Abstract
FcεRI engagement in mast cells (MC) induces the activation of two distinct sphingosine kinase isoforms (SphK1 and SphK2) to produce sphingosine-1-phosphate, a mediator essential for MC responses. While embryonic derived SphK2-null MC showed impaired responses to antigen, RNA silencing studies on other MC types indicated a dominant role for SphK1. Given the known functional heterogeneity of MC, we explored whether the reported differences in SphK1 or SphK2 usage could be reflective of phenotypic differences between MC populations. Using lentiviral-based short hairpin RNA to silence SphK1 or SphK2 we found that SphK2 is required for murine MC degranulation, calcium mobilization, cytokine and leukotriene production irrespective of the tissue from which the MC progenitors were derived, the stage of MC granule maturity, or the conditions used for differentiation. This was consistent with the lack of a full allergic response in SphK2 -null mice challenged to undergo passive cutaneous anaphylaxis. A redundant role for both SphKs was uncovered, however, in chemotaxis towards antigen in all MC types tested and in TNF-α production in certain MC types. In contrast, human MC (HuMC) responses were dependent only on SphK1, associating with a more robust expression of this isoform and a more varied representation of SphK variants relative to murine MC. The findings show that the function of SphK1 and SphK2 can be interchangeable in MC; however, an important determinant of SphK isoform usage is the species of origin and an influencing factor, the tissue from which MC may be derived and/or their differentiation state.
INTRODUCTION
Two mammalian sphingosine kinase isoforms (SphK13 and SphK2) are responsible for the phosphorylation of sphingosine to generate sphingosine-1-phosphate when cells are activated by a variety of stimuli (1). Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator of diverse biological functions, including the regulation of vascular permeability and vascular tone (2, 3), modulation of immune cell trafficking and function (4, 5) and regulation of numerous disease processes (6-10). S1P generated during activation of SphKs may bind and regulate its intracellular targets, or once transported out of the cells, bind and engage its membrane receptors (S1PR1 through 5), thus mediating complex arrays of responses (reviewed in (9)). In part, the mode of action of S1P depends on the location where it is produced, the regulation of its levels by enzymes involved in its degradation, and the coupling of its synthesis to either its export via lipid transporters or to specific signaling pathways (9, 11, 12). Each individual isoform of SphK may also contribute to the type of cellular actions S1P is able to elicit (12, 13). SphK1 and SphK2 share high degree of structural homology, but differ considerably in their overall sequence, tissue distribution, biochemical properties and in the cellular functions they can mediate. There is evidence for unique, redundant or even opposing roles for SphK1 and 2 (12-14). This versatility may be attributed to their relative expression in the cell, their subcellular redistribution under a particular stimulus or their impact on other bioactive sphingolipid metabolites. Furthermore, splicing variants for both isoforms have been described (14-18), although their specific function in cells is largely unknown. While the function and mechanism of activation of SphK1 have been investigated in several systems, those for SphK2 remain largely unexplored. The emerging view, gathered from the accumulated studies, suggests that there is a preferential use for one of the isoforms of SphK in a particular cell, stimulus and type of response. Dominance of SphK1 function is most common and consistent amongst mammalian systems, while the SphK2 function is more variable with an apparent dependence on where it may be localized in a given cell type.
Mast cells are key effector cells of allergic responses, characterized by the constitutive expression of the high affinity IgE receptor, FcεRI, on their surface. Allergen-mediated cross-linking of FcεRI results in a cascade of signaling events that culminates in the secretion of preformed mediators and the production of a variety of cytokines and lipid mediators, all of which promote allergic and inflammatory responses in vivo (19, 20). Engagement of the IgE receptor by antigen in MC induces the activation of both SphK1 and SphK2 and the production of S1P, which promotes the release of MC-derived mediators (21-26). S1P is also secreted by activated mast cells in considerable amounts to the extracellular medium by the ABCC-1 transporter (27). Since S1P has been found elevated at sites of inflammation in diseases where mast cells may play important roles (i.e, asthma and arthritis) (28, 29), it is possible that, during allergic and inflammatory processes, MC produce S1P in the tissue environment that can affect the pathological course of these diseases (10, 11). Furthermore, since the generation of S1P is intrinsically important for MC responses, an understanding of the specific role for each isoform of SphKs in early or late phase MC responses is essential and could provide novel therapeutic targets for specific diseases.
SphK isoform dominance in MC function is not completely understood. Evidence for SphK1 and SphK2, either individually or jointly, in MC effector functions has been reported (21, 26, 30-32). However, a number of discrepancies are found in these studies that may arise from differences in the experimental approaches, the species of origin of the MC used, the tissue from which MC progenitors are derived and/or the conditions used for differentiation of MC, all of which can influence the MC phenotype. Using embryonic liver-derived MC from SphK1- and SphK2-deficient mice, we previously found that SphK2 was the major source of S1P and was essential for MC effector responses. Others have reported, using the rat cell line RBL-2H3 cells (30) or murine bone marrow derived MC (BMMC) (32), that siRNA silencing of SphK1, but not SphK2, resulted in impaired degranulation in MC. In HuMC, SphK1 was also reported to be required for MC degranulation (26, 31) whereas both SphK1 and SphK2 were involved in cytokine production (31).
In the present study, we thus explored the relative roles of SphK isoforms in MC function. We employed a short hairpin (sh) RNA silencing strategy to achieve selective silencing of each SphK isoform in contrast to the previously described (32) transient silencing (si) RNA strategies, which we found to be neither sufficient nor selective to cause effective silencing of individual SphKs in murine MC. In addition, we used MC from different species or derived from various tissues as well as MC differentiated under different culture conditions to explore the role of the SphK isoforms in heterogeneous populations of MC. Our findings indicate that a major determinant of the relative importance of each SphK isoform in MC function depends on the species of origin (SphK1 for human and SphK2 for mouse), which in part reflects the relative abundance of SphK1 and SphK2 expression. Despite the dominance of SphK2 in the murine system, there was redundancy among the isoforms in their role in migration of mouse MC towards antigen and in cytokine production, although the latter was restricted to a particular type of MC. Our findings also suggest that murine MC constitute an ideal cellular system to investigate the possible post-translational mechanisms in the activation of SphK2, which have largely been elusive.
MATERIALS AND METHODS
Mice and Cell Culture
SphK1- and SphK2-null mice (C57BL/6 × 129/Sv, N5) were generated as described previously (33, 34) and wild-type (WT) mice were on the same genetic background. Animals were maintained and used according to National Institutes of Health guidelines and a National Institute of Arthritis and Musculoskeletal and Skin Diseases-approved animal study proposal A010-04-03. Bone marrow-derived MC (BMMC) were generated by flushing MC progenitors from the femurs of 6-8 week old WT mice and culturing these cells as previously described (35). Peritoneum-derived MC (PDMC) were obtained from the peritoneal lavage of adult WT mice and cultured in media containing 20% FBS as previously described (36). Cells were used when greater than 95% of the population expressed both FcεRI and KIT as measured by flow cytometry (35). Ear skin MC were obtained from WT, SphK1-, and SphK2-null mice. Briefly, ears were split into dorsal and ventral halves and placed in 2 ml of 1% FBS in RPMI containing 0.1 mg/ml DNase I (Sigma-Aldrich) and 0.2 mg/ml Liberase TL (Roche Diagnostics). Following a 1 h incubation at 37°C, the digested tissue was placed into a 70 mm cell strainer (BD Biosciences), and teased through the mesh. The strainers were washed with 2 ml of 1% FBS, 2 mM EDTA in PBS (Gibco) (37). All cells were cultured in RPMI media (Invitrogen) supplemented with 10% (BMMC) or 20% (PDMC and ear mast cells) FBS (Invitrogen) and 20 ng/ml each of recombinant mouse IL-3 and stem cell factor (SCF) (Peprotech, Rocky Hill, NJ). Primary human (Hu)MC were prepared from CD34+ peripheral blood progenitors isolated from healthy volunteers following informed consent under a protocol (NCT00001756) approved by the NIH internal review board (38). 293LTV cells (Cells Biolabs) used for viral production were cultured in DMEM media supplemented with 10% FBS (Invitrogen).
shRNA construction and gene transduction
A lentiviral based transduction system was used for shRNA-mediated gene knockdown of SphK1 and SphK2 in mouse and human MC. Bacterial glycerol stocks of shRNA constructs for mouse and human SphK1 and SphK2 were purchased from Sigma Aldrich. The shRNA constructs used were as follows: mSphK1 (TRCN0000024684), mSphk2 (TRCN0000024632), huSphK1 (TRCN0000036966) and huSphK2 (TRCN0000036972). Nontarget shRNA (SHC002) was used as a negative control.
To generate virus, 293LTV cells were cotransfected with 3.9 μg shRNA vector and 39 μl lentiviral packaging mix (Sigma Aldrich) using Fugene HD (Roche). The viral supernatants were collected 72 h post transfection and concentrated by centrifugation at 20,000 × g for 2 h. The viral pellet was resuspended and used to transduce 107 fully differentiated BMMC or PDMC or 5×106 HuMC. The efficiency of lentiviral transduction was greater than 90% as determined by the GFP expression cassette pNUTS (data not shown). Two days post-transduction, cells were changed to virus-free medium and, following an additional two-day recovery period, selection was started using 3 μg/ml (BMMC), 1.5 μg/ml (PDMC), or 0.2 μg/ml (HuMC) puromycin (Sigma). Cells were kept in selection media for 5-7 d and allowed to recover for at least two days in puromycin-free media before being used for the experiments.
Real-time PCR and Western blot
To assess mRNA knockdown, total RNA was isolated from cells (1 to 4×106) using the RNeasy Kit (Qiagen) per the manufacturer's instructions. RNA was converted into cDNA using the SuperScript III First Strand Synthesis System for qRT-PCR (Invitrogen). Quantitative RT-PCR was performed using the ABI PRISM 7500 sequence-detection system (Applied Biosystems). Taqman gene expression assays for SphK1 and SphK2 were purchased from Applied Biosystems. Relative levels of mRNA were calculated based on ΔCt using GAPDH as endogenous control gene. The levels of expressions of the different SphK isoform variants were determined as described in Supplemental Table I. Western blots were performed to measure the effectiveness of protein knockdown. Cells were lysed in borate-buffered saline containing 1% triton-X 100, 60 mM octyl-β-glucoside, 2 mM PMSF, 10 μg/ml of aprotinin, 2 μg/ml of leupeptin and pepstatin, 5 mM sodium pyrophosphate, 50 mM NaF and 1 mM sodium orthovanadate as described (22). Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and the resolved protein was identified using antibodies for SphK2 (22). Available antibodies to SphK1 failed to selectively detect this isoform in murine MC as assessed by comparison with lysates from SphK1-deficient cells. This may be due to low expression of this isoform in murine mast cells and/or specificity of the antibodies. IR-dye labeled secondary antibodies were used for the detection of proteins in Western blots and the immunocomplexes were imaged and quantified using an Odyssey Infrared Imaging System (Li-cor Biosciences).
Sphingosine kinase activity assay
The enzymatic reaction of converting sphingosine to sphingosine-1-phosphate is catalyzed by SphKs in the presence of ATP-MgCl2+. Briefly, cells (4×106) were harvested and lysed by freeze thawing in SphK buffer (50 mM Tris [pH 7.4], 100 mM KCl, 10% glycerol, 1 mM mercaptoethanol, 1 mM EDTA, 10 μg/ml pepstatin, 1 mM PMSF, 0.5 mM 4-deoxypyridoxine, and 10 μl/ml Halt protease and phosphatase inhibitor cocktail (ThermoScientific)). Cell samples were incubated with 50 μM sphingosine, [γ-32P]-ATP (10 μCi), 1mM ATP and 10 mM MgCl2+. SphK1 and SphK2 activities can be selectively measured using conditions that favor each individual kinase (with about a 30% overlap). SphK1 activity was measured in SphK buffer containing sphingosine- Triton X-100 (5%), which is inhibitory to SphK2 activity. For SphK2 activity measurements, sphingosine was presented as BSA complexes (50 μM sphingosine in 4 mg/ml BSA) instead of in a micellar form and the SphK buffer contained 1 M KCl, which conversely inhibits SphK1 activity. Following a 30-min incubation at 37°C, the reactions stopped, radiolabeled lipids extracted, and S1P separated on TLC plates, as described (22). Both SphK1 and SphK2 activities were linear from 2.5 to 100 μg protein, indicating the validity of these assays for assessing SphK expression.
Measurement of mast cell effector responses
Murine MC were sensitized with 1 μg/ml anti-DNP IgE (H1-DNP-ε–26.82; (39)) in Hepes-BSA buffer (37°C, 10 mM Hepes, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4, 5.6 mM glucose, 1.8 mM CaCl2, and 1.3 mM MgSO4 and 0.04% fatty acid free bovine serum albumin (BSA)) for one hour at room temperature. HuMC were sensitized with 100 ng/ml biotinylated IgE (40) in cytokine-free media or complete media (cytokine production) overnight at 37°C. For degranulation experiments, murine and human MC were stimulated for 30 min with the indicated concentration of DNP-HSA (antigen) (Sigma-Aldrich) or streptavidin (SA) (Sigma-Aldrich), respectively and the release of β-hexosaminidase into the incubation media was determined using a colorimetric assay as previously described (40). The extent of degranulation was calculated as the percent of β-hexosaminidase found in the supernatants following challenge versus the total content found in the cells. Cytokine production was measured from BMMC (4×106 cells) stimulated with 25 ng/ml of antigen for 3 h. Alternatively, HuMC (1×106 cells) were stimulated with 100 ng/ml SA for 6 h. Production of IL-6 and TNF-α for murine MC or IL-8 and TNF-α for HuMC was determined by Bio-Plex Pro cytokine assays (BioRAD). For leukotriene production, cells were stimulated with the indicated concentrations of antigen for 30 minutes at 37°C in Hepes-BSA buffer containing 10 μg/ml indomethacin (Sigma Aldrich). The amount of LTB4 in the supernatants was measured using a competitive ELISA (R&D Systems).
Passive cutaneous anaphylaxis
The ears of WT, SphK1- or SphK2-null mice were sensitized subcutaneously with either anti-DNP IgE (75 ng in 20 μl saline) or the same volume of saline in the contralateral ear. The next day, mice were challenged i.v. with antigen (250 μg) dissolved in 0.5% Evans blue/PBS. After 30 minutes, the mice were euthanized and the ears were collected and minced. The minced ears were incubated in formamide for 1 h at 55°C to extract the Evans blue, and the amount of dye was measured by absorbance at 620 nm (41).
Calcium mobilization
Calcium mobilization was measured using a Zeiss LSM-510 Meta confocal microscope. Cells were sensitized with 1 μg/ml anti-DNP IgE for 1 h in IL-3 only-containing media and then plated in Lab-TEK chamber slides (ThermoScientific) coated with poly-L-lysine (Sigma Aldrich). Cells were then loaded with Fluo4-AM (2 μM) and FuraRed-AM (10 μM) for 30 min at 37°C. Cells were carefully washed three times using Tyrodes-BSA buffer and stimulated with 10 ng/ml antigen. Fluorescent images were collected every 5 s, and the intensity of fluorescence was quantified using Zeiss LSM-510 Meta software. Responses are shown as a ratio of intensity of Fluo4-AM/FuraRed-AM as previously described (42).
Chemotaxis assay
Chemotaxis assays were performed using Transwell® permeable support with a 5.0 μM pore polycarbonate membranes on 6.5 mm inserts (Costar) placed within 24-well polystyrene plates. Cells were sensitized with 1 μg/ml anti-DNP IgE overnight in full media. Following three washings with Hepes-BSA buffer, cells (3×105 cells/100 μl) were placed in the upper chamber insert and preincubated in 600 μl Hepes buffer for 30 minutes. The upper chambers were then placed in contact with the lower chambers containing antigen (10 ng/ml or 30 ng/ml). After incubation for 4 h at 37°C, cells migrating to the lower chambers were transferred to 96-well flat bottom plate. Cell pellets were frozen and the relative numbers of migrated cells was determined using Cyquant cell proliferation assay (Invitrogen). A linear standard curve with serial dilutions of the cells, ranging from 50 to 5000 cells, was included to convert the relative fluorescence intensity to cell numbers. Fluorescence was measured using a Perkin Elmer Victor2 microplate reader.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was determined by unpaired, two-tailed Student's t test. Each culture represents cells derived from either an individual mouse or human subject. Each knockdown experiment was repeated in 3 to 10 separate cultures as indicated in the legend to figures. Whenever more than one knockdown experiment was performed from a single culture, it was averaged and considered as one.
RESULTS
MC degranulation is impaired in SphK2-null connective tissue MC in vitro and in vivo
Our previous work (21) had established an essential function for SphK2 in embryonic liver-derived cultured mast cells (LDMC). While MC derived from the bone marrow of SphK1- or SphK2-null adult mice also showed a role for SphK2, the results were less convincing given the high degree of variability from culture to culture (21). Unlike other hematopoietic cells, MC precursors from the bone marrow migrate through the blood to the tissues where they differentiate, responding to tissue microenvironment cues that ultimately influence their phenotype (43, 44). In vitro, the MC phenotype may also vary depending on the tissue from where progenitors are derived, the culture conditions, or the species of origin (44). Given the MC heterogeneity in vivo and in vitro, we sought to determine if the dominance of SphK2 over SphK1 observed in the LDMC and BMMC was representative of other more mature MC populations or of in vivo MC. BMMC grown in the presence of IL-3 and SCF are thought to be an immature connective tissue-like mast cell (CTMC) model, with no known tissue equivalent. However, PDMC, which differentiate and mature in vivo, are a more mature CTMC, based on their staining properties and morphology (45). In agreement, PDMC had a denser granule content (data not shown) and expressed higher levels of mMCP-1, 2, 4, 5 and 9 than BMMC (Supplemental Fig. 1A). They also expressed mMCP5 and CPA3, proteases that characterize CTMC (Supplemental Fig. 1B and C)
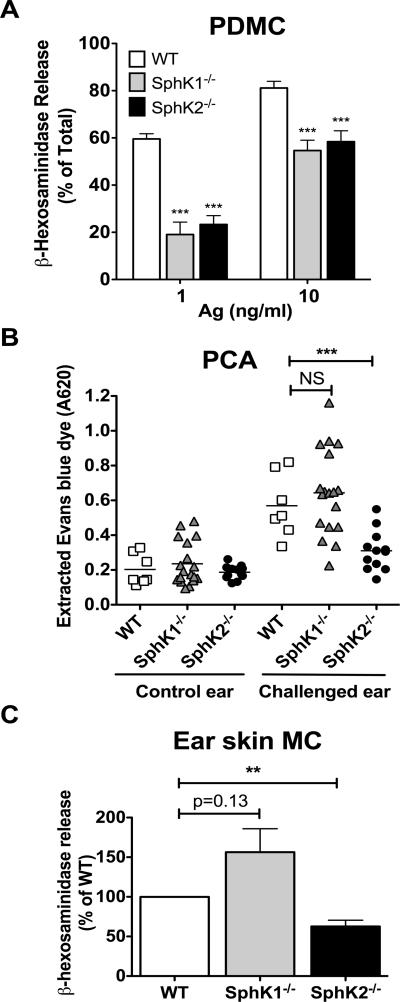
Consistent with results in LDMC and BMMC, SphK2 deficiency in PDMC resulted in impaired degranulation (Fig. 1A). However, a role for SphK1 in the degranulation response was also seen in SphK1-deficient PDMC (Fig. 1A). To determine whether the dependence of both SphK1 and SphK2 in degranulation is a characteristic of mature CTMC or specific for PDMC, we tested the responsiveness of skin mast cells in vivo using the MC-dependent model of passive cutaneous anaphylaxis (PCA). Skin MC is another CTMC type and shows similar content and distribution of the CTMC proteases mMCP5 and CPA3 as PDMC (Supplemental Fig. 1B and C). Local sensitization and challenge of skin MC resulted in similar responses in WT and SphK1-null mice but a reduced response was observed in SphK2-null mice, as evidenced by decreased Evans blue extravasation (Fig. 1B). This was not due to differences in MC numbers in the ears, as all genotypes expressed similar cell numbers (Supplemental Fig. 1D). Skin MC from SphK2-deficient mice showed a reduction in degranulation as observed from MC in the ears of mice upon PCA challenge in vivo (Fig. 1C). Furthermore, the difference in Evans blue extravasation was not due to alterations in blood vessel reactivity in SphK2-null mice since we have shown that induction of PCA by compound 48/80 (a mast cell secretagogue) in mice deficient for each kinase results in similar vascular permeability for all genotypes (41). The PCA responses together with the in vitro experiments, provides evidence for an essential role for SphK2 in mediating murine MC degranulation, particularly the CTMC type, regardless of the tissue origin of the progenitor from which they are derived. However, our previous findings in a systemic challenge (passive systemic anaphylaxis, PSA) indicated that histamine release in SphK2-null mice was normal (21), suggesting that under a systemic challenge, other in vivo compensatory factors (i.e., levels of circulating S1P) may influence mast cell responses, as previously reported (10, 21).
Figure 1. Genetic deletion of SphK2 results in impaired degranulation in mature connective tissue mast cells in vitro and in vivo.
(A) MC were obtained by peritoneal lavage from WT and SphK1- and SphK2-null mice. After expansion of PDMC for 11-20 days, β-hexosaminidase release was measured from supernatants of cells challenged with the indicated concentrations of antigen. Data represents the average of ten individual cultures ± S.E. Statistical significance compared to WT mice, *** P<0.001. (B) Degranulation of skin MC in vivo upon passive cutaneous anaphylaxis challenge. WT and SphK1- and SphK2-null mice ears were locally sensitized with saline or anti-DNP IgE (75ng) in the contralateral ear. The next day, mice were challenged systemically with antigen dissolved in Evans Blue (0.5%). Following a 30 min incubation, mice were euthanized and Evans Blue dye was extracted from the ears and absorbance was measured at 620 nm, as described in the materials and methods. Statistical significance compared to WT mice, *** P<0.001. (C) MC were obtained from the ear skin of WT and SphK1- and SphK2-null mice and cultured in vitro. β-hexosaminidase release was measured using 10 ng/ml of antigen as in (A). Statistical significance between SphK2-null ear mast cells and WT, ** P<0.01. Data represents the average of 3 separate experiments ± S.E. Each experiment had duplicate cultures for all genotypes and each culture was from 4 separate ears. Data is expressed as percentage of WT degranulation in each separate experiment.
Lentiviral shRNA silencing of SphK1 and SphK2 in murine PDMC and BMMC shows a dominant role for SphK2
Because PDMC from SphK1 and SphK2-deficient mouse showed a dependence for both SphK isoforms while other MC populations showed an essential role for SphK2 alone, we explored if the alteration in MC function could be due to environment or epigenetic changes associated with the genetic deletion model. To address this issue, we used RNA silencing to inhibit SphK1 or SphK2 expression in fully differentiated MC. Previously published (32) along with commercially available siRNA sequences were used; however, in our hands the previously published SphK2 siRNA sequences did not achieve selective silencing of SphK2, knocking down both SphK1 and 2 (Supplemental Fig. 2A). Furthermore, neither these sequences nor the commercially available sequences were very efficient in silencing SphK2 (~40%) after multiple attempts to use such strategies (Supplemental Fig. 2A, 2B, respectively). It is important to note that the overall levels of SphK2 in these studies were about 6.5 fold higher than those of SphK1 (Fig. 2 and Supplemental Fig. 2A). Hence, the ~40% decrease in SphK2 expression may not constitute a significant enough reduction in activity to impair the biological response (37 pmol/mg/min of activity remaining). Nevertheless, transient silencing of SphK1 also had no impact on degranulation, a result that contrasts with a previous report by Pushparaj et al (32). The reasons for these discrepancies are not entirely clear. However, unlike for SphK2, incomplete knockdown of SphK1 is unlikely to explain the discrepancies with the previous study since the amount of SphK1 activity detected in the cells following siRNA knockdown was negligible (<5 pmol/mg/min) and similar to the activity reported in SphK1-knockout mast cells (22, 46) (this background activity probably represents SphK2 activity; see method section).
Figure 2. Specific knockdown of SphK1 and SphK2 in rodent mast cells.
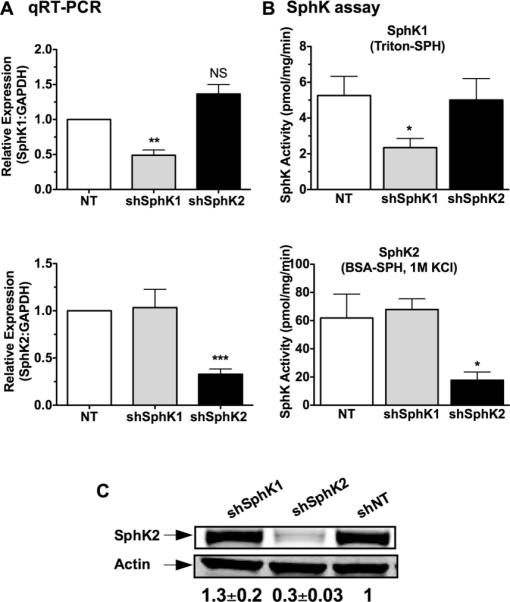
MC were transduced with lentivirus containing shRNA sequences specific for SphK1, SphK2, or nontarget (NT) shRNA control. Efficient shRNA silencing of each isoenzyme was determined by real- time PCR (A) or by enzymatic assays (B). (A) Gene expression assays specific for SphK1 (upper panel) and SphK2 (lower panel) were used to determine the relative mRNA levels after shRNA silencing. A specific probe for GAPDH was used as an internal control. Each measurement for SphK1 and SphK2 was normalized to GAPDH (ΔCt) and measurements were then compared to the nontarget (NT) values. (B) Cells transduced with the various shRNA constructs were assayed for SphK1 (upper panel) or SphK2 (lower panel) activities as described in methods. Specific assay conditions allowed for the selective detection of SphK1 activity in the presence of triton-sphingosine micelles and SphK2 activity in the presence of a buffer containing 1M KCl as described in the Methods section. Shown in A and B are results from BMMC; similar results were obtained in PDMC. Data are the average ± S.E. of at least four individual cultures. Statistical significance compared to nontarget control, * P<0.05; **P<0.01, *** P<0.001. (C) Protein confirmation of shRNA-mediated SphK2 silencing by Western blot analysis. Equal protein loading was confirmed using Actin as a loading control and quantified by densitometry. Shown is a representative blot from PDMC lysates out of at least 3 separate experiments; very similar results were obtained in BMMC. The values underneath the blots represent the average densitometry values (n=7 including BMMC and PDMC blots) corrected by actin and normalized to NT control and are expressed as average ± S.E.
We therefore attempted to more effectively reduce the activity of the SphK isoforms by using lentiviral-based shRNA silencing, which allows for stable and high expression of shRNA. To assess the selectivity and efficiency of all shRNA constructs at the RNA and protein levels, we used real time PCR, western blot, and measured SphK1 and SphK2 activity; the latter of which can be distinguished in vitro due to low cross-reactivity of the assay (22, 46). This multilayered strategy identified the shRNA sequences showing the highest degree of selective silencing for SphK1 and SphK2 with no apparent effect on the other isoform. As shown in Fig. 2, we achieved a reasonable degree of selective mRNA reduction (>60% on average for SphK1 and >70% for SphK2) for each of the targeted kinases as measured by real-time PCR (Fig. 2A). This was verified by the selective reduction of the enzymatic activity of each isoform (63% for SphK1 and 72% for SphK2) (Fig. 2B). Regrettably, immunoblotting for SphK1 protein expression was not possible, given that the available antibodies failed to selectively detect this isoform in murine MC. However, cells targeted with SphK2 shRNA showed a 70% reduction in SphK2 protein expression whereas cells expressing shRNA for SphK1 or control shRNA showed no effect on SphK2 protein expression (Fig. 2C).
shRNA-mediated silencing of SphK2 in fully differentiated MC resulted in decreased (35-40%) IgE-dependent degranulation in PDMC and BMMC (Fig. 3A, 3C, respectively) at antigen concentrations ranging from 1 ng/ml (data not shown) to 100 ng/ml. This was consistent with our previous findings (21) in SphK2-/- MC. However, unlike PDMC derived from SphK1-null mice (Fig. 1A), silencing of SphK1 expression in PDMC or BMMC did not alter β-hexosaminidase release, indicating that SphK1 activity was less critical for degranulation in this MC population. Although the discrepancies between the SphK1-null PDMC and SphK1 knockdown PDMC could indicate that complete depletion of SphK1 is required to manifest its role in degranulation of PDMC, this is unlikely since PDMC obtained from mice in which SphK1 was conditionally deleted after birth (2, 3) had no defect in degranulation (Supplemental Fig. 3). In addition, conditional deletion of SphK1 in SphK2-/- mice did not result in any further reduction in the degranulation of SphK2-deficient PDMC or BMMC (Supplemental Fig. 3A and B). Thus, a more likely scenario is that general knockout of SphK1 (in contrast to SphK1 conditional knockout mostly in the hematopoietic compartment (2, 3)) causes developmental alterations that may phenotypically affect this mast cell population. The defective response in SphK1-null PDMC, and perhaps other mast cell subtypes, may explain, at least in part, why histamine release after systemic antigen challenge (PSA), but not PCA, in these mice was found to be lower than expected (21).
Figure 3. shRNA-mediated silencing of SphK2, but not SphK1, results in impaired degranulation and calcium responses in PDMC and BMMC.
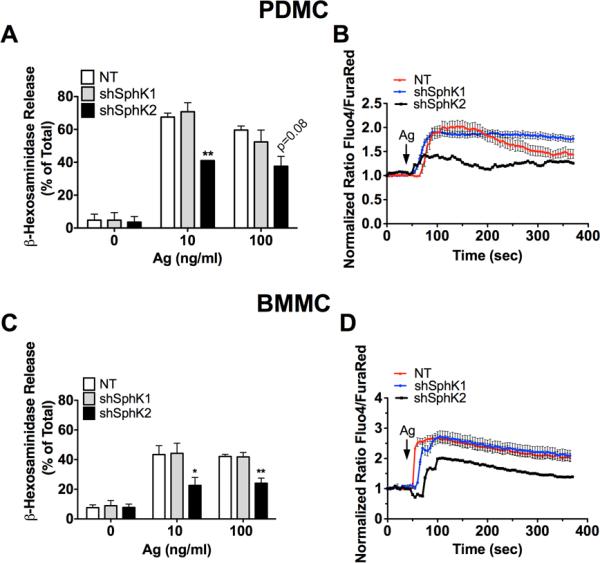
Lentivirus transduced PDMC (A, B) or BMMC (C, D) were sensitized with anti-DNP IgE as described in methods. Cells were washed and then stimulated with the indicated concentrations of antigen. (A, C) Degranulation was calculated as the percentage of β-hexosaminidase released to the incubation media as compared to the total β-hexosaminidase cellular content. Data shown are the average of 3-5 independent cultures done in triplicate ± S.E. Statistical significance compared to nontarget control, * P<0.05, **P<0.01. (B, D) Lentivirus transduced cells were loaded with Fluo4-AM and FuraRed-AM. Following antigen (Ag) stimulation, calcium responses were measured using confocal microscopy and are shown as a ratio of intensity of Fluo4-AM/FuraRed-AM. One representative experiment is shown averaging 15 to 20 individual cells/ time point. Data shown are a mean ± S.E. Similar results were obtained in 3 independent cultures measuring calcium as the ratio of emission at 510 nm when FURA-2AM loaded cells were excited at 340 and 380 nm in populational analysis (data not shown).
Calcium is needed for degranulation and SphKs have been linked to calcium responses in IgE/Ag-activated MC (21, 24, 26, 47). Consistent with the effect on degranulation, calcium responses were reduced in both PDMC and BMMC (Fig. 3B, 3D, respectively) when SphK2, but not SphK1, was silenced.
To further explore the apparent selectivity of SphK2 in murine MC responses, we also measured inflammatory mediators that are produced and secreted at later times following antigen stimulation. Silencing of SphK2 resulted in a significant impairment in production of IL-6 and TNF-α in both PDMC (Fig. 4A, 4B) and BMMC (Fig. 4D, 4E). Leukotriene B4 (LTB4) production was also significantly impaired in PDMC in which SphK2 was silenced (Fig. 4C); however, only a slight reduction in the secretion of LTB4 was seen in BMMC (Fig. 4F), suggesting that SphK2 may not be required for production of this lipid mediator in this population of MC. Silencing of SphK1 in BMMC also resulted in a trend towards reduced cytokine production, particularly IL-6, suggesting a possible role for SphK1 in cytokine production in this type of MC, although significance was not achieved (Fig. 4D, 4E). Collectively, the shRNA-mediated silencing of SphK1 and Sphk2 in fully differentiated BMMC and PDMC established a predominant role for SphK2 in regulating Ag-induced MC effector functions, akin to the results seen in genetically altered mouse MC (21). These findings indicate that the absence of SphK2 causes an intrinsic defect in MC function and resolves the muddling issue of potential environmental or developmental defects in genetically-deleted SphK1 and 2 MC (Fig. 1 and (21)).
Figure 4. shRNA-mediated silencing of SphK2 results in impaired cytokine and LTB4 production, while partial effects are observed by silencing SphK1 in BMMC.
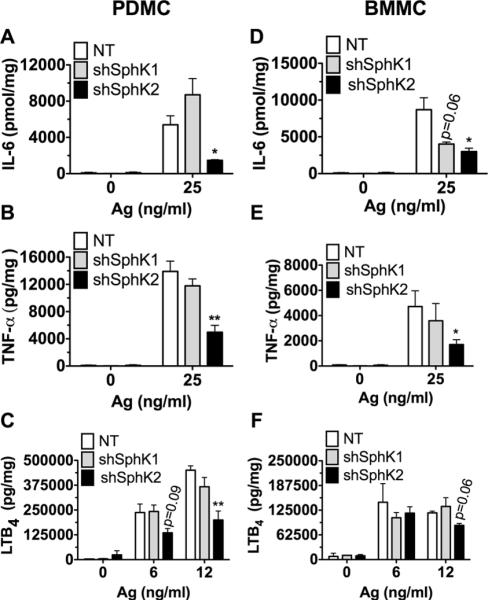
Lentivirus transduced PDMC (A-C) and BMMC (D-F) were sensitized with anti-DNP IgE and stimulated with antigen (DNP-HSA) for 3 h for cytokine and 30 minutes for leukotriene B4 production. The amount of IL-6 (A and D) and TNF-α (B and E) released into the media was determined using Bio-Plex magnetic beads based cytokine assays. LTB4 released into the media was quantified by ELISA (C and F). Data represent the mean ± S.E. of 3-5 individual cultures. Statistical significance by an unpaired two-tailed Student's t test compared to nontarget control, * P<0.05, **P<0.01.
SphK1 and SphK2 are required for migration of murine MC towards antigen
MC migrate towards a variety of stimuli, such as SCF, highly cytokinergic IgE, antigen, S1P, as well as cytokines and chemokines (30, 48, 49). Jolly et al. reported that MC migration to antigen was mediated through FcεRI-mediated activation of SphK1, which leads to the formation of S1P and to the subsequent transactivation of S1PR1 receptor (30). This model of S1P-dependent migration was also shown for HuMC (31). Using LDMC and BMMC derived from SphK1 and SphK2-null mice, we found that both SphK isoforms were required for MC migration towards antigen in a transwell migration assay (Fig. 5A, 5B) as well as migration towards SCF (30 ng/ml, data not shown). Furthermore, the absence of both SphK1 and SphK2 in LDMC did not show additional reduction (Fig. 5A). Similarly, shRNA silencing of SphK1 and SphK2 also caused a marked impairment in MC migration in both PDMC and BMMC, indicating a role for both isoenzymes in MC migration (Fig. 5C, 5D) that contrasted with the dominant role for SphK2 in murine MC degranulation and cytokine production.
Figure 5. MC migration requires both SphK1 and SphK2.
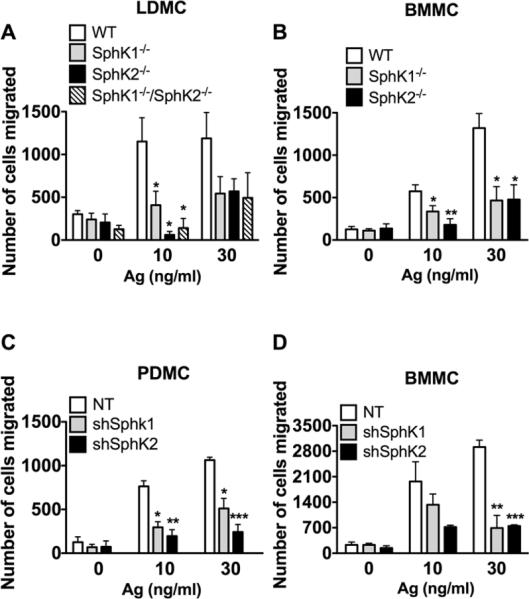
Fetal liver (A) and bone marrow (B) MC were derived from SphK1-, SphK2- mice (A, B) or SphK1/SphK2 double-deficient (A) mice. Alternatively, shRNA was used to silence SphK1 and SphK2 in peritoneal (C) and bone marrow-derived (D) MC. The ability of MC to migrate towards increasing concentrations of antigen was measured using transwell assays. Cells were placed in the upper inserts and the indicated concentrations of antigen were placed in the lower chamber. Cells were allowed to migrate for three hours. The number of cells migrated was quantified using Cyquant-GR dye. Data represent the mean ± S.E. of at least three individual cultures. Statistical significance compared to WT (A and B) or nontarget (NT) control (C and D), * P<0.05, **P<0.01, ***P<0.001. Despite the trends, some comparisons did not achieve statistical significance due to variability in the total of cells migrating between different cultures. However, in each experiment silencing or genetic deletion of SphK1 or SphK2 showed an average of at least a 50% reduction in mast cell migration regardless of the MC type. Deletion or silencing of SphK1 or SphK2 also resulted in impairment of MC migration towards SCF (30 ng/ml), a potent MC chemoattractant. In LDMC migration to SCF was as follows: WT: 3402±823 ; SphK1-/-:1450± 650 ; SphK2-/-: 719±167; SphK1-/- /SphK2-/-: 473 ± 148 (mean+ SEM).
(mean+ SEM).
SphK1 is necessary for effector responses in human MC
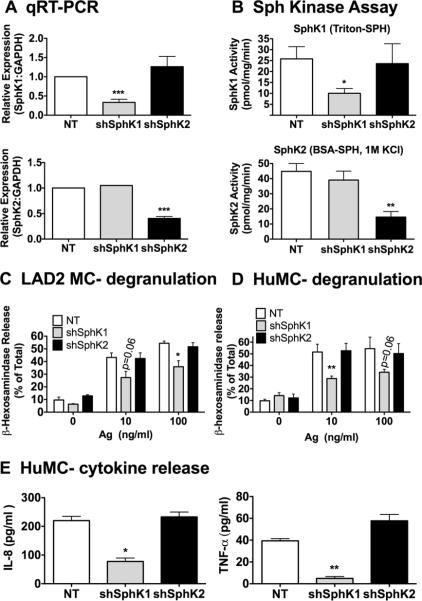
Studies using human bone marrow MC and cord blood-derived MC have demonstrated that SphK1 is the dominant isoform in HuMC function (26, 31). Given that such studies employed transient siRNA for silencing SphK1 and 2, we wished to confirm the reported findings using shRNA to selectively target SphK1 and SphK2 in LAD2 cells, an SCF-dependent human MC line, and in HuMC. LAD2 cells are immortalized cells that, unlike the primary HuMC, can be sustained in long-term cultures, (50), and therefore provided an ideal system to screen the efficacy of various shRNA constructs. As in mouse MC, the efficiency of RNA and protein silencing of SphK1 and 2 was between 60-75% as determined by real time PCR and the respective enzymatic activities (Fig. 6A, 6B). Similar knockdown efficiencies were obtained in HuMC using these constructs (data not shown).
Figure 6. SphK1 is required for IgE/Ag mediated responses in human MC.
LAD2 and CD34+-derived HuMC were transduced with lentivirus containing shRNA sequences specific for SphK1, SphK2, or nontarget shRNA control. Specific knockdown of SphK1 (upper panels) or SphK2 (lower panels) in LAD2 cells were confirmed at the mRNA level using qRT-PCR (A) and by measuring enzymatic activity (B) as described above. Degranulation was determined in LAD2 MC (C) and CD34+-derived HuMC (D) transduced with the indicated SphK isoform shRNA. MC were sensitized with 100 ng/ml biotinylated IgE overnight, stimulated for 30 min with the indicated concentrations of streptavidin and the release of β-hexosaminidase into the media was determined as described in methods. (E) The effect of silencing SphK1 or SphK2 on IL-8 (left) and TNF-α (right) production was also determined in CD34+-derived HuMC. HuMC sensitized as in C and D were stimulated with 100 ng/ml SA for 6 h and the amount of cytokines released was determined by Bio-Plex Pro cytokine assays. Data represent the mean ± S.E. of 2-3 individual cultures. Statistical significance compared to nontarget control, * P<0.05, **P<0.01.
The shRNA mediated silencing of SphK1 resulted in the reduced degranulation of LAD2 cells, while SphK2 silencing had no effect (Fig. 6C). Similarly, shRNA silencing of SphK1 in HuMC resulted in impaired MC degranulation whereas silencing of SphK2 did not (Fig. 6D). This is consistent with the previous reports of the requirement for SphK1 in cord blood-derived HuMC responses (26, 31). A drawback of the LAD2 cell line is that cytokine secretion is poor (31) and it is an immortalized cell line, thus, we used the CD34+ derived primary HuMC to study the effect of SphK1 and SphK2 on cytokine secretion as a potentially more accurate model of human MC function. Similar to the degranulation responses, silencing of SphK1 resulted in a reduction of IL-8 and TNF-α secretion, whereas silencing of SphK2 had no effect (Fig. 6E). These results indicate that in HuMC SphK1 is dominant and further demonstrate that the species of origin appears to be determinant of which isoform of SphK is dominant in MC function.
SphK1 splice variants have been described in both mouse and human cells whereas SphK2 variants have been described in human cells (14-18). Because these variants may differ in their cellular localization and thus potential function (12, 14), we sought to explore whether human and mouse MC showed differences in the composition and abundance of these variants. Murine PDMC (Supplemental Table I) or BMMC (data not shown) expressed only the SphK1a and/or SphK1a2 variant but, consistent with the SphK enzymatic activity data (Fig. 2B), these cells predominantly expressed SphK2 (all SphK2 transcripts in mouse code for the same protein). In HuMC, the overall expression of SphK1 and 2 was quantitatively similar to Sphk2 (as supported by the SphK activity data shown in Fig. 6B) and equally distributed between the variants 1a and 1c for SphK1, and 2a(short) and 2b(long) for SphK2 (Supplemental Table I). This more complex mixture of SphK variants in HuMC suggests the potential for greater diversity in SphK usage by HuMC relative to mouse MC.
DISCUSSION
Sphingosine kinases and their product, sphingosine-1-phosphate (S1P), have been implicated in regulating MC responses. Sphingosine kinases (SphK1 and SphK2) are activated upon FcεRI activation of MC (22-26). In mouse MC, this activation is dependent on the Src family protein tyrosine kinase Lyn and Fyn, which function to promote translocation of both SphK1 and SphK2 as well as enhance their enzymatic activity (22, 51). Translocation of these kinases to the plasma membrane enables the formation of S1P, which is vital for both immediate and delayed MC responses including degranulation, cytokine production, arachidonic acid release and leukotriene production. However, the specific contribution of each SphK isoform to the various MC functions is controversial (10). Here we found that in a particular MC type, one SphK isoform appeared to be dominant for most MC responses while the other isoform did not contribute noticeably to each measured response. Furthermore, the dominance of a particular SphK isoform depended on the species of MC origin. Thus, SphK1 is key for regulating most human MC responses whereas SphK2 is generally predominant in most mouse MC responses. In addition, we found that, in the same species, the contributions of a given isoform may differ depending on its tissue of origin. While in most murine MC studied SphK2 is functionally required, in BMMC, a less differentiated type of MC, SphK1 also seemed to contribute to cytokine production. Interestingly, SphK1 and SphK2 also showed redundancy in mouse MC migration regardless of the tissue origin of a given MC.
Mast cells are heterogeneous cells. Their phenotype varies depending on the in vivo microenvironment where they differentiate or on the in vitro experimental culture conditions used (19). The expression of SphK1 and 2 varies among tissues and cells and during development (14), although how their transcription is regulated is not well understood. Thus, the expression and usage of one or the other isoform of SphK may depend on the developmental and environmental cues to which the MC is exposed during its ontogeny and tissue differentiation. It has been reported that human MC derived from the bone marrow express only SphK1 (26). On the other hand, cord blood-derived HuMC express both SphK1 and SphK2 (31). Consistent with this latter report, we demonstrated that HuMC derived from CD34+ cells express comparable amounts of SphK1 and SphK2 enzymatic activity (Fig. 6B) and mRNA (Supplemental Table 1). Therefore, it appears reasonable to conclude that the expression of SphK isoforms depends on the tissue origin and possibly the differentiation state of the human MC. In contrast to HuMC, murine MC showed markedly different levels of SphK1 and SphK2 expression and activity, with the latter being an order of magnitude higher ((21) Fig. 2B and Supplemental Table I). This difference was independent of the tissue from which progenitors were derived (bone marrow vs. embryonic liver) or the conditions of differentiation (stem cell factor and IL-3 or differentiated in the peritoneum in vivo and expanded in vitro). This suggests that, unlike in HuMC, expression of SphKs in the mouse is less dependent on environmental signals.
The higher proportion of SphK2 activity in murine MC appears to correlate with its functional dominance, yet the division of function of these isoforms may not be related solely to their relative expression. In fact, we show that SphK1 has a role in chemotaxis in murine MC despite its lower expression suggesting that this isoform is more signaling-efficient in regulating chemotaxis. Additional evidence for this view is provided by our findings with CD34+-derived HuMC, which have equivalent levels of SphK1 and SphK2 activity (Fig. 6B), and yet, in response to IgE/Ag, showed dependence on SphK1 but not on SphK2. One hypothesis for the differential use of SphK isoforms in HuMC may be the intracellular location of these enzymes, which may differ between species. Translocation of SphKs from cytosol to the plasma membrane (12), and, in some instances, their relocation to lipid microdomains (12, 52), seems to be crucial for their signaling roles. We previously reported that in IgE/Ag-stimulated mouse BMMC, both SphK1 and SphK2 translocated to the plasma membrane in proximity to the FcεRI in lipid rafts (22, 51). Translocation of these isoforms in HuMC remains to be explored, however, it may be possible that SphK1 (26, 31) but not SphK2 translocate in these cells following FcεRI engagement. Interestingly, HuMC express a variant of SphK1 not found in mouse MC, SphK1c, which showed enhanced plasma membrane localization in human endothelial cells (18), raising the possibility of a preferential membrane association of SphK1 in HuMC when compared to mouse MC. Along these lines, the localization of SphK2 in human cells has often been described as vesicular or nuclear (17, 18, 53, 54), particularly the long isoform (2b), and the only example of receptor mediated activation of SphK2 in human cells was in cell types where SphK2 was mostly localized at the plasma membrane (55). Thus, investigation of the differences in SphK isoform or variant localization in mouse and human MC warrants future investigation. Another possible factor determining the preference for SphK1 or 2 could be that the signaling mechanisms facilitating translocation/activation of each isoform may differ depending on the species, the cell type or the stimulus, thus favoring one isoform over the other. While phosphorylation of Ser225 in SphK1 has been linked to plasma membrane association and biological functions, this site is not conserved in SphK2 (12, 56), and thus the mechanism of phosphorylation/activation of SphK2 is likely to differ. Due to the predominance of Sphk2 activity in mouse MC (Fig. 2B), this may prove a valuable cellular system for an in-depth evaluation of the molecular mechanisms regulating SphK2 activity.
Despite the overall dominance of function for one or the other isoenzyme in a particular MC type, we also found evidence for the redundant function of SphK1 and SphK2 in cytokine production and chemotaxis. This was not unexpected, since there are multiple examples of SphK1 and 2 showing overlapping functions. It has been shown that both SphK genes are needed for proper neurotube and vascular formation during embryogenesis (34); both isoforms are also required for migration in MDA-MB-453 breast cancer cells (55) and for survival of cardiomyocytes and cardioprotection by ischemic preconditioning (57, 58). In addition, both isoforms seem to contribute to glioblastoma cell growth (59) and to resistance to drug treatment in human colon cancer cells (60). In our study, the joint involvement of SphK1 and SphK2 in the regulation of cytokines was restricted to only BMMC cultures (Fig. 4D), while no involvement for SphK1 was observed in PDMC or MC derived from embryonic liver progenitors. This supports the concept that differences in phenotype between MC populations can contribute to determining which SphK isoforms are involved in certain MC responses. Both isoforms of SphK were also required for migration towards antigen in murine MC, regardless of their progenitor or phenotype (Fig. 5). Nevertheless, their contribution to cell migration was neither synergistic nor redundant since deletion of both kinases did not have additional effects on migration responses of mast cells and the lack of one isoform could not compensate for the lack of the other one. In agreement, neither SphK1- nor SphK2- deficient mice failed to accumulate MC in the gastric mucosa after induction of food allergy (61). It should be noted that MC derived from closely related species may differ in SphK usage since only SphK1 has been involved in cell migration toward antigen in the rat MC line RBL-2H3 cells (30). In this regard RBL-2H3 cells are more akin to the HuMC line LAD2 as well as cord-blood derived HuMC (31), which showed dependency on SphK1 in their migration to antigen. However, migration of HuMC to antigen, unlike rodent MC migration, was slow (within 24 h) (31) and was not observed within 4 h, a time period long enough for their robust migration to SCF (data not shown). This suggest that the dependency on SphK1 in HuMC, unlike RBL-2H3 cells, may not be a direct consequence of FcεRI engagement but of secreted chemokines/cytokines. Thus the mechanisms and regulation of migration towards antigen vary between species and the involvement of SphK isoform usage is also highly complex within and across species.
In summary, our findings conclusively demonstrate a more general role for SphK2 in murine MC function in vitro and in vivo whereas HuMC revealed a dominant role for SphK1. However, considering that the expression and/or function of SphK1 and SphK2 can be influenced by environmental cues, one cannot conclude that the dominance of SphK1 or SphK2 in human or mouse MC, respectively, can be generalized to all MC under all circumstances and for all mast cell responses. It has been well documented that mature in vivo-differentiated MC differ functionally from in vitro-differentiated MC presumably due to environmentally driven phenotypic differences and maturation (62, 63). Studies to define SphK expression in in vivo differentiated human MC would be of considerable value in determining SphK isoform usage in specific tissues. Based on such studies, specific inhibitors for SphK1 or inhibitors for both SphK1 and SphK2 could deserve some consideration in blocking allergic responses.
Supplementary Material
Figure 7. Model of SphK1 and SphK2 involvement in human and murine MC responses.
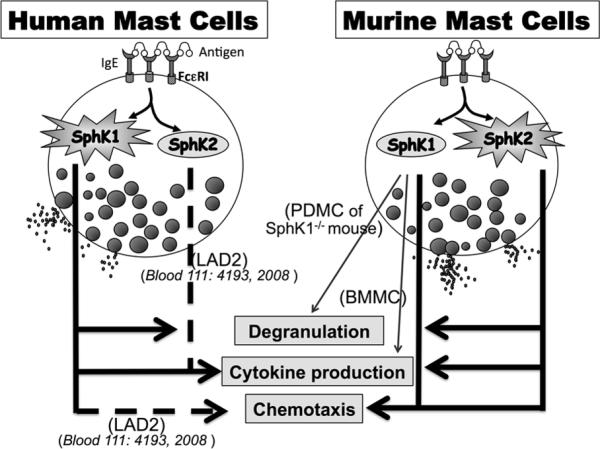
Engagement of FcεRI results in the activation of SphK1 and 2 in both human and murine MC. While SphK1 in human MC mediates degranulation and cytokine production, SphK2 is crucial for these processes in murine MC. SphK1 in human MC regulates chemotaxis towards antigen as demonstrated previously (31), while SphK1 and SphK2 jointly regulate chemotaxis of murine MC. However, the role for SphK1 or SphK2 cannot be generalized to all MC for all mast cell responses, even within the same species, since SphK1 also seems to play a role in cytokine production in BMMC and in the degranulation of PDMC from the SphK1-/- mouse (see results section and discussion), and SphK2 has a role in cytokine production in the immortalized human MC line LAD2. A changed environment (as in the complete absence of SphK1 in the mouse) or changes in the progenitors may alter the functional requirements for these kinases during allergic activation. Dashed lines represent previously reported findings, as indicated.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of the animal care program and the light image section of the Office of Science and Technology, NIAMS.
Footnotes
This work was supported by the Intramural Research Programs of NIAMS, NIDDK and NIAID, NIH.
Abbreviations used in this article:
BMMC bone marrow derived mast cell
CPA3 carboxypeptidase A3
CTMC connective tissue-like mast cell
LDMC liver derived mast cell
LTB4 leukotriene- B4
PDMC peritoneal derived mast cell
MC mast cell
mMCP murine mast cell protease
HuMC human mast cell
PCA passive cutaneous anaphylaxis
SA streptavidin
SCF stem cell factor
S1P sphingosine-1-phosphate
SphK1 sphingosine kinase 1
SphK2 sphingosine kinase 2
REFERENCES
- 1.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 2.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 5.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aktas O, Kury P, Kieseier B, Hartung HP. Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol. 2010;6:373–382. doi: 10.1038/nrneurol.2010.76. [DOI] [PubMed] [Google Scholar]
- 7.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin Immunopathol. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivera A, Rivera J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv Exp Med Biol. 2011;716:123–142. doi: 10.1007/978-1-4419-9533-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivera A. Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model. Prostaglandins Other Lipid Mediat. 2008;86:1–11. doi: 10.1016/j.prostaglandins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2010;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr., Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 14.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 15.Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 16.Kihara A, Anada Y, Igarashi Y. Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J Biol Chem. 2006;281:4532–4539. doi: 10.1074/jbc.M510308200. [DOI] [PubMed] [Google Scholar]
- 17.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 18.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 21.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 23.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after FcεRI triggering. J. Exp. Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 25.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 27.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 29.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 30.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, Milstien S, Spiegel S. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105:4736–4742. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushparaj PN, Manikandan J, Tay HK, H'Ng S C, Kumar SD, Pfeilschifter J, Huwiler A, Melendez AJ. Sphingosine kinase 1 is pivotal for Fc epsilon RI-mediated mast cell signaling and functional responses in vitro and in vivo. J Immunol. 2009;183:221–227. doi: 10.4049/jimmunol.0803430. [DOI] [PubMed] [Google Scholar]
- 33.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. The Journal of biological chemistry. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 34.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 36.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O'Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, Rivera J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–571. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im0737s90. Chapter 7:Unit 7 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 40.Kuehn HS, Radinger M, Gilfillan AM. Measuring mast cell mediator release. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im0738s91. Chapter 7:Unit7 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, Watford W, Meylan F, Diesner SC, Li L, Schnermann J, Proia RL, Rivera J. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki R, Liu X, Olivera A, Aguiniga L, Yamashita Y, Blank U, Ambudkar I, Rivera J. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J Leukoc Biol. 2010;88:863–875. doi: 10.1189/jlb.0510253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, Befus AD. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010;3:111–128. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- 45.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daeron M. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465–6475. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 47.Ryu SD, Lee HS, Suk HY, Park CS, Choi OH. Cross-linking of FcepsilonRI causes Ca2+ mobilization via a sphingosine kinase pathway in a clathrin-dependent manner. Cell Calcium. 2009;45:99–108. doi: 10.1016/j.ceca.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collington SJ, Williams TJ, Weller CL. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011;32:478–485. doi: 10.1016/j.it.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Ishizuka T, Okajima F, Ishiwara M, Iizuka K, Ichimonji I, Kawata T, Tsukagoshi H, Dobashi K, Nakazawa T, Mori M. Sensitized mast cells migrate toward the antigen: a response regulated by p38 mitogen-activated protein kinase and Rho-associated coiled-coil-forming protein kinase. J Immunol. 2001;167:2298–2304. doi: 10.4049/jimmunol.167.4.2298. [DOI] [PubMed] [Google Scholar]
- 50.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 51.Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hengst JA, Guilford JM, Fox TE, Wang X, Conroy EJ, Yun JK. Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Arch Biochem Biophys. 2009;492:62–73. doi: 10.1016/j.abb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, Dent P, Milstien S, Spiegel S. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 54.Don AS, Rosen H. A lipid binding domain in sphingosine kinase 2. Biochem Biophys Res Commun. 2009;380:87–92. doi: 10.1016/j.bbrc.2009.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 56.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 57.Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc Res. 2009;82:184–192. doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 60.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, Banno Y. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diesner SC, Olivera A, Dillahunt S, Schultz C, Watzlawek T, Forster-Waldl E, Pollak A, Jensen-Jarolim E, Untersmayr E, Rivera J. Sphingosine-kinase 1 and 2 contribute to oral sensitization and effector phase in a mouse model of food allergy. Immunol Lett. 2012;141:210–219. doi: 10.1016/j.imlet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.