Abstract
Purpose
To report the long-term results of the Intergroup Radiation Therapy Oncology Group 91-11 study evaluating the contribution of chemotherapy added to radiation therapy (RT) for larynx preservation.
Patients and Methods
Patients with stage III or IV glottic or supraglottic squamous cell cancer were randomly assigned to induction cisplatin/fluorouracil (PF) followed by RT (control arm), concomitant cisplatin/RT, or RT alone. The composite end point of laryngectomy-free survival (LFS) was the primary end point.
Results
Five hundred twenty patients were analyzed. Median follow-up for surviving patients is 10.8 years. Both chemotherapy regimens significantly improved LFS compared with RT alone (induction chemotherapy v RT alone: hazard ratio [HR], 0.75; 95% CI, 0.59 to 0.95; P = .02; concomitant chemotherapy v RT alone: HR, 0.78; 95% CI, 0.78 to 0.98; P = .03). Overall survival did not differ significantly, although there was a possibility of worse outcome with concomitant relative to induction chemotherapy (HR, 1.25; 95% CI, 0.98 to 1.61; P = .08). Concomitant cisplatin/RT significantly improved the larynx preservation rate over induction PF followed by RT (HR, 0.58; 95% CI, 0.37 to 0.89; P = .0050) and over RT alone (P < .001), whereas induction PF followed by RT was not better than treatment with RT alone (HR, 1.26; 95% CI, 0.88 to 1.82; P = .35). No difference in late effects was detected, but deaths not attributed to larynx cancer or treatment were higher with concomitant chemotherapy (30.8% v 20.8% with induction chemotherapy and 16.9% with RT alone).
Conclusion
These 10-year results show that induction PF followed by RT and concomitant cisplatin/RT show similar efficacy for the composite end point of LFS. Locoregional control and larynx preservation were significantly improved with concomitant cisplatin/RT compared with the induction arm or RT alone. New strategies that improve organ preservation and function with less morbidity are needed.
INTRODUCTION
Over the last decade, results of prospective, randomized controlled trials have changed the standard of care and clinical practice for management of locally advanced head and neck cancer. One of these trials, Radiation Therapy Oncology Group (RTOG) 91-11, for resectable stage III and IV cancer of the larynx, led to a change in the treatment paradigm for larynx preservation from induction cisplatin and fluorouracil (PF) followed, in good responders, by radiotherapy (RT) to concomitant cisplatin/RT.1,2
In 2003, we published the results of RTOG 91-11, a comparison of induction PF followed by RT, concomitant cisplatin/RT, and RT alone, after a median follow-up of 3.8 years.1 The goals of this trial were to determine the contribution of chemotherapy added to RT and the optimal sequencing of chemotherapy and RT to achieve larynx preservation. Induction PF was the control group based on the results of the Veterans Administration Laryngeal Study Group trial that compared induction PF followed by RT with laryngectomy followed by RT.2 We now report the long-term update (5- and 10-year results) and analyses of pattern of failure, cause of death, and late effects.
PATIENTS AND METHODS
The details of eligibility, chemotherapy, and RT are provided in the previous report1 and are summarized briefly here.
Patient Population
Eligible patients had stage III or IV squamous cell cancer of the supraglottic or glottic larynx curable with laryngectomy and RT. T1 primaries and high-volume T4 primaries (invasion > 1 cm into the base of tongue or penetration through cartilage) were excluded.
Random Assignment and Treatment
Patients were stratified for site (glottis or supraglottis), T stage (T2, T3 with fixed cord, T3 with no cord fixation, or T4), and N stage (N0-1 or N2-3) and then randomly assigned to one of three regimens. Group 1 (induction, control arm) received up to three cycles of PF (cisplatin 100 mg/m2 on day 1 and fluorouracil 1,000 mg/m2 per day for 5 days) every 3 weeks. Responders (≥ 50% reduction of the primary tumor and at least stable disease in the neck) received RT (2 Gy per fraction in 35 treatments to 70 Gy). Group 2 (concomitant) received cisplatin 100 mg/m2 on days 1, 22, and 43 of RT (70 Gy). Group 3 (RT alone) received RT (70 Gy). Salvage surgery was performed for patients in group 1 who achieved less than a partial response at the primary site or who experienced progression in the neck after two cycles of PF or progression at any time during induction; salvage surgery was performed in all groups in patients with biopsy-proven persistent disease after completing RT or for subsequent recurrence. A planned neck dissection was recommended for patients with N2 or N3 disease at initial staging. Patients underwent a comprehensive head and neck examination approximately 8 weeks from completion of RT, every 3 months thereafter for 1 year, semi-annually during years 2 and 3, and then annually thereafter. Imaging of the neck was performed at the 8-week follow-up visit and then as clinically indicated.
Statistical Design and Analysis
The primary objective was maximizing survival with preservation of laryngeal function, with the control group being induction PF followed by RT. This primary end point was assessed through determination of survival with a preserved larynx (laryngectomy-free survival [LFS]), overall survival, disease-free survival, laryngectomy rate, pattern of relapse, acute and late effects, and quality-of-life measures. For LFS, disease-free survival, and overall survival, the Kaplan-Meier method was used to estimate the failure rates, and the log-rank test was used to compare patient groups.3,4 For other study end points with death as the only competing risk, the cumulative incidence method was used to estimate failure rates, and Gray's test was used to compare groups.5 All efficacy end points were measured from the date of random assignment to the date of the event or competing risk; otherwise, patients were censored at the date of their last follow-up visit. To account for differences in the timing of protocol-specified disease assessments between groups, patients with recurrence or censored before 6 months after random assignment were counted as having treatment failure or censored at 6 months, for efficacy end points other than overall survival. Cox proportional hazards models6 were used to compare patient groups. Eligible patients who received protocol treatment were included in the toxicity analysis, whereas all eligible patients were included in the efficacy analysis.
The cutoff for designating acute and late effects was 90 days after the end of radiotherapy. Speech and swallowing effects were updated yearly and were based on the percentage of patients who were disease free with intact larynx and with speech or swallowing information reported.
To explore the causes of death, an analysis of study cancer mortality and non–study cancer mortality, per the methods of Peto et al,6a has been added. Deaths attributed to causes other than study cancer were categorized as deaths not caused by study cancer. All other deaths were categorized as study cancer deaths. To prevent late recurrences from biasing the analyses of cause-specific mortality, the log-rank analysis of non–study cancer mortality covered only the period before recurrence (ie, data are censored at the first recurrence).
RESULTS
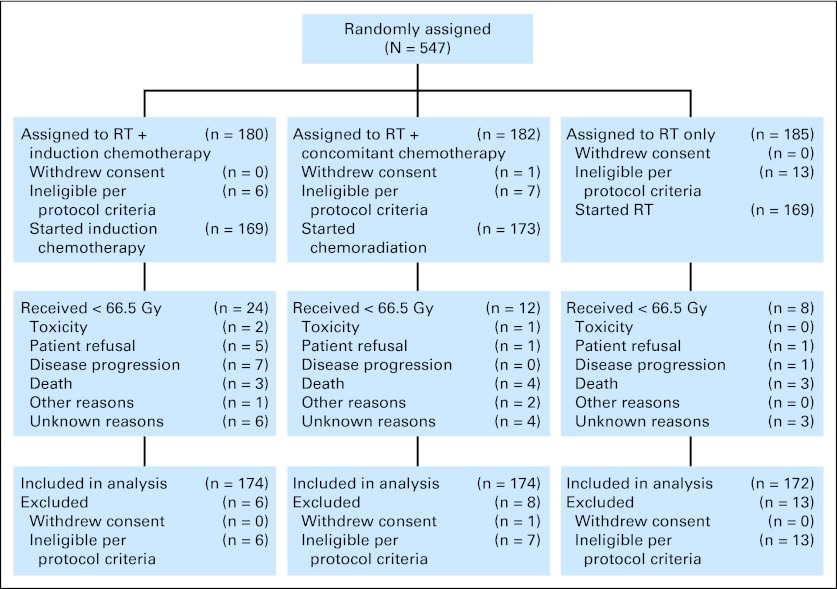
A total of 547 patients were enrolled between August 1992 and May 2000 (Fig 1). This analysis was performed after a median follow-up for surviving patients of 10.8 years (range, 0.07 to 17 years); only 11.7% of patients were alive with less than 10 years of follow-up. One patient withdrew consent and 26 patients were retrospectively declared ineligible, for a total of 520 analyzable patients (174 assigned to induction, 174 assigned to concomitant, and 172 assigned to RT alone). Sixty-four percent of patients had stage III disease; primary site was supraglottic in 69% of patients; and T and N stages were distributed as follows: T2, 11%; T3, 79%; T4, 10%; N0, 50%; N1, 21%; N2, 28%; and N3, 2% (American Joint Committee on Cancer TNM staging system).
Fig 1.
CONSORT diagram. RT, radiation therapy.
Late Toxicity
Appendix Table A1 (online only) lists the worst late toxicity using the RTOG late toxicity scoring system. Subcutaneous, salivary gland, pharynx/esophagus, and larynx toxicities were the most frequent serious events. These complications led to fatal events in all groups (four deaths, three deaths, and one death in induction, concomitant, and RT alone arms, respectively). The 10-year cumulative rates of grade 3 to 5 late toxicity were 30.6%, 33.3%, and 38% in induction, concomitant, and RT alone arms, respectively. We did not detect any significant differences in cumulative incidence between treatment groups.
Preservation of the Larynx
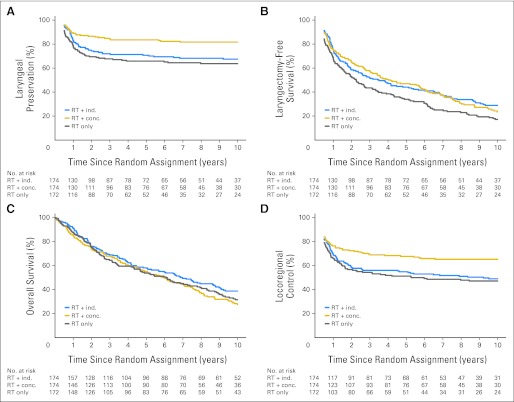
The 5- and 10-year results are listed in Table 1; hazard ratios (HRs) are listed in Table 2. A total of 148 patients underwent laryngectomy, including 55 (67.5% larynx preservation), 32 (81.7%), and 61 patients (63.8%) in the induction, concomitant, and RT alone arms, respectively; 80% of laryngectomies were performed during the first 2 years (84 during year 1 and 35 during year 2). Laryngectomy was performed for persistent or recurrent disease except for three patients who were noncompliant with their assigned treatment and nine patients with laryngeal dysfunction (four patients in induction PF arm, two patients in concomitant cisplatin/RT arm, and three patients in RT alone arm). The significant advantage of concomitant cisplatin/RT for larynx preservation (compared with the induction and RT alone treatment groups) persists (Fig 2A). Concomitant cisplatin/RT resulted in a 54% relative reduction in risk of laryngectomy compared with RT alone (HR, 0.46; 95% CI, 0.30 to 0.71; P < .001) and a 42% reduction compared with the induction arm (HR, 0.58; 95% CI, 0.37 to 0.89; P = .005). There was no significant difference when comparing RT alone with induction (HR, 1.26; 95% CI, 0.88 to 1.82; P = .35).
Table 1.
The 5- and 10-Year Estimates of Efficacy End Points
| End Point | RT + Induction Chemotherapy |
RT + Concomitant Chemotherapy |
RT Alone |
|||
|---|---|---|---|---|---|---|
| Estimate (%) | 95% CI (%) | Estimate (%) | 95% CI (%) | Estimate (%) | 95% CI (%) | |
| Laryngectomy-free survival | ||||||
| 5 years | 44.1 | 36.6 to 51.6 | 47.0 | 39.5 to 54.5 | 34.0 | 26.8 to 41.3 |
| 10 years | 28.9 | 21.9 to 36.0 | 23.5 | 16.8 to 30.3 | 17.2 | 11.2 to 23.3 |
| Larynx preservation | ||||||
| 5 years | 70.8 | 63.9 to 77.6 | 83.6 | 78.1 to 89.2 | 65.8 | 58.7 to 73.0 |
| 10 years | 67.5 | 60.4 to 74.6 | 81.7 | 75.9 to 87.6 | 63.8 | 56.5 to 71.1 |
| Local control | ||||||
| 5 years | 58.2 | 50.8 to 65.6 | 71.1 | 64.3 to 77.9 | 53.6 | 46.1 to 61.1 |
| 10 years | 53.7 | 46.1 to 61.2 | 69.2 | 62.3 to 76.1 | 50.1 | 42.5 to 57.7 |
| Locoregional control | ||||||
| 5 years | 54.8 | 47.3 to 62.3 | 67.7 | 60.7 to 74.7 | 51.2 | 43.7 to 58.8 |
| 10 years | 48.9 | 41.3 to 56.5 | 65.3 | 58.1 to 72.4 | 47.2 | 39.6 to 54.8 |
| Distant control | ||||||
| 5 years | 85.3 | 79.9 to 90.6 | 86.4 | 81.2 to 91.6 | 78.0 | 71.7 to 84.3 |
| 10 years | 83.4 | 77.7 to 89.0 | 83.9 | 78.2 to 89.5 | 76.0 | 69.4 to 82.5 |
| Disease-free survival | ||||||
| 5 years | 37.7 | 30.4 to 45.0 | 38.0 | 30.8 to 45.3 | 28.0 | 21.1 to 34.8 |
| 10 years | 20.4 | 14.0 to 26.7 | 21.6 | 15.2 to 28.0 | 14.8 | 9.2 to 20.3 |
| Overall survival | ||||||
| 5 years | 58.1 | 50.6 to 65.5 | 55.1 | 47.6 to 62.6 | 53.8 | 46.1 to 61.4 |
| 10 years | 38.8 | 31.2 to 46.3 | 27.5 | 20.4 to 34.5 | 31.5 | 24.1 to 39.0 |
Abbreviation: RT, radiation therapy.
Table 2.
Hazard Ratios for Efficacy End Points
| End Point | Hazard Ratio* | 95% CI | P |
|---|---|---|---|
| Laryngectomy-free survival | |||
| RT + concomitant v RT + induction | 1.05 | 0.83 to 1.34 | .68 |
| RT alone v RT + induction | 1.33 | 1.05 to 1.69 | .02 |
| RT + concomitant v RT alone | 0.78 | 0.61 to 0.98 | .03 |
| Laryngeal preservation | |||
| RT + concomitant v RT + induction | 0.58 | 0.37 to 0.89 | .005 |
| RT alone v RT + induction | 1.26 | 0.88 to 1.82 | .35 |
| RT + concomitant v RT alone | 0.46 | 0.30 to 0.71 | < .001 |
| Local control | |||
| RT + concomitant v RT + induction | 0.66 | 0.47 to 0.93 | .006 |
| RT alone v RT + induction | 1.18 | 0.87 to 1.60 | .50 |
| RT + concomitant v RT alone | 0.57 | 0.40 to 0.80 | < .001 |
| Locoregional control | |||
| RT + concomitant v RT + induction | 0.66 | 0.48 to 0.92 | .0037 |
| RT alone v RT + induction | 1.13 | 0.84 to 1.52 | .72 |
| RT + concomitant v RT alone | 0.59 | 0.43 to 0.82 | .0015 |
| Distant control | |||
| RT + concomitant v RT + induction | 1.11 | 0.66 to 1.86 | .88 |
| RT alone v RT + induction | 1.59 | 0.99 to 2.58 | .06 |
| RT + concomitant v RT alone | 0.69 | 0.43 to 1.11 | .08 |
| Disease-free survival | |||
| RT + concomitant v RT + induction | 0.98 | 0.78 to 1.24 | .88 |
| RT alone v RT + induction | 1.26 | 1.00 to 1.58 | .06 |
| RT + concomitant v RT alone | 0.78 | 0.62 to 0.98 | .04 |
| Overall survival | |||
| RT + concomitant v RT + induction | 1.25 | 0.98 to 1.61 | .08 |
| RT alone v RT + induction | 1.15 | 0.89 to 1.47 | .29 |
| RT + concomitant v RT alone | 1.08 | 0.85 to 1.39 | .53 |
Abbreviation: RT, radiation therapy.
Hazard ratio = hazard for first treatment listed/hazard for second treatment listed.
Fig 2.
(A) Laryngeal preservation, (B) laryngectomy-free survival, (C) overall survival, and (D) locoregional control according to treatment group. conc., concomitant; ind., induction; RT, radiation therapy.
Speech and Swallowing
We analyzed speech and swallowing based on symptom scales reported in patients alive and disease free with a retained larynx. Impaired speech or voice quality described as “moderate difficulty saying some words, and cannot use the phone; only family and/or friends can understand me; or cannot be understood” was reported during years 2 to 5 in 3% to 9% of patients in the induction group, 4% to 8.5% of patients in the concomitant group, and 5% to 8.5% of patients in the RT alone group.
Swallowing dysfunction classified as “can only swallow soft foods” or worse reported over the same time interval fluctuated between 13% and 14% of patients in the induction group, 17% to 24% of patients in the concomitant group, and 10% to 17% of patients in the RT alone group. The ability to swallow only liquids was reported in less than 4% of patients in all groups, and inability to swallow was reported in less than 3% of patients in all groups at any time point. In view of diminishing numbers of patients and missing information, the interpretation of these data is limited but does not show any substantive differences in quality of function based on treatment.
For the composite end point of LFS, there is no difference between the concomitant arm (23.5% at 10 years) versus the induction arm (control arm; 28.9%), with an HR of 1.05 (95% CI, 0.83 to 1.34; P = .68). However, LFS is significantly worse for the RT alone group (17.2%) versus both the induction group (HR, 1.33; 95% CI, 1.05 to 1.69; P = .02) and concomitant group (HR, 1.29; 95% CI, 1.03 to 1.63; P = .03; Fig 2B).
Survival Outcomes
Overall survival (Fig 2C) did not differ in any of the treatment comparisons, with 5- and 10-year estimates of 58% and 39% for induction, 55% and 28% for concomitant, and 54% and 32% for RT alone, respectively. After about 4.5 years, the curves begin to separate favoring induction, although the difference is not statistically significant. The HRs for the overall survival comparisons of all randomly assigned patients (intent to treat) were similar to those shown in Table 2 for the analysis of eligible patients (concomitant v induction: HR, 1.25; 95% CI, 0.98 to 1.61; P = .08; RT alone v induction: HR, 1.15; 95% CI, 0.89 to 1.47; P = .29; concomitant v RT alone: HR, 1.08; 95% CI, 0.85 to 1.39; P = .53).
There was no significant difference in overall survival comparing patients who did and did not undergo salvage laryngectomy within 1 year of completing treatment (P = .21; Fig 3A). An exploratory analysis showed a significantly worse survival for patients who had a laryngectomy in both chemotherapy groups compared with those who did not (induction, P = .03; concomitant, P = .01) and no difference for RT alone (P = .20; Figs 3B to 3D). For disease-free survival, only the comparison of concomitant chemotherapy versus RT alone reaches statistical significance (HR, 0.78; 95% CI, 0.62 to 0.98; P = .04), although the comparison of RT versus induction is close (HR, 1.26; 95% CI, 1.00 to 1.58; P = .06).
Fig 3.
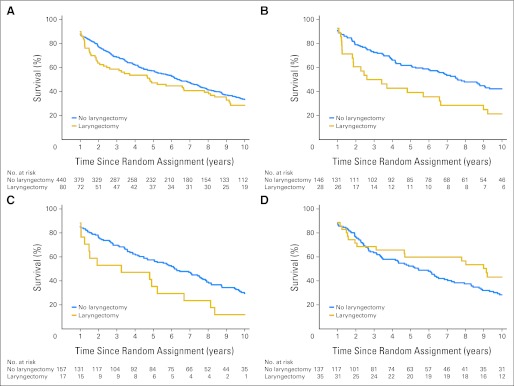
Survival according to whether or not a laryngectomy was performed in the first year: (A) all treatment arms combined (P = .21); (B) induction arm (P = .03); (C) concomitant arm (P = .01); and (D) radiation therapy–only arm (P = .20).
Pattern of First Failure
The outcome for 444 patients (induction, n = 144; concomitant, n = 152; RT alone, n = 148) who were disease free after completing protocol radiation is shown in Appendix Table A2 (online only). Two hundred twenty-eight patients (51%) died without recurrence or are alive without recurrence. The proportions of patients in the induction, concomitant, and RT alone groups with recurrence and the site as a component of recurrence were as follows: local, 33.3%, 22.3%, and 35.8%; regional, 7.6%, 3.3%, and 11.5%; and distant, 10.4%, 11.2%, and 14.9%, respectively. The results for locoregional control (Fig 2D) parallel those for larynx preservation. Concomitant cisplatin/RT resulted in a 41% reduction in risk of locoregional failure compared with RT alone (HR, 0.59; 95% CI, 0.43 to 0.82; P = .0015) and a 34% reduction compared with induction (HR, 0.66; 95% CI, 0.48 to 0.92; P = .0037).
Distant Metastases
Chemotherapy given concomitantly or as induction showed similar benefit for achieving distant control (7% to 8% difference compared with RT alone), but neither comparison reached statistical significance (P = .08 and P = .06, respectively). The distant recurrence rate was low, with 28, 29, and 41 patients experiencing distant recurrence in the induction, concomitant, and RT alone groups, respectively.
Cause of Death
A total of 374 patients have died. The RT alone group had the highest proportion of deaths attributed to larynx cancer, and the concomitant group had the lowest (Table 3). More deaths were classified as unrelated to either larynx cancer or treatment in the concomitant group (30.8%) compared with the induction group (20.8%) and the RT alone group (16.9%), whereas the proportion of deaths classified as unknown/not reported was similar for all groups (17.7%, 19.2%, and 16.1%, respectively).
Table 3.
Cause of Death
| Cause of Death | RT + Induction Chemotherapy |
RT + Concomitant Chemotherapy |
RT Alone |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Cancer under study | 45 | 37.5 | 38 | 29.2 | 60 | 48.4 |
| Second malignancy | 15 | 12.5 | 18 | 13.8 | 15 | 12.1 |
| Complications of protocol treatment | 9 | 7.5 | 9 | 6.9 | 5 | 4.0 |
| Complications of other treatment | 3 | 2.5 | 2 | 1.5 | 3 | 2.4 |
| Unrelated to cancer or treatment | 25 | 20.8 | 40 | 30.8 | 21 | 16.9 |
| Unknown/not reported | 23 | 19.2 | 23 | 17.7 | 20 | 16.1 |
| Total deaths | 120 | 130 | 124 | |||
Abbreviation: RT, radiation therapy.
We conducted an exploratory analysis of death caused by study cancer and death not caused by study cancer (Figs 4A and 4B). There were no statistically significant pairwise differences between the treatment groups for death caused by the study cancer. However, for death from causes not related to the study cancer, there was a significant disadvantage for the concomitant group compared with the induction group (52.8% v 69.8%, respectively, at 10 years; P = .03).
Fig 4.
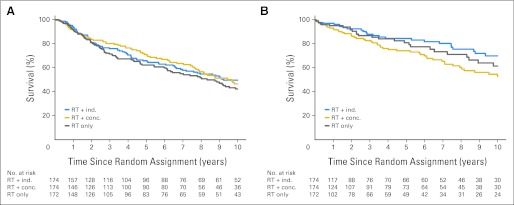
Survival, limited to (A) deaths from study cancer and (B) deaths not caused by study cancer according to treatment group. conc., concomitant; ind., induction; RT, radiation therapy.
DISCUSSION
Intergroup trial RTOG 91-11, designed more than two decades ago, compared concomitant cisplatin/RT and RT alone with the control group of induction PF followed by RT. The composite end point of LFS was chosen as the primary end point. For this outcome, treatment with concomitant cisplatin/RT compared with induction is not significantly different and treatment with RT alone is significantly inferior. However, when larynx preservation and survival are separately analyzed, the significant advantage of concomitant cisplatin/RT for larynx preservation and securing locoregional control is confirmed; induction PF was not better than RT alone. With longer follow-up, differences in overall survival were not statistically significant, but there was a suggestion of better survival in patients treated with induction PF. After 4.5 years, the survival curves show some separation, and the death rate in the concomitant treatment group remains constant. Deaths in later years of follow-up resulted in LFS changing from nonsignificant to significant for induction PF compared with RT alone (and obscure the significantly inferior larynx preservation achieved by induction PF compared with concomitant treatment). More deaths in the concomitant group were unrelated to larynx cancer. This occurred with no apparent increase in late effects. This raises the possibility of fatal treatment-related episodes not identified with the current system for monitoring and grading late effects.
Function, as assessed through patient-reported outcomes, was excellent. The results for each treatment group were consistent over time. Although we did not collect information on gastrostomy tube usage, few patients (< 5%) were limited to liquids or unable to swallow.
The composite end point of LFS was chosen as the primary end point for sample size calculation in 1990 using an estimate of 66% (based on the crude rate of 52 of 79 patients reported alive with their larynx in the Veterans Administration Laryngeal Study Group report).2 However, LFS had not previously been used or reported. In this report, induction and concomitant treatments show similar LFS. This composite end point obscures the significant difference in local control and larynx preservation rates and underscores what are now recognized as significant limitations of an ill-considered end point.7 Evaluating each end point separately (eg, survival, larynx preservation, locoregional control) provides the clearest information and does not equally weight death and loss of one's larynx.8
For stage III or IV larynx cancer studied in this trial (T1 and high-volume T4 primary tumors excluded), the larynx can be preserved in the majority of patients with any of the three nonsurgical approaches tested; however, nearly twice as many patients will undergo laryngectomy if treated with induction PF or RT alone instead of concomitant cisplatin/RT. The results also suggest that improved local control and high rates of larynx preservation achievable with concomitant high-dose cisplatin/RT may be accompanied by increased risk of serious late effects perhaps as a consequence of more acute mucosal toxicity.1 Whether that risk would be the same with today's RT techniques (three-dimensional or intensity-modulated RT), which provide much better conformality and normal tissue sparing, is unknown. Most patients in RTOG 91-11 were treated with simple two-dimensional plans. The reason for the increase in late noncancer deaths in the concomitant group remains speculative.
The finding that induction PF did not add to treatment with RT alone for achieving locoregional control or larynx preservation was consistent with previous trials of induction PF and with the updated Meta-Analysis of Chemotherapy for Head and Neck Cancer, which showed no effect of induction PF on local control, contrasted with an absolute difference in local failure of −13.5 ± 2.8% for concomitant treatment.9 A phase III comparison of alternating PF and RT versus induction PF followed by RT for larynx and hypopharynx cancers postulating improved outcomes with the alternating regimen showed no difference in larynx preservation.10 Lower doses of chemotherapy and RT in the alternating treatment regimen (in an effort to reduce toxicity) may have contributed to the negative result.
Strategies for further investigation include RT with a different radiosensitizing agent (with or without a more effective induction regimen). However when compared with PF induction, no differences were detected for local, locoregional, and distant failure or (in subset analyses) for overall survival for larynx cancer, stage III cancers, T3 and T4 cancers, and N0 and N1 cancers for taxane plus PF (TPF) followed by carboplatin/RT.11 The Groupe Oncologie Radiothérapie Tête et Cou (GORTEC) 2000-0112 trial investigated TPF induction compared with PF induction followed by RT for larynx preservation in patients with T3 and selected T4 cancers of the larynx or hypopharynx. The preliminary findings showed higher rates of larynx preservation with induction TPF, likely because of the better response rate with TPF (80% v 59% for PF; P = .002), which was the decision point for radiation or laryngectomy. On the basis of this result, induction TPF is considered a standard treatment option by European investigators. The findings were not reported separately for these two biologically different primary sites, so the specific benefit for larynx cancer is uncertain.
A randomized trial comparing RT with or without cetuximab in patients with locally advanced, stage III or IV head and neck cancer showed significantly improved overall and locoregional failure-free survival (a composite end point) but no difference in distant metastasis.13 Locoregional control was not reported. Subset analyses suggested that the benefit of cetuximab was limited to patients with oropharynx cancer. The RTOG has evaluated RT/cisplatin with or without cetuximab and found no improvement in any outcome with the addition of cetuximab14; however, a direct comparison of cetuximab/RT and cisplatin/RT is only now being undertaken in patients with human papillomavirus–related cancers of the oropharynx (RTOG 1016). This is a relevant question for intermediate-stage larynx cancer, where cetuximab/RT would be an attractive alternative, if efficacy were comparable.
Integrating chemotherapy and conservation laryngeal surgery in selected patients with T3 disease warrants investigation.15,16 Our exploratory analysis of the outcome after salvage laryngectomy for patients receiving induction or concomitant chemotherapy suggests that early identification of patients who will eventually experience failure with nonsurgical therapy may be important for long-term survival.
In conclusion, the long-term results of Intergroup RTOG 91-11 show that for the composite end point of LFS, induction and concomitant treatments had similar efficacy. However, locoregional control and larynx preservation were significantly improved with concomitant treatment compared with induction or RT alone. No differences in late toxicity or speech or swallowing function were demonstrated, but there was an unexplained increase in deaths unrelated to cancer in patients who received concomitant cisplatin/RT. This may be responsible for the absence of a survival advantage for the concomitant treatment group. For intermediate-stage larynx cancer, new strategies focusing on improved locoregional control should be undertaken, and better assessment of late events should be performed.
Appendix
Table A1.
Patients With a Late Toxicity by Type and Grade
| Late Toxicity | No. of Patients |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT + Induction Chemotherapy (n = 154) |
RT + Concomitant Chemotherapy (n = 157) |
RT Alone (n = 158) |
|||||||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hematologic NOS | 14 | 4 | 0 | 0 | 0 | 21 | 6 | 3 | 0 | 0 | 16 | 3 | 1 | 0 | 0 |
| Skin (within RT field) | 51 | 16 | 5 | 1 | 1 | 58 | 16 | 1 | 0 | 0 | 59 | 20 | 2 | 1 | 0 |
| Mucous membrane/stomatitis | 32 | 23 | 5 | 0 | 0 | 40 | 23 | 3 | 0 | 0 | 42 | 25 | 3 | 1 | 0 |
| Subcutaneous tissue | 33 | 34 | 11 | 1 | 0 | 46 | 34 | 9 | 1 | 0 | 40 | 35 | 9 | 2 | 0 |
| Salivary gland | 30 | 72 | 9 | 0 | 0 | 52 | 53 | 9 | 0 | 0 | 39 | 72 | 6 | 0 | 0 |
| Pharynx/esophagus | 38 | 19 | 15 | 3 | 2 | 39 | 20 | 22 | 3 | 1 | 34 | 30 | 22 | 2 | 0 |
| Larynx | 43 | 41 | 10 | 6 | 1 | 50 | 40 | 17 | 6 | 1 | 27 | 52 | 21 | 8 | 0 |
| Upper GI | 4 | 4 | 2 | 0 | 0 | 8 | 4 | 0 | 0 | 0 | 8 | 3 | 0 | 0 | 0 |
| GU/renal | 6 | 2 | 0 | 0 | 0 | 12 | 4 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | 0 |
| Spinal cord | 5 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Neurologic | 10 | 6 | 0 | 0 | 0 | 20 | 5 | 3 | 0 | 0 | 15 | 3 | 1 | 1 | 0 |
| Bone | 2 | 1 | 1 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 7 | 3 | 0 | 0 | 0 |
| Joint | 8 | 0 | 2 | 0 | 0 | 11 | 2 | 0 | 0 | 0 | 8 | 3 | 1 | 0 | 0 |
| Other | 18 | 27 | 1 | 2 | 1 | 20 | 36 | 7 | 1 | 1 | 14 | 26 | 12 | 1 | 1 |
Abbreviations: GU, genitourinary; NOS, not otherwise specified; RT, radiation therapy.
Table A2.
Pattern of First Failure
| Treatment Failure | RT + Induction Chemotherapy (n = 144) |
RT + Concomitant Chemotherapy (n = 152) |
RT Alone (n = 148) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| No failure, alive | 34 | 23.6 | 35 | 23.0 | 26 | 17.6 |
| No failure, dead | 38 | 26.4 | 60 | 39.5 | 35 | 23.6 |
| Local only | 45 | 31.3 | 32 | 21.1 | 45 | 30.4 |
| Regional only | 8 | 5.6 | 3 | 2.0 | 7 | 4.7 |
| Local and regional | 3 | 2.1 | 1 | 0.7 | 8 | 5.4 |
| Local and/or regional, and distant | 0 | 2 | 1.3 | 2 | 1.4 | |
| Distant only | 15 | 10.4 | 15 | 9.9 | 20 | 13.5 |
| Dead, study cancer NOS | 1 | 0.7 | 4 | 2.6 | 5 | 3.4 |
NOTE. Does not include second primary tumors; includes all patients with no evidence of disease at the completion of protocol therapy.
Abbreviations: NOS, not otherwise specified; RT, radiation therapy.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 833 and articles on pages 840 and 853; listen to the podcast by Dr Harari at www.jco.org/podcasts
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661, Community Clinical Oncology Program Grant No. U10 CA37422, and Eastern Cooperative Oncology Group Grants No. CA16116 and CA21115 from the National Cancer Institute.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Arlene A. Forastiere, Qiang Zhang, Randal S. Weber, Helmuth Goepfert, Thomas F. Pajak, John F. Ensley, Jay S. Cooper
Administrative support: John F. Ensley
Provision of study materials or patients: Arlene A. Forastiere
Collection and assembly of data: Thomas F. Pajak, Wade Thorstad
Data analysis and interpretation: Arlene A. Forastiere, Qiang Zhang, Randal S. Weber, Moshe H. Maor, Helmuth Goepfert, Thomas F. Pajak, William Morrison, Bonnie Glisson, Andy Trotti, John A. Ridge, Henry Wagner
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 2.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer: The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 4.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 5.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 6.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 6a.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, II: Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forastiere AA, Trotti AM. Searching for less toxic larynx preservation: A need for common definitions and metrics. J Natl Cancer Inst. 2009;101:129–131. doi: 10.1093/jnci/djn490. [DOI] [PubMed] [Google Scholar]
- 8.List MA, Stracks J, Colangelo L, et al. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–884. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142–152. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorch JH, Goloubeva O, Haddad RI, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: Long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–159. doi: 10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 13.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 14.Ang K. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):360s. doi: 10.1200/JCO.2013.53.5633. abstr 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holsinger FC, Kies MS, Diaz EM, Jr, et al. Durable long-term remission with chemotherapy alone for stage II to IV laryngeal cancer. J Clin Oncol. 2009;27:1976–1982. doi: 10.1200/JCO.2008.17.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forastiere AA, Shaha AR. Chemotherapy alone for laryngeal preservation: Is it possible? J Clin Oncol. 2009;27:1933–1934. doi: 10.1200/JCO.2008.20.9445. [DOI] [PubMed] [Google Scholar]