Abstract
Ethnopharmacological relevance
Extracts of leaves from different species of the genus Alchornea have been used for centuries to treat a variety of medicinal problems in tropical Africa. However, little is known about the high-molecular weight active components conferring therapeutic properties to these extracts.
Objective
The aim of this study was to evaluate the immunomodulatory activity of polysaccharides isolated from the leaves of Alchornea cordifolia.
Materials and methods
Water-soluble polysaccharides from leaves of A. cordifolia were extracted and fractionated by DEAE-cellulose, Diaion HP-20, and size-exclusion chromatography. Molecular weight, sugar analysis, and other physical and chemical characterization of the fractions were performed. Immunomodulatory activity of the polysaccharide fractions was evaluated by determining their ability to induce monocyte/macrophage nitric oxide (NO) and cytokine production. Activation of mitogen activated protein kinases (MAPK) was also assessed using a phospho-MAPK array. Activation of nuclear factor κB (NF-κB) was measured using an alkaline phosphatase reporter gene assay in THP1-Blue monocytic cells.
Results
Six polysaccharide fractions from A. cordifolia were isolated. Fractions containing type II arabinogalactan had potent immunomodulatory activity. Particularly, the parent fraction AP-AU and its high-molecular weight sub-fraction AP-AU1 (average Mr was estimated to be 39.5 kDa) induced production of NO and cytokines [interleukin (IL)-1β, -6, -10, tumor necrosis factor (TNF)-α, and granulocyte macrophage-colony stimulating factor (GM-CSF)] in human peripheral blood mononuclear cells and human and murine monocyte/macrophages cell lines in vitro. Furthermore, treatment with AP-AU1 induced phosphorylation of Akt2, p38δ/p38γ, p70S6K1, RSK2, and mTOR, as well as stimulation of NF-κB transcriptional activity.
Conclusion
Our results provide a molecular basis to explain a portion of the beneficial therapeutic properties of water extracts from A. cordifolia leaves in traditional folk medicine of Africa.
Keywords: Alchornea cordifolia, polysaccharide, macrophage, cytokine, immunomodulation, alternative medicine
Introduction
Complementary and alternative medicines have been used historically around the world to treat many diseases. Hence, mechanistic studies and the identification of active compounds of these medicines could lead to new discoveries in the biological and biomedical sciences. Modern translational research on herbal medicines beyond basic science and clinical perspectives could contribute to the development of new therapeutics. Polysaccharides, one of main classes of bioactive substances from fungi, algae, and higher plants, have been demonstrated to exhibit a wide range of pharmacological activities, including broad immunomodulatory and antitumor effects (Jiang et al., 2010; Schepetkin and Quinn, 2011). For example, mushroom and plant polysaccharides identified through complementary and alternative medicine are undergoing scientific analysis and development to treat cancer (Pelley and Strickland, 2000; Zaidman et al., 2005; Ramberg et al., 2010; Ren et al., 2012). In addition, some polysaccharides have been tested for their therapeutic properties in human clinical trials (Leung et al., 2006).
Alchornea cordifolia (Schum. & Thonn.) Muell. Arg., which belongs to the family Euphorbiaceae, grows as a shrub or small tree and is distributed throughout tropical Africa in secondary forests, usually near water or marshy places (Dalziel, 1956). A. cordifolia is known by many traditional healers to be a plant with a variety of medicinal properties. For example, extracts obtained by boiling A. cordifolia leaves in water are used as a remedy for stomach ulcers, venereal disease, cough, bronchial troubles, malaria, fever, rheumatic pain, sores, and toothache (Dalziel, 1956; Le Grand A., 1989; Ogungbamila and Samuelsson, 1990; Agbor et al., 2004; Adeshina et al., 2008). In addition, the local and systemic anti-inflammatory properties of various extracts from A. cordifolia have been validated in recent pharmacological studies (Ajali, 2000; Tona et al., 2000; Osadebe and Okoye, 2003; Agbor et al., 2004; Osadebe et al., 2008; Manga et al., 2004; Mavar-Manga et al., 2008; Pesewu et al., 2008; Mesia et al., 2008; Okoye et al., 2011; Ayisi et al., 2011). Thus, A. cordifolia extracts have a very broad spectrum of activity and have also been suggested to be useful for treatment of various microbial infections (Okeke et al., 1999). Phytochemical screening of A. cordifolia extracts revealed the presence of tannins, flavonoids, glycosides, resins and carbohydrates (Adeshina et al., 2007; Adeshina et al., 2010).
Although biologically-active substances with low molecular weight, including alkaloids, terpenoids, saponins, and flavonoids in medicinal herbs have been well studied and characterized, they often do not account for all of the clinical effects achieved. Among the high molecular weight components of medicinal herbs, polysaccharides have been shown to possess a variety of pharmacological activities (Schepetkin and Quinn, 2011). Although, there are several reports on the medicinal properties of polysaccharides isolated from plants belonging to the Euphorbiaceae family (Milo et al., 2002; Mellinger et al., 2005; Yu et al., 2006), there are no reports regarding isolation and characterization of polysaccharides from any of the seventy known tropical Alchornea species. Thus, we performed studies to investigate the immunomodulatory properties of Alchornea polysaccharides isolated by hot water extraction, which is the most frequently used preparation reported in our ethnopharmacological analysis. Alchornea polysaccharides were fractionated and analyzed for potential immunomodulatory properties in mouse and human monocyte/macrophage cell lines and human peripheral blood mononuclear cells (PBMCs). To investigate the molecular mechanisms responsible for the polysaccharide-induced immunomodulatory responses, the roles of signaling molecules, including mitogen-activated protein kinases (MAPKs) and nuclear factor κB (NF-κB), were evaluated. Our results suggest that Akt/NF-κB and p38 signaling pathways participate in Alchornea polysaccharide-stimulated cytokine production. Thus, polysaccharides present in Alchornea extracts likely contribute to their therapeutic properties.
1. Materials and Methods
1.1. Fractionation of polysaccharides
Leaves of A. cordifolia were collected in the Bingerville areas of Cote d’Ivoire and were taxonomically confirmed. Dried and ground leaves (500 g) were extracted with 3 L boiling distilled H2O for 1 hr, and the aqueous extracts were centrifuged at 2,500 × g for 15 min. A four fold volume of ethanol was added to each supernatant to precipitate the polysaccharides overnight at 4°C. The precipitates were pelleted by centrifugation, dissolved in distilled H2O, centrifuged at 80,000 × g for 1 hr, and re-precipitated with a four-fold volume of ethanol. The precipitates were re-dissolved in distilled H2O, filtered through a 0.22 μm filter, and concentrated in an Amicon concentrator with a 1 kDa PLAC membrane (Millipore, Biillerica. MA) to obtain crude polysaccharide extracts. The crude extracts were further purified using ion-exchange chromatography on a DEAE-cellulose column equilibrated with 0.05 MTris-HCl buffer (pH 8.0). For each fractionation, the column was washed with equilibration buffer to obtain the crude neutral polysaccharide fraction. The bound material was eluted with equilibration buffer containing 2 M NaCl. The eluates were concentrated in an Amicon concentrator with a 1 kDa PLAC membrane to obtain the crude neutral and acid polysaccharide fractions. The obtained fractions were then fractionated on a Diaion HP-20 absorbent resin column (2.5 × 20 cm). For each fractionation, the column was eluted with distilled H2O and lyophilized to obtain unbound fractions (designated as AP-NU and AP-AU). Bound polysaccharides were eluted with methanol and dried.
The unbound fraction AP-AU was further fractionated by size-exclusion chromatography on a Sepharose-6B column (2.5×95 cm) eluted with distilled H2O at a flow rate of 21 ml/hr. The carbohydrate elution profile was determined by the phenol-H2SO4 method (Dubois et al., 1956), modified to a microplate format, and absorbance was measured at 488 nm using a SpectraMax Plus microplate reader (Molecular Devices, Palo Alto, CA). The relevant fractions were pooled and concentrated. Buffer salts were removed from the polysaccharide samples by repeated (6×) concentration in an Amicon concentrator (1 kDa cut-off PLAC membrane) and dilution with a 10-fold volume of distilled H2O. For analysis of biological activity, the fractions were diluted in Hanks balanced-salt solution without Ca2+ and Mg2+ (HBSS−) to a concentration of 5 mg/ml and filtered through sterile 0.22 μm filters.
1.2. Characterization of polysaccharide fractions
1.2.1. NMR analysis
For nuclear magnetic resonance (NMR) analysis, samples (5 mg) were dissolved in deuterium oxide (0.5 ml), and 1H NMR spectra were recorded on a Bruker DRX-600 spectrometer (Bruker BioSpin, Billerica, MA) at 20°C using 3-(trimethylsilyl)-propionic 2,2,3,3,-d4 acid sodium salt as an internal reference (δ 0.00 ppm).
1.2.2. High-performance liquid chromatography (HPLC) analysis
The homogeneity and average Mr of the polysaccharide fractions were determined by high performance size-exclusion chromatography (HP-SEC) using a Shimadzu Class VP HPC and ShodexOHpak SB-804 HQ column (8 mm × 300 mm) eluted with 50 mM sodium citrate buffer, pH 7.5, containing 0.15 M NaCl and 0.01% NaN3 at a flow rate of 0.3 ml/min. Peaks were detected using a refractive index detector (RID-10A; Shimadzu, Torrance, CA). Average molecular weights of the polysaccharide fractions were estimated by comparison with retention times of pullulan standards P-100, 50, 20, 10, and 5 (Phenomenex, Torrance, CA), which have molecular weights of 112, 47.3, 22.8, 11.8, and 5.9 kDa, respectively. Reproducibility of the retention times was typically >98%.
1.2.3. Detection of arabinogalactan type II
The presence of arabinogalactan in the samples was detected by single radial gel diffusion in 1% agarose gels containing 100 μg/ml β-glucosyl Yariv reagent, which selectively interacts with and precipitates compounds containing type II arabinogalactan structures. Four μl of polysaccharide samples (10 mg/ml; w/v) were loaded into the wells, and the samples were incubated at 25°C for 24 hr in a humid atmosphere. A positive reaction was identified by a reddish circle (halo) around the well, and arabic gum (4 mg/ml) (FlukaChemie GmbH, Germany) served as a positive control.
1.2.4. Carbohydrate and polyphenol determination
Carbohydrate content was determined by the phenol-sulfuric acid method, modified to a microplate format (Masuko et al., 2005) with glucose as a standard, and absorbance was measured at 490 nm using a SpectraMax Plus microplate reader.
The total amount of polyphenols in the polysaccharide fractions was determined by the Folin-Ciocalteu assay (Singleton and Rossi, 1965). Briefly, 250 μL of Folin’s phenol reagent was added to the samples dissolved in 500 μL distilled water. After 3 min at room temperature, 1.25 mL of 20% sodium carbonate was added, mixed, and the mixture was allowed to stand for 40 min. The absorbance was measured at 750 nm in a SpectraMax Plus microplate reader. Tannic acid (TA) from Rhussemialata (Sigma-Aldrich, St. Louis, MO) was used to generate a standard curve.
1.2.5. Limulus Amebocyte Lysate (LAL) assay
The LAL assay was used to estimate the amount of endotoxin in the polysaccharide fractions. Analyses of endotoxin concentration were performed via the kinetic method (ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit, GenScript, Piscataway, NJ) using a SpectraMax Plus microplate reader.
1.2.6. Monosaccharide analysis
For monosaccharide analysis, the polysaccharide fractions were lyophilized and submitted for analysis to the Oklahoma Center for Glycobiology Analytical Core Lab (Oklahoma City, OK). The polysaccharide samples or background blanks were subjected to methanolysis (methanolic 2 M HCl, 16 hr, 80°C), followed by acid hydrolysis (2 M trifluoroacetic acid, 4 hr, 100°C), and the resulting monosaccharide mixtures were analyzed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a Dionex DX-600 HPAEC system equipped with an ED50 detector (Dionex Corporation, Sunnyvale, CA). The samples were separated on a DionexCarboPac PA20 column eluted isocratically with 12 mM NaOH at a flow rate of 1 ml/min at 22°C. For analysis of uronic acids, the column was eluted with 10 mM NaOH for 20 min, followed by a gradient of 100 mM NaOH/150 mM sodium acetate (0–100% in 45 min). Background signals were subtracted from all samples, and individual components were quantified based on electrochemical detection relative to known standards.
1.3. Cell cultures
Murine macrophage J774.A1 cells were cultured in DMEM supplemented with 10% (v/v) heat-inactivated, endotoxin-free FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin. Cells were grown to confluence in sterile tissue culture flasks and gently detached by scraping. Human monocyte-macrophage MonoMac6 cells (DSMZ, Germany) were grown in RPMI 1640 supplemented with 10% (v/v) endotoxin-free FBS, 10 μg/ml bovine insulin, 100 μg/ml streptomycin, and 100 U/ml penicillin. Human monocytic THP1-Blue cells obtained from In vivo Gen (San Diego, CA) were cultured in RPMI 1640 medium supplemented with 10% (v/v) endotoxin-free FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, 100 μg/ml zeocin, and 10 μg/ml blasticidin S. These cells are stably transfected with a secreted embryonic alkaline phosphatase gene that is under the control of a promoter inducible by nuclear factor κB (NF-κB).
PBMCs were purified from human blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of erythrocytes. Blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montan State University, Bozeman, MT.
All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. Cell number and viability were assessed microscopically using trypan blue exclusion.
1.4. Analysis of nitric oxide (NO) production
J774.A1 cells were plated at a density of 1.5 × 105 cells/well in a final volume of 200 μl in 96-well flat-bottom tissue culture plates and incubated in medium alone or medium containing various concentrations of polysaccharide fractions or E. coli lipopolysaccharide (LPS) as a positive control. Cells were incubated at 37 °C in the presence of 5% CO2 for 24 h, and 100 μl of the cell culture supernatants were removed and analyzed for nitrite using a colorimetric method with NaNO2 as the standard. Briefly, supernatants were mixed with an equal volume of Griess reagent, which was prepared by mixing one part of 0.1% (w/v) N-(1-naphthyl) ethylenediamine with one part of 1% (w/v) sulfanilamide in 5% phosphoric acid. After 20 min, absorbance was measured at 540 nm using a SpectraMax Plus microplate reader.
1.5. Determination of cytokine production
Cells were incubated for 24 h in culture medium supplemented with 3% (v/v) endotoxin-free fetal bovine serum, with or without polysaccharide fractions or LPS as a positive control. J774.A1 cells, human PBMCs, and human MonoMac-6 cells were plated in 96-well plates at a density 2×105 cells in 100 μL per well and incubated for 24 h. Enzyme-linked immunosorbent assay (ELISA) kits for human TNF-α and GM-CSF, or human and mouse IL-6 (all from Biolegend) were used to test the protein levels in the cell supernatants. A human cytokine Multi-Analyte ELISArray™ Kit (SABiosciences Corporation; Frederick, MD) was utilized to evaluate various cytokines [interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17A, interferon-γ (IFNγ), tumor necrosis factor α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF)] in supernatants of PBMCs.
1.6. Analysis of NF-κB activation
Activation of NF-κB was measured using an alkaline phosphatase reporter gene assay in THP1-Blue cells. Polysaccharide fractions or LPS (100 ng/ml) were added, and the cells (2 × 105 cells/well) were incubated for 24 h. Alkaline phosphatase activity was measured in cell supernatants using QUANTI-Blue mix (InvivoGen). Activation of NF-κB is reported as absorbance at 655 nm and compared with positive control samples (LPS).
1.7. Cytotoxicity assay
Cytotoxicity was analyzed with a Cell Titer-Glo Luminescent Cell Viability Assay Kit (Promega), according to the manufacturer’s protocol. Following treatment, the cells were allowed to equilibrate to room temperature for 30 min, substrate was added, and the samples were analyzed with a Fluoroscan Ascent FL.
1.8. Mitogen-activated protein kinase (MAPK) profile
Analysis of the phosphorylation states of all MAPKs was performed using a human phospho-MAPK array kit (R&D Systems, Minneapolis, MN). Human PBMCs were incubated for 60 min with selected polysaccharide fraction or negative control (HBSS). The cells were rinsed with HBSS and lysed with the buffer provided. Arrays were incubated overnight at 4°C with lysates obtained from 3 × 106 cells for each sample. The arrays were washed three times with 20 ml of wash buffer provided and incubated for 2 h with the provided detection antibody cocktail containing phospho-site-specific MAPK biotinylated antibodies. The wash steps were repeated, after which the arrays were exposed to chemiluminescent reagents and the signal was monitored with an Alpha Innotech FluorChem FC2 imaging system.
2. Results
2.1. Ethno pharmacological information
The leaves, roots and stem bark extracts of A. cordifolia (local name: Djeka) are used extensively in traditional medicine for treating many diseases in Côte d’Ivoire. To assess the importance of this plant in traditional medicine, 76 healers from 3 different markets of traditional medicines in Abidjan (Abobo, Adjamé, and Yopougon) were interviewed in 2010 and 2012 regarding their use of A. cordifolia.
Interviewees indicated that leaves are the part A. cordifolia most commonly used for preparing treatments and that a decoction of the leaves in water is the most common extraction method. Oral and topical applications are the mode of administration of these medicines. Topical application was reported to be used for conjunctivitis and female urogenital infection. A decoction of fresh A. cordifolia leaves was used topically as a disinfectant for wounds and the vaginal tract, where it also has an astringent effect. Juice from young leaves was recommended for treatment of skin ulcers and dermatoses, and ground leaves were reported to be useful for uterine wounds after delivery. Oral administration of hot water extracts of A. cordifolia leaves was recommended for treatment of a wide range of medical problems, including colic, colitis, enteritis, gastroenteritis, dysentery, helminthiasis, vomiting, dermatosis, dermatitis, paludism, anaemia, gingivitis, child convulsion, anorexia, toothache, febrile convulsions, and bronchitis. Hot water extracts of A. cordifolia leaves were also recommended as restorative and fortifying remedies. In addition to leaves, decoctions of other plant parts were also reported. For example, a decoction of A. cordifolia roots has been used for dysentery, leprosy, respiratory diseases, pain, and as a disinfectant for haemorrhoids. Likewise, a paste of crushed root bark has been applied as a poultice to treat thromboses. A. cordifolia is sold at all plant material markets, and the applications described above were known by all traditional healers and most of the villagers.
2.2. Preparation and partial characterization of Alchornea polysaccharide fractions
Raw polysaccharides from A. cordifolia were obtained by ethanol precipitation and then fractionated using DEAE-cellulose. The resulting fractions were designated as AP-N (Alchornea polysaccharide, neutral fraction) and AP-A (Alchornea polysaccharide, acid fraction). These fractions were further fractionated using Diaion HP-20 resin to obtain four Alchornea-derived fractions (AP-AB, AP-AU, AP-NB, and AP-NU) (Table 1). AP-AU was further fractionated by preparative Sepharose 6B size-exclusion chromatography to obtain two sub-fractions, which were selected based on the total carbohydrate and polyphenol elution profiles (designated as AP-AU1 and AP-AU2) (Figure 1).
Table 1.
Molecular weights and chemical properties of A. cordifolia polysaccharide fractions fractionated sequentially on DEAE-cellulose, Diaion HP-20, and Sepharose 6B
| Fraction Name | Molecular Weight (kDa) | Arabinogalac-tan Type II | Polyphenol Content (%) | ||
|---|---|---|---|---|---|
| DEAE cellulose | Diaion HP-20 | Sepharose 6B | |||
| AP-A (parent fraction) | AP-AB | - | 8.9 | Negative | 19.6 ± 0.8 |
| AP-AU (parent fraction) | 6.6 | Negative | 15.1 ± 0.9 | ||
| AP-AU1 | 39.5 | Positive | 2.7 ± 0.1 | ||
| AP-AU2 | 5.9 | Negative | 15.6 ± 0.4 | ||
| AP-N (parent fraction) | AP-NB | - | 6.2 | Negative | 1.7 ± 0.1 |
| AP-NU | - | 4.9 | Positive | 4.2 ± 0.1 | |
Figure 1.
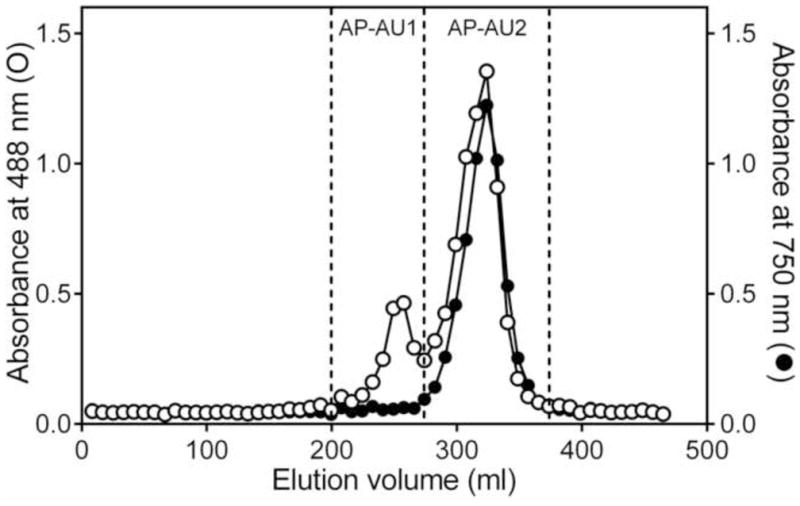
Fractionation of Alchornea polysaccharide fraction AP-AU by size-exclusion chromatography. Polysaccharides from crude A. cordifolia were isolated by sequential DEAE-cellulose and Diaion HP-20 chromatography. Fraction AP-AU was further fractionated using Sepharose 6B column chromatography into two sub-fractions (AP-AU1 and AP-AU2). Total carbohydrate content of the fractions was determined by the phenol-sulfuric acid method, measured at 488 nm (left axis, ○), and polyphenol content was determined by the Folin-Ciocalteu assay, measured at 750 nm (right axis, ●).
Analytical HP-SEC of the individual fractions showed that each fraction was represented by a single, generally symmetrical peak on the chromatogram, suggesting the polysaccharide fractions were relatively homogeneous. Based on calibration curves derived from analysis of pullulan standards, we determined average molecular weights of the polysaccharide fractions (Table 1). Sequential fractionation of A. cordifolia extract by DEAE-cellulose and Diaion HP-20 gave parent fractions with relatively low molecular weights in the range of 4.9 kDa (fraction AP-NU) to 8.9 kDa (fraction AP-AB). Material that bound to Diaion had higher molecular weight in comparison with the unbound fractions (compare 8.9 vs. 6.6 kDa for and 6.2 vs.4.9 kDa). Only fraction AP-AU1 had a relatively high molecular weight of 39.5 kDa (Table 1). The samples were also tested for possible endotoxin contamination using the LAL assay. All fractions contained less than 0.2 ng endotoxin/mg of polysaccharide, which is comparable with endotoxin levels reported for polysaccharides isolated from Aconitum kusnezoffii (Gao et al., 2011), and is considered to be insignificant for various bioactive products (Catchpole et al., 2002; Brito and Singh, 2011).
Our analysis indicated that polyphenols were a minor component of both neutral fractions from A. cordifolia (AP-NB and AP-NU); where as, the two acid parent fractions AP-AB and AP-AU were found to contain a relatively high amount of polyphenols (19.6 and 15.1%, respectively). Polyphenol content was correlated with color of the fractions, which was light-brown in the fractions with lower polyphenol content (AP-NB) and dark-brown for the fraction with the highest polyphenol content (AP-AB). The polyphenols in AP-AU were associated with the low-molecular weight sub-fraction AP-AU2, as polyphenols were a minor component of high-molecular weight sub-fraction AP-AU1 (Table 1).
Analysis of the fractions using the Yariv test showed that the fractions that did not bind to the Diaion HP-20 resin (AP-AU1 and AP-NU) contained type II arabinogalactan; whereas, fractions that bound to the resin (AP-AB and AP-NB), unbound fraction AP-AU, and its low molecular sub-fraction AP-AU2 tested negative for the presence of type II arabinogalactan (Table 1); however, the presence of type I arabinogalactan in the fractions was not excluded. Thus, it appears that the relative amount of type II arabinogalactan in parent fraction AP-AU was too dilute to be detected by the Yariv reagent and that it became concentrated enough to be detected in the high-molecular weight sub-fraction AP-AU1 after chromatography.
Sugar composition analysis revealed that the polysaccharides from A. cordifolia consisted primarily of galactose, arabinose, glucose, mannose, rhamnose, galacturonic and glucuronic acids (Table 2). Small amounts of fucose and xylose were also detected in the fractions. In all fractions, arabinose and galactose were the major monosaccharides found, supporting the conclusion that arabinogalactan is a main macro molecule of Alchornea-derived polysaccharides. Monosaccharide content in the Diaion-bound and -unbound fractions differed by several-fold for mannose, galactosamine, galacturonic, and glucuronic acids, where as glucosamine was found only in the Diaion-bound fraction.
Table 2.
Monosaccharide composition of A. cordifolia polysaccharide fractions
| Monosaccharide | AP-AU | AP-AB | AP-NU | AP-AU1 |
|---|---|---|---|---|
| Mol % | ||||
| Galactose | 43.2 | 42.1 | 38.5 | 44.0 |
| Arabinose | 21.5 | 24.7 | 23.1 | 28.6 |
| Glucose | 9.3 | 16.4 | 11.1 | 4.6 |
| Galacturonic Acid | 8.2 | 2.4 | 8.2 | 5.2 |
| Mannose | 7.9 | 2.8 | 9.5 | 8.1 |
| Rhamnose | 6.1 | 3.6 | 6.4 | 4.7 |
| Glucuronic Acid | 2.0 | 0.8 | 1.5 | 2.6 |
| Fucose | 1.1 | 1.7 | 1.0 | 1.1 |
| Xylose | 0.6 | 2.1 | 0.6 | 1.2 |
| Lyxose | 0 | 0 | 0 | 0 |
| Glucosamine | 0 | 3.4 | 0 | 0 |
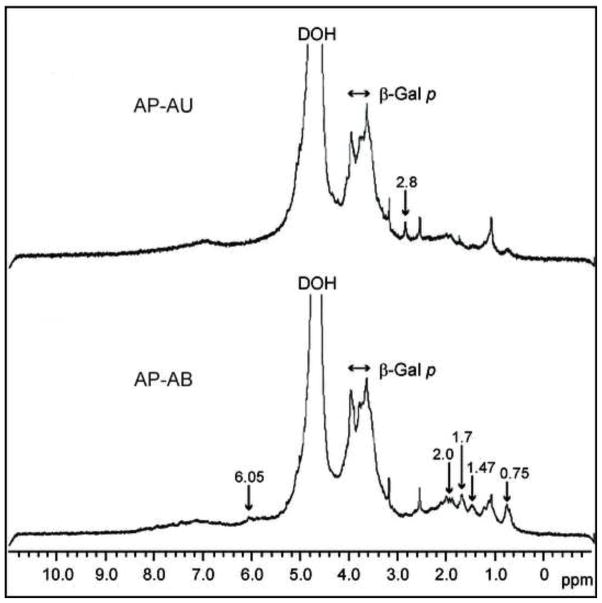
Very-high-field (600 MHz) 1H NMR was used to characterize the structure of native A. cordifolia polysaccharides. The spectra from both acidic fractions, AP-AB and AP-AU, were similar to each other in the range of 3.00–4.00 ppm, suggesting a common backbone structure (Figure 2). The strong signals at 3.6–3.9 ppm are consistent with the presence of β-galactopyranose (β-Galp) (Polle et al., 2002), one of the main structural units of arabinogalactan types I and II. Fraction AP-AB is enriched by aliphatic molecular fragments with terminal methyl groups giving signals at 0.7–0.8 ppm and 1.4–1.5 ppm and acetyl groups (O-Ac or N-Ac) with resonance near 2 ppm (Schiller et al., 1994). Unlike the Diaion-unbound fractions, the Diaion-bound fraction contained glucosamine (Table 2), which together with specific 1H-NMR signals for acetyl groups may indicate the presence of N-acetyl-D-glucosamine (GlcNAc) in this polysaccharide fraction. The relatively high content of reducing sugars (glucose or glucosamine) in the Diaion-bound fractions (Table 2) suggests the presence of products of the Maillard reaction. Indeed, characteristic signals for side chains of amino acid residues are present in the spectra of AP-AB in the region of 0.9–2.8 ppm and may represent such products. The broad weak signal around 7 ppm in both fractions is in agreement with the presence of aromatic moieties. The resonances near 2.8 ppm (AP-AU) and 6.05 ppm (AP-AB), which are not characteristic of polysaccharides, likely correspond to phenyl ring substituents, e.g. of (di) methylamine or olefinic nature, respectively.
Figure 2.
1H NMR spectra of Alchorneapolysaccharide fractions AP-AU and AP-AB. The polysaccharide fractions were dissolved in D2O, and the spectra were recorded at 20 °C.
These results indicate that the individual Alchornea polysaccharide fractions contain different structures, as determined by molecular size, reactivity with Yariv reagent, sugar content, and 1H-NMR. Furthermore, acidic fractions (AP-AB and AP-AU) were enriched with polyphenols (increased by 20%), whereas the neutral fractions (AP-NB and AP-NU) had a minimal amount of polyphenols (1.7–4.2%).
2.3. Effects of Alchornea polysaccharide fractions on macrophage NO production
To characterize the general effectiveness of the polysaccharide fractions in a macrophage cell model, we evaluated their effects in murine J774.A1 macrophages. While fraction AP-NB and background control (medium alone) did not induce NO production, fractions AP-AU, AP-NU, AP-AU2, and AP-AB all stimulated NO production by J774.A1 macrophages, although the response was lower than that induced by LPS (Figure 3).
Figure 3.
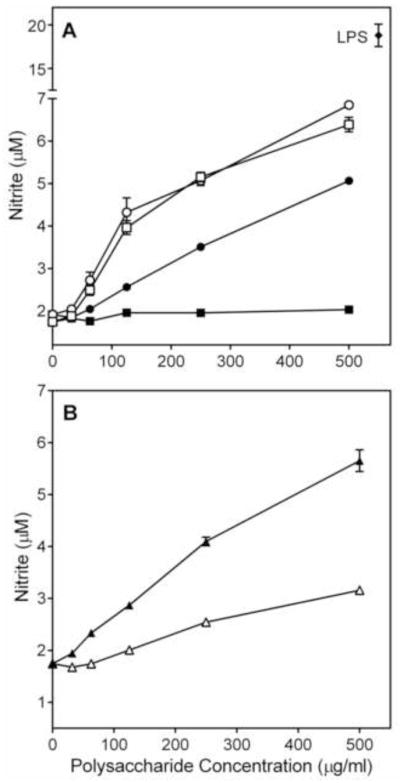
Effect of Alchornea polysaccharide fractions on murine macrophage nitric oxide production. Murine J774.A1 macrophages were incubated for 24 h with the indicated concentrations of polysaccharide fractions AP-AU (○), AP-NU(□), AP-AB (●), AP-NB (■), AP-AU1 (△), AP-AU2 (▲), or 500 ng/ml LPS (panel A, ◆). NO production was quantified by measuring nitrite in the cell-free supernatants. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments.
2.4. Effects of Alchornea polysaccharide fractions on phagocyte cytokine production
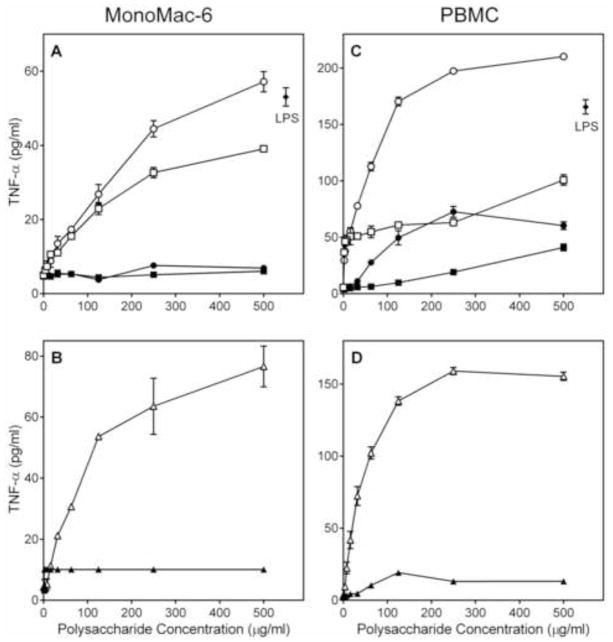
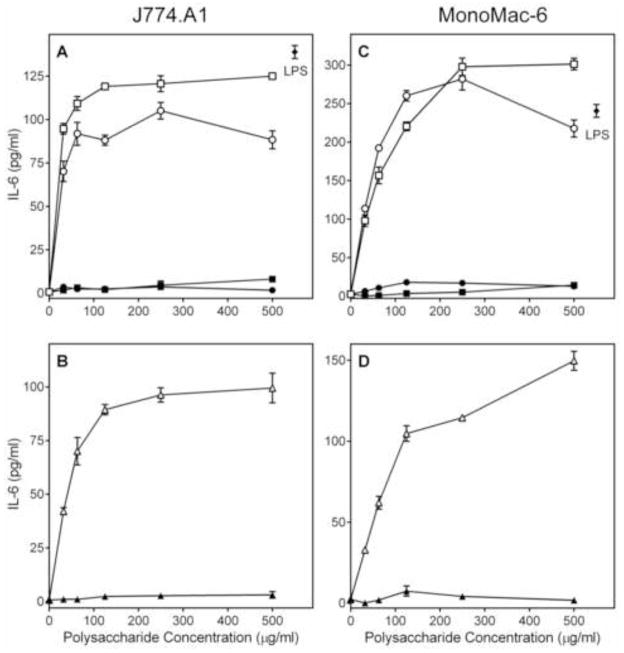
We analyzed the ability of the Alchornea polysaccharide fractions to stimulate TNF-α, IL-6, and GM-CSF production by phagocytes, including human monocytic Mono Mac 6 cells, murine J774.A1 macrophages, and human PBMCs. We found that both Diaion-unbound fractions (AP-AU and AP-NU) dose-dependently stimulated TNF-α (Figure 4), IL-6 (Figure 5), and GM-CSF (Figure 6) production by these cells. In contrast, the Diaion-bound fractions (AP-AB and AP-NB) were not active (Figures 4–6). Furthermore, analysis of the AP-AU sub-fractions showed that the low molecular weight sub-fraction AP-AU2 (Mr 5.9 kDa) was inactive, whereas the high-molecular weight sub-fraction AP-AU1 (Mr 39.5 kDa) dose-dependently stimulated TNF-α (Figure 4), IL-6 (Figure 5), and GM-CSF (Figure 6) production. Fractions AP-AU and AP-NU induced similar levels of TNF-α and IL-6 as positive control LPS (Figures 4 and 5, respectively), whereas LPS did not induce GM-CSF in either Mono Mac-6 cells or PBMCs (Figure 6).
Figure 4.
Effect of Alchornea polysaccharide fractions on monocyte/macrophage TNF-α production. Human MonoMac6 cells (Panels A and B) or PBMCs (Panels C and D) were incubated for 24 h with the indicated concentrations of polysaccharide fractions AP-AU (○), AP-NU (□), AP-AB (●), AP-NB (■), AP-AU1 (△), AP-AU2 (▲), or 500 ng/ml LPS (panels A and C, ◆). Cell-free supernatants were collected, and extra cellular TNF-α was quantified by ELISA. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of three independent experiments.
Figure 5.
Effect of Alchornea polysaccharide fractions on monocyte/macrophage IL-6 production. Murine J774.A1 macrophages (Panels A and B) or human MonoMac-6 monocyte/macrophages (Panels C and D) were incubated for 24 h with the indicated concentrations of polysaccharide fractions AP-AU (○), AP-NU (□), AP-AB (●), AP-NB (■), AP-AU1 (△), AP-AU2 (▲), or 500 ng/ml LPS (panels A and C, ◆). Cell-free supernatants were collected, and extra cellular IL-6 was quantified by ELISA. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of three independent experiments.
Figure 6.
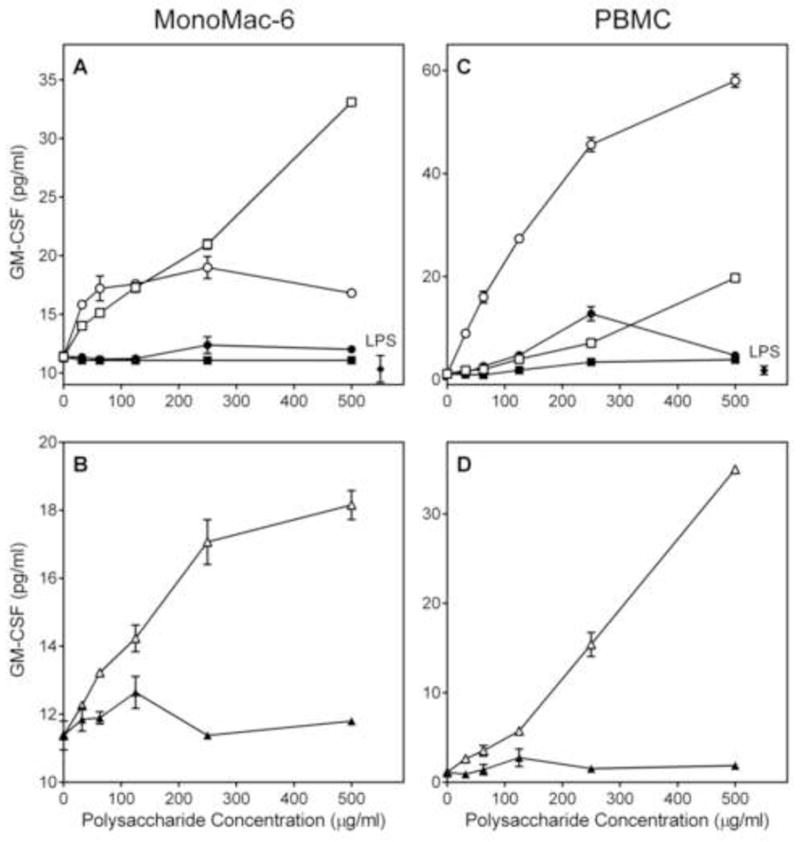
Effect of Alchornea polysaccharide fractions on GM-CSF production. Human MonoMac6 (Panels A and B) or PBMCs (Panels C and D) were incubated for 24 h with the indicated concentrations of polysaccharide fractions AP-AU (○), AP-NU (□), AP-AB (●), AP-NB (■), AP-AU1 (△), AP-AU2 (▲) or 500 ng/ml LPS (panels A and C, ◆). Cell-free supernatants were collected, and extra cellular GM-CSF was quantified by ELISA. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of three independent experiments.
As shown in Figures 4–6, fraction AP-AU was highly active in stimulating phagocyte cytokine production at concentrations ≤ 250 μg/ml, and it appears that its high-molecular weight sub-fraction AP-AU1 was responsible for essentially all activity of the parent fraction. Thus, to determine whether AP-AU1 induced production of other pro-inflammatory and/or anti-inflammatory cytokines, conditioned medium from AP-AU1-treated PBMCs was analyzed using a cytokine ELISA semi quantitative array. Among the 12 cytokines analyzed (Figure 7), six were consistently induced in PBMCs (>5-fold) by 100 μg/ml AP-AU1, as compared to control (HBSS-treated) cells. These included IL-1α [Fold Increase (FI) = 10.0], IL-1β (FI = 44.4), IL-6 (FI = 47.8), IL-10 (FI = 7.4), TNF-α (FI = 7.4), and GM-CSF (FI = 5.2) (Figure 7). IL-8 production was inconclusive because of the high background production by PBMCs (data not shown), a problem which has been documented previously [e.g., (Kikkert et al., 2008)]. Importantly, these results confirmed our ELISA studies, showing induction of TNF-α, IL-6, and GM-CSF production by this polysaccharide fraction, while revealing additional modulatory effects on IL-1α, IL-1β, and IL-10 production.
Figure 7.
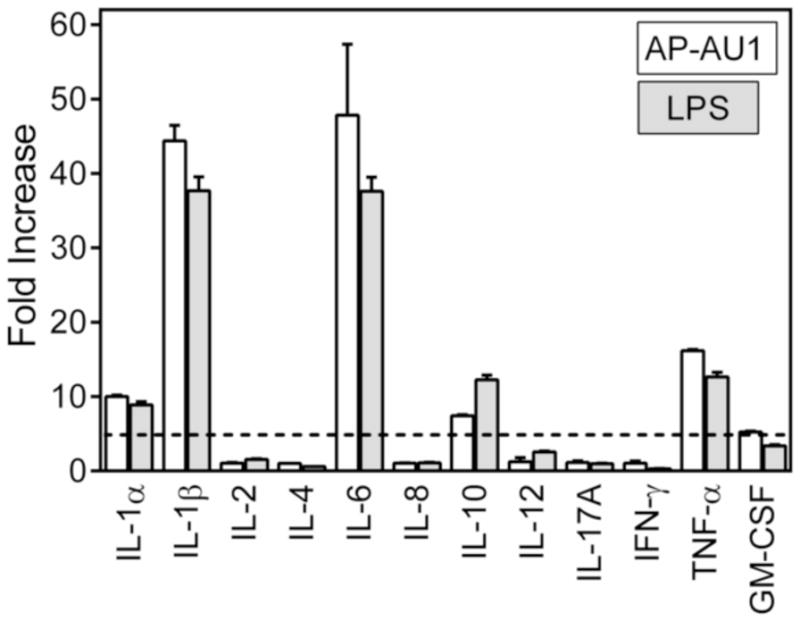
Effect of Alchornea polysaccharide sub-fraction AP-AU1 on cytokine production by human PBMCs. PBMCs were incubated for 24 h with 250 μg/ml of sub-fraction AP-AU1 (open bars) or 500 ng/ml LPS (positive control, shaded bars), and production of cytokines in the supernatants was evaluated using a Multi Analyte ELISArray kit. Cytokine expression is shown as fold increase compared to background measured in supernatants from PBS-treated cells. The data are presented as mean ± SD of duplicate samples from one experiment that is representative of two independent experiments.
2.5. Effect of Alchornea sub-fraction AP-AU1 on MAPK phosphorylation and NF-κB activity
Stimulation of cytokine production in monocyte/macrophages depends on multiple signaling pathways, including MAPKs (Guha and Mackman, 2001). To evaluate effects of sub-fraction AP-AU1 on the activation status of all three major MAPKs, extra cellular-signal regulated kinases (ERK1/ERK2), c-Jun N-terminal kinases (JNK 1–3), and different p38 MAPK isoforms (α, β, δ, and γ), as well as other intracellular kinases, such as MSK2, mTOR, CREB, HSP27, p53, Akt, glycogen synthase kinase (GSK-3), p90 ribosomal S6 kinase (RSK) 1/2, MAP kinase kinases (MKK2, MKK3, and MKK6), and p70 S6 kinase 1 (p70S6K1) in hPBMCs, we utilized a human phospho-MAPK array (Proteome Profiler; R&D Systems), which simultaneously evaluates phosphorylation (i.e., the activation state) of these intracellular kinases. Out of the signaling molecules screened, treatment with AP-AU1 was found to induce phosphorylation specifically of Akt2 (Ser474; FI = 1.5), p38δ (Thr180/Tyr182; FI = 1.4) p38γ (Thr183/Tyr185; FI = 1.9), p70S6K1 (Thr421/Ser424; FI = 1.4), RSK2 (Ser386; FI = 2.5), and mTOR (Ser2448; FI = 1.7) in human PBMCs (Figure 8).
Figure 8.
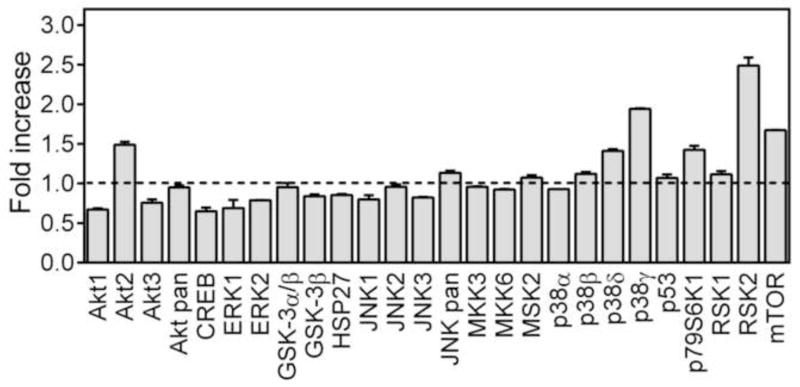
Effect of Alchornea polysaccharide sub-fraction AP-AU1 on MAP kinase phosphorylation. PBMCs were incubated for 30 min with 250 μg/ml of fraction AP-AU1, and levels of kinase phosphorylation were evaluated using a phospho-MAPK array kit. The data are presented as mean ± SD of duplicate samples from one experiment.
To evaluate activation of NF-κB by Alchornea sub-fraction AP-AU1, we utilized a transcription factor-based bioassay in human THP-1 Blue cells. Sub-fraction AP-AU1 dose-dependently stimulated NF-κB transcription activity (Figure 9). At high concentrations, this activity was similar to that induced by LPS (500 ng/ml). In contrast and consistent with our cytokine data, the low-molecular weight sub-fraction AP-AU2 did not induce NF-κB activity (Figure 9).
Figure 9.
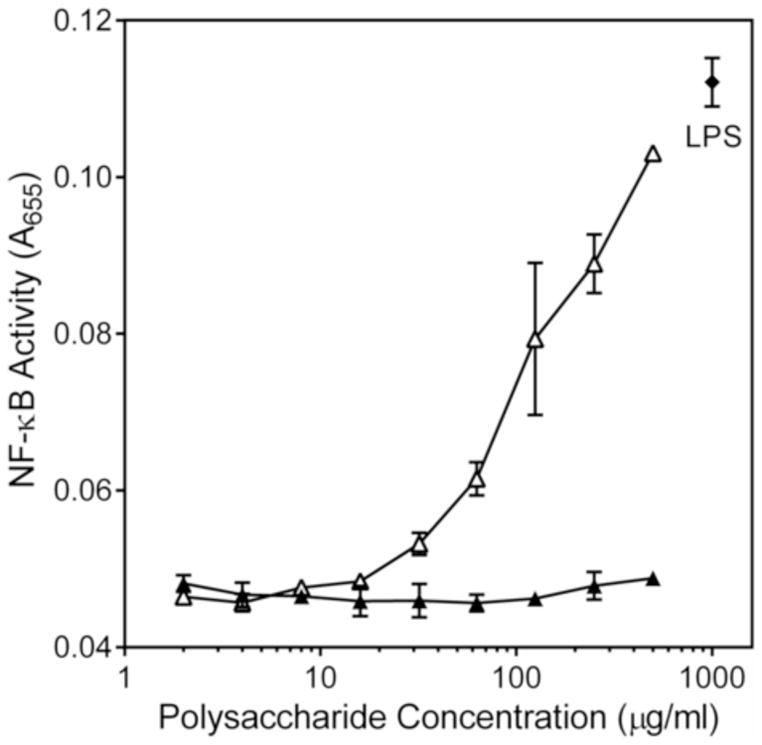
Effect of Alchornea polysaccharide sub-fraction AP-AU1 on NF-κB activation. Human THP-1 monocytes were incubated for 24 h with the indicated concentrations of polysaccharide AP-AU1 (△), AP-AU2 (▲), or 500 ng/ml LPS (positive control, ◆). Alkaline phosphatase activity was analyzed spectrophotometrically (absorbance at 655 nm) in the cell supernatants, as described. Values are the mean ± S.D. of triplicate samples from one experiment, which is representative of two independent experiments.
2.6. Effect of Alchornea polysaccharide fractions on cell viability
Although our functional assays suggested that the polysaccharides extracted from A. cordifolia were relatively non-toxic, we evaluated the potential cytotoxic effects of these polysaccharides to determine if the results might be influenced by background toxicity. We found that none of the polysaccharides significantly affected viability of THP-1 Blue cells in the concentration range 10–250 μg/ml of polysaccharides verifying that these polysaccharides were not cytotoxic (data not shown).
3. Discussion
Historically, A. cordifolia has been used in traditional African medicine for treating a number of diseases and conditions. However, despite the widespread traditional use of A. cordifolia in tropical countries, little is known regarding the active components responsible for its therapeutic properties. Previous studies indicated that some of the biological properties of A. cordifolia extracts, including hepatoprotection, antibacterial, antifungal, and anti-inflammatory activities were due to the presence of low-molecular weight compounds (Ogungbamila and Samuelsson, 1990; Lamikanra et al., 1990; Ebi, 2001; Banzouzi et al., 2002; Osadebe and Okoye, 2003; Manga et al., 2004; Mavar-Manga et al., 2008; Osadebe et al., 2012). Recently, it was reported that a flavonoid-rich fraction of A. cordifolia extract had immunostimulatory and adjuvant properties (Nworu et al., 2010a; Nworu et al., 2010b); however, immunomodulatory activity of Alchornea polysaccharides has not been studied. In the present work, we provide evidence suggesting that polysaccharides of A. cordifolia have potent immunomodulatory properties that can enhance monocyte/macrophage function and propose that these properties may contribute, at least in part, to the therapeutic potential of A. cordifolia-derived medicines.
Our ethno botanical survey supported that decoction of A. cordifolia leaves in water is the mode of extraction most commonly used to prepare remedies in African folk medicine. Based on this observation, we isolated five polysaccharide fractions from the hot-water extract of A. cordifolia leaves and provide initial structural and pharmacological characterization. Average molecular weights of the fractions were in range from 4.9 to 39.5 kDa. We found that the high-molecular weight sub-fraction AP-AU1 (Mr 39.5 kDa) was highly active in biological tests with phagocytes, whereas the low-molecular weight sub-fraction AP-AU2 was inactive or minimally active in the same cells. This may be a common feature of plant polysaccharides that modulate macrophage functions, as we found previously in a number of studies that biological activity correlated with increased molecular weight of plant polysaccharides (Schepetkin et al., 2005; Xie et al., 2007; Xie et al., 2008; Schepetkin et al., 2008).
Active and inactive polysaccharide fractions also contained polyphenols over a broad concentration range. Polyphenols have been previously identified in polysaccharide fractions of various plants [e.g., (Smiderle et al., 2011; Holderness et al., 2011)] and it has been reported that polysaccharides are able to bind to polyphenols by intermolecular interactions (Renard et al., 2001), changing the molecular conformation of carbohydrates. Therefore, the presence of polyphenols in polysaccharide fractions should be considered. However, inmost of our biological studies, activities of the polysaccharide fractions was not associated with polyphenol content, as polyphenol-enriched fraction AP-AB was inactive and the highly-active sub-fraction AP-AU1 had the lowest polyphenol content. Note, however, this paradigm was not universal for all species and/or cell types, as fraction AP-AB was more active than sub-fraction AP-AU1 for stimulating NO production by murine J774.A1 macrophages. Thus, further studies are needed to clarify this issue.
We found that both Diaion-bound fractions (AP-AB and AP-NB) were inactive or had very low activity in biological assays. 1H-NMR spectroscopy data showed that Diaion-bound fractions were enriched with CH3 and acetyl groups (O-Ac or N-Ac). These signals were also previously noticed by us in relatively low-molecular weight polysaccharides isolated from Tanacetum vulgare (Xie et al., 2007) and Artemisia tripartite (Xie et al., 2008). Consistent with the current results, these fractions from T. vulgare were also non-active or had very low activity in most of biological studies (Xie et al., 2007). In any case, it is likely that a Maillard reaction between carbonyl groups of sugar residues and the nucleophilic amino group of amino acids could occur in A. cordifolia leaves under physiological and tropical conditions (Njoroge and Monnier, 1989; Jokic et al., 2004).
Analysis of Alchornea polysaccharide fractions using the Yariv reagent demonstrated that two fractions (AP-AU1 and AP-NU) contained detectable levels of type II arabinogalactan. In addition, the most potent immunomodulatory properties were associated with these fractions, with exception of NO production by murine J774.A1 macrophages, suggesting that type II arabinogalactans may be one of the main structures responsible for macrophage-activating properties of A. cordifolia-derived polysaccharides, although presence of type I arabinogalactanin the fractions could not be excluded. Thus, Diaion HP-20 resin could be used to enrich bioactive polysaccharides, removing inactive material that binds to the resin. Note, we also evaluated if the inactive Diaion-bound material might possibly have inhibitory activity by assessing its ability to inhibit LPS-induced cytokine production but found that this material was inactive, having no effect on LPS induced cytokine production (data not shown). Future chemical and physical analysis of these fractions will be necessary to characterize Diaion-bound and unbound polysaccharide fractions and determinae the nature of the polysaccharide sub molecules responsible for immunomodulatory activities of these structures.
The most active polysaccharide sub-fraction AP-AU1 contained potent immunomodulatory activity, as demonstrated by induction of monocyte/macrophage NO and cytokine production. These cytokines included inflammatory cytokines IL-1β, IL-6, TNF-α, and GM-CSF, and the anti-inflammatory cytokine IL-10. Alchornea-derived polysaccharides seem to be unique compared to LPS in their ability to enhance GM-CSF production. Indeed, even high concentrations of LPS (up to 10 μg/ml) were unable to stimulate GM-CSF production in human PBMCs (data not shown). However, AP-AU at even lower concentrations (~30 μg/ml) significantly activated production of this cytokine in MonoMac-6 cells and PBMCs.
Differences in intracellular signaling were observed between Alchornea polysaccharides and previously reported botanical polysaccharides. For example, polysaccharides from algae Capsosiphon fulvescens induced ERK1/2, but not p38 (Go et al., 2011), whereas polysaccharide AP-1 from Platycodon grandiflorum activated phosphorylation of all three MAPKs (Yoon et al., 2004). Here, we demonstrated that Alchornea polysaccharide sub-fraction AP-AU1 induced phosphorylation of the Akt2, p38δ/p38γ, p70S6K1, RSK2, and mTOR, but not JNK1/2/3 and ERK1/2 in human peripheral mononuclear cells. Thus, it is clear that various polysaccharides can exert their biological activities through different signaling pathways, and a better understanding of these signaling events will be important as we expand our knowledge of such complementary and alternative medicines.
In summary, the present study demonstrates polysaccharides extracted from leaves of A. cordifolia are not cytotoxic and activate human and murine monocyte/macrophages, resulting in modulation of NO and cytokine production. Our data provide a molecular basis to explain at least part of the beneficial therapeutic effects for hot-water extracts of A. cordifolia and suggest that macrophage stimulation by Alchornea polysaccharides might enhance resistance to infection. Future studies are now in progress to determine complete carbohydrate structure of these polysaccharides and evaluate which receptor(s) are essential to the expression of the various immunomodulatory effects observed for these macromolecules.
Acknowledgments
We would like to thank Professor Andrei Khlebnikov, Altai State Technical University, Barnaul, Russia for help in interpretation of NMR spectra and Drs. Christopher West and Christa L. Feasley, Oklahoma Center for Medical Glycobiology Core Laboratory, Oklahoma City, OK for expert monosaccharide analysis. This work was supported in part by National Institutes of Health grants GM103500 and AT04986, United States Department of State Fulbright Visiting Scholar Program (Grant ID 68110029), an equipment grant from the M. J. Murdock Charitable Trust, and the Montana State University Agricultural Experimental Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeshina GO, Jegede IA, Kunle OF, Odama LE, Ehinmidu JO, Onaolapo JA. Pharmacognostic studies of the leaf of Alchornea cordifolia (Euphobiaceae) found in Abuja Nigeria. Journal of Pharmaceutical Science. 2008;7:29–35. [Google Scholar]
- Adeshina GO, Onaolapo JA, Ehinmidu JO, Odama LE. Phytochemical and antimicrobial studies of the ethyl acetate extract of Alchornea cordifolia leaf found in Abuja, Nigeria. Journal of Medicinal Plants Research. 2010;4:649–658. [Google Scholar]
- Adeshina GO, Onaolapo JA, Ehinmidu JO, Odama LE, Kunle OF. Phytochemical and toxicologic activity of the leaf extracts of Alchornea cordifolia (Schum and Thonn) Muell. Arg. (Euphorbiaceae) Nigerian Journal of Pharmacological Research. 2007;6:19–24. [Google Scholar]
- Agbor GA, Leopold T, Jeanne NY. The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytotherapy Research. 2004;18:873–876. doi: 10.1002/ptr.1446. [DOI] [PubMed] [Google Scholar]
- Ajali U. Antibacterial activity of Alchornea cordifolia stem bark. Fitoterapia. 2000;71:436–438. doi: 10.1016/s0367-326x(00)00131-3. [DOI] [PubMed] [Google Scholar]
- Ayisi NK, Appiah-Opong R, Gyan B, Bugyei K, Ekuban F. Plasmodium falciparum: Assessment of selectivity of action of chloroquine, Alchornea cordifolia, Ficus polita, and other drugs by a tetrazolium-based colorimetric assay. Malaria Research Treatment. 2011;2011:816250. doi: 10.4061/2011/816250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzouzi JT, Prado R, Menan H, Valentin A, Roumestan C, Mallie M, Pelissier Y, Blache Y. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. Journal of Ethnopharmacology. 2002;81:399–401. doi: 10.1016/s0378-8741(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Brito LA, Singh M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. Journal of Pharmaceutical Science. 2011;100:34–37. doi: 10.1002/jps.22267. [DOI] [PubMed] [Google Scholar]
- Catchpole B, Hamblin AS, Staines NA. Autologous mixed lymphocyte responses in experimentally-induced arthritis of the Lewis rat. Autoimmunity. 2002;35:111–117. doi: 10.1080/08916930290016619. [DOI] [PubMed] [Google Scholar]
- Dalziel JM. Useful Plants of West Africa. Crown Agents for Oversea Government and Administration; Millbank, London: 1956. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Ebi GC. Antimicrobial activities of Alchornea cordifolia. Fitoterapia. 2001;72:69–72. doi: 10.1016/s0367-326x(00)00254-9. [DOI] [PubMed] [Google Scholar]
- Gao T, Ma S, Song J, Bi H, Tao Y. Antioxidant and immunological activities of water-soluble polysaccharides from Aconitum kusnezoffii Reichb. International Journal of Biological Macromolecules. 2011;49:580–586. doi: 10.1016/j.ijbiomac.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Go H, Hwang HJ, Nam TJ. Polysaccharides from Capsosiphon fulvescens stimulate growth of IEC-6 cells by activating the MAPK signaling pathway. Marine Biotechnology. 2011;13:433–440. doi: 10.1007/s10126-010-9314-y. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cellular Signalling. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Holderness J, Schepetkin IA, Freedman B, Kirpotina LN, Quinn MT, Hedges JF, Jutila MA. Polysaccharides isolated from Acai fruit induce innate immune responses. PLoS One. 2011;6:e17301. doi: 10.1371/journal.pone.0017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MH, Zhu L, Jiang JG. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opinion on Therapeutic Targets. 2010;14:1367–1402. doi: 10.1517/14728222.2010.531010. [DOI] [PubMed] [Google Scholar]
- Jokic A, Wang MC, Liu C, Frenkel AI, Huang PM. Integration of the polyphenol and Maillard reactions into a unified abiotic pathway for humification in nature: the role of δ-MnO2. Organic Geochemistry. 2004;35:747–762. [Google Scholar]
- Kikkert R, de Groot ER, Aarden LA. Cytokine induction by pyrogens: comparison of whole blood, mononuclear cells, and TLR-transfectants. Journal of Immunological Methods. 2008;336:45–55. doi: 10.1016/j.jim.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Lamikanra A, Ogundaini AO, Ogungbamila FO. Antibacterial constituents of Alchornea cordifolia leaves. Phytotherapy Research. 1990;4:198–200. doi: 10.1002/(SICI)1099-1573(199902)13:1<67::AID-PTR366>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Le Grand A. Anti-infective phytotherapies of the tree-savannah, Senegal (occidental Africa). III: A review of phytochemical substances and the antimicrobial activity of 43 species. Journal of Ethnopharmacology. 1989;25:315–338. doi: 10.1016/0378-8741(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Leung MY, Liu C, Koon JC, Fung KP. Polysaccharide biological response modifiers. Immunology Letters. 2006;105:101–114. doi: 10.1016/j.imlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Manga HM, Brkic D, Marie DE, Quetin-Leclercq J. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Mull. Arg. (Euphorbiaceae) Journal of Ethnopharmacology. 2004;92:209–214. doi: 10.1016/j.jep.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mavar-Manga H, Haddad M, Pieters L, Baccelli C, Penge A, Quetin-Leclercq J. Anti-inflammatory compounds from leaves and root bark of Alchornea cordifolia (Schumach. & Thonn.) Mull. Arg. Journal of Ethnopharmacology. 2008;115:25–29. doi: 10.1016/j.jep.2007.08.043. [DOI] [PubMed] [Google Scholar]
- Mellinger CG, Carbonero ER, Noleto GR, Cipriani TR, Oliveira MB, Gorin PA, Iacomini M. Chemical and biological properties of an arabinogalactan from Phyllanthus niruri. Journal of Natural Products. 2005;68:1479–1483. doi: 10.1021/np050129s. [DOI] [PubMed] [Google Scholar]
- Mesia GK, Tona GL, Nanga TH, Cimanga RK, Apers S, Cos P, Maes L, Pieters L, Vlietinck AJ. Antiprotozoal and cytotoxic screening of 45 plant extracts from Democratic Republic of Congo. Journal of Ethnopharmacology. 2008;115:409–415. doi: 10.1016/j.jep.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Milo B, Risco E, Vila R, Iglesias J, Canigueral S. Characterization of a fucoarabinogalactan, the main polysaccharide from the gum exudate of Croton urucurana. Journal of Natural Products. 2002;65:1143–1146. doi: 10.1021/np010188f. [DOI] [PubMed] [Google Scholar]
- Njoroge FG, Monnier VM. The chemistry of the Maillard reaction under physiological conditions: a review. Progress in Clinical Biological Research. 1989;304:85–107. [PubMed] [Google Scholar]
- Nworu CS, Esimone CO, Tenbusch M, Nabi G, Proksch P, Uberla K, Temchura VV. Adjuvant properties of AcF1, an immunostimulant fraction of Alchornea cordifolia extract. Immunological Investigations. 2010a;39:132–158. doi: 10.3109/08820130903496793. [DOI] [PubMed] [Google Scholar]
- Nworu CS, Temchura V, Okoye FB, Akah PA, Esimone CO, Uberla K. Activation of murine lymphocytes and modulation of macrophage functions by fractions of Alchornea cordifolia (Euphorbiaceae) leaf extract. Immunopharmacology and Immunotoxicology. 2010b;32:28–36. doi: 10.3109/08923970903062587. [DOI] [PubMed] [Google Scholar]
- Ogungbamila FO, Samuelsson G. Smooth muscle relaxing flavonoids from Alchornea cordifolia. Acta Pharma Nordica. 1990;2:421–422. [PubMed] [Google Scholar]
- Okeke IN, Ogundaini AO, Ogungbamila FO, Lamikanra A. Antimicrobial spectrum of Alchornea cordifolia leaf extract. Phytotherapy Research. 1999;13:67–69. doi: 10.1002/(SICI)1099-1573(199902)13:1<67::AID-PTR366>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Okoye FB, Osadebe PO, Nworu CS, Okoye NN, Omeje EO, Esimone CO. Topical anti-inflammatory constituents of lipophilic leaf fractions of Alchornea floribunda and Alchornea cordifolia. Natural Product Research. 2011;25:1941–1949. doi: 10.1080/14786419.2010.512272. [DOI] [PubMed] [Google Scholar]
- Osadebe PO, Ebi GC, Okoye FB. Anti-inflammatory effects of triterpenoids from Alchornea cordifolia leaves. Recent Progress in Medicinal Plants. 2008;22:571–577. [Google Scholar]
- Osadebe PO, Okoye FB. Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. Journal of Ethnopharmacology. 2003;89:19–24. doi: 10.1016/s0378-8741(03)00195-8. [DOI] [PubMed] [Google Scholar]
- Osadebe PO, Okoye FB, Uzor PF, Nnamani NR, Adiele IE, Obiano NC. Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pacific Journal of Tropical Medicine. 2012;5:289–293. doi: 10.1016/S1995-7645(12)60041-8. [DOI] [PubMed] [Google Scholar]
- Pelley RP, Strickland FM. Plants, polysaccharides, and the treatment and prevention of neoplasia. Critical Review in Oncogenesis. 2000;11:189–225. [PubMed] [Google Scholar]
- Pesewu GA, Cutler RR, Humber DP. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. Journal of Ethnopharmacology. 2008;116:102–111. doi: 10.1016/j.jep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Polle AY, Ovodova RG, Chizhov AO, Shashkov AS, Ovodov YS. Structure of tanacetan, a pectic polysaccharide from tansy Tanacetum vulgare L. Biochemistry. 2002;67:1371–1376. doi: 10.1023/a:1021810010222. [DOI] [PubMed] [Google Scholar]
- Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutrition Journal. 2010;9:54. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Perera C, Hemar Y. Antitumor activity of mushroom polysaccharides: a review. Food & Function. 2012;3:1118–1130. doi: 10.1039/c2fo10279j. [DOI] [PubMed] [Google Scholar]
- Renard CM, Baron A, Guyot S, Drilleau JF. Interactions between apple cell walls and native apple polyphenols: quantification and some consequences. International Journal of Biological Macromolecules. 2001;29:115–125. doi: 10.1016/s0141-8130(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Schepetkin IA, Faulkner CL, Nelson-Overton LK, Wiley JA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. International Immunopharmacology. 2005;5:1783–1799. doi: 10.1016/j.intimp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Schepetkin IA, Quinn MT. Immunomodulatory effects of botanical polysaccharides. In: Popa V, editor. Polysaccharides in Medicinal and Pharmaceutical Applications. 35. Smithers Rapra; Shawbury, UK: 2011. pp. 988–994. [Google Scholar]
- Schepetkin IA, Xie G, Kirpotina LN, Klein RA, Jutila MA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. International Immunopharmacology. 2008;8:1455–1466. doi: 10.1016/j.intimp.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Arnhold J, Grunder W, Arnold K. The action of hypochlorous acid on polymeric components of cartilage. Biological Chemistry Hoppe-Seyler. 1994;375:167–172. doi: 10.1515/bchm3.1994.375.3.167. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Smiderle FR, Ruthes AC, van AJ, Chanput W, Iacomini M, Wichers HJ, Van Griensven LJ. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complementary and Alternative Medicine. 2011;11:58. doi: 10.1186/1472-6882-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona L, Kambu K, Ngimbi N, Mesia K, Penge O, Lusakibanza M, Cimanga K, De BT, Apers S, Totte J, Pieters L, Vlietinck AJ. Antiamoebic and spasmolytic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa, Congo. Phytomedicine. 2000;7:31–38. doi: 10.1016/S0944-7113(00)80019-7. [DOI] [PubMed] [Google Scholar]
- Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. International Immunopharmacology. 2007;7:1639–1650. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Schepetkin IA, Siemsen DW, Kirpotina LN, Wiley JA, Quinn MT. Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochemistry. 2008;69:1359–1371. doi: 10.1016/j.phytochem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YD, Kang JS, Han SB, Park SK, Lee HS, Kang JS, Kim HM. Activation of mitogen-activated protein kinases and AP-1 by polysaccharide isolated from the radix of Platycodon grandiflorum in RAW 264.7 cells. International Immunopharmacology. 2004;4:1477–1487. doi: 10.1016/j.intimp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Yu F, Lu S, Yu F, Feng S, McGuire PM, Li R, Wang R. Protective effects of polysaccharide from Euphorbia kansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Canadian Journal of Physiology and Pharmacology. 2006;84:1071–1079. doi: 10.1139/y06-052. [DOI] [PubMed] [Google Scholar]
- Zaidman BZ, Yassin M, Mahajna J, Wasser SP. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Applied Microbiology and Biotechnology. 2005;67:453–468. doi: 10.1007/s00253-004-1787-z. [DOI] [PubMed] [Google Scholar]