Abstract
As a result of intense genetic studies of families with specific mutations, the road to better therapeutic intervention for pheochromocytoma (PHEOs) and parangangliomas (PGLs) has more recently become populated with several promising molecular targets. Consequently a change in paradigm from a previous view on nonspecific therapy has shifted towards more selective molecular targeted therapies. In particular, malignant PHEOs/PGLs, more specifically the tumors that result from mutations in succinate dehydrogenase subunit B (SDHB), are a clear concern, and novel therapies should be developed to address this problem. Here we summarize current and future therapeutic approaches.
Keywords: Pheochromocytoma, Paraganglioma, SDHB, Therapy
INTRODUCTION
Pheochromocytomas (PHEOs) are catecholamine-producing tumors that are most commonly located in the adrenal gland; extra-adrenal tumors are classified as paragangliomas (PGLs) [1]. Recently, several outstanding discoveries have been made that have substantially improved and changed our approach to understanding the pathogenesis, genetics, diagnosis, localization, and experimental treatment options of these tumors. Metastatic PHEOs or PGLs are diagnosed based on the presence of tumor cells of chromaffin origin in a location outside the site of normal development (adrenal medulla and the sympathetic chain), including mainly the lungs, spleen, bones, and liver [2]. Although frequently non-metastatic and slow growing in most cases, in selected patient populations (especially those with SDHB mutations), up to 90% of patients can develop metastatic disease. PHEOs or PGLs presenting with various succinate dehydrogenase B (SDHB) gene mutations have a very high likelihood to develop metastatic disease, commonly in the bones, lymphatic nodes, lungs, and liver [2]. Other hereditary PHEOs or PGLs, mainly those related to neurofibromatosis type 1 (NF1) and multiple endocrine neoplasia type 2 (MEN 2), have much lower tendencies to metastasize [3]. Despite a vast interest and effort to develop new therapeutic approaches to treat metastatic PHEO and PGL, data is either limited or still at an experimental level, although there are some promising results and observations. In general, current data suggests there is no long term survival benefit between CVD chemotherapy-treated and untreated patients [4]; although there is an initial benefit from this regimen in malignant PHEOs (with a response rate of about 70%–80%), relapse occurs most of the time [5].
The use of novel targeted therapy has been explored with different results. For example, disappointing results has been observed with the use of the mTORC1 inhibitor everolimus [6]; at the same time, investigation, especially in advanced disease, of the oral multitarget inhibitor sunitinib has gained some interest, and clinical studies are underway to define its role in the treatment of PHEO and PGL.
In an effort to understand the malignant transformation of PHEO cells, we can distinguish two main forces which confer a survival advantage of these tumor cells over other cell populations: 1) induction of proliferation and 2) inhibition of apoptosis. In the following paragraphs we will attempt to illustrate how these two heavily regulated events can direct strategies in our search for effective treatment for malignant PHEOs and PGLs.
Current Therapeutic Approaches
Surgical removal of primary tumors and metastatic foci is a mainstay in the management of malignant PHEOs and PGLs [7]. For unresectable tumors (i.e. bone metastasis; encroachment of a major vascular branch), radioactive isotope treatment with [131I]-MIBG could be given [8]. A 30% tumor response rate, 45% biochemical response rate, and 76% rate of symptomatic relief has been reported with a mean of 158 mCi single therapy dose and a mean of 3.3 +/− 2.2 doses administered [9]. An increase in the dose to 492–1,160 mCi has yielded a better tumor response rate of 67% and a 5-year survival rate of 75%, but also increased the myelosuppresive side effect [10]. Only those with positive MIBG uptake will respond to MIBG treatment, but unfortunately not every lesion will be identified by the uptake of the isotope; consequently this treatment option is limited and dependent on positive uptake on the initial scan [11, 12].
Patients with a substantial number of metastases (multiple metastatic foci) or with recurrent disease will require systemic chemotherapeutic intervention. Of all the possible combinations that have been tried, chemotherapy with combined cyclophosphamide, vincristine, and dacarbazine (CVD) has emerged as the best option [13]. A tumor response rate of 55% and biochemical response rate of 72% was reported in a 22-year follow-up analysis of 18 patients performed at the National Institutes of Health [4]. However, this moderate response rate still does not meet the real clinical needs of these patients, and more effective options should be sought and explored.
Future Therapeutic Options
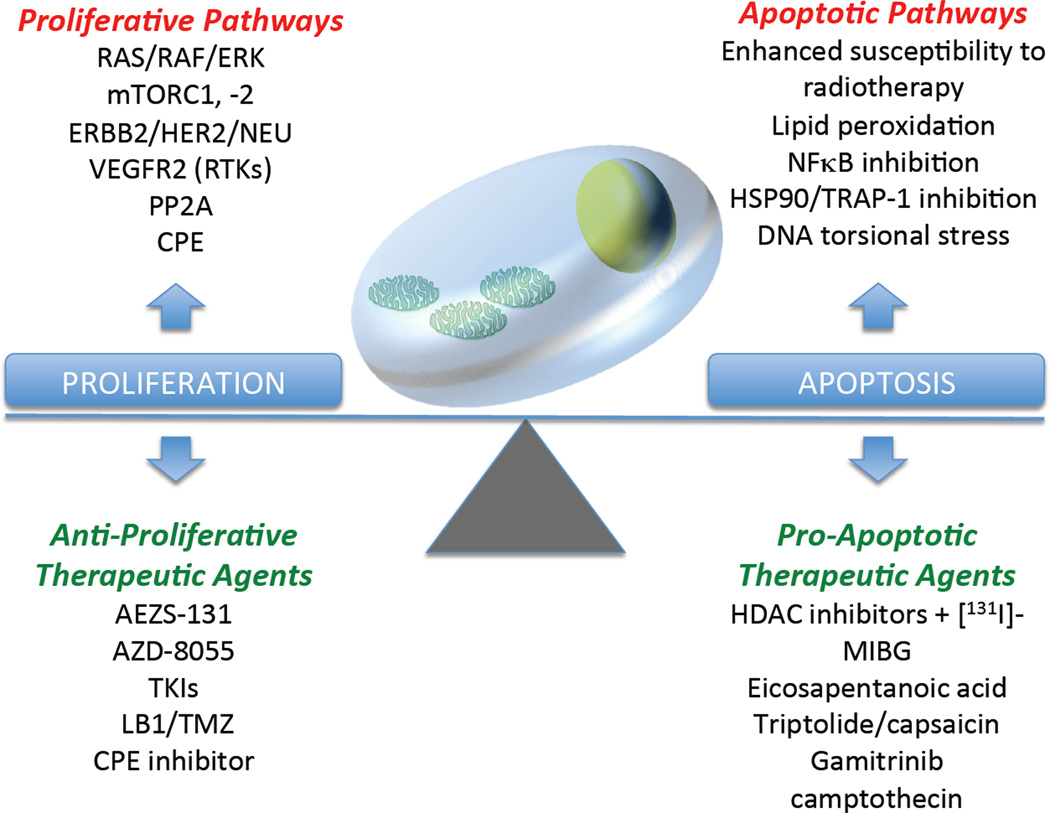
Our better understanding of the biology of these tumors opens the possibility of using more selective targeted therapeutic options, with the promise of superior efficacy and substantially reduced adverse effects. We can schematically classify these novel therapeutic strategies as either preventing the transformed cells from dividing indefinitely (anti-proliferative therapeutic strategies) or enhancing programmed cell death (pro-apoptotic therapeutic strategies) (Figure 1).
Figure 1.
Normal tissue development is dependent on careful balance between proliferative and apoptotic factors. Enhancement of proliferative activity and/or inhibition of pro-apoptotic pathways tilt this balance towards oncogenesis. Restoration of this balance can be achieved by strategically utilizing therapeutic agents that would inhibit unrestrained proliferation to occur or allow cell death to proceed.
1) Anti-proliferative therapeutic strategies
Several pathways are involved in increased proliferative activity in different tissue environment conditions and are potential and promising therapeutic targets for PHEOs and PGLs. Two major clusters can be derived from transcriptome profiling of different PHEO and PGL tumor tissues [14]. One of them, identified as Cluster 2 (which includes RET, NF1, TMEM127, and MAX), is believed to increase HIFα expression by increasing signal transduction in the PI3K/AKT/mTORC1 pathway [15–18]. The other cluster, identified as Cluster 1, increases HIFα accumulation either by interrupting ubiquitylation (SDHx and SDHAF2) or as a result of a direct defect in the ubiquitylation system (VHL), thereby inhibiting its proteosomal degradation. For the chromaffin cells that develop into PHEOs and PGLs, HIFα stabilization seems to be a recurrent motif and an important unifying phenomenon at the intersection of all the genes described so far in the hereditary forms of the disease. However, much remains to be clarified about the biology of HIFα induction. Common and specific target genes have been described for HIF1α and HIF2α [19–22], the two main isoforms described, but experimental work also suggests that some functions of these two isoforms may be opposing [23, 24]. Care must be taken in deciding which one to block, as it appears that different PHEO/PGL genotypes preferentially express either of the isoforms, and even possibly at various titer ranges [25]. It is worth noting that although cluster 1 was grouped based on the pseudohypoxic signature of these tumors, PHEOs/PGLs with SDHB mutations appear to have more HIF2α, in contrast to those with VHL mutations, where HIF1α and its common target genes predominate [26]. This finding is consistent with a recent analysis of expression profiles of patient subsets with defined gene mutations (Pacak, unpublished).
Unfortunately, at the moment there are no clinically available selective compounds directly targeting only the HIF protein, but several approaches targeting proteins connected to the HIF pathway have been investigated. These indirect approaches to target the HIF protein include inhibitors of mTOR. In particular, mTORC1, which is a complex of mTOR with raptor, Pras40, and mLST8, is activated by Akt through the upstream activation of PI3K, leading to p70S6 phosphorylation and stimulating cell proliferation and pro-survival signaling. Under hypoxic conditions (which upregulate HIFα) or in patients with SDHB mutations, the same pathway is activated. More recently, mutations in TMEM127, which is associated with mTORC1 downregulation, have been demonstrated in patients affected by pheochromocytoma [27]. Moreover, in vitro experiments demonstrated that mTORC1 inhibition leads to a repression of HIFα stabilization [28, 29]. Together, these findings support the use of inhibitors of this important pathway. However, early clinical experience with the mTORC1 inhibitor everolimus in a small number of patients with progressive malignant PHEOs resulted in disease progression [6]. Two potential explanations for this treatment failure have been proposed. Firstly, mTORC1 repression only inhibits HIF1α but appears to have no effect on HIF2α [30], and the latter is generally regarded as the more oncogenic of the two isoforms [31]. Secondly, a compensatory activation of a parallel signaling pathway involved in cell growth, RAS/RAF/ERK, was noted when mTORC1 was inhibited [32]. To address these concerns, an agent that inhibits both mTORC-1 and -2 (e.g. AZD-8055) can potentially be a rewarding option. Alternatively, considering the compensatory ERK activation through PI3K, a combination with an ERK inhibitor (e.g. AEZS-131) to prevent any feedback loop that the transformed cells could use to their advantage is a viable choice. Combination treatment, like the ones mentioned, can also potentially decrease the likelihood of the cancer cells developing resistance over time. Increased dependence on glycolysis, which has been observed in several tumors and constitutes another important hallmark of cancer, is currently under investigation, with the hope to select a few molecular targets for future therapy.
Other main players in cell proliferation are the tyrosine kinase receptors (RTKs), including RET (Rearranged during Transformation), platelet-derived growth factor receptor (PDGFR), KIT (v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog), and epidermal growth factor receptor (EGFR). Activation of downstream signaling molecules, such as Ras and BRAF (v-raf murine sarcoma viral oncogene analog B1), can be involved in their pathogenesis as well. Much attention has been devoted recently to these proteins, because they are potential targets for targeted molecular therapy by novel drugs. Some identified genes of susceptibility for hereditary PHEOs/PGLs are RTKs or directly involved with RTKs, which naturally puts them into consideration in planning therapeutic strategies.
Among RTKs a prominent role is played by the protooncogene RET, which is primarily expressed in neural crest cells (including parasympathetic and sympathetic ganglion cells) and urogenital cells [33]. Its ligands are growth factors of the glial cell line-derived neurotrophic factor (GDNF) family, including GDNF, artemin, neurturin, and persephin; these ligands bind and activate RET in conjunction with one of the GPI-linked co-receptors, identified as GDNF-family alpha (GFRα) receptors [34]. The formation of this three-part complex (ligand, Ret, and GFRα receptors), with subsequent dimerization, induces the autophosphorylation of the RET receptor on several intracellular tyrosine residues. Consequently, several intracellular adaptors dock on specific phosphotyrosine residues, connecting the receptor with several cellular pathways, including nodal mediator of tumor cell migration and metastasis [35].
Mutations in the RET gene, and subsequent ligand-independent activation of the gene product, cause multiple endocrine neoplasia type 2 (MEN 2), a hereditary cancer syndrome classified into three subtypes based on clinical presentation: 1) MEN 2A; 2) MEN 2B; and 3) familiar medullary thyroid carcinoma (FMTC). PHEO develops in about 50% of patients with MEN 2A and MEN 2B, with a strong correlation between the position of the RET mutation and the clinical phenotype [36]. High levels of RET have been identified in a murine PHEO cell line (MPC) derived from neurofibromatosis knockout mice [37], and these represent one of the best cellular models we have for testing novel therapeutic strategies. In comparison, normal mouse chromaffin cells express very low levels of RET. In the MPC cell line, the receptor was found to be highly respondent to the ligand GDNF, with an effect comparable to those obtained in primary human PHEO cell cultures.
Overall, RET represents a potential target in patients affected by PHEO. At the moment several tyrosine kinase inhibitors of the RET receptor are in clinical development [38]. These drugs target other kinases beside RET, with the advantage of simultaneously blocking several pathways involved in tumorigenesis. In particular the drug ZD6474 (vandetanib) has demonstrated promising results in vitro and in vivo [39]. This agent is able to inhibit other RTKs at the same time, including VEGFR2 (KDR), VEGFR3 (Flt-4), and VEGFR1 (Flt-1), and at higher concentrations, EGFR. Recently Vitagliano et al. [40] demonstrated the effect of this drug in medullary thyroid carcinoma cells harboring RET mutations. The co-existence of MTC and PHEO in MEN 2 syndrome suggests that ZD6474 is a good candidate drug to test in PHEO tumors as well.
Angiogenesis is a major player in the development of several tumors, and in particular in the formation of tumor metastases, which frequently develop in patients carrying SDHB mutations. The vascular endothelial growth factor (VEGFR) family is a major player in angiogenesis, and its role in several cancers is well established. PHEOs are highly vascularized tumors, and angiogenesis in these tumors has been associated with a malignant phenotype [41]. The rat PHEO cell line PC12, which has been a valuable model for this disease for several years, as well as human PHEO specimens, express vascular endothelial growth factor (VEGF), and follow-up studies have been shown to mediate angiogenesis in this tumor, both in vitro and in vivo, via VEGF [42, 43].
More recently, Takekoshi et al. (REF) have analyzed the expression of VEGF and its receptors in several PHEOs; compared with normal adrenomedullary tissue, these tumors expressed higher levels of Flk-1/KDR and Flt-1, suggesting that expression of both the receptors and the ligands have an important role in the pathogenesis of these tumors. Interestingly, using antibodies against VEGF was able to reduce tumor angiogenesis and tumor proliferation in a xenograft mouse model of PHEOs [44].
The tyrosine kinase c-Kit is another example of an RTK involved in the pathogenesis of PHEOs and PGLs. C-Kit (also identified as cluster of differentiation or CD117) is the receptor for stem cell factor (SCF), and altered forms of this receptor have been associated with some types of cancers, including leukemias, gastrointestinal stromal tumors (GIST), seminomas, and melanomas. Matsude et al. have demonstrated the presence of c-Kit in normal adult adrenal medullary tissue and positivity in one PHEO sample [45]. More recently, Tavanger et al. [46] have demonstrated no correlation between c-Kit immunohistochemical staining and tumor malignancy. Consistent with these findings, the use of the tyrosine kinase inhibitor imatinib has demonstrated no activity in several neuroendocrine tumors, including PHEOs, raising questions about the utility of such inhibitors in these tumors [47].
Another notable PHEO/PGL malignant marker is v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 or ERBB2 (Her2/Neu), an RTK involved in cell growth and differentiation. ERBB2 was reported to induce bilateral PHEOs in mice [15], and overexpression of ERBB2 is found in human malignant PHEOs [48, 49]. No clinical study to date, however, has been done to evaluate ERBB2 as a treatment target in malignant PHEOs, but several molecular therapies selective for this receptor are already available and can potentially be implemented in mini-clinical trials.
Recently Sunitinib, a potent multi-RTK inhibitor FDA-approved for metastatic renal cell carcinoma (RCC), has been used in patients with PHEOs associated with VHL mutations [50]. However, disappointing results with Sunitinib have been observed among patients with SDHB mutations, probably due to the lack of overexpression of the molecular targets of this drug compared to patients with VHL mutations (unpublished observations). Lesions with defective VHL protein have a predominant overexpression of HIF1α [26], as in clear cell RCC [51], and subsequently increased RTKs involved in neoangiogenesis [52]. Considering reports describing xenograft experiments using several receptor tyrosine kinase inhibitors (TKIs) that have demonstrated the ability to block metastatic foci in mouse models [53, 54], we expect growth in the number of clinical trails using RTKs and their ligands as targets, either alone or in combination with traditional chemotherapy or radiotherapy.
Another interesting strategy, currently under investigation, has recently emerged, which takes advantage of the capacity of chaperone proteins to correctly fold therapeutically relevant oncoproteins. The proteins, collectively called heat shock proteins (HSPs), are cellular chaperones that are important for housekeeping functions, including protein transportation, assembly, and folding [55]. HSPs are frequently overexpressed and activated in cancer cells, suggesting a role in the process of malignant transformation. While HSP90 is present in a latent, uncomplexed state in normal cells [56], it is completely activated and part of multichaperone complexes in cancer cells [57]. HSP90 is a key protein in several pathways of cell proliferation, survival, and tumor progression, and consequently it has been recognized as a central nodal target for cancer therapy [58]. In particular, overexpression of HSP90 has been observed in malignant PHEOs compared with benign disease [59], and it has been identified as a promising therapeutic target for PHEOs [60, 61]. Several HSP90 inhibitors have been developed in the last decade, and several clinical trials are ongoing and could potentially include patients with malignant PHEOs/PGLs.
More recently, we investigated the ability of LB1, a small molecule inhibitor of serine/threonine protein phosphatase 2A (PP2A), to inhibit mouse PHEO cells (MPC) in vitro and in vivo in a mouse model of metastatic PHEO alone and in combination with temozolomide (TMZ) [62]. When LB1 or TMZ were used separately or in combination, they only modestly inhibited MPC cell proliferation. However, in vivo, the combination of both drugs markedly reduced the rate of increase of metastatic (hepatic) tumor volume in all animals. There was a slight or no inhibitory effect on tumor growth in vivo when these drugs were used separately. Inhibition of PP2A was associated with the prevention of G1/S phase arrest and of mitotic arrest mediated by Plk-1. We proposed that a transient pharmacologic inhibition of PP2A could represent a new approach for enhancing the efficacy of non-specific cancer chemotherapy regimens against a broad spectrum of low growth fraction of PHEO/PGL tumors commonly resistant to cytotoxic drugs.
Carboxypeptidase E is another molecular target we could use in the treatment of metastatic PHEO/PGL [63]. We found an increased expression of an N-terminal splice isoform (CPE-DN) in various human metastatic tumor cell lines, and suppression of this gene in these cell lines with siRNA led to inhibition of growth and invasion in xenograft mouse models [64]. It appears that CPE-DN increases neural precursor expressed, developmentally down-regulated 9 or NEDD9 by interacting with histone deacetylase 1 and -2 (HDAC 1 and -2), another potential molecular target for metastatic PHEO. As a side note, NEDD9 has been associated with tumor progression on various cancer types [65]. High copy numbers of CPE-DN mRNA were observed in metastatic PHEO/PGL, and interestingly high copy numbers in benign tumors are able to predict recurrence or metastatic disease.
2) Pro-apoptotic therapeutic strategies
Despite the two major clusters derived from transcriptome profiling of different PHEO and PGL tissues [14], as described above, other groups have suggested that the susceptibility genes described so far converge into a common pathway [66]. In this model, mutations in the PHEO susceptibility genes RET, VHL, NF1 and SDHx are responsible for a defective apoptotic pathway in neuronal progenitor cells, a process that usually occurs upon withdrawal of nerve growth factor (NGF) during embryogenesis and is mediated by c-Jun. This finding is consistent with an increase in JunB, modulator of c-JUN, among cells with defective pVHL. JunB also promotes survival in rat PHEO cells after NGF withdrawal. In addition, SDHx mutations, as observed in PHEO/PGL, cause accumulation of succinate, which in turn inhibits EGLN3; this process subsequently inhibits apoptosis [66]. It is interesting to note that the kinesin KIF1Bbeta, whose loss of function has been described to cause one rare form of hereditary PHEO/PGL, was described to act downstream of EGLN3 [67]. Several molecules along this developmental pathway are potential drug targets that should be further explored for induction of cell-specific apoptosis in PHEOs/PGLs. The escape from programmed cell death through inhibition of HIF-prolyl hydroxylase 3 (PHD3) or EGLN3 establishes an important link between the two pathways. This is an example of how better knowledge of the molecular pathways involved in pathogenesis of PHEO can guide the selection of successful drug combinations.
Many different types of experimental therapeutic agents, either alone or in combination with other therapeutic options, have been shown to have a significant effect on PHEO cell apoptosis. For example, the HDAC inhibitors romidepsin and trichostatin A were studied to assess their ability to enhance apoptosis induced by [123I]-MIBG uptake in a mouse model of metastatic PHEO and found to profoundly increase the expression of the norepinephrine transporter system (NET) and the uptake of [123I]-MIBG in vitro and in vivo [68]. We concluded that HDAC inhibitors can enhance the therapeutic efficacy of [131I]-MIBG treatment in patients with metastatic PHEO/PGL.
In another recent study we demonstrated that NFkB inhibition using either triptolide or capsaicin led to NET upregulation and apoptosis induction in the three available PHEO cell lines: MTT, MPC, and PC12 [69]. Moreover, NFkB inhibition with triptolide resulted in a significant reduction of liver metastases in an animal model of metastatic PHEO.
Apoptosis can also be induced in PHEOs/PGLs via inhibition of TNF receptor-associated protein 1 (TRAP-1) [70]. This mitochondrial-specific HSP90 homolog is found to be elevated among SDHx-related PHEO/PGLs. MPC expresses high levels of TRAP-1, and treatment with gamitrinib, an HSP90 inhibitor, resulted in 90 to 95% cell death in two weeks (Powers et al, USCAP 2011).
Somatostatin analogs are also promising agents in the treatment of PHEOs/PGLs, not only because of their established use in neuroendocrine tumors, but more importantly because it has been shown that PHEO tissues express more than one type of somatostatin receptor [71]. A study reported a more potent inducer of apoptosis, SOM230, in comparison to octreotide [72]. SOM230 also markedly suppressed intracellular catecholamine levels. The superior response profile of SOM230 is attributed to its ability to block 4 out of 5 somatostatin receptor compared to octreotide or lanreotide, which are known to have high affinity to somatostatin receptor 2 (sst2) [72].
Nutritional therapy for cancer has also been gaining momentum. Eicosapentanoic acid (EPA), a polyunsaturated fatty acid sourced from aquatic animals, has been found to induce apoptosis in the rat PHEO cell line PC12 [73]. It was postulated that lipid peroxidation with subsequent cellular and DNA damage could account for the anticancer properties of EPA. It is important to outline that, unlike the more widely used and approved chemotherapeutic agents, EPA was reported to cause only negligible side effects [74], outlining the importance of exploring this class of therapy more deeply.
Lastly, highly proliferative cells such as cancer cells are dependent on topoisomerases that release torsional stress on the DNA double strand during replication. This presents a potential drug target, whereby inhibition of this enzyme (e.g. with compounds such as camptothecin) leads to irreversible DNA strand breaks and subsequent activation of apoptotic pathways [75].
In conclusion, our clinical experience and the use of murine models of metastatic PHEOs have helped to introduce new experimental treatment options, using proliferation inhibition or apoptosis as a way to reduce tumor burden. First, using a combination of microCT and [18F]-FDA PET in a mouse model of PHEOs [76], we optimized a useful approach to assess various organs for metastatic lesions. More recently we introduced an animal model that takes advantage of bioluminescence technology for the rapid in vivo investigation and implementation of therapeutic options for PHEOs [77]. These models will greatly help the PHEO community explore novel targeted therapies that have already shown promising results in several other tumor types.
REFERENCES
- 1.Timmers HJ, Taieb D, Pacak K. Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012;44(5):367–372. doi: 10.1055/s-0031-1299712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fliedner SM, Lehnert H, Pacak K. Metastatic paraganglioma. Seminars in oncology. 2010;37(6):627–637. doi: 10.1053/j.seminoncol.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenhofer G, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clinical chemistry. 2011;57(3):411–420. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113(8):2020–2028. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholz T, et al. Clinical review: Current treatment of malignant pheochromocytoma. The Journal of clinical endocrinology and metabolism. 2007;92(4):1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 6.Druce MR, et al. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001) Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009;41(9):697–702. doi: 10.1055/s-0029-1220687. [DOI] [PubMed] [Google Scholar]
- 7.Adjalle R, et al. Treatment of malignant pheochromocytoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009;41(9):687–696. doi: 10.1055/s-0029-1231025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro B, et al. Radiopharmaceutical therapy of malignant pheochromocytoma with [131I]metaiodobenzylguanidine: results from ten years of experience. Journal of nuclear biology and medicine. 1991;35(4):269–276. [PubMed] [Google Scholar]
- 9.Loh KC, et al. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. Journal of endocrinological investigation. 1997;20(11):648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 10.Gonias S, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(25):4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havekes B, et al. Detection and treatment of pheochromocytomas and paragangliomas: current standing of MIBG scintigraphy and future role of PET imaging. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine. 2008;52(4):419–429. [PubMed] [Google Scholar]
- 12.Fonte JS, et al. False-negative (1)(2)(3)I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocrine-related cancer. 2012;19(1):83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grogan RH, Mitmaker EJ, Duh QY. Changing paradigms in the treatment of malignant pheochromocytoma. Cancer control : journal of the Moffitt Cancer Center. 2011;18(2):104–112. doi: 10.1177/107327481101800205. [DOI] [PubMed] [Google Scholar]
- 14.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An Update on the Genetics of Paraganglioma, Pheochromocytoma, and Associated Hereditary Syndromes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012 doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 15.Burnichon N, et al. A novel TMEM127 mutation in a patient with familial bilateral pheochromocytoma. European journal of endocrinology / European Federation of Endocrine Societies. 2011;164(1):141–145. doi: 10.1530/EJE-10-0758. [DOI] [PubMed] [Google Scholar]
- 16.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. The Journal of biological chemistry. 2000;275(5):3568–3576. doi: 10.1074/jbc.275.5.3568. [DOI] [PubMed] [Google Scholar]
- 17.Wall M, et al. Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood. 2008;112(6):2305–2317. doi: 10.1182/blood-2007-09-111856. [DOI] [PubMed] [Google Scholar]
- 18.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nature reviews. Molecular cell biology. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 19.Jain S, et al. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mechanisms of development. 1998;73(1):117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 20.Raval RR, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Molecular and cellular biology. 2005;25(13):5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta D, et al. Activation of the VEGFR1 chromatin domain: an angiogenic signal-ETS1/HIF-2alpha regulatory axis. The Journal of biological chemistry. 2008;283(37):25404–25413. doi: 10.1074/jbc.M804349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bras A, et al. HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26(53):7480–7489. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 23.Eubank TD, et al. Opposing roles for HIF-1alpha and HIF-2alpha in the regulation of angiogenesis by mononuclear phagocytes. Blood. 2011;117(1):323–332. doi: 10.1182/blood-2010-01-261792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors--similar but not identical. Molecules and cells. 2010;29(5):435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 25.Maher ER. Genetics of phaeochromocytoma. British medical bulletin. 2006;79–80:141–151. doi: 10.1093/bmb/ldl002. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Jimenez E, et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Molecular endocrinology. 2010;24(12):2382–2391. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nature genetics. 2010;42(3):229–233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson CC, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Molecular and cellular biology. 2002;22(20):7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. The Journal of biological chemistry. 2007;282(28):20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 30.Toschi A, et al. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. The Journal of biological chemistry. 2008;283(50):34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldewijns MM, et al. VHL and HIF signalling in renal cell carcinogenesis. The Journal of pathology. 2010;221(2):125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 32.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 34.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4–5):441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Panta GR, Nwariaku F, Kim LT. RET signals through focal adhesion kinase in medullary thyroid cancer cells. Surgery. 2004;136(6):1212–1217. doi: 10.1016/j.surg.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Yip L, et al. Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch Surg. 2003;138(4):409–416. doi: 10.1001/archsurg.138.4.409. discussion 416. [DOI] [PubMed] [Google Scholar]
- 37.Powers JF, et al. High-level expression of receptor tyrosine kinase Ret and responsiveness to Ret-activating ligands in pheochromocytoma cell lines from neurofibromatosis knockout mice. Molecular and cellular neurosciences. 2002;20(3):382–389. doi: 10.1006/mcne.2002.1139. [DOI] [PubMed] [Google Scholar]
- 38.Mologni L. Development of RET kinase inhibitors for targeted cancer therapy. Current medicinal chemistry. 2011;18(2):162–175. doi: 10.2174/092986711794088308. [DOI] [PubMed] [Google Scholar]
- 39.Brave SR, et al. Vandetanib inhibits both VEGFR-2 and EGFR signalling at clinically relevant drug levels in preclinical models of human cancer. International journal of oncology. 2011;39(1):271–278. doi: 10.3892/ijo.2011.1022. [DOI] [PubMed] [Google Scholar]
- 40.Vitagliano D, et al. The tyrosine kinase inhibitor ZD6474 blocks proliferation of RET mutant medullary thyroid carcinoma cells. Endocrine-related cancer. 2011;18(1):1–11. doi: 10.1677/ERC-09-0292. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, et al. Tumor angiogenesis in pheochromocytomas and paragangliomas. Surgery. 1996;120(6):938–942. doi: 10.1016/s0039-6060(96)80037-7. discussion 942-3. [DOI] [PubMed] [Google Scholar]
- 42.Claffey KP, Wilkison WO, Spiegelman BM. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992;267(23):16317–16322. [PubMed] [Google Scholar]
- 43.Middeke M, et al. In vitro and in vivo angiogenesis in PC12 pheochromocytoma cells is mediated by vascular endothelial growth factor. Exp Clin Endocrinol Diabetes. 2002;110(8):386–392. doi: 10.1055/s-2002-36424. [DOI] [PubMed] [Google Scholar]
- 44.Zielke A, et al. VEGF-mediated angiogenesis of human pheochromocytomas is associated to malignancy and inhibited by anti-VEGF antibodies in experimental tumors. Surgery. 2002;132(6):1056–1063. doi: 10.1067/msy.2002.128613. discussion 1063. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda R, et al. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol. 1993;142(1):339–346. [PMC free article] [PubMed] [Google Scholar]
- 46.Tavangar SM, et al. Immunohistochemical expression of Ki67, c-erbB-2, and c-kit antigens in benign and malignant pheochromocytoma. Pathol Res Pract. 2010;206(5):305–309. doi: 10.1016/j.prp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Koch CA, et al. Does the expression of c-kit (CD117) in neuroendocrine tumors represent a target for therapy? Ann N Y Acad Sci. 2006;1073:517–526. doi: 10.1196/annals.1353.055. [DOI] [PubMed] [Google Scholar]
- 48.Tavangar SM, et al. Immunohistochemical expression of Ki67, c-erbB-2, and c-kit antigens in benign and malignant pheochromocytoma. Pathology, research and practice. 2010;206(5):305–309. doi: 10.1016/j.prp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Yuan W, et al. Overexpression of ERBB-2 was more frequently detected in malignant than benign pheochromocytomas by multiplex ligation-dependent probe amplification and immunohistochemistry. Endocrine-related cancer. 2008;15(1):343–350. doi: 10.1677/ERC-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimenez C, et al. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94(2):386–391. doi: 10.1210/jc.2008-1972. [DOI] [PubMed] [Google Scholar]
- 51.Wiesener MS, et al. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer research. 2001;61(13):5215–5222. [PubMed] [Google Scholar]
- 52.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews. Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 53.Osusky KL, et al. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis. 2004;7(3):225–233. doi: 10.1007/s10456-004-3149-y. [DOI] [PubMed] [Google Scholar]
- 54.Huang D, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer research. 2010;70(3):1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 55.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin SH, et al. Independent ATPase activity of Hsp90 subunits creates a flexible assembly platform. J Mol Biol. 2004;344(3):813–826. doi: 10.1016/j.jmb.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 57.Kamal A, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425(6956):407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 58.Trepel J, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boltze C, et al. Expression profile of the telomeric complex discriminates between benign and malignant pheochromocytoma. J Clin Endocrinol Metab. 2003;88(9):4280–4286. doi: 10.1210/jc.2002-021299. [DOI] [PubMed] [Google Scholar]
- 60.Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45(2):65–90. doi: 10.4149/endo_2011_02_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grogan RH, Mitmaker EJ, Duh QY. Changing paradigms in the treatment of malignant pheochromocytoma. Cancer Control. 2011;18(2):104–112. doi: 10.1177/107327481101800205. [DOI] [PubMed] [Google Scholar]
- 62.Martiniova L, et al. Pharmacologic modulation of serine/threonine phosphorylation highly sensitizes PHEO in a MPC cell and mouse model to conventional chemotherapy. PloS one. 2011;6(2):e14678. doi: 10.1371/journal.pone.0014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cellular and molecular neurobiology. 2010;30(8):1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Lee TK, et al. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. The Journal of clinical investigation. 2011;121(3):880–892. doi: 10.1172/JCI40433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Ahn J, Sanz-Moreno V, Marshall CJ. The metastasis gene NEDD9 product acts through integrin beta3 and Src to promote mesenchymal motility and inhibit amoeboid motility. Journal of cell science. 2012;125(Pt 7):1814–1826. doi: 10.1242/jcs.101444. [DOI] [PubMed] [Google Scholar]
- 66.Lee S, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer cell. 2005;8(2):155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Schlisio S, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes & development. 2008;22(7):884–893. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martiniova L, et al. Increased uptake of [(1)(2)(3)I]meta-iodobenzylguanidine, [(1)(8)F]fluorodopamine, and [(3)H]norepinephrine in mouse pheochromocytoma cells and tumors after treatment with the histone deacetylase inhibitors. Endocrine-related cancer. 2011;18(1):143–157. doi: 10.1677/ERC-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pacak K, et al. NF-kappaB inhibition significantly upregulates the norepinephrine transporter system, causes apoptosis in pheochromocytoma cell lines and prevents metastasis in an animal model. International journal of cancer. Journal international du cancer. 2012 doi: 10.1002/ijc.27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chemistry & biology. 2007;14(11):1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Mundschenk J, et al. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. The Journal of clinical endocrinology and metabolism. 2003;88(11):5150–5157. doi: 10.1210/jc.2003-030262. [DOI] [PubMed] [Google Scholar]
- 72.Pasquali D, et al. Effects of somatostatin analog SOM230 on cell proliferation, apoptosis, and catecholamine levels in cultured pheochromocytoma cells. Journal of molecular endocrinology. 2008;40(6):263–271. doi: 10.1677/JME-08-0012. [DOI] [PubMed] [Google Scholar]
- 73.Li M, Kong ZM, Liu ZL. Antioxidant enzyme activities and lipid peroxidation induced by eicosapentaenoic acid (EPA) in PC12 cells. Cell biology and toxicology. 2006;22(5):331–337. doi: 10.1007/s10565-006-0060-x. [DOI] [PubMed] [Google Scholar]
- 74.Senzaki H, et al. Dietary effects of fatty acids on growth and metastasis of KPL-1 human breast cancer cells in vivo and in vitro. Anticancer research. 1998;18(3A):1621–1627. [PubMed] [Google Scholar]
- 75.Sordet O, et al. Apoptosis induced by topoisomerase inhibitors. Current medicinal chemistry. Anti-cancer agents. 2003;3(4):271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- 76.Martiniova L, et al. Usefulness of [18F]-DA and [18F]-DOPA for PET imaging in a mouse model of pheochromocytoma. Nuclear medicine and biology. 2012;39(2):215–226. doi: 10.1016/j.nucmedbio.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giubellino A, et al. Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer letters. 2012;316(1):46–52. doi: 10.1016/j.canlet.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]