Abstract
The genomes of all living organisms are exposed to a wide spectrum of insults. To maintain genomic integrity, eukaryotes have evolved an elaborate surveillance mechanism - DNA damage checkpoint signaling - to detect damaged DNA and to arrest cell cycle progression, allowing time to process and repair DNA damage. TopBP1 plays multiple roles in the regulation of DNA damage checkpoint signaling. However, the molecular mechanism of how TopBP1 regulates ATR-mediated Chk1 phosphorylation is poorly understood. In this communication, we demonstrate (1) that the Chk1 activation domain of TopBP1 is critical in response to several different types of DNA damage; (2) that WD40-repeat protein WDR18 associates with the C-terminus of TopBP1 in vitro and in vivo; (3) that the association between WDR18 and TopBP1 is required for AT70-induced Chk1 phosphorylation; (4) and that WDR18 itself is required for AT70-triggered Chk1 phosphorylation. In addition, WDR18 associates with Chk1 in vitro. These data suggest that WDR18 facilitates ATR-dependent Chk1 phosphorylation via interacting with both C-terminus of TopBP1 and Chk1. Our findings indicate that WDR18 is a bona fide checkpoint protein and that WDR18 works together with TopBP1 to promote DNA damage checkpoint signaling.
Keywords: ATR, Chk1, DNA damage checkpoint, TopBP1, WD40, WDR18
1. Introduction
All organisms are exposed to a barrage of endogenous and exogenous damaging agents that stress genomic DNA, thereby generating DNA damage such as inter-strand crosslinks (ICLs) and double-strand breaks (DSBs) [1,2]. To respond to these genomic insults, cells activate DNA damage checkpoint signaling pathway to coordinate lesion repair, transcription activation and cell cycle arrest [3,4]. Failure to trigger the DNA damage checkpoint when necessary will lead to genomic instability, one emerging hallmark and enabling characteristic of cancer [5,6]. There are two key sensor kinases: ATM (Ataxia telangiectasia mutated) and ATR (ATM and Rad3-related), which share biochemical and functional similarities. However, ATR, but not ATM, is essential for the viability of replicating human and mouse cells. Whereas ATM primarily responds to DSBs, ATR can respond to a diverse group of lesions such as ICLs as well as replication stress [4,7]. ATR kinase is activated by primed single-stranded DNA (ssDNA) structure [7]. Additionally, ATR activation requires many factors: 9-1-1 complex (Rad9-Rad1-Hus1), ATRIP (ATR-interacting protein), Claspin, RPA (Replication Protein A) and TopBP1 (Topoisomerase II β Binding Protein 1) [8,9,10,11,12]. Upon activation, ATR can phosphorylate its substrates. Chk1, an ATR substrate, is subsequently phosphorylated on Ser317 and Ser345 in humans and Ser314 and Ser344 in Xenopus [13]. Phosphorylated Chk1 is the activated form that can phosphorylate its downstream target proteins such as Cdc25, thereby regulating cell cycle progression [14].
TopBP1 (also known as Rad4, Cut5, Dpb11, and Mus101) contains eight BRCT (BRCA1 C-terminus domain) domains and plays multiple functions in chromosome metabolism including DNA replication, DNA damage checkpoint signaling, and transcriptional regulation [12,15]. The first five BRCT domains of TopBP1 are sufficient for DNA replication initiation. Notably, TopBP1 regulates ATR-Chk1 checkpoint signaling in several different ways: (I) TopBP1 is a direct activator of ATR kinase through a domain between BRCT 6 and 7 known as ATR activation domain (AAD) [16,17]; (II) The interaction between the N-terminus of TopBP1 and the 9-1-1 complex is essential for ATR activation [18,19]; (III) TopBP1 is critical for the ATM-dependent activation of ATR following production of DSBs [20]; (IV) TopBP1 cooperates with 53BP1 in the G1 DNA damage checkpoint [21]; (V) An interaction between TopBP1 and BACH1/FANCJ helicase is likely required for ssDNA extension and RPA loading in response to replication stress [22]; (VI) TopBP1 collaborates with MDC1 in DNA replication checkpoint control [23].
To better understand the role of TopBP1 in ATR-dependent checkpoint signaling, we have conducted a series of studies using Xenopus egg extracts [24,25,26,27]. We previously demonstrated that TopBP1 is a bona fide checkpoint protein and that TopBP1 is required for the recruitment of polymerase α and Rad9-Rad1-Hus1 to stalled DNA replication fork [24,25]. We identified a Chk1 activation domain (CAD) containing BRCT 7&8 in the C-terminus of TopBP1 that is required for Chk1 phosphorylation (Fig. 1A) [24]. We characterized two inhibitors of TopBP1: a dominant negative fragment CT333 and a neutralizing antibody, which diminish Chk1 phosphorylation via an interaction with the CAD on TopBP1 (Fig. 1A). Both inhibitors prevent Chk1 phosphorylation induced by annealed synthetic oligonucleotides poly (dA) 70 and poly (dT) 70 (designated as AT70), a widely used checkpoint-activating DNA structure [11,24]. The CAD domain of TopBP1 is required for Claspin-bound Chk1 phosphorylation by AT70-induced ATR kinase. We hypothesize that TopBP1 CAD functions at a late phase by recruiting an interacting protein which acts as a scaffold to bring Claspin-bound Chk1 to the vicinity of the ATR activation domain (AAD). Once the AAD of TopBP1 activates ATR, Chk1 can then be phosphorylated by activated ATR. We propose WDR18 is a good candidate to functionally interact with the CAD domain of TopBP1.
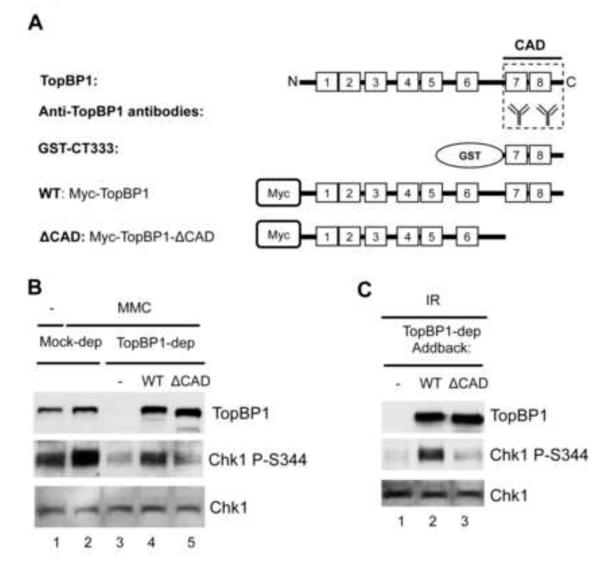
Fig. 1.
Chk1 activation domain of TopBP1 is critical for the DNA damage checkpoint. (A) Schematic representation of TopBP1 domains and antibodies. Numbered blocks represent BRCT domains. CAD represents Chk1 Activation Domain. GST-CT333 is the C-terminus 333 amino acids fragment of TopBP1 tagged with GST. Anti-TopBP1 antibodies were raised against GST-CT333. WT represents wild type TopBP1 with Myc-tag. ΔCAD is the CAD deletion mutant of Myc-tagged TopBP1. (B) CAD of TopBP1 is required for MMC-triggered Chk1 phosphorylation. Mock- or TopBP1-depleted egg extracts were supplemented with sperm chromatin and optionally with MMC. TopBP1-depleted egg extracts were added back with buffer (-), WT TopBP1 or ΔCAD TopBP1. Extracts were probed for TopBP1, Chk1 P-S344 (short for Chk1 phosphorylation at Ser344), and Chk1 via immunoblotting. (C) CAD of TopBP1 is required for IR - triggered Chk1 phosphorylation. IR-treated sperm chromatin (γ-irradiation, 20 Gy) was added into TopBP1-depleted egg extracts supplemented with buffer (-), WT TopBP1 or ΔCAD TopBP1. The extracts were examined as (B).
WDR18, WD40-repeat protein 18, comprises seven WD40 repeats (Fig. 2A). WD40-repeat is a conserved motif of 44-60 residues with the GH dipeptide 11-24 residues from its N-terminus and the WD dipeptide at the C-terminus [28]. WD40-repeat proteins are very abundant in eukaryotic organisms and participate in diverse functions including signal transduction, cell cycle control, cytoskeleton assembly, apoptosis, chromatin dynamics, and transcriptional regulation [29]. Structural studies have shown that each WD40-repeat consists of a four-stranded anti-parallel β–sheet and seven WD40-repeats form a seven-bladed β–propeller like structure. WD40-repeat proteins function as scaffolds to interact with proteins, peptides, or nucleic acids [30]. For example, human WDR18 is involved in ribosome biogenesis via a complex with its partners Rix1, PELP and TEX10 [31,32]. Crb3 (Cut5-repeat binding protein 3), the fission yeast homologue of WDR18, was first found to associate with Cut5 (TopBP1) through two-hybrid screening [33]. In S. pombe, Cut5/Rad4-c11TopBP1 (a mutant TopBP1 in the third BRCT domain) abolishes the DNA damage checkpoint response, but not DNA replication, and fails to associate with Crb3 in two-hybrid assays [34]. One copy deletion of Crb3 from diploid yeast cells compromises the maintenance of DNA damage checkpoint signaling. However, a molecular mechanism elucidating how WDR18 regulates the DNA damage checkpoint is largely unknown. In this study, we provide evidence showing an interaction between WDR18 and TopBP1 in Xenopus egg extracts and the TopBP1-WDR18 interaction is functionally required for DNA damage checkpoint signaling.
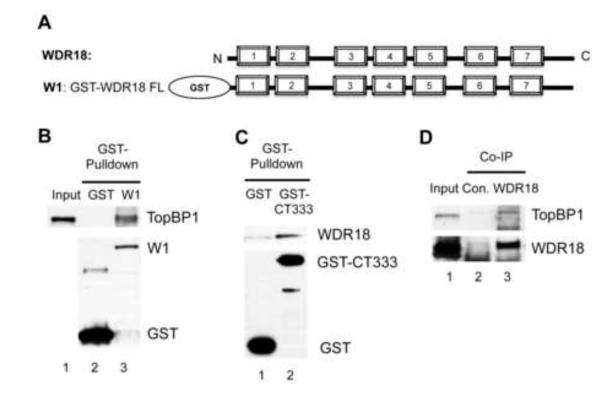
Fig. 2.
WDR18 interacts with the C-terminus of TopBP1. (A) Schematic representation of WDR18. Numbered blocks represent WD40 repeats. W1 is the GST-tagged full length WDR18. (B) GST or W1 (GST-WDR18) was added to egg extracts supplemented with Glutathione beads. The GST pulldowns were examined via immunoblotting. Input represents 1 μl of egg extract. (C) GST or GST-CT333 was incubated in egg extract complemented with Glutathione sepharose. The bead-bound fractions were examined via immunoblotting. (D) Anti-WDR18 or control antibodies (2μg) was added into egg extracts for immunoprecipitation (Co-IP) assay. TopBP1 and WDR18 in the immune-complexes were examined via immunoblotting.
2. Materials and Methods
2.1 Xenopus egg extracts and related procedures
Xenopus egg extracts and sperm chromatin were prepared as described previously [24,27,35]. Sperm chromatin was added to egg extracts at a concentration of ~4000 sperm/μl. AT70 and TopBP1 depletion were performed as previously described [24]. WDR18 depletion was done in a similar way as TopBP1 depletion. In addition, Mitomycin C (EMD) was used at a final concentration of 0.75 μM.
2.2 Expression vectors, recombinant proteins, and antibodies
GST-WDR18 (W1), GST-WDR18 1-250 (W2), GST-WDR18 251-428 (W3), GST-WDR18 161-428 (W4) and GST-WDR18 161-250 (W5) were cloned into the pGEX-4T1 expression vector and correspond to X. laevis WDR18 (BC097753, MGC: 115442) nucleotide 7-1293, 7-756, 757-1293, 487-1293, and 487-756, respectively. WT and ΔCAD of TopBP1 correspond to TopBP1 nucleotide 1-4542 and 1-3837, respectively, and were subcloned into pCS2+MT as described previously [24]. 35S-labeled recombinant TopBP1 was prepared using TnT SP6 Quick Coupled Transcription/Translation System (Promega) according to manufacturer’s protocol. Recombinant Chk1-ΔKD-TH and GST-CT333 were prepared as previously described [24,36]. Anti-TopBP1 antibodies were used as described previously [24]. WDR18 antibody was raised in rabbit against GST-WDR18 (Cocalico Biologicals). Antibodies against Chk1 (Santa Cruz), Chk1 P-S344 (Cell Signaling), GST (Santa Cruz), Myc (Santa Cruz) and T7 (Novagen) were purchased from their vendors and used as described previously (Yan and Michael, 2009).
2.3 Protein-protein interaction assays
For the GST-pulldown experiments (Fig. 2B and 2C), GST (5μg), GST-WDR18 (5μg) or GST-CT333 (5μg) was added to 100 μl Xenopus egg extracts. The extract mixtures were diluted with 200 μl Interaction Buffer A (100mM NaCl, 5mM MgCl2, 10% Glycerol, 0.1% NP-40, 20mM Tris-HCl, pH 8.0) supplemented with 30 μl of Glutathione, respectively. After one-hour incubation at room temperature, bead-bound fractions were analyzed via immunoblotting as indicated. 1 μl of egg extract was used as “Input”.
For the Co-IP experiment in Fig. 2D, we added either control antibodies (Con., 1 μg) or anti-WDR18 antibodies (WDR18, 1 μg) to egg extracts supplemented with 15 μl of Protein A beads. After one-hour incubation, the extract mixtures were diluted with 200 μl IP Buffer (500mM NaCl, 1% NP-40, 2.5mM EGTA, 20mM β-glycerophosphate, 10mM HEPES, pH7.5). The bead-bound fractions (Co-IP) were eluated and examined via Western blotting with anti-TopBP1 and anti-WDR18 antibodies. 1 μl of egg extract was used as “Input”.
For the GST-pulldown experiment in Fig. 3B, GST, or W1, W2, W3, W4, or W5 (5μg each) was added to 200 μl Interaction Buffer A supplemented with 35S-labled recombinant TopBP1 and 30 μl of Glutathione. After one-hour incubation at room temperature, bead-bound fractions were analyzed for TopBP1 via a Phosphorimager method as described previously [24]. 1/20th of 35S-labled recombinant TopBP1 was used as “Input”. GST or GST-tagged proteins on the beads were examined via immunoblotting.
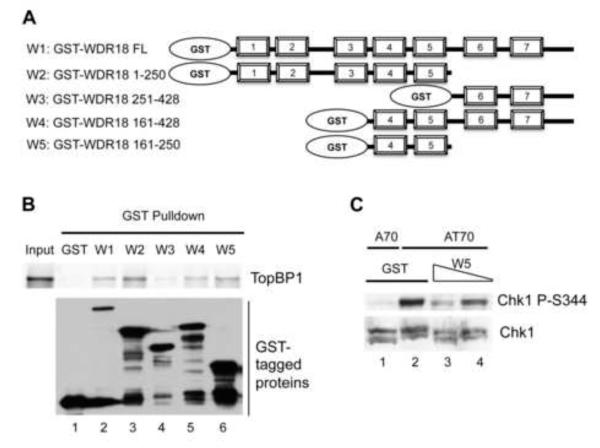
Fig. 3.
TopBP1-interacting domain of WDR18 dominantly compromises AT70-induced Chk1 phosphorylation. (A) Schematic representations of GST-tagged full length and different fragments of WDR18. (B) WDR18 161-250 is necessary and sufficient for TopBP1 interaction. (C) W5 (WDR18 161-250) over-expression inhibits AT70-induced Chk1 phosphorylation in a dose-dependent manner.
For Fig. 4B, either GST (5μg) or W1 (GST-WDR18, 5μg) was added to 200 μl Interaction Buffer B (150mM NaCl, 0.5% NP-40, 2.5mM EGTA, 20mM β-glycerophosphate, 50mM Imidazole, 10mM HEPES, pH7.5) containing 2.5 μg of Chk1-ΔKD-TH. 1/20th of these mixtures were used as “Input”. Then 20 μl of Ni-NTA beads were added to these protein-buffer mixtures, respectively. After one-hour incubation, the beads were washed by Interaction Buffer B. The bead-bound fractions were examined via immunoblotting with anti-GST or anti-T7 antibodies.
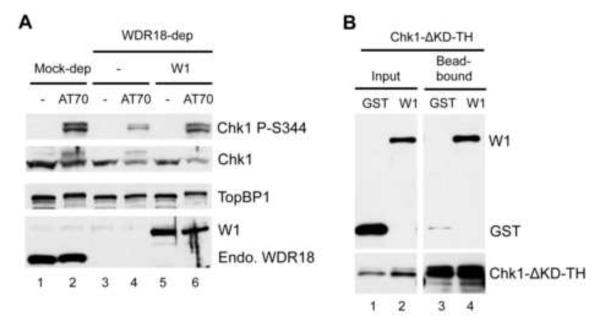
Fig. 4.
Functional analysis of WDR18 in ATR-Chk1 checkpoint signaling. (A) WDR18 is required for AT70-triggered Chk1 phosphorylation. Mock or WDR18-depleted egg extracts were supplemented with either buffer (-) or AT70. Recombinant WDR18 (W1) was also added back to WDR18-depleted egg extracts. The samples were probed for TopBP1, WDR18, Chk1 P-S344, and Chk1 by immunoblotting. “Endo. WDR18” represents endogenous WDR18 while W1 represents GST-WDR18.
3. Results
3.1 Chk1 activation domain (CAD) of TopBP1 is critical for DNA damage triggered Chk1 phosphorylation
To test the idea that TopBP1 CAD is required for Chk1 activation in response to more general DNA damage (i.e. not only AT70), we initially asked if TopBP1 CAD is required for Chk1 phosphorylation induced by Mitomycin C (MMC, a DNA cross-linking reagent). We added sperm chromatin to either mock or TopBP1-depleted egg extracts supplemented with MMC. In TopBP1-depleted extracts, we added back buffer (-), recombinant wild type (WT) or CAD deletion mutant TopBP1 (ΔCAD, Fig. 1A-B). After 1-hour incubation, samples were resolved by SDS-PAGE gel and probed for TopBP1, Chk1, or Chk1 P-S344 via Western blotting. Phosphorylation of Chk1 at S344 (Chk1 P-S344) indicates ATR is activated. As shown in Fig. 1B, TopBP1 depletion compromised the MMC-induced Chk1 phosphorylation. WT, but not ΔCAD, rescued the MMC-induced Chk1 phosphorylation when endogenous TopBP1 was absent (Fig. 1B). This result suggests that the CAD domain of TopBP1 is critical for MMC-induced Chk1 phosphorylation. A similar observation was obtained when γ-irradiation was used to trigger Chk1 phosphorylation (Fig. 1C). These data demonstrate that the CAD of TopBP1 is a critical domain required for not only AT70 but also MMC and IR triggered Chk1 phosphorylation.
3.2 WDR18 associates with TopBP1 C-terminus
To better understand the molecular mechanism underlying the role of the CAD domain of TopBP1 in ATR-Chk1 signaling, we hypothesize that a TopBP1 interacting protein brings Chk1 to activated ATR. In our quest to characterize a possible TopBP1-interacting protein, we chose WDR18 (a Xenopus homologue of Crb3). First, we asked whether WDR18 associates with TopBP1 in Xenopus. GST-WDR18 (W1), but not GST, associated with endogenous TopBP1 in Xenopus egg extracts in GST pulldown assays (Fig. 2B). Next, purified recombinant GST-WDR18 was used for antibody production. Our anti-WDR18 antibodies recognize endogenous WDR18 and GST-WDR18 (data not shown). GST pulldown assays showed that GST-CT333, but not GST, pulled down endogenous WDR18 from Xenopus egg extracts (Fig. 2C). This finding also indicates that the CAD domain of TopBP1 is sufficient for WDR18 interaction. Finally, we tested whether WDR18 and TopBP1 associate with each other in vivo. Anti-WDR18 antibodies, but not Control antibodies, efficiently immunoprecipitated WDR18 and TopBP1 from Xenopus egg extracts (Fig. 2D). We also did a reciprocal immunoprecipitation experiment with anti-TopBP1 antibodies. WDR18 was not found in the immune-complex using anti-TopBP1 antibodies (data not shown). We reasoned that anti-TopBP1 antibodies might bind to the CAD domain of TopBP1 thereby preventing WDR18’s interaction with TopBP1. This result indirectly indicates why anti-TopBP1 antibodies have an inhibitory effect on Chk1 phosphorylation as shown in our previous study: the interaction between TopBP1 and WDR18 is compromised when anti-TopBP1 antibodies are present in the egg extract [24]. Therefore, these data suggest that WDR18 associates with TopBP1 C-terminal CAD domain.
3.3 TopBP1-interacting domain of WDR18 inhibits AT70-induced Chk1 phosphorylation in a dominant negative manner
To determine which region in WDR18 is responsible for TopBP1 interaction, full length and truncated fragments of WDR18 were expressed in DE3 E. coli and purified according to manufacturer’s protocol (Fig. 3A). Then GST or GST-tagged full length or fragments of WDR18 were added into Interaction Buffer A containing 35S-labeled recombinant TopBP1 and Glutathione beads for GST pulldown assays. GST and GST-tagged proteins on glutathione beads were shown via immunoblotting using anti-GST antibodies. TopBP1 bound to glutathione beads was visualized using a Phosphorimager. Full length (W1), WDR18 1-250 (W2), WDR18 161-428 (W4) and WDR18 161-250 (W5), but not GST and WDR18 251-428 (W3), could pulldown TopBP1 (Fig. 3B). Therefore, W5 of WDR18 is necessary and sufficient for TopBP1 interaction.
We then wanted to determine if the interaction between TopBP1 and WDR18 is biologically relevant. In our previous work, we reported that over-expression of GST-CT333, but not GST, inhibited AT70-induced Chk1 phosphorylation [24], indicating the CAD domain of TopBP1 is essential for Chk1 activation. We tested whether over-expression of W5 in egg extract attenuates AT70-induced Chk1 phosphorylation. The idea is that the interaction between endogenous TopBP1 and endogenous WDR18 could be blocked if a TopBP1 interaction fragment of WDR18 sequesters the endogenous TopBP1. Addition of W5 (300nM and 150nM), but not GST (300nM), compromised AT70-induced Chk1 phosphorylation (Fig. 3C). A70 (synthetic oligonucleotide poly (dA)-70) was used as negative control and did not trigger Chk1 phosphorylation in the presence of GST. Notably, the higher concentration of W5 caused reduction of Chk1 phosphorylation in a concentration-dependent manner. Total Chk1 was used as loading control. These data suggest that WDR18 161-250 containing WD40-repeat 4&5 is a TopBP1-interacting domain, and that WDR18 161-250 inhibits AT70-induced Chk1 phosphorylation in a dominant-negative manner.
3.4 WDR18 deletion compromises AT70-induced Chk1 phosphorylation
The data we obtained so far suggest that the interaction between WDR18 and TopBP1 is essential for Chk1 phosphorylation. We also wanted to determine if WDR18 itself is required for Chk1 phosphorylation induced by AT70. To test this directly, we added buffer or AT70 to mock- or WDR18-depleted egg extracts. TopBP1, WDR18, and Chk1 phosphorylation were examined via western blotting. As shown in Fig. 4A, endogenous WDR18 was removed from egg extract successfully after three rounds of immunodepletion using anti-WDR18 antibodies. There was no noticeable change on TopBP1 when WDR18 was removed by immunodepletion, indicating TopBP1 was not co-depleted by anti-WDR18 antibodies. AT70 addition to mock-depleted egg extract triggered Chk1 phosphorylation. However, AT70-triggered Chk1 phosphorylation was compromised when WDR18 was not present, suggesting WDR18 is required for AT70-induced Chk1phosphorylation. More importantly, this phenotype is rescued by adding recombinant GST-WDR18 to WDR18-depleted egg extracts (Fig. 4A). Taken together, TopBP1 can’t fully conduct its Chk1 activation function when WDR18 is not present or when its interaction with WDR18 is attenuated.
3.5 WDR18 associates with Chk1
The above findings from our study led us to hypothesize that WDR18 may associate with Chk1. To test this hypothesis, we performed an in vitro protein-protein interaction assay using purified recombinant proteins. As shown in Fig. 4B, GST-WDR18, but not GST, associated with Chk1-ΔKD-TH (Kinase domain deletion of Chk1 with T7 and His tags). This result suggests that WDR18 may bring Chk1 closer to activated ATR via interacting with TopBP1 C-terminus.
4. Discussion
In this study, we sought to elucidate the molecular mechanism of how the TopBP1 C-terminus contributes to the DNA damage checkpoint signaling. Our series of studies support the notion that the CAD domain of TopBP1 is required for the DNA damage checkpoint in response to DNA damage (i.e. MMC-induced interstrand crosslinks and IR-induced DSBs) or a DNA damage-mimicking structure (i.e. AT70) (Fig. 1B-C) [24]. From protein-protein interaction assays, we demonstrate that WDR18 associates with the TopBP1 C-terminus in vitro and in vivo (Fig. 2). We further show that TopBP1 associates with WD40-repeat 4&5 of WDR18 (Fig. 3A). Our two findings have elucidated the biological significance of the interaction between TopBP1 and WDR18: (1) over-expression of TopBP1-interacting domain in WDR18 (i.e. W5 in this study) inhibits AT70-induced Chk1 phosphorylation (Fig. 3B-3C); and (2) over-expression of WDR18-interacting domain in TopBP1 (i.e. GST-CT333) compromises AT70-triggered Chk1 phosphorylation [24]. In addition, removing endogenous WDR18 from egg extracts compromises AT70-triggered Chk1 phosphorylation and this phenotype can be rescued by adding recombinant protein W1 (Fig. 4A), indicating WDR18 is a bona fide checkpoint protein.
Through different BRCT-mediated or non-BRCT-mediated function, TopBP1 regulates the activation of ATR-Chk1 checkpoint signaling at several levels [7,12,15]. In summary, there are at least three different types of molecular mechanisms: (1) TopBP1 is a direct activator of ATR kinase activity [16]; (2) TopBP1 acts as a scaffold for all necessary proteins for ATR activation, such as the 9-1-1 complex and ATR-ATRIP complex, to trigger the DNA damage checkpoint signaling [17,18]; and (3) TopBP1 can regulate late stage of the DNA damage checkpoint by facilitating Chk1 phosphorylation by activate ATR [24]. Our current study extends our mechanistic understanding of TopBP1 in ATR-Chk1 checkpoint signaling: WDR18 facilitates ATR-dependent Chk1 phosphorylation by interacting with both C-terminal end of TopBP1 and Chk1. In summary, we conclude that WDR18 is a bona fide checkpoint protein and that WDR18 collaborates with TopBP1 to promote DNA damage checkpoint signaling.
Highlights.
The Chk1 activation domain (CAD) of TopBP1 is critical in DNA damage response.
WD40-repeat protein WDR18 associates with TopBP1 in vitro and in vivo.
WDR18 and TopBP1 association is required for AT70-induced Chk1 phosphorylation.
WDR18 is required for AT70-triggered Chk1 phosphorylation.
WDR18 associates with Chk1 in vitro.
Acknowledgements
We thank Dr. W.M. Michael for reagents and encouragement. We also thank the UNC Charlotte Vivarium staff and Drs. N. Lefebvre, C.D. Williams, and Y.M. Huet for caring our frogs. This study was supported in part by funds provided by University of North Carolina at Charlotte and a grant from the NIGMS/NIH (R15 GM101571) to S. Yan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- [2].Kastan MS, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- [3].Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu. Rev. Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- [4].Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- [5].Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland J, Lukas C, Orntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- [6].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [7].Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choi JH, Sancar A, Lindsey-Boltz LA. The human ATR-mediated DNA damage checkpoint in a reconstituted system. Methods. 2009;48:3–7. doi: 10.1016/j.ymeth.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [9].Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPAssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- [10].Lieberman HB. Rad9, an evolutionarily conserved gene with multiple functions for preserving genomic integrity. J Cell. Biochem. 2006;97:690–697. doi: 10.1002/jcb.20759. [DOI] [PubMed] [Google Scholar]
- [11].Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- [12].Garcia V, Furuya K, Carr AM. Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 2005;4:1227–1239. doi: 10.1016/j.dnarep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- [13].Chen Y, Poon RY. The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front. Biosci. 2008;13:5016–5029. doi: 10.2741/3060. [DOI] [PubMed] [Google Scholar]
- [14].Boddy MN. Replication Checkpoint Enforced by Kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- [15].Sokka M, Parkkinen S, Pospiech H, Syvaoja JE. Function of TopBP1 in genome stability. Subcell. Biochem. 2010;50:119–141. doi: 10.1007/978-90-481-3471-7_7. [DOI] [PubMed] [Google Scholar]
- [16].Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- [17].Mordes DA, Glick GG, Zhao R, Cortez D. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes & Dev. 2008;22:1478–1489. doi: 10.1101/gad.1666208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes & Dev. 2007;21:1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee J, Kumagai A, Dunphy WG. The Rad9-Hus1-Rad1 checkpoint clamp regulates interaction of TopBP1 with ATR. J. Biol. Chem. 2007;282:28036–28044. doi: 10.1074/jbc.M704635200. [DOI] [PubMed] [Google Scholar]
- [20].Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J. Biol. Chem. 2007;282:17501–17506. doi: 10.1074/jbc.M701770200. [DOI] [PubMed] [Google Scholar]
- [21].Cescutti R, Negrini S, Kohzaki M, Halazonetis TD. TopBP1 functions with 53BP1 in the G1 DNA damage checkpoint. EMBO J. 2010;29:3723–3732. doi: 10.1038/emboj.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gong Z, Kim JE, Leung CC, Glover JN, Chen J. BACH1/FANCJ acts with TopBP1 and participates early in DNA replication checkpoint control. Mol. Cell. 2010;37:438–446. doi: 10.1016/j.molcel.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang J, Gong Z, Chen J. MDC1 collaborates with TopBP1 in DNA replication checkpoint control. J. Cell Biol. 2011;193:267–273. doi: 10.1083/jcb.201010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan S, Lindsay HD, Michael WM. Direct requirement for Xmus101 in ATRmediated phosphorylation of Claspin bound Chk1 during checkpoint signaling. J. Cell Biol. 2006;173:181–186. doi: 10.1083/jcb.200601076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yan S, Michael WM. TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J. Cell Biol. 2009;184:793–804. doi: 10.1083/jcb.200810185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Van C, Yan S, Michael WM, Waga S, Cimprich KA. Continued primer synthesis at stalled replication forks contributes to checkpoint activation. J. Cell Biol. 2010;189:233–246. doi: 10.1083/jcb.200909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Willis J, Destephanis D, Patel Y, Gowda V, Yan S. Study of the DNA damage checkpoint using Xenopus egg extracts. J. Vis. Exp. 2012:e4449. doi: 10.3791/4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- [29].Stirnimann CU, Petsalaki E, Russell RB, Muller CW. WD40 proteins propel cellular networks. Trends Biochem. Sci. 2010;35:565–574. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [30].Xu C, Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Finkbeiner E, Haindl M, Muller S. The SUMO system controls nucleolar partitioning of a novel mammalian ribosome biogenesis complex. EMBO J. 2011;30:1067–1078. doi: 10.1038/emboj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Castle CD, Cassimere EK, Denicourt C. LAS1L interacts with the mammalian Rix1 complex to regulate ribosome biogenesis. Mol. Biol. Cell. 2012;23:716–728. doi: 10.1091/mbc.E11-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes & Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Taricani L, Wang TS. Rad4TopBP1, a scaffold protein, plays separate roles in DNA damage and replication checkpoints and DNA replication. Mol. Biol. Cell. 2006;17:3456–3468. doi: 10.1091/mbc.E06-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lebofsky R, Takahashi T, Walter JC. DNA replication in nucleus-free Xenopus egg extracts. Methods Mol. Biol. 2009;521:229–252. doi: 10.1007/978-1-60327-815-7_13. [DOI] [PubMed] [Google Scholar]
- [36].Michael WM. Activation of the DNA Replication Checkpoint Through RNA Synthesis by Primase. Science. 2000;289:2133–2137. doi: 10.1126/science.289.5487.2133. [DOI] [PubMed] [Google Scholar]