Abstract
Invariant NKT (iNKT) cells are a subset of T lymphocytes that recognize glycolipid antigens presented by the MHC class I-related protein CD1d. Activation of iNKT cells with glycolipid antigens such as the marine sponge-derived reagent α-galactosylceramide (α-GalCer) results in the rapid production of a variety of cytokines and activation of many other immune cell types. These immunomodulatory properties of iNKT cells have been exploited for the development of immunotherapies against a variety of autoimmune and inflammatory diseases but mechanisms by which activated iNKT cells confer disease protection have remained incompletely understood. Here, we demonstrate that glycolipid-activated iNKT cells cooperate with myeloid-derived suppressor cells (MDSCs) in protecting mice against the development of experimental autoimmune encephalomyelitis (EAE) in mice, an animal model for multiple sclerosis (MS). We showed that α-GalCer induced the expansion and immunosuppressive activities of MDSCs in the spleen of mice induced for development of EAE. Disease protection in these animals also correlated with recruitment of MDSCs to the central nervous system. Depletion of MDSCs abrogated the protective effects of α-GalCer against EAE and, conversely, adoptive transfer of MDSCs from α-GalCer-treated mice ameliorated passive EAE induced in recipient animals. The cytokines GM-CSF, IL-4 and IFN-γ, produced by activated iNKT cells, and inducible nitric oxide synthase, arginase-1 and IL-10 produced by MDSCs, contributed to these effects. Taken together, our findings have revealed cooperative immunosuppressive interactions between iNKT cells and MDSCs that might be exploited for the development of improved immunotherapies for MS and other autoimmune and inflammatory diseases.
Introduction
Invariant natural killer T (iNKT) cells are a subset of T lymphocytes that express a semi-invariant T cell receptor (TCR), Vα14-Jα18/Vβ8.2-, 7 or -2 in mice or Vα24-Jα18/Vβ11 in humans, multiple activation markers such as CD25, CD69 and CD122, and markers of the natural killer (NK) cell lineage such as NK1.1 and Ly49 (1-4). The TCR of iNKT cells recognizes glycolipid antigens presented by the MHC class I-related protein CD1d (2). Following TCR engagement, iNKT cells can rapidly mount an effector T cell response characterized by production of a wide variety of cytokines and cytotoxicity, making them a crucial component of the innate immune response (5, 6). During this activation process, iNKT cells also interact with other cells of the immune system such as NK cells, dendritic cells (DCs), B cells and conventional T cells, resulting in their activation (7, 8). Activation of iNKT cells can also influence the differentiation of Th cells, typically skewing the response towards Th2 cytokine production (9). Owing to their capacity to produce a mixture of cytokines and to interact with a variety of other cell types of the immune system, iNKT cells can either promote or suppress immune responses in different diseases (10). They confer natural immunity against cancer, provide protective immunity to various infectious agents, generally play a suppressive role during autoimmune responses and graft-versus-host disease, and contribute to the development of allergic airway disease, contact hypersensitivity, hepatitis, ischemia-reperfusion injury, atherosclerosis and obesity-associated disease (9, 11, 12). Because iNKT cells display such a wide variety of versatile functions, they are often referred to as the ‘Swiss army knife of the immune system’ (6).
Multiple sclerosis (MS) is a chronic inflammatory disease that causes demyelination of the neurons in the central nervous system (CNS), resulting in muscular weakness, loss of coordination, and speech and visual disturbances, ultimately leading to paralysis. Experimental autoimmune encephalomyelitis (EAE) in mice is an experimental model frequently employed to study MS. Our laboratory has previously shown that iNKT cell activation by their prototypical agonist, α-galactosylceramide (α-GalCer), prevents the development of EAE (13, 14), and similar results have been obtained by other research groups (15-17). However, the mechanism of this protection remains ill-defined (7, 9). The available evidence suggests that the cytokines IL-4 and IL-10, which are secreted by iNKT cells and are critical for the protective effects of α-GalCer against EAE (13), influence Th cell differentiation, leading to an overall deviation towards Th2 cytokine production and suppression of pathogenic T cell responses. Surprisingly, however, recent studies have also provided evidence for a critical role of IFN-γ in these activities of α-GalCer (18, 19), suggesting that Th2 cell deviation may not be the dominant mechanism of protection involved. These paradoxical findings led us to search for additional cellular targets for the cytokines released by iNKT cells that could play a critical role in protection against EAE. We focused on myeloid-derived suppressor cells (MDSCs), a regulatory cell population that exhibits strong T cell suppressive functions in vitro and in vivo (20-24).
MDSCs are a major population of cells that regulate immune responses during inflammation. These cells accumulate in various organs such as spleen, liver, bone marrow and peripheral blood in response to inflammatory conditions such as cancer, autoimmunity, sepsis, trauma and chronic infections (20-24). They have a strong capacity to suppress T cell responses in vitro and in vivo, a property that has been exploited to prevent the generation of autoimmunity. MDSCs are a heterogeneous population of cells that consist of myeloid progenitor cells and immature myeloid cells. In normal mice, these cells make up to 1-2% of the spleen and 20-30% of the bone marrrow (21, 22). Under steady state conditions, these cells lack immune suppressive properties and quickly differentiate into various myeloid cells such as macrophages, DCs and granulocytes. Only during pathologic conditions do these cells expand vigorously, retain their immature phenotype, and acquire immunosupressive functions (21, 22, 25). MDSCs in mice are mainly defined as cells that co-express CD11b and Gr1 markers. The immunosuppressive functions of these cells have been associated with high expression of enzymes involved in arginine metabolism, including inducible nitric oxide synthase (iNOS or NOS2) and arginase-1 (Arg1) (23, 26).
Here we demonstrate that α-GalCer-mediated protection against EAE involves the immunosuppressive functions of MDSCs. Our findings provide evidence for cooperative interactions between iNKT cells and MDSCs that could be exploited for the development of immunotherapies for MS and other autoimmune diseases.
Methods
Mice
C57BL/6 (B6), B6.129P2-Nos2tm1Lau/J (iNOS−/−) mice were purchased from The Jackson Laboratory, and B6.129P2-Il10tm1Cgn/J (IL-10−/−) mice were obtained from Dr. Fang Yan (Vanderbilt University School of Medicine, Nashville, TN), who originally obtained these animals from The Jackson Laboratory. B6.CD1d−/− mice were generated in our laboratory (27). All breeders and experimental mice were housed in specific pathogen-free conditions in compliance with guidelines from the Institutional Animal Care and Use Committee at Vanderbilt University.
Reagents
α-GalCer was purchased from Funakoshi Co. Mouse CD1d monomers were obtained from the National Institutes of Health Tetramer Facility (Emory University, Atlanta, GA) and were used to prepare α-GalCer/CD1d-tetramers. Fluorescently labeled antibodies against TCR-β, B220, CD11b, iNOS, CD11c, F4/80, MHC class II, CD8α, CD4, Ly6G, Ly6C, Gr1, CD69, CD25, CD45.1, CD45.2, NK1.1, IFN-γ, IL-4, TNF-α, IL-17A, CD1d and BrdU were obtained from BD Biosciences or eBioscience, and fluorescently labeled antibodies against CD204 and CD206 were from Serotec. Recombinant GM-CSF was obtained from Peprotec, neutralizing anti-IL-10 and anti-GM-CSF antibodies from eBioscience, neutralizing anti-IFN-γ and anti-IL-4 antibodies from BD Biosciences, complete Freund adjuvant (CFA) from Difco, pertussis toxin from Calbiochem, and gemcitabine, ionomycin (ION), phorbol myristate acetate (PMA), BrdU and S-(2-boronoethyl)-L-cysteine (BEC) from Sigma.
Induction and evaluation of active EAE (aEAE)
Female mice at 8 to 10 weeks of age were immunized s.c. with 200 μg of MOG35–55 peptide (MOGp; BioMatik Corporation) emulsified in CFA on days 0 and 7. Mice also received 250 ng of pertussis toxin i.p. on days 0 and 2 and 2 μg of α-GalCer or vehicle on days 0, 4, and 7 by i.p. injection. Clinical symptoms were monitored every other day after the first immunization. The clinical scores were graded as follows: 0, no disease; 1, tail limpness; 2, hind-limb weakness; 3, hind-limb paralysis; 4, forelimb weakness; 5, quadriplegia; and 6, death. Mice exhibiting a score of 5 were sacrificed and retained that score for the remainder of the experiment (11, 14).
Induction of passive EAE (pEAE)
Passive EAE was induced in B6 mice as described before (28). Briefly, active EAE was induced in B6 mice as described above. At day 11, draining lymph nodes (LN) were harvested and single cell suspensions were prepared. As indicated in some experiments, splenocytes depleted of CD11b+ cells were used. The cells were stimulated with 50 μg/ml MOGp at 1×107 cells/ml in 10 ml RPMI 1640 medium containing 10% FBS (complete medium), in a 25 cm2 flask. After 72 hrs, dead cells were removed by Histopaque®-1077 (Sigma) and the live cells were washed and resuspended in PBS for adoptive transfer. Recipient mice (5-6 weeks old) were irradiated sublethally at 400 rads to generate a lymphopenic environment 6 hrs prior to injection of MOGp-specific T cells. Live cells from the restimulation cultures were injected i.v. at 5×106-107 cells per mouse. The clinical scores were graded similarly to aEAE.
Adoptive transfer of MDSCs to modulate EAE
MDSCs generated during aEAE were used to prevent pEAE in an adoptive transfer experiment as described before in a different model (29). Mice induced with aEAE were treated with vehicle or α-GalCer and sacrificed 11 days later. MDSCs were enriched from the spleen on the basis of CD11b and Gr1 marker expression using magnetic sorting. Sequential purification of Gr1+ and CD11b+ cells was carried out by positive sorting using an anti-phycoerythrin multisort kit (Miltenyi Biotec) according to the manufacturer’s protocol. MDSCs thus obtained were pulsed with MOGp at 100 μg/ml for 1 hr before adoptive transfer. pEAE was then induced as described above by transfer of MOGp-specific T cells in irradiated mice and peptide-pulsed MDSCs (5×106) were transferred on days 1, 4 and 9 after transfer of MOGp-specific T cells.
Gemcitabine-mediated depletion of MDSCs during EAE
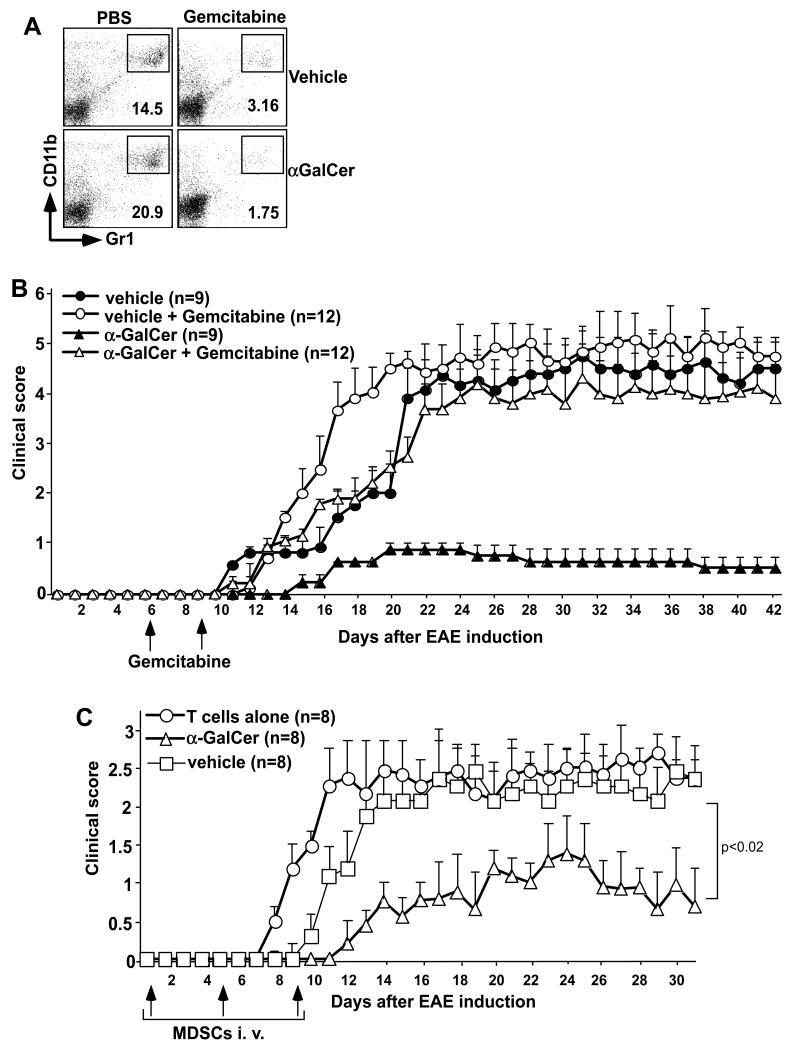
MDSCs were depleted (or functionally inactivated) from mice as described before in a cancer model (30, 31). Briefly, B6 mice were induced with aEAE as above and gemcitabine was administered on days 6 and 9 at 20 mg/kg body weight. Such treatment routinely depleted >80% of MDSCs in the spleen (Fig. 4A).
FIGURE 4.
Effects of MDSC depletion or adoptive transfer on EAE. (A,B) EAE was induced in B6 mice and these animals were treated with vehicle or α-GalCer. Mice were then treated at days 6 and 9 after EAE induction with PBS or gemcitabine at 20 mg/kg body weight by i.p. injection. (A) At day 11 after EAE induction, spleen cells were analyzed for the prevalence of MDSCs. Note that >80% depletion was observed. (B) EAE clinical scores were determined as described in Methods. (C) Mice induced with aEAE were treated with vehicle or α-GalCer and sacrificed 11 days after EAE induction. MDSCs were enriched from the spleen using magnetic sorting, pulsed with MOGp at 100 μg/ml for 1 hr and 5×106 cells were adoptively transferred into B6 mice on days 1, 4 and 9 following induction of pEAE with MOGp-specific T cells. EAE clinical scores were determined as described for active EAE. Combined data for two experiments with 4 mice in each group are shown.
Generation of bone marrow chimeras
B6 (CD45.2) mice were lethally irradiated (1000 cGy) and 6 hours CD1d̄ later wild-type (CD45.1) and (CD45.2) bone marrow cells (107 cells) mixed at a 1:1 ratio were injected into these animals. The mice were housed in nude housing conditions for 6-8 weeks before use in experiments. Transfer of control wild-type (CD45.1) bone marrow cells alone was used to confirm >96% chimerism in the recipient mice (data not shown).
Enrichment of MDSC subsets and evaluation of nuclear morphology
Female B6 mice were induced with aEAE as described above and at day 11, CD11b+ cells were positively sorted by magnetic sorting (Miltenyi Biotec). The cells were stained with anti-Ly6G and -Ly6C antibodies, and Ly6GhiLy6C− granulocytic (G)-MDSCs and Ly6G−Ly6Chi monocytic (M)-MDSCs were sorted by preparative grade flow cytometry using a FACSAria III cell sorter (BD Biosciences) after doublets were excluded from the analyses. More than 96% of pure cells were routinely obtained. The G-MDSCs (103 cells) were subjected to cytospin for analysis of nuclear morphology. The cytospun cells were air dried and stained with HEMA3® staining kit (Fisher Diagnostics) according to the manufacturer’s protocol.
T cell suppression assay
We employed an in vitro assay in which MOGp-specific, IL-17A-producing T cells were used to determine the immunosuppressive activity of MDSCs. aEAE was induced in B6 mice and 11 days later, splenic CD4+ T cells were purified by anti-CD4 microbeads and DCs by anti-CD11c microbeads using positive sorting (Miltenyi Biotec). 2×105 CD4+ T cells were activated with 2×104 DCs in the presence or absence of 50 μg/ml MOGp for 3 days in complete medium in a U-bottomed 96-well plate. MDSCs purified from mice treated with vehicle or α-GalCer during induction of EAE were added to these cultures at varying ratios to prevent the activation of MOGp-specific T cells. IL-17A production was measured in the culture supernatant by ELISA as a readout of Th17 cell activation. In some experiments neutralizing anti-cytokine antibodies or their respective isotype controls were used to restore the activity of MOGp-specific T cells. A chemical inhibitor of arginase activity, BEC, was used in cultures of MDSCs and Th cells in HL-1 medium containing 1% FBS (BioWhittaker).
Flow cytometry
Single cell suspensions of the spleen and LN were prepared and stained with fluorescently labeled monoclonal antibodies as described (11). In all experiments, dead cells were excluded from the analysis by electronic gating. The iNKT cell population was identified as B220− tetramer+TCRβ+ cells. For intracellular staining, cells were stimulated with 1 μg/ml ION and 50 ng/ml PMA in the presence of Golgi Plug™ (BD Pharmingen) for 5 hrs. Intracellular cytokine staining was performed with Cytofix/Cytoperm reagents (BD Pharmingen) according to the manufacturer’s protocol. MDSCs were identified based on simultaneous expression of CD11b and Gr1 markers. CD11b+Ly6Ghi cells were considered G-MDSCs and CD11b+Ly6Chi cells were considered M-MDSCs (32). Data acquisition was performed on a FACSCalibur instrument (BD Biosciences) and data were analyzed by FlowJo software (Tree Star, Inc.).
ELISA assay
A standard sandwich ELISA was performed to measure mouse IL-17A, IFN-γ and IL-4, using OptEIA™ kit (BD Biosciences). For detection, streptavidin-horse radish peroxidase (HRP) conjugate (Zymed Laboratories) was used, and the color was developed with the substrate 3,3′,5,5′-tetramethylbenzidine (DAKO) in the presence of H2O2.
ELISPOT assay
ELISPOT assays were carried out to determine the frequency of MOGp-specific Th17 cells in the spleen and LN. EAE was induced in B6 mice and these animals were treated with vehicle or α-GalCer. Mice were sacrificed at time points indicated in figure legends, and CD4+ T cells and CD11c+ DCs were purified. ELISPOT plates were coated with IL-17A cytokine capture antibodies (eBioscience) under sterile conditions. 5×105 CD4+ T cells were activated with 5×104 DCs and 50 μg/ml MOGp in duplicate wells coated with cytokine capture antibodies for 24 hrs. The plates were washed and developed further according to the manufacturer’s protocol (eBioscience). The spots were counted on an immunospot image analyzer (Cellular Technology).
Isolation of CNS infiltrates
Spinal cords were dissected from the vertebral column by perfusion of PBS, cut into small pieces and then digested with collagenase D (Roche) at 0.2 mg/ml along with 0.05% DNase I (Sigma) for 45 min at 37°C. The tissue was mechanically disrupted with the help of a 21-gauge needle and spun down. The cells were resuspended in 40% Percoll™ (GE Healthcare) and underlayed with 70% Percoll™. After centrifugation at 700g for 15 min, the cells at the interface were collected, washed and stained with antibodies against CD4 or surface markers of MDSCs.
In vivo BrdU labeling
BrdU (1 mg/dose) was injected into B6 mice twice a day for two days starting from day 7 after EAE induction. Five hrs after the last BrdU injection, the mice were sacrificed and splenic cells were stained with markers of MDSCs as described above. The cells were fixed with 2% paraformaldehyde for 2 hrs at room temperature and permeabilized with Cytoperm reagents (BD Pharmingen). The cells were treated with DNase I solution in PBS (2.5 kU/ml) for 30 min at 37°C and washed again with Cytoperm buffer. The cells were then stained with isotype control or anti-BrdU antibodies for 45 min at 4°C. The cells were analyzed by flow cytometry as described above.
Quantitative real-time PCR
RNA was purified using Trizol® reagent (Invitrogen) according to the manufacturer’s instructions, including a DNase I digestion step. cDNA was prepared from 1-2 μg RNA using ThermoScript™ Reverse Transcriptase system (Invitrogen). Real-time PCR was performed using Sybr green master mix (Biorad) and 20 ng of cDNA per well in duplicates for each sample on a CFX96 real-time PCR machine (Biorad). The CT values were collected for the housekeeping gene β-actin and the genes of interest during the log phase of the cycle. Gene of interest levels were normalized to β-actin for each sample and compared with the values obtained for the test sample. Each gene was compared with every normalizer in succession and the ΔCt was calculated (ΔCT = CT Gene of interest – CT Normalizer). The normalized expression (ΔΔC(t)) of the gene of interest was calculated using the CFX manager software (Biorad). The following PCR primers were used: IL-12 P35: forward, 5′-AAATGAAGCTCTGCATCCTGC-3′ and reverse, 5′-TCACCCTGTTGATGGTCACG-3′; IL-23 P19, forward, 5′-TATCCAGTGTGAAGATGGTTGTG-3′ and reverse, 5′-CACTAAGGGCTCAGTCAGAGTTG-3′; iNOS: forward, 5′-CTGGCTCGCTTTGCCACGGA-3′ and reverse, 5′-TGCGACAGCAGGAAGGCAGC-3′; IL-6: forward, 5′-GAGGATACCACTCCCAACAGACC-3′ and reverse, 5′-AAGTGCATCATCGTTGTTCATACA-3′; TGFβ; forward:, 5′-TGACGTCACTGGAGTTGTACGG-3′ and reverse, 5′-GGTTCATGTCATGGATGGTGC-3′; IL-10, forward, 5′-ATGCTGCCTGCTCTTACTGACTG-3′ and reverse, 5′-CCCAAGTAACCCTTAAATCCTGC-3′; Arg1: forward, 5′-ACAGTCTGGCAGTTGGAAGCATC-3′ and reverse, 5′-GGGAGTCCCCAGGAGAATCCT-3′; β-actin: forward: 5′-TACAGCTTCACCACCACAGC-3′ and reverse, 5′-AAGGAAGGCTGGAAAAGAGC-3′.
Statistical analyses
Statistical significance between the groups was determined by application of an unpaired 2-tailed Student’s t test using Graphpad Prism software. A P value less than 0.05 was considered significant.
Results
Pathogenic IL-17A-producing T cells in MOGp-challenged and α-GalCer-treated mice are actively suppressed
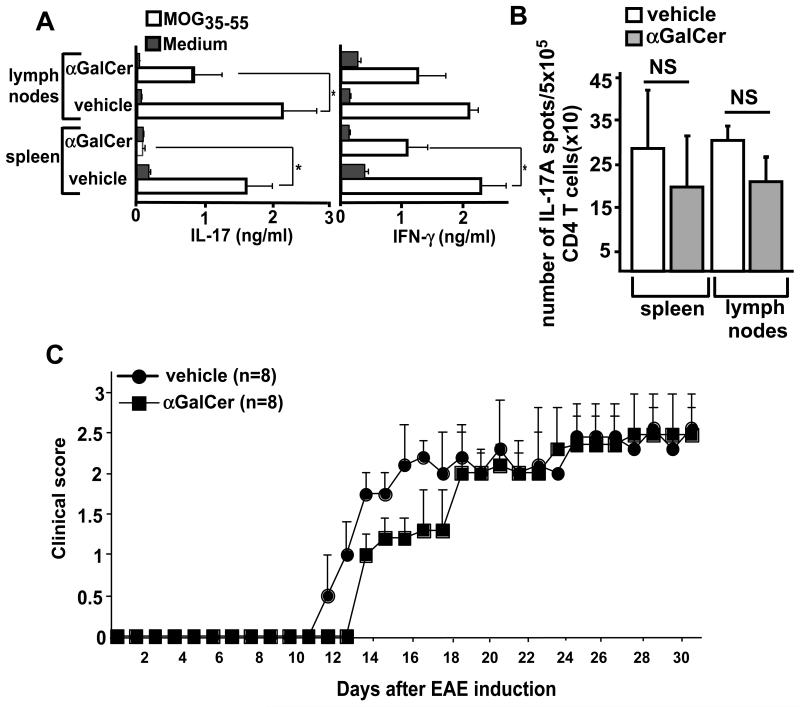
It is now generally accepted that Th17 cells specific for the immunizing antigen are required for the progression of EAE disease in B6 mice (33-37). Consequently, mice deficient in IL-23, a cytokine that is required for expansion of Th17 cells, are resistant to the development of EAE (38). In sharp contrast, IL-12 and IFN-γ, which are critical for the development and effector functions of Th1 cells, might in some situations exhibit a protective role during the development of EAE, as mice deficient in either of these cytokines exhibit exacerbated and fulminant EAE (34). We therefore measured production of the cytokines IL-17A and IFN-γ by MOGp-stimulated splenocytes or LN cells from vehicle- or α-GalCer-treated mice at day 11 after EAE induction. Compared with vehicle-treated mice, splenocytes and to a lesser extent LN cells from α-GalCer-treated mice produced profoundly reduced levels of IL-17A (Fig. 1A). IFN-γ production in these cultures was less affected by α-GalCer treatment (Fig. 1A), which is consistent with the non-pathogenic role of IFN-γ-producing T cells in this EAE model. However, we considered that the pathogenic activities of IL-17A-producing MOGp-specific T cells might be actively suppressed. Therefore, we measured the frequency of IL-17A-producing CD4+ T cells among purified CD4+ T cells stimulated with MOGp-pulsed DCs by ELISPOT assay. We found that such cells were induced in the spleen and LN of vehicle- and α-GalCer-treated mice at comparable levels (Fig. 1B). To further evaluate the pathogenic potential of MOGp-specific T cells in α-GalCer-treated mice, we tested the capacity of CD4+ T cells from these animals to induce disease in a passive EAE (pEAE) model. For this purpose, splenic cells from MOGp-challenged mice treated with vehicle or α-GalCer were depleted of CD11b+ cells and restimulated with MOGp in vitro for 3 days. The T cells obtained from these cultures were then used to induce pEAE in sublethally irradiated B6 mice. Results showed that T cells obtained from vehicle- and α-GalCer-treated mice were equally competent in causing EAE disease in recipient mice, suggesting that Th17 cells with pathogenic potential are induced in the spleen of α-GalCer-treated mice but that these cells are rendered incapable of causing EAE, possibly by accessory cells that keep the pathogenic T cells in check.
FIGURE 1.
Pathogenic T cells in MOGp-challenged and α-GalCer-treated mice are actively suppressed. B6 mice were induced with EAE, treated with vehicle or α-GalCer and sacrificed 11 days later. (A) Splenocytes or draining LN cells were stimulated in vitro with or without MOGp, and the cytokines IL-17A and IFN-γ in the supernatant were measured 3 days later by ELISA. *p<0.05. (B) To determine the frequency of MOGp-specific Th17 cells by ELISPOT assay, CD4+ T cells were purified and stimulated with DCs and MOGp in anti-IL-17A coated ELISPOT plates for 24 hrs. The plates were developed and the spots were counted on an automated immunosplot image analyzer. NS, not significant. (C) Splenocytes derived from vehicle- or α-GalCer-treated mice were depleted of CD11b+ cells and were stimulated with MOGp for 72 hrs in vitro. Live cells (5×106) were adoptively transferred into irradiated B6 mice to induce passive EAE. EAE clinical scores were determined as described in Methods.
α-GalCer promotes expansion of MDSCs early after EAE induction
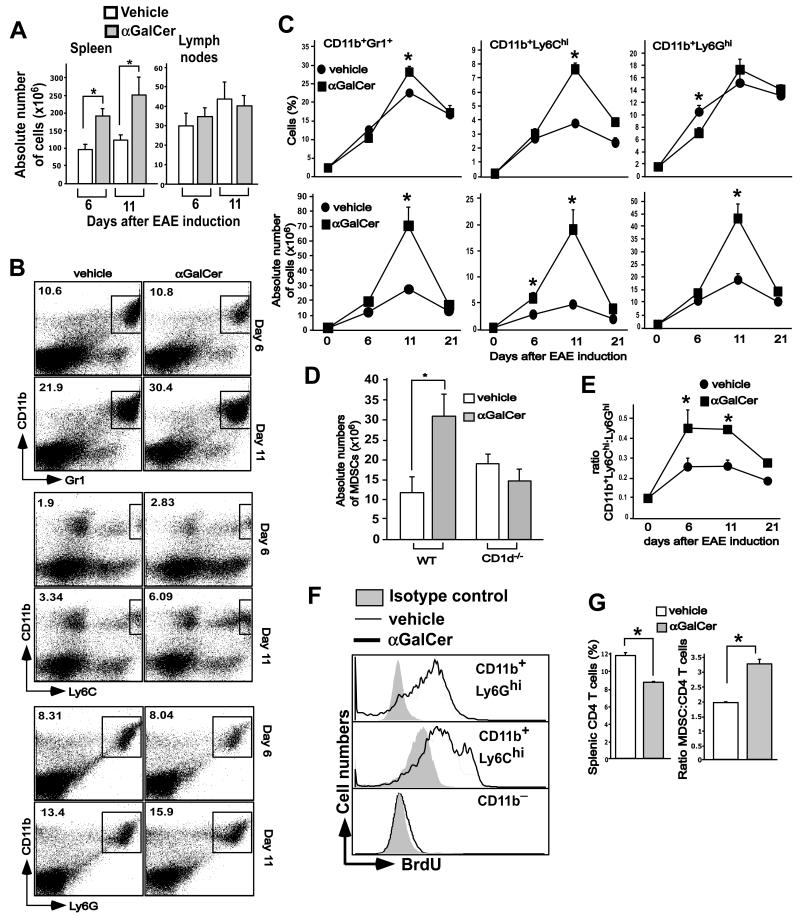
We previously observed splenic hyperplasia in α-GalCer- but not vehicle-treated animals between days 6-12 after EAE induction (Fig. 2A). As these effects of α-GalCer might provide a clue to the underlying mechanism of protection, we analyzed the cells that contributed to this transient splenomegaly. We found that cells co-expressing the CD11b and Gr1 markers, a surface phenotype characteristic of MDSCs (22, 39), contributed significantly to the splenic cellularity (Fig. 2B and C). At day 6 after EAE induction, the percentages of MDSCs in vehicle- and α-GalCer-treated mice were comparable, but absolute numbers of these cells were slightly increased in α-GalCer-treated mice as compared with vehicle-treated mice (Fig. 2B and C). At day 11 after EAE induction, at the peak of the pathogenic T cell response, both the percentage and the absolute numbers of MDSCs were significantly increased in α-GalCer-treated mice as compared with vehicle controls. By day 21, such expanded MDSCs in α-GalCer-treated mice had returned to levels found in vehicle-treated mice (Fig. 2B and C). The cellularity of the draining LN was comparable between the groups (Fig. 2A). The capacity of α-GalCer to cause expansion of MDSCs was dependent on iNKT cells, as such expansion was not observed in CD1d−/− mice (Fig. 2D).
FIGURE 2.
α-GalCer-mediated expansion of MDSCs in EAE-induced mice. B6 mice were induced with EAE and treated with vehicle or α-GalCer. (A) Splenic and lymph node cellularity in vehicle- or α-GalCer-treated mice induced for development of EAE. B6 mice were induced with EAE and treated with vehicle or α-GalCer. At the indicated times the cellularity of spleen and lymph nodes was determined. The data presented are the mean ± SEM of 6 mice per group and representative of at least 3 individual experiments. *p<0.05. (B) At the indicated times after EAE induction, splenocytes were stained with anti-CD11b and -Gr1 antibodies, or with anti-CD11b, -Ly6G and -Ly6C antibodies, as indicated. CD11b+Ly6GhiLy6C− cells represent G-MDSCs and CD11b+Ly6G−Ly6Chi cells represent M-MDSCs. (C) The absolute numbers of CD11b+Gr1+ MDSCs, CD11b+Ly6Ghi G-MDSCs and CD11b+Ly6Chi M-MDSCs were determined based on splenic cellularity. *p<0.05. (D) Absolute numbers of MDSCs in wild-type and of MDSCs in wild-type and mice at day 11 after EAE induction. *p<0.05. (E) The ratio of absolute numbers of CD11b+Ly6Chi M-MDSCs to CD11b+Ly6G+ G-MDSCs was calculated at various time points after EAE induction. *p<0.05. (F) BrdU was injected in mice starting from day 7 after EAE induction, twice a day for two days. The cells were stained with anti-CD11b, -Ly6G and -Ly6C antibodies followed by staining with anti-BrdU antibody or its isotype control antibody. Representative plots of 3 independent experiments are shown. (G) Percent of CD4+ T cells and the ratio of MDSCs to CD4+ T cells are depicted. The results are plotted as the mean ± SEM of 6 mice and are representative of 5 independent experiments. *p<0.05.
CD11b+Gr1+ MDSCs can be further divided into subpopulations based on their expression of the Ly6G and Ly6C surface markers (22, 39). CD11b+Ly6GloLy6Chi cells are monocyte-like cells and are therefore called monocytic MDSCs (M-MDSCs), whereas CD11b+Ly6GhiLy6Clo cells have a granulocyte-like polymorphonuclear phenotype and are therefore termed granulocytic MDSCs (G-MDSCs) (22, 39). We evaluated these MDSC subpopulations in vehicle- and α-GalCer-treated mice after EAE induction. We observed expansion of both subpopulations of MDSCs with preferential expansion of M-MDSCs in α-GalCer-treated mice (Fig. 2B and C). Thus, the ratio of M-MDSCs to G-MDSCs was higher in α-GalCer-treated than vehicle-treated mice (Fig. 2E). Consistent with these findings, in vivo BrdU-labeling experiments during EAE induction showed preferential labeling of MDSCs, particularly M-MDSCs, while CD11b− cells containing mostly T and B cells incorporated little BrdU (Fig. 2F). We further found that the increased expansion of MDSCs in α-GalCer-treated mice at day 11 after EAE induction correlated with reduced expansion of CD4+ T cells in these animals as compared with vehicle-treated mice (Fig. 2G). Consequently, the ratio of MDSCs to CD4+ T cells calculated based on percent of cells was much higher in α-GalCer- than vehicle-treated mice at day 11 after EAE induction (Fig. 2G).
Collectively, these results demonstrate that α-GalCer-activated iNKT cells promote expansion of MDSCs early after EAE induction.
Infiltration of CD4+ T cells and MDSCs into the central nervous system
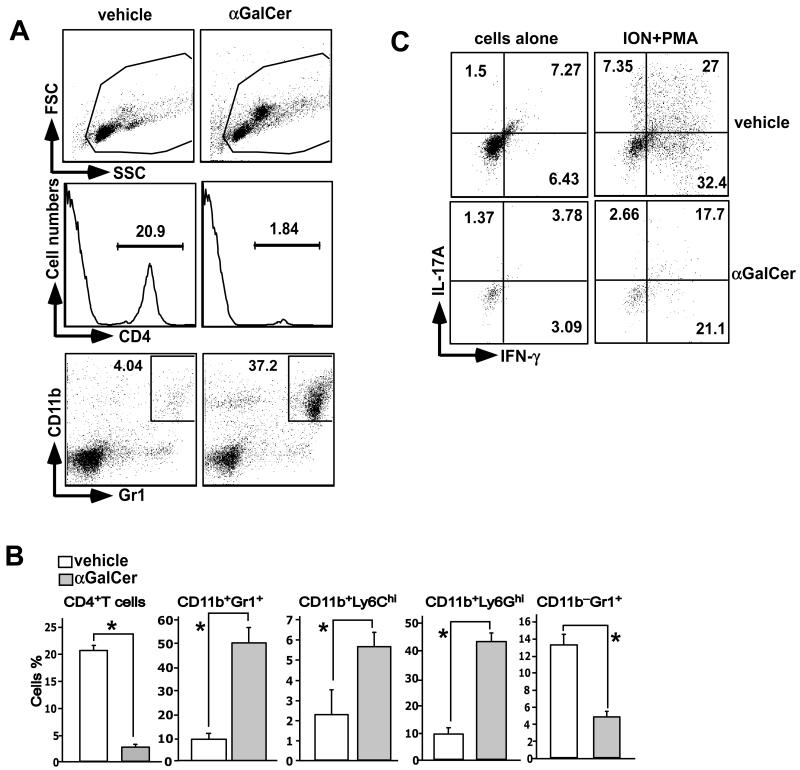
Next, we assessed infiltration of cells into the central nervous system (CNS) at day 21 when vehicle-treated, MOGp-challenged mice had fulminant EAE disease with hind limb paralysis (clinical score ~4), but α-GalCer-treated mice had few signs of EAE (clinical score ~1). We found that the percentage of CD4+ T cells was 10 times lower in α-GalCer-treated mice as compared with vehicle-treated mice (Fig. 3A and B). CD4+ T cells that infiltrated the spinal cord of α-GalCer-treated mice produced lower levels of IL-17A and/or IFN-γ than those in vehicle-treated mice (Fig. 3C). Interestingly, CD11b+Gr1+ MDSCs were found to infiltrate the spinal cords of α-GalCer-treated mice, which is consistent with the higher scattered profile of cells from α-GalCer-treated mice (Fig. 3A). We also found that mature CD11b−Gr1+ granulocytes were reduced in the spinal cord of α-GalCer-treated compared with vehicle-treated mice (Fig. 3B). These results suggest that MDSCs induced following iNKT cell activation infiltrate the CNS.
FIGURE 3.
Infiltration of MDSCs and CD4 T cells into the CNS. B6 mice were induced with EAE, treated with vehicle or α-GalCer, sacrificed 21 days later, and cells infiltrating the spinal cord were isolated. (A,B) Cells were stained with anti-CD4 antibodies, anti-CD11b and -Gr1 antibodies, or anti-CD11b anti-Ly6G and -Ly6C antibodies. Representative data are shown in (A). Note the change in the scatter profile of cells isolated from α-GalCer-treated mice (top panels), which is predominantly due to infiltration of MDSCs. A summary of the percentage of cells from 6 individual mice from 3 independent experiments is shown. *p<0.05. (C) Cells were stimulated with ionomycin (ION) plus PMA in the presence of Golgi Plug™ for 5 hrs. The cells were then surfaced-stained with anti-CD4 antibody followed by intracellular staining with anti-IL-17A and -IFN-γ antibodies. A representative of 3 experiments is shown.
Effects of MDSC depletion or adoptive transfer
Next we performed experiments to test whether selective depletion of MDSCs modulates protection against EAE conferred by iNKT cell activation. Selective depletion or functional inactivation of MDSCs can be achieved with the chemotherapeutic agent gemcitabine (30, 31, 40). To ensure that such treatment does not affect the pathogenic T cells, we treated animals at days 6 and 9 after EAE induction, at a dose (20 mg/kg body wt) that is much lower than that used for treating cancer (~100-120 mg/kg body wt) (30, 31). We found that such treatment consistently depleted >80% of MDSCs in both vehicle- and α-GalCer-treated mice (Fig. 4A). Treatment with gemcitabine for such a short time period specifically depleted MDSCs without noticeable effects on other cell populations in the spleen and LN when measured 12 days after EAE induction (Table I). The results showed that depletion of MDSCs by gemcitabine in vehicle-treated mice modestly exacerbated EAE disease (Fig. 4B), which is consistent with the documented regulatory role of these cells in EAE (21, 32, 41, 42). In α-GalCer-treated mice, MDSC depletion completely abrogated disease protection conferred by iNKT cell activation (Fig. 4B).
Table I. Effect of gemcitabine on MDSCs and other cells of the immune system.a.
| Vehicle + PBS |
Vehicle + Gemcitabine |
α-GalCer + PBS |
α-GalCer + Gemcitabine |
|
|---|---|---|---|---|
| LN CD11b+Gr1+cells | 1.175 ± 0.125 | 0.11 ± 0.013* | 2.02 ± 0.076 | 0.13 ± 0.027* |
| spleen CD11b+Gr1+ cells | 22.13 ± 0.436 | 2.61 ± 0.28* | 27.73 ± 1.325 | 1.63 ± 0.275* |
| LN NK cells | 1.32 ± 0.254 | 1.19 ± 0.245 | 0.83 ± 0.083 | 0.83 ± 0.04 |
| spleen NK cells | 2.96 ± 0.226 | 2.88 ± 0.361 | 2.49 ± 0.226 | 2.3 ± 0.086 |
| LN B cells | 53.85 ± 2.00 | 54.32 ± 3.29 | 53.9 ± 2.565 | 54.1 ± 3.57 |
| spleen B cells | 39.12 ± 0.961 | 40.27 ± 5.577 | 38.6 ± 4.12 | 33.85 ± 3.09 |
| LN CD4+ T cells | 18.95 ± 2.26 | 20.65 ± 2.364 | 19.82 ± 1.024 | 17.67 ± 0.757 |
| spleen CD4+ T cells | 15.32 ± 2.01 | 15.8 ± 1.277 | 15.92 ± 0.370 | 13.52 ± 1.084 |
| LN CD11b−Gr1+ cells | 7.7 ± 0.779 | 5.66 ± 0.581 | 6.42 ± 0.742 | 6.54 ± 0.374 |
| spleen CD11b−Gr1+ cells | 3.602 ± 0.103 | 3.167 ± 0.231 | 3.057 ± 0.381 | 3.45 ± 0.122 |
EAE was induced in B6 mice and these animals were treated with vehicle or α-GalCer. Mice were also treated at days 6 and 9 after EAE induction with PBS or gemcitabine at 20 mg/kg body weight by i.p. injection. At day 12, mice were sacrificed and the splenic and LN cells were stained with antibodies against MDSCs, NK cells, B cells, CD4+ T cells and neutrophils. The data presented are the mean ± SEM percentage of cells from 6 mice and are representative of 3 independent experiments.
p<0.05, compared with PBS treatment.
To complement the depletion studies, we next tested the capacity of MDSCs expanded during EAE to protect against induction of pEAE in an adoptive transfer model. The results showed that adoptive transfer of MDSCs derived from α-GalCer-treated mice significantly prevented induction of pEAE while no protection was observed with MDSCs derived from vehicle-treated mice (Fig. 4C).
Collectively, these results provide strong evidence that MDSCs induced in α-GalCer-treated mice exhibit immune suppressive functions and play a critical role in protection against EAE.
MDSCs suppress pathogenic T cells
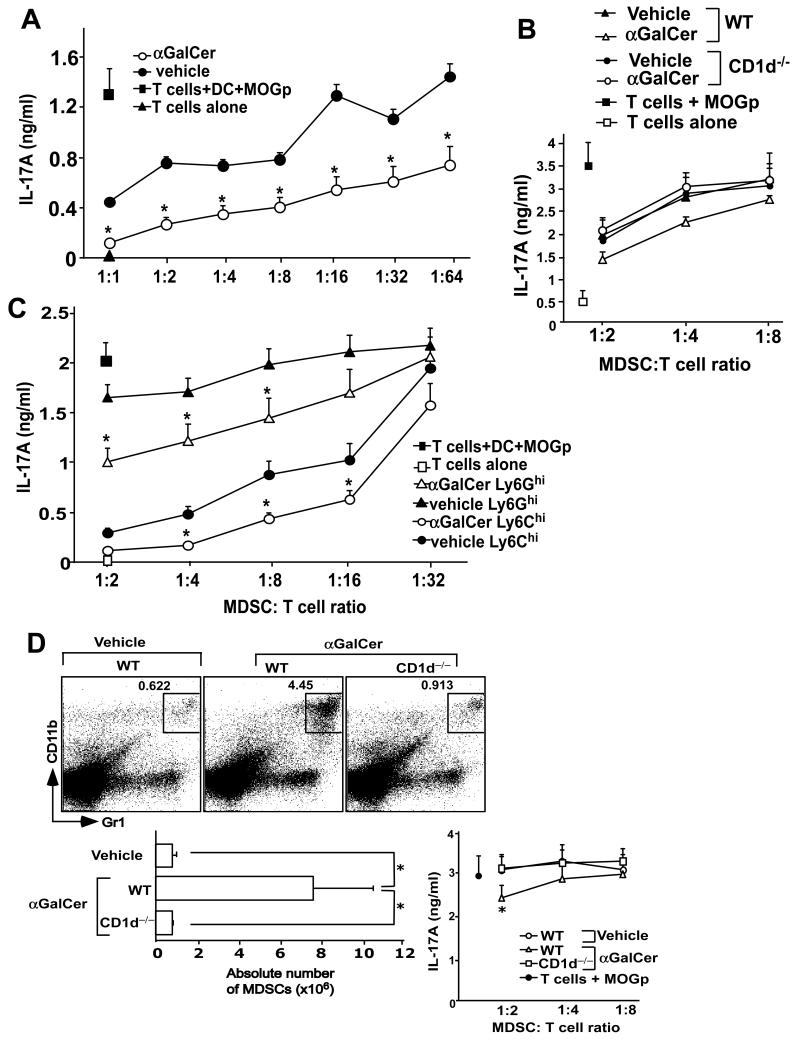
Next we tested the capacity of MDSCs derived from vehicle- or α-GalCer-treated mice to suppress MOGp-specific, IL-17A-producing CD4+ T cells ex vivo. Purified CD4+ T cells from MOGp-immunized mice were stimulated with DCs and MOGp in the presence of varying numbers of MDSCs isolated from MOGp-immunized mice treated with vehicle or α-GalCer. Results showed that MDSCs from α-GalCer-treated mice exhibited superior capacity to suppress pathogenic T cells (Fig. 5A). These effects of α-GalCer were absent in CD1d−/− mice, indicating that α-GalCer-mediated induction of suppressive MDSCs requires iNKT cells (Fig. 5B). Both M-MDSCs and G-MDSCs had such capacity to suppress IL-17A production by pathogenic T cells (Fig. 5C). These results indicated that α-GalCer-treated mice, compared with vehicle-treated mice, contain not only increased numbers of MDSCs but that these cells have superior capacity to suppress pathogenic T cells.
FIGURE 5.
Inhibition of MOGp-specific Th17 cells by MDSCs. CD4+ T cells were isolated at day 11 after EAE induction and were stimulated with DCs and MOGp in the presence of varying numbers of total MDSCs from wild-type mice (A), total MDSCs derived from wild-type or CD1d̄ mice (B), or G-MDSCs or M-MDSCs (C) derived from MOGp-challenged mice treated with vehicle or α-GalCer. IL-17A production in the culture supernatants was measured by ELISA. (D) Naïve B6 mice were injected i.p. with 2 μg α-GalCer at days 0, 4, and 7. At day 11 the percentage (top) and absolute numbers (bottom left) of MDSCs, and the ability of these cells to inhibit MOGp-specific Th17 cells (bottom right) was determined. The results are the mean ± SEM of 3 mice and are a representative of at least 3 experiments. *p<0.05.
We also tested whether induction of MDSCs by α-GalCer was limited to an inflammatory context such as CFA. For this purpose, we treated naïve B6 mice with α-GalCer at 0, 4 and 7 days, and tested the expansion and suppressive functions of MDSCs at day 11. Results showed that repeated α-GalCer treatment was able to expand MDSCs with superior suppressive functions, in an iNKT cell-dependent manner (Fig. 5D). These results suggest that expansion of MDSCs and acquisition of immune suppressive functions in these cells by activated iNKT cells can occur in the absence of an inflammatory context.
MDSCs in α-GalCer-treated mice are enriched for expression of immune suppressive markers
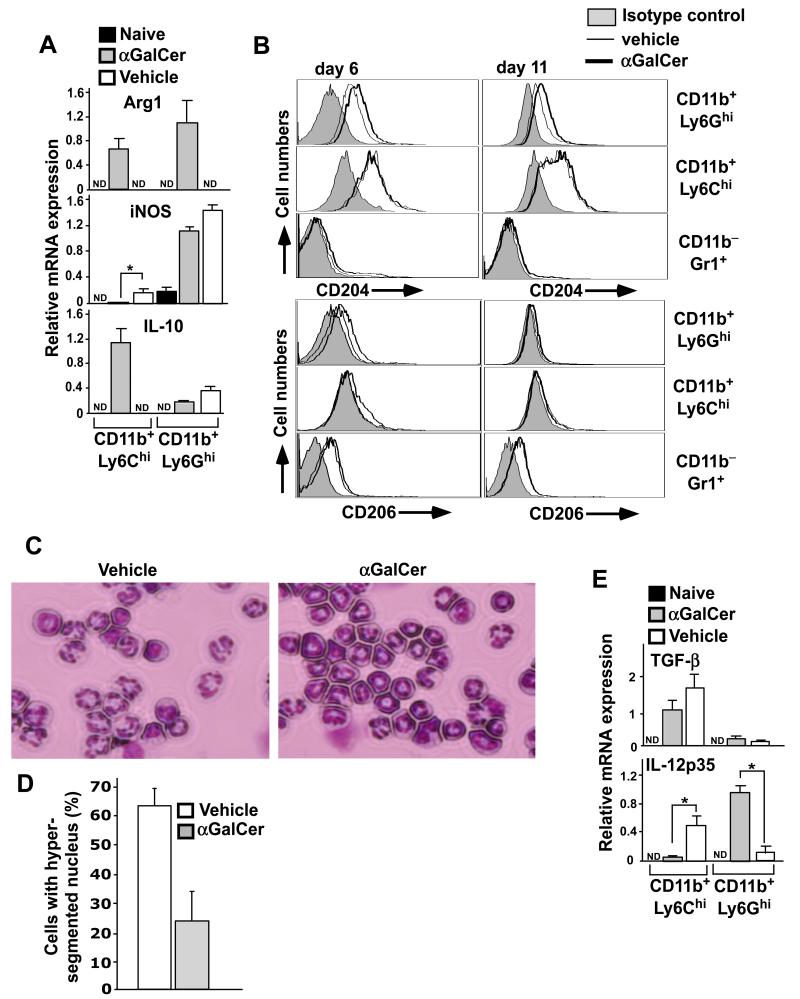
It has been shown that the immunosuppressive functions of MDSCs are associated with high expression of enzymes involved in arginine metabolism, including iNOS and Arg1 (23, 26). To understand the mechanism by which MDSCs from α-GalCer-treated mice exert superior immune suppressive activities, we measured mRNA expression levels of Arg1 and iNOS in M- and G-MDSCs derived from MOGp-immunized mice treated with vehicle or α-GalCer. We found that at day 11 after EAE induction, iNOS was expressed at high levels by G-MDSCs from both vehicle- and α-GalCer-treated mice, whereas M-MDSCs from both vehicle- and α-GalCer-treated mice expressed low levels of iNOS (Fig. 6A). Interestingly, both populations of MDSC from vehicle-treated mice lacked Arg1 expression, but its expression was induced at high levels in MDSCs from α-GalCer-treated mice (Fig. 6A).
FIGURE 6.
Expression of anti-inflammatory proteins by MDSCs from α-GalCer-treated mice. (A) At day 11 after EAE induction, G- and M-MDSCs were enriched by preparative flow cytometry and RNA was prepared. Relative mRNA levels of Arg1, iNOS and IL-10 were measured by real-time PCR assay. The mean ± SEM of 4 mice is shown. *p<0.05; ND, not detected. (B) At day 6 and 11 after EAE induction, splenocytes were stained with different combinations of anti-CD11b, -Gr1, -Ly6G, -Ly6C, -CD204 and -CD206 antibodies. Representative plots are shown from a total of 6 individual mice from two independent experiments. (C) G-MDSCs enriched from vehicle- or α-GalCer-treated mice were cytospun on slides, stained with HEMA3 staining kit and the nuclear morphology was examined (magnification: 40×). (D) Cells were prepared as in (C) and the number of cells containing hypersegmented nuclei was counted manually and plotted as percent of cells. Nearly 50 cells were counted from randomly chosen fields and a total of at least 300 cells were counted from each mouse. The data presented are the mean ± SEM of 2 mice and representative of 4 mice in each group. (E) Relative mRNA levels of IL-12 p40 and TGF-β were measured by real-time PCR assay as in (A). The mean ± SEM of 4 mice is shown. *p<0.05; ND, not detected.
Such high expression of Arg1 is characteristically observed in anti-inflammatory M2-type macrophages (43). Based on these results we measured additional markers such as IL-10, CD204 (43) and CD206 (44), associated with an M2-phenotype. IL-10 expression was barely detected in G-MDSCs in both groups of mice and in M-MDSCs from vehicle-treated mice (Fig. 6A). However, upon α-GalCer treatment, high expression of IL-10 was observed in M-MDSCs (Fig. 6A). Further, we found increased levels of CD204 expression on G-MDSCs in α-GalCer-treated mice compared with vehicle-treated mice at days 6 and 11 after EAE induction, whereas no significant differences were observed in M-MDSCs (Fig. 6B). CD206 expression was increased in both G- and M-MDSCs at day 6 after EAE induction in α-GalCer treated mice, and no expression was observed at day 11 after EAE induction in either experimental group (Fig. 6B).
G-MDSCs are often referred to as immature neutrophils and may adopt an N1 or N2 phenotype, analogous to the M1 or M2 phenotype of macrophages (45). It has been proposed that N1-type cells have a hypersegmented and lobular nuclear morphology, whereas N2-type cells have an immature nuclear morphology (45). We therefore enriched G-MDSCs from vehicle- or α-GalCer-treated mice at day 11 after EAE induction and determined their nuclear morphology after cytospin followed by Giemsa staining. The results showed that the majority of G-MDSCs derived from vehicle-treated mice exhibited a hypersegmented nuclear morphology that is reminiscent of the N1-like phenotype (Fig. 6C and D). In contrast, the majority of G-MDSCs isolated from α-GalCer-treated mice exhibited a ring-shaped nucleus (Fig. 6C and D), a phenotype that is typically seen in CD11b+Gr1+ immature cells of the bone marrow during steady state conditions (46), and suggestive of an N2-like phenotype. These results suggested that both populations of MDSCs from α-GalCer-treated mice are enriched in cells with a suppressive M2/N2-like phenotype.
We also measured mRNA expression levels of cytokines relevant to EAE, including IL-12, IL-23 and IL-6, and the inhibitory cytokine TGF-β in both populations of MDSCs. We found that IL-12p35 expression was greatly induced in G-MDSCs from α-GalCer-treated mice, while the opposite was observed for M-MDSCs (Fig. 6E). TGF-β expression was mainly restricted to M-MDSCs and its expression in these cells was only modestly upregulated in M-MDSCs from α-GalCer-treated mice (Fig. 6E). IL-23p19 and IL-6 mRNA were not detected in either MDSC population (data not shown).
Role of factors expressed by MDSCs and iNKT cells
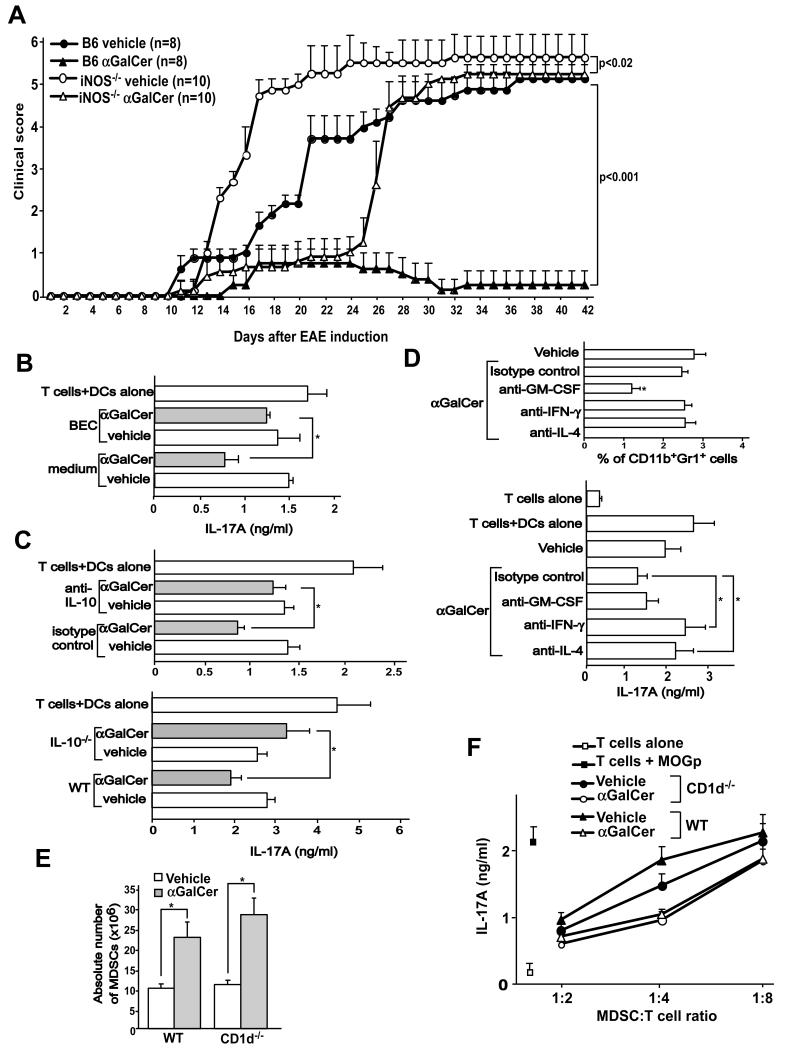
High expression of iNOS and Arg1 in MDSCs is associated with the immunosuppressive functions of these cells (23, 26). We therefore tested the role of these factors in the capacity of α-GalCer to protect mice against EAE. First, we tested the course of EAE in iNOS−/− mice treated with vehicle or α-GalCer. Vehicle-treated iNOS−/− mice developed fulminant EAE. α-GalCer treatment resulted in a delay in the onset of EAE in iNOS−/− mice but severity of disease was unaffected (Fig. 7A). Since we observed high expression of Arg1 in MDSCs derived from α-GalCer-treated mice, we employed the chemical inhibitor of this enzyme, BEC, in an attempt to restore the function of pathogenic T cells in an in vitro culture assay. We enriched CD4+ T cells from MOGp-immunized mice and stimulated these cells in vitro with DCs and MOGp in the presence of MDSCs derived from MOGp-challenged mice treated with vehicle or α-GalCer. The results showed significant restoration of IL-17A production by CD4+ T cells cultured with MDSCs from α-GalCer-treated mice in the presence of BEC, whereas such treatment had no effect on cultures with MDSCs from vehicle-treated mice (Fig. 7B). These results suggested that both enzymes involved in arginine metabolism, iNOS and Arg1, play an important role in the capacity of α-GalCer to protect mice against EAE.
FIGURE 7.
Role of iNOS, Arg1 and cytokines in the protective effects of α-GalCer against EAE. (A) B6 and iNOS−/− mice were induced with EAE and treated with vehicle or α-GalCer. The course of EAE disease was monitored and clinical scores were determined. (B) CD4+ T cells were isolated at day 11 after EAE induction in B6 mice and were stimulated with DCs and MOGp in the presence of MDSCs derived from mice treated with vehicle or α-GalCer at a MDSC:T cells ratio of 1:4 in HL-1 medium alone or medium containing BEC (50 μM). IL-17A production was measured in the culture supernatant by ELISA as a readout of Th17 cell activation. (C) CD4+ T cells were isolated at day 11 after EAE induction and were stimulated with DCs and MOGp in the presence of M-MDSCs derived from mice treated with vehicle or α-GalCer at a M-MDSC:T cell ratio of 1:4 in complete medium containing isotype control or anti-IL-10 antibody (10 μg/ml) (top panel). M-MDSCs derived from wild-type or IL-10−/− mice induced for EAE and treated with vehicle or α-GalCer were cultured as above at a M-MDSC:T cell ratio of 1:4 (bottom panel). IL-17A production was measured in the culture supernatant by ELISA as a readout of Th17 cell activation. *p<0.05. (D) B6 mice were treated with CFA and 3 days later splenocytes were activated with α-GalCer for 24 hours in the presence of anti-GM-CSF, -IL-4 or -IFN-γ neutralizing antibodies. The cultures were harvested and the percentage of MDSCs was determined (top panel). MDSCs from these cultures were enriched and were tested for their ability to inhibit MOGp-specific Th17 cells at a MDSC:T cell ratio of 1:2. The data presented are the mean ± SEM of 3 mice and representative of two individual experiments. (E) Mixed bone marrow chimeras were generated using a 1:1 mixture of bone marrow from wild-type (CD45.1) and CD1d−/− (CD45.2) bone marrow injected into B6 mice. After 6-8 weeks, mice were induced with EAE and treated with vehicle or α-GalCer. Wild-type and CD1d−/− hematopoietic cells were distinguished using the congenic markers and the absolute numbers of MDSCs at day 11 after EAE induction were determined. (F) Following induction of EAE and treatment with α-GalCer, wild-type and CD1d−/− MDSCs were enriched from the chimeric animals and their ability to suppress MOGp-specific Th17 cells was determined. The data presented are the mean ± SEM of 4 mice and representative of two individual experiments.
Because we found high expression of IL-10 mRNA in M-MDSCs of α-GalCer-treated mice (Fig. 6A), we tested whether anti-IL-10 antibodies restored the function of Th17 cells in vitro. Results showed that anti-IL-10 antibodies significantly restored IL-17A production by MOGp-specific T cells in cultures with M-MDSCs derived from α-GalCer-treated mice. Anti-IL-10 antibodies had no effect on IL-17A production in cultures containing M-MDSCs from vehicle-treated mice (Fig. 7C, top panel) or G-MDSCs derived from either treatment group (data not shown). Further, M-MDSCs derived from IL-10−/− mice were significantly impaired in their capacity to suppress pathogenic Th17 cells in vitro (Fig. 7C, bottom panel). These results are consistent with a role of IL-10 in the immunosuppressive functions of MDSCs in α-GalCer-treated mice.
To further understand the mechanism of MDSC activation in α-GalCer-treated mice, we next assessed the role of cytokines, including IL-4, IFN-γ and GM-CSF, produced by iNKT cells, in inducing immunosuppressive functions in MDSCs. For this purpose, we treated naïve B6 mice with CFA and stimulated splenocytes from these animals 3 days later in vitro with α-GalCer for 24 hours in the presence of neutralizing antibodies against these cytokines. MDSCs were then enriched and tested for their capacity to suppress pathogenic Th17 cells. Results showed that anti-GM-CSF antibodies significantly reduced numbers of MDSCs in this culture system but had no effect on the suppressive functions of these cells (Fig. 7D). In contrast, anti-IFN-γ and anti-IL-4 antibodies did not affect MDSC numbers but significantly impaired the suppressive activities of these cells (Fig. 7D).
Finally, we assessed the contribution of direct contact between iNKT cells and MDSCs for the generation of functionally suppressive MDSCs in α-GalCer-treated mice. For this purpose, we generated mixed bone marrow chimeras, using bone marrow from wild-type and CD1d−/− donor mice and wild-type B6 recipient mice. CD1d−/− iNKT cells in such animals can develop by interacting with CD1d expressed by wild-type thymocytes (47) (Fig. 7E). We then induced EAE in these animals, sacrificed them 11 days later, and compared the capacity of wild-type and CD1d−/− MDSCs to suppress pathogenic Th17 cells in vitro. The results showed that α-GalCer induced similar expansion of wild-type and CD1d−/− MDSCs in these animals (Fig. 7E). Further, wild-type and CD1d−/− MDSCs enriched from α-GalCer- or vehicle-treated chimeric mice using congenic markers were equally effective in suppressing pathogenic Th17 cells (Fig. 7F). Collectively, these results suggest that cytokines secreted by iNKT cells play a critical role, whereas direct contact between iNKT cells and MDSCs may not be required for the induction of suppressive MDSCs by α-GalCer.
Discussion
Glycolipid-activated iNKT cells produce a wide variety of cytokines and interact with a number of other cell types of the immune system, permitting these cells to regulate immune responses in different disease conditions (10, 12). Prior studies have suggested a role for several subsets of the myeloid lineage, including tolerogenic DCs (48) and suppressive macrophages (49) in iNKT cell-mediated protection against EAE. Here, we provide strong evidence for cooperation between iNKT cells with MDSCs, a heterogeneous population of immature myeloid cells with potent immunosuppressive properties, in protection against autoimmunity in the CNS conferred by the iNKT cell agonist α-GalCer.
Activation of iNKT cells in mice induced for EAE resulted in the expansion of both subpopulations of MDSCs that possess superior capacity to suppress pathogenic T cells. Because 70-80% of MDSCs in these animals were G-MDSCs, it is likely that this MDSC subset plays a dominant role in preventing autoimmunity in our model. The pathogenic T cell suppressive function of MDSCs in α-GalCer-treated mice is consistent with the expression of MHC class II molecules by MDSCs, which permits these cells to actively interact with antigen-specific CD4+ T cells (50). We further found that MDSCs express CD1d (data not shown), but we found that CD1d expression by these cells was not required for their expansion or induction of suppressive functions in response to α-GalCer stimulation. Nevertheless, we cannot exclude that interactions between iNKT cells and MDSCs via other surface molecules are required for the capacity of α-GalCer to induce the generation of functional MDSCs.
Our findings are consistent with prior studies reporting a protective role of MDSCs in CNS autoimmunity (21, 32, 41, 42, 50, 51). Studies by Zhu et al. (32, 42) showed a predominant role for M-MDSCs and iNOS in the capacity of MDSCs to regulate autoimmunity and these investigators showed that activated M-MDSCs can prevent EAE upon adoptive transfer. On the other hand, Ioannou et al. (41) described a role of G-MDSCs expressing program death ligand-1(PD-L1) in suppressing pathogenic T cells in vitro and in vivo (41). Our findings similarly demonstrated that both subpopulations of MDSCs possess immunosuppressive functions and that iNKT cell activation induces increased numbers of both subtypes of MDSCs with superior capacity to suppress pathogenic T cells.
M-MDSCs and G-MDSCs express different sets of anti-inflammatory proteins, and therefore utilize different mechanisms to suppress T cell functions (21). We found high expression of Arg1 in both M- and G-MDSCs from α-GalCer treated mice, whereas iNOS and IL-12 were predominantly expressed by G-MDSCs, and IL-10 and TGF-β by M-MDSCs. These results therefore suggested that the suppressive effects of G-MDSCs predominantly involve iNOS, Arg1 and IL-12, whereas those of M-MDSCs mostly involve Arg1, IL-10 and TGF-β. We further demonstrated a direct role of iNOS expression in the capacity of α-GalCer to protect mice against EAE, and a role for Arg1 and IL-10 in the capacity of MDSCs from α-GalCer-treated mice to suppress pathogenic T cells.
MDSCs are derived from hematopoietic bone marrow progenitor cells in response to inflammatory stimuli (20-24). Consistently, evidence suggests that iNKT cells influence and regulate hematopoiesis in vivo and in vitro (52, 53). It has been shown that a single injection of α-GalCer in B6 mice results in rapid secretion of GM-CSF and IL-3 by iNKT cells, and increased colony-forming unit frequency in peripheral blood and spleen (54). These studies therefore suggest that GM-CSF and IL-3 secreted by iNKT cells may directly influence hematopoiesis and promote the recruitment of MDSCs to the spleen during EAE. Consistent with this notion, we found that GM-CSF neutralization in vitro inhibited the capacity of activated iNKT cells to promote MDSC survival. In addition to GM-CSF and IL-3, additional cytokines produced by glycolipid-activated iNKT cells could contribute to the expansion of MDSCs and induction of immunosuppressive functions in these cells. MDSCs that are recruited to tumor cells were shown to require expression of IL-4Rα for their immunosuppressive function and expansion (55). We similarly observed IL-4Rα expression on MDSCs from vehicle- and α-GalCer-treated mice induced for EAE (data not shown). Therefore, it is tempting to speculate that IL-4 secreted by iNKT cells could directly be involved in activating MDSCs to expand these cells in the spleen and promote their immunosuppressive activities. This possibility is also consistent with the critical role of IL-4 in the capacity of α-GalCer to protect mice against MOG-p-induced EAE (13). Similarly, IFN-γ released by iNKT cells and by NK cells activated in response to α-GalCer treatment, was shown to be required for the protective effects of α-GalCer against EAE (18, 19). Furthermore, the protective effects of IFN-γ in EAE have been shown to directly relate to iNOS expression and nitric oxide (NO) production (32), a finding that was also confirmed in our studies. Consistent with this proposed scenario, in vitro antibody blockade of IL-4 or IFN-γ in our studies suggested a direct role of these cytokines in the induction of suppressive functions in MDSCs. Thus, a variety of cytokines produced by activated iNKT cells might contribute to the recruitment of MDSCs to the spleen and in inducing the immunosuppressive activities of these cells.
A few previous studies have reported interactions between iNKT cells and MDSCs (56-58). De Santo et al. (56) showed that the presence of iNKT cells suppressed the development of MDSCs during influenza virus infection, and studies by Ko et al. (57) and Kmieciak et al. (58) showed that the immunosuppressive activities of MDSCs that accumulated in tumor-bearing mice could be overcome by activated iNKT cells. While these studies appear inconsistent with the findings reported here, we treated mice with α-GalCer either under steady-state conditions or during inflammation induced by MOGp emulsified in CFA. Thus, the timing of iNKT cell activation, the absence of an inflammatory response, and the context of the inflammatory response induced during infections, autoimmunity or cancer, likely all contribute to the effects of iNKT cells on MDSCs. These divergent effects of iNKT cells on MDSCs are consistent with the well-documented notion that iNKT cells can exhibit both pro- and anti-inflammatory properties that are influenced by the immunological context in which these cells are present (1-9).
While our studies provide strong evidence that α-GalCer-activated iNKT cells protect against EAE in a mechanism that involves MDSCs, it remains unclear whether similar mechanisms are involved in other autoimmune and inflammatory conditions where α-GalCer confers disease protection. The involvement of MDSCs is likely dependent upon the particular experimental model involved. Many autoimmune models involve immunization in the context of CFA, a known inducer of MDSCs (50, 59, 60). Thus, the capacity of α-GalCer to protect mice against experimental autoimmune uveitis (61), autoimmune myasthenia gravis (62) and collagen-induced arthritis (63) likely involves the generation of functional MDSCs as reported here for EAE. Additional studies will be needed to investigate the contributions of MDSCs to the immunomodulatory activities of activated iNKT cells in the context of different diseases.
Acknowledgments
We thank the NIH tetramer facility for providing CD1d-monomers and Dr. Fang Yan for providing IL-10-deficient mice.
This work was supported by NIH grants AI070305 (to L.V.K.), HL089667 (to L.V.K.), DK081536 (to L.W. and L.V.K.), DK053620 (to K.T.W.), and AT004821 (to K.T.W.), a Merit Review Grant from the Office of Medical Research, Department of Veterans Affairs (to K.T.W.), and a postdoctoral fellowship from the National Multiple Sclerosis Society of America (to V.V.P.).
Abbreviations used in this article
- aEAE
active EAE
- α-GalCer
α-galactosylceramide
- Arg1
arginase-1
- BEC
S-(2-boronoethyl)-L-cysteine
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- G-MDSC
granulocytic MDSC
- iNKT
invariant natural killer T
- iNOS
inducible nitric oxide synthase
- MDSC
myeloid-derived suppressor cell
- MOG
myelin oligodendrocyte glycoprotein
- M-MDSC
monocytic MDSC
- MOGp
MOG peptide
- pEAE
passive EAE
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 4.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 5.Parekh VV, Lalani S, Van Kaer L. The in vivo response of invariant natural killer T cells to glycolipid antigens. Int Rev Immunol. 2007;26:31–48. doi: 10.1080/08830180601070179. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Kaer L, Parekh VV, Wu L. Invariant NK T cells: potential for immunotherapeutic targeting with glycolipid antigens. Immunotherapy. 2011;3:59–75. doi: 10.2217/imt.10.85. [DOI] [PubMed] [Google Scholar]
- 8.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2010;343:43–55. doi: 10.1007/s00441-010-1023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 11.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Kaer L, Parekh VV, W. L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2012 doi: 10.1016/j.it.2012.08.009. prepub (doi: 10.1016/j.it.2012.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 16.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal E, Tabira T, Kawano T, Taniguchi M, Miyake S, Yamamura T. Costimulation-dependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of V alpha 14 NK T cells. J Immunol. 2001;166:662–668. doi: 10.4049/jimmunol.166.1.662. [DOI] [PubMed] [Google Scholar]
- 18.Furlan R, Bergami A, Cantarella D, Brambilla E, Taniguchi M, Dellabona P, Casorati G, Martino G. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur J Immunol. 2003;33:1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 19.Mars LT, Araujo L, Kerschen P, Diem S, Bourgeois E, Van LP, Carrie N, Dy M, Liblau RS, Herbelin A. Invariant NKT cells inhibit development of the Th17 lineage. Proc Natl Acad Sci U S A. 2009;106:6238–6243. doi: 10.1073/pnas.0809317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 24.Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Samantha S, Mandruzzato S, Bronte V. Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Rev. 2011;30:27–43. doi: 10.1007/s10555-011-9268-1. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 28.Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- 29.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 31.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 32.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6Chi suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 33.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 36.Segal BM. Th17 cells in autoimmune demyelinating disease. Semin Immunopathol. 2010;32:71–77. doi: 10.1007/s00281-009-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 39.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2012 doi: 10.1038/nm.2999. prepub (doi: 10.1038/nm.2999) [DOI] [PubMed] [Google Scholar]
- 41.Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A, Boumpas D, Verginis P. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 42.Zhu B, Kennedy JK, Wang Y, Sandoval-Garcia C, Cao L, Xiao S, Wu C, Elyaman W, Khoury SJ. Plasticity of Ly-6Chi myeloid cells in T cell regulation. J Immunol. 2011;187:2418–2432. doi: 10.4049/jimmunol.1100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40–55. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkotter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65:217–231. doi: 10.1002/jlb.65.2.217. [DOI] [PubMed] [Google Scholar]
- 47.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, Nakayama T, Taniguchi M. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J Immunol. 2005;175:3648–3655. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- 49.Denney L, Kok WL, Cole SL, Sanderson S, McMichael AJ, Ho LP. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551–557. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- 50.Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol. 2011;11:789–793. doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotsianidis I, Silk JD, Spanoudakis E, Patterson S, Almeida A, Schmidt RR, Tsatalas C, Bourikas G, Cerundolo V, Roberts IA, Karadimitris A. Regulation of hematopoiesis in vitro and in vivo by invariant NKT cells. Blood. 2006;107:3138–3144. doi: 10.1182/blood-2005-07-2804. [DOI] [PubMed] [Google Scholar]
- 53.Broxmeyer HE, Christopherson K, Hangoc G, Cooper S, Mantel C, Renukaradhya GJ, Brutkiewicz RR. CD1d expression on and regulation of murine hematopoietic stem and progenitor cells. Blood. 2012;119:5731–5741. doi: 10.1182/blood-2012-01-404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leite-de-Moraes MC, Lisbonne M, Arnould A, Machavoine F, Herbelin A, Dy M, Schneider E. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: relevance to peripheral myeloid recruitment. Eur J Immunol. 2002;32:1897–1904. doi: 10.1002/1521-4141(200207)32:7<1897::AID-IMMU1897>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 55.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 58.Kmieciak M, Basu D, Payne KK, Toor A, Yacoub A, Wang XY, Smith L, Bear HD, Manjili MH. Activated NKT cells and NK cells render T cells resistant to myeloid-derived suppressor cells and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J Immunol. 2011;187:708–717. doi: 10.4049/jimmunol.1100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntosh KR, Drachman DB. Induction of apoptosis in activated T cell blasts by suppressive macrophages: a possible immunotherapeutic approach for treatment of autoimmune disease. Cell Immunol. 1999;193:24–35. doi: 10.1006/cimm.1998.1445. [DOI] [PubMed] [Google Scholar]
- 60.Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31:354–361. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Oh K, Byoun OJ, Ham DI, Kim YS, Lee DS. Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol. 2011;41:392–402. doi: 10.1002/eji.201040569. [DOI] [PubMed] [Google Scholar]
- 62.Liu R, La Cava A, Bai XF, Jee Y, Price M, Campagnolo DI, Christadoss P, Vollmer TL, Van Kaer L, Shi FD. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 63.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50:305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]