Abstract
Despite high rates of risky behavior among patients, many drug abuse treatment programs do not provide on-site HIV testing. This secondary analysis examined differences in outcome by program modality from a multi-site trial in which 1,281 HIV-negative patients in 3 methadone programs, 7 non-methadone outpatient programs, and 3 residential programs were randomly assigned to: (1) off-site referral for HIV risk reduction counseling and testing; or on-site rapid testing (2) with or (3) without risk reduction counseling. The parent study using generalized estimating equations with site as a cluster variable found significantly higher rates of HIV testing and feedback of results by 1 month post-enrollment for the combined on-site conditions compared to the offsite condition (RR=4.52, 97.5% CI (3.57, 5.72). Utilizing the same statistical approach, we found neither significant treatment modality nor significant treatment modality by testing condition interaction effects either for receipt of HIV test results at 1 month or for sexual or drug use HIV-risk behaviors at 6-month follow-up. On-site HIV testing is effective across treatment modalities for achieving high rates of testing and results feedback. All programs should be encouraged to adopt or expand this service.
Keywords: HIV rapid testing, drug treatment programs, HIV risk behavior
1. Introduction
There are several distinct types of drug treatment programs in the United States that serve a wide range of patients in diverse settings. Residential programs provide short-term (e.g. 28 days or less) or longer-term (up to 6 months) inpatient care generally for individuals with severe levels of substance use. In contrast, opioid treatment programs (OTP) are specifically licensed to provide methadone and/or buprenorphine for opioid dependent patients on an outpatient basis. Outpatient programs treat patients who may be dependent on alcohol or any drug. Such programs may also provide buprenorphine through an authorized physician. Despite this heterogeneity among programs, they all treat individuals who are at elevated risk for HIV infection compared to the general population, as injection drug use remains an important vector for HIV infection and sexual transmission of HIV is prevalent in the alcohol and drug treatment population (Brown et al., 2006; Murril et al., 2001; Shah et al., 2000; Sorensen, Masson, & Perlman, 2002).
In recognition of the heightened risk of HIV infection among substance using patients and the need to expand access to HIV testing, the Centers for Disease Control and Prevention (CDC) called for expanding HIV testing in substance use treatment programs and other healthcare settings (Branson et al., 2006). Yet, despite the high risk of HIV infection among substance using patients, a recent national survey reported that only 27.7% of the nation’s treatment programs offered HIV testing onsite (NSSATS, 2010). This is consistent with other national studies that show less than optimal delivery of on-site HIV testing in substance use treatment programs (Brown et al., 2006; Pollack & D’aunno, 2010).
Studies that have examined the availability of HIV testing in substance abuse treatment programs have found that residential and methadone treatment programs were more likely than outpatient programs to offer HIV testing (Polinksy et al, 1998; Pollack & D’Aunno, 2010). Programs that were of greater size, received public funding, offered medical services onsite, and had Joint Commission accreditation, were more likely to offer HIV testing (Knudson & Oser, 2006; Pollack et al., 2006; Strauss et al., 2003).
Identified barriers to providing HIV testing in substance abuse treatment programs include lack of funding and/or reimbursement for services, lack of medical staff, and lack of staff training (Abraham et al., 2012; Bini et al., 2011; Brown et al., 2006; 2007; Knudsen & Oser, 2008). Haynes and colleagues (2011) pointed out the need for a phlebotomist as well as the requirement for pre- and post-test risk reduction counseling (codified in some state regulations) constitute two additional barriers to the provision of testing. The availability of rapid onsite oral fluid testing, which does not require phlebotomy, and the 2006 revision of the CDC’s testing guidelines which no longer recommend routine HIV risk reduction counseling at the time of HIV testing (except in cases of positive tests) raised two questions. The first question is whether on-site HIV testing in substance use treatment programs, as compared to the usual practice of off-site referral for testing, would increase testing and the receipt of testing results and second, whether there would be any additional benefit in terms of reduced risk behaviors to providing risk reduction counseling with testing. Although methadone programs have been found to be more likely than other outpatient programs to provide HIV testing (Polinksy et al., 1998; Pollack & D’Aunno, 2010), we did not hypothesize that methadone programs would have higher rates of testing and receipt of testing because the study design addressed some of the major barriers to implementation by providing adequate resources and training to provide testing in clinics willing to participate in the study.
The overall goal of the parent study was to evaluate the more effective strategy (referral, onsite rapid testing, onsite rapid testing + counseling) to (1) increase HIV testing acceptance and receipt of results and (2) decrease HIV sexual risk behaviors. The National Institute on Drug Abuse Clinical Trials Network (CTN) conducted a multi-site, three-arm, random assignment study (Metsch et al., 2012) in which 1,281 HIV-negative (or unknown sero-status) patients in 12 drug treatment programs throughout the US, were randomly assigned to: (1) off-site referral for HIV risk reduction counseling and testing; (2) on-site rapid testing without risk reduction counseling; and (3) on-site rapid testing with risk reduction counseling. Overall, participants who received on-site testing were more likely to report receiving HIV test results at one month follow-up than those referred off-site (82.2% versus 18.4%, p < 0.001; adjusted RR 4.53, 97.5% CI (3.57, 5.72). Groups did not differ in unprotected intercourse or needle sharing at 6 months, but those who received onsite testing with counseling were more likely to discontinue needle sharing (p = 0.044). The effect of substance use treatment modality on these outcomes, however, was not assessed. Given the considerable differences among types of drug treatment modalities in terms of length of stay, locus of care, and characteristics of patient populations and the relative lack of attention in the literature to the impact of these differences on HIV testing and risk behavior, we conducted a planned secondary analysis to assess differences by treatment modality in: (1) receipt of HIV testing and feedback results during the study; and (2) HIV risk behaviors at baseline and at 6-month follow-up.
2. Methods
2.1. Parent Study
The parent study (described elsewhere in detail, Metsch et al., 2012) was a three-arm random assignment study conducted between January and December 2009 in 12 US drug treatment programs that had not been providing HIV testing prior to the study’s start. Research sites included 3 residential, 3 opioid treatment, and 7 outpatient programs. Participants were recruited from among both newly-admitted and active adult patients who reported being HIV negative or who were not aware of their sero-status.
Consenting participants were stratified by site, gender and race and randomly assigned to one of three conditions. The first condition consisted of referral for off-site HIV testing. The second condition consisted of on-site rapid testing with risk-reduction counseling based on Project RESPECT-2 (Metcalf et al., 2005). Counseling sessions were of planned 30 minutes duration and included information about the routes of HIV transmission, HIV testing procedure, interpretation of the test results, and the creation in partnership with the participant of a personalized risk reduction plan. Testing was offered and conducted onsite using the OraQuick Advance Rapid HIV-1/2 Antibody test. The third condition consisted of on-site rapid testing offered along with information describing the test but without any risk reduction counseling.
2.1.1. Measures
At baseline, audio computer assisted self interviews (ACASIs) were conducted to obtain participant demographic characteristics, HIV testing history, substance use (frequency and amount of use), injection risk behavior, and sex risk behavior for the 6 months prior to the interview. At 1-month follow-up, participants indicated through ACASI whether they had been HIV tested and received their results, and at 6 months post-baseline participants reported through ACASI their sexual and injection risk behaviors in the 6 months prior to the follow-up interview.
OraQuick ADVANCE HIV-1/2 Antibody oral swab was used to conduct HIV testing for all participants in the two on-site testing arms. If oral swab test results were positive or invalid, OraQuick ADVANCE HIV-1/2 Antibody whole blood fingerstick was performed. Participants with positive results on either test had an additional oral swab collected for confirmatory testing with Orasure Western blot analyzed by an external laboratory. These participants were also provided with post-test counseling on sexual and injection risk behaviors, and the importance of receiving appropriate medical assessment and care.
In the current analysis, we examined the relationship between substance use treatment modality and the receipt of testing results at 1-month follow-up, and the reduction in HIV sexual risk behaviors from baseline to 6-month follow-up and specifically tested whether the impact of assigned intervention group differed by treatment modality. A secondary outcome of needle sharing was also examined in this fashion. Both sexual and needle sharing HIV risk behaviors were examined in the analysis because they were the focus of the counseling session in one of the three study conditions and we were interested in examining changes in these behaviors by treatment modalities given the different patient populations and treatment approaches across modalities.
Initially, demographics and baseline characteristics were examined and compared across the three drug treatment modalities using chi-square analysis for binary indicators. Wilcoxon-rank sum tests were used to test whether baseline level of sexual risk behavior differed across drug treatment modality. The present study utilized score tests to assess treatment group differences by fitting generalized estimating equations with site as a cluster variable and adjustment for race and gender strata. The receipt of HIV test results outcome utilized a logit link function with a binomial error distribution as did the needle risk behavior outcome. Number of risky sexual behaviors outcome utilized a log link function with a negative binomial error distribution. Main effects of drug treatment modality and its interaction with testing condition were added to these models to specifically address the questions of the present analysis. The score tests used for the interaction are distributed as a chi-square with 4 degrees of freedom.
3. Results
3.1. Participant Characteristics
A total of 1,281 participants were randomly assigned to condition. While participant characteristics in the parent study were comparable across conditions (Metsch et al., 2012), as shown in Table 1, there were significant differences across treatment modalities in age, gender, race, marital status, income and education. OTP participants were older than both outpatient and residential participants. Residential as compared to outpatient and OTP participants were more frequently male. As shown in Table 2, expected differences in self-reported use of alcohol and drugs during the 6 months prior to baseline by treatment modality were observed (e.g., higher rates of opioid use in OTPs), reflecting the different types of populations treated in the three treatment modalities. The 1- and 6-month follow-up rates were 99.2%, and 93.7%, respectively.
Table 1.
Participant Demographics, Across Drug Abuse Treatment Modality
| Residential (N=318) | Outpatient (N=619) | OTP (N=344) | Total (N=1281) | Chi-square | Sig. | |
|---|---|---|---|---|---|---|
|
| ||||||
| % | % | % | % | |||
| Race/Ethnicity | 48.99 | <.001 | ||||
| Hispanic | 2.8 | 11.6 | 19.2 | 11.5 | ||
| Black | 21.1 | 23.9 | 20.3 | 22.2 | ||
| White | 69.5 | 57.5 | 52.6 | 59.2 | ||
| Other | 6.6 | 6.9 | 7.8 | 7.1 | ||
| Gender | 106.47 | <.001 | ||||
| Male | 84.0 | 56.7 | 46.2 | 60.7 | ||
| Female | 16.0 | 43.3 | 53.8 | 39.3 | ||
| Marital Status | 27.15 | <.001 | ||||
| Married/Cohabiting | 23.3 | 24.4 | 28.5 | 25.2 | ||
| Widowed | 1.6 | 3.1 | 7.3 | 3.8 | ||
| Divorced/Separated | 24.5 | 30.2 | 26.5 | 27.8 | ||
| Single | 50.6 | 42.3 | 37.8 | 43.2 | ||
| Education | 42.52 | <.001 | ||||
| Less than High School | 30.5 | 22.9 | 33.1 | 27.6 | ||
| High School | 43.1 | 30.9 | 32.3 | 34.3 | ||
| More than High School | 26.4 | 46.2 | 34.6 | 38.2 | ||
| Income | 17.20 | <.001 | ||||
| $0 to $5000. | 42.2 | 46.1 | 45.6 | 45.0 | ||
| $5001 to $20,000 | 39.4 | 33.6 | 43.2 | 37.6 | ||
| More than $20,000 | 18.4 | 20.3 | 11.2 | 17.4 | ||
| Age (Mean, SD) | 36.15 (10.620) | 39.97 (11.247) | 43.61 (10.654) | 40.00 (11.252) | 38.44* | <.001 |
Note: Test Statistics is an F(2, 1278).
Table 2.
Participant Baseline Characteristics across Drug Abuse Treatment Modality (Categorical Indicators).
| Residential | Outpatient | OTP | Total | Chi-square | Sig. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | % | N | % | N | % | N | % | |||
|
| ||||||||||
| Ever HIV Tested | 155/318 | 48.7 | 437/619 | 70.6 | 304/344 | 88.4 | 896/1281 | 69.9 | 123.70 | <.001 |
| Ever Injected drugs | 138/318 | 43.4 | 196/617 | 31.8 | 288/344 | 83.7 | 622/1279 | 48.6 | 243.29 | <.001 |
| Inject in Last 6 Months | 69/318 | 21.7 | 78/618 | 12.6 | 117/343 | 34.1 | 264/1279 | 20.6 | 62.47 | <.001 |
| Shared needle/works in past 6 months | 28/318 | 8.8 | 22/6618 | 3.6 | 44/344 | 12.8 | 94/1280 | 7.3 | 29.00 | <.001 |
| Court Mandated Treatment | 119/317 | 37.5 | 148/615 | 24.1 | 21/344 | 6.1 | 288/1276 | 22.6 | 94.80 | <.001 |
| Alcohol Binge/Problem in past 6 months | 276/318 | 86.8 | 464/619 | 75.0 | 180/344 | 52.3 | 920/1281 | 71.8 | 102.83 | <.001 |
| Any Opioid use in past 6 months | 125/318 | 39.3 | 177/619 | 28.6 | 172/344 | 50.0 | 474/1281 | 37.0 | 44.43 | <.001 |
| Any Cocaine use in past 6 months | 146/318 | 45.9 | 233/619 | 37.6 | 123/344 | 35.8 | 502/1281 | 39.2 | 8.35 | <.016 |
| Any Amphetamine use in past 6 months | 56/318 | 17.6 | 33/619 | 5.3 | 43/344 | 12.5 | 132/1281 | 10.3 | 36.72 | <.001 |
Note: All tests are 2 df. If three groups were significantly different then all pair-wise comparisons were significantly different except for OTP and Residential on Amphetamine use, Outpatient and OTP on Cocaine use, and Residential and OTP on needle sharing.
3.2. Baseline HIV Risk Behaviors
There were significant differences in prior history of HIV testing at baseline, with highest rates seen in the OTP and outpatient participants and lowest rates in residential programs (88.4%, 70.6%, and 48.7%, respectively; p < .001). At baseline, participants enrolled in OTP had significantly higher rates of ever injecting drugs as well as of injecting in the last 6 months as compared to the outpatient and residential groups (ever injecting: 83.7%, 31.7%, and 43.4% and last 6 months: 34.0%, 12.6%, and 21.7%, respectively, both p< .001). Of the 69 participants who reported having injected drugs in the 6 months prior to baseline, data were available for 66 participants on whether they shared needles or works during that time. While the overall rates of sharing were relatively high (38.5%), there were no significant differences across treatment modality groups.
As shown in Table 3, the median number of acts of unprotected vaginal and/or anal intercourse and number of those behaviors while high on drugs or alcohol in the 6 months prior to baseline were significantly different across treatment modalities (both p < .001). For both the number of acts of unprotected sex and for the number of those behaviors while high, residential participants reported a higher number of these behaviors as compared to outpatient, which in turn, had higher rates than participants in OTP treatment. Although medians for both outpatient and OTP are zero, OTP had a much higher proportion at the median, than did outpatient.
Table 3.
Baseline Past 6-month Sexual Risk Indicators (Skewed, Count of Unprotected Sexual Episodes)
| Residential | Outpatient | OTP | Total | Chi-square | Asymp. Sig. | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median [p25, p75] | N | Median [p25, p75] | N | Median [p25, p75] | N | |||
| Unprotected Vaginal and/or Anal Intercourse | 318 | 12 [0, 51] | 619 | 5 [0, 30] | 344 | 0 [0, 14] | 1281 | 60.55 | <.001 |
| Unprotected Vaginal and/or Anal Intercourse While High on Alcohol or Drugs | 318 | 12 [0, 25] | 619 | 0 [0, 7] | 344 | 0 [0, 1] | 1281 | 90.93 | <.001 |
Note: Tested using Kruskal-Wallis test (nonparametric ANOVA). All tests are 2 df.
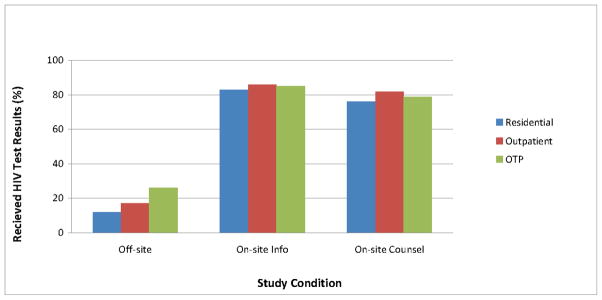
3.3. Receipt of HIV Testing Results at 1-month follow-up
There was neither a significant treatment modality main effect nor a significant treatment modality by testing condition interaction effect for receiving HIV test results by the 1–month follow-up, with all treatment modality groups receiving test results at rates ranging from 12% to 86% (p=.54 and 0.42, respectively). However, post-hoc testing within the off-site referral condition revealed that OTP participants were more likely than participants in the outpatient and residential groups to have received testing results (26.1%, 17.1%, and 12.0%, respectively; p = .03).
3.4. HIV Sexual Risk Behavior at 6-month follow-up
Similarly, there was neither a significant treatment modality main effect nor a significant modality by testing condition interaction effect for the number of unprotected acts of vaginal and anal intercourse at 6 month follow-up (ps=.07 and 0.79, respectively). However, the median number of unprotected sex acts reported during the 6 months post-baseline was lower for OTP participants (Mdn = 0 acts) than for outpatient and residential groups, (Mdn = 2, and Mdn = 2, acts, respectively). Post-hoc testing revealed that the number of unprotected acts was significantly lower for participants enrolled in OTP compared with those receiving outpatient (p < .001) and residential treatment (p < .001).
3.5. Needle Risk Behavior at 6-month follow-up
Due to the small number of participants reporting needle risk behaviors, it was not possible to include the interaction term with all three drug treatment modalities. Therefore, the difference between OTP treatment and the other two treatment modalities combined was examined. There was neither a significant treatment modality main effect nor a significant treatment modality by testing condition interaction effect for the needle sharing outcome (ps = .18 and .67, respectively). Additional tests of significance showed that across testing conditions the three treatment modalities differed by the proportion of individuals reporting injection drug use between baseline and 6 months (p < .001; OTP = 19.7%, outpatient = 8.4% and residential = 6.2%). Treatment modalities also differed in the proportion reporting needle sharing between baseline and 6 months (OTP = 3.3%, outpatient = 1.6% and residential = 0.7%, p = .049).
4. Discussion
Despite the availability of rapid oral fluid HIV testing and the CDC’s and the US National HIV/AIDS Policy’s (http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf, 2010) calls to expand HIV testing in drug treatment programs and other non-traditional settings, the majority of drug treatment programs in the U.S do not provide HIV testing (NSATTS, 2009). This systems level data was not reflected in our individual level baseline data, where prior experience with HIV testing was reported by 88.4%, 70.6%, and 48.7% of study participants enrolled in OTP, outpatient and residential participants, respectively; p < .001). Our data does not refute the systems level data, but suggests that other factors may be at work. For example, participants may have been exposed to multiple clinics in the past, some of which offered testing. It is also possible that our sample of OTP and outpatient clinics in particular may have been biased toward those that had offered testing in the past.
The current analysis found that there was no significant difference in the percentage of participants who received their HIV test results at 1-month post-randomization when testing was offered on-site. The lack of significant differences found in the present study in receipt of HIV test results may be attributed to the fact that the study design addressed major barriers to testing found in prior research including lack of funding and training (Abraham et al., 2012; Bini et al., 2011; Brown et al., 2006; 2007; Knudsen & Oser, 2008). Nevertheless, these findings of cross modality effectiveness of on-site testing, are very encouraging in light of the differences in implementation of HIV testing across modalities found in prior survey research (Brown et al., 2007; Polinksy et al, 1998; Pollack & D’Aunno, 2010). These results should encourage substance use treatment providers in all three of these treatment modalities to expand their onsite testing.
When referred for offsite HIV testing, participants treated in OTPs were more likely to obtain their test results than participants treated in residential and outpatient treatment facilities. This finding may be attributable to baseline characteristic differences in the patient populations treated in these three types of programs. For example, the OTP participants were older, more likely to be injection drug users, and to have previously been HIV tested than the other groups. In contrast, this may have been attributable in part to higher treatment retention rates in OTPs as compared to other treatments (Mattick, Breen, Kimber, & Davoli, 2009). Thus, OTP participants may have been encouraged by their drug abuse counselors to follow-up on their testing results, while participants in other modalities may no longer have been engaged in treatment. Finally, OTPs have been shown in prior research to be more likely to provide HIV testing than outpatient programs (Brown et al., 2007). It is possible that OTP staff may have been more comfortable with obtaining HIV testing for their patients and therefore were more likely to encourage them to receive their off-site test results. Nevertheless, only 26.1% of the OTP participants who were referred off-site for testing received their results, leaving much room for improvement. The current investigation shows that moving to an on-site testing program utilizing a rapid HIV test would provide significant improvement regardless of treatment modality. For sites where this would not be possible, it might prove beneficial to focus performance improvement efforts on increasing the number of patients who seek HIV testing and follow through to receive the results.
With regard to sexual risk behavior, an interesting finding was that the OTP participants reported a significantly lower median number of unprotected sex acts at both baseline and 6 months follow-up compared to the other two treatment modalities, a difference however that was not significant as a main effect in the replication model which included treatment modality (p = .07 in the full model; and p < .001 in post-hoc comparisons). An exploratory multivariate analysis revealed that none of the treatment modality differences shown in Tables 1 and 2, explained the difference in sexual risk of OTP patients versus the other two modalities (though age did explain the significant difference in sexual risk of residential and outpatient participants). The lower sexual risk of OTP patients may be attributable to the noted side effect of methadone and other opioids which may decrease libido and cause erectile dysfunction (Lott, Strain, Brooner, Bigelow, & Johnson, 2006). The addition of risk reduction counseling did not differentially impact sexual risk behavior across treatment modalities, a finding that is consistent with its lack of effectiveness overall.
There were also no treatment modality differences in the impact of testing condition on needle sharing. This may be attributable to the lack of impact of the single session risk-reduction counseling afforded by the study (in the context of drug abuse treatment as usual) on such behavior, to the relatively small number of needle sharers in the study, or to other factors that impact on needle sharing, such as lack of access to needles and frequent cocaine injection (Wood et al., 2002). As expected given the patient populations, there are differences in the amount of injection drug use and needle sharing across modality, however, with the highest proportion of needle sharers found in OTPs (3.3%), followed by outpatient (1.6%), and residential programs (0.7%).
There are a number of limitations to this analysis. The sensitive subject matter introduces the possibility of reporting bias. Participants were asked to report on potentially stigmatizing behaviors including drug and alcohol use and acts of sexual intercourse, which increased the risk of underreporting. To decrease this risk questions were administered in an ACASI format, which has been shown to elicit increased reporting of drug use and sex risk behaviors when compared to face to face interviews (Des Jarlais et al., 1999; Metzger et al., 2000; Perlis, Des Jarlais, Friedman, Arasteh, & Turner, 2004; Turner et al., 1998). Additionally, there is no reason to suspect that reporting bias varied by treatment modality. Finally, the study was a secondary analysis of data and hence was not powered to determine differences by treatment modality, although the total sample size of the parent study (N = 1,281) was robust.
Our findings demonstrate the feasibility of achieving high rates of receipt of HIV testing results when HIV testing is provided onsite at a variety of treatment program types. This finding strengthens the rationale for widespread implementation of routine HIV testing within such programs. As sexual risk reduction counseling appeared to have no effect on sexual risk behaviors at 6-month follow-up, the inability to provide sexual risk counseling should not serve as a barrier to providing HIV testing. Staff training in HIV testing for counselors and program administrators of drug treatment programs should be made widely available. Such training could be delivered through the State or Local Health Departments’ AIDS Administrations, through local medical schools, or through the regional Center for Substance Abuse Treatment’s Addiction Technology and Transfer Centers (Abraham et al., 2012). Funding is also needed to support testing. Program administrators should contact their State and Local Health Departments to determine the availability of grant funding for this purpose as it has been found that substance abuse programs are not always aware of existing funding for HIV testing in their state (Abraham et al., 2012; Bini et al., 2011).
Medicaid is another important source of funding for drug abuse treatment and HIV testing. While all states pay for “medically necessary” HIV testing for individuals at high risk (such as intravenous drug users, men who have sex with men, those with certain heterosexual risk factors), only about half of the states pay for “routine” HIV testing for individuals who are not considered to be at high risk (Kaiser Family Foundation, 2012). Should the US Prevention Services Task Force revise its recommendation on HIV testing to recommend “routine”, HIV testing would be required and incentivized under the Affordable Care Act of 2010 (Kaiser Family Foundation, 2012). This would help some drug treatment providers to overcome the reimbursement barrier for patients with Medicaid and possibly for those with private insurances as well.
Figure 1.
Percentage of Participants Receiving HIV Testing Results Within 1 Month Post-Randomization
Acknowledgments
Funding for this study and analysis was provided by the National Drug Abuse Treatment Clinical Trials Network (CTN) under the following Cooperative Agreements, awards and contracts: U10 DA013720, U10DA13720-09S, U10 DA020036, U10DA15815, U10DA13034, U10DA013038, U10 DA013732, U10 DA13036, U10 DA13727, U10DA015833, HHSN271200522081C, HHSN271200522071C, R01DA027379 and K23DA019809.
Site PIs: David Avila, Michael DeBernardi, Lillian Donnard, Antoine Douaihy, Louise Haynes, Ray Muszynski, Patricia E. Penn, Ned Snead, Kevin Stewart, Robert C. Werstlein and Katharina Wiest. Site PIs’ contributions to the work reported in this manuscript include: directing all aspects of the proposed study at their site(s), having overall responsibility for achieving the specific aims of the study, maintaining the proposed study schedule and budget, supervising the project staff, and ensuring quality control over all aspects of this study.
Staff: Walitta Abdullah, Elizabeth Alonso, Anika Alvanzo, Anna Amberg, Holly Angel, Rebekka (Peggy) M Arias, Natasha Arocho, Carolyn Baron-Myak, Sarah Battle, Melissa Beddingfield, Dan Blazer, Stacy Botex, Sarah Bowles, Audrey Brooks, Elizabeth Buttrey, Betty Caldwell, Lynn Calvin, Maria Campanella, Sarah Carney, Angela Casey-Willingham, Jack Chally, Roberta Chavez, Nicholas Cohen, Zoe Cummings, Elisa Cupelli, Dennis Daley, Meredith Davis, Kay Debski, Andrea Dedier, Ashley Dibble, Bruce Dillard, Debbie Drosdick, Monica Eiden, Matthew Elmore, Sarah Essex, Laura Feldberg, Elizabeth Ferris, Lauren Thomas, John Gary, Daniel Gerwien, Marisa Gholson, Melissa Gordon, Lauren Griebel, Laurel Hall, Stephanie Hart, Joshua Hefferen, Beverly Holmes, Christine Horne, Alice Huang, Aleks Jankowska, Beth Jeffries, Kristen Jehl, Eve Jelstrom, Andrew Johnson, Jacob Johnson, Shanna Johnson, Emily Kinsling-Law, Amy Knapp, Eric Kohler, Beatrice Koon, Andrzej Kosinski, Emily Kraus, Lynn Kunkel, Robert Kushner, Diane Lape, Theresa Latham, Larry Lee, Carol Luna-Anderson, Sue McDavit, Michael McKinney, Cindy Merly, Melody Mickens, Jenni Mulholland, Roger Owen, Barbara Paschke, Wayne Pennachi, Sharon Pickrel, Kimberly Pressley, John Reynolds, Gillian Rossman, Lauretta Safford, Christine Sanchez, Lynn Sanchez, Dorothy Sandstrom, Carmel Scharenbroich, Robert Schwartz, Nicolangelo Scibelli, Michael Shopshire, Jessica Sides, Eugene Somoza, Maxine Stitzer, Joseph Sullivan, Krishna Suwal, Danielle Terrell, Rena Treacher, Dominic Usher, Angel Valencia, Tammy Van Linter, Rosa Verdeja, Joanne Weidemann, Brandi Welles, Lindsay Worth and Pamela Yus.
Site Staff contributions to the work reported in this manuscript include: conducting recruitment and enrollment activities, performing assessment interviews, conducting study interventions, performing quality assurance monitoring activities, performing data entry and completing other day to day study activities that led to the collection of the study data.
National Institute on Drug Abuse, Office of the Director, AIDS Research Program: We also would like to acknowledge Jacques Normand and Lynda Erinoff for their contributions to protocol development.
Footnotes
All subjects provided informed consent and study procedures were in accord with the standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975.
All authors have seen and approved the manuscript, have contributed significantly to the work, and have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AJ, O’Brien LA, Knudsen HK, Bride BE, Smith GR, Roman PM. Patient characteristics and availability of onsite non-rapid and rapid HIV testing in US substance use disorder treatment programs. J Subst Abuse Treat. 2012 Apr 24;2012 doi: 10.1016/j.jsat.2012.03.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini EJ, Kritz S, Brown LS, Jr, Robinson J, Alderson D, Rotrosen J. Barriers to providing health services for HIV/AIDS, hepatitis C virus infection and sexually transmitted infections in substance abuse treatment programs in the United States. J Addict Dis. 2011;30(2):98–109. doi: 10.1080/10550887.2011.554780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE Centers for Disease Control Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. [Practice Guideline] MMWR Recommendations and Reports. 2006;55:1–17. quiz CE11-14. [PubMed] [Google Scholar]
- Brown LS, Jr, Kritz S, Goldsmith RJ, Bini EJ, Robinson J, Alderson D, Rotrosen J. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Rep 2007. 2007 Jul-Aug;122(4):441–51. doi: 10.1177/003335490712200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LS, Kritz SA, Goldsmith RJ, Bini EJ, Rotrosen J, Baker S, Robinson J, McAuliffe P. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: The National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2006;30:315–321. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, Shi Q, Hagan H, Friedman SR. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: A quasi-randomised trial. Lancet. 1999;353:1657–1661. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- Haynes LF, Korte JE, Holmes BE, Gooden L, Matheson T, Feaster DJ, Leff JA, Wilson L, Metsch LR, Schackman BR. HIV rapid testing in substance abuse treatment: Implementation following a clinical trial. Evaluation and Program Planning. 2011;34:399–406. doi: 10.1016/j.evalprogplan.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation. Fact Sheet, State Medicaid Coverage of Routine HIV Screening. Vol. 2012. The Henry Kaiser Family Foundation; 2012. Mar, [Accessed on August 6, 2012]. HIV/AIDS Policy. http://www.kff.org/hivaids/upload/8286.pdfwww.kff.org. [Google Scholar]
- Knudsen HK, Oser CB. Availability of HIV-related health services in adolescent substance abuse treatment programs. AIDS Care. 2009;21(10):1238–46. doi: 10.1080/09540120902803182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: A comparison of levomethadyl acetate [corrected] buprenorphine, and methadone. Journal of Substance Abuse Treatment. 2006;31:187–194. doi: 10.1016/j.jsat.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Systematic Reviews. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Metcalf CA, Malotte CK, Douglas JM, Paul SM, Dillon BA, Cross H, Brookes LC, Deaugustine N, Lindsey CA, Byers RH, Peterman TA RESPECT-2 Study Group. Efficacy of a booster counseling session 6 months after HIV testing and counseling: A randomized, controlled trial. Sexually Transmitted Diseases. 2005;32:123–129. doi: 10.1097/01.olq.0000151420.92624.c0. [DOI] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden L, Matheson T, Mandler RN, Haynes L, Tross S, Kyle T, Gallup D, Kosinski AS, Douaihy A, Schackman BR, Das M, Lindblad R, Erickson S, Korthuis PT, Martino S, Sorensen JL, Szapocznik J, Walensky R, Branson B, Colfax GN. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: Results of a randomized trial. American Journal of Public Health. 2012;102(6):1160–7. doi: 10.2105/AJPH.2011.300460. Epub 2012 Apr 19. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Koblin B, Turner C, Navaline H, Valenti F, Holte S, Gross M, Sheon A, Miller H, Cooley P, Seage GR., 3rd Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. American Journal of Epidemiology. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- Murrill CS, Prevots DR, Miller MS, Linley LA, Royalty JE, Gwinn M Seroincidence Study Group. Incidence of HIV among injection drug users entering drug treatment programs in four US cities. [Multicenter Study] Journal of Urban Health. 2001;78:152–161. doi: 10.1093/jurban/78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National HIV/AIDS Strategy for the United States. [August 6, 2012];The White House Office of National AIDS Policy. 2010 Jul;2010 Accessed at http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. [Google Scholar]
- National Survey of Substance Abuse Treatment Services (N-SSATS) 2010 State Profile -- United States. Rockville, MD: Substance Abuse and Mental Health Services Administration. Office of Applied Studies; 2010. [accessed August 3, 2012]. http://wwwdasis.samhsa.gov/10nssats/nssats2010web.pdf. [Google Scholar]
- Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:885–896. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- Polinsky ML, Hser YI, Anglin MD, Maglione MA. Drug-user treatment programs in a large metropolitan area. Subst Use Misuse. 1998;33(8):1735–61. doi: 10.3109/10826089809058953. [DOI] [PubMed] [Google Scholar]
- Pollack HA, D’Aunno T, Lamar B. Outpatient substance abuse treatment and HIV prevention: an update. J Subst Abuse Treat 2006. 2006 Jan;30(1):39–47. doi: 10.1016/j.jsat.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pollack HA, D’Aunno T. HIV testing and counseling in the nation’s outpatient substance abuse treatment system, 1995–2005. Journal of Substance Abuse Treatment. 2010;38:307–316. doi: 10.1016/j.jsat.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Shah NG, Celentano DD, Vlahov D, Stambolis V, Johnson L, Nelson KE, Strathdee SA. Correlates of enrollment in methadone maintenance treatment programs differ by HIV-serostatus. AIDS. 2000;14:2035–2043. doi: 10.1097/00002030-200009080-00020. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Masson CL, Perlman DC. HIV/Hepatitis prevention in drug abuse treatment programs: Guidance from research. Science and Practice Perspectives. 2002;1:4–11. doi: 10.1151/spp02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SM, Falkin GP, Vassilev Z, Des Jarlais DC, Astone J. A nationwide survey of hepatitis C services provided by drug treatment programs. J Subst Abuse Treat. 2002;22(2):55–62. doi: 10.1016/s0740-5472(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. [August 4, 2012];HIV Screening. Accessed at http://www.uspreventiveservicestaskforce.org/uspstf05/hiv/hivrs.htm.
- Wood E, Tyndall MW, Spittal PM, Li K, Hogg RS, Montaner JSG, O’Shaughnessy MV, Schechter MT. Factors associated with persistent high-risk syringe sharing in the presence of an established needle exchange programme. J AIDS. 2002;16(6):941–943. doi: 10.1097/00002030-200204120-00021. [DOI] [PubMed] [Google Scholar]


