Abstract
This review addresses the latest advances in our understanding of the regulation of a novel Ca2+ signal called L-type Ca2+ channel sparklets in arterial smooth muscle. L-type Ca2+ channel sparklets are elementary Ca2+ influx events produced by the opening of a single or a small cluster of L-type Ca2+ channels. These Ca2+ signals were first visualized in the vasculature in arterial smooth muscle cells. In these cells, L-type Ca2+ channel sparklets display two functionally distinct gating modalities that regulate local and global intracellular Ca2+ concentration ([Ca2+]i). The activity of L-type Ca2+ channel sparklets varies regionally within a cell depending on the dynamic activity of a cohort of protein kinases and phosphatases recruited to L-type Ca2+ channels in the arterial smooth muscle sarcolemma in a complex coordinated by the scaffolding molecule A kinase anchoring protein 150 (AKAP150). We also described a mechanism whereby clusters of L-type Ca2+ channels gate cooperatively to amplify intracellular Ca2+ signals with likely pathological consequences.
Introduction
The function of arteries is to deliver blood to organs and tissues. Arterial diameter is a critical regulator of blood flow (26) and is largely determined by the contractile state of smooth muscle cells lining the walls of arteries. Classic work by Bayliss (5) demonstrated that arteries respond to increases in intravascular pressure and stretch by constricting, thereby maintaining constant blood flow. This process is called the myogenic response, which is an inherent property of arterial smooth muscle cells (14, 25, 46).
Generally accepted models for the development of myogenic tone indicate a critical role for Ca2+ influx via L-type Ca2+ channels in the sarcolemma of arterial smooth muscle cells (28, 31, 41). In contrast to cardiac myocytes and many other excitable tissues, in arterial smooth muscle L-type Ca2+ channels do not contribute to or are subjected to the influence of action potentials. Rather, L-type Ca2+ channels respond to graded changes in membrane potential (41), such as those resulting from alteration of intravascular pressure (27). While the importance of L-type Ca2+ channels in arterial smooth muscle is well established, examination of the spatiotemporal organization of these channels has only recently been possible (32, 43, 44). Advances in imaging technologies during the last decade have allowed for optical recording of subcellular Ca2+ signals produced by Ca2+ influx through single L-type Ca2+ channels (i.e. L-type Ca2+ channel sparklets). The superior temporal and spatial resolution provided by emerging Ca2+ imaging technologies (e.g. confocal and total internal reflection fluorescence (TIRF)) have redefined our understanding of mechanisms underlying Ca2+ influx in arterial smooth muscle (15, 32, 50, 53). Such recordings of L-type Ca2+ channel sparklets have allowed investigators to unravel regulatory mechanisms and dynamics of underlying L-type Ca2+ channels with exquisite detail, revealing important features about their activity, functional localization and impact on excitation-contraction (EC) and excitation-transcription (ET) coupling in arterial smooth muscle (17, 33–35). Here, these findings, and their implications are discussed in the context of an experimental model based on local regulation of L-type Ca2+ channel activity in these cells.
Imaging L-type Ca2+ channel sparklets in arterial smooth muscle
Ca2+ influx through L-type Ca2+ channels plays a vital role in the regulation of arterial smooth muscle excitability, contractility and gene expression (6, 22, 37). Due to the ubiquity of Ca2+ with respect to cellular signaling, changes in Ca2+ concentration must be spatially restricted in order to regulate different cell signaling cascades. Thus, the spatial and temporal profile of Ca2+ signals produced by L-type Ca2+ channels is crucial for appropriate cell function. Experimental limitations precluded direct investigation of different Ca2+ signaling in arterial smooth muscle until recently. Our ability to characterize various Ca2+ signals in arterial smooth muscle was revolutionized with recent advances in imaging and fluorescence Ca2+ indicator technology (4, 32, 53). Accordingly, optical recording of Ca2+ permeable channels offers complementary yet, distinct advantages over the patch-clamp technique in that: 1) it simultaneously monitors the activity of multiple channels at high speed, and 2) it provides important information about the localization and mobility of functional channels (16, 32).
The first optical recordings of Ca2+ influx via sarcolemmal Ca2+ permeable channels (e.g. caffeine and stretch-sensitive channels) in smooth muscle cells were performed by Joshua Singer’s group using widefield fluorescence microscopy (53, 55). These optical recordings of submembrane Ca2+ signals were limited as they suffered from limited spatial and temporal resolution due to Ca2+ diffusion from the point of origin and the large focal plane of the widefield microscope (45). Experimental limitations associated with widefield microscopy have been ameliorated with the use of confocal (50) and TIRF (15, 32) microscopy, which limit the cytosolic volume that is imaged. In addition, imaging of Ca2+ influx via single L-type Ca2+ channels was greatly improved with the use of low affinity fluorescence Ca2+ indicators in combination with high affinity, and non-fluorescence Ca2+ buffers. These experimental conditions restrict the fluorescence signal to areas surrounding the pore of the channel (51). The use of the aforementioned approaches in combination with high-speed electron multiplying charged-coupled device (EMCCD) cameras allowed capture of two-dimensional images with ultra-high speed and sensitivity. Due to space limitations, a more extensive discussion of these issues can be found in (43, 45).
Using this approach, the Santana group was the first to record fluorescence Ca2+ influx events via single or small clusters of L-type Ca2+ channels in the sarcolemma of arterial smooth muscle cells (Figure 1A) (32). These fluorescent events were called L-type Ca2+ channel sparklets, following the name coined by Heping Cheng and colleagues, who were the first to optically record the activity of L-type Ca2+ channels in cardiac cells (50).
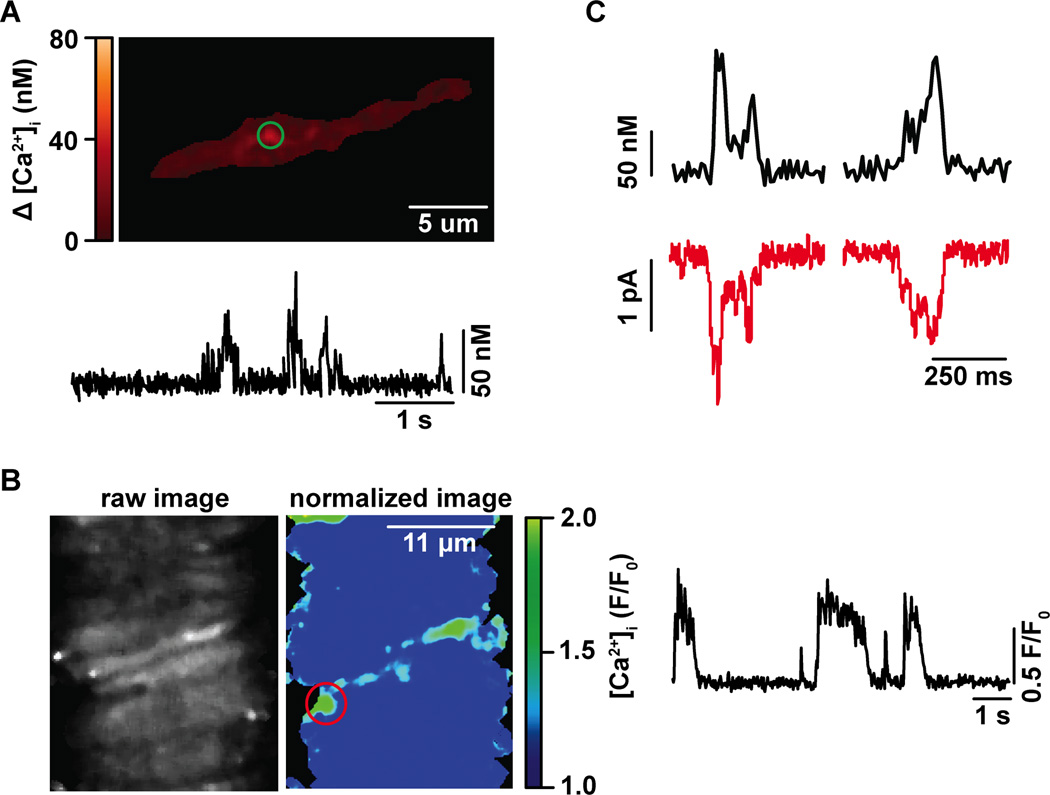
Figure 1. L-type Ca2+ channel sparklets in arterial smooth muscle and intact cerebral arteries.
A) Representative TIRF image of a cerebral arterial smooth muscle cell exhibiting L-type Ca2+ channel sparklets. The trace below the image shows the time course of [Ca2+]i in the area highlighted by the green circle. B) Representative confocal image of an intact cerebral artery. The trace on the right side panel shows the time course of [Ca2+]i in the site marked by the red circle in the normalized image. C) Simultaneous recording of L-type Ca2+ channel sparklets (black traces) and L-type Ca2+ currents (red traces).
General properties of L-type Ca2+ channel sparklets in arterial smooth muscle
In arterial smooth muscle cells, L-type Ca2+ channel sparklets are small with an average area of ~ 0.8 µm2. These optical events have variable amplitudes ranging from 20 nM to several hundred nM Ca2+, depending on the extracellular Ca2+ concentration and membrane potential. However, the amplitude of L-type Ca2+ channel sparklets is quantal in nature (e.g. ~38 nM Ca2+ with 20 mM Ca2+ at −70 mV), and varies depending on the number of quantal events activated (Table 1).
Table 1.
General properties of L-type Ca2+ channel sparklets in arterial smooth muscle
| Parameters | L-type Ca2+ channel Ca2+ sparklet |
|---|---|
| spatial spread (µm2) | 0.8a |
| quantal level (nM) | 38a |
| amplitude range (nM) | 20–500a |
| voltage dependency | Yesa,b |
| inhibition by nifedipine (10 µM) | Yesa |
| activation by Bay-K8644 (500 nM) | Yesa |
| activity (nPs) | low nPs = 0.07 |
| high nPs = 1.25a | |
| Ca2+ flux (fC) | low nPs = 122 |
| high nPs = 270c | |
| duration (ms) | low nPs τ = 23 |
| high nPs τfast = 42 | |
| high nPs τslow = 104c |
Data were obtained from L-type Ca2+ channel sparklets recorded in rat arterial smooth muscle.
Navedo et al (32); quantal level was determined with 20 mM Ca2+ at −70 mV,
Navedo et al (33),
Amberg et al (2). The signal mass and duration of L-type Ca2+ channel sparklets were recorded at the physiological membrane potential of −40 mV and using 2 mM extracellular Ca2+.
As expected from events resulting from Ca2+ influx via L-type Ca2+ channels, sparklets are activated by dihydropyridine agonists such as Bay-K 8644 and are inhibited by dihydropyridine antagonists such as nifedipine. The kinetics, voltage dependency and duration of L-type Ca2+ channel sparklets closely reflect those of underlying L-type Ca2+ currents (Figure 1C). Indeed, the signal mass of fluorescence events follows a linear relationship when compared to L-type Ca2+ currents in arterial smooth muscle.
L-type Ca2+ channel sparklet activity, as determined by analysis of nPs values (where n is the number of quantal levels and Ps is the probability of sparklet occurrence) is bimodal. Accordingly, sparklet sites display either low activity (i.e. nPs between 0.0 and 0.2), or high activity (i.e. nPs > 0.2; Table 1). High activity sparklets are also known as persistent L-type Ca2+ channel sparklets. Consistent with the level of activity in different L-type Ca2+ channel sparklet sites, low activity sparklets had shorter duration and lower levels of Ca2+ flux (i.e. signal mass) than persistent sparklet sites (for more detail about the pharmacological, molecular and biophysical properties of vascular sparklets see Table 1). It is important to note that L-type Ca2+ channel sparklets with indistinguishable biophysical properties have also been recorded in in situ smooth muscle contained in isolated intact arterial segments (Figure 1B). The recording of L-type Ca2+ channel sparklets in intact arteries will further improve our understanding of the impact of this novel Ca2+ signal on arterial excitation-contraction and excitation-transcription coupling during physiological and pathological conditions in a physiological setting.
Heterogeneous L-type Ca2+ channel sparklet spatial distribution and activity in arterial smooth muscle: A model for local regulation of L-type Ca2+ channels
An important feature of L-type Ca2+ channels that could only be revealed by optical recording methods is the variable nature of L-type Ca2+ channel sparklet activity and spatial distribution throughout the arterial smooth muscle sarcolemma (29, 32, 49). Multiple lines of evidence support this assessment. First, L-type Ca2+ channel sparklet activity (e.g. nPs) in arterial smooth muscle cells is bimodal, reflecting distinct gating modalities of L-type Ca2+ channels (7, 33). Second, analysis of Poisson distribution indicated that the spatial distribution of L-type Ca2+ channel sparklet sites within an arterial smooth muscle cell was not random, but rather restricted to certain regions (32) (Table 1). These results were unexpected since several reports suggested that L-type Ca2+ channels were broadly distributed throughout the sarcolemma and display stochastic activity in arterial smooth muscle cells (30, 41).
A snapshot of the molecular mechanisms underlying subcellular variation in L-type Ca2+ channel sparklet activity and spatial distribution in arterial smooth muscle has emerged in recent years. Based on biochemical, immunofluorescence, electrophysiological and optical clamping data, a subpopulation of L-type Ca2+ channels associate with the scaffolding protein A-kinase anchoring protein 150 (AKAP150) in complex with protein kinase C α (PKCα), protein kinase A (PKA) and the phosphatase calcineurin (35, 36, 39). Once targeted to the vicinity of L-type Ca2+ channels in the sarcolemma, kinases and phosphatase activity can dynamically regulate the activity of AKAP150-associated channels, thus inducing heterogeneous activation of L-type Ca2+ channels that is differentially modulated during physiological and pathological conditions (see below for details).
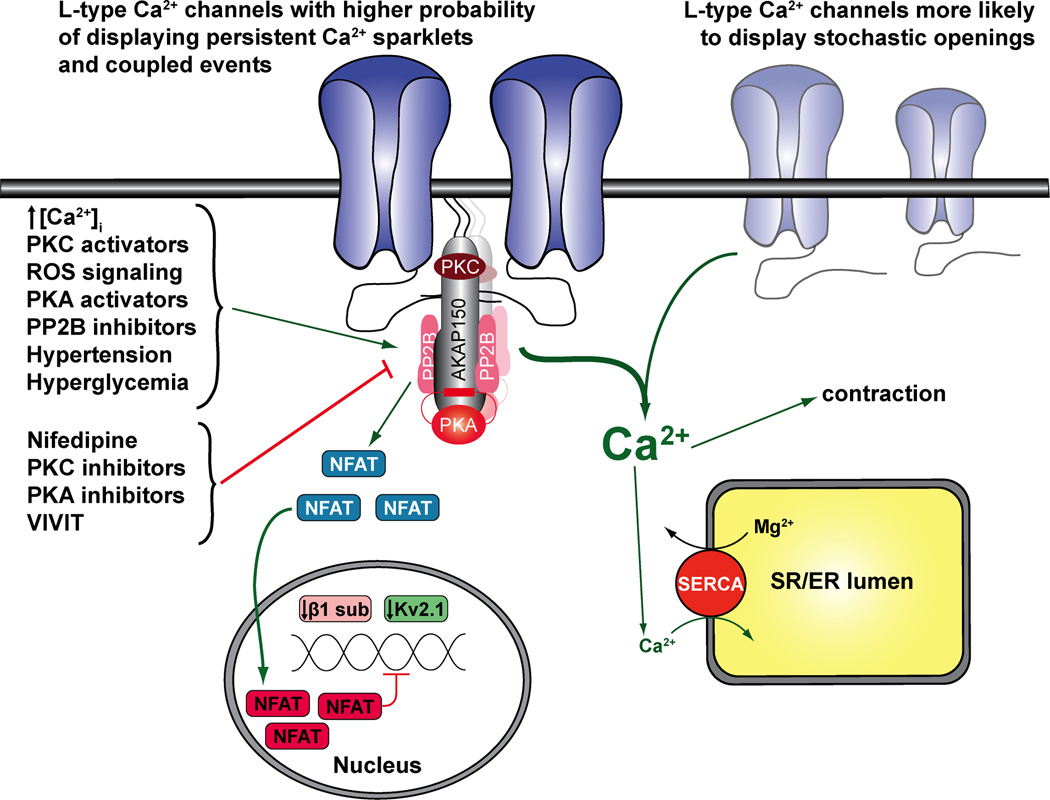
Figure 2 illustrates a proposed model for subcellular variation in L-type Ca2+ channel sparklet activity in arterial smooth muscle. It proposes that AKAP150 targets PKCα, PKA, and calcineurin to a subpopulation of L-type Ca2+ channels. These protein kinases and phosphatase have been shown to regulate L-type Ca2+ channels (11, 24, 42, 52). Under physiological conditions, activation of AKAP150-targeted PKCα by an increases in diacylglycerol or [Ca2+]i underlies persistent L-type Ca2+ channel sparklet activity. Interestingly, PKCα-dependent L-type Ca2+ channel sparklets not only correlate with, but also require concomitant increases in localized hydrogen peroxide-dependent changes in redox status following angiotensin II exposure (1, 8). AKAP150-targeted PKA does not appear to play a role in the induction of persistent L-type Ca2+ channel sparklet activity in arterial smooth muscle under basal conditions (33, 36). Instead, evidence suggests that AKAP-targeted PKA underlies enhanced L-type Ca2+ channel sparklet activity during diabetes (see below) (36). The actions of PKCα on L-type Ca2+ channels are opposed by activation of AKAP150-targeted calcineurin. In this model, the level of sparklet activity under physiological conditions varies regionally depending on the relative activities of AKAP150-targeted PKCα and calcineurin in arterial smooth muscle.
Figure 2. Proposed model for local regulation of L-type Ca2+ channels, excitation-contraction coupling and excitation-transcription coupling in arterial smooth muscle.
In this model, L-type Ca2+ channels are broadly distributed in the sarcolemma of arterial smooth muscle. However, a subpopulation of these channels can form clusters of varied sized driven by their interaction with the scaffolding protein A kinase anchoring protein 150 (AKAP150) in discrete regions of the sarcolemma. AKAP150 coordinate the assembly of a regulatory signaling complex form by PKA, PKC and calcineurin near AKAP150-associated L-type Ca2+ channels to promote heterogeneous channel activity and coupled gating. Conversely, L-type Ca2+ channels not associated with AKAP150 will likely exhibited stochastic channel openings. Heterogeneous L-type Ca2+ channel activity and coupled events are enhanced upon increase in [Ca2+]i, with activators of PKC, PKA or ROS signaling, with calcineurin inhibitors, or during hypertension and hyperglycemia. Meanwhile, in our model, these characteristics are minimized with application of nifedipine, or inhibitors of PKA ad PKC. The formation of this signaling unit controls local and global [Ca2+]i, which modulates the contractile machinery, SR Ca2+ refilling, and NFATc3-dependent gene expression in arterial smooth muscle. Green arrows indicate activation; red arrow indicates inhibition/downregulation.
Data in support of this model is compelling (35). First, immunofluorescence data indicate that AKAP150 and PKCα colocalize to similar regions in the sarcolemma of arterial smooth muscle. Consistent with this, knock down of AKAP150 prevents PKCα targeting to the membrane and abolishes persistent L-type Ca2+ channel sparklets. Second, inhibition of calcineurin with cyclosporine A increased persistent L-type Ca2+ channel sparklet activity in wild type cells, but was without effects in arterial smooth muscle from AKAP150-null mice. Third, persistent L-type Ca2+ channel sparklets were readily observed under control conditions, and further enhanced by application of PKC activators or calcineurin inhibitors in smooth muscle cells from a genetically modified AKAP150 mouse that does not anchor PKA. Altogether, data indicate that, under physiological conditions, the dynamic activity of AKAP150-targeted PKCα and calcineurin, but not PKA, underlies heterogeneous L-type Ca2+ channel sparklet activity in arterial smooth muscle.
The aforementioned model also helps explain mechanisms underlying subcellular variations in L-type Ca2+ channel sparklet spatial distributions. In addition to targeting kinases and phosphatases near L-type Ca2+ channels in the sarcolemma, AKAP150 also binds directly to the channel (12, 21, 39). Interestingly, not all L-type Ca2+ channels are associated with AKAP150, as the expression pattern of this scaffolding protein seems to be restricted to defined regions, while that of the channel seems to be broadly distributed in the sarcolemma of arterial smooth muscle cells (30, 35). Thus, subcellular variations in L-type Ca2+ channel sparklet spatial distribution may result from the association of a subpopulation of L-type Ca2+ channels with AKAP150 in specific regions in the sarcolemma of arterial smooth muscle cells (Figure 2). Future studies should determine the mechanisms regulating the expression pattern of AKAP150 in these cells, and whether this model also applies to the regulation of L-type Ca2+ channels in other cells, as well as other ion channels.
Coupled gating of L-type Ca2+ channel sparklets in arterial smooth muscle
Another interesting feature uncovered by the optical recording of L-type Ca2+ channel activity is that small clusters of channels can open and close cooperatively (i.e. coupled gating) in arterial smooth muscle cells (34). This assessment is based on multiple lines of evidence. First, while the amplitude of the majority of L-type Ca2+ channel sparklets follows a binomial distribution, the observed amplitude distribution from a subpopulation of sparklets diverged significantly from the expected outcome. Accordingly, all-point amplitude histograms for a group of L-type Ca2+ channel sparklet sites and L-type Ca2+ current obtained in cell-attached patches exhibited a higher frequency of multichannel events than that of events resulting from the simultaneous opening of fewer channels (34). Second, implementation of a simplified coupled Markov chain model (10) to quantitatively determine the coupling coefficient or strength (κ) between L-type Ca2+ channels revealed that numerous sparklet and current events were produced by L-type Ca2+ channels gating in unison. Accordingly, while the majority of L-type Ca2+ channel sparklets and Ca2+ currents had κ values of 0 (e.g. independently gating channels), a subpopulation of sparklets and currents exhibited κ values that ranged from 0.1 to 1 (e.g. transient to tightly coupled channels) (34). These results are consistent with the binomial distribution data above, and together suggested that although the majority of L-type Ca2+ channels in arterial smooth muscle cells may open stochastically, a subpopulation of channels can gate coordinately. Third, given the nonlinear nature of fluorescent signals produced by single wavelength Ca2+ indicators, the acquisition of optical as well as electrical recording is necessary for the accurate identification of coupled events, especially in circumstances where the optical signals have not been calibrated. Thus, employment of optical and electrical recordings together with these stringent analytical criteria is not only complementary, but also necessary for the accurate identification of coupled events. Interestingly, coupled gating is not a unique feature of L-type Ca2+ channels in arterial smooth muscle. Indeed, coupled gating has been observed for L-type Ca2+ channels in skeletal and cardiac myocytes (9, 23) and more recently for transient receptor potential channels in vascular endothelial cells (47).
Mechanisms underlying coupled gating of L-type Ca2+ channel sparklets in arterial smooth muscle are the subject of intense investigation. A model has been proposed whereby rearrangements of the ubiquitous calcium sensor and regulatory molecule calmodulin (CaM) from its putative binding motif (i.e. IQ domain) in L-type Ca2+ channels are important for coupled gating. Accordingly, displacement of CaM from the IQ domain of L-type Ca2+ channels promotes transient interactions between the carboxy terminals of a variable number of channels, thus inducing coupled gating. Activation of PKCα, PKA (see below) or inhibition of calcineurin increases coupled gating of L-type Ca2+ channels (34). In support of this model, inhibition of CaM or activation of PKCα displaced CaM from the IQ domain of L-type Ca2+ channels, thus increasing coupled gating activity. In addition, Förster resonance energy transfer (FRET) analysis revealed that the carboxy terminal of L-type Ca2+ channels comes into close juxtaposition of each other under conditions that favor coupled gating. Consistent with this hypothesis, an L-type Ca2+ channel construct lacking most of the carboxy terminal showed no coupled gating activity. Interestingly, this construct also eliminates the section of the channel that interacts with AKAP150, suggesting that this scaffolding protein may also play an important role in coupled gating of L-type Ca2+ channels. Accordingly, the gating mode of L-type Ca2+ channels in arterial smooth muscle cells from an AKAP150-null mice was mostly stochastic even under conditions that favor coupled gating in cells from wild type animals (e.g PKC activation) (9, 34). Finally, novel optogenetic approaches demonstrated that light-induced fusion of the carboxy tail of wild type L-type Ca2+ channels promoted coupled gating (17). Altogether, these results suggest that coupled gating of L-type Ca2+ channel sparklets results from CaM-dependent transient contacts between the carboxy terminals of a subpopulation of AKAP150-associated L-type Ca2+ channels in arterial smooth muscle (Figure 2). Thus, coupled gating of L-type Ca2+ channels may represent a novel mechanism for the regulation and amplification of Ca2+ signals in arterial smooth muscle cells with important physiological and pathological consequences. At present however, the model of coupled gating of L-type Ca2+ channels in arterial smooth muscle remains largely untested. Future studies should examine this model in further detail.
Physiological role of L-type Ca2+ channel sparklets in arterial smooth muscle
Figure 2 shows an illustration depicting a model for the regulation of EC and ET coupling by L-type Ca2+ channel sparklets in arterial smooth muscle. EC and ET coupling refers to mechanisms by which increases in intracellular Ca2+ induce muscle contraction or changes in gene expression, respectively. Let us discuss the role of L-type Ca2+ channel sparklets on EC coupling first.
As mentioned above, L-type Ca2+ channel sparklet activity is bimodal, with sites exhibiting either low activity or persistent activity (43). An important question that arises from this observation is whether L-type Ca2+ channel sparklet activity and gating modalities are physiologically relevant in arterial smooth muscle. This issue has been systematically addressed in isolated arterial smooth muscle cells and in intact arteries (2). Using a signal mass analytical approach (54), it was found that Ca2+ influx was greater via persistent L-type Ca2+ channel sparklets than via low activity sparklets. In addition, membrane depolarization to physiological potentials increased Ca2+ influx via low activity and persistent L-type Ca2+ channel sparklets. Accordingly, data indicate that Ca2+ entering via persistent L-type Ca2+ channel sparklets accounts for ~50% of the total dihydropyridine-sensitive Ca2+ influx component in arterial smooth muscle cells. Consistent with this, loss of persistent L-type Ca2+ channel sparklet activity by ablation of PKCα decreased whole-cell L-type Ca2+ currents and global intracellular Ca2+ in dissociated arterial smooth muscle cells and intact arteries by ~50%. Importantly, and in agreement with these data and the local control model, myogenic tone was nearly abolished in AKAP150- and PKCα-null mice. Remarkably, only 1–2 persistent sparklet sites, produced by activation of 2–8 L-type Ca2+ channels out of ~5,000–10,000 expressed channels, are seen at any given time under basal conditions in arterial smooth muscle (2, 32, 35, 41). Thus, although persistent L-type Ca2+ channel sparklets account for less than 1% of total channels in the sarcolemma, they are major contributors to Ca2+ influx in arterial smooth muscle cells. Altogether, these results suggest that low activity and persistent L-type Ca2+ channel sparklets control local and global elevations in intracellular Ca2+ in arterial smooth muscle cells, and consequently regulate arterial excitability.
Another mechanism by which L-type Ca2+ channel sparklets could regulate arterial excitability is by modulating sarcoplasmic reticulum (SR) Ca2+ load and/or release (e.g Ca2+ sparks) via the mechanisms of Ca2+-induced Ca2+ release (CICR). Data indicate that L-type Ca2+ channel sparklets coincide with junctional SR regions expressing proteins involved in SR Ca2+ load (e.g. Ca2+-ATPase SERCA) and release (ryanodine receptors (RyRs)). Moreover, L-type Ca2+ channel sparklets occur in areas near Ca2+ sparks (48). However, L-type Ca2+ channel sparklets do not modulate SR Ca2+ content or Ca2+ spark activity, nor was L-type Ca2+ channel sparklet activity regulated by SR Ca2+ concentration. Nonetheless, L-type Ca2+ channel sparklet activity was required for faster SR Ca2+ refilling. These data are consistent with the idea that RyRs and L-type Ca2+ channels are loosely coupled in arterial smooth muscle (13, 19). Furthermore, data support a model in which L-type Ca2+ channel sparklets induce Ca2+ influx into a global cytosolic Ca2+ pool from which the SR can draw to accelerate store refilling in arterial smooth muscle (Figure 2).
The local control model proposed above also helps explain the role of L-type Ca2+ channel sparklets in ET coupling in arterial smooth muscle. Accordingly, local Ca2+ signaling via L-type Ca2+ channels has been shown to stimulate activation of several transcription factors, including NFATc3 (18, 37, 39). Furthermore, it has been found that formation of a signaling pentad composed of AKAP150, PKCα, PKA, calcineurin and L-type Ca2+ channels contributed to activation of NFAT (37, 39). Based on these data, we propose that Ca2+ influx via a subpopulation of L-type Ca2+ channels associated with AKAP150 promotes the activation of AKAP150-targeted calcineurin. Once activated, calcineurin dephosphorylates NFAT, thus allowing translocation of this transcription factor into the nucleus where it can regulate gene expression. In this model, NFAT activity is regulated by the levels of L-type Ca2+ channel activity and nuclear NFAT export rates (20).
Role of L-type Ca2+ channel sparklets in vascular dysfunction
The findings described above suggest that persistent L-type Ca2+ channel sparklets and functional cooperativity of L-type Ca2+ channels may have profound functional implications on EC and/or ET coupling in arterial smooth muscle. A surge in the frequency of persistent L-type Ca2+ channel sparklets and/or coupled events could result in an increase in Ca2+ influx and consequently, global [Ca2+]i that may further activate the contractile machinery in arterial smooth muscle. Indeed, upregulation of the expression and activity of L-type Ca2+ channels has been associated with elevated arterial tone during hypertension (40). Consistent with this, persistent L-type Ca2+ channel sparklet activity and the frequency of coupled events are increased in arterial smooth muscle during hypertension and in type II diabetes (36, 37).
An interesting concept raised by the aforementioned findings is that increased L-type Ca2+ channel sparklet activity and/or coupled events is not a common phenomenon throughout the sarcolemma of arterial smooth muscle cells. Rather, data suggest that additional activation of a limited number of channels account for the majority of the Ca2+ influx underlying abnormal arterial smooth muscle contraction and tone during pathological conditions. This observation is consistent with the proposed model for local regulation of L-type Ca2+ channels in arterial smooth muscle. Thus, it seems as if arterial smooth muscle cells functionally operate on the edge between beneficial (e.g. physiological) and detrimental (e.g. pathological) conditions, in which a stimuli that enhances persistent L-type Ca2+ channel sparklet activity and/or coupled gating events in a small cluster of L-type Ca2+ channels is a critical initial step in the chain of events leading to the development of pathological conditions such as hypertension and diabetic vascular dysfunction.
The mechanisms underlying increased persistent L-type Ca2+ channel sparklet activity during hypertension and type II diabetes vary depending on the pathological conditions (Figure 2). While it seems that an AKAP is a critical component for the induction of persistent L-type Ca2+ channel sparklets during each pathological condition, enhanced sparklet activity and coupled events during hypertension results primarily from increased channel expression, AKAP150-targeted PKCα activity (37) and likely oxidative stress (1, 8). Meanwhile, activation of AKAP-targeted PKA contributes to higher persistent L-type Ca2+ channel sparklet activity and possibly coupled events during hyperglycemia and type II diabetes (36). This finding was surprising since multiple studies have suggested that PKA activation relaxes arterial smooth muscle by reducing global [Ca2+]i. A potential answer to this conundrum was provided by the Santana and Navedo groups (36). According to the local control model, a stimulus that primarily activates AKAP-targeted PKA to regions near a subpopulation of AKAP-associated L-type Ca2+ channels could preferentially increase persistent L-type Ca2+ channel sparklet activity, without the hyperpolarizing effects of global PKA activation (e.g. activation of large conductance, Ca2+-activated K+ channels). Ultimately, this will increase global [Ca2+]i in arterial smooth muscle and consequently, enhanced arterial tone during type II diabetes. Altogether, these findings suggest that AKAPs are responsible for the organization of a macromolecular signaling complex that is capable of differentially modulating the activity of a select subpopulation of L-type Ca2+ channels to promote persistent L-type Ca2+ channel sparklet activity and coupled events during different pathological conditions.
Increased persistent L-type Ca2+ channel sparklet activity and frequency of coupled events during hypertension and likely type II diabetes may also regulate ET coupling in arterial smooth muscle (3, 37). Indeed, local Ca2+ signals via L-type Ca2+ channels activate the prohypertensive calcineurin/NFATc3 signaling cascade (37) thus leading to downregulation of several K+ channel subunits in arterial smooth muscle during hypertension (3, 38). On the basis of data described above, the following model for activation of this signaling cascade during hypertension and possibly type II diabetes is proposed (Figure 2). Activation of AKAP150-targeted PKCα or AKAP150-targeted PKA induces persistent L-type Ca2+ channel sparklets and coupled gating events during hypertension and type II diabetes, respectively. This produces an amplification of local Ca2+ influx, which specifically activates nearby AKAP150-targeted calcineurin. Once activated, calcineurin dephosphorylates NFATc3. Upon dephosphorylation, NFATc3 translocates to the nucleus where it can modify the expression of voltage-dependent (Kv) and large conductance, Ca2+-activated K+ (BK) channel subunits. Downregulation of Kv and/or BK channel function depolarizes arterial smooth muscle, thus further activating L-type Ca2+ channels. This will ultimately promote elevation of global [Ca2+]i and vasoconstriction thus effectively perpetuating the cycle. This positive feedback loop may be interrupted by pharmacological blockade of L-type Ca2+ channels, or by inhibition of calcineurin or NFAT. Furthermore, and consistent with the local control model, ablation of AKAP150 or PKCα protects against the development of angiotensin II-induced hypertension (35, 38). While this model has been described for an angiotensin II-induced hypertension model, the role of AKAP150 and relevance of this model to vascular dysfunction during type II diabetes requires confirmation.
Conclusions
The past few years have provided important new insights into the behavior and regulation of L-type Ca2+ channels in arterial smooth muscle. The use of optical recording approaches to study L-type Ca2+ channels in native cells and tissues has been instrumental to advancing our understanding of the dynamics of these channels and how they modulate arterial smooth muscle excitability. Indeed, several paradigm-shifting observations have been made since the first optical recording of L-type Ca2+ channels in the sarcolemma of arterial smooth muscle. Perhaps, one of the most important discoveries is that the activity of L-type Ca2+ channels is heterogeneous throughout the sarcolemma of arterial smooth muscle. This finding raises an interesting issue in that a limited number of channels account for 50% of the total dihydropyridine-sensitive Ca2+ influx in these cells, and enhanced Ca2+ influx during pathological conditions. The use of optical recording approaches and unique analytical strategies have also helped delineate a local control model for the regulation of L-type Ca2+ channels, and unexpectedly revealed a second paradigm-shifting observation: L-type Ca2+ channels can open in unison. In this model, AKAP150 plays a central role, both because of 1) its well-documented ability to target a specific cohort of regulatory proteins near L-type Ca2+ channels and 2) its capacity to promote functional coupling between a subpopulation of adjacent channels. This latter point has important functional implications. Accordingly, coupled gating of L-type Ca2+ channels may result in the amplification of Ca2+ influx with significant consequences for EC and ET coupling in arterial smooth muscle.
Acknowledgements
The authors would like to thanks Drs. Madeline Nieves-Cintrón and Matthew A. Nystoriak for critically reading this manuscript. This work was supported by grants from the American Heart Association – Scientist Development Grant 0735251N and National Institute of Health 1R01HL098200 (to MFN), and the Pew Scholars Program and the Colorado State University College Research Council (to GCA).
References
- 1.Amberg GC, Earley S, Glapa SA. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ Res. 2010;107:1002–1010. doi: 10.1161/CIRCRESAHA.110.217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium Sparklets Regulate Local and Global Calcium in Murine Arterial Smooth Muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 Regulates Kv2.1 Expression in Arterial Smooth Muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 4.Axelrod D. Selective imaging of surface fluorescence with very high aperture microscope objectives. J Biomed Opt. 2001;6:6–13. doi: 10.1117/1.1335689. [DOI] [PubMed] [Google Scholar]
- 5.Bayliss WM. On the local reaction of the arterial wall to changes in internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse : roles of ryanodine receptors and voltage- dependent Ca2+ channels. Circ Res. 2000;86:760–767. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 8.Chaplin NL, Amberg GC. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels. Am J Physiol Cell Physiol. 2012;302:C1382–C1393. doi: 10.1152/ajpcell.00222.2011. [DOI] [PubMed] [Google Scholar]
- 9.Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, Scott JD, Santana LF. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ Res. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung SH, Kennedy RA. Coupled Markov chain model: characterization of membrane channel currents with multiple conductance sublevels as partially coupled elementary pores. Math Biosci. 1996;133:111–137. doi: 10.1016/0025-5564(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 11.Cobine CA, Callaghan BP, Keef KD. Role of L-type Calcium Channels and PKC in Active Tone Development in Rabbit Coronary Artery. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01261.2006. [DOI] [PubMed] [Google Scholar]
- 12.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 13.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115:653–662. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox RH, Rusch NJ. New Expression Profiles of Voltage-gated Ion Channels in Arteries Exposed to High Blood Pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 15.Demuro A, Parker I. Imaging the activity and localization of single voltage-gated Ca2+ channels by total internal reflection fluorescence microscopy. Biophys J. 2004;86:3250–3259. doi: 10.1016/S0006-3495(04)74373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demuro A, Parker I. "Optical patch-clamping": single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. J Gen Physiol. 2005;126:179–192. doi: 10.1085/jgp.200509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon RE, Yuan C, Cheng EP, Navedo M, Santana LF. Calcium signaling amplification by oligomerization of L-type Cav1.2 channels. Proc Natl Acad Sci U S A. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 19.Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and RyR to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol. 2007 doi: 10.1113/jphysiol.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez MF, Bosc LV, Stevenson AS, Wilkerson MK, Hill-Eubanks DC, Nelson MT. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 and Crm-1. J Biol Chem. 2003;278:46847–46853. doi: 10.1074/jbc.M304765200. [DOI] [PubMed] [Google Scholar]
- 21.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 22.Harder DR, Gilbert R, Lombard JH. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am J Physiol. 1987;253:F778–F781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- 23.Hymel L, Striessnig J, Glossmann H, Schindler H. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc Natl Acad Sci U S A. 1988;85:4290–4294. doi: 10.1073/pnas.85.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res. 1993;73:1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- 25.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- 27.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langton PD, Standen NB. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. The Journal of physiology. 1993;469:535–548. doi: 10.1113/jphysiol.1993.sp019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarron JG, Olson ML, Currie S, Wright AJ, Anderson KI, Girkin JM. Elevations of intracellular calcium reflect normal voltage-dependent behavior, and not constitutive activity, of voltage-dependent calcium channels in gastrointestinal and vascular smooth muscle. J Gen Physiol. 2009;133:439–457. doi: 10.1085/jgp.200810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore ED, Voigt T, Kobayashi YM, Isenberg G, Fay FS, Gallitelli MF, Franzini-Armstrong C. Organization of Ca2+ release units in excitable smooth muscle of the guinea-pig urinary bladder. Biophys J. 2004;87:1836–1847. doi: 10.1529/biophysj.104.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. Embo J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navedo MF, Amberg G, Votaw SV, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms Underlying Heterogeneous Ca2+ Sparklet Activity in Arterial Smooth Muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 36.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C211–C220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 Down-regulates the β1 Subunit of Large Conductance, Calcium-activated K+ Channels in Arterial Smooth Muscle and Contributes to Hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 39.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesic A, Madden JA, Pesic M, Rusch NJ. High Blood Pressure Upregulates Arterial L-Type Ca2+ Channels. Is Membrane Depolarization the Signal? Circ Res. 2004 doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 41.Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol. 2009;47:436–444. doi: 10.1016/j.yjmcc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santana LF, Navedo MF, Amberg GC, Nieves-Cintron M, Votaw VS, Ufret-Vincenty CA. Calcium sparklets in arterial smooth muscle. Clin Exp Pharmacol Physiol. 2008;35:1121–1126. doi: 10.1111/j.1440-1681.2007.04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuai J, Parker I. Optical single-channel recording by imaging Ca2+ flux through individual ion channels: theoretical considerations and limits to resolution. Cell Calcium. 2005;37:283–299. doi: 10.1016/j.ceca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda Y, Nystoriak MA, Nieves-Cintron M, Santana LF, Navedo MF. Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle. Am J Physiol Heart Physiol. 2011;301:H2285–H2294. doi: 10.1152/ajpheart.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nature Chem Biol. 2007;3:423–431. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 51.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong J, Hume JR, Keef KD. Anchoring protein is required for cAMP-dependent stimulation of L-type Ca2+ channels in rabbit portal vein. Am J Physiol. 1999:C840–C844. doi: 10.1152/ajpcell.1999.277.4.C840. [DOI] [PubMed] [Google Scholar]
- 53.Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Imaging Ca2+ entering the cytoplasm through a single opening of a plasma membrane cation channel. J Gen Physiol. 1999;114:575–588. doi: 10.1085/jgp.114.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Using total fluorescence increase (signal mass) to determine the Ca2+ current underlying localized Ca2+ events. J Gen Physiol. 2004;124:259–272. doi: 10.1085/jgp.200409066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Visualization of Ca2+ entry through single stretch-activated cation channels. Proc Natl Acad Sci U S A. 2002;99:6404–6409. doi: 10.1073/pnas.092654999. [DOI] [PMC free article] [PubMed] [Google Scholar]