Abstract
Purpose
The purpose of this study was to determine remission induction frequency when bortezomib was combined with daunorubicin and cytarabine in previously untreated older adults with acute myeloid leukemia (AML) and safety of bortezomib in combination with consolidation chemotherapy consisting of intermediate-dose cytarabine (Int-DAC).
Patients and Methods
Ninety-five adults (age 60 to 75 years; median, 67 years) with previously untreated AML (including therapy-related and previous myelodysplastic syndrome) received bortezomib 1.3 mg/m2 intravenously (IV) on days 1, 4, 8, and 11 with daunorubicin 60 mg/m2 on days 1 through 3 and cytarabine 100 mg/m2 by continuous IV infusion on days 1 through 7. Patients who achieved complete remission (CR) received up to two courses of consolidation chemotherapy with cytarabine 2 gm/m2 on days 1 through 5 with bortezomib. Three cohorts with escalating dose levels of bortezomib were tested (0.7, 1.0, and 1.3 mg/m2). Dose-limiting toxicities were assessed during the first cycle of consolidation. The relationship between cell surface expression of CD74 and clinical outcome was assessed.
Results
Frequency of CR was 65% (62 of 95), and 4% of patients (four of 95) achieved CR with incomplete platelet recovery (CRp). Eleven patients developed grade 3 sensory neuropathy. Bortezomib plus Int-DAC proved tolerable at the highest dose tested. Lower CD74 expression was associated with CR/CRp (P = .04) but not with disease-free or overall survival.
Conclusion
The addition of bortezomib to standard 3 + 7 daunorubicin and cytarabine induction chemotherapy for AML resulted in an encouraging remission rate. The maximum tested dose of bortezomib administered in combination with Int-DAC for remission consolidation was 1.3 mg/m2 and proved tolerable. Further testing of this regimen is planned.
INTRODUCTION
Remission induction chemotherapy with cytarabine and an anthracycline has been a standard treatment for newly diagnosed acute myeloid leukemia (AML) for more than 30 years. The complete remission (CR) rate for patients with AML age > 60 years is 40% to 60%, and several strategies have been employed to intensify the induction regimen in the hope of improving the remission rate; however, none have provided a clear advantage to induction therapy with cytarabine and an anthracycline.1–3 Therefore, agents capable of improving on existing regimens with minimal added toxicity are clearly needed for this age group.
Bortezomib is a potent, reversible, and specific inhibitor of the proteasome, a target for antineoplastic agents.4 By inhibiting the proteasome, bortezomib has been demonstrated to inhibit the master transcription factor nuclear factor-kappa B (NF-κB), which is increased in the active form in AML cells5–7 and leukemia stem cells (LSCs).5,8
We previously demonstrated that bortezomib can be safely added to induction chemotherapy in patients with relapsed or refractory AML, resulting in an encouraging remission rate.9 We also previously demonstrated that expression of CD74, the major histocompatibility complex class II chaperone molecule and receptor for macrophage migration inhibitory factor (MIF), which signals via NF-κB and other molecules,10 was associated with clinical outcome in patients with AML treated with bortezomib, idarubicin, and cytarabine.9,11
The goal of this study was to determine the CR rate in older patients with AML treated with bortezomib plus daunorubicin and cytarabine and the maximum-tolerated dose (MTD) of bortezomib when added to intermediate-dose cytarabine (Int-DAC). We also sought to determine the disease-free (DFS) and overall survival (OS) of patients in this study and the relationship between CD74 expression and clinical outcome.
PATIENTS AND METHODS
Eligibility for the study was restricted to patients age 60 to 75 years with previously untreated AML. An unequivocal histologic diagnosis of AML based on WHO criteria (≥ 20% blasts in bone marrow) was required. Acute promyelocytic leukemia was excluded. Patients with therapy-related AML and previous myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN) were eligible. Additional eligibility information is provided in the Appendix (online only).
Treatment
For remission induction, bortezomib 1.3 mg/m2 was administered by rapid intravenous (IV) bolus (3 to 5 seconds) on days 1, 4, 8, and 11. Bortezomib was the first drug to be administered on day 1, 1 hour before daunorubicin. It was recommended that bortezomib be administered at the same time on days 4, 8, and 11 as on day 1 ± approximately 2 hours. Daunorubicin 60 mg/m2/d was administered by IV injection or short IV infusion on days 1 through 3 at approximately the same time daily. The dose of daunorubicin was reduced if the bilirubin level was elevated (daunorubicin was reduced by 25% for total bilirubin between 2 and 3 mg/dL and by 50% for total bilirubin > 3 mg/dL). Cytarabine 100 mg/m2/d was administered by continuous IV infusion on days 1 through 7 and was started after bortezomib and daunorubicin were administered on day 1. Bone marrow examination was performed on day 18 ± 1 day. Day 18, rather than day 14, was selected to allow for an interval of 7 days from the last chemotherapy administration (day 11 bortezomib) to the assessment of bone marrow. Patients with residual leukemia received a second course of remission induction chemotherapy, which consisted of bortezomib 1.3 mg/m2 on days 1 and 4, daunorubicin 60 mg/m2/d on days 1 through 2, and cytarabine 100 mg/m2/d by continuous IV infusion on days 1 through 5. Bone marrow examination (aspirate and biopsy) was required within 1 week of recovery of the absolute neutrophil count ≥ 1,000/μL and platelets ≥ 100,000/μL, but no later than day 42 of the final induction course.
Patients who achieved CR or partial remission (PR; defined as meeting criteria for CR but with reduction in bone marrow blasts by ≥ 50% to 5% to 25%) were eligible to receive consolidation therapy, as were patients achieving CR with incomplete platelet recovery (CRp; defined as meeting criteria for CR but with platelet count < 100,000/μL) who recovered platelets to ≥ 100,000/μL within 4 weeks of achieving CRp. Patients were reregistered through the Alliance Statistics and Data Center for the phase II portion of the trial.
During consolidation chemotherapy, the dose of bortezomib was escalated in cohorts. Bortezomib dose cohorts were assigned at the time of registration to remission consolidation therapy. Bortezomib was administered on days 1, 4, 8, and 11 and was the first drug to be administered on day 1. Beginning 1 hour after bortezomib on day 1, cytarabine 2 g/m2 by IV infusion over 3 hours once daily was administered on days 1 through 5. Before the cytarabine infusion, corticosteroid ophthalmic solution two drops to each eye 4× daily was initiated and continued for at least 24 hours after the final dose of cytarabine.
Patients were enrolled in one of three consecutive bortezomib dose cohorts, each consisting of three to six patients. The three dose cohorts of bortezomib assessed were: 0.7, 1.0, and 1.3 mg/m2. Enrollment in a cohort ceased until all three patients in a cohort could be fully assessed for treatment-related toxicities. If a cohort already contained three patients currently undergoing evaluation for dose-limiting toxicity (DLT), subsequent patients were treated at the previous cohort dose of bortezomib already tested. If no DLTs were experienced by the first three patients, the dose was escalated to the next level. If one DLT was experienced among the first three patients, an additional three patients were enrolled at the same dose level. If < two DLTs were experienced among these six patients, dose escalation was permitted. If ≥ two DLTs were experienced among three to six patients at a given dose level, the previous lower dose was declared the MTD. Once the maximally tolerated, or maximally tested, dose of bortezomib was identified, at least 10 additional patients were to be treated with Int-DAC in combination with bortezomib at this dose. The maximum planned dose of bortezomib was 1.3 mg/m2.
Patients who experienced bortezomib-related neuropathic pain and/or peripheral sensory neuropathy were managed according to dose-reduction and discontinuation guidelines set forth in the protocol. DLTs were considered only during the first cycle of consolidation therapy and included grade 3 or 4 sensory or autonomic neuropathy, persistent grade 4 thrombocytopenia or neutropenia at day 42 in the absence of AML, any grade 4 or 5 nonhematologic toxicity, and any grade 3 nonhematologic toxicity (excluding neuropathy and toxicities secondary to neutropenia and sepsis) that did not resolve to grade 2 by day 42 unless attributable to persistent or recurrent AML. Grade 4 anorexia (requiring total parenteral nutrition) and grade 4 fatigue (requiring bed rest) are commonly observed in older patients during AML treatment and were not considered DLTs.
For patients who completed the first consolidation cycle without DLT and without development of new grade ≥ 3 nonhematologic or infectious toxicities, a second cycle of consolidation chemotherapy was administered using the same dose of bortezomib used in the first cycle. Some patients discontinued protocol treatment to pursue allogeneic hematopoietic cell transplantation (alloHCT). All treated patients were observed for DFS, OS, and event-free survival (EFS).
Statistical Analyses
There were two treatment components to this phase II/I trial: one, remission induction chemotherapy consisting of bortezomib at a previously established dose combined with daunorubicin and cytarabine (ie, 3 + 7), followed by two, remission consolidation chemotherapy with a dose escalation of bortezomib administered with Int-DAC.
The primary clinical end point for the remission induction portion of the trial was complete response (CR and CRp). The primary end point of the dose-escalation portion of the trial was the determination of DLT and identification of the MTD of bortezomib when combined with Int-DAC. The secondary clinical end points were: one, to define the toxicities of bortezomib when combined with intermediate-dose cytarabine, and two, to define DFS and OS of patients treated in this study.
Regarding the induction phase, previous studies have suggested a 50% CR rate for a similar older patient population treated with comparable 3 + 7 chemotherapy.12 Therefore, the phase II portion of this trial was designed to discriminate between true CR rates of no more than 45% and at least 65%. Regarding the consolidation phase, an initial dose finding phase was used to establish the MTD combination of bortezomib and Int-DAC. Information regarding definitions, cytogenetic analysis, assessment of CD74 expression levels, and statistical analyses is provided in the Appendix (online only).
RESULTS
Patient Characteristics
Ninety-eight patients age 60 to 75 years were enrolled between September 15, 2008, and February 26, 2010, at 15 CALGB (Cancer and Leukemia Group B) member institutions and their affiliated hospitals. Three patients did not begin treatment and were excluded from all analyses. Baseline characteristics for the 95 treated patients are listed in Table 1.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 67 | |
| Range | 60–75 | |
| Male sex | 53 | 56 |
| WBC | ||
| Median | 4.7 | |
| Range | 0.5–230.7 | |
| Performance status | ||
| 0 | 31 | 33 |
| 1 | 55 | 58 |
| 2 | 9 | 10 |
| Race* | ||
| White | 86 | 95 |
| Black | 3 | 3 |
| Asian | 1 | 1 |
| Pacific Islander | 1 | 1 |
| Other | 4 | 4 |
| AML type | ||
| De novo | 74 | 78 |
| Therapy related | 7 | 7 |
| After MDS/MPN | 14 | 15 |
| ELN genetic group† | ||
| Favorable | 10 | 11 |
| Intermediate-1 | 17 | 18 |
| Intermediate-2 | 33 | 35 |
| Adverse | 11 | 12 |
Abbreviations: AML, acute myeloid leukemia; ELN, European LeukemiaNet; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
Total may exceed 100% because of rounding.
Available for 71 patients.
Response to Induction Chemotherapy
Among the first 45 patients, 30 (67%) achieved CR, thereby excluding a true CR incidence of < 45%. Among all 95 patients, CR frequency was 65% (62 of 95); an additional 4% of patients (four of 95) achieved CRp. Twenty-nine patients (31% of total) received a second cycle of induction therapy; 17 of these patients (59% of this group) achieved CR. Response rates by type of AML and ELN (European LeukemiaNet) genetic group classification are shown in Tables 2. There was no statistically significant difference in CR rate by AML type according to ELN group. Of eight patients with FLT3-ITD, six (75%) achieved CR.
Table 2.
Response Rate According to Type of AML and ELN Genetic Group Classification
| Category | No. of Patients | CR |
CRp |
PR |
|||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Type of AML | |||||||
| De novo | 74 | 48 | 65 | 3 | 4 | 1 | 1 |
| Therapy related | 7 | 6 | 86 | 1 | 14 | 0 | 0 |
| After MDS/MPN | 14 | 8 | 57 | 0 | 0 | 1 | 7 |
| All | 95 | 62 | 65 | 4 | 4 | 2 | 2 |
| ELN genetic group | |||||||
| Favorable | 10 | 9 | 90 | 0 | 0 | 0 | 0 |
| Intermediate-I | 17 | 12 | 71 | 1 | 6 | 0 | 0 |
| Intermediate-II | 33 | 17 | 52 | 2 | 6 | 0 | 0 |
| Adverse | 11 | 5 | 45 | 1 | 10 | 0 | 0 |
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; CRp, complete remission with incomplete platelet recovery; ELN, European LeukemiaNet; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PR, partial response.
Consolidation Treatment
Of the 62 patients who achieved CR, 41 received at least one cycle of consolidation. Twenty-five patients received two cycles of consolidation. One patient whose best response was PR also received one cycle of consolidation.
During consolidation, there were no DLTs within each of the first two three-person cohorts treated with bortezomib 0.7 and 1.0 mg/m2, respectively. One of the first three patients treated with Int-DAC and bortezomib 1.3 mg/m2 sustained a DLT of grade 3 sensory neuropathy. There were no DLTs in the second cohort of three patients treated with bortezomib 1.3 mg/m2 and Int-DAC. Therefore, an additional 11 patients were treated with 1.3 mg/m2 in consolidation.
Treatment Toxicities
In induction, 96% of patients had grade 4 hematologic toxicity during induction. In addition, there were 51 patients with grade 3 and three patients with grade 4 febrile neutropenia. There were 40 patients with grade 3 and two patients with grade 4 clinically documented infections. There were eight deaths in induction, six in primary induction (one resulting from pulmonary hemorrhage; three, infection; one, cerebral vascular accident; and one, respiratory failure), and two in reinduction (both resulting from infection).
In consolidation, 98% of patients had grade 4 hematologic toxicity. There were 15 patients with grade 3 and one patient with grade 4 febrile neutropenia and 12 patients with grade 3 and two patients with grade 4 clinically documented infections. There was one treatment-related death resulting from infection during consolidation. Nonhematologic and noninfectious toxicities of grade ≥ 3 occurring in at least 10% of patients are listed in Table 3.
Table 3.
Maximal Grade ≥ 3 Nonhematologic and Noninfectious Toxicities Occurring in ≥ 10% of Patients
| Treatment Phase | Grade 3 |
Grade 4 |
Grade 5 |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Induction (n = 95) | ||||||
| Rash | 12 | 13 | ||||
| Diarrhea | 13 | 14 | ||||
| Hypoalbuminemia | 12 | 13 | ||||
| Hypophosphatemia | 14 | 15 | ||||
| Hypokalemia | 12 | 13 | 3 | 3 | ||
| Dyspnea | 11 | 12 | 2 | 2 | ||
| Hypoxia | 6 | 6 | 5 | 5 | ||
| Consolidation one (n = 41) | ||||||
| Hypotension | 5 | 12 | 1 | 2 | ||
| Fatigue | 4 | 10 | ||||
| Muscle weakness | 4 | 10 | ||||
| Pain | 5 | 12 | ||||
Neuropathy
All patients received bortezomib during cycle one of induction, and 97% of the 29 patients receiving a second induction course received bortezomib (3% did not receive bortezomib because of neuropathy). Table 4 indicates all grade neuropathies reported. There were 11 patients who developed grade 3 sensory neuropathy at some point during therapy. Six of these were during the first induction cycle, three during the second induction cycle, and two during consolidation. In general, investigators reported that neuropathy lessened or resolved within weeks of treatment; however, long-term grade 1 sensory neuropathy persisted in several patients.
Table 4.
All Grade Neuropathies According to Treatment Phase
| Treatment Phase | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Induction (n = 95) | ||||||||||
| Cranial | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Motor | 0 | 0 | 0 | 0 | 4 | 4 | 1 | 1 | 0 | 0 |
| Sensory | 9 | 10 | 4 | 4 | 6 | 6 | 0 | 0 | 0 | 0 |
| Re-induction (n = 29) | ||||||||||
| Cranial | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Motor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensory | 5 | 17 | 2 | 7 | 3 | 10 | 0 | 0 | 0 | 0 |
| Consolidation one (n = 41) | ||||||||||
| Cranial | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Motor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensory | 5 | 12 | 5 | 12 | 2 | 5 | 0 | 0 | 0 | 0 |
| Consolidation two (n = 25) | ||||||||||
| Cranial | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Motor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensory | 4 | 16 | 4 | 16 | 1 | 4 | 0 | 0 | 0 | 0 |
Survival and Long-Term Follow-Up
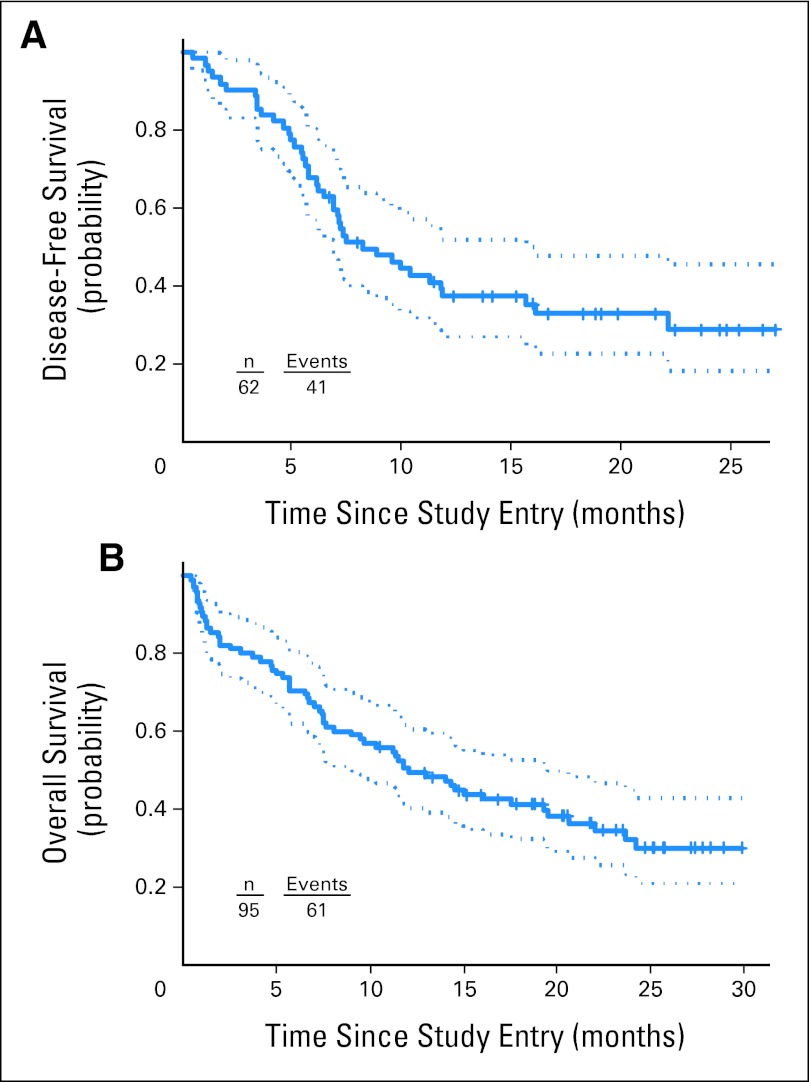
Median follow-up time for the 34 patients still alive was 22 months (range, 11 to 30 months). Treatment outcomes and progression to consolidation and alloHCT are provided in Figure 1. Median DFS and OS for patients on this study were 8 and 12 months, respectively (Fig 2).
Fig 1.
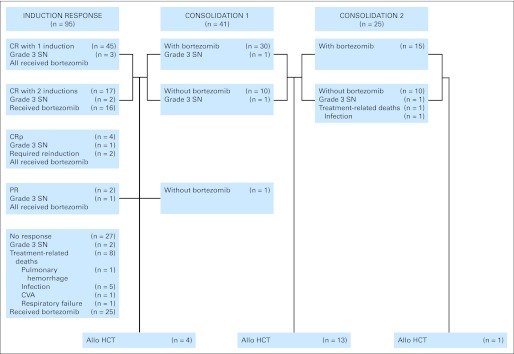
Flow chart indicating patient outcome, treatment-related deaths, and occurrence of grade 3 sensory neuropathy. Allo HCT, allogeneic hematopoietic cell transplantation; CR, complete remission; CRp, complete remission with incomplete platelet recovery; CVA, cerebral vascular accident; PR, partial response; SN, sensory neuropathy.
Fig 2.
Survival plots and 95% CIs indicating (A) disease-free and (B) overall survival of patients treated in this study.
Twenty-five patients received an allogeneic transplant. Of these 25 patients, 18 underwent alloHCT while in first CR, and their estimated DFS and OS at 24 months are 54% (95% CI, 28% to 74%) and 52% (95% CI, 25% to 73%), respectively.
For the 48 patients who achieved CR or CRp and did not undergo alloHCT, median DFS and OS were 7.4 and 17.5 months, respectively. The estimated OS at 24 months is 36% (95% CI, 20% to 51%); the estimated DFS at 24 months is 19% (95% CI, 7% to 36%). ELN genetic group was not significantly correlated with EFS, DFS, or OS.
Relationship of CD74 Expression to Clinical Outcome
The median percentage of CD74+ cells (28%; range, 0% to 95%) was significantly lower in patients with CR/CRp than in those who did not respond to therapy (52%; range, 2% to 97%; P = .040). The median CD74 molecules of equivalent fluorescein (MEFS) for patients who achieved CR/CRp (7,232; range, 15 to 223,987) was also lower compared with patients who did not respond (16,551; range, 929 to 201,985; P = .086). Neither CD74 expression nor MEFS was correlated with patient age, WBC, or percentage of blood or bone marrow blasts. Neither CD74 expression nor MEFS was correlated with DFS, OS, or EFS.
CD74 expression on blast cells by MEFS, although not the percentage of cells expressing CD74, was associated with ELN genetic group (P = .045 v .166). Given the relatively small number of patients within each genetic group, we combined favorable and intermediate-I into one combined group and intermediate-II and adverse into a second combined group for the purpose of determining if increased CD74 expression correlated with progressively worse combined group. In this analysis, increased CD74 by both percentage of cells expressing CD74 (P = .034) and CD74 expression according to MEFS (P = .015) was significantly associated with worse combined group.
DISCUSSION
In this study, which enrolled older adults with newly diagnosed and secondary AML, we assessed the CR rate when bortezomib was added to standard induction chemotherapy consisting of daunorubicin and cytarabine and determined the safety of bortezomib when added to intermediate-dose cytarabine chemotherapy in consolidation. We also assessed the relationship between CD74 expression by flow cytometry and clinical outcome.
The combination of bortezomib with daunorubicin and cytarabine in induction resulted in an encouraging remission frequency of 65%, with an additional 4% CRp. A similar remission frequency was seen in our previous study of bortezomib combined with idarubicin and cytarabine in patients with relapsed AML and older adults with de novo disease, in which CR and CRp rates of 61% and 10% were observed, respectively. These remission frequencies are high relative to those in recent studies12 and may have resulted from the addition of bortezomib to the 3 + 7 regimen. Bortezomib is able to induce cytotoxicity in vitro in AML cells resistant to daunorubicin, and furthermore, bortezomib induces synergistic cytotoxicity when combined with anthracyclines13 and antimetabolites.14,15 In addition, proteasome inhibition has been demonstrated to induce LSC cytotoxicity.8 Whether one of these mechanisms or others may have contributed to the favorable CR rate observed in this study will need to be explored in future clinical trials.
We established that bortezomib up to 1.3 mg/m2 has an acceptable toxicity profile when combined with Int-DAC during consolidation. This observation is consistent with other studies in patients with mantle-cell lymphoma, in which bortezomib was well tolerated when added to single-agent cytarabine16 and the R-HyperCVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) regimen.17
Grade 3 sensory neurotoxicity was observed in 11 patients. Neurotoxicity is frequently observed with bortezomib in multiple myeloma, but typically only after multiple cycles. Possible explanations for the neurotoxicity observed in this study may relate to the more intensive treatment required for AML; coadministration with cytarabine, which is potentially neurotoxic at higher doses; or effects of concomitant medications such as antifungal agents used in the treatment of AML. Strategies such as altering the route of administration of bortezomib from IV to subcutaneous, changing the schedule from twice to once per week, or using other proteasome inhibitors with less neurotoxicity, such as carfilzomib, may reduce neurotoxicity without sacrificing clinical efficacy; these approaches warrant investigation in future clinical studies.
We assessed the relationship between CD74 expression by flow cytometry and found that lower levels were significantly correlated with increased CR/CRp and favorable ELN genetic groups. We used flow cytometry to assess CD74 expression rather than gene expression because flow is routinely conducted in the workup of AML and is less time consuming than microarray gene expression profiling. The relationship between CD74 and CR/CRp differs with that in a previous smaller study of 31 patients with AML treated with bortezomib, idarubicin, and cytarabine,6 possibly because of differences between intracellular and cell surface expression of CD74. In other studies, increased CD74 expression by microarray has been significantly correlated with adverse DFS/OS,11 as has increased expression of the CD74 fragment CLIP.18,19 In this study, we did not observe a significant relationship between CD74 and DFS/OS, possibly because a substantial number of patients went on to undergo alloHCT. Taken together, however, our current findings raise the possibility that the CD74 expression levels on AML blasts at diagnosis may potentially serve as a novel marker capable of identifying patients likely to achieve CR/CRp with the combination of daunorubicin, cytarabine, and bortezomib. Additional studies will be required to determine if CD74 is associated with ELN genetic group.
We examined the safety and efficacy of bortezomib in combination with AML induction and consolidation chemotherapy because bortezomib interacts synergistically with chemotherapeutic agents to induce apoptosis in leukemia cells and because proteasome inhibition has been demonstrated to target LSCs in vitro and in models of LSC function. We demonstrated that the combination of bortezomib with 3 + 7 chemotherapy results in an encouraging remission frequency, that bortezomib 1.3 mg/m2 has acceptable toxicity with Int-DAC in consolidation, and that CD74 expression by flow cytometry is inversely correlated with complete remission. A phase III study in which patients are randomly assigned to receive bortezomib or a double-blinded placebo would be required to define the specific clinical benefit of bortezomib with induction and consolidation chemotherapy. Because bortezomib was recently demonstrated to downregulate FLT3 expression,20 this study design would also permit testing the effect of bortezomib on FLT3 levels and LSC populations.
Acknowledgment
We thank Kathleen Donohue and Allison Booth in the Alliance Statistics and Data Center for their assistance in the conduct of this trial. We would also like to thank the patients, nurses, and physicians who participated. The following institutions participated in this study: Christiana Care Health Services, Wilmington, DE, Stephen Grubbs, MD, supported by Grant No. CA45418; Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD, supported by Grant No. CA32291; Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD, supported by Grant No. CA47577; Georgetown University Medical Center, Washington, DC, Minetta C. Liu, MD, supported by Grant No. CA77597; Monter Cancer Center of North Shore–LIJ Health Systems, Lake Success, NY, Daniel Budman, MD, supported by Grant No. CA35279; Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, MD, supported by Grant No. CA32291; Mount Sinai School of Medicine, New York, NY, Lewis R. Silverman, MD, supported by Grant No. CA04457; Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD, supported by Grant No. CA59518; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD, supported by Grant No. CA77658; University of Chicago, Chicago, IL, Hedy L. Kindler, MD, supported by Grant No. CA41287; University of Iowa, Iowa City, IA, Daniel A. Vaena, MD, supported by Grant No. CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD, supported by Grant No. CA31983; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by Grant No. CA47559; and Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, MD, supported by Grant No. CA0392.
Appendix
Eligibility
Patients with therapy-related acute myeloid leukemia (AML; defined as occurring after chemotherapy and/or irradiation for another malignancy or autoimmune disorder) were eligible for treatment in this trial, as were patients with an antecedent hematologic disorder, such as myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN), provided they had not received cytotoxic chemotherapy, including azacitidine and decitabine, for their preleukemic disorder. Antecedent MDS was defined by cytopenias and bone marrow morphology establishing a diagnosis of MDS at least 3 months before diagnosis of AML. Emergency leukapheresis and hydroxyurea were permitted before protocol treatment. Patients with grade ≥ 2 ataxia, cranial or peripheral neuropathy, left ventricular ejection fraction < 40% by echocardiogram or nuclear scan, or abnormal lung function (diffusion capacity [corrected for hemoglobin] < 50%) were excluded. There were no eligibility requirements involving performance status. The protocol was approved by institutional review boards, and protocol-specific written consent was obtained from patients according to institutional guidelines. Patient registration and data collection were conducted by the Alliance Statistics and Data Center.
As part of the quality assurance program of CALGB (Cancer and Leukemia Group B), members of the audit committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such onsite review of medical records was performed for a subgroup of 12 (12%) of the 98 patients registered to this study.
Definitions
Complete remission (CR) required all of the following: absolute neutrophil count ≥ 1000/μL, platelet count ≥ 100,000/μL, cellular bone marrow with maturation of all cell lines, and < 5% blast cells (Cheson et al: J Clin Oncol 21:4642-4649, 2003). CR with incomplete platelet recovery (CRp) was defined as CR except platelets < 100,000/μL without need for platelet transfusion. Partial response (PR) met all criteria for CR except that bone marrow blasts decreased by > 50% to 5% to 25%. Treatment failure was defined as no achievement of CR, CRp, or PR. Relapse of AML was defined as > 5% marrow blasts not attributable to another cause or development of extramedullary disease in a patient previously in CR. Disease-free survival (DFS) was measured as the interval from achievement of CR until relapse or death, regardless of cause, and overall survival (OS) as the interval from the on-study date until death. Event-free survival was measured from study entry until failure of treatment to result in achievement of CR, relapse, or death. Patients alive at last follow-up were censored for both DFS and OS. Survival function estimates were computed using the product-limit method, and survival distributions were compared using the log-rank test. Patients who underwent allogeneic hematopoietic cell transplantation were not censored at the time of transplantation. Data were updated as of October 2011. All analyses were performed by the Alliance for Clinical Trials in Oncology Statistics and Data Center.
Cytogenetic Analysis
Cytogenetic analysis was performed as part of a prospective karyotyping study, CALGB (Alliance) 8461 (Mrózek et al: Int J Oncol 33:239-244, 2008), which was a mandatory companion for the current study. Analyses used the European LeukemiaNet Classification cytogenetic risk groups (Döhner et al: Blood 115:453-474, 2010).
Measurement of CD74 Expression on Bone Marrow and Peripheral Blood Samples
Bone marrow (or peripheral blood blasts if bone marrow was not available) was evaluated for expression of CD74 antigen using qualitative and quantitative flow cytometric analysis. Flow studies were performed in a clinical flow cytometry laboratory (G.L., Ohio State University). Viable cells were analyzed based on CD45 and light side scatter characteristics to allow separation of blasts, and the percent of CD74 positive blasts was determined as the percentage of cells relative to isotype control. The quantitative determination of CD74 antibody binding per cell was performed using Quantum Simply Cellular antimouse immunoglobulin G kit (Bangs Laboratories, Fishers, IN). The antibody bound per cell (ABC) values were derived by plotting the value of mean fluorescence channel intensity for CD74 stained cells on the standard curve derived from five level capture beads stained with this antibody at a saturating concentration. The final CD74 ABC value was calculated by subtracting the ABC value for the matching isotype from the ABC for anti-CD74 stained cells for populations of blasts, monocytes, and lymphocytes.
The relationship between CD74 expression on myeloblasts and response to induction chemotherapy was conducted treating CD74 expression as a continuous variable. The P values are two-sided P values using the Wilcoxon rank sum test. The Spearman correlation coefficient was used to assess the relationship between percent CD74 and CD74 molecules of equivalent fluorescein, patient age, WBC, and percentage of marrow and blood blasts at baseline.
Statistical Analyses
Regarding the induction phase, a maximum sample size was set at 45 evaluable patients. If at least 25 CRs were observed among the 45 evaluable patients, this regimen was to be considered worthy of further testing. If no more than 12 CRs were observed among the initial 25 patients, the study was to be terminated early. This design yields at least 90% power to detect a true response rate of at least 65%. It yields at least 0.91 probability of a negative result if the true response rate is no more than 45%, with at least 0.69 probability of early negative stopping.
In the consolidation phase, the probability of ≥ one of the first three patients having a dose-limiting toxicity is approximately 0.66 if the individual probability of a treatment-related death is 0.3. The probability of < one treatment-related death in the first three patients is 0.51 if the true treatment-related mortality is no more than 0.20.
Results
There were 81 patients with CD74 data and 80 with MEF counts. Among these patients, there were 54 patients achieving CR, three achieving CRp, two achieving PR, and 22 who did not respond (18 stable, one progression, and three unevaluable).
Footnotes
Listen to the podcast by Dr Liesveld at www.jco.org/podcasts
Written on behalf of the Alliance for Clinical Trials in Oncology.
Supported by Grants No. CA32291 (E.C.A., P.C.A.); CA32291 (M.W., D.J.D., R.M.S.); CA33601 (J.L.J., B.K.M.); CA03927 (B.L.P.); CA47559 (P.M.V.); CA77658, CA101140, CA140148, and CA16058 (G.L., W.B., G.M., C.D.B.); CA35279 (J.E.K.); CA59518 (E.S.W.); CA41287 (R.A.L.); CA31946 to the Alliance for Clinical Trials in Oncology; and CA33601 to the Alliance Statistical Center from the National Cancer Institute; by the Leukemia Clinical Research Foundation (G.L., W.B., G.M., C.D.B.); and in part by Grant No. NIH-5K23CA118419-05 (E.C.A.) from the National Institutes of Health.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010, and 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00742625.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard M. Stone, Novartis (C) Stock Ownership: None Honoraria: None Research Funding: Richard M. Stone, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eyal C. Attar, Philip C. Amrein, Gerard Lozanski, Daniel J. DeAngelo, Richard M. Stone, Guido Marcucci, Clara D. Bloomfield, Barry K. Moser, Richard A. Larson
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Castaigne S, Chevret S, Archimbaud E, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: Long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104:2467–2474. doi: 10.1182/blood-2003-10-3561. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK, Milligan D, Goldstone A, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: The results of the LRF AML14 trial. Br J Haematol. 2009;145:318–332. doi: 10.1111/j.1365-2141.2009.07604.x. [DOI] [PubMed] [Google Scholar]
- 3.Brunnberg U, Mohr M, Noppeney R, et al. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: A randomized phase II trial in elderly patients. Ann Oncol. 2012;23:990–996. doi: 10.1093/annonc/mdr346. [DOI] [PubMed] [Google Scholar]
- 4.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 5.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 6.Ciftci O, Ullrich O, Schmidt CA, et al. Regulation of the nuclear proteasome activity in myelomonocytic human leukemia cells after adriamycin treatment. Blood. 2001;97:2830–2838. doi: 10.1182/blood.v97.9.2830. [DOI] [PubMed] [Google Scholar]
- 7.Brach MA, Kharbanda SM, Herrmann F, et al. Activation of the transcription factor kappa B in human KG-1 myeloid leukemia cells treated with 1-beta-D-arabinofuranosylcytosine. Mol Pharmacol. 1992;41:60–63. [PubMed] [Google Scholar]
- 8.Guzman ML, Swiderski CF, Howard DS, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attar EC, De Angelo DJ, Supko JG, et al. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14:1446–1454. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 10.Starlets D, Gore Y, Binsky I, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 11.Attar E, Maharry K, Mrozek K, et al. 1616: Increased expression of macrophage migration inhibitory factor (MIF) receptor CD74 is associated with inferior outcome in younger patients (pts) with cytogenetically normal acute myeloid leukemia (CN-AML): A Cancer and Leukemia Group B (CALGB) study. Presented at the 51st American Society of Hematology Meeting and Exposition; December 5–8, 2009; New Orleans, LA. [Google Scholar]
- 12.Baer MR, George SL, Sanford BL, et al. Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B study 9720. Leukemia. 2011;25:800–807. doi: 10.1038/leu.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matondo M, Bousquet-Dubouch MP, Gallay N, et al. Proteasome inhibitor-induced apoptosis in acute myeloid leukemia: A correlation with the proteasome status. Leuk Res. 2010;34:498–506. doi: 10.1016/j.leukres.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Minderman H, Zhou Y, O'Loughlin KL, et al. Bortezomib activity and in vitro interactions with anthracyclines and cytarabine in acute myeloid leukemia cells are independent of multidrug resistance mechanisms and p53 status. Cancer Chemother Pharmacol. 2007;60:245–255. doi: 10.1007/s00280-006-0367-6. [DOI] [PubMed] [Google Scholar]
- 15.Liesveld JL, Rosell KE, Bechelli J, et al. Proteasome inhibition in myelodysplastic syndromes and acute myelogenous leukemia cell lines. Cancer Invest. 2011;29:439–450. doi: 10.3109/07357907.2011.590567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigert O, Weidmann E, Mueck R, et al. A novel regimen combining high dose cytarabine and bortezomib has activity in multiply relapsed and refractory mantle cell lymphoma - long-term results of a multicenter observation study. Leuk Lymphoma. 2009;50:716–722. doi: 10.1080/10428190902856790. [DOI] [PubMed] [Google Scholar]
- 17.Romaguera JE, Fayad LE, McLaughlin P, et al. Phase I trial of bortezomib in combination with rituximab-HyperCVAD alternating with rituximab, methotrexate and cytarabine for untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;151:47–53. doi: 10.1111/j.1365-2141.2010.08315.x. [DOI] [PubMed] [Google Scholar]
- 18.Chamuleau ME, Souwer Y, Van Ham SM, et al. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–5550. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 19.van Luijn MM, van den Ancker W, Chamuleau ME, et al. Absence of class II-associated invariant chain peptide on leukemic blasts of patients promotes activation of autologous leukemia-reactive CD4+ T cells. Cancer Res. 2011;71:2507–2517. doi: 10.1158/0008-5472.CAN-10-3689. [DOI] [PubMed] [Google Scholar]
- 20.Blum W, Schwind S, Tarighat SS, et al. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood. 2012;119:6025–6031. doi: 10.1182/blood-2012-03-413898. [DOI] [PMC free article] [PubMed] [Google Scholar]