Abstract
The cellular development of resistance to chemotherapy contributes to the high mortality noted in patients affected by ovarian cancer. Novel compounds that specifically target cellular drug resistance in ovarian cancer are therefore highly desired. Previous epidemiological studies indicate that consumption of green tea and cruciferous vegetables is inversely associated with occurrence of ovarian cancer. Therefore revealing the effects and mechanisms of major components of green tea (epigallocatechin gallate, EGCG) and cruciferous vegetables (sulforaphane, SFN) on ovarian cancer cells will provide necessary knowledge for developing potential novel treatments for the disease. In this study, EGCG or SFN was used to treat both paclitaxel-sensitive (SKOV3-ip1) and -resistant (SKOV3TR-ip2) ovarian cancer cell lines alone or in combination. We found that SFN inhibits cell viability of both ovarian cancer cell lines time- and dose-dependently and that EGCG potentiates the inhibiting effect of SFN on ovarian cancer cells. Cell cycle analysis indicates SFN can arrest ovarian cancer cells in G2/M phase, while EGCG and SFN co-treatment can arrest cells in both G2/M and S phase. Combined EGCG and SFN treatment increases apoptosis significantly in paclitaxel-resistant SKOV3TR-ip2 cells after 6 days of treatment, while reducing the expression of hTERT, the main regulatory subunit of telomerase. Western blotting also indicates that SFN can down-regulate Bcl-2 (a gene involved in anti-apoptosis) protein levels in both cell types. Cleaved poly(ADP-ribose) polymerase (PARP) becomes up-regulated by 6 days of treatment with SFN and this is more pronounced for combination treatment indicating induction of apoptosis. Furthermore, phosphorylated H2AX is up-regulated after 6 days of treatment with SFN alone, and EGCG can potentiate this effect, suggesting that DNA damage is a potential cellular mechanism contributing to the inhibiting effect of EGCG and SFN combination treatment. Taken together, these results indicate that EGCG and SFN combination treatment can induce apoptosis by down-regulating of hTERT and Bcl-2 and promote DNA damage response specifically in paclitaxel-resistant ovarian cancer cell lines and suggest the use of these compounds for overcoming paclitaxel resistance in ovarian cancer treatment.
Keywords: ovarian cancer, SKOV3, epigallocatechin gallate, sulforaphane, paclitaxel
Introduction
Ovarian cancer is the leading cause of death from gynecologic cancers in the United States and is the fifth leading cause of cancer death among American women. Most ovarian cancer patients are diagnosed at advanced stages due to the lack of effective screening strategies and specific symptoms associated with early stages. Currently, the preferred treatment is surgical excision followed by platinum/taxane combination chemotherapy. Although most ovarian cancers respond to first-line chemotherapy, recurrence occurs in up to 75% of ovarian cancer patients, most of whom will ultimately succumb to their disease [1]. Thus, novel therapies that can reverse drug resistance or kill drug resistant ovarian cancer cells directly are highly desired.
Green tea is the most popular beverage next to water worldwide. Epidemiological studies have revealed an inverse correlation between the dietary intake of green tea and the risk for certain types of cancers, including ovarian cancer [2–4]. Epigallocatechin gallate (EGCG), as a major component of green tea, is generally accepted to be the most effective constitute that contributes to the anti-cancer effect of green tea. It has been reported that EGCG can induce apoptosis in breast cancer cells [5], and EGCG can target cancer cells through a variety of mechanisms, including decreasing expression of hTERT, the major catalytic subunit of telomerase [6].
Additionally, consumption of cruciferous vegetables such as broccoli, Brussels sprouts or cabbage has also been linked with low occurrence of lung, stomach, colon, rectal, prostate, endometrial and ovarian cancer [7]. Sulforaphane (SFN), as the major component of these vegetables, has received considerable attention in the past due to its anti-cancer effect in numerous cancer cells including ovarian cancer. Mechanistic studies reveal that SFN can target cancer cells through Nrf2-mediated induction of phase 2 detoxification enzymes which can elevate the cellular defense against oxidative damage and promote the removal of carcinogens [8]. SFN has also been observed to suppress cytochrome P450 enzymes [9], induce apoptotic pathways [10], suppress cell cycle progression [10], and inhibit angiogenesis and inflammatory response [11, 12]. More recently, SFN has been shown to inhibit histone deacetylase (HDAC) activity leading to reactivation of tumor suppressor genes and silencing of oncogenes [13].
Late stages of ovarian cancer are characterized by resistance to conventional platinum based chemotherapy as aforementioned. Although drug resistance can be mediated by individual genes, such as overexpression of ABC transporters or enhanced DNA repair genes, or down-regulation of apoptosis-promoting genes, alteration in multiple pathways concurrently are commonly observed. Treatments that can target multiple pathways involved in drug resistance or can target multiple intact cancer cell survival pathways are promising strategies. Combination treatment with different compounds that target several different pathways is therefore a promising direction. One frequent obstacle to this approach, however is increased toxicity that accompanies targeting multiple genes. Therefore drugs that have minimal side effects are of course preferred. Although the anti-cancer effects of EGCG or SFN alone are well known, and both extracts are well-tolerated, it is not clear whether the combination of these two compounds can exert a stronger anti-cancer effect. Additionally, although several studies have attempted to investigate the effect of EGCG and SFN on ovarian cancer cells, the doses in those studies were too high to be reached physiologically [14]. Therefore revealing the effects and mechanisms of EGCG and SFN on ovarian cancer within a reasonable dose range will provide critical knowledge for developing potential novel treatments for the disease.
The present study was undertaken to evaluate the effect of EGCG and SFN combination treatment on ovarian cancer cells, especially paclitaxel-resistant ovarian cancer cells, and to elucidate the potential mechanisms responsible for the effect. Our results indicate that SFN with EGCG can inhibit both sensitive and resistant ovarian cancer cells while exerting a much stronger inhibiting effect on the paclitaxel-resistant ovarian cancer cells. Mechanistic studies reveal that EGCG and SFN combination treatment can cause DNA damage and decrease hTERT (a protein involved in cancer cell survival) and Bcl-2 (a protein involved in anti-apoptosis) expression in these ovarian cancer cells. These findings reveal for the first time that EGCG and SFN combination treatment can inhibit ovarian cancer cells by creating DNA damage through decreasing hTERT and Bcl-2 expression.
Materials and Methods
Cell culture
SKOV3-ip1 and SKOV3TR-ip2 cells were grown in RPMI 1640 medium (Mediatech Inc, Manassas, VA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin (Mediatech). SKOV3TR-ip2 cells were maintained with the addition of 150 ng/mL of paclitaxel [15]. EGCG (Sigma) or R,S-sulforaphane (LKT Laboratories, Minneapolis, MN) was dissolved in DMSO and stored at a stock concentration of 100 mM/L at −20°C. After seeding the cells and culturing for 24 h, EGCG and/or SFN was added to the culture medium at indicated concentrations and the maximum concentration of DMSO was 0.1% (v/v) in the medium. Cells treated only with DMSO served as a vehicle control. Cells were treated with fresh EGCG and/or SFN every 24 h.
MTT assay
The viability of ovarian cancer cells was examined using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Approximately 5000 ovarian cancer cells were plated in 96- well plates and allowed to attach to the bottom overnight. Cells were then treated with increasing doses of EGCG (5, 10, 20, 30 µmol/L) or SFN (7.5, 10, 15 µmol/L) or combination of EGCG and SFN (5+7.5, 10+7.5, 20+7.5, 30+7.5; 10+10, 20+10, 30+10; 10+10, 20+10, 30+10 µmol/L) for 24, 48 and 72 h. At the end of each treatment, cells were incubated with 1 mg/mL MTT for 2 hours at 37°C; 200 µl DMSO was then added to each to dissolve the formazan crystals. Dye absorbance in viable cells was measured at 595 nm, with 630 nm as a reference wavelength. Cell viability was estimated as a percentage of the value of untreated controls.
Morphological analysis
Cells were plated at a density of 2 × 105 cells/2 ml medium in 6 well plates, and were treated 24 h after seeding. Medium containing freshly added EGCG (20 µmol/L) and/or SFN (10 µmol/L) was changed daily for 6 days. Morphology of cells was observed under phase contrast microscope with magnification of 100. Images of cells were taken with a Nikon Coolpix 990 (Nikon, Tokyo, Japan).
Clonogenic assay
Cells were seeded at 2 × 105 /well in 6 well plates and allowed to adhere overnight. Cell culture medium was changed and cells were treated with 20 µmol/L EGCG, 10 µmol/L SFN or in combination in triplicate for each group. After 24 h, cells were split and approximately 400 cells were seeded into sixwell plates. The cells were allowed to incubate at 37°C in the incubator undisturbed for 15 days. During this period each individual surviving cell would proliferate and form colonies. On day 15, the colonies were washed with cold phosphate buffer saline, fixed with cold 70% ethanol and stained with 0.25% trypan blue solution. The colonies that had ≥50 cells/colony were counted and expressed as percent control.
Analysis of cell cycle progression
Cells were plated at a density of 2 × 105 cells/2 ml medium in 6 well plates, and were treated 24 h after seeding. Medium containing freshly added EGCG (20 µmol/L) and/or SFN (10 µmol/L) was changed daily. After 2 days of treatment, cells were removed from culture dishes by trypsinization and centrifugation. After washing with PBS, they were fixed in 70% ethanol at −20°C overnight. Cells were then centrifuged and washed with PBS the second day followed by suspension in PBS containing 0.1% Triton X-100, 0.1% RNase and 50 µg/ml propidium iodide. DNA contents in stained nuclei were analyzed with flow cytometry.
Analysis of apoptosis
Ovarian cancer cells were seeded at a density of 2 × 105 cells / well in 6 well plates. Cells were treated with EGCG (20 µmol/L) and/or SFN (10 µmol/L) for 6 days. Cells were then washed with PBS; they were stained with Annexin V and PI (Invitrogen) in the binding buffer for 15 min in the dark. Cells were then analyzed through flow cytometry.
Paclitaxel treatment
Ovarian cancer cells were seeded at 2 × 105 cells / well in 6 well plates. Cells were then treated with 50 nmol/L paclitaxel, 20 µmol/L EGCG and 10 µmol/L SFN for 3 days. Proteins were harvested for western blotting analysis.
Western blotting
Protein extracts were prepared by RIPA Lysis Buffer (Upstate Biotechnology, Charlottesville, VA) according to the manufacturer's protocol. Proteins (20 µg) were electrophoresed on 10% SDS-polyacrylamide gels and transferred to PDVF membranes. Membranes were probed with antibodies to hTERT (Millipore, CA), Phosphor H2AX (Cell signaling), PARP (Cell signaling), DNMT1 (ABcam), Bcl-2 (Cell signaling), and β-actin antibody (Cell signaling). Molecular weight markers were run on each gel to confirm the molecular size of the immunoreactive proteins. Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (Santa Cruz Biotechnology) following the protocol of the manufacturer.
Telomerase activity assay
Telomerase activity was measured using TeloTAGGG telomerase PCR ELISA kit (Roche applied science, Indianapolis, IN) according to the manufacturer's protocol. Protein from total cell lysates (3 µg) was added to the reaction mixture, and the generated telomere product was PCR amplified using 30 cycles (25°C for 20 min, 94°C for 5 min, 94°C for 30 sec, 50°C for 30 sec, 72°C for 90 sec and 72°C for 10 min). PCR amplified products (5 µL) were used for ELISA assays, and the level of telomerase activity was expressed as an arbitrary unit of absorbance at OD 450.
Statistical analyses
All the data were derived from at least three independent experiments. Statistical significance among treatments was evaluated using one way ANOVA followed by Tukey test. P < 0.05 was considered significant.
Results
EGCG and SFN can inhibit viability of SKOV3-ip1 and SKOV3TR-ip2 cells
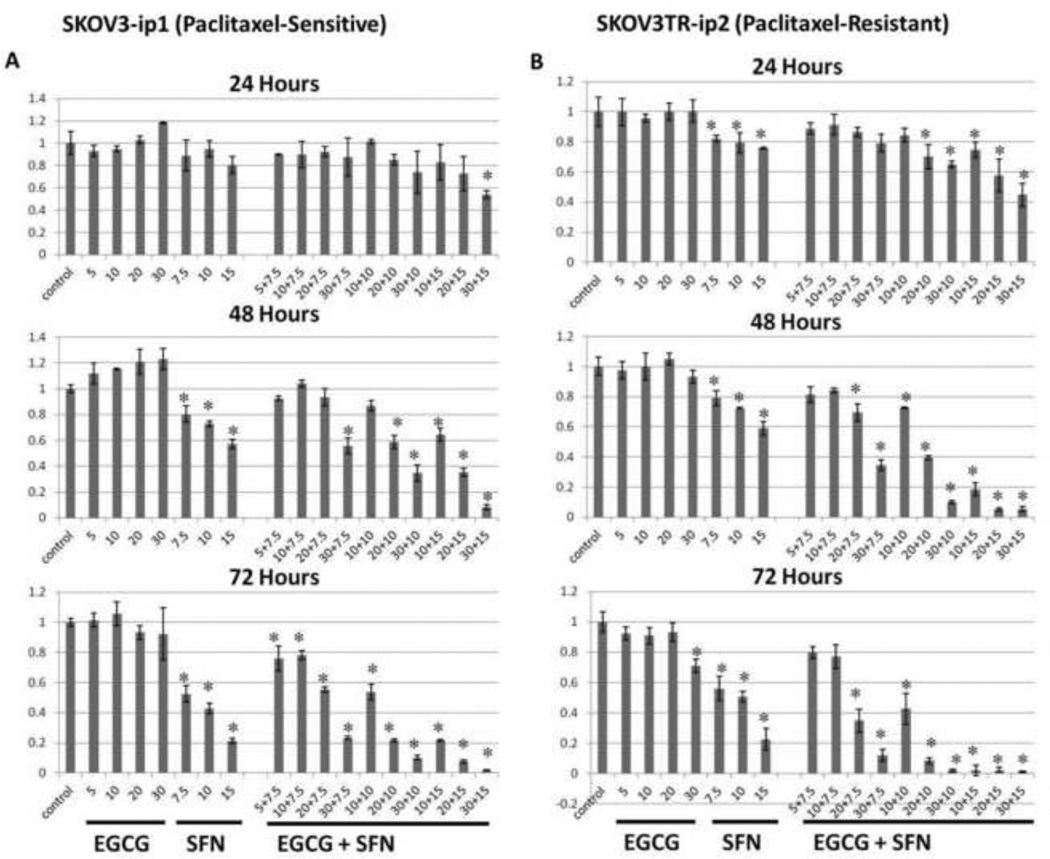
To determine the effective dose of EGCG and/or SFN on ovarian cancer cells, we first performed MTT assays to measure cell proliferation status. As shown in Fig.1, human paclitaxel-sensitive ovarian cancer SKOV3-ip1 (Figure 1A) and paclitaxel-resistant SKOV3TR-ip2 (Figure 1B) cells were treated with the indicated concentrations of EGCG and SFN for 24, 48 and 72 h. We observed a dose- and time-dependent cell growth inhibition with SFN or EGCG and SFN combination treatment both in SKOV3-ip1 and SKOV3TR-ip2 cells. These ovarian cancer cells are not responsive to EGCG treatment alone; however, 10 µM SFN is effective in inhibiting both cell types. Furthermore, the addition of 20 µM EGCG to 10 µM SFN exhibits much stronger inhibiting effect compared with 10 µM SFN alone.
Figure 1.
EGCG and SFN combination treatments decrease viability of SKOV3 cells. Viability of cells was measured through MTT assay. A. EGCG and SFN combination treatment decrease viability of SKOV3-ip1 cells. B. EGCG and SFN combination treatment decrease viability of SKOV3TR-ip2 cells. P<0.05.
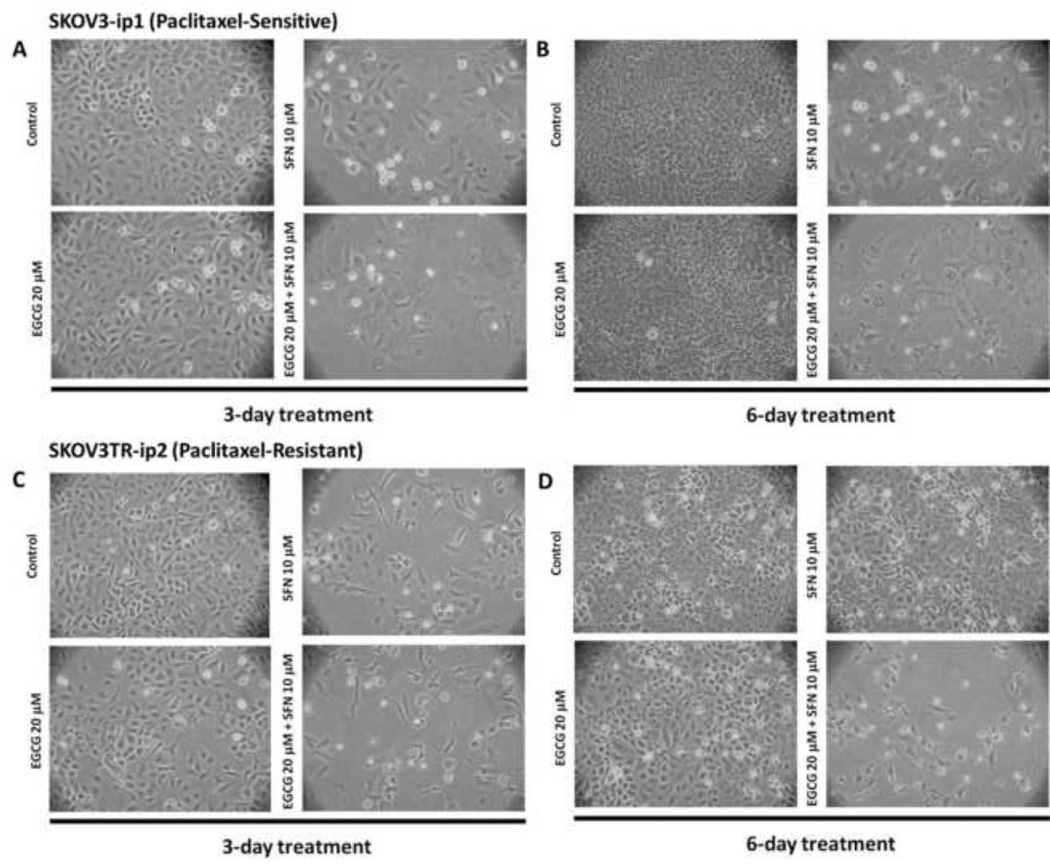
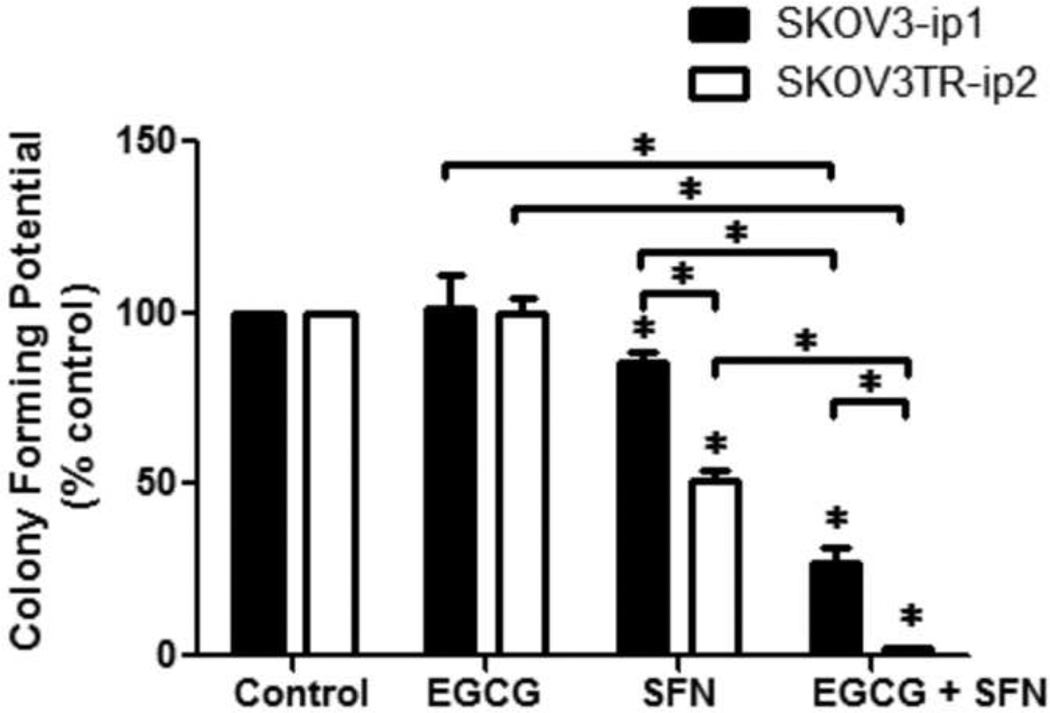
The morphology of human ovarian cancer cells under treatment were thus further studied with optimal dosage of 20 µM EGCG and 10 µM SFN combination treatment. As shown in Figure 2, SFN-treatments or EGCG and SFN combination treatments clearly inhibited cellular proliferation in these ovarian cancer cells. We have also performed colonogenic assays with 20 µM EGCG and 10 µM SFN combination treatments. We found that treatment with SFN significantly reduces SKOV3-ip1 and SKOV3TR-ip2 cell proliferation at doses of 10 µM (Figure 3). Furthermore, EGCG and SFN combination treatments have an even stronger inhibiting effect on these ovarian cancer cells. Perhaps most importantly, the inhibiting effect is particularly strong in paclitaxel-resistant ovarian cancer cells (SKOV3TR-ip2) (Figure 3).
Figure 2.
Morphology of ovarian cancer cells after treatments observed under phase contrast microscope. Cells were visualized at 100× magnification. A. SKOV3-ip1 3 day treatment. B. SKOV3-ip1 6 day treatment. C. SKOV3TR-ip2 3 day treatment. D. SKOV3TR-ip2 6 day treatment.
Figure 3.
EGCG and SFN combination treatment can inhibit growth of ovarian cancer cells m colonogenic assays. SFN (10 µmol/L) treatment alone can significantly inhibit colony formation compared with control. EGCG (20 µmol/L) and SFN (10 µmol/L) combination treatment can significantly inhibit colony formation compared with SFN treatment alone. The inhibition effect of EGCG and SFN combination treatment or SFN alone treatment is significantly stronger in paclitaxel-resistant ovarian cancer cells (SKOV3TR-ip2) than in sensitive cells (SKOV3-ip1). * P<0.05.
Arrest of SKOV3-ip1 and SKOV3TR-ip2 cells in G2/M and S phase by EGCG and SFN
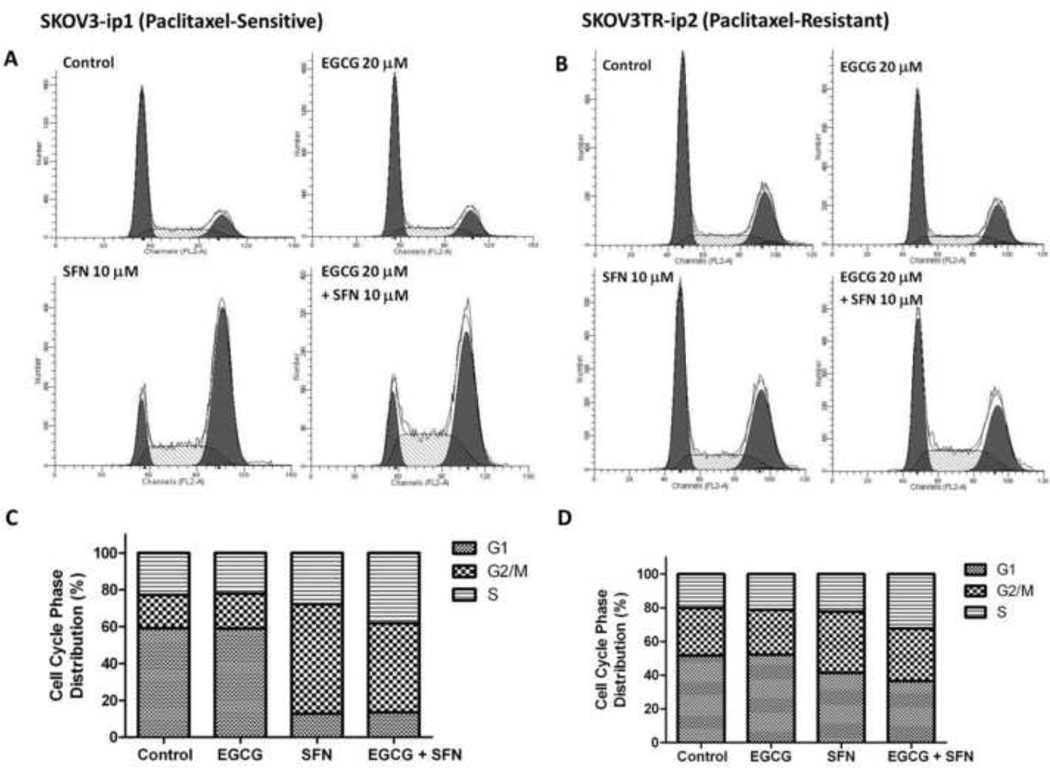
To determine the potential mechanisms by which EGCG and SFN inhibit viability of ovarian cancer cells, cell cycle phase distribution of SKOV3 cells before and after treatment was analyzed by flow cytometry following PI staining. As shown in Figure 4, SFN treatment alone can arrest cells in G2/M phase, while EGCG and SFN combination treatment led to an arrest cells in both G2/M and S phases.
Figure 4.
EGCG and SFN treatment can arrest SKOV3-ip1 and SKOV3TR-ip2 cells at G2/M and S phase. A, C. SKOV3-ip1 cells were treated with EGCG (20 µmol/L) or SFN (10 µmol/L) for 2 days. B, D. SKOV3TR-ip2 cells were treated with EGCG (20 µmol/L) or SFN (10 µmol/L) for two days. The first peak represents cells in G1 phase, the second peak represents cells in G2/M phase, and the area between the two peaks represents S phase. The histograms were obtained using PI staining followed by flow cytometry analysis.
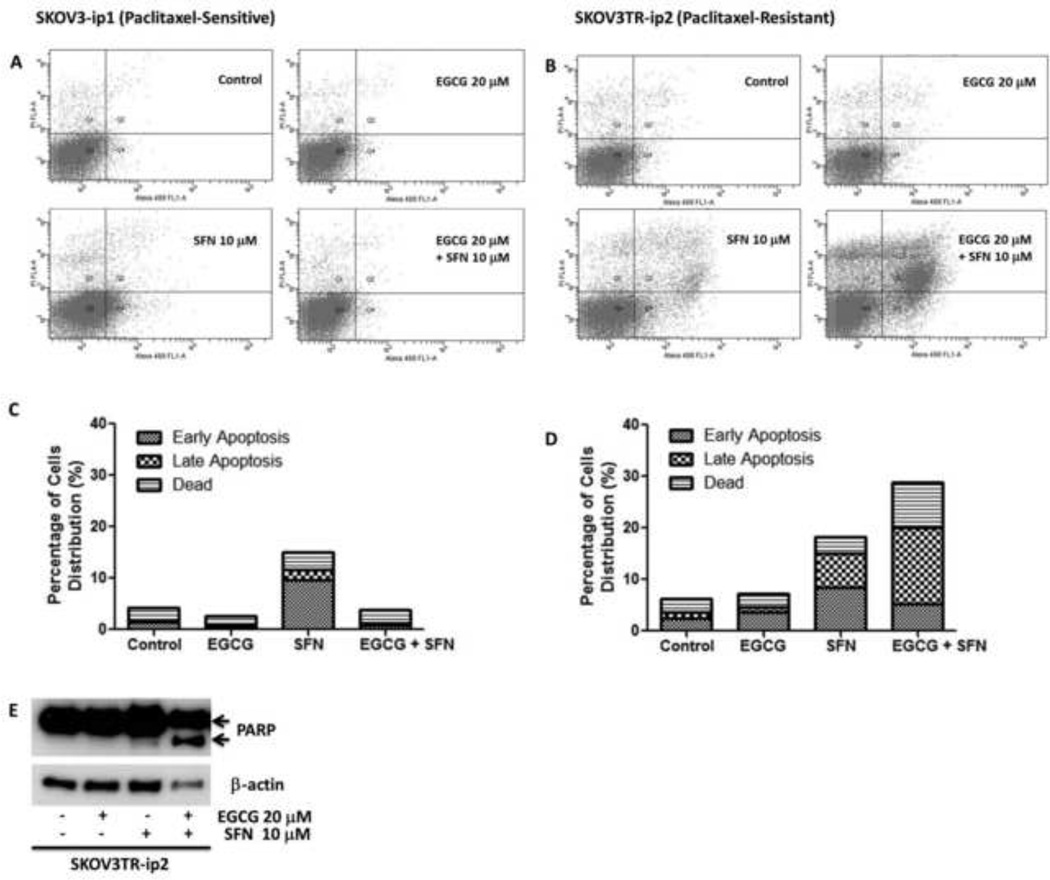
EGCG and SFN can induce apoptosis of paclitaxel-resistant ovarian cancer cells
Suppression decrease in cell viability can be achieved by inhibiting cell cycle progression or by inducing cellular apoptosis [16, 17]. To further determine if EGCG and/or SFN treatment also induce cellular apoptosis, percentages of apoptotic cells in SKOV3 cells before and after treatment were analyzed by flow cytometry following staining with Annexin V-FITC and PI. Apoptotic cells were significantly increased in both the late phase (upper right panel) and the early phase (lower right panel). Treatment of SKOV3TR-ip2 cells with SFN resulted in a marked induction of apoptosis at both the early (8.5%) and late (6.6%) stages of apoptosis (Figure 5B and D). Cleaved PARP protein was also noted to be enhanced in response to EGCG and SFN combination treatment (Figure 5E). These observations suggest that SFN induces cell apoptosis in SKOV3TR-ip2 cells, and EGCG can significantly potentiate the apoptosis induction.
Figure 5.
SFN can induce apoptosis of SKOV3TR-ip2 cells, and EGCG can potentiate the effect of SFN on apoptosis induction. A, C. Effect of EGCG and SFN treatment on apoptosis of SKOV3-ip1 cells. B, D. Effect of EGCG and SFN treatment on apoptosis of SKOV3TR-ip2 cells. E. Cleaved PARP has been increased in response to EGCG and SFN combination treatment in SKOV3TR-ip2 cells. Cells were treated with 20 µmol/L EGCG or 10 µmol/L SFN for 6 days. The cytograms were obtained by Annexin V and PI staining.
Induction of DNA damage response in SKOV3-ip1 and SKOV3TR-ip2 cells by EGCG and SFN
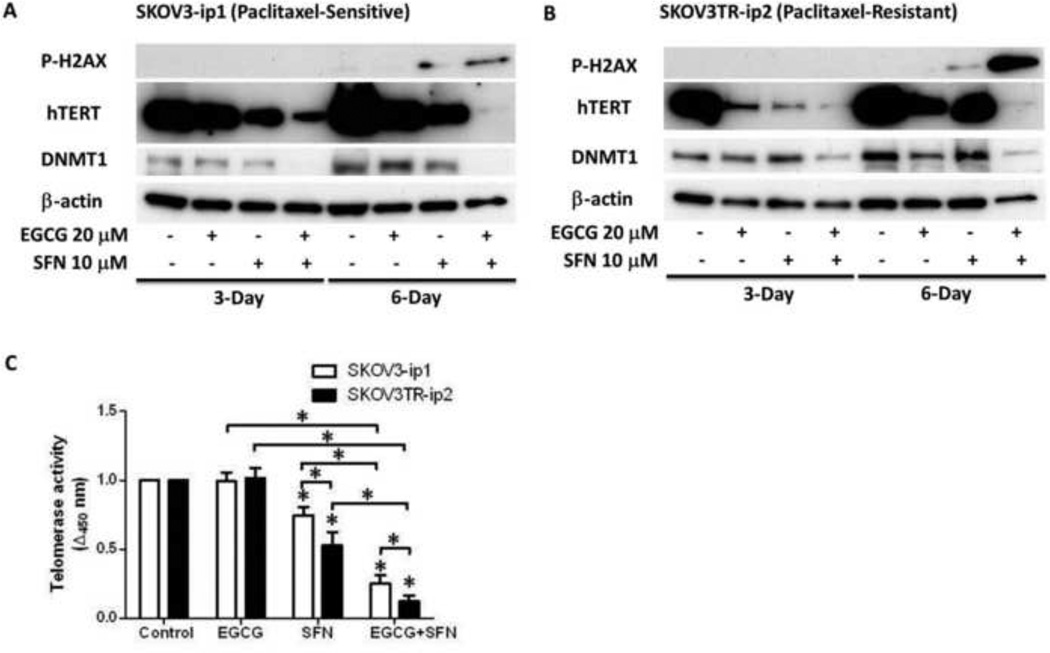
To reveal mechanisms behind EGCG and SFN-mediated G2/M and S phase arrest and apoptosis of ovarian cancer cells, the level of the DNA damage marker phosphor-H2AX was analyzed (Fig. 6A, B) since SFN has been indicated to cause DNA damage in a number of cancer cells [18, 19]. Compared with vehicle and each drug alone, DNA damage response with the combination of EGCG and SFN was significantly increased in both SKOV3-ip1 and SKOV3TR-ip2 (Fig. 6A, B).
Figure 6.
EGCG and SFN combination treatment can cause DNA damage and decrease expression level of hTERT and DNMT1. A. SFN treatment alone can cause DNA damage in SKOV3-ip1 cells, while EGCG can enhance the DNA damage induction effect of SFN. EGCG and SFN combination can decrease the expression levels of hTERT and DNMT1. B. SFN treatment alone can cause DNA damage in SKOV3TR-ip2 cells, while EGCG can enhance the DNA damage induction effect of SFN. EGCG and SFN combination can decrease the expression level of hTERT and DNMT1. C. SFN treatment alone can significantly inhibit telomerase activity compared with control. EGCG and SFN combination treatment can significantly inhibit telomerase activity compared with SFN treatment alone. The inhibition effect of EGCG and SFN combination treatment or SFN alone treatment is significantly stronger in paclitaxel-resistant ovarian cancer cells (SKOV3TR-ip2) than in sensitive cells (SKOV3-ip1). Cells were treated with 20 µmol/L EGCG or 10 µmol/L SFN. * P<0.05. These figures are representative of three experiments.
EGCG and SFN can decrease hTERT and DNA methyltransferase 1 (DNMT1) expression in SKOV3 cells
To explore the mechanism of the effect of EGCG and SFN, the expression level of hTERT and epigenetic modification enzyme, DNMT1, were analyzed. These genes have previously been noted to be under epigenetic regulation [20]. As shown in Figure 6A, B, hTERT expression level is down-regulated after EGCG and SFN combination treatment. Furthermore, telomerase activity assay indicates that SFN can decrease telomerase activity in both cell types; EGCG can further potentiate the inhibiting effect of SFN on hTERT (Figure 6C). Additionally, the expression level of DNMT1 was also decreased by EGCG and SFN combination treatment.
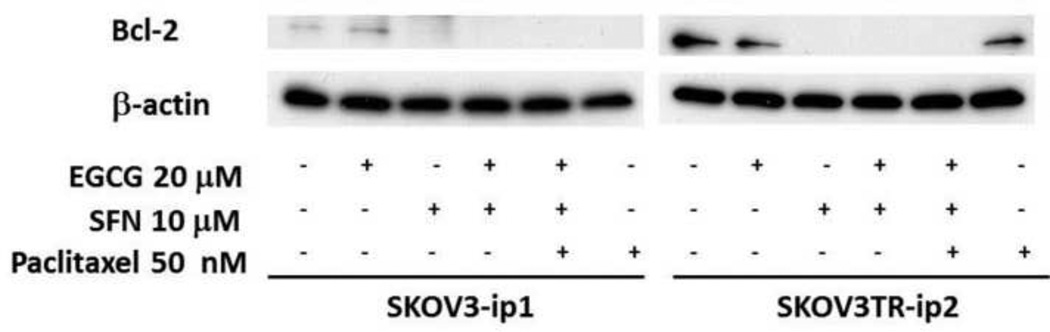
SFN can decrease Bcl-2 level in SKOV3 cell lines
To target the paclitaxel resistance problem of ovarian cancer, paclitaxel was also incorporated into the treatment; and the expression of a critical anti-apoptosis molecule, Bcl-2, was examined. As shown in Figure 7, paclitaxel can decrease Bcl-2 level in SKOV3-ip1 cells, while it fails to decrease Bcl-2 level in paclitaxel-resistant SKOV3TR-ip2 cells. EGCG has no apparent effect on Bcl-2 levels in both cell types, while SFN can decrease Bcl-2 levels in both paclitaxel-sensitive and resistant- cells. Real time PCR indicates no alteration on the mRNA level of Bcl-2 (data not shown), suggesting the expression regulation occurs at the protein level.
Figure 7.
Bcl-2 expression is regulated by paclitaxel and SFN in ovarian cancer cells. Paclitaxel alone can decrease Bcl-2 in paclitaxel-sensitive ovarian cancer cells but fails to decrease Bcl-2 in paclitaxel-resistant ovarian cancer cells. SFN can decrease Bcl-2 in both paclitaxel-sensitive and –resistant ovarian cancer cells. Ovarian cancer cells were treated with 50 nmol/L paclitaxel, 20 µmol/L EGCG and 10 µmol/L SFN for 3 days.
Discussion
Since high grade serous epithelial ovarian cancers account for most of the mortality from ovarian cancer, we selected human epithelial ovarian cancer SKOV3 cells as a model for this study. These cells were isolated from the ascitic fluid of a patient with ovarian serous adenocarcinomas of Grade 2/3. To evaluate efficacy in a drug resistant model, we used both paclitaxel-sensitive (SKOV3-ip1) and paclitaxel-resistant (SKOV3TR-ip2) ovarian cancer cells for analyses. SKOV3-ip1 and its resistant counterpart SKOV3TR-ip2 is a pair of cell lines which have been well characterized as a proper model for study the paclitaxel resistance problem [15, 21]. The SKOV3-ip1 cell line was generated from ascites developed in nu/nu mouse by administering an intraperitoneal injection of SKOV3. SKOV3-ip1 is more invasive and metastatic compared with its parental line SKOV3. The SKOV3TR-ip2 cell line was generated by sequential exposure to increasing concentrations of chemotherapy. These two cell lines are p53- and p16 (INK4a)-null which is common in drug resistant cancer cells. Two strategies, including resensitization of paclitaxel-resistant ovarian cancer cells to paclitaxel and inhibition of paclitaxel-resistant ovarian cancer cells directly, have been tested for the potential of EGCG and SFN combination treatment as an effective solution to the paclitaxel resistance problem. We have demonstrated that SFN or EGCG and SFN combination treatment has a clear and dramatic apoptotic effect on paclitaxel-resistant ovarian cancer cells.
The goal of this study is to determine the effect of EGCG and SFN combination treatment on both paclitaxel-sensitive (SKOV3-ip1) and resistant (SKOV3TR-ip2) ovarian cancer cells and to reveal potential mechanisms responsible for the effect. Our results indicate for the first time that EGCG and SFN combination treatment can inhibit proliferation of ovarian cancer cells through cell cycle arrest and apoptosis induction within a 6 day period of treatment with relative low doses. We have also shown that SFN can inhibit the viability of these two ovarian cancer cell lines. Interestingly, EGCG and SFN combination treatments exhibit a much stronger inhibiting effect on paclitaxel-resistant ovarian cancer cells (SKOV3TR-ip2), suggesting the usefulness of combining EGCG and SFN for treatment in paclitaxel-resistant ovarian cancer patients. Previously, EGCG and SFN combination treatment has been shown to inhibit viability of HT-29 AP-1 human colon carcinoma cells [22], but its role in ovarian cancer, which confers high mortality in women, has not previously been explored and the mechanisms of action are unknown.
Progression through each phase of the cell cycle is regulated carefully to avoid proliferation or mitosis when adverse conditions exist. Cells can be arrested in G1, S and G2/M phases to prevent replication of damaged DNA or to prevent aberrant mitosis. p53 is required for cells to be arrested in G1 phase but not for S and G2/M phases. Our results indicate that SFN treatment can arrest most ovarian cancer cells in G2/M phase with a slight S phase arrest, while EGCG and SFN combination treatments arrest less cells at G2/M phase but more in S phase compared with SFN alone. The cell cycle arrest at G2/M and S phase is also consistent with the defective p53 status of SKOV3-ip1 and SKOV3TR-ip2 cells. Furthermore, apoptosis analysis revealed that EGCG can enhance the apoptosis in paclitaxel-resistant ovarian cancer cells while no enhancement was detected in paclitaxel-sensitive ovarian cancer cells. These results indicate EGCG and SFN combination treatment exerts its anti-cancer effect primarily through inhibiting cell cycle progression in paclitaxel-sensitive ovarian cancer cells, but mainly through induction of apoptosis in paclitaxel-resistant ovarian cancer cells.
hTERT is important in the survival of cancer cells, in part due to its role in elongating the telomeres of chromosomes which can solve the end replication problem. However, its extra-telomeric role has also been revealed in recent studies [23], which is probably more universal than its telomere elongation role in short-term effects as observed in this investigation. We found that the viability of ovarian cancer cells was inhibited within a short period of EGCG and SFN combination treatment. With consideration of the relatively short time frame of the results, an extra-telomeric role of hTERT is more reasonable in the effect mediated by EGCG and SFN combination treatment.
As the catalytic subunit of telomerase, which is the main rate-limiting subunit, expression of hTERT is strictly regulated. Numerous treatments against cancers have been developed based on the expression discrepancy of hTERT between normal somatic cells and cancer cells [24]. Recently, it has been shown that targeted inhibition of telomerase activity with the BIBR1532 small molecule telomerase inhibitor combined with chemotherapy synergistically eliminated ovarian cancer spheroid-forming cells which indicates targeting telomerase activity may be a promising strategy against ovarian cancer [25]. In this study, we show for the first time that EGCG and SFN combination treatment can down-regulate hTERT expression as evidenced by both western blotting and telomerase activity assay. Additionally, the coincidence of S phase arrest and reduced expression of hTERT is consistent with a previous investigation by Luo et al [26]. Furthermore, we described that DNMT1 expression was down-regulated in response to EGCG and SFN combination treatments, suggesting epigenetic mechanisms such as DNA methylation regulation may be involved in hTERT expression in ovarian cancer cells. Down-regulation of DNMT1 expression may further lead to hypomethylation of the first exon of hTERT which actually results in down-regulation of hTERT. This piece of data is consistent with what we have observed in the regulation of hTERT expression by SFN in MCF-7 and MDA-MB-231 breast cancer cells [20].
To reveal the mechanism of the effect of EGCG and SFN combination treatment on paclitaxel-resistant ovarian cancer cells, paclitaxel was also involved in the treatment. Bcl-2 has been indicated to play an important role in anti-apoptosis. Here in this study, we found that paclitaxel can decrease Bcl-2 levels in the paclitaxel-sensitive cell line (SKOV3-ip1), while fails to decrease Bcl-2 levels in the paclitaxel-resistant cell line (SKOV3TR-ip2), suggesting loss of down-regulation of Bcl-2 levels in response to paclitaxel may be a factor contributing to the resistance of SKOV3TR-ip2 to paclitaxel. This observation is consistent with a previous study that overexpression of Bcl-2 is associated with resistance to paclitaxel in multiple myeloma cells [27, 28]. Consistent with this observation, SFN, which has been shown to have a strong inhibition effect on both ovarian cancer cell types, can reduce Bcl-2 levels in both cell types.
The dose of EGCG (20 µM) and SFN (10 µM) adopted in this investigation is physiologically achievable. It has been demonstrated that the peak plasma concentration achievable with SFN in rats was 20 µM after oral administration which is higher than the dose we used here [29]. In addition, only 50 µM of SFN was toxic to HepG2 C8 cells, whereas SFN 10 µM was suboptimal in its efficacy [30]. Data from our previous study also indicates up to 40 µg/ml EGCG and 20 µM SFN combination treatment is not toxic to normal human breast cells [31]. Our results here also indicate that EGCG and SFN combination treatment is an effective way to minimize the toxic effect of applying each single agent alone.
In this study we have also shown that SFN alone can cause DNA damage after 6 days of treatment, while EGCG and SFN combination treatment can cause even more DNA damage to the ovarian cancer cells. This is in consistent with our cell cycle analysis results that SFN can arrest cells slightly in S phase while EGCG and SFN combination treatment can arrest more cells in S phase, indicating the DNA damage-causing role of SFN or EGCG and SFN combination treatment in cell cycle arrestment. It is possible that inhibition of telomerase contributes to induction of DNA damage response and leads to cell cycle arrest and apoptosis of ovarian cancer cells.
Conclusions
Our results indicate that SFN with EGCG can inhibit both paclitaxel-sensitive and -resistant ovarian cancer cells while exerting a much stronger inhibiting effect on the paclitaxel-resistant ovarian cancer cells through both G2/M phase cell cycle arrest and apoptosis induction. Mechanistic studies reveal for the first time that EGCG and SFN combination treatment can cause DNA damage and decrease hTERT expression, and SFN can decrease Bcl-2 expression in these ovarian cancer cells. This study provides support for the use of EGCG and SFN in cancer therapy trials for chemo-resistant ovarian cancer.
Highlights.
Epigallocatechin gallate (EGCG) plus sulforaphane (SFN) inhibit resistant cancer
This inhibition of resistant ovarian cancer cells causes G2/M arrest and apoptosis
EGCG and SFN treatment causes DNA damage to the ovarian cancer cells
These treatments also decrease hTERT and Bcl-2 expression in ovarian cancer cells
EGCG and SFN are effective in treating paclitaxel-resistant ovarian cancer cells
Acknowledgements
This work was supported by grant from the NIH (RO1 CA129415), the Norma Livingston Foundation and the American Institute for Cancer Research.
Abbreviations
- EGCG
epigallocatechin gallate
- SFN
sulforaphane
- PARP
poly(ADP-ribose) polymerase
- HDAC
histone deacetylase
- DMSO
dimethyl sulfoxide
- DNMT1
DNA methyltransferase 1
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- PI
propidium iodide
- PCR
polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Binns CW, Lee AH. Tea consumption and ovarian cancer risk: a case-control study in China. Cancer Epidemiol Biomarkers Prev. 2002;11:713–718. [PubMed] [Google Scholar]
- 3.Song YJ, Kristal AR, Wicklund KG, Cushing Haugen KL, Rossing MA. Coffee, tea, colas, and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:712–716. doi: 10.1158/1055-9965.EPI-07-2511. [DOI] [PubMed] [Google Scholar]
- 4.Nagle CM, Olsen CM, Bain CJ, Whiteman DC, Green AC, Webb PM. Tea consumption and risk of ovarian cancer. Cancer Causes Control. 2010;21:1485–1491. doi: 10.1007/s10552-010-9577-7. [DOI] [PubMed] [Google Scholar]
- 5.Mittal A, Pate M, Wylie R, Tollefsbol T, Katiyar S. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int J Oncol. 2004;24:703–710. [PubMed] [Google Scholar]
- 6.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–168. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- 8.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 9.Langouët S, Furge LL, Kerriguy N, Nakamura K, Guillouzo A, Guengerich FP. Inhibition of human cytochrome P450 enzymes by 1,2-dithiole-3-thione, oltipraz and its derivatives, and sulforaphane. Chem Res Toxicol. 2000;13:245–252. doi: 10.1021/tx990189w. [DOI] [PubMed] [Google Scholar]
- 10.Gamet Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 11.Bertl E, Bartsch H, Gerhäuser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 12.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 13.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 14.Huh SW, Bae SM, Kim YW, Lee JM, Namkoong SE, Lee IP, Kim SH, Kim CK, Ahn WS. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol Oncol. 2004;94:760–768. doi: 10.1016/j.ygyno.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Landen CN, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. Proc Natl Acad Sci U S A. 1997;94:2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 18.Sestili P, Paolillo M, Lenzi M, Colombo E, Vallorani L, Casadei L, Martinelli C, Fimognari C. Sulforaphane induces DNA single strand breaks in cultured human cells. Mutat Res. 2010;689:65–73. doi: 10.1016/j.mrfmmm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 20.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steg AD, Katre AA, Bevis KS, Ziebarth A, Dobbin ZC, Shah MM, Alvarez RD, Landen CN. Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol Cancer Ther. 2012;11:1587–1597. doi: 10.1158/1535-7163.MCT-11-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair S, Hebbar V, Shen G, Gopalakrishnan A, Khor TO, Yu S, Xu C, Kong AN. Synergistic effects of a combination of dietary factors sulforaphane and (-) epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm Res. 2008;25:387–399. doi: 10.1007/s11095-007-9364-7. [DOI] [PubMed] [Google Scholar]
- 23.Lai SR, Cunningham AP, Huynh VQ, Andrews LG, Tollefsbol TO. Evidence of extra-telomeric effects of hTERT and its regulation involving a feedback loop. Exp Cell Res. 2007;313:322–330. doi: 10.1016/j.yexcr.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Li Y, Tollefsbol TO. Strategies targeting telomerase inhibition. Mol Biotechnol. 2009;41:194–199. doi: 10.1007/s12033-008-9117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng E, Taylor B, Ray A, Shevde LA, Rocconi RP. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol Oncol. 2012;124:598–605. doi: 10.1016/j.ygyno.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y, Yi Y, Yao Z. Growth arrest in ovarian cancer cells by hTERT inhibition short-hairpin RNA targeting human telomerase reverse transcriptase induces immediate growth inhibition but not necessarily induces apoptosis in ovarian cancer cells. Cancer Invest. 2009;27:960–970. doi: 10.3109/07357900802491451. [DOI] [PubMed] [Google Scholar]
- 27.Gazitt Y, Rothenberg ML, Hilsenbeck SG, Fey V, Thomas C, Montegomrey W. Bcl-2 overexpression is associated with resistance to paclitaxel, but not gemcitabine, in multiple myeloma cells. Int J Oncol. 1998;13:839–848. doi: 10.3892/ijo.13.4.839. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Gazitt Y. Potentiation of dexamethasone-, paclitaxel-, and Ad-p53-induced apoptosis by Bcl-2 antisense oligodeoxynucleotides in drug-resistant multiple myeloma cells. Blood. 2003;101:4105–4114. doi: 10.1182/blood-2002-10-3067. [DOI] [PubMed] [Google Scholar]
- 29.Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong AN. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–271. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- 30.Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, Kong AN. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003;63:7520–7525. [PubMed] [Google Scholar]
- 31.Meeran SM, Patel SN, Li Y, Shukla S, Tollefsbol TO. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One. 2012;7:e37748. doi: 10.1371/journal.pone.0037748. [DOI] [PMC free article] [PubMed] [Google Scholar]