Abstract
A sizable segment of addiction research investigates the effects of persuasive message appeals on risky and deleterious behaviors. However, to date, little research has examined how various forms of message framing and corresponding behavioral choices might by mediated by risk-related brain regions. Using event-related functional magnetic resonance imaging, we investigated brain regions hypothesized to mediate the influence of message appeals on decision making in substance-dependent (SD) compared to non-substance-dependent (non-SD) individuals. The Iowa Gambling Task (IGT) was modified to include positively-framed, negatively-framed, and control messages about long-term deck payoffs. In the positively-framed condition, the SD and non-SD groups showed improved decision-making performance that corresponded to higher risk-aversion-related brain activity in the anterior cingulate cortex (ACC) and anterior insula (AI). In contrast, in the negatively-framed condition, the SD group showed poorer performance that corresponded to lower risk-aversion-related brain activity in the AI region. In addition, only the non-SD group showed a positive association between decision quality and greater risk-related activity in the ACC, regardless of message type. The findings suggest substance-dependent individuals may have reduced neurocognitive sensitivity in the ACC and AI regions involved in risk perception and aversion during decision-making, especially in response to framed messages that emphasize reduced prospects for long-term gains.
Keywords: cognitive control, informative messages, substance dependence, anterior cingulate
Introduction
Substance abuse and related problems are among society’s most pervasive health and social concerns (United States Department of Health and Human Services [USDHHS], 2010). To reduce the health-related risk associated with these behaviors, substantial effort is being made to construct persuasive messages that promote healthy behaviors while discouraging risky ones. Such health-related information is often described in terms of costs and benefits, based on the assumption that people respond differentially to information presented as gains and losses (Rothman & Salovey, 1997). However, the effects of message framing on health behaviors have yielded inconsistent results (Fishbein et al., 2002). In particular, the issue of how to best frame information aimed at persuading targeted clinical populations, such as substance-dependent individuals, to resist or desist from a health-degrading behavior remains an open question. This study investigates the brain regions mediating the influence of message appeals and examines how the link between persuasion and brain activity can be used to describe the impact of targeted behavior-changes in at-risk populations.
Using a young adult sample with a heterogeneous prevalence of substance dependence (SD), the present study investigates addiction-related differences in brain activity that are hypothesized to mediate the influence of message appeals on risky decision making. We used the Iowa Gambling Task (IGT) (Bechara, Damasio, Damasio, & Anderson, 1994), a model of decision making under uncertainty (Bechara, Damasio, Tranel, & Damasio, 1997), as the context for understanding the neural basis of message framing. To examine message-framing brain activation differences between the SD and non-SD groups, positively- and negatively-framed messages regarding the long-term outcomes of deck choices were used in a rapid event-related functional magnetic resonance imaging (fMRI) design (see Figure 1A).
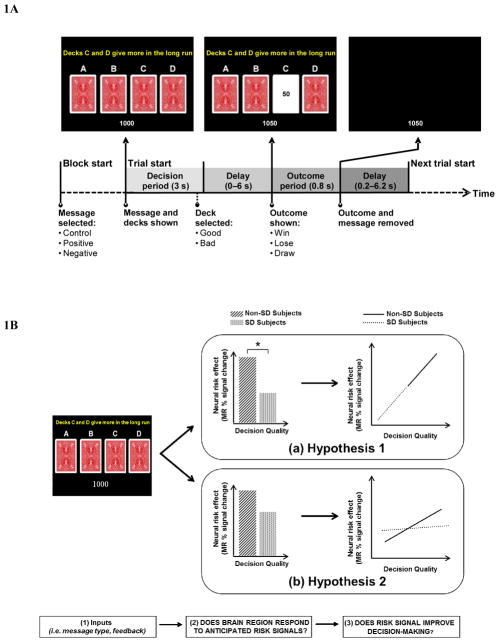
Figure 1.
Figure 1A. Schematic of a trial of the Iowa Gambling Task (Bechara, et al., 1997) with informative messages in a rapid event-related fMRI design. Images across the top represent the visual layout of the display at various times during the trial. Diagram at the bottom indicates trial timecourse with durations and relevant events as classified for fMRI analysis.
Figure 1B. Depiction of the two hypotheses about the underlying neural correlates of informative messages on choice during risky decision-making in SD group compared non-SD group: (a) is an illustration of the first hypothesis postulating the SD group to suffer from a weakened neural response to anticipated risk effects, however, showing similar correlational strengths between brain signals and behavior compared to the non-SD group; whereas (b) is a representation of the second hypothesis postulating similar brain activation levels between the two groups, but having significantly different strengths in their relationship between brain activity and task performance.
Message Framing, Addiction-related Decision Behavior, and the IGT
Cognitive neuroscience approaches, including fMRI, have provided new insights into the underlying neural mechanisms of message effectiveness on decision behavior. Neuroimaging research has focused on persuasion-induced changes in risky choice behavior (rather than addictive behavior per se) by examining brain regions involved in the influence of persuasive messages on decision making under uncertainty (Krawitz, Fukunaga, & Brown, 2010), as well as on health-related behavior change (Falk, Berkman, Mann, Harrison, & Lieberman. 2010; Mann, Sherman, & Updegraff, 2004). Relevant to the current study are findings from the health communication literature that include reports of a small but significant advantage for gain-framed over loss-framed messages to encourage disease prevention behaviors (O’Keefe & Jenson, 2007). Additionally, some studies have found conditions in which gain-framed messages are not optimally effective unless it is congruent with the individual’s motivational orientation (Mann et al. 2004; Sherman, Mann & Updegraff, 2006; Updegraff, Sherman, Luyster, & Mann, 2007). And in our previous fMRI work, we found a significant improvement in choice behavior correlated with enhanced risk-related activity for the positively-framed, but not the negatively-framed message (Krawitz et al. 2010).
Clinical studies have found the IGT particularly useful for examining deficits in decision-making processes attributed to the persistent maladaptive behavioral choices made by those with substance-use disorders (SUDs) (Bechara et al., 2001; Grant, Contoreggi, & London, 2000; Stout Busemeyer, Lin, Grant, & Bonson, 2004; Tanabe et al., 2007; Verdejo-Garcia et al., 2007; Whitlow et al., 2004). Those with SUDs consistently perform more poorly on the IGT than those without SUDs despite receiving feedback to improve their performance. Moreover, past studies show it is possible to discriminate individuals with SUDs from those without SUD problems based on brain activation differences (Tanabe et al. 2007; Ersche et al. 2005; Fishbein et al. 2005). These brain activation differences may account for impairments in decision-making involving risk. More generally, deficits in decision making among SUD individuals have been marked by hypoactivity in the orbitofrontal, anterior cingulate, and ventral medial prefrontal cortices; regions critically involved in a variety of cognitive processes, including inhibitory decision-making, cue reactivity, and craving (Dom, Sabbe, Hulstijn, & van den Brink, 2005; Tanabe et al., 2007).
The Neural Correlates of IGT Message Framing and Corresponding Decision Behavior
The primary candidate regions that may show addiction-related group differences in persuasion-induced risk appraisal in the IGT are the anterior cingulate cortex (ACC) and anterior insula (AI). The ACC is known to be critically involved in cognitive control, including performance monitoring processes, which has been found to play a key role in decision making (Li, Lu, A’Argembeau, Ng, & Bechara, 2010; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 1998; Gehring & Knight, 2000). A study of framing effects found greater ACC activity when participants made decisions based on frames that were more likely to result in loss (De Martino, Kumaran, Seymour, & Dolan, 2006). Other results have identified the ACC to be active in driving loss avoidance (Magno, Foxe, Molholm, Robertson, & Garavan, 2006), further supported by findings of increased risk-taking behaviors in drug abusers with blunted ACC responses (Fishbein et al., 2005). Recent work also has shown ACC dysregulation during reward-seeking behavior to be associated with excessive alcohol consumption (Bogg, Fukunaga, Finn, & Brown, 2012). These findings are consistent with previous work that has implicated performance-monitoring processes in ACC as a critical signal for learning the likelihood of an error (Brown & Braver, 2005) and the potential consequences of risky behavior (Brown & Braver, 2007; Brown & Braver, 2008).
In the AI, as in the ACC, activations have been identified during decision making processes (Lin, Chiu, Cheng, & Hsieh. 2008). This region has been associated with a wide range of effects including error awareness (Hester, Nestor, & Garavan, 2009), risk predictions and error in those predictions (Preuschoff, Quartz, & Bossaerts, 2008), error-specific responses (Magno et al., 2006), and making bad decisions compared to good decisions (Lawrence, Jollant, O’Daly, Zelaya, & Phillips, 2009). Neuroimaging studies also have found greater AI activation to precede decisions to avoid risk (Kuhnen & Knutson, 2005) and to drive harm avoidance during a risky response (Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003). Recently, activity in the insula has been associated with subjective cue-induced drug urges that result in subjective experiences of “craving” (Naqvi & Bechara, 2009; Naqvi, Rudrauf, Damasio, & Bechara, 2007; Verdejo-García & Bechara, 2009).
These previous findings collectively suggest the influence of messages in improving choice behavior will be correlated with risk effects in the ACC and AI. The present study, however, not only examines the brain regions implicated in the influence of message appeals, but it aims to describe the differential impact message content has on brain activity and behavior compared across substance dependent (SD) and non-SD groups. To do so, we focus on two main contrasts of interest (for details see Methods): First, we define risk effects as the difference in brain activity associated with choosing a bad deck relative to choosing a good deck, with greater neural activity reflecting both greater perceived risk and a corresponding drive to avoid such risks. Second, we define heightened-risk effects as greater neural activity reflecting greater perceived risk for a particular message appeal (e.g. positive-frame or negative-frame) compared to the control message. We used these contrasts to test our two main hypotheses:
-
H1)
Generalized Input Insensitivity Hypothesis: This hypothesis proposes SD individuals, compared to non-SD individuals, suffer from an overall insensitivity to stimulus inputs, but not from a weakened association between brain signals and behavior (see Figure 1B). Specifically, we examined whether SD individuals show patterns of hypo/hyper-activity in risk-aversion-related brain regions relative to non-SD individuals. We predicted the SD group to have a weaker ‘risk effect’ by showing a smaller difference between the risky option and safe option brain activations compared to the non-SD group, which we expected would show a larger ‘risk effect’ resulting in a greater bias toward selecting the safe option (Fukui, Murai, Fukuyama, Hayashi, & Hanakawa, 2005; Paulus et al., 2003). Similarly, we predicted weaker ‘heightened-risk effects’ in the SD group compared to the non-SD group. We predicted the non-SD group would show stronger input sensitivity to message appeals which would confer more optimal modulation of risk-aversion-related brain activity. This hypothesis is motivated by previous findings that strongly suggest that a substance user’s inability to effectively modulate risk may be related to deficits in the activation of risk-aversion-related brain regions, which is believed to lead to a greater bias toward making riskier decisions (Fishbein et al. 2005; Hester et al. 2009).
-
H2)
Weakened Brain-Behavior Association Hypothesis: This hypothesis proposes group differences in the correlational strengths between risk-aversion-related brain activity and task performance (see Figure 1B). This alternative hypothesis assumes both SD and non-SD groups share similar brain activation levels but show significantly different strengths in their relationship between brain activity and task performance. We predicted a weaker correlational strength for both the ‘risk effect’ and ‘heightened-risk effect’ in the SD group compared to the non-SD group, suggesting that despite having similar risk-aversion-related brain activation across groups, the groups differ on how message appeals influence the brain-behavior relationship. This hypothesis was motivated by findings that in some cases of substance dependence (e.g. nicotine), brain regions including the rostral ACC remain sensitive to outcomes but lose their correlation with behavior (Chiu, Lohrenz, & Montague, 2008).
Methods
All procedures were approved by the Indiana University Bloomington Institutional Review Board. Several components of the methods have been reported in our previous study (Krawitz et al., 2010)
Participants
A total of 47 subjects participated in the present study and provided written informed consent. All subjects were required to be at least 18 years of age, right-handed, and to meet standard health and safety requirements, including no history of neurological problems or claustrophobia, weigh less than 440 lbs., and have no metallic implants, for entry into the magnetic resonance imaging scanner. They were paid $25/hour for participation, plus performance bonuses based on points earned during the task.
Non-Substance-Dependent (Non-SD) Group
Non-SD subjects (n=25) were initially recruited for an earlier version of this study, and the results have been published previously (Krawitz et al. 2010). Here we use the same data to compare against the SD participants. The non-SD participants are therefore representative of the general population with a relatively low rate of substance dependence, but the original study did not explicitly exclude for current or past alcohol abuse or other substance abuse or dependence. By contrast, all subjects in the SD group were subject to a separate exclusion criteria. We further address the potential limitation of the differing screening procedures in the discussion section.
Substance-Dependent (SD) Group
SD subjects (n=22) were recruited using advertisements placed around campus and the Bloomington community (see Finn et al., 2009, for specifics about the recruitment strategy). The initial inclusion criteria for the SD group required participants to meet additional eligibility requirements: (a) be between the ages of 18 – 30 years, (b) be able to read and speak English (whether as a native or second language), (c) have at least a sixth grade level of education, (d) have consumed alcohol, (e) have no reports of suffering from any serious head injuries, (f) have no major cognitive impairments and; (f) have no history of psychotic symptoms. Individuals who met this preliminary criteria were administered a diagnostic interview, using the Semi-Structural Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994) to ascertain diagnoses for substance use disorders using DSM-IV diagnostic criteria (American Psychiatric Association [APA], 1994). All SD subjects met DSM-IV criteria for either: (a) alcohol dependence and no drug abuse (n = 9); (b) marijuana dependence and no polydrug abuse (n = 7); (c) marijuana or other drug dependence (apart from alcohol) and polydrug abuse (n = 6). Some of the subjects in (b) and (c) also met DSM-IV criteria for alcohol dependence. Due to the relatively low numbers of individuals reported for each subgroup, subjects were combined during the analysis, enabling the maximization of statistical power. All SD participants were asked to refrain from using either alcohol or drugs for at least 12 hours prior to their scheduled fMRI session.
Design and Procedure
Participants performed the IGT for three blocks of 100 trials. During each block, the same hint message was presented to the participant for each trial. The message was a control message, “Some decks are better than others,” a positively-framed informative message, “Decks C and D give more in the long run,” or a negatively-framed informative message, “Decks A and B give less in the long run.” The order of the messages was counter-balanced across participants. The rationale for presenting the message types by block was motivated by our intent to preserve the exploratory learning process of the IGT. The specific sequences of gains and losses for each deck were the same as in the original task design (Bechara et al., 1994). However, unlike in the original design, each trial outcome was presented as a net gain, draw, or loss, the participant started with an initial sum of 1000 points, and the entire task was performed on a computer using E-Prime 1.2 (Psychology Software Tools Inc., 2006).
The timing and presentation of a trial is presented schematically in Figure 1A. The participant’s running point total was displayed throughout the block at the bottom of the screen. At the start of a trial, the current hint message and the 4 decks of cards were presented. If the participant failed to respond within 3 seconds, then the trial was considered a no-response trial. Following a variable delay, a card from the chosen deck was flipped over to reveal the outcome as a negative, zero, or positive point value, and the running total was updated. On no-response trials, the outcome was always a loss of 100 points. The feedback remained visible for 0.8 seconds, after which the message, cards, and outcome were removed. Temporal jittering between choice and outcome and between trials was used to allow separate estimation of brain activation for decision making versus outcome evaluation (Dale, 1999). Details concerning the variable length delays can be found in our earlier fMRI study (Krawitz et al. 2010).
Scoring
Decision quality is defined as the normalized difference in the number of selections from good decks and bad decks, with −1 indicating all bad deck selections, 1 indicating all good deck selections, and 0 indicating an equal number of selections from bad and good decks. To compare performance with a framed informative message to that with the control message, message effectiveness is defined as the difference in decision quality between trial blocks with different messages. Decision quality and message effectiveness are robust against variance in the total number of decisions across blocks and participants, and provide a relative measure of the number of good and bad decisions independent of the actual points earned.
fMRI Analysis
Imaging acquisition and preprocessing
Imaging data were collected on a Siemens Magnetom Trio at 3.0 Tesla MRI scanner and an 8-channel head coil. For each participant, functional blood oxygenation-level dependent (BOLD) data were collected using echo planar imaging with free induction decay for 3 blocks of 360 whole brain volumes (echo time (TE) = 25 ms, repetition time (TR) = 2000 ms, flip angle = 70°) with 33 axial slices (field of view: matrix, 64 × 64; voxel size, 3.4 × 3.4 × 3 mm, thickness, 3-mm; gap, 1 mm spacing; interleaved order). A structural scan was collected at the end of each session using three-dimensional MP-RAGE imaging using a high resolution T1 weighted imaging sequence, (TE = 3.93 ms, TR = 2300 ms, flip angle 12°) with nonselective excitation consisting of 160 sagittal slices (field of view: matrix, 512 × 448; voxel size, 0.5 × 0.5 × 1.0 mm, thickness, 1-mm).
Preprocessing was done using SPM5 (Wellcome Trust Centre for Neuroimaging, 2005) except where otherwise specified. Functional data were spike-corrected on a voxel-by-voxel basis to reduce the impact of artifacts using AFNI’s 3dDespike. The structural scan was skull-stripped using FSL’s BET2 with default parameters (Péchaud, Jenkinson, & Smith, 2006). The functional images were slice-timing corrected using sinc-interpolation (Oppenheim, Schafer, & Buck, 1999), motion corrected by means of a least-squares 6-parameter rigid-body transformation, and coregistered with the structural scan. Once the structural scan was normalized to the SPM MNI template, the normalized images were smoothed with an 8 mm3 FWHM isotropic Gaussian kernel.
Intrasubject analysis
Event-related responses were estimated using a general linear model (GLM) approach and analyses conducted using SPM5 and the Marsbar toolkit for ROI analyses (Brett, Anton, Valabregue, & Poline, 2002). A general linear model (GLM) was estimated for each participant using a total of 20 regressors: a constant term, 6 motion regressors using the parameters of the motion correction performed during preprocessing, and 13 regressors for experimental conditions during the decision period and the outcome period. Furthermore, to rule out potential effects of time on task on regions heavily active during choice behavior (Grinband et al. 2011), we conducted additional analyses by directly controlling for differences in reaction time by treating it as a nuisance covariate in all of our contrasts of interest. Specifically, for each subject, we created new GLMs with an additional regressor that was active from the onset of each choice cue until a response was made.
The decision period for each trial was classified on whether the message for the block was the control (Control), positively-framed (Positive), or negatively-framed (Negative) message and whether the deck selected was good (Good) or bad (Bad). This provided six regressors (ControlGood, ControlBad, PositiveGood, PositiveBad, NegativeGood, and NegativeBad) plus a seventh regressor (NoResponse) for trials in which no response was made, regardless of the message. The decision-making events were aligned to the time of response. The outcome period for each trial was classified on whether a good (Good) or bad (Bad) deck was selected and whether the actual outcome was a gain (Win), a draw (Draw), or a loss (Lose). Note that draws were only possible after good decisions due to the design of the decks. This provided five regressors (GoodWin, BadWin, GoodLose, BadLose, and GoodDraw) plus a sixth regressor (NoResponseOutcome) for trials in which no response was made. The outcome events were aligned to the time of presentation of the outcome.
Contrasts of interest
Contrasts of interest were defined for changes in brain activity during the decision-making period. The risk effect was defined as the difference in brain activation associated with choosing a bad deck relative to choosing a good deck. For comparison of activations with a framed message to those with the control message, the heightened-risk effect was defined as the difference in the risk effect for a framed-message block compared to the control-message block. Finally, to determine whether the heightened-risk effect was driven by changes in brain activation associated with choosing the good or bad decks, deck-specific contrasts compared activations between message blocks for only good or bad decisions.
Individual-group analysis
Second-level analyses used linear regression on the per-participant measures with ReML estimation in SPM5. To identify regions whose activity related to choice behavior without informative messages, a correlation was calculated across subjects in control-message blocks between decision quality and the risk effect. To identify regions whose activity related to changes in choice behavior due to the informative messages compared to the control message, correlations were computed between message effectiveness and the heightened-risk effect. Finally, to identify regions showing a behaviorally-relevant risk effect across all messages, correlations were computed between average decision quality across blocks and the average risk effect. The statistical threshold for significance was p < 0.05, with False Discovery Rate (FDR) correction, and only voxels contiguous with those passing this corrected threshold were reported.
Between-group analysis
A between-group analysis was performed by entering the t-contrast images for each subject into a random effects two-sample t-test detecting for statistically significant differences in mean signal values between the two groups. An analysis of covariance (ANCOVA) also was conducted to evaluate activation differences between the two groups with the t-contrast images as the dependent variable, group as the independent variable, and the subjects’ performance as the covariate. A separate slope and intercept was estimated for each group. A total of 4 contrasts and Z maps were generated for the SPM analysis, one for each tail for the difference between the intercepts, and one for each tail for the difference between the slopes.
Results
Group Demographics
Demographic characteristics of the two groups are given in Table 1. Pearson’s chi-square tests showed no significant differences between the non-SD and SD groups in terms of gender, race, socioeconomic status (SES; based on father’s level of education), and age.
Table 1.
Group Demographics. Demographic characteristics of the study comparing the non-SD group to the SD group.
Demographics of subjects a
| non-SD Group (N = 25) | SD Group (N = 22) | Analysis | ||
|---|---|---|---|---|
| χ2 | p | |||
| % | 53.2 | 46.8 | ||
| Age | 22.3 ± 0.6 | 21.5 ± 0.5 | 1.68 | .17 |
| Gender | ||||
| Female | 11 | 12 | .18 | .67 |
| Male | 14 | 10 | ||
| Father’s education (level) b | ||||
| Graduate school | 8 | 4 | 1.59 | .66 |
| Standard college | 11 | 10 | ||
| High School graduate | 4 | 6 | ||
| Other | 2 | 2 | ||
| Ethnicity | ||||
| African American | 0 | 3.5 | 10.22 | .03 |
| Caucasian | 6 | 0 | ||
| Asian | 15 | 16.5 | ||
| Hispanic | 3 | 1 | ||
| Other | 1 | 1 | ||
| Race | ||||
| Hispanic or Latino | 3 | 0 | 1.17 | .28 |
| Not Hispanic or Latino | 22 | 22 | ||
Data are represented as means ± standard errors.
Information about father’s education was obtained from all subjects in each group.
Behavioral Results
Non-SD Group
Decision quality for the non-SD group was significantly above chance with the control message (M = 0.261, SE = 0.062), t(24) = 4.209, p = 0.0003, indicating that even with the control message, participants picked more good decks than bad decks. Compared to the control message, decision quality was significantly higher with both the positively-framed message (M = 0.46, SE = 0.075), t(24) = −2.4, p = 0.024 and the negatively-framed message (M = 0.53, SE = 0.071), t(24) = −3.27, p = 0.003, indicating that both framed messages were effective in improving participants’ decision-making behavior. Decision quality did not differ between the positively and negatively framed messages, t(24) = −0.98, p = 0.34.
A three-way within-subject ANOVA with message, block position within session, and epoch within block as factors was conducted to evaluate whether the influence of informative messages and the knowledge gained from experience interacted in their effect on decision quality. The main effects were significant, but not the interactions, suggesting the effectiveness of the messages was additive, with learning from experience occurring within and across trial blocks (see Supplemental Table 1A).
SD Group
Decision quality for the SD group was not significantly above chance with the control message (M = 0.07, SE = 0.07), t(21) = 1.143, p = 0.266. Compared to the control message, decision quality was significantly higher with both the positively-framed message (M = 0.33, SE = 0.061), t(21) = −3.423, p = 0.003, and the negatively-framed message (M = 0.32, SE = 0.075), t(21) = −2.659, p = 0.015, and did not differ between message frames, t(21) = 0.12, p = 0.909. Main effects were significant in a three-way within-subject ANOVA with message, block position within session, and epoch within block as factors, but the interaction effects were not significant (see Supplemental Table 1B).
Group Comparison
A two-factor analysis of variance (ANOVA) revealed a significant main effect for group, F(1, 135) = 9.492, p < 0.005, and message type, F(2, 135) = 8.081, p < 0.001, such that performance scores differed significantly between groups for the control message (t(45) = 1.99, p < 0.03) and negatively-framed message (t(45) = 2.03, p < 0.03; See Figure 3). Behavioral scores did not significantly differ between groups in the positively-framed message (t(45) = 1.34, p > 0.05), however, there was a trend toward poorer performance in the SD group compared with the non-SD group (see Figure 2) The interaction effect of group and message type was non-significant, F(2, 135) = 0.174, p > .05, although as we found below, the neural activity does differ between the groups and also predicts choice behavior.
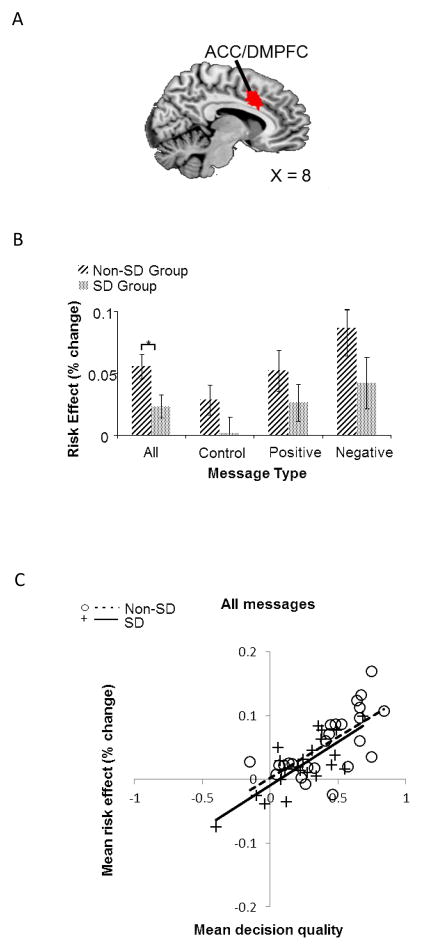
Figure 3.
Behaviorally-relevant neural risk effect averaging across all messages in ACC and DMPFC. (A) Shown in red is a region of the right ACC/DMPFC (BA 24/32, peak voxel: MNI 2, 20, 34), where averaging across messages, there is a correlation between decision quality (DQ) and the risk effect. Sagittal section of the human brain (MNI x = 8). (B) Within the region shown in A (using voxels that passed at p <0.05, FDR), follow-up ROI analysis found risk-aversion-related brain activity to be significantly lower in the SD group when averaged across messages. (C) The correlation of DQ and the risk effect averaged across messages. For panel C, each point represents a participant from either the non-SD or SD group and the solid/dotted lines show the best-fit linear regressions by group.
Figure 2.
Group differences in decision quality (DQ). Scores differed significantly between groups for the negatively-framed message and the control message. There also was a trend toward poorer performance in the positively-framed message in the SD group compared to the non-SD group. Error bars indicate within-subject standard error of the mean (SEM) data (Loftus & Masson, 1994).
fMRI Results: Behaviorally-Relevant Neural Risk Effects
Our analysis began by collapsing across groups and identifying brain regions showing brain-behavior correlations across subjects, i.e. behaviorally-relevant neural risk effects (see Supplemental Text for reporting of the neural risk effects for the control message; see also Supplemental Table 2 and the Supplemental Text for reporting of main effects).
Averaging across messages
We identified brain areas showing a positive correlation between the risk effect and decision quality averaged across all three messages. The risk effect was defined as the difference in brain activation associated with choosing a bad deck relative to choosing a good deck. Four brain regions met this criterion: the right ACC/DMPFC (BA 24/32, peak voxel: MNI 2, 20, 34), t(45)= 6.12, p <0.05, FDR (see Figure 3A), the right INS/IFG (BA 47, peak voxel: MNI 38, 22, 0), t(45)= 6.71, p <0.05, FDR (see Figure 4A), the bilateral PRE (BA 7, peak voxel: MNI -10, -70, 44), t(45)= 7.74, p <0.05, FDR, the left Middle Frontal Gyrus (MFG) (BA 6, peak voxel: MNI -30, -6, 48), t(45)= 6.12, p <0.05, FDR and the left INS/IFG (Inferior Frontal Gyrus) (BA 47, peak voxel: MNI -30, 20, 6), t(45)= 5.60, p <0.05, FDR (see Supplemental Text).
Figure 4.
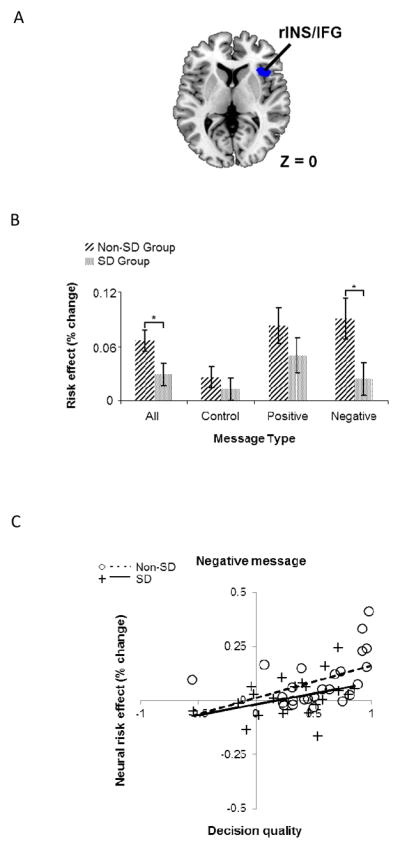
Behaviorally-relevant neural risk effect averaging across all messages in right INS and IFG. (A) Shown in blue is a region of the right INS/IFG (BA 47, peak voxel: MNI 38, 22, 0), where averaging across messages, there is a correlation between decision quality (DQ) and the risk effect. Transverse section of the human brain (MNI z = 0). (B) Within the region shown in A (using voxels that passed at p <0.05, FDR), follow-up ROI analysis found risk-aversion-related brain activity to be significantly lower in the SD group for the negatively-framed message and when averaged across messages. (C) The correlation of DQ and the risk effect with the negative message. For panel C, each point represents a participant from either the non-SD or SD group and the solid/dotted lines show the best-fit linear regressions by group.
Follow-up ROI analysis in the ACC/DMPFC region showed that, averaging across all of the blocks, greater neural risk effects correlated with higher decision quality, r(45) = 0.75. This suggests that greater activity in this region is associated with better decisions, for example by signaling risk- or loss-aversion (Krawitz et al., 2010). The correlation held separately for the SD group, r(20) = 0.77, p < 0.05, and the non-SD group, r(23) = 0.68, p < 0.05 (see Figure 3C), which is inconsistent with the weakened brain-behavior association hypothesis (H2). When collapsed across groups, a positive correlation was found for the risk effect when considering the control message blocks, r(45) = 0.58, the positive message blocks, r(45) = 0.51, and the negative message blocks, r(45) = 0.59. We then correlated the heightened-risk effect, defined as the increase in the neural risk effect for a framed-message block compared to the control-message block, with message effectiveness, which is the improvement in decision quality in a framed-message block relative to a control-message block. Correlations between the heightened-risk effect and message effectiveness were significant with both the positively-framed message, r(45)=0.47, p < 0.05, and the negatively-framed message, r(45) = 0.47, p < 0.05. Also, the neural risk magnitudes across these conditions did not differ within the ROI (t(46) = 1.36, p > 0.05). We also conducted independent main effects analysis testing for group differences in the level of risk-aversion related brain activity within the ROIs that showed behaviorally-relevant risk effects. Consistent with the generalized input insensitivity hypothesis (H1), a two-sample t-test revealed significant group differences only for the neural risk effect when averaged across all the blocks (t(45) = 2.38, p < 0.05) (see Figure 3B)
Follow-up ROI analysis in the right INS showed results that are largely similar to those of the ACC/DMPFC above. Specifically, averaging across all of the blocks, there was a positive correlation of neural risk effect and decision quality, r(45) = 0.62, which also separately held for the SD group, r(20) = 0.68, p < 0.005, and the non-SD group, r(23) = 0.49, p < 0.05. Combining across groups, the correlations between the neural risk effect and decision quality were significant when restricted to the positively-framed message block, r(45) = 0.38, p < 0.05, and the negatively-framed message block, r(45) = 0.51 p < 0.05 (see Figure 4C). In contrast however with the ACC/DMPFC results, the correlations of the heightened-risk effect and message effectiveness were not significant for the positively-framed message, r(45)=0.26, p > 0.05, nor for the negatively-framed message, r(45) = 0.03, p > 0.05. In addition, the correlations considering the control block were not significant within the INS ROI (r(45) = 0.17, p > 0.05. However, in support of the generalized input insensitivity hypothesis (H1), a two-sample t test revealed significant group differences in the neural risk effect with greater neural risk effects for the non-SD group compared to the SD group when collapsed across all messages, (t(45) = 2.27, p < 0.03) and also in the negatively-framed message condition, (t(45) = 2.29, p < 0.03), in particular (See Figure 4B).
Additional ROI analysis in the remaining three brain regions found comparable results to the ACC/DMPFC and anterior insula regions above. The correlation between greater risk effects and higher decision quality held across all message types and groups (see the Supplemental Text for details).
Having first collapsed across groups, we next conducted two between-groups analyses by performing two-sample t tests to test for significant activation differences, as well as ANCOVA analyses to test for correlational differences between the two groups. The two-sample t tests revealed a general pattern of significantly greater neural risk effects in the non-SD group, which is consistent with the generalized input insensitivity hypothesis (H1); specifically for the negatively-framed message and when averaged across all the blocks. Whereas, when we conducted ANCOVA analyses to test for different strengths in the correlations between brain activity and behavior on the individual ROIs, we found no significant differences in the slopes or intercepts estimated for each group, thus failing to support the weakened brain-behavior association hypothesis (H2). These results were not surprising given that the brain regions were selected based on a shared correlation between the neural risk effect and decision quality across all subjects. But alternatively, we conducted an independent ANCOVA analysis, taking an exploratory approach searching for regions passing a whole-brain FDR-corrected threshold and found a significantly greater slope for the non-SD group between brain activity and behavioral performance in the bilateral Middle Frontal Gyrus (MFG) (BA 9, peak voxels: MNI -42, 28, 34 and 36, 26, 44), t(43)= 5.97 and t(43)= 5.31, p <0.05, FDR, and the right Medial Frontal Gyrus (MedFG) (BA 6, peak voxels: MNI 14, -18, 64 and 36, 26, 44), t(43)= 5.38, p <0.05.
Positively-framed message
In the analysis above, we identified regions that showed a correlation between neural risk effects and decision quality. Here, we directly searched for brain regions that show message influences on behavior, i.e. a significant positive correlation between message effectiveness and the heightened-risk effect for the positively-framed message. The brain regions were located in the right CG (BA 32, peak voxel: MNI 0, 2, 46), t(45)=4.96, p <0.05, FDR and the bilateral PRE (BA 7/7, peak voxel: MNI -12, -64, 48 and 20, -62, 44), t(45)= 5.33 and 5.56, p <0.05, FDR. Based on two-sample t tests, we found no significant group differences in the framed neural-risk effect for the positively-framed message. Overall, the ROI analysis within these regions, for the positively-framed message compared to the control message, found a greater increase in the risk effect to be associated with a greater increase in decision quality: r(45)=0.67 in the right CG, r(45)=0.63 in the left PRE, and r(45)=0.64 in the right PRE. In other words, the right CG and bilateral PRE reflects the relationship between the influence of the positively-framed message and the resulting improvement in decision quality for both groups (see Supplemental Figure 1).
Negatively-framed message
No brain regions showed a positive correlation between message effectiveness and the heightened-risk effect for the negatively-framed message when we used the FDR correction. Only when an uncorrected test was used were active regions identified in left MeFG/CG (BA 8/32, peak voxel: MNI -2, 26, 44), t(45)=4.99, p <0.0001, uncorrected, left MFG (BA 6, peak voxel: MNI -26, 2, 62), t(45)=4.87, p <0.0001, uncorrected (see Supplemental Figure 2), and the left INS (BA, peak voxel: MNI -32, 14, 6), t(45)=4.79, <0.0001, uncorrected.
Discussion
This study tested two distinct hypotheses: For the generalized input insensitivity hypothesis (H1), we anticipated the SD group to show weaker activation patterns in risk-aversion-related brain regions compared to the non-SD group, and the prediction largely held, especially in the ACC/DMPFC and anterior insula. For the weakened brain-behavior association hypothesis (H2), we predicted a potential group difference in the correlational strengths between risk signals and task performance, reflecting a weakened effect of brain activity on risk avoidance. Interestingly, none of our direct group comparisons (e.g. ANCOVA analyses on previously defined ROIs) yielded significant differences between the SD and non-SD groups in the slopes or intercepts of the relationships between brain activity and task performance. This strongly suggests that both groups have similar strengths in their correlations of higher risk-aversion-related activity associated with better decision-making performance, which is inconsistent with our second hypothesis (see Figure 1B). Nevertheless, we cannot entirely rule out the second hypothesis as other regions did show significant ANCOVA effects in an exploratory whole-brain analysis.
Lastly, we also inquired whether specific message frames could strengthen avoidance behavior for one or both groups based on signal changes in the modulation of risk-aversion-related brain activation. With the exception of the ACC/DMPFC and PRE, the risk-aversion-related brain activity mainly differed for the negative messages, but not the positive messages between the two groups. The negative message, but not the positive message, was significantly less effective at increasing risk-related activation in the SD group relative to the non-SD group.
Reduced risk sensitivity in substance dependence
As we predicted within the ACC/DMPFC and right INS regions, we found significant group differences in the neural risk effect across messages. The SD group compared to the non-SD group had lower risk-aversion-related brain activity, which correlated with poorer behavioral performance scores. This is consistent with previous neuroimaging studies suggesting that greater ACC activity predicts more normative (i.e., optimal) decision making behavior (Paulus & Frank, 2006) and is a crucial component for making advantageous decisions (Lin et al., 2008), as well as action-relevant evaluation of risk and its avoidance (Brown & Braver, 2008; Magno et al., 2006), and also for integrating risk appraisals related to informative messages. Similar to Chua’s findings on tailored message effectiveness in MPFC regions (Chua et al., 2011; Mann et al., 2004), we also found (although more posterior) MPFC neural risk effects across messages. Our findings also support various studies that strongly suggest a substance user’s inability to modulate risk may be related to the failures of activating the ACC and INS, which is characterized by impairments in error awareness, which in turn are associated with higher levels of risky decision making (Fishbein et al., 2005; Hester et al., 2009; Krawitz et al., 2010) and erroneous behaviors (Garavan & Stout, 2005; Kaufman, Ross, Stein, & Garavan, 2003). In drug abusers, reduced AI activation during decision making under uncertainty also has shown to predict later relapse (Paulus, Tapert, & Schuckit, 2005).
When we conducted an independent whole-brain ANCOVA analysis for the decision contrast averaging across message type, we found a significantly greater slope for the non-SD group between brain activity and behavioral performance in the right Medial Frontal Gyrus (MedFG). This seems to suggest that SD individuals are not as effective as non-SD individuals in perceiving less informative messages (i.e., control message) to be still self-relevant enough in order to guide their risky behavior (Chua, Liberzon, Welsh, & Strecher, 2009). This interpretation seems to support the growing literature on how individual differences in the self-salience of information may play a pivotal role in attitude and behavioral change (Brinol & Petty, 2005), as well as in influencing which motivational and behavioral representations guide behavior (Wheeler, Liberzon, Welsh, & Strecher, 2007).
Taken together, at-risk populations, such as substance users, may suffer from deficits in encoding message relevance, which may point to why individually-tailored messages, which typically include references to the individual’s needs, interests, and obstacles preventing desired change, are much more effective in eliciting health-related behavior change (Chua et al., 2011; Mann et al., 2004).
Limitations and Alternative Explanations
There are several limitations to this current study (see Supplemental Text for details). First, because we did not screen the non-SD group for drug use history, there is a possibility the non-SD group may consist of subjects meeting the SSAGA diagnostic inclusion criteria. If the non-SD group includes subjects meeting the SD group criteria, we would predict a group comparison to show weaker differences in behavioral performances or BOLD signal changes in brain regions involved in risky decision-making. Despite this potential limitation, we observed significant differences between the non-SD group and SD group in both their behavioral data and fMRI results during performance on the gambling task, suggesting drug use history was not a significant feature of the non-SD group. Similarly, we did not obtain details concerning the length of abstinence from substance use in both groups prior to scanning.
Secondly, we did not intend to explicitly explore the various proposed definitions of risk (Yates, 1992). This paper referred to the two bad decks (i.e., cumulative loss) of the IGT as being riskier than the two good decks (i.e., cumulative win). However, risk is a concept with multiple meanings (e.g. variance, probability of loss, probability of harm), and its meaning depends greatly on who is using the term (Lupton, 1993). While we recognize the importance of this topic and appreciate that the various constructs related to risk and loss avoidance are not completely independent across the IGT task conditions, we believe that identifying brain regions showing changes in risk appraisal due to informative messages in substance-dependent individuals is an intriguing research question in its own right.
Our study also is limited in terms of directly addressing the precise neural mechanisms of cessation message interventions in changing risky health-related behaviors. The present study demonstrated a significant group difference in the level of neural risk effects due to informative messages by focusing the analyses on the period of choice selection. However, developing an overall understanding of persuasive messages will require future research to focus on the encoding process of persuasion-based communication, which is not limited to, but could include, the role of source, channel, receiver, content, and intentions (i.e., attitude change) (Lasswell, 1948). Finally, health communication studies have illustrated the power of tailored message interventions in producing significant behavioral changes. Therefore, we believe future work will be needed to elucidate the specific neural mechanisms involved in the differential processing of tailored messages based on the perceptions of message (self) relevance (Chua et al., 2011; Chua et al., 2009), as well as showing how tailored messages (compared to generic messages) may differ in the neural processing of risk appraisal for targeted behaviors in specific at-risk populations.
Finally, we explored several alternative explanations for the risk effects and heightened-risk effects, which we detail in the Supplemental Text.
Conclusion
This study presents evidence showing differential effects of informative messages on risk-related activation between SD and non-SD groups. One possible implication of our findings is in the context of health media campaigns based on negative appeals (Mann et al., 2004), namely that highlighting the negative consequence of risky behaviors may not be as effective in substance users, especially at the neural level. In particular, we found negative messages to be less effective in strengthening avoidance-related brain activity in SDs relative to non-SDs (e.g. Fig. 5B), which is consistent with the generalized input insensitivity hypothesis (H1). Nevertheless, we cautiously point out that our results do not suggest positive messages instead will lead to higher risk-aversion-related brain activity; positive messages did not differ in their effectiveness between the two groups, either behaviorally or at the neural level. The evidence for our second hypothesis, the weakened brain-behavior association hypothesis (H2), was much more limited in comparison with the evidence for H1. More broadly, the findings in support of the generalized input insensitivity hypothesis are consistent with a pattern of phenotypic expression that shows individuals with externalizing psychopathology (including substance dependence) tend to have reduced cognitive capacity in a variety of forms, including intelligence, short-term memory, and working memory (Bogg & Finn, 2010; Endres, Rickert, Bogg, Lucas, & Finn, 2011; Finn et al., 2009). The results suggest generalized reduced signal sensitivity to informational inputs might be a neural link that can help explain observed patterns of co-varying reduced cognitive capacity and externalizing psychopathology.
To our knowledge, this is the first fMRI study to compare SD and non-SD groups in a choice behavior task showing how informative messages interact with risk processing. This study has shown differential neural effects of informative messages on risk-related activation in the ACC and INS regions, when comparing the SD group to the non-SD group in a choice behavior task. Our results identify candidate neural deficits in the processing of persuasive messages against risky behavior in a substance-dependent group.
Supplementary Material
Acknowledgments
This research was supported in part by National Institutes of Health R03 DA023462-01 (JWB), R01 DA026457 (JWB), R00 AA017877 (TB), R01 DA017924 (PRF), Air Force Office of Scientific Research FA9550-07-1-0454 (JWB), a 2005 NARSAD Young Investigator Award (JWB), the Sydney R. Baer, Jr. Foundation (JWB), and the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc. We thank Srikanth Padmala for helpful comments, Adam Krawitz for help with fMRI analysis, and Elizabeth Dinh for assistance with data collection.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding Advantageously Before Knowing the Advantageous Strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bogg T, Finn PR. A self-regulatory model of behavioral disinhibition in late adolescence: Integrating personality traits, externalizing psychopathology, and cognitive capacity. Journal of Personality. 2010;78:441–470. doi: 10.1111/j.1467-6494.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Fukunaga R, Finn PR, Brown JW. Cognitive control links alcohol use, trait disinhibition, and reduced cognitive capacity: Evidence for medial prefrontal cortex dysregulation during reward-seeking behavior. Drug and Alcohol Dependence. 2012;122:112–118. doi: 10.1016/j.drugalcdep.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Marseille Boite a Region d’Interet (MarsBaR) 2002 From http://marsbar.sourceforge.net/
- Brinol P, Petty RE. Individual differences in attitude change. In: Albarracin D, Johnson BT, Zanna MP, editors. The Handbook of Attitudes and Attitude Change. Mahwah: Elbaum; 2005. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Research. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A New, Semi-Structured Psychiatric Interview for Use in Genetic Linkage Studies: A Report on the Reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Lohrenz TM, Montague PR. Smokers’ brain compute, but ignore, a fictive error signal in a sequential investment task. Nat Neurosci. 2008;11:514–520. doi: 10.1038/nn2067. [DOI] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Liberzon I, Welsh RC, Strecher VJ. Neural Correlates of Message Tailoring and Self-Relatedness in Smoking Cessation Programming. Biological Psychiatry. 2009;65:165–168. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, Biases, and Rational Decision-Making in the Human Brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex. The British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Endres MJ, Rickert ME, Bogg T, Lucas J, Finn PR. Externalizing psychopathology and behavioral disinhibition: Working mmory mediates signal discriminability and reinforcement moderates response bias in approach–avoidance learning. Journal of Abnormal Psychology. 2011;120:336–351. doi: 10.1037/a0022501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche K, Fletcher P, Lewis S, Clark L, Stocks-Gee G, London M, Sahakian B. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005;180(4):612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting Persuasion-Induced Behavior Change from the Brain. The Journal of Neuroscience. 2010;30(25):8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, Cantrell H. Reduced cognitive ability in alcohol dependence: Examining the role of covarying externalizing psychopathology. Journal of Abnormal Psychology. 2009;118:100–116. doi: 10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Cognitive Brain Research. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa gambling task. NeuroImage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teicher T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex in sensitive to time on task, not response conflict or error likelihood. NeuroImage. 2011;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate crtex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz A, Fukunaga R, Brown JW. Anterior insula activity predicts the influence of positively framed messages on decision making. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:392–405. doi: 10.3758/CABN.10.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lasswell HD. The structure and function of communication in society. In: Bryson L, editor. The Communication of Ideas, a Series of Addresses. New York: Harper; 1948. [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct Roles of Prefrontal Cortical Subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Z-L, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2010;31(3):410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Chiu Y-C, Cheng C-M, Hsieh J-C. Brain maps of Iowa gambling task. BMC Neuroscience. 2008;9(1):72–87. doi: 10.1186/1471-2202-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lupton D. Risk as moral danger: The social and political functions of risk discourse in public health. International Journal of Health Services. 1993;23:425–435. doi: 10.2190/16AY-E2GC-DFLD-51X2. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T, Sherman D, Updegraff J. Dispositional motivations and message framing: a test of the congruency hypothesis in college students. Health Psychol. 2004;23:330–334. doi: 10.1037/0278-6133.23.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe DJ, Jensen JD. The Relative Persuasiveness of Gain-Framed Loss-Framed Messages for Encouraging Disease Prevention Behaviors: A Meta-Analytic Review. Journal of Health Communication. 2007;12:623–644. doi: 10.1080/10810730701615198. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. 2. Upper Saddle River: Prentice Hall; 1999. [Google Scholar]
- Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. NeuroImage. 2006;30(2):668–677. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Péchaud M, Jenkinson M, Smith S. Brain Extraction Tool (BET) [Software] Oxford: Oxford University Centre for Functional MRI of the Brain; 2006. [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools Inc. E-Prime [Software] Pittsburgh: Psychology Software Tools Inc; 2006. [Google Scholar]
- Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: The role of message framing. Psychological Bulletin. 1997;121:178–183. doi: 10.1037/0033-2909.121.1.3. [DOI] [PubMed] [Google Scholar]
- Sherman D, Mann T, Updegraff J. Approach/avoidance motivation, message framing, and health behavior: understanding the congruency effect. Motivation and Emotion. 2006;30(2):164–168. doi: 10.1007/s11031-006-9001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychonomic Bulletin & Review. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff JA, Sherman DK, Luyster FS, Mann TL. The effects of message quality and congruency on perceptions of tailored health communications. Journal of Experimental Social Psychology. 2007;43(2):249–257. doi: 10.1016/j.jesp.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Service. Healthy People 2010: Understanding and improving health. Washington, DC: Author; 2010. [Google Scholar]
- Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla K. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Centre for Neuroimaging. Statistical Parametric Mapping (SPM) [Software] London: Wellcome Trust Centre for Neuroimaging; 2005. [Google Scholar]
- Wheeler SC, DeMarree KG, Petty RE. Understanding the Role of the Self in Prime-to-Behavior Effects: The Active-Self Account. Personality and Social Psychology Review. 2007;11:234–261. doi: 10.1177/1088868307302223. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Brooke Livengood L, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Yates JF. Risk-Taking Behavior. Chichester: John Wiley & Sons; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.