
| Range | 4 – 56 |
| Gender-number (%) | |
| Male-number (%) | 40 (51.9) |
| Female-number (%) | 37 (48.1) |
| Oral antiepileptic drugs * | |
| 1-number (%) | 22 (28.6) |
| 2-number (%) | 37 (48.1) |
| 3-number (%) | 16 (20.8) |
| 4-number (%) | 2 (2.6) |
| VCI score (60 patients) | |
| Mean | 84.5 |
| Standard deviation | 17.7 |
| VIQ score (6 patients) | |
| Mean | 71.7 |
| Standard deviation | 10.5 |
| PPVT score (47 patients) | |
| Mean | 82.6 |
| Standard deviation | 18.7 |
| CELF score (23 patients) | |
| Mean | 75.5 |
| Standard deviation | 23.1 |
| Handedness | |
| Right-number (%) | 62 (80.5) |
| Left-number (%) | 14 (18.2) |
| Ambidextrous-number (%) | 1 (1.3) |
| Subdural electrodes | |
| Mean | 107.2 |
| Standard deviation | 24.4 |
| Number of analyzed trials | |
| Mean | 81.5 |
| Standard deviation | 28.9 |
The following neuropsychological measures were obtained preoperatively.
VCI: Verbal Comprehension Index. VIQ: Verbal IQ. PPVT: Peabody Picture Vocabulary Test. CELF: Clinical Evaluation of Language Fundamentals.
The number of patients taking each oral antiepileptic drug is listed as follows. Oxcarbazepine: 41, Levetiracetam: 31, Lamotrigine: 19, Lacosamide: 14, Valproic acid: 11, Topiramate: 9, Carbamazepine: 9, Phenytoin: 6, Zonisamide: 3, Clobazam: 3, Ethosuximide: 1, Vigabatrin: 1, Clonazepam:
2.2. Definition of anatomical regions of interest
The anatomical regions of interest included: the superior-temporal region (Brodmann Area [BA] 22/41/42), inferior-frontal region (inferior frontal gyrus involving BA 44/45), dorsolateral-premotor region (dorsolateral portion of BA 6), and inferior-Rolandic region (BA 4/3/1/2 not more than 4 cm superior from the sylvian fissure; Haseeb et al., 2007; Fukuda et al., 2008; Figure 1). The summation of these four regions was referred to here as ‘canonical language regions’. The present study will determine whether gamma-augmentation in ‘canonical language regions’ could predict postoperative language deficits. In addition, the present study will describe the spatial-temporal characteristics of less-frequently studied regions: the middle-temporal region (defined as the middle temporal gyrus involving BA 21/37), the medial-temporal region (defined as BA 27/28/34/35/36) and the medial-frontal region (medial portion of BA 6/8 and posterior portion of BA 24/32/33), bilaterally (Brodmann, 1909; Mitelman et al., 2003; Figure 1). Regions outside of the aforementioned seven regions were collectively defined as ‘other’, in each hemisphere (Figures 1 and 2).
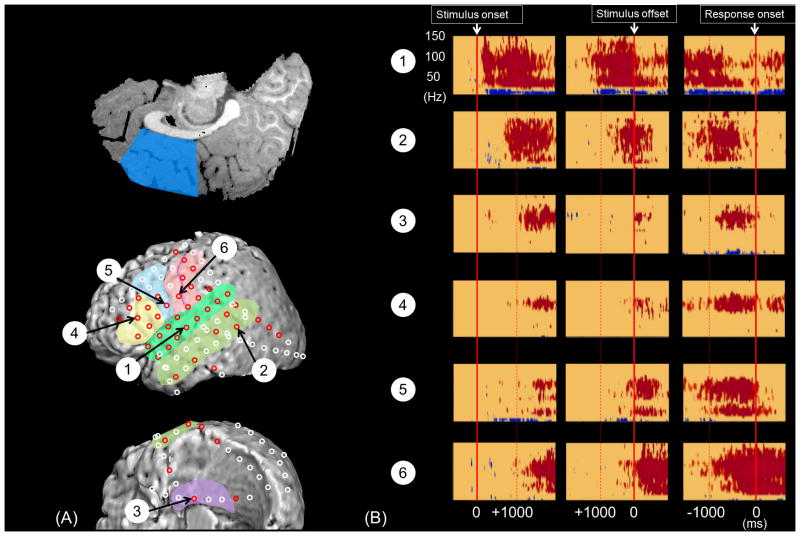
Figure 1. Locations of subdural electrodes and auditory-language-related gamma-augmentation.
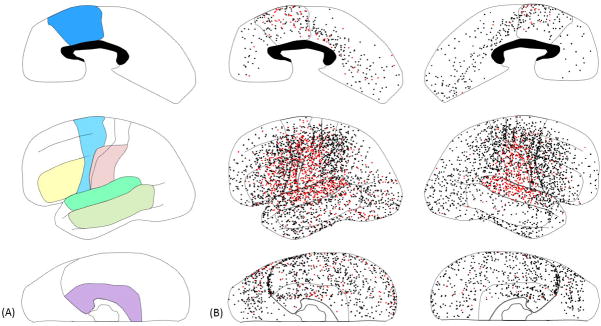
(A) Regions of interest were color-coded as follows. Green: Superior-temporal region (superior-temporal gyrus involving BA 22/41/42). Yellow: Inferior-frontal region (inferior-frontal gyrus involving BA 44/45). Light-blue: Dorsolateral-premotor region (dorsolateral portion of BA 6). Pink: Inferior-Rolandic region (BA 4/3/1/2 not more than 4 cm superior from the sylvian fissure). These four regions of interest were analyzed in our previous study of 13 patients with left-hemispheric language dominance on Wada test (Kojima et al., 2012). Light-green: Middle-temporal region (middle-temporal gyrus involving BA 21/37). Purple: Medial-temporal region (parahippocampal gyrus, hippocampus, and uncus involving BA 27/28/34/35/36). Blue: Medial-frontal region (medial portion of superior-frontal and anterior-cingulate gyri involving the posterior portion of BA 24/32/33). White: ‘other’ regions. The ‘canonical language regions’ were defined as a summation of superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions in the present study. (B) Subdural electrodes on each region of interest in the individual brain surface image are superimposed on the given region of interest of this schematic illustration. Red dots indicate the sites showing significant gamma-augmentation (i.e.: ‘language-related gamma sites’). Black dots indicate all remaining sites analyzed. Seizure-onset sites are not shown in this figure. The presented data are derived from 73 patients who were assumed to have essential language function remaining in the left hemisphere.
Figure 2. The effects of seizure onset and sampling location on auditory-language-related gamma-augmentation.
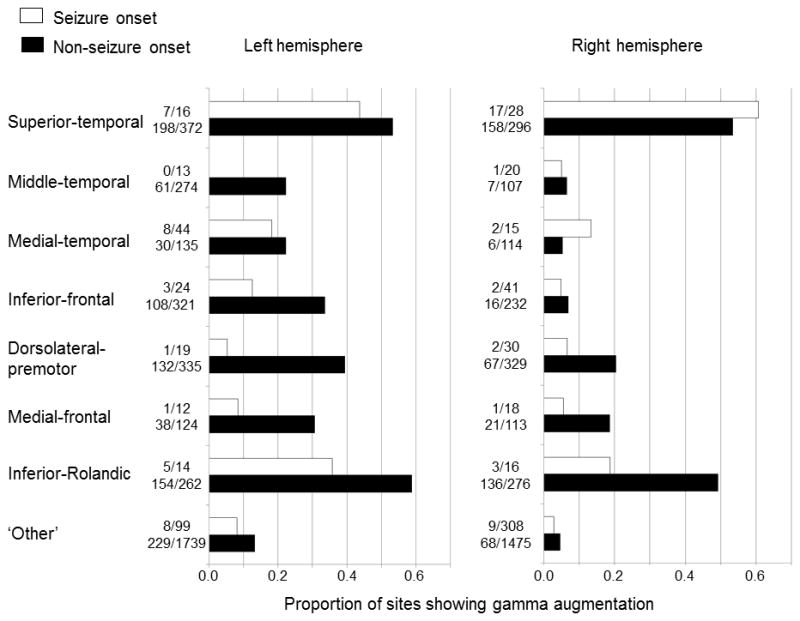
The proportion of sites showing significant gamma-augmentation was calculated in 14 regions of interest plus 2 ‘other’ regions. The Wilcoxon signed rank test compared such a proportion in a total of 16 regions between seizure onset and non-seizure onset sites and found that the proportion of sites showing significant gamma-augmentation was smaller in the seizure onset sites across regions (p=0.004). The Fisher’s exact probability test was repeated eight times for each region of interest and suggested that significant gamma-augmentation was more frequently noted in the left compared to the right hemisphere (FDR-corrected p<0.05) except for the superior-temporal region (FDR-corrected p>0.05). The presented data are derived from 73 patients who were assumed to have essential language function remaining in the left hemisphere.
2.3. Preoperative estimation of reorganization of essential language function to the right hemisphere
Based on handedness and neuroimaging data obtained during Phase-I presurgical evaluation (Asano et al., 2009a), the language-dominant hemisphere was assumed for each patient; namely, left-handed patients with left-sided seizure focus and early-onset left-sided neocortical lesions (defined as either congenital or perinatal lesions such as dysplasia, encephalomalacia and porencephaly) were assumed to have right-hemispheric language dominance (Rasmussen and Milner, 1977; Akanuma et al., 2003; Möddel et al., 2009). All remaining patients with left-sided seizure focus were assumed to have either left-sided language dominance or bilateral language representation regardless of handedness; thus, the surgical hemisphere was assumed to still contain essential language cortex. Those with a right-sided seizure focus were assumed to have left-sided language dominance, regardless of handedness. We recognize that there is no ideal gold-standard to determine the language dominant hemisphere. The majority of young children with epilepsy cannot complete the Wada test, fMRI, or magnetoencephalography (MEG) in a cooperative fashion. The ultimate goal of this study is to determine whether and how well a new language deficit requiring postoperative speech therapy was predicted by a logistic regression model not incorporating the results of the Wada test, fMRI, or MEG.
2.4. Subdural electrode placement
Platinum macro-electrodes (intercontact distance: 10 mm; median: 112 electrodes per patient [standard deviation: 24.4]) were surgically placed in the subdural space over left, right, or bilateral cortical regions. The number of implanted electrodes was less than 50 in 3 patients in which the extent of surgical resection was expected to be small preoperatively. Placement of intracranial electrodes was guided by the results of Phase-I presurgical evaluation including: scalp video-EEG recording, MRI, and 2-deoxy-2-[18F] fluoro-D-glucose (FDG) positron emission tomography (PET) (Asano et al., 2009a). All electrode plates were stitched to adjacent plates or the edge of dura mater, to avoid movement of subdural electrodes after intracranial implantation. In all patients, intraoperative photographs were taken with a digital camera before dural closure as well as after re-opening during the second stage of surgery. All electrodes were displayed on the three-dimensional brain surface reconstructed from high-resolution MRI, as previously described in detail (Alkonyi et al., 2009; Wu et al, 2011). We confirmed the spatial accuracy of electrode display on the three-dimensional brain surface by using intraoperative digital photographs (Wellmer et al., 2002; Dalal et al., 2008).
2.5. Extraoperative video-ECoG recording
ECoG signals were obtained for 3–5 days with a sampling rate of 1,000 Hz, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Band-pass: 0.08–300 Hz; Nihon Kohden America Inc, Foothill Ranch, CA, USA). The averaged voltage of ECoG signals derived from the fifth and sixth intracranial electrodes on the amplifier was used as the original reference; ECoG signals were then re-montaged to a common average reference. Channels contaminated with large interictal epileptiform discharges or artifacts were visually identified and excluded from the average (Laufs et al., 2006), in order to minimize their influence on the results. Usage of a common average reference is a widely-accepted practice in assessment of event-related gamma-augmentation recorded on subdural grid electrodes; its advantages and limitations were previously discussed (Crone et al., 2001; Asano et al., 2009b; Nagasawa et al., 2011; Kojima et al., 2012). Surface electromyography electrodes were placed on the left and right deltoid muscles, and electrooculography electrodes were placed 2.5 cm below and 2.5 cm lateral to the left and right outer canthi. ECoG traces were visually inspected with a low-frequency filter at 53 Hz and a sensitivity of 20 μV/mm; thereby, irregular broadband signals synchronized with facial and ocular muscle activities seen on electrooculography electrodes were treated as artifacts (Otsubo et al., 2008; Jerbi et al., 2009a; Kovach et al., 2011; Kojima et al., 2012). Seizure onset sites were clinically determined (Asano et al., 2009a).
2.6. Language mapping using gamma-augmentation
While awake and comfortably seated with intracranial electrodes in place, each patient submitted to an auditory-naming task (Brown et al, 2012; Kojima et al., 2012). Patients received a series of question-and-answer trials. Question stimuli ranged from 1-to 2.5-s in duration. Patients were instructed to overtly verbalize a one- or two-word answer (e.g.: ‘Ears’) to a given auditory question (e.g.: ‘What do you hear with?). Source analysis was not conducted in the present study. Each ECoG trial was transformed into the time-frequency domain using complex demodulation (Papp and Ktonas, 1977) via BESA® software (BESA GmbH, Gräfelfing, Germany; Hoechstetter et al., 2004). The ECoG signal at each channel was assigned an amplitude (a measure proportional to the square root of power) as a function of time and frequency (in steps of 10 ms and 5 Hz). The time-frequency transform was obtained by multiplication of the time-domain signal with a complex exponential, followed by a band-pass filter. The band-pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. The filter had a full width at half maximum of 2 × 15.8 msec in the temporal domain and 2 × 7.1 Hz in the frequency domain. The corresponding time-frequency resolution was ±15.8 msec and ±7.1 Hz (defined as the 50% power drop of the finite impulse response filter). ECoG traces were aligned to: (i) stimulus (question) onset; (ii) stimulus offset; and (iii) response (answer) onset. We determined ‘when,’ ‘where,’ and ‘how much’ gamma activity at 50–120 Hz averaged across trials (Kojima et al., 2012) were augmented compared to the resting periods. Further methodological details (such as the reference periods) are described in Figure S1 on the website. We also determined whether the degree of such gamma-augmentation reached significance using studentized bootstrap statistics followed by Simes’ correction (Brown et al., 2008; Koga et al., 2011). Sites surviving correction showing significant gamma-augmentation spanning (i) at least 20-Hz in width and (ii) at least 20-msec in duration (Kojima et al., 2012) were defined as ‘language-related gamma sites’. We previously discussed the advantage and limitation of this analytic approach (Wu et al., 2011; Brown et al., 2012). We previously reported that the sites showing significant event-related gamma-augmentation agreed with the sensory and motor symptoms elicited by electrical stimulation (Fukuda et al., 2008; Nagasawa et al., 2010a; 2010b). We also reported that ‘language-related gamma sites’ determined by this procedure predicted postoperative language deficits requiring speech therapy in 13 epileptic patients with left-hemispheric language dominance suggested by the Wada test (Kojima et al., 2012).
2.7. Language mapping using electrical stimulation
Following the above-mentioned auditory-language task, language mapping by cortical stimulation was performed (Kojima et al., 2012). Patients and Clinical Neuropsychologists (R.R. and D.F.) were blinded to, but epileptologists (A.S., M.A., M.B. and E.A.) were aware of, the results of time-frequency analyses prior to electrical stimulation. A pulse-train of repetitive electrical stimuli was delivered to subdural electrode pairs, using the Grass constant-current stimulator (Astro-Med, Inc, West Warwick, RI, USA). The stimulus frequency was 50 Hz, the pulse duration was 300 μsec, and the train duration ranged from 5 to 10 sec. Initially, stimulus intensity was set to 3 mA, and stimulus intensity was increased up to 9 mA in a stepwise manner until a clinical response or after-discharge was observed. We rarely increased the stimulus intensity above 9 mA, since such stimulation frequently elicits after-discharges. During each period of stimulation, each patient was asked to answer brief auditory questions similar to those used for ECoG mapping. Other tasks such as picture naming, counting, and reciting ABC’s were also performed. Sites at which stimulation reproducibly resulted in auditory perceptual changes, failure to verbalize correct responses, or sensorimotor symptoms involving the mouth or throat were determined by at least two investigators, always including at least one Clinical Neuropsychologist, and defined as ‘language-related stimulation sites’ (Kojima et al., 2012). It is plausible to assume that auditory perceptual changes induced by stimulation would reflect the cortical function of auditory analysis and stimulation-induced failure to verbalize correct responses would reflect the cortical functions necessary for naming; stimulation-induced sensorimotor symptoms involving the mouth or throat would reflect the cortical function involved in articulation. Sites were declared ‘not proven to be language-related’ if after-discharges were elicited without a clinical symptom, clinical responses failed to be elicited by maximal stimulation, or baseline language performance between electrical stimuli was too poor to clearly distinguish inducible language deficits.
2.8. Assessment of the effect of seizure onset zone on auditory-language-related gamma-augmentation
Here, we empirically determined whether significant gamma-augmentation would be less frequently elicited in the seizure onset compared to the non-seizure onset sites. Initially, we compared the proportion of seizure onset sites showing significant gamma-augmentation with that of non-seizure onset sites using the chi-square test. Subsequently, we determined the effect of clinical classification as ‘seizure onset’ on the proportion of significant gamma-augmentation, after controlling for electrode site locations. In each region of interest (e.g.: in the left superior-temporal region; Figure 1), we separately calculated the proportion of seizure onset and non-seizure onset sites showing significant gamma-augmentation. Then, we determined whether the median proportion of sites showing significant gamma-augmentation across all regions of interest differed between the seizure onset and non-seizure onset sites (Wilcoxon signed rank test; Figure 2).
2.9. Assessment of the effect of age on the spectral frequency of auditory-language-related gamma-augmentation
We determined whether age would correlate with the spectral frequency of peak gamma-augmentation. Initially, the spectral frequency band showing the maximal augmentation was determined on each electrode site showing significant gamma-augmentation. Subsequently, the median peak frequency band was determined among electrode sites in each region of interest for each patient. Coefficients of correlation between age and median peak frequency band were measured for all regions of interest, using the Spearman’s rank test. Finally, we determined the effect of age on peak spectral frequency of gamma-augmentation across all regions of interest (one-sample t-test). Statistical analysis was conducted by excluding seizure onset sites in order to control for the potential effects of the seizure onset zone on the spectral characteristics of gamma-augmentations.
2.10. Assessment of the effect of age on the spatial pattern of auditory-language-related gamma-augmentation
We determined which regions of interest would show a different proportion of significant gamma-augmentation between patients above 10 years and those younger (chi-square test). Statistical analysis was conducted while excluding seizure onset sites. A previous study of epileptic patients showed that electrical stimulation was insensitive to language-related sites in patients of 10 years and younger (Schevon et al., 2007). Behavioral studies of healthy children reported age-related linear increase in performance of verbal working memory and phonological processing between 6 and 10 years of age (Gaulin and Campbell, 1994; Korkman et al., 2001).
2.11. Delineation of the temporal characteristics of auditory-language-related gamma-augmentation
Initially, we determined the moment of peak gamma-augmentation in each electrode site, and plotted the temporal profile of gamma-augmentation in each region of interest in each hemisphere. We then determined whether gamma-augmentations in the middle-temporal, medial-temporal, inferior-frontal, dorsolateral-premotor, and medial-frontal regions occurred following that of the superior-temporal region but before that of the inferior-Rolandic region. Analysis was conducted by excluding seizure onset sites.
2.12. Prediction of a new language deficit requiring postoperative speech therapy
The extent of cortical resection was determined after the team had extensive discussion with the patient or the legal guardian(s), regarding the risks and benefits of surgical resection of eloquent areas including ‘language-related stimulation sites’ (Asano et al., 2009a). The results of language mapping using gamma-augmentation were not used for surgical decision-making in this study period; each ‘language-related gamma site’ was considered to be involved but not necessarily essential for language function unless cortical stimulation proved it to be essential (Crone et al., 2006).
We determined whether the language outcome would be predicted by a logistic regression model incorporating the extent of resection of ‘language-related gamma sites’ but not incorporating the results of Wada testing or electrical stimulation. The outcome measure of interest was a categorical variable for the occurrence of a post-operative language deficit which was not explained by the effects of medications or altered consciousness and which required speech therapy. Disturbance in verbal comprehension, naming, repetition, articulation or fluency was evaluated by the speech therapist as well as the family member or legal guardian of the patient. In this study, we specifically determined whether the language outcome was predicted by the number of resected ‘language-related gamma sites’ in the ‘canonical language regions’ on the left hemisphere assumably containing essential language function. The present study was not designed to correlate the predictor measures with the postoperative neuropsychological measures, since neuropsychological data were not systematically collected before and after surgery.
Even if the univariate logistic regression model incorporating ‘language-related gamma sites’ turned out to predict the language outcome with statistical significance, this observation does not rule out the possibility that its prediction performance was simply attributed to the extent of resection in the aforementioned regions of interest. Also, it does not suggest that ECoG provides an improvement over electrical stimulation data. Furthermore, a previous study using electrical stimulation suggested that removal of hippocampus in the dominant hemisphere was a predictor of postoperative language declines detected by neuropsychological assessment (Hamberger et al., 2010). Therefore, we determined whether ECoG’s prediction performance remained significant even if the extent of resection, electrical stimulation or such hippocampal resection was incorporated into the logistic regression model at each time. In addition to the p-value, the r2 value was also used to estimate the prediction performance of each logistic regression model. The extent of resection was calculated using the number of resected electrode sites, which is readily available prior to dural closure (Jacobs et al., 2010; Akiyama et al., 2011; van’t Klooster et al., 2011). All statistical tests were two-tailed with a significance threshold of 0.05.
3. RESULTS
3.1. Preoperative estimation of language dominant hemisphere
Four left-handed children had early-onset neocortical lesions (cortical dysplasia with or without encephalomalacia) in the left-hemisphere preoperatively suggested on neuroimaging. According to the definition described earlier, these four children were assumed to have essential language function reorganized to the right hemisphere in this study. None of these four patients underwent a Wada test.
The remaining 73 patients were assumed to have essential language function represented in the left hemisphere or in both hemispheres. Thirty-one patients underwent a Wada test (Shah et al., 2010; Kojima et al., 2012). Left-hemispheric language dominance was suggested in 26 patients and bilateral language representation was suggested in 2 patients. The language-dominant hemisphere failed to be satisfactorily evaluated in the remaining three patients, due to a lack of sufficient hemiparesis induced by the maximal dose of sodium amobarbital (2 mg/kg; maximum 125 mg per side); these patients were, therefore, treated as the others who went without Wada testing.
3.2. The effect of seizure onset zone on auditory-language-related gamma-augmentation
Significant gamma-augmentation was elicited in 70 out of 740 analyzed seizure onset sites and in 1485 out of 6910 remaining sites in all 77 patients. In 73 patients with essential language function assumed to remain in the left hemisphere, gamma-augmentation was elicited in 70 out of 717 analyzed seizure onset sites and in 1429 out of 6504 remaining sites (9.8% vs 22.0%). The chi-square test suggested that gamma-augmentation was less frequently elicited in the seizure onset compared to the non-seizure onset sites (p<0.0001). We subsequently considered the proportion of sites showing gamma-augmentation in each region of interest in the same 73 patients (Figure 2). The Wilcoxon signed rank test, when employed across the regions of interest, suggested that the median proportion of sites showing gamma-augmentation was smaller in the seizure onset sites (n=16 regions of interest; p=0.004). Taken together, the seizure onset sites were found to elicit gamma-augmentation less frequently compared to non-seizure onset sites.
3.3. The spatial characteristics of auditory-language-related gamma-augmentation
Figure 2 shows the proportion of sites showing significant gamma-augmentation in each region of interest across the 73 patients with essential language function assumed to remain in the left hemisphere. Gamma-augmentation was elicited at 49–59% of the superior-temporal and inferior-Rolandic sites, bilaterally. Conversely, gamma-augmentation was elicited at about 22–39% of the middle-temporal, medial-temporal, inferior-frontal, dorsolateral-premotor, and medial-frontal sites on the left hemisphere and at only 5–20% of those regions on the right hemisphere. We also compared the proportion of sites showing gamma-augmentation between the hemispheres (Figure 2). The Fisher’s exact probability test suggested that gamma-augmentation was elicited more frequently in the left compared to the right hemisphere (false discovery rate [FDR]-corrected p-value<0.05) except for the superior temporal regions (FDR-corrected p>0.05).
3.4. Effect of age on the spatial pattern of auditory-language-related gamma-augmentation
Out of 73 patients with essential language function assumed to remain in the left hemisphere, 20 patients were 10 years old or younger and the remaining 53 were older than 10 years (Figure S2 on the website). The Fisher’s exact probability test was employed to 16 regions of interest, and revealed that gamma-augmentation in the left dorsolateral-premotor, right inferior-frontal, and left ‘other’ regions (Table 2; Figures 1 and 2) was more frequently noted in patients above 10 years old compared to those younger (FDR-corrected p<0.05; Figure 3). These results cannot be simply attributed to the difference in the number of analyzed trials between the two age groups, since there was no detectable difference (mean trial number: 80.9 in the younger group and 81.9 in the older group; p=0.90 on t-test). Likewise, there was no difference in verbal comprehension index between the two age groups (mean: 83.8 in the younger group and 85.0 in the older group; p=0.79).
Table 2.
Spectral frequency of auditory-language-related gamma-augmentation
| Left hemisphere | Right hemisphere | |||
|---|---|---|---|---|
| Region of interest | Number of patients | Median of peak frequency bands (95%CI) | Number of patients | Median of peak frequency bands (95%CI) |
| Superior-temporal | 40 | 95.0 Hz (90.9 to 99.1) | 29 | 100.0 Hz (95.0 to 100.5) |
| Middle-temporal | 20 | 90.0 (79.3 to 100.7) | 3 | 95.0 (58.8 to 131.2) |
| Medial-temporal | 12 | 87.5 (74.9 to 100.1) | 3 | 80.0 (46.7 to 113.3) |
| Inferior-frontal | 26 | 95.0 (88.2 to 101.8) | 7 | 90.0 (55.6 to 124.4) |
| Dorsolateral-premotor | 31 | 90.0 (85.2 to 94.8) | 26 | 97.5 (92.5 to 102.5) |
| Medial-frontal | 17 | 87.0 (78.1 to 95.9) | 11 | 82.5 (76.6 to 88.4) |
| Inferior-Rolandic | 36 | 90.0 (86.0 to 94.0) | 32 | 90.0 (84.4 to 95.6) |
| ‘Other’ regions | 27 | 90.0 (83.5 to 96.5) | 13 | 95.0 (80.6 to 109.4) |
The median frequency band of peak gamma-augmentations on each region of interest is presented. ‘Other’ regions were defined as the entire regions outside the aforementioned seven regions in each hemisphere. These data are derived from 73 patients with essential language function assumed to remain in the left hemisphere. The seizure onset sites were excluded.
Figure 3. The effect of patient age on the spatial pattern of auditory-language-related gamma-augmentation.
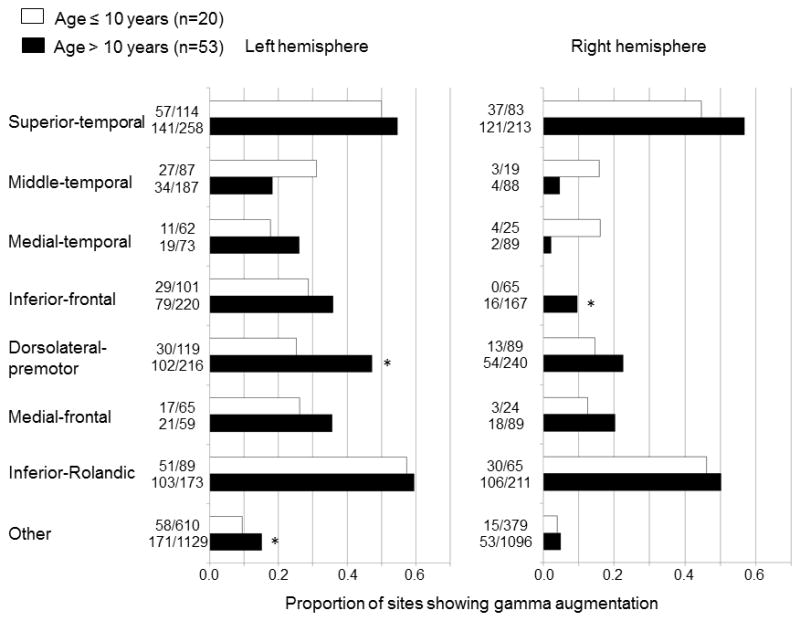
The proportion of sites showing significant gamma-augmentation was calculated in 14 regions of interest plus 2 ‘other’ regions in each age group (white bar: 10 years old or younger; black bar: over 10 years old). The Fisher’s exact probability test was repeated for 16 regions of interest in 73 patients with essential language function assumed to remain in the left hemisphere; the proportion of sites showing significant gamma-augmentation in the left dorsolateral-premotor, right inferior-frontal, and left ‘other’ regions was greater in the older age group (*: FDR-corrected p<0.05 on Fisher’s exact probability test). The distribution of sites showing gamma-augmentation on each age group is presented in Figure S2 on the website.
Patient age was positively correlated to the proportion of sites showing gamma-augmentation in the left dorsolateral-premotor region (rho=0.32; p=0.04 on Spearman’s rank test) and right inferior-frontal region (rho=0.62; p=0.001) but not to that in the left ‘other’ region (rho=0.12; p=0.41).
3.5. Effect of age on spectral frequency of auditory-language-related gamma-augmentation
Regardless of age or sampled hemisphere (Table 2), significant gamma-augmentation most commonly involved the 80–100 Hz frequency band, as reported in other investigators’ studies of mostly adult populations (Sinai et al., 2005; Tanji et al., 2005; Towle et al., 2008; Jerbi et al., 2009b; Flinker et al., 2010; McDonald et al., 2010; Wu et al., 2010; Conner et al., 2011; Miller et al., 2011). Figure 4 shows the relationship between patient age and the median frequency bands of peak gamma-augmentation elicited in the left inferior-Rolandic region. The Spearman’s rank test, employed to all regions of interest in the 73 patients with essential language function assumed to remain in the left hemisphere, failed to find an overall correlation between patient age and median frequency band of peak gamma-augmentation (mean rho-value across regions of interest: +0.06; 95% confidence interval [95%CI]: −0.07 to +0.19).
Figure 4. The effects of patient age and the peak frequency band of auditory-language-related gamma-augmentation in the left inferior-Rolandic region.
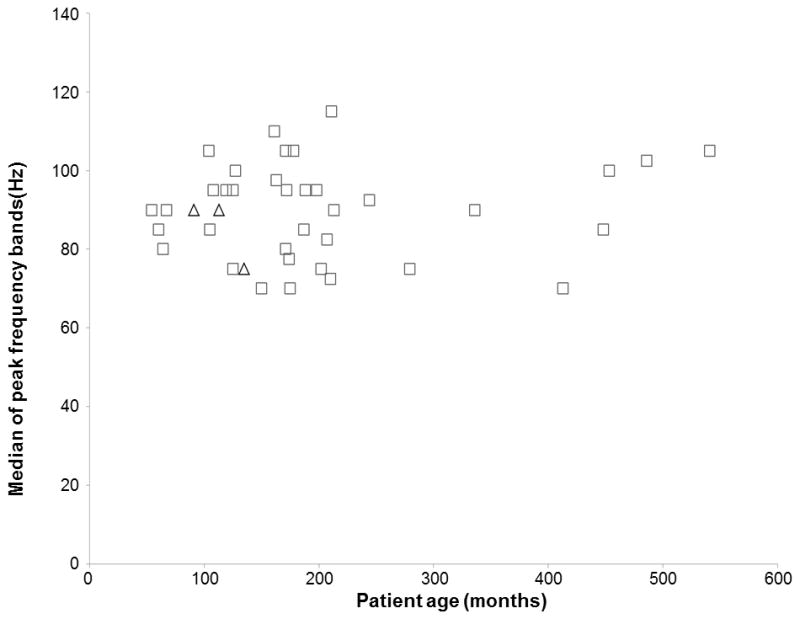
Each square indicates the median of peak frequency bands of gamma-augmentations in the left inferior-Rolandic region in each of patients assumed to have essential language function remaining in the left hemisphere. Each triangle indicates that of patients assumed to have essential language function reorganized to the right hemisphere. The median of peak frequency bands of gamma-augmentations in each region of interest is listed in Table 2.
3.6. Temporal characteristics of auditory-language-related gamma-augmentation
Figure 5 shows the temporal characteristics of gamma-amplitudes at 80–100 Hz in each region of interest of the left hemisphere of patients with essential language function assumed to remain in the left hemisphere. The detailed temporal profiles of gamma-augmentations at each region of interest on each hemisphere are presented in Tables S1–S3 on the website. In short, according to 95%CI of peak latencies, gamma-augmentation in both hemispheres initially involved the superior-temporal regions, subsequently the middle-temporal, medial-temporal, inferior-frontal, dorsolateral-premotor, and medial-frontal regions with considerable temporal overlap across regions, and finally involved the inferior-Rolandic regions.
Figure 5. The temporal characteristics of auditory-language-related gamma-augmentation in the left hemisphere.
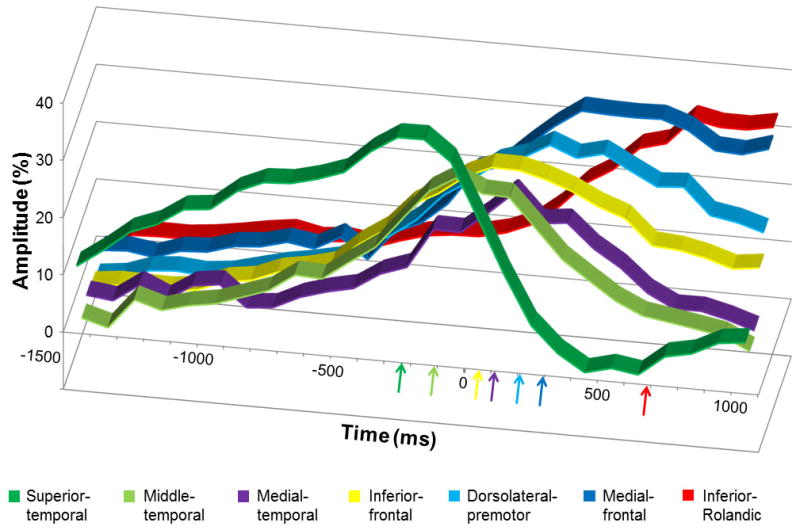
The X-axis shows the time in ms, and +/− 0 ms reflects the offset of question. The Y-axis shows the average percent change of gamma-amplitudes at 80–100 Hz compared to those during the reference period 3000 to 2600 msec prior to the offset of question. Gamma-augmentation reached the peak sequentially in the superior-temporal (−230 msec prior to the stimulus offset), middle-temporal (−110 msec), inferior-frontal (50 msec), medial-temporal (110 msec), dorsolateral-premotor (210 msec), medial-frontal (290 msec), and inferior-Rolandic regions (680 msec). The presented data are derived from patients with essential language function assumed to remain in the left hemisphere.
3.7. Prediction of language outcome
A total of 15 out of all 77 patients developed a post-operative language deficit requiring speech therapy following resection of the presumed epileptogenic zone. The incidence of such a deficit, the primary outcome measure of this study, was not associated with patient age at surgery (p=0.6 on the Mann-Whitney U test).
The univariate logistic regression model suggested that the language deficit was predicted by the number of resected ‘language-related gamma sites’ in the ‘canonical language regions’ in the left hemisphere assumed to contain essential language function (r2=0.59; p=0.001; odds ratio=6.04 [95%CI: 2.26 to 16.15]). This finding indicates that a more extensive resection involving such ‘language-related gamma sites’ was associated with a greater chance of post-operative language deficits, and that 58.7% of the outcome measures can be explained by this univariate model. Another univariate model incorporating the number of resected electrode sites in ‘canonical language regions’ in the left hemisphere containing essential language function also predicted the postoperative language deficit (r2=0.51; p=0.001; odds ratio=1.54 [95%CI: 1.20 to 1.97]). Furthermore, the multiple logistic regression model incorporating both predictors suggested that the prediction performance of such ‘language-related gamma sites’ remained significant (p=0.04), but that of extent of resection did not (p=0.81).
The results of electrical stimulation mapping are presented in Figure S3 on the website. The univariate model incorporating ‘language-related stimulation sites’ in the ‘canonical language regions’ in the left hemisphere containing essential language function predicted the language deficits (r2=0.41; p=0.02; odds ratio=16.8 [95%CI: 1.65 to 172.0]). The multiple logistic regression model suggested that the prediction performance of ‘language-related gamma sites’ remained significant (p=0.005), but that of ‘language-related stimulation sites’ did not (p=0.24).
A total of 17 patients had resection involving the hippocampus in the left hemisphere assumed to contain essential language function. The univariate model incorporating such hippocampal resection failed to predict the language deficits (r2=0.005; p=0.6; odds ratio=1.37 [95%CI: 0.37 to 5.02]). The multiple logistic regression model suggested that the prediction performance of ‘language-related gamma sites’ remained significant (p=0.002), but that of such hippocampal resection failed to reach significance (p=0.24).
Since the number of resected ‘language-related gamma sites’ in the ‘canonical language regions’ in the left hemisphere turned out to be a significant predictor of language outcome, we explored how well ‘language-related gamma sites’ in the left middle-temporal, medial-temporal, or medial-frontal region also predicted the language outcome, in a post-hoc fashion. The univariate logistic regression model suggested that the postoperative language deficit was predicted by the total number of resected ‘language-related gamma sites’ in the ‘canonical language regions’ plus the middle-temporal region in the left hemisphere assumed to contain essential language function (r2=0.59; p=0.001; odds ratio=5.0 [95%CI: 2.18 to 11.32]). Likewise, the language outcome was predicted by the total number of resected ‘language-related gamma sites’ in the ‘canonical language regions’ plus the medial-temporal region (r2=0.42; p=0.001; odds ratio=2.3 [95%CI: 1.47 to 3.57]) and that in the ‘canonical language regions’ plus the medial-frontal region (r2=0.58; p=0.001; odds ratio=5.84 [95%CI: 2.22 to 15.38]). These findings suggest that ‘language-related gamma sites’ in the left middle-temporal and medial-frontal regions (but not necessarily in the left medial-temporal region) were also important in prediction of the new/acute language deficit requiring speech therapy.
4. DISCUSSION
4.1. Significance of auditory-language-related gamma-augmentation
In the present study, all 77 patients, including a 4-year-old patient, completed a given auditory naming task (Figure 6) and showed significant gamma-augmentations in variable areas as summarized in Figure 1. We found that the logistic regression model incorporating gamma-augmentation predicted a postoperative language deficit. Furthermore, the predictive performance of this logistic regression model incorporating gamma-augmentation remained significant after controlling for the extent of resection, electrical stimulation data or resection of the hippocampus in the hemisphere assumed to contain essential language function. Since there is no added risk in the employment of auditory naming tasks, measurement of auditory-language-related gamma-augmentation may be warranted as a part of presurgical evaluation of epileptic patients who undergo extraoperative ECoG recording.
Figure 6. Auditory-language-related gamma-augmentation in a 4-year-old boy with epilepsy.
(A) Red circles indicate ‘language-related gamma sites’ (showing significant gamma-augmentation at 50–120 Hz, spanning at least 20-Hz in width and at least 20-msec in duration). (B) The results of time-frequency analyses are shown. Red: significant amplitude-augmentation prior to the preceding resting period. Blue: significant amplitude-attenuation. Significant gamma-augmentation sequentially involved the left superior-temporal (Channel #1), middle-temporal (Channel #2), medial-temporal (Channel #3), inferior-frontal (Channel #4), dorsolateral-premotor (Channel #5) and inferior-Rolandic regions (Channel #6). The left medial-frontal region was not sampled.
The outcome measure of interest in the present study was an acute language deficit requiring speech therapy. It remains uncertain whether gamma-augmentation can predict the long-term language outcome following surgery. The assumption of full recovery from postoperative language deficit may or may not be feasible even in pediatric patients (Sherman et al., 2011). Thus, it is expected that there will be considerable inter-subject variability in the degree of improvement in language skills following speech therapy, whereas we can report that such improvement was observed in all patients who developed a language deficit in the present study. It is quite possible that some patients might have had minor language impairment following surgery that went clinically undetected. A previous study of preadolescent children with temporal lobe epilepsy showed that left-sided resection was associated with a chronic decline in verbal memory (Szabó et al., 1998). Another study of adults with temporal lobe epilepsy showed that resection of the hippocampus in the dominant hemisphere resulted in a chronic decline in verbal memory performance detected by neuropsychological assessment (Hamberger et al., 2010). Studies incorporating systematically obtained pre- and postoperative neuropsychological measures are warranted to determine whether and how well gamma-augmentation can predict long-term language outcome.
We still do not fully understand which language task or combination of tasks would maximize the performance of prediction of language outcomes. While the auditory-naming task used in the present study requires semantic/lexical and syntactic processing, a word association task would require semantic/lexical but less syntactic processing (Thampratankul et al., 2010), and a phoneme repetition task would least require either (Fukuda et al., 2010). Combinations of tasks may segregate the underlying function of a site showing gamma-augmentation specifically elicited by one or a set of tasks but not by others. A number of previous studies employed visually-triggered language production tasks (Sinai et al., 2005; Tanji et al., 2005; Wu et al., 2010; Pei et al., 2011; Wu et al., 2011), as opposed to entirely auditory tasks like the one described here. Further studies are warranted to determine the spatial characteristics of common and differential gamma-augmentations elicited by different language tasks and to determine their performances of outcome prediction.
4.2. Developmental changes in auditory-language-related gamma-augmentation
In the present study, adults and children over the age of 10 had gamma-augmentation elicited more extensively in the left dorsolateral-premotor region especially in the portion involving the posterior portion of the left middle-frontal gyrus (Figures 3 and S2 on the website). Likewise, correlation with age was found in the right inferior-frontal region. These observations are consistent with fMRI observations suggesting that adults and older children, compared to younger children, showed larger augmentation of language-related blood oxygenation level-dependent (BOLD) responses in these regions (Adleman et al., 2002; Gaillard et al., 2003; Brown et al., 2005; Chou et al., 2006; Szaflarski et al., 2006). Previous studies reported that the amplitude of gamma-activity at >50 Hz on ECoG was tightly correlated to BOLD responses on fMRI (Niessing et al., 2005; Scheeringa et al., 2011). A potential explanation of less extensive gamma-augmentation in the aforementioned region in the younger age group is that language is less proficiently processed by an immature brain. A volumetric MRI study reported that white matter volume in the frontal lobes continues to increase until age 12 (Giedd et al., 1999). Significance of gamma-augmentation in the right inferior-frontal region could be of nonlinguistic nature, since functional imaging studies previously reported that tasks requiring identification of pitch patterns were associated with increased blood flow in this region (Zatorre et al., 1992; Wong et al., 2004).
Although some might expect that the frequency band of peak gamma-augmentation increases with age, this was not the case in the present study. Instead, regardless of age, patients showed significant gamma-augmentation most commonly involving 80–100 Hz frequency band (Figure 4). This observation is consistent with those in previous ECoG studies that various sensorimotor and cognitive tasks elicit gamma-augmentation commonly involving this frequency band in human neocortex (Axmacher et al., 2006; Crone et al., 2006; Jensen et al., 2007; Tallon-Baudry, 2009). It still remains unknown at what point each brain region begins to show language-related gamma-augmentation maximally involving 80–100 Hz. Our recent ECoG study of a 10-month-old patient with right-sided seizure focus reported that spontaneous cooing and babbling elicited gamma-augmentation ranging up to 100 Hz in the right inferior-Rolandic and superior-temporal regions (Cho-Hisamoto et al., 2012). Since few infants have the capability to complete a naming task, studies including infants using passive auditory or somatosensory tasks may be warranted to determine the developmental changes of event-related gamma-augmentation.
4.3. Effects of seizure focus on auditory-language-related gamma-augmentation
In the present study, the seizure onset sites were found to elicit gamma-augmentation less frequently compared to the non-seizure onset sites. The seizure onset zone often includes lesions such as cortical dysplasia with disorganized laminar architecture (Palmini et al., 1995; Bast et al., 2004). A previous study reported that lesioning resulted in decreased amplitude of physiological cortical gamma activity in rats (Bragin et al., 1995). One of the possible explanations for less frequent gamma-augmentation in the seizure onset zones is dysfunction associated with macro- or microscopic lesions in the seizure onset zone. Another possible explanation is temporal inhibition of gamma-augmentation by interictal epileptiform discharges. A previous ECoG study using micro- and macro-electrodes reported that subsets of neurons were suppressed for 100–1300 ms following single pulse electrical stimulation (Alarcón et al., 2012). A previous fMRI study showed that interictal spike-and-slow wave discharges on scalp EEG recording, especially those with large slow wave components, were followed by a lingering decline in cerebral blood flow (Kobayashi et al., 2006). Lastly, it is also possible that paroxysmal epileptiform gamma-band activity at >40 Hz (Andrade-Valenca et al., 2011) may contaminate ECoG signals during the reference period, effectively ‘hiding’ physiological gamma activity from our analysis. It is very difficult to completely exclude the effects of such contamination on the measurement of language-related gamma-augmentation, since physiological gamma activity is also spontaneously generated by non-epileptogenic neocortex (Csercsa et al., 2010; Le Van Quyen et al., 2010; Nagasawa et al., 2012). Further studies using a trial-by-trial analysis are warranted to determine the transient effects of interictal epileptiform discharges on language-related gamma-augmentation.
4.4. Temporal characteristics of auditory-language-related gamma-augmentation
According to 95%CI of peak latencies (Tables S1–S3 on the website), gamma-augmentation initially involved the superior-temporal regions bilaterally during the questions. The superior-temporal regions may be involved in acoustic and phonetic analyses as well as semantic/lexical comprehension (Crone et al., 2001; Poeppel et al., 2004). Previous fMRI studies of healthy volunteers indicated that greater BOLD responses in the right more than left superior-temporal region was noted during discrimination of pitch and duration, while greater responses in the left more than right superior-temporal region was noted during categorical perception of syllables, and lexical decision (Poeppel et al., 2004; Reiterer et al., 2005; Hyde et al., 2008).
The left middle-temporal region may be also involved in semantic/lexical comprehension. Gamma-augmentation in the left middle-temporal region reached the maximum value immediately prior to stimulus offset (Figure 5). Previous fMRI studies reported that BOLD responses in both left superior- and middle-temporal regions were larger when word stimuli were given compared to when nonlinguistic control stimuli were provided (Scott and Wise, 2004; Rimol et al., 2006).
Gamma-augmentation in the left medial-temporal region reached the maximum value around stimulus offset (Figure 5). The roles of the medial-temporal structure in language processing are less fully understood in humans, though it is considered to be essential for verbal memory. A study of adults who underwent selective amygdalohippocampectomy revealed that four out of the 10 patients postoperatively developed a minor decline in linguistic functions characterized by reduced language comprehension and fluency, well-articulated speech, and frequent word-finding difficulties (Bartha et al., 2004). A PET study of healthy volunteers showed that successful word retrieval following repeated encoding was associated with increased blood flow in the left medial-temporal region (Heckers et al., 2002). Previous ECoG studies reported that successful recalls of stimuli such as word, letter or face were predicted by the gamma-amplitudes following stimulus presentation (Sederberg et al., 2007) as well as during a nap between stimulus presentation and recalls (Axmacher et al., 2008); furthermore, such gamma-amplitudes were increased with memory load (van Vugt et al., 2010). Taken together with the results of the present study, gamma-augmentation observed in the left medial-temporal region may play a role in encoding and retrieval of contextually-mediated episodic memories as well as working memory, but loss of function in the left medial-temporal region may not be necessarily associated with acute major language deficits.
Gamma-augmentation in the left inferior-frontal region reached the maximum value around stimulus offset, which was immediately followed by the peak gamma-augmentation in the left dorsolateral-premotor region (Figure 5). These structures are likely to be involved in encoding of given stimuli into working memory (Howard et al., 2003; Mainy et al., 2007), generation of semantically, syntactically and phonologically appropriate responses (Mainy et al., 2008; Sahin et al., 2009), and selection of a single response from a range of possible options (Koga et al., 2011). Considerable temporal overlap in gamma-augmentation was noted between the temporal and frontal lobes at both intra- and inter-individual levels (Figure S4 on the website; Brown et al., 2008; Kojima et al., 2012). This observation supports but does not sufficiently prove the notion that both frontal and temporal language networks interact bi-directionally in real-time rather than a unidirectional, linear transfer of information from the temporal to the frontal lobe.
Gamma-augmentation in the medial-frontal regions reached the maximum between stimulus offset and response onset (Figure 5). This finding is consistent with the notion that this region is involved in voluntary control over the initiation and suppression of articulation (Jürgens, 2002). A study of adults with focal epilepsy reported that electrical stimulation of the pre-supplementary motor area induced various forms of clinical responses including vocalization, speech arrest, and slowing of speech (Fried et al., 1991).
Immediately prior to and during responses, gamma-augmentation finally involved bilateral inferior-Rolandic regions, which is thought to continuously determine and monitor the dynamic positioning of the mouth and throat (Sinai et al., 2005; Towle et al., 2008; Fukuda et al., 2010).
4.5. Methodological issues
Inherent limitations of our ECoG study include cross-sectional measurement, which is less sensitive to the detection of small magnitude developmental changes compared to longitudinal measurement. We cannot exclude the effects of confounding factors that are difficult to control for, such as different type and number of oral antiepileptic drugs (Chen et al., 1997; Zijlmans et al., 2009) and variance in IQ across subjects. Another limitation of ECoG and stimulation-based language mapping is limited spatial sampling. Since it is impossible to sample ECoG from all cortical regions in a single individual, we cannot rule out the possibility of variations in latencies of peak gamma amplitudes across patients. Auditory-language-related gamma-augmentation may be an alternative, useful biomarker of underlying language function, but should be interpreted alongside other clinical variables, such as patient handedness and neuroimaging.
HIGHLIGHTS.
An auditory naming task elicited augmentation of gamma activity on intracranial ECoG in epileptic patients.
The incidence of a post-operative language deficit requiring speech therapy was predicted by the number of resected sites with gamma-augmentation in the superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions of the left hemisphere assumed to contain essential language function.
Regardless of age, auditory-language-related gamma-augmentation most commonly involved the 80–100 Hz frequency band.
Acknowledgments
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano) as well as Japan Foundation for Neuroscience & Mental Health, Japan Epilepsy Research Foundation, and Japan-North America Medical Exchange Foundation (to K. Kojima). We are grateful to Harry T. Chugani, MD, Csaba Juhász, MD, PhD, Sarah Minarik, RN, BSN, Carol Pawlak, REEG/EPT, and Farah Huq at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Akanuma N, Alarcón G, Lum F, Kissani N, Koutroumanidis M, Adachi N, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–418. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–11. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- Alarcón G, Martinez J, Kerai SV, Lacruz ME, Quiroga RQ, Selway RP, et al. In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–31. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009a;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009b;45:477–89. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res Rev. 2006;52:170–82. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–17. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Bartha L, Trinka E, Ortler M, Donnemiller E, Felber S, Bauer G, et al. Linguistic deficits following left selective amygdalohippocampectomy: a prospective study. Epilepsy Behav. 2004;5:348–57. doi: 10.1016/j.yebeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, et al. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004;45:621–31. doi: 10.1111/j.0013-9580.2004.56503.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, remollissement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bull Soc Anthropol. 1861;2:235–8. [Google Scholar]
- Brodmann K. Vergleichende Lokalizationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth; 1909. [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, et al. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–31. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Matsuzaki N, Juhasz C, Shah AK, et al. Evaluating reverse speech as a control task with language-related gamma activity on electrocorticography. Neuroimage. 2012;60:2335–45. doi: 10.1016/j.neuroimage.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–3. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- Cho-Hisamoto Y, Kojima K, Brown EC, Matsuzaki N, Asano E. Cooing- and babbling-related gamma-oscillations during infancy: intracranial recording. Epilepsy Behav. 2012;23:494–6. doi: 10.1016/j.yebeh.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, Cone NE. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006;29:1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J Neurosci. 2011;31:12855–65. doi: 10.1523/JNEUROSCI.1457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–95. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Csercsa R, Dombovári B, Fabó D, Wittner L, Eross L, Entz L, et al. Laminar analysis of slow wave activity in humans. Brain. 2010;133:2814–29. doi: 10.1093/brain/awq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–15. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT. Single-trial speech suppression of auditory cortex activity in humans. J Neurosci. 2010;30:16643–50. doi: 10.1523/JNEUROSCI.1809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–66. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–45. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–85. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin CA, Campbell TF. Procedure for assessing verbal working memory in normal school-age children: some preliminary data. Percept Mot Skills. 1994;79:55–64. doi: 10.2466/pms.1994.79.1.55. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Language and the brain. Sci Am. 1972;226:76–83. doi: 10.1038/scientificamerican0472-76. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, McKhann GM., 2nd Does cortical mapping protect naming if surgery includes hippocampal resection? Ann Neurol. 2010;67:345–52. doi: 10.1002/ana.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseeb A, Asano E, Juhász C, Shah A, Sood S, Chugani HT. Young patients with focal seizures may have the primary motor area for the hand in the postcentral gyrus. Epilepsy Res. 2007;76:131–9. doi: 10.1016/j.eplepsyres.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Weiss AP, Alpert NM, Schacter DL. Hippocampal and brain stem activation during word retrieval after repeated and semantic encoding. Cereb Cortex. 2002;12:900–7. doi: 10.1093/cercor/12.9.900. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Peretz I, Zatorre RJ. Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia. 2008;46:632–9. doi: 10.1016/j.neuropsychologia.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ishitobi M, Nakasato N, Suzuki K, Nagamatsu K, Shamoto H, Yoshimoto T. Remote discharges in the posterior language area during basal temporal stimulation. Neuroreport. 2000;11:2997–3000. doi: 10.1097/00001756-200009110-00034. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, et al. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topogr. 2009a;22:18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Minotti L, Kahane P, Berthoz A, Lachaux JP. Watching brain TV and playing brain ball exploring novel BCI strategies using real-time analysis of human intracranial data. Int Rev Neurobiol. 2009b;86:159–68. doi: 10.1016/S0074-7742(09)86012-1. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–58. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27:488–97. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Rothermel R, Juhasz C, Nagasawa T, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a stroop task-intracranial recording in epileptic patients. Hum Brain Mapp. 2011;32:1580–91. doi: 10.1002/hbm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, et al. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331–54. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, 3rd, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, et al. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31:1408–18. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Staba R, Bragin A, Dickson C, Valderrama M, Fried I, et al. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30:7770–82. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Webber WR, Crone N, Miglioretti DL, Lesser RP. When is electrical cortical stimulation more likely to produce afterdischarges? Clin Neurophysiol. 2010;121:14–20. doi: 10.1016/j.clinph.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser RP, Lüders H, Morris HH, Dinner DS, Klem G, Hahn J, et al. Electrical stimulation of Wernicke’s area interferes with comprehension. Neurology. 1986;36:658–63. doi: 10.1212/wnl.36.5.658. [DOI] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–93. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, et al. Cortical dynamics of word recognition. Hum Brain Mapp. 2008;29:1215–30. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–9. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, et al. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage. 2010;53:707–17. doi: 10.1016/j.neuroimage.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–90. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–68. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhász C, Fukuda M, Nishida M, Akiyama T, et al. Cortical gamma-oscillations modulated by auditory-motor tasks-intracranial recording in patients with epilepsy. Hum Brain Mapp. 2010a;31:1627–42. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by visuomotor tasks: Intracranial recording in patients with epilepsy. Epilepsy Behav. 2010b;18:254–61. doi: 10.1016/j.yebeh.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhász C, Hanazawa A, Shah A, Mittal S, et al. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. Neuroimage. 2011;58:1101–9. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: Intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33:569–83. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, et al. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol. 2008;119:862–8. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Pei X, Leuthardt EC, Gaona CM, Brunner P, Wolpaw JR, Schalk G. Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage. 2011;54:2960–72. doi: 10.1016/j.neuroimage.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR. Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia. 2004;42:183–200. doi: 10.1016/j.neuropsychologia.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–43. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reiterer SM, Erb M, Droll CD, Anders S, Ethofer T, Grodd W, et al. Impact of task difficulty on lateralization of pitch and duration discrimination. Neuroreport. 2005;16:239–42. doi: 10.1097/00001756-200502280-00007. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Specht K, Hugdahl K. Controlling for individual differences in fMRI brain activation to tones, syllables, and words. Neuroimage. 2006;30:554–62. doi: 10.1016/j.neuroimage.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Sahin NT, Pinker S, Cash SS, Schomer D, Halgren E. Sequential processing of lexical, grammatical, and phonological information within Broca’s area. Science. 2009;326:445–9. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–83. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Carlson C, Zaroff CM, Weiner HJ, Doyle WK, Miles D, et al. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48:539–45. doi: 10.1111/j.1528-1167.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17:1190–6. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Shah AK, Atkinson MD, Gupta P, Zak I, Watson CE, Rothermel R, et al. Transient shivering during Wada test provides insight into human thermoregulation. Epilepsia. 2010;51:745–51. doi: 10.1111/j.1528-1167.2009.02398.x. [DOI] [PubMed] [Google Scholar]
- Sherman EM, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52:857–69. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–70. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Szabó CA, Wyllie E, Stanford LD, Geckler C, Kotagal P, Comair YG, et al. Neuropsychological effect of temporal lobe resection in preadolescent children with epilepsy. Epilepsia. 1998;39:814–9. doi: 10.1111/j.1528-1157.1998.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C. The roles of gamma-band oscillatory synchrony in human visual cognition. Front Biosci. 2009;14:321–32. doi: 10.2741/3246. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–93. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thampratankul L, Nagasawa T, Rothermel R, Juhasz C, Sood S, Asano E. Cortical gamma oscillations modulated by word association tasks: intracranial recording. Epilepsy Behav. 2010;18:116–8. doi: 10.1016/j.yebeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, et al. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–9. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Klooster MA, Zijlmans M, Leijten FS, Ferrier CH, van Putten MJ, Huiskamp GJ. Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain. 2011;134:2855–66. doi: 10.1093/brain/awr211. [DOI] [PubMed] [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, et al. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–50. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- Wernicke C. The symptom of complex aphasia. In: Church AE, editor. Diseases of the nervous system. New York: Appleton; 1911. pp. 265–324. [Google Scholar]
- Wong PC, Parsons LM, Martinez M, Diehl RL. The role of the insular cortex in pitch pattern perception: the effect of linguistic contexts. J Neurosci. 2004;24:9153–9160. doi: 10.1523/JNEUROSCI.2225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, et al. Gamma-oscillations modulated by picture naming and word reading: intracranial recording in epileptic patients. Clin Neurophysiol. 2011;122:1929–42. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–86. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]