Abstract
IL-17C is a functionally distinct member of the IL-17 family that binds IL-17RE/A to promote innate defense in epithelial cells and regulate Th17 cell differentiation. We demonstrate that IL-17C (not IL-17A) is the most abundant IL-17 isoform in lesional psoriasis skin (1058pg/ml vs. 8pg/ml; p<0.006) and localizes to keratinocytes (KCs), endothelial cells (ECs) and leukocytes. ECs stimulated with IL-17C produce increased TNFα and KCs stimulated with IL-17C/TNFα produce similar inflammatory gene response patterns as those elicited by IL-17A/TNFα, including increases in IL-17C, TNFα, IL-8, IL-1α/β, IL-1F5, IL-1F9, IL-6, IL-19, CCL20, S100A7/A8/A9, DEFB4, LCN2 and PI3 (p<0.05); indicating a positive pro-inflammatory feedback loop between the epidermis and ECs. Psoriasis patients treated with etanercept rapidly decrease cutaneous IL-17C levels, suggesting IL-17C/TNFα-mediated inflammatory signaling is critical for psoriasis pathogenesis. Mice genetically engineered to overexpress IL-17C in KCs develop well-demarcated areas of erythematous, flakey “involved” skin adjacent to areas of normal appearing “uninvolved” skin despite increased IL-17C expression in both areas (p<0.05). Uninvolved skin displays increased angiogenesis and elevated S100A8/A9expression (p<0.05) but no epidermal hyperplasia; whereas involved skin exhibits robust epidermal hyperplasia, increased angiogenesis and leukocyte infiltration and upregulated TNFα, IL-1α/β, IL-17A/F, IL-23p19, VEGF, IL-6 and CCL20 (p<0.05) suggesting that IL-17C, when coupled with other pro-inflammatory signals, initiates the development of psoriasiform dermatitis. This skin phenotype was significantly improved following 8 weeks of TNFα inhibition. These findings identify a role for IL-17C in skin inflammation and suggest a pathogenic function for the elevated IL-17C observed in lesional psoriasis skin.
Keywords: Psoriasis, Inflammation, cytokines, transgenic mouse
Introduction
Considerable data has been developed in the past two decades regarding IL-17 cytokine family members and the initiation of inflammation by IL-17, specifically IL-17A and IL-17F, cytokines produced by Th17 cells, mast cells, neutrophils and γδT cells(1). IL-17C is a newly cloned member of the IL-17 family, sharing 23% homology with IL-17A, but maps to a different chromosome location(2). Unlike IL-17A, little is currently known about the biological role or importance of IL-17C in promoting inflammation, although several recent reports identified key roles for IL-17C and its receptors IL-17 receptor E (RE)/RA in regulating innate immune function in epithelial cells(3–7), promotion of mucosal barrier maintenance(8), promoting Th17 cell responses (9), and potentially, in the skin disease psoriasis (5, 10).
Johansen et al. (10)initially reported increases in IL-17C mRNA and protein in lesional compared to nonlesionalhuman psoriasis skin. IL-17C was rapidly decreased following TNFα inhibition(11), possibly as a result of NFκB signaling downstream of TNFα(4). More recent reports have demonstrated an amelioration of imiquimod-induced psoriasiform skin inflammation in IL-17C- and IL-17RE/RA- deficient mice(5, 12), suggesting that IL-17C contributes to the initiationand/or maintenance ofpsoriatic skin.Others have also identified a>50-fold increase in keratinocyte (KC)-derived IL-17C following combined IL-17A/TNFα stimulation(13) along with increases in ~160 other genes that respond synergistically and ~196 genes that respond additively that are also found in the human psoriasis transcriptome. These findings suggest that psoriasis therapies targeting IL-17A, in addition to proven TNFα-targeted biologics, should lead to a significant downregulationin psoriasis-related genes. Indeed, recent clinical reports demonstrate IL-17A-targeted therapeutics are efficacious in psoriasis patients(using ixekizumab; formerly, LY2439821; 75mg) leading to significant clinical improvement, with 83% of patients achieving a 75% reduction in disease severity (PASI75) and 38% of patients achieving complete clearance (14) by week 12 of the study. Interestingly, targeting IL-17RA with the antibody brodalumab (formerly, AMG827; 210mg)resulted in 82% of patients achieving PASI75 and 62% of patients achieving complete clearance (15). The observation that IL-17C and IL-17A share IL-17RA as a co-receptor(5, 6, 9) may provide rationale for why patient responses to brodalumab are closer to optimal and provide contextual relevance for targeting IL-17C-signaling in psoriasis.
Given the shared receptors for IL-17 family proteins, it is likely that IL-17 family members collaborate to enhance inflammation by affecting distinct cellular targets. To further examine the importance of IL-17C in psoriasis pathogenesis, we compared gene and protein expression levels of IL-17 family members in psoriasis and control patient skin, examined primary human KCs stimulated with IL-17C/TNFα for changes in key psoriasis transcriptome genes and engineered mice to overexpress KC-specific IL-17C (K5-IL-17C), modeling the epidermal-specific overexpression of IL-17C observed in human lesional psoriasis skin.
Materials and Methods
Study population
Eight individuals with chronic plaque psoriasis and 8 normal controls were enrolled (age range 18–75 years). Inclusion criteria included one or more well-demarcated, scaly, erythematous psoriatic plaques that were not limited to the scalp and no systemic anti-psoriatic treatments for 2 weeks before biopsy. Biopsy sites varied between patients depending on active plaques, whereas biopsies of uninvolved and control skin were from the buttocks.
For the etanercept study, 18 individuals with chronic plaque psoriasis were enrolled (age range 18–75 years). Entry criteria included age greater than 18 years and stable plaque-type psoriasis involving at least 10% body surface area. Exclusion criteria included use of systemic psoriasis therapy within 4 weeks, topical therapy within 2 weeks, or severe co-morbid diseases. For 12 weeks, subjects received etanercept (Enbrel®) 50mg twice a week subcutaneously (open-label). At baseline, 6 mm punch biopsies were obtained under local anesthesia (lidocaine) from uninvolved skin and a target plaque. Subsequent biopsies were taken on days 1, 3, 7 and 14 of therapy from the same target plaque.
Informed consent was obtained from all subjects under protocols approved by the Institutional Review Board of the University of Michigan or University Hospitals Case Medical Center/Case Western Reserve University. This study was conducted in compliance with good clinical practice and according to the Declaration of Helsinki Principles.
Primary human dermal microvascular endothelial cells
Endothelial cells (ECs) were purchased from Lonza (Hopkinton, MA) and grown according to the manufacturer’s instructions. Confluent cells were stimulated in triplicate with recombinant IL-17C (200ng/ml; R&D Systems, Minneapolis, MN) for 6hrs. Experiments were reproduced independently at least three times.
Primary human keratinocytes
Normal human KC cultures were established from adult human skin and grown for stimulation experiments as previously described (16–18). Recombinant human TNFα (2ng/ml) and IL-17C (200 ng/ml) were obtained from R&D Systems. Experiments were reproduced independently using KCs grown from at least three different patients.
Transgenic mice
To overexpress murine IL-17C, we PCR cloned HindIII and XhoI sites onto IL-17C using pCR4-IL17C as the template (Source BioScience, Nottingham, UK), and then subcloned the HindIII and XhoIenzyme digested product into pSecTag2B (Life Technologies, Grand Island, NY) to introduce a secretory signal and a MycHis epitope. The sequence of this construct was confirmed and a Sal1 digested fragment was then inserted into the pTetos vector using methods previously published (19, 20). Expression was validated in vitro in 293T cells co-transfected with Tetos-IL-17C and CMV-tTA plasmid DNA using electrophoresis and Western blotting on proteins isolated from cells and conditioned media. IL-17C and Myc/His protein expression were confirmed in both cells and supernatants. The backbone of the plasmid was removed using PvuI, and the DNA insert was prepared using standard techniques. The oocyte injection of the construct was completed as described previously (21). One set of injections was completed, with five founder mice being born. These mice were mated to a keratin 5 (K5tTA) keratinocyte-specific driver line (22) (generously provided by Dr. Adam Glick; Pennsylvania State University, PA) which we backcrossed to the C57Bl/6 background strain for >10 generations prior to use in these experiments. Offspring were genotyped by polymerase chain reaction (PCR) using DNA extracted from ear biopsies and tTA transgene primers as previously described (23). Primers for PCR genotyping of the pTETosfwd 5'-ACC ATG TTC ATG CCT TCT T-3’ and IL17c rev 5'-ACT TCG AGT TAG CAG GTG T-3’. Control littermates were mice that had inherited one or no transgenes. All cloning protocols and animal protocols were approved by the Institutional Biosafety Committee and the Case Western Reserve University Institutional Animal Care and Use Committee and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Systemic TNFα inhibition
TNFα inhibition was completed using a rat/mouse chimeric monoclonal IgG2a,κ antibody specific for murine TNFα (CNTO5048) generously provided by Dr. David Shealy (Johnson & Johnson's Janssen Research and Development). Adult mice with established skin disease were injected with either anti-TNFα (n=4) or negative control murine IgG2a,k monoclonal antibodies (n=4) at 5mg/kg IP, once a week for an eight week period.
RNA and real-time quantitative RT-PCR
After removal from the skin, human skin biopsies were snap-frozen in liquid nitrogen and stored at −80°C until use. RNA was isolated from human and mouse skin, primary human ECs and KCs and reverse transcribed as previously described (17, 18)and transcripts amplified and quantitated using a Prism 7700 Sequence Detector (Applied Biosystems; Carlsbad, California). TaqMan primer sets and probes were purchased from Applied Biosystems by Life Technologies. All values were normalized to the expression of the housekeeping gene RPLP0 (KCs) or 18S (ECs). For mouse experiments, mouse skin mRNA was extracted, reverse transcribed, and transcripts quantified as previously described (23). All values were normalized to GAPDH.
ELISA
Frozen human skin biopsies had protein isolated as previously described (17). Human IL-17A and IL-17C protein expression was quantitated in patient samples using enzyme linked immunosorbant assay (ELISA) approaches according to manufacturer’s instructions (R&D Systems). Frozen mouse skin from control mice (n=4), and uninvolved and involved skin of K5-IL-17C mice (n=4-7) underwent protein isolation as previously described (23) and murine VEGF-A, IL-17A, IL-6 and TNFα protein expression was quantitated using ELISA following the manufacturer’s instructions (R&D Systems).
Western blotting and Protein expression array
For western blotting analyses, equal amounts of protein/sample from control (n=3) and K5-IL-17C uninvolved and involved skin (n=3) or from human dermal microvascular ECs stimulated with/without IL-17C (200ng/ml) was separated on an SDS-Page polyacrylamide gel and transferred onto PVDF membrane (Millipore, Temecula, CA). Using methods published previously by our group (23), membranes were probed using antibodies targeting murine S100A8/A9, IL-17A, IL-17C, IL-12/23p40 (R&D Systems) and β-actin (Sigma Aldrich, St. Louis, MO)or against human TNFα (R&D Systems) and β-actin (Sigma Aldrich, St. Louis, MO). Differences in pro-inflammatory cytokine expression were also examined using a commercially available protein array (R&D Systems; ARY006; n=2 each controls and K5-IL-17C involved skin), following the manufacturer’s instructions.
Immunohistochemistry
Human skin biopsies were formalin fixed and paraffin embedded, sectioned at 5µm and then immunohistochemically stained using standard approaches (23) with goat anti-human IL-17Cor matched goat IgG (1µg/ml; AF1234; R&D Systems) or anti-IL17A polyclonal goat IgGor matched goat IgG (2µg/ml; AF317; R&D Systems). Biopsies from human sigmoid colon diagnosed with Crohn’s disease were used as a positive control for IL-17A staining.
Adult mice were euthanized, their hair shaved, and skin from the back and ear was processed as previously described in detail (23). H&E staining and immunohistochemistry using antibodies specific for CD4, CD8, CD11c (BD Biosciences, San Jose, CA), F4/80 (eBioscience, San Diego, CA), mouse endothelial cell antigen-32 (MECA-32, Developmental Studies Hybridoma Bank, Iowa City, Iowa), Ki67 (Dako, Carpinteria, CA), Loricrin (Covance, Princeton, NJ), and VEGF-A (A20, Santa Cruz)were also completed as described previously (23).
Epidermal thickness measures and immune cell quantitation were completed as previously described (23).
Statistics
All data are presented as mean ± SEM. Data were tested for normality and statistical significance calculated using either a Student t test, Mann–Whitney U test or Friedman’s test, as appropriate. Significance was defined as P≤0.05.
Results
IL-17C is the most abundant IL-17 cytokine in inflamed skin
Expression of IL-17 family genes were compared in lesional (PP) and uninvolved (PN) skin of psoriasis patients as well as skin from healthy controls (NN) using qRT-PCR (Figure 1A). Consistent with previous reports (10) we identified significant increases in IL-17A (p<0.02), IL-17C (P<0.01) and IL-17F (P<0.002) gene expression in PP compared to NN and PN skin (n=8 each), and significant decreases in IL-17B (P=0.05) and IL-17D (P=0.002) in PP compared to NN skin. IL-17RA/B/E expression was not significantly different between NN, PN or PP skin, whereas IL-17RC (P=0.02) and IL-17RD (P=0.01) both decreased in PP compared to control skin.
Figure 1. IL-17C RNA and protein are increased in lesional psoriasis skin.
Real-time quantitative PCR analyses of IL-17 ligand and receptor family members in control patient (NN) and psoriasis patient uninvolved (PN) and involved lesional (PP) skin (A). ELISA analyses of IL-17A and IL-17C demonstrates significant increases in both cytokines, but IL-17C protein expression is ~125-fold higher than IL-17A in lesional skin (B). IL-17C and IL-17A immunohistochemistry in control and psoriasis skin shows IL-17C protein localized to epidermal, vasculature and dermal areas and IL-17A localized to very few cells in the dermal papillae(C). Cutaneous IL-17C mRNA significantly decreases in responding patients 72 hours following etanercept treatment (D). * P<0.05 compared to NN in (A) or Day 0 of treatment in (D); ** P<0.05 compared to PN in (A) or as indicated in (B).
To explore the significance of these changes in gene expression, we measured IL-17A and IL-17C protein by ELISA (Figure 1B) and determined both to be significantly increased in PP compared with PN and NN skin (P values as indicated). Interestingly, IL-17C protein expression was ~4-fold greater in PP skin (P=0.002) and ~125-fold greater than IL-17A protein (P=0.006). These results demonstrate similar mRNA transcript abundance of IL-17A and IL-17C but also illustrate that IL-17C protein is the most highly expressed IL-17 family member found in human involved psoriasis skin (Figure 1B, 1058 ± 133 pg/mg IL-17C vs. 8 ± 2 pg/mg IL-17A protein). Immunohistochemical staining of healthy control and involved and uninvolved psoriasis skin confirmed the increases in IL-17C and IL-17A protein and demonstrated significantly more IL-17C protein present in psoriasis skin than IL-17A (Figure 1C). IL-17C co-localized with many cells, including KCs, dermal ECs and skin resident and infiltrating leukocytes; IL-17A-positive cells were only observed in the dermal papillae (Figure 1C). No IL-17A staining was observed in control patient skin.
Despite its gross over-expression in lesional psoriasis skin, we observed that IL-17C is one of the most rapidly down-regulated transcripts in the skin of psoriasis patients treated with the anti-TNFα agent etanercept. Seventy-two hours following etanercept treatment, and prior to any changes in clinical disease, cutaneous IL-17C mRNA expression was significantly suppressed (n=18; Figure 1D, P=0.019).
Endothelial cells and keratinocytes produce pro-inflammatory molecules in response to IL-17C and IL-17C and TNFα respectively
Others have previously demonstrated direct effects of IL-17C on fibroblasts, monocytic cells and peritoneal exudates (2, 24)and IL-17C has the capacity to elicit anti-microbial peptides from epithelial cells (5, 6); more recent studies have revealed IL-17C regulates Th17 cell differentiation and Th17-cell derived IL-17A and IL-17F (9). To further identify cell responsiveness to IL-17C, we stimulated human dermal microvascular ECs with IL-17C and observed statistically significant increases in TNFα and IL-6 mRNA (P<0.05; Figure 2A), with no significant effect on IL-1α/β, IL-8 or IL-17RE/A/C. The increase in TNFα was further confirmed at the protein level (Figure 2B), identifying a novel cellular target for IL-17C promotion of inflammation.
Figure 2. IL-17C and TNFα induce characteristic psoriasis-related transcriptome genes in both additive and synergistic manners.
Human dermal microvascular endothelial cells stimulated with IL-17C (200ng/ml) for 6 hours increase mRNA expression of TNFα and IL-6 (A). Representative western blots of cell lysates confirm the increase in TNFα protein (B). Real-time quantitative PCR analyses of key psoriasis-transcriptome genes in human primary epidermal keratinocytes stimulated with IL-17C (200ng/ml), TNFα (2ng/ml) or both as indicated for 24 hrs (C). In vitro experimental data are representative of at least three independent experiments. * P<0.05 compared to control.
Given that KCs are the major source of IL-17C in the skin, and that others have recently identified increases in S100A8/A9, RegIIIβ/γ, hBD2 and hG-CSF in epithelial cells following IL-17C exposure (5, 6), we sought to further investigate the role of IL-17C in primary human KCs. Primary human KCs failed to respond poorly to IL-17C alone (Figure 2C), however, the addition of sub-optimal TNFα (2ng/ml) lead to a significant induction of candidate genes previously identified to respond either synergistically or additively in response to IL-17A/TNFα(13) (Figure 2C) and known to contribute to psoriasis pathogenesis. Additive increases in KC-derived IL-17C, TNFα, IL-8, IL-1α/β, IL-1F5, IL-6, S100A8/A9 and lipocalin 2 (LCN2) were observed along with synergistic increases in KC-derived IL-1F9, IL-19, CCL20, S100A7, hBD2 (DEFB4) and peptidase inhibitor 3 (PI3) following stimulation with IL-17C/TNFα.
K5-IL-17C mice develop a psoriasiform skin phenotype
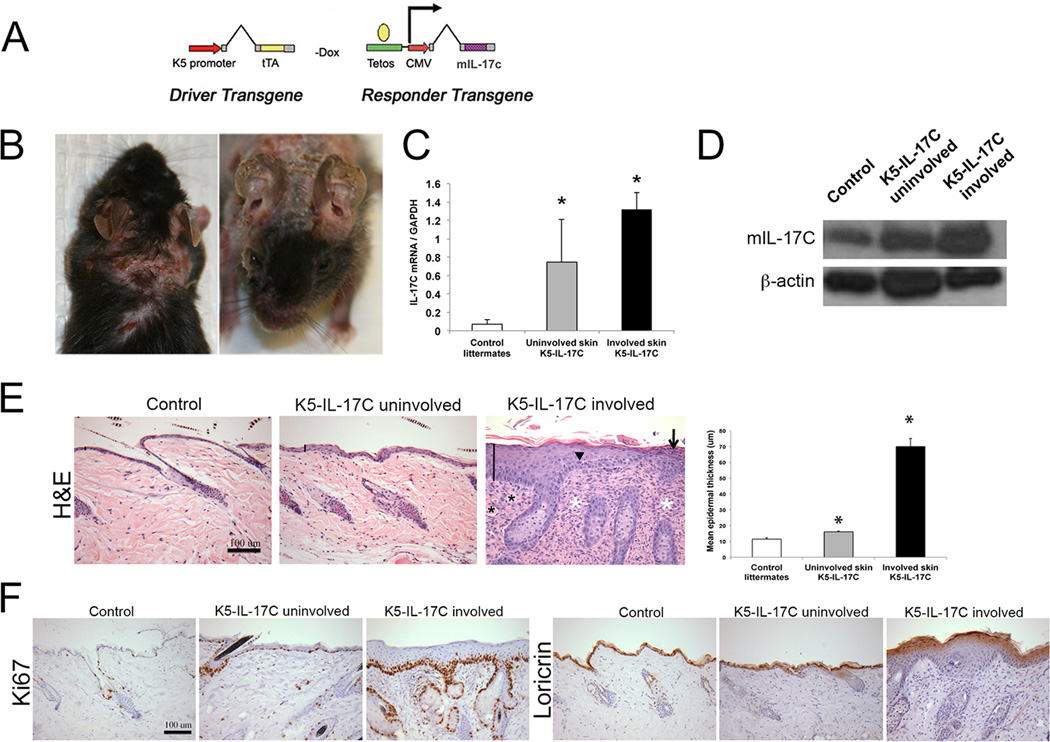
In psoriasis patients there is ~125-fold more IL-17C than IL-17A protein in lesional skin (Figure 1B) and IL-17C is localized principally to activated KCs (Figure 1C). To model this increase and to test the hypothesis that IL-17C plays a contributing and critical role in psoriasis pathogenesis, we genetically engineered mice to overexpress murine IL-17C in KCs using a conditional tetracycline repressible binary approach (Figure 3A) similar to models we have previously published (23). This controlled system allows us to modulate increases in IL-17C and recapitulate levels observed in lesional human psoriasis (Figure 1C). K5-IL-17C double transgenic mice appeared normal at birth, however as early as 8 weeks of age well demarcated areas of dorsal skin began to develop a thickened appearance with sloughing of epidermis and erythema, while adjacent areas of skin appeared relatively normal (Figure 3B). In severely affected K5-IL-17C animals, alopecia was observed. Almost all mice developed ear involvement by the time they were 12 weeks of age. Quantitative RT-PCR of uninvolved and involved skin from K5-IL-17C mice revealed ~11- and ~18-fold increases in IL-17C gene expression in K5-IL-17C uninvolved and involved skin, respectively compared to littermate controls (n=9-10 each group; P=0.04 and 0.009 Figure 3C). Western blotting confirmed the increases in cutaneous IL-17C protein in uninvolved and involved K5-IL-17C skin compared to control mice (Figure 3D).
Figure 3. K5-IL-17C transgenic mice develop a psoriasiform skin phenotype.
A tetracycline-repressible binary mouse molecular genetics approach similar to that previously described (23) was utilized to genetically overexpress IL-17C in a keratinocyte-specific manner using the K5 promoter (A). Mice spontaneously develop regions of affected (involved) and unaffected (uninvolved) skin with involved skin characterized by hyperkeratosis (B). Real-time quantitative PCR analyses of IL-17C gene expression of control mouse and uninvolved and involved skin of K5-IL-17C mice shows significant increases in IL-17C gene expression in K5-IL-17C mice (C). Representative western blot demonstrates increases in IL-17C protein in uninvolved and involved K5-IL-17C skin (D). Hematoxylin & eosin-stained dorsal skin of control mouse skin and K5-IL-17C mouse uninvolved and involved skin and epidermal thickness quantitation (E). Ki67 and loricrin immunostained dorsal skin demonstrates increases in cell proliferation and loss of terminal differentiation between control and K5-IL-17C mouse skin (F). Solid lines in D highlight the epidermis; arrow is pointing at a parakeratotic scale; arrowhead is pointing at a mitotic body; black asterisks are placed on dermal blood vessels; white asterisks are placed on foci of inflammatory infiltrate. * P<0.05 compared to control mouse. Scale bar is 100µm.
Histological examination of uninvolved K5-IL-17C dorsal skin compared to littermate control skin revealed modest increases in epidermal thickness (acanthosis) in uninvolved skin (11.5±0.9µm control skin vs. 16.1±0.6µm; P=0.006). In contrast, involved skin of K5-IL-17C animals exhibited robust epidermal hyperplasia (70.0±5.2µm; P<0.001 vs. controls and uninvolved K5-IL-17C skin), loss of the granular cell layer, thickening of the interfollicular epidermal layers, and confluent parakeratotic scale (Figure 3E). This increase in epidermal thickness occurred concurrent with increases in cell proliferation, indicated by Ki67 staining, and decreases in KC terminal differentiation, indicated by loricrin in uninvolved skin and involved K5-IL-17C skin compared to control mouse skin (Figure 3F). H&E stained skin sections also revealed an apparent increase in dermal blood vessels and a dense immune cell infiltrate (Figure 3E).
To further explore these changes, dermal angiogenesis was examined using mouse endothelial cell antigen (MECA) staining. Increases in the MECA-positive staining was observed between control and uninvolved K5-IL-17C skin which was further amplified in involved skin (Figure 4A). VEGF protein increased between control and uninvolved K5-IL-17C skin (79.9±6.5pg/ml control vs. 127.9±11.4pg/ml uninvolved K5-IL-17C; P=0.07) and involved K5-IL-17C skin (258.9±54.1pg/ml; P=0.02 vs. control) and was localized to KCs, infiltrating leukocytes, and cutaneous nerves (Figure 4B). Primary human KCs and dermal microvascular ECs both directly responded to stimulation with IL-17C by producing 52% (P=0.05) and 20% more (P=0.04) VEGF mRNA (Figure 4C), respectively. No differences in angiopoietin or Tie2 expression were observed.
Figure 4. K5-IL-17C mouse skin has increases in dermal angiogenesis and VEGF.
Mouse endothelial cell antigen (MECA)-immunostained dorsal skin of control mouse skin and K5-IL-17C mouse uninvolved and involved skin (A). VEGF ELISA and immunohistochemistry demonstrate increases in VEGF protein in uninvolved and involved K5-IL-17C skin compared to littermate control skin (B). qRT-PCR of primary human KCs and ECs stimulated with IL-17C (200ng/ml) demonstrates significant increases in VEGF mRNA. * P<0.05 compared to control mouse. ** P<0.05 compared to uninvolved K5-IL-17C skin (B). * P<0.05 compared to control stimulated (C). Scale bar as indicated.
Cell type specific examination and quantification of the inflammatory infiltrate in K5-IL-17C mouse skin revealed significant increases in CD4+ T cells, CD8+ T cells, CD11c+ myeloid dendritic cells and F4/80+ macrophages in K5-IL-17C involved skin compared to both control mouse skin and to K5-IL-17C uninvolved skin (Figure 5). Modest, but insignificant (p=0.09) increases in CD8+ T cells were seen in uninvolved K5-IL-17C skin compared to controls. Similar observations were seen in the affected ear skin of K5-IL-17C animals (Supplemental Figure 1).
Figure 5. Characterization of the immune cell infiltrate in K5-IL-17C mouse skin.
Representative photographs of immunohistochemistry and cellular quantitation of CD4-, CD8-, CD11c- and F4/80- positive cells in control mouse dorsal skin and K5-IL-17C uninvolved and involved dorsal skin. * P<0.05 compared to control mouse. ** P<0.05 compared to uninvolved K5-IL-17C skin. Scale bar is 100µm.
To explore the molecular signature of K5-IL-17C skin, we examined changes in candidate cytokines, chemokines, and innate defense molecules directly related to the histological and immunophenotypic changes observed in the skin (Figure 5) and that have been proposed to contribute to the pathogenesis of psoriasis (25, 26). Very few significant gene changes were observed between control littermate skin and uninvolved K5-IL-17C skin, but increases in transcript level were observed for S100A8 (P=0.04; n=4–6) and S100A9 (P=0.02) and IL-17F (P=0.04; Figure 6A), most likely derived from IL-17C direct effects on KCs(5) and T cells (9)(Figures 3 and 5).Comparison between involved K5-IL-17C skin with littermate control skin revealed significant increases in many of the hallmark psoriasis-transcriptome genes, including the pro-inflammatory cytokines and chemokines, TNFα, IL-1α, IL-1β, IL-6 and CCL20; Th1 and Th17 derived cytokines IFNγ and IL-17A, and the myeloid derived cytokines, IL-12 and IL-23with sustained expression of the innate defense markers DefB3 (the murine homologue of HBD2), S100A8 and S100A9 (Figure 6A; P<0.05). Genes that changed significantly between uninvolved and involved skin included IL-6 (P=0.05), IL-1β (P=0.004), IL-23p19, DefB3, S100A8, and S100A9 (all P=0.03).
Figure 6. Psoriasis-related innate defense and cytokines/chemokines are increased in K5-IL-17C skin.
Real-time quantitative PCR analyses of key innate defense molecules and psoriasis-related pro-inflammatory cytokines/chemokines demonstrates significant increases in S100A8/S100A9 and IL-17F in K5-IL-17C uninvolved mouse skin compared to control mouse skin and significant increases in these and other key pro-inflammatory molecules in involved skin compared to littermate control animals (A). Representative western blots confirming increases in pro-inflammatory cytokines and innate defense molecules (B). ELISA analyses of TNFα, IL-6 and IL-17A confirm the increases in gene expression observed between control and K5-IL-17C animals (C). Representative protein expression array confirms increases in IL-1α and IL-1β protein in involved K5-IL-17C and control mouse skin and identifies increases in other known psoriasis-related molecules. Asterisks represent reference spots (D). *P<0.05 compared to control littermates; ** P<0.05 compared to K5-IL-17C uninvolved skin or as indicated.
Gene expression changes between control mouse skin and involved K5-IL-17C skin were further validated at the protein level (Figure 6B–D). Increases in IL12/23p40, S100A8 and S100A9 proteins were observed (Figure 6B) as well as increases in TNFα, IL-6 and IL-17Aproteins (Figure 6C) between uninvolved K5-IL-17C and control mouse skin. Moreover, additional inflammatory molecules: C5/C5A, CD54, IL-16, CXCL1, CCL2, CCL3, CXCL2, TIMP-1 and TREM-1 also increased between involved K5-IL-17C skin and control mouse skin (Figure 6D).
TNFα inhibition improves the K5-IL-17C mouse skin phenotype
Psoriasis patients treated with the TNFα inhibitor etanercept show significant improvement in disease severity (27–29), and have rapid decreases in IL-17C (Figure 1D). To examine the effects of TNFα inhibition in K5-IL-17C mice, animals were systemically treated with either TNFα or negative control murine IgG2a,κ monoclonal antibodies for 8 weeks as described previously (30). Significant improvement in the gross appearance of individual animals treated with TNFα inhibitors was observed (Figure 7A), although some level of skin disease was still present. Significant decreases in epidermal thickness and infiltrating CD4+ and CD8+ T cell numbers were identified (Figure 7B), although these failed to return to control mouse levels. Decreases in neither CD11c+ nor F4/80+ cells were seen (data not shown). Examination of elevated pro-inflammatory cytokines in treated mice demonstrated sustained levels of IL-17C, as expected based on its genetic overexpression (Figure 7C), and robust decreases to control levels for IFNγ, IL-6 and IL-1β and more modest decreases in S100A8 and DefB3. CCL20, IL-17A and IL-1α levels remained elevated (Figure 7C).
Figure 7. Systemic inhibition of TNFα in K5-IL-17C mice leads to improvement in disease severity.
Photographs of the same severely affected K5-IL-17C mouse before and after 8 weeks of TNFα antibody treatment (A). Representative images of hematoxylin & eosin-stained and CD4- and CD8-immunostained dorsal skin of K5-IL-17C involved skin treated with either anti-TNFα antibodies or IgG and quantitation of epidermal thickness and CD4+ and CD8+ cell numbers. The hatched line represents control mouse levels. * P<0.05 compared to K5-IL-17C involved + IgG (B). Real-time quantitative PCR analyses of key innate defense molecules and psoriasis-related pro-inflammatory cytokines/chemokines demonstrates decreases in IFNγ, IL-6 and IL-1β in involved skin between K5-IL-17C+ IgG and K5-IL-17C+ TNFα treated mice. *P<0.05 compared to control mice (C).
Discussion
In this study we have identified IL-17C as a contributing pro-inflammatory cytokine critical for psoriasis pathogenesis. This conclusion is supported by the following data: 1.) IL-17C is the most abundantly expressed IL-17 cytokine at the protein level in lesional psoriasis skin and co-localizes with KCs, ECs and skin resident and infiltrating leukocytes; 2.) IL-17C elicits increases in TNFα from human dermal microvascular ECs and when combined with TNFα, produces additive and synergistic increases in key psoriasis-related pro-inflammatory cytokines, chemokines and innate defense molecules from human KCs; 3.) Mice engineered to overexpress IL-17C in KCs develop a psoriasis-like dermatitis containing clinical, histological, cellular and molecular changes that mimic human psoriasis, that are improved with TNFα inhibition; 4.) K5-IL-17C mice provide in vivoevidence demonstrating synergistic effects of IL-17C and TNFα/IL-17A in promoting psoriasiform skin disease.
The biological relevance of increased IL-17C in psoriasis is poorly understood and the role of IL-17C in psoriasis pathogenesis remains unclear. One potential mechanism by which IL-17C-mediated skin inflammation may occur is by activating host defense pathways in human epidermal KCs, as others have recently identified increases in expression of hBD2, S100A7/8/9, CXCL1/2/3, CCL20, TNFAIP6 and TNIP3 at levels similar to or greater than those elicited by IL-17A(5). These gene changes are reminiscent of those previously identified from human KCs following stimulation with IL-17A/TNFα, as part of the signature psoriasis transcriptome(13, 31). In our experiments, we failed to identify significant changes in these transcripts in primary human KCs following IL-17C stimulation alone (Figure 2C), perhaps reflective of differences in stimulation conditions (24 vs 48hrs). However, increases in the anti-microbial transcripts, S100A8 and S100A9 were observed in uninvolved skin of K5-IL-17C mice (Figure 6), prior to increases in other pro-inflammatory cytokines, and models transcriptional gene changes we have observed previously in uninvolved psoriasis patient skin(32). Moreover, IL-17C stimulation lead to increases in EC-derived pro-inflammatory cytokines, including TNFα (Figure 2A–B); and primary human KCs stimulated with both IL-17C and sub-threshold levels of TNFα produced significant increases in these, as well as other key innate defense, chemokine and pro-inflammatorycytokine transcripts (Figure 2C). These findings suggest a potential feedback loop occurs between KCs (producing IL-17C) and ECs (responding by producing TNFα), which may serve to promote self-sustaining inflammation. In vivo support for this feedback loop is evidenced by the increases in dermal angiogenesis in uninvolved skin of K5-IL-17C mice (Figure 4) that precedes increases in cutaneous TNFα expression and the development of involved skin lesions and the upregulation of the these same KC-derived and psoriasis-related transcripts (Figure 6). Our in vitro data suggest that IL-17C may indirectly promote dermal angiogenesis by eliciting VEGF from both ECs and KCs (Figure 4), which in turn promotes angiogenesis, and additional TNFα production.
The similarity between genes upregulated by IL-17A and IL-17C suggests they may be functionally redundant on epithelial cells; and although transcript levels of IL-17A and IL-17C suggest IL-17A may be expressed more robustly in lesional psoriasis tissue, our protein data demonstrate there is ~125 fold more IL-17C protein present than IL-17A and differences in signaling strength based solely on the local concentration of ligand, and the number of cells producing and responding to the ligand cannot be overlooked (Figure 1B–C). The discrepancy between cytokine mRNA and protein level correlation is consistent with a recent report (33) and may reflect differences in non-translational regulation, cytokine storage and cellular source.
Previous reports have identified the importance of synergistic and additive responses to IL-17A and TNFα as a hallmark of IL-17A biology and of psoriasis (13). Recently similar synergies have been identified between IL-17C/TNFα on hBD2 expression by primary human KCs (5) and IL-17C/IL-22 synergism drives S100A8/A9 production in colonic epithelial cells (6). This phenomenon is recapitulated here (Figure 2C) with 16 gene transcripts being either additively or synergistically induced by IL17C/TNFα.
Studies examining IL-17C-IL-17RE interactions have recently revealed that IL-17C regulates Th17cell differentiation and production of IL-17A and IL-17F (9) cytokines known to promote skin inflammation in the imiquimod model of psoriasiform dermatitis (5) and that are upregulated in psoriasis lesional skin (Figure 1A). These findings suggest synergy may occur not only between IL-17C and TNFα but also IL-17C and IL-17A; such that the pro-inflammatory feedback loop may include the epidermis, the vasculature and also TH17 cells. In K5-IL-17C skin, increases in IL-17A/F, TNFαand IL-6 were found in uninvolved skin compared to control mouse skin (Figure 6), perhaps reflective of IL-17C direct effects on ECs (Figure 2A–B) and T cells (9). Whether IL-17A/F and IL-6 also synergize with IL-17C, similar to that of TNFα has yet to be explored. Taken together, these data support the idea of a crucial role for IL-17/TNFα synergism (IL-17A and IL-17C) in the molecular fingerprint of psoriasis and demonstrate the capacity of IL-17C to augment an immune response concurrent with IL-17A/TNFα. This concept is supported by clinical observations that psoriasis patients treated with the anti-TNFα agent etanercept exhibit rapid decreases in cutaneous IL-17C expression (within 72 hours; Figure 1D), prior to skin improvement and prior to reported decreases in circulating levels of IL-17A and IL-22 (34). Others have reported similar outcomes in psoriasis patients treated with the TNFα inhibitor, adalimumab, where IL-17C gene expression decreased within 4 days of the initial treatment, whereas IL-17A/IL-17F and IL-22 failed to decrease significantly until 14 days post-treatment, and IL-23p19 and IFNγ did not drop significantly until 84 days (11). Moreover, recent clinical trials targeting the common IL-17A/C receptor IL-17RA (using Brodalumab; formally AMG841), also demonstrated rapid decreases in IL-17C mRNA (within two weeks) (35), that occurred prior to clinical disease improvement and preceded the gradual decreases of IL-17A and IL-22, which reached baseline nonlesional levels by 6 weeks(36).
K5-IL-17C mice treated with TNFα inhibitors also showed significant improvement in their disease severity (Figure 7), although disease was not reversed. This most likely reflects the sustained levels of IL-1α and IL-17A that may synergize with sustained level of IL-17C. TNFα is known to regulate IL-17C expression via NF-κB (p65/p50) (4); therefore it’s possible that efficacy of TNFα inhibition in psoriasis patients actually reflects decreases in IL-17C, thus TNFα inhibition may not be completely effective in K5-IL-17C mice because of the high levels of IL-17C, which are being genetically expressed and cannot be regulated by blockade of TNFα.
In conclusion, our results suggest that IL-17C is a highly sensitive member of the IL-17 family and that IL-17C may serve as a novel mechanism for amplifying Th17/Th22/TNFα-mediated inflammatory signaling critical for psoriasis pathogenesis. Support for the sensitivity of IL-17C in other models of inflammatory disease are provided by kinetic studies showing that IL-17C induction precedes that of IL-17A and other Th17/Th22cytokines (IL-22) in a murine colitis model (5). These data support the concept of a crucial role for both IL-17A and IL-17C synergizing with TNFα to generate the molecular fingerprint of psoriasis. Here, we propose that IL-17C serves as a critical cytokine mediating psoriasis, and that psoriasiform skin inflammation is likely to be driven by the amount of signal due to numerous KCs activated in psoriasis tissue rather than the comparatively modest numbers of IL-17A producing cells that likely initiate the primary response and then feed into the self-sustaining pro-inflammatory cascade of events. The increases in cytokines are likely reflective of the increased presence of skin infiltrating immune cells and proliferating KCs and signal a synergistic immune response between IL-17C with TNFα and IL-17A (Figure 2)(5). This synergistic increase may also reflect changes associated with IL-1β which we found elevated compared to control skin, supporting and elaborating recent work illustrating similar IL-17C-IL-1β and IL-17C-TNFα synergy(5).
Although the cause of psoriasis is unknown, the currently proposed hypothesis is that in patients with a susceptible genetic background, some stimulus, perhaps an infection, leads to a coordinated series of events involving cutaneous cells and cytokines that once started initiates a self-sustained vicious pro-inflammatory signal resulting in KC hyperproliferative cell cycle response. Recent reports have identified IL-17C as a key innate immune defense cytokine that is rapidly and robustly upregulated following infection or TLR stimulation (5, 6, 9). Our data support the supposition of a crucial role for IL-17/TNFα synergism (IL-17A and IL-17C) in the molecular fingerprint of psoriasis and when taken together provide evidence indicating that IL-17C synergizes with TNFα as well as IL-17A to initiate and sustain KC-activation and promote epidermal hyperplasia. Our results demonstrate a potential inflammatory feedback loop between the endothelium, the epidermis and Th17 cells that can be amplified by IL-17C. Once triggered, this loop is sufficient to promote chronic skin inflammation and acanthosis, two cutaneous characteristics of psoriasis. Because IL-17C appears to act upstream of many pro-inflammatory cytokines critical in psoriasis pathogenesis, including IL-1β, IL-17A/F, IL-22, IL-6, IL-8, VEGF and TNFα (our data and others, (2, 5, 6, 9, 24), targeting IL-17C as an early upstream regulator of these cytokines may provide a more encompassing therapeutic strategy for the effective treatment of psoriasis. Taken together, our findings support prior work (5) demonstrating a need for IL-17C signaling for imiquimod-elicited psoriasiform skin inflammation and demonstrate a pathogenic role for the elevated IL-17C observed in lesional psoriasis skin.
Supplementary Material
Acknowledgements
The authors recognize the excellent technical assistance of Marybeth Riblett, Candace Loyd, Alexander Foster and Xianying Xing. We thank Dr. David Shealy from Johnson & Johnson's Janssen Research and Development team for providing the murine monoclonal antibodies targeting TNFα and guidance on dosing and administration.
Grant numbers and sources of support:
The American Skin Association (NLW, AJ, JEG), National Psoriasis Foundation (AJ, JEG, NLW), Babcock Endowment Fund (AJ, JEG), National Institutes of Health (P30AR39750, P50AR05508; RO1AR063437 NLW, TSM and K08AR060802, JEG), Murdough Family Center for Psoriasis (NLW, TSM), A. Alfred Taubman Medical Research Institute Frances and Kenneth Eisenberg Emerging Scholar Award (JEG).
References
- 1.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordon Y. Cytokines, IL-17C joins the family firm. Nat Rev Immunol. 2011;11:805. doi: 10.1038/nri3118. [DOI] [PubMed] [Google Scholar]
- 4.Johansen C, Riis JL, Gedebjerg A, Kragballe K, Iversen L. Tumor necrosis factor alpha-mediated induction of interleukin 17C in human keratinocytes is controlled by nuclear factor kappaB. The Journal of biological chemistry. 2011;286:25487–25494. doi: 10.1074/jbc.M111.240671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de Voss J, Balazs M, Gonzalez L, Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 6.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 7.Im E, Jung J, Rhee SH. Toll-Like Receptor 5 Engagement Induces Interleukin-17C Expression in Intestinal Epithelial Cells. Journal of interferon & cytokine research, the official journal of the International Society for Interferon and Cytokine Research. 2012 doi: 10.1089/jir.2012.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds JM, Martinez GJ, Nallaparaju KC, Chang SH, Wang YH, Dong C. Cutting Edge, Regulation of Intestinal Inflammation and Barrier Function by IL-17C. J Immunol. 2012;189:4226–4230. doi: 10.4049/jimmunol.1103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35:611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. The British journal of dermatology. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 11.Johansen C, Vinter H, Soegaard, Madsen L, Olsen LR, Steiniche T, Iversen L, Kragballe K. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. British Journal of Dermatology. 2010;163:1194–1204. doi: 10.1111/j.1365-2133.2010.10036.x. [DOI] [PubMed] [Google Scholar]
- 12.El Malki K, Karbach SH, Huppert J, Zayoud M, Reissig S, Schuler R, Nikolaev A, Karram K, Munzel T, Kuhlmann CR, Luhmann HJ, von Stebut E, Wortge S, Kurschus FC, Waisman A. An Alternative Pathway of Imiquimod-Induced Psoriasis-Like Skin Inflammation in the Absence of Interleukin-17 Receptor A Signaling. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.318. [DOI] [PubMed] [Google Scholar]
- 13.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 15.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 16.Elder JT, Fisher GJ, Zhang QY, Eisen D, Krust A, Kastner P, Chambon P, Voorhees JJ. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991;96:425–433. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- 17.Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011;131:329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, Wohn C, Prens EP, Wang F, Maier LE, Kang S, Voorhees JJ, Elder JT, Gudjonsson JE. IL-1F5, -F6, -F8, -F9, a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward NL, Slyke PV, Dumont DJ. Functional inhibition of secreted angiopoietin, a novel role for angiopoietin 1 in coronary vessel patterning. Biochemical and biophysical research communications. 2004;323:937–946. doi: 10.1016/j.bbrc.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 20.Bureau W, Van Slyke P, Jones J, Han RNN, Ward NL, Stewart DJ, Dumont DJ. Chronic systemic delivery of angiopoietin-2 reveals a possible independent angiogenic effect. Am J Physiol Heart Circ Physiol. 2006;291:H948–H956. doi: 10.1152/ajpheart.00734.2005. [DOI] [PubMed] [Google Scholar]
- 21.Logan C, Khoo WK, Cado D, Joyner AL. Two enhancer regions in the mouse En-2 locus direct expression to the mid/hindbrain region and mandibular myoblasts. Development. 1993;117:905–916. doi: 10.1242/dev.117.3.905. [DOI] [PubMed] [Google Scholar]
- 22.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, Askew D, Gilliam AC, McCormick TS, Ward NL. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 25.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–244. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 26.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 27.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis, a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, Gaspari AA, Ling M, Weinstein GD, Nayak A, Gordon KB, Zitnik R. A randomized trial of etanercept as monotherapy for psoriasis. Archives of dermatology. 2003;139:1627–1632. 1632. doi: 10.1001/archderm.139.12.1627. discussion. [DOI] [PubMed] [Google Scholar]
- 29.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 30.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. The British journal of dermatology. 2011;164:750–758. doi: 10.1111/j.1365-2133.2010.10129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, Pensabene C, Coats I, Novitskaya I, Lowes MA, Krueger JG. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. The British journal of dermatology. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudjonsson JE, Ding J, Li X, Nair RP, Tejasvi T, Qin ZS, Ghosh D, Aphale A, Gumucio DL, Voorhees JJ, Abecasis GR, Elder JT. Global Gene Expression Analysis Reveals Evidence for Decreased Lipid Biosynthesis and Increased Innate Immunity in Uninvolved Psoriatic Skin. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shebl FM, Pinto LA, GarcíLa-Piñeres A, Lempicki R, Williams M, Harro C, Hildesheim A. Comparison of mRNA and Protein Measures of Cytokines following Vaccination with Human Papillomavirus-16 L1 Virus-like Particles. Cancer Epidemiology Biomarkers & Prevention. 2010;19:978–981. doi: 10.1158/1055-9965.EPI-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis, a randomized-controlled trial. J Clin Immunol. 2009;29:210–214. doi: 10.1007/s10875-008-9233-0. [DOI] [PubMed] [Google Scholar]
- 35.Russell CB, Kerkof K, Bigler J, Timour M, Diaz RL, Symons A, Rand H, Krueger JG, Martin DA. IL-17 Receptor A subunit is a central hub for inflammatory signaling in human psoriasis. Journal of Investigative Dermatology. 2012;132:S7. [Google Scholar]
- 36.Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, Dong H, Krueger JG, Russell CB, Martin DA. Anti-IL-17 Receptor Antibody AMG 827 Leads to Rapid Clinical Response in Subjects with Moderate to Severe Psoriasis, Results from a Phase I, Randomized, Placebo-Controlled Trial. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.163. advance online publication 24 May 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.