Abstract
IL-35 is a member of the IL-12 family of cytokines consisting of IL-12 p35 subunit and IL-12 p40-related protein subunit, EBV-induced gene 3 (EBI3). IL-35 functions through IL-35R and has a potent immune suppressive activity. Although IL-35 has been demonstrated to be produced by regulatory T cells, gene expression analysis has revealed that IL-35 is likely to have wider distribution including expression in cancer cells. In this study we have demonstrated that IL-35 is produced in human cancer tissues such as large B cell lymphoma, nasopharyngeal carcinoma and melanoma. In order to determine the roles of tumor-derived IL-35 in tumorigenesis and tumor immunity, we generated IL-35 producing plasmacytoma J558 and B16 melanoma cells, and observed that the expression of IL-35 in cancer cells does not affect their growth and survival in vitro, but stimulates tumorigenesis in both immune competent and Rag1/2 deficient mice. Tumor-derived IL-35 increases CD11b+Gr1+ myeloid cell accumulation in tumor microenvironment, and thereby promotes tumor angiogenesis. In immune competent mice, spontaneous CTL responses to tumors are diminished. IL-35 does not directly inhibit tumor antigen specific CD8+ T cell activation, differentiation and effector functions. However, IL-35-treated cancer cells had increased expression of gp130 and reduced sensitivity to CTL destruction. Thus, our study indicates novel functions of IL-35 in promoting tumor growth via enhancing myeloid cell accumulation, tumor angiogenesis and suppression of tumor immunity.
Introduction
Interleukin-35 (IL-35) is a dimeric protein composed of IL-12α and IL-27β chains, which are encoded by two separate genes IL12A and EBI3, respectively (1–3). A recent study has revealed that IL-35 signals through a unique heterodimer of receptor chains IL-12Rβ2 and gp130 or the homodimers of each chain in target cells (4). Although the expression pattern of IL-35 may differ in humans (5–6), IL-35 has been shown to be secreted by FoxP3+CD4+CD25+ Treg cells in mice (3) or “iTr35” cells, a regulatory T-cell population induced by IL-35 (7). IL-35 has also been shown to expand Foxp3+ Treg cells (2). IL-35-deficient (either deficient for EBI3 or P35) Treg cells have significantly reduced regulatory activity in vitro and fail to control homeostatic proliferation or cure inflammatory bowel disease (IBD) in vivo (3). Recombinant IL-35 suppresses T cell proliferation (2–3), Th17, Th2 responses and experimental arthritis (2), and airway inflammation (8). Ectopic expression of IL-35 in pancreatic β cells, prevents development of diabetes mellitus in NOD mice (9). Thus, IL-35 is a novel regulatory cytokine that has potent inhibitory effects on T cell responses.
Although the expression and function of IL-35 have only been demonstrated in Treg cells, gene expression analysis has revealed that IL-35 may have much broader tissue distribution (10). Reports indicate up-regulation of EBI3 and IL-12 p35 expressions in placental trophoblasts (11) and EBI3 associates with p35 in the extract of the trophoblastic components of human full-term normal placenta (1). EBI3 is also expressed in Hodgkin lymphoma cells (12), acute myeloid leukemia cells (13) and lung cancer cells (14). IL-12p35 (12), but not IL-27p28 (15) was detectable in EBI3-positive tumor cells, therefore it is likely that some cancer cells can produce IL-35 but not IL-27. In the tumor microenvironment, Foxp3+ Treg cells and other regulatory T cells are frequently demonstrated (16–17) and thus can provide another source of IL-35. In addition, tumor infiltrating dendritic cells were also found to express EBI3 (12, 15) and that could be additional source of IL-35. Taken together, IL-35 could be an important factor in the tumor microenvironment, which impacts tumor specific T cell responses and tumor progression.
The regulatory T cell-derived IL-35 has been shown to inhibit anti-tumor T cell response (7). siRNA silencing of EBI3 in lung cancer cells, inhibits cancer cell proliferation, whereas stable expression of EBI3 in lung cancer cells confers growth promoting activity in vitro (14). Moreover, high EBI3 gene expression in human lung cancer cells has been shown to be associated with poor prognosis (14). However, it is unclear if the observed effect was due to the production of the IL-35 heterodimer. Overall, little is known about the roles of tumor-derived IL-35 in tumorigenesis and anti-tumor CTL response. Based on the known roles of IL-35, we hypothesized that IL-35 production in the tumor microenvironment could contribute to tumor progression. To test this hypothesis, we generated IL-35 producing cancer cells and found that expression of IL-35 significantly increased tumorigenesis. IL-35 in the tumor microenvironment significantly increased the numbers of CD11b+Gr1+ myeloid cells in tumors and subsequently promoted tumor angiogenesis. Although tumor-derived IL-35 inhibits T cell responses in tumors in immune competent mice, IL-35 has no direct effects in stimulating tumor antigen specific CD8+ T cells. However, IL-35 up-regulates gp130 and renders cancer cells less susceptible to CTL destruction. Our results thus indicate novel functions of IL-35 in the tumor microenvironment.
Materials and Methods
Mice
BALB/c, C57BL/6 and Rag1−/−C57BL/6 mice were originally purchased from The Jackson Laboratories. Rag2−/−BALB/c mice were purchased from Taconic Farms (Germantown, New York, USA). Transgenic mice expressing a TCR specific for the tumor antigen P1A (P1CTL), whose TCR recognizes H-2Ld:P1A35-43 complex, have been described (18). All animal experiments were performed after approval by the Institutional Animal Care and Use Committee.
Cancer cell lines and tumor establishment in mice
Mouse plasmacytoma J558 cells (H-2Ld) have been described (19). Mouse plasmacytoma J558 cells or B16F10 melanoma cells were co-transfected with an expression vector pORF9-mIL-35elasti (InvivoGen) and a selection vector (pcDNA3-neo) or the control expression vector pORF9 (InvivoGen) and pcDNA3-neo. Thereafter, stable cell lines resistant to G418 were generated. RT-PCR was used to screen IL-35-positive cell lines and the primers used were: EBI3: 5’- ACG TCC TTC ATT GCC ACT TAC AGG CT-3’(forward), 5’-AGG GAG GCT CCA GTC ACT TGG TTT-3’(reverse). IL12A: 5'-AGG TGT CTT AGC CAG TCC CGA AAC C-3' (forward), 5'-CTG AAG GCG TGA AGC AGG ATG CAG A-3' (reverse). RT-PCR was also used to determine the expression of IL-35R subunits (IL-12Rβ2 and gp130) in IL-35-treated or IL-35-positive and negative tumor cells. The following primers were used: IL-12Rβ2: 5’-GTA TGA CCT TGT TTG TCT GCA AGC-3’ (forward), and 5’-CTG TAA ACG GTC TCA GAT CTC GCA-3’ (reverse); gp130: 5’-TGT CAC GTT CAC AGA CGT GGT CCT-3’ (forward), and 5’-CCA AGT TGA GGT ATC TTT GGT CCT-3’ (reverse). HPRT gene was amplified for PCR loading control, and the primers used were: 5’-GTC GTG ATT AGC GAT GAT GAA CCA-3’ (forward), and 5’-CAC CAG CAA GCT TGC AAC CTT AAC-3’ (reverse). The generated J558-IL-35, J558-Ctrl or B16-IL-35, B16-Ctrl cells were maintained in RPMI 1640 medium (Gibco) supplemented with 100 µg/ml penicillin, 100 µg/mL streptomycin, and 5% FBS. To establish tumors in mice, 5×106 J558-IL-35, J558-Ctrl or 0.1×106 B16-IL-35, B16-Ctrl cells were subcutaneously (s.c.) inoculated into the flank. The length (a) and width (b) of each tumor were measured using a digital caliper every 2 or 3 days. The tumor volume was calculated according to the formula V = ab2/2, as described (18–21).
Immunohistochemistry and Immunofluorescence
Immunohistochemistry (IHC) was used for the staining of formalin-fixed, paraffin-embedded cancer tissues. Immunostaining were performed on cancer tissues using 4 µg/ml of mouse anti-human IL-35 IgG1 (15K8D10, Imgenex, CA) for 120 min. Ab binding was detected using anti-mouse peroxidase conjugated EnVision reagent and diaminobenzidine as chromogen. Sections were also counterstained with Mayer hematoxylin. Standard H&E staining was performed on each serial cancer tissues.
Immunofluorescence staining was performed on tissues of mouse tumors. Briefly, established mouse tumors were harvested and frozen in Tissue-Tek OCT media (Sakura Finetek), and 10-µm-thick slices were prepared. Tissue sections were fixed in ice cold acetone for 30 seconds and were then stained with the corresponding fluorescent antibodies overnight at 4°C. The antibodies used for fluorescence staining of tumor sections were the following: FITC-anti-CD31, FITC-anti-VEGF, PE-anti-Gr1 and FITC-anti-CD11b. After washing with phosphate-buffered saline (PBS), slides were mounted with DAPI-containing Vectashield mounting medium (Vector Laboratories). For detection of apoptotic cells in tumor tissues, a TUNEL apoptosis detection kit (GenScript, NJ) containing biotin-11-dUTP and streptavidin-FITC was used by following a labeling protocol from the vendor. Immunofluorescence labeled slides were examined and photographed on an inverted three-color fluorescence microscope system (Nikon Ti-U). Images were analyzed and quantified using the ImageJ software (NIH).
Antibodies and flow cytometry
FITC-, PE-, APC- or PerCP- labeled antibodies to CD4, CD8α, Vα8.3, FoxP3, IFN-γ and isotype-matched control antibodies were purchased from BD Biosciences (San Diego, CA) or eBiosciences (San Diego, CA). For staining of cell surface markers, cells (cultured lymphocytes and single cell suspensions of tumors) were stained with various antibodies in staining buffer (PBS with 1% FCS) and incubated on ice for 30 min. After washing with staining buffer, cells were fixed in 1% Paraformaldehyde in PBS. For intracellular cytokine staining, cells were stimulated in culture medium for 4 h with 100 ng/ml of phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml of ionomycin in the presence of Golgistop (1:1500; BD Biosciences). Viable cells were first stained for cell surface markers and then fixed in IC fixation buffer (eBioscience), permeabilized with 1×permeabilization buffer (eBiosciece) and stained with respective antibodies. Staining for FoxP3 was performed according to manufacturer’s protocol (BD Biosciences). Stained cells were analyzed on a FACSCalibur flow cytometer and data were analyzed using the flowjo software (Tree Star, Inc., OR).
IL-35 ELISA
A mouse IL-35 ELISA kit (MyBioSource, San Diego, CA) was used to detect IL-35 concentration in the supernatants of cell cultures or protein lysates of established IL-35-positive and negative J558 tumors.
MTT assay
To determine proliferation and survival of IL-35-positive and negative tumor cells, a MTT assay kit (ATCC) was used by following the instructions of the manufacturer.
3H-Thymidine incorporation assay and 51Cr-release assay
For measuring P1CTL cells proliferation, 0.3 × 106/ml of spleen and lymph node cells from P1CTL mice were cultured in click’s EHAA medium (Invitrogen) containing 100 µg/ml penicillin and 100 µg/ml streptomycin, 1 mM 2-ME, 5% fetal bovine serum (FBS), and 0.2 µg/ml P1A35-43 peptide in the absence or presence of IL-35 containing culture supernatants from J558 cells in 96-well U-bottomed plates. For detection of proliferation of P1CTL cells, 3H-Thymidine was added in the culture for the last 12 h and incorporation of 3H-Tritium was quantified using a scintillation counter.
To determine cytotoxicity of cultured P1CTL cells, splenocytes from P1CTL TCR transgenic mice were stimulated with P1A peptide (0.1 µg/ml) for 5 days in the presence or absence of IL-35 and used as effectors. 51Cr-labeled tumor cells with or without IL-35 incubation were used as targets. The effector T cells and the targets were incubated together for 6 h, and the percentages of specific lysis were calculated based on the following formula: specific lysis % = 100 × (cpmsample−cpmmedium)/(cpmmax−cpmmedium).
Cell migration assay
CD11b+ or Gr1+ cells were purified from spleens of J558 tumor-bearing Rag2−/− mice or bone marrows of normal mice. Macrophage cell lines Raw264.7 (H-2b) and P338D1 (H-2d) were cultured in complete RPMI 1640 medium. 2×105 cells in 200 µl of medium were added to the upper chamber of a transwell system (3.0–8.0 micron PET, BD Biosciences) and inserted into a 24-well plate. The lower chambers of each transwell were filled with 800 µl of culture medium with or without the presence of IL-35. The cells were incubated for 24 h at 37°C, then medium was discarded and the trans-membranes were washed and numbers of cells migrated into the bottom surface of the trans-membranes were counted under microscope.
Statistical analysis
Data are expressed as mean ± SD. Two-tailed Student’s t-test was used for statistical analysis. p<0.05 was considered significant.
Results
1. IL-35 is produced in human cancer tissues
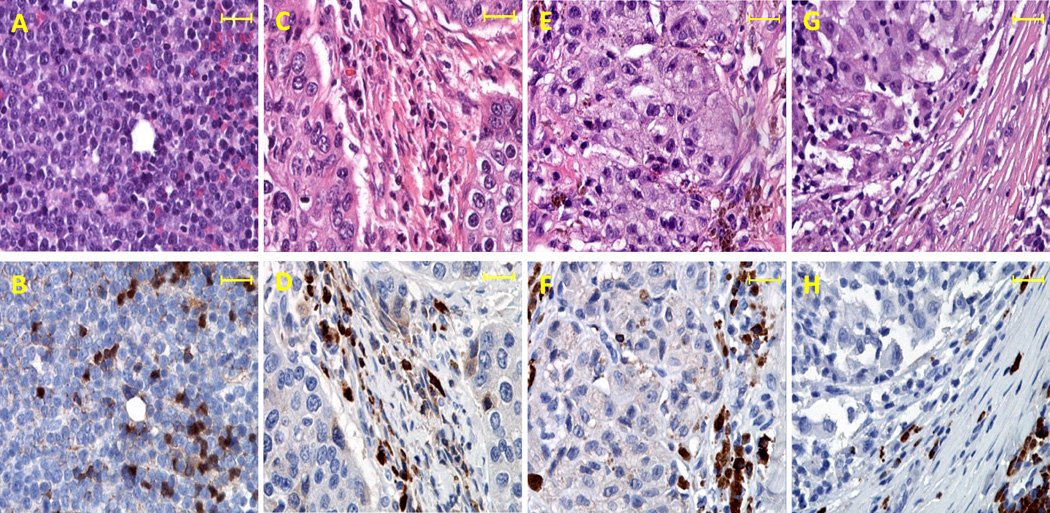
Accumulating evidences suggest that human cancer tissues may produce IL-35. IHC studies have revealed that EBI3 protein can be detected in Hodgkin lymphoma cells (12), nasopharyngeal carcinoma cells (12) and lung cancer cells (14). IL-12p35 (12), but not IL-27p28 (15) was detectable in EBI3-positive tumor cells. Therefore it is likely that some cancer cells can produce IL-35. Moreover, tumor infiltrating dendritic cells (TIDC) also express EBI3 protein (12, 15), thus TIDC could be an additional source of IL-35. Furthermore, Foxp3+ Treg cells are frequently found in human cancer tissues (16–17) and could be an additional source of IL-35. Accordingly, it is possible that IL-35 could be detected in human cancer tissues. To test this hypothesis, we performed IL-35 IHC analysis on human large B cell lymphoma (Fig.1A–B), nasopharyngeal carcinoma (Fig.1C–D), two known cancer types that are associated with EBV infection. In addition, we also examined IL-35 expression in non-EBV related cancer tissues such as melanoma. As shown in Fig.1B, some large B cell lymphoma cells showed positive staining of IL-35. In other cancer types, IL-35 positive staining was mainly detected in the stromal cells rather than cancer cells (Fig.1D, 1F and 1H). In addition, IL-35-positive cells also exhibited various shapes (Fig.1D, 1F and 1H). Thus, IL-35 can be readily detected in various human cancer tissues and are likely of multiple cellular sources.
Fig.1. Expression of IL-35 in human cancer tissues.
H&E staining and IHC were performed on paraffin-embedded serial tissue sections of human large B cell lymphoma (A–B), nasopharyngeal carcinoma (C–D), skin melanoma (E–F) and lymph node metastatic melanoma (G–H). The anti-human IL-35 mAb 15K8D10 (Imgenex, CA) was used to stain human cancer tissues. Images shown in the upper panel (A, C, E and G) are H&E staining, and images shown in the lower panel (B, D, F and H) are IL-35 specific staining. Scale bars, 100µm.
2. IL-35 production in the tumor microenvironment stimulates tumor growth
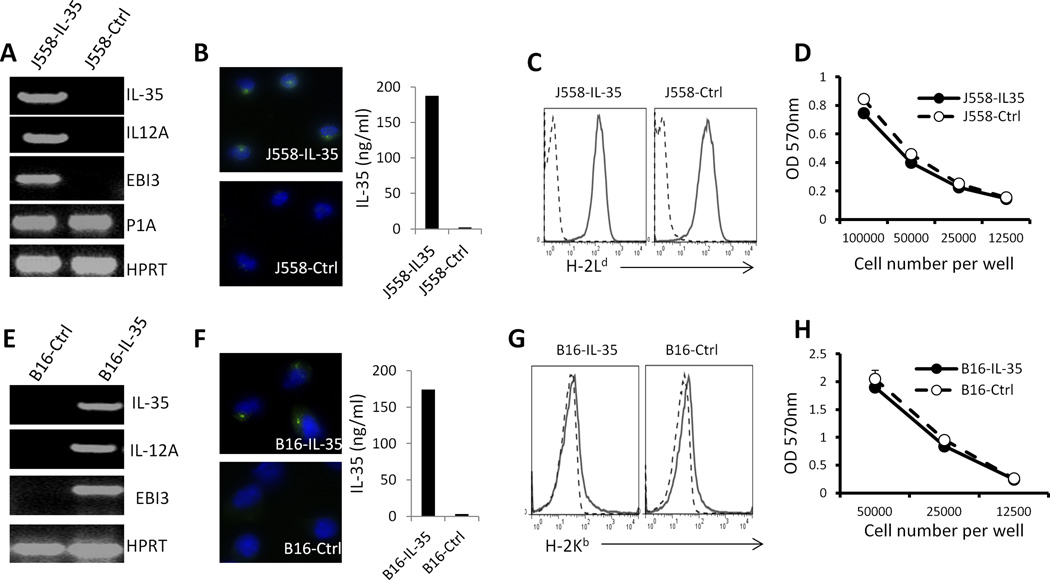
To determine the roles of IL-35 in the tumor microenvironment, we generated IL-35-positive and negative mouse plasmacytoma J558 cells and B16 melanoma cells by transfecting J558 and B16 melanoma cells with an IL-35 expression vector or a control expression vector. RT-PCR analysis revealed that J558-IL-35 and B16-IL-35 cells expressed both EBI3 and IL35A mRNA (Fig.2A and 2E). Moreover, RT-PCR assay using EBI3 forward primer and P35 reverse primer detected the recombinant RNA encoding both EBI3 and IL-35A subunits (Fig.2A and 2E). Thus, IL-35 molecule was stably expressed in J558-IL-35 and B16-IL-35 cells. Immunocytochemistry staining of cells and ELISA assay of supernatants from cell cultures revealed that IL-35 protein was produced by J558 (Fig.2B) and B16 (Fig.2F) cells. Expression of IL-35 in cancer cells did not alter the expression of MHC class I (Fig.2C and 2G) and tumor antigen P1A (Fig.2A) in J558 cells. MTT assay revealed that IL-35 expression did not affect J558 and B16 cell growth in vitro (Fig.2D and 2H).
Fig.2. Generation of IL-35 producing J558 and B16.F10 cells.
Mouse plasmacytoma J558 cells or B16F10 melanoma cells were co-transfected with an expression vector pORF9-IL-35 (Invivogen) and a selection vector (pCDNA-neo) or the control expression vector pORF9 (Invivogen) and pCDNA-neo. Stable cell lines that were resistant to G418 were generated. RT-PCR was used for detecting the expression of transcripts for recombinant IL-35, IL-12A, EBI3 and tumor antigen P1A in J558 cells (A) and IL-35, IL-12A and EBI3 transcripts in B16F10 cells (E). Immunofluorescence staining and ELISA revealed that IL-35 protein was produced by the generated J558 (B) and B16.F10 (F) cells. Flow cytometry was used for the analysis of MHC class I expression on the generated J558 cells (C) and B16 cells (G). MTT proliferation assay (MTT kit, ATCC) was used to measure growth and proliferation of J558 cells (D) and B16 cells (H). Bars indicate SD of triplicates.
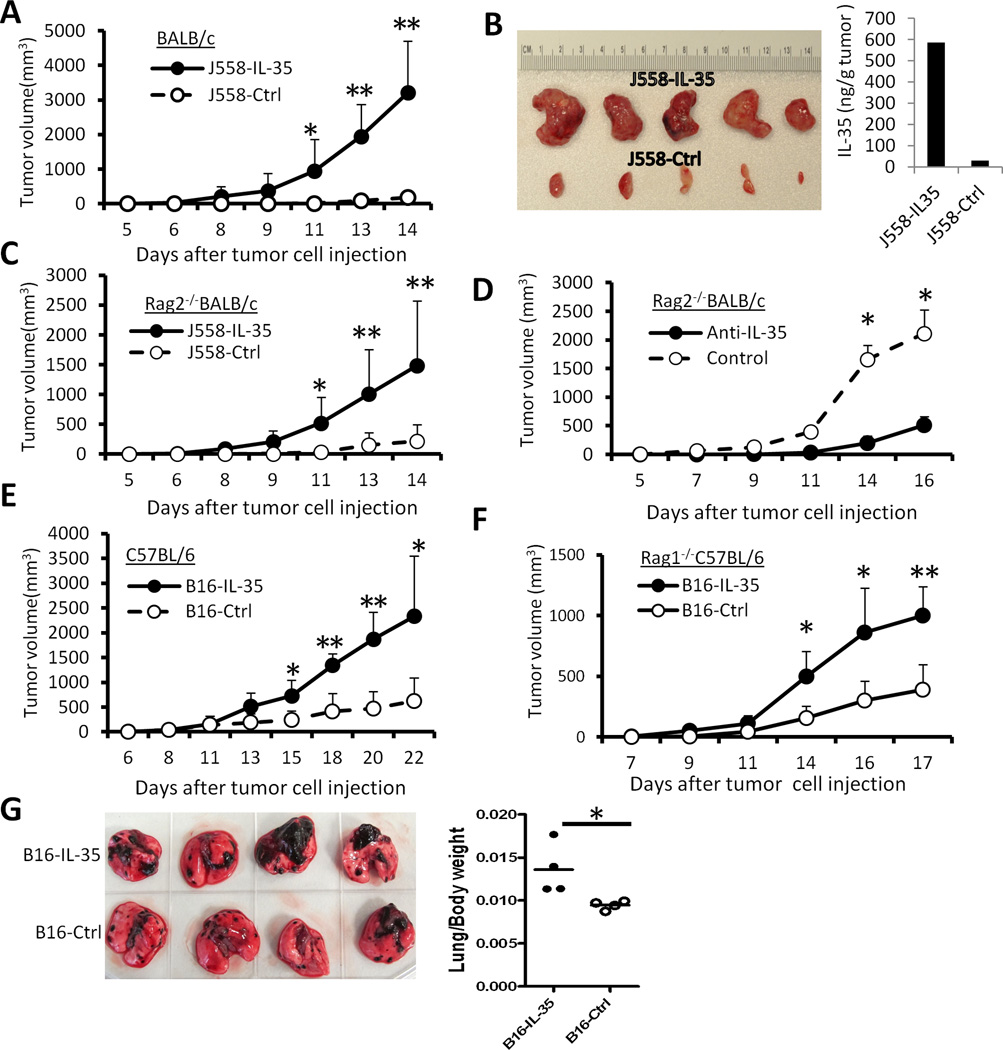
To determine if the expression of IL-35 in tumor cells affects tumor growth in vivo, 5 × 106 IL-35-positive or IL-35-negative J558 cells were injected s.c. into each BALB/c mice. As shown in Fig.3A, IL-35-negative J558 cells induced tumors in normal BALB/c mice 10–14 days after tumor cell injection, J558-IL-35 tumors grew progressively in BALB/c mice and within two weeks tumors grew to a size that required euthanasia. Our results indicated that ex vivo J558-IL-35 tumors were significantly bigger than ex vivo J558-ctrl tumors by day 15 after tumor cell injection and high concentration of IL-35 was detected in protein lysates of IL-35-positive tumors (Fig.3B).
Fig.3. Expression of IL-35 in the tumor microenvironment enhances tumorigenesis.
5 × 106 J558-IL-35 or J558-Ctrl cells were injected into each BALB/c (A) or Rag2−/−BALB/c mouse (C) s.c. The tumor growth was observed over time, and at the end of experiments, tumors were removed from sacrificed mice and photographed. Shown in photo (B) are J558-IL-35 and J558-Ctrl tumors removed from BALB/c mice. ELISA was used to quantify IL-35 concentration in lysates of representative tumors (B). Each Rag2−/−BALB/c mouse was inoculated with 5 × 106 J558-IL-35 cells s.c. in the presence of anti-IL-35 (V1.4C4.22, Shenandoah Biotechnology) or an isotype-matched control mAb (IgG2b, BioXcell) at a concentration of 50 µg/ml. Mice were observed for tumor growth over time. Bars indicate SD of 3 mice in each group and data shown represent two experiments with similar results. 1 × 105 B16-IL-35 or B16-Ctrl cells were injected into each C57BL/6 (E) or Rag1−/−C57BL/6 mouse (F) s.c. The tumor growth was observed over time. Bars in A, C, D, E and F indicate SD of 5 mice in each group. Data shown represents three to five experiments with similar results. (G) 1 × 105 B16-IL-35 or B16-Ctrl cells were injected into each C57BL/6 i.v. Eighteen days after tumor cell injection, lungs from the recipient mice were removed, photographed (left) and weighed, and lung/body weight ratios were calculated and plotted in the right panel. *P<0.05; **P<0.01 by student’s t test.
To determine if IL-35 enhances tumor growth via inhibiting adaptive immune response, we injected J558-IL-35 or J558-ctrl cells into Rag2−/−BALB/c mice that lack T and B lymphocytes. As shown in Fig.3C, J558-IL-35 tumors grew much faster compared to J558-ctrl cells in Rag2−/−BALB/c mice. To determine if the tumor enhancement was IL-35 specific, an IL-35 neutralizing mAb or an isotype matched control mAb was co-injected with J558-IL-35 cells into Rag2−/−BALB/c mice, and we found that IL-35 neutralizing mAb abrogated tumor enhancement (Fig.3D).
Similarly, we found that B16-IL-35 tumors grew much faster than B16-ctrl tumors in both C57BL/6 mice (Fig.3E) and Rag1−/−C57BL/6 mice (Fig.3F). In addition, when injected intravenously, B16-IL-35 cells established lung foci faster than B16-ctrl cells (Fig.3G). Thus, IL-35 production in the tumor microenvironment stimulates tumor growth.
3. IL-35 production in the tumor microenvironment increases myeloid cell accumulation and tumor angiogenesis
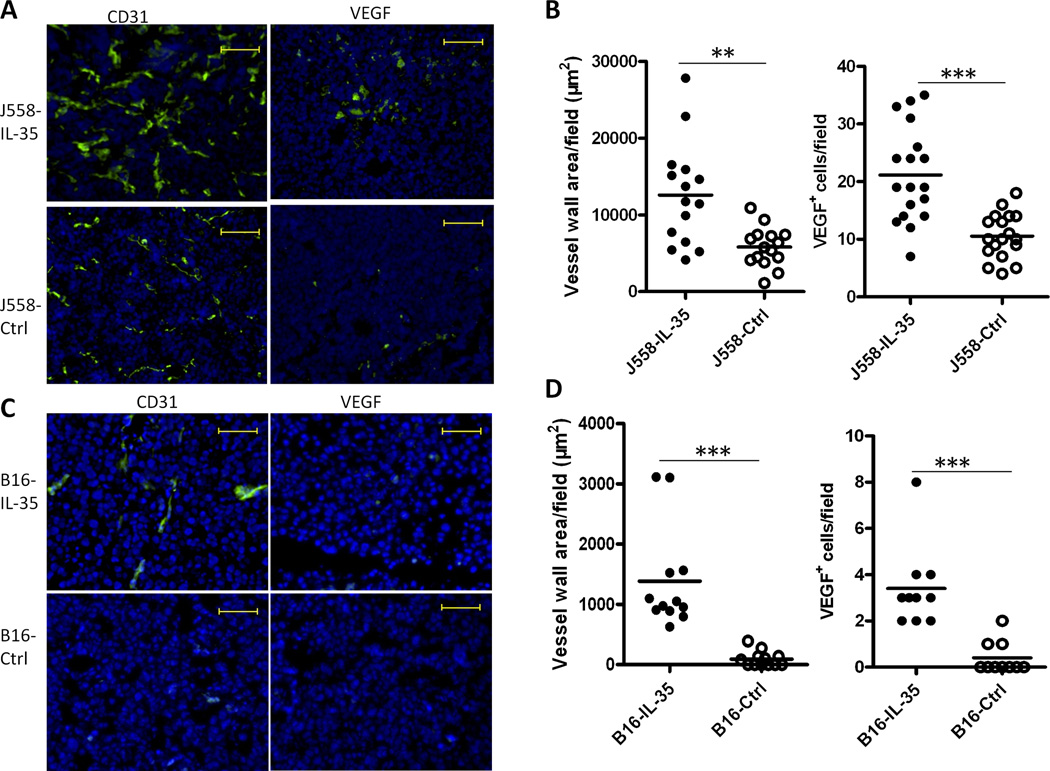
To determine the mechanisms by which IL-35 expression promote tumor growth, we first examined tumor angiogenesis in established J558-IL-35 and J558-ctrl tumors collected from Rag2−/−BALB/c mice. Tumor angiogenesis, as determined by CD31 expression and expression of VEGF, was significantly increased in J558-IL-35 tumors in comparison to J558-ctrl tumors (Fig.4A and Fig.4B). Increased tumor angiogenesis was also found in B16-IL-35 tumors (Fig.4C and Fig.4D).
Fig.4. IL-35 production in the tumor microenvironment enhances angiogenesis.
J558 tumors (A–B) from Rag2−/− mice and B16 tumors from C57BL6 mice (C–D) were analyzed for the expression of CD31 and VEGF by immunofluorescent staining and microscopy. Scale bars, 200µm. Mean vessel wall area and numbers of VEGF-positive cells for each tumor were analyzed and quantified using the ImageJ software (NIH). Three random fields from each slide/tumor were analyzed, and each dot represents values from one microscope field. *P<.05; **P=0.001, ***P<.0001 by student’s t test.
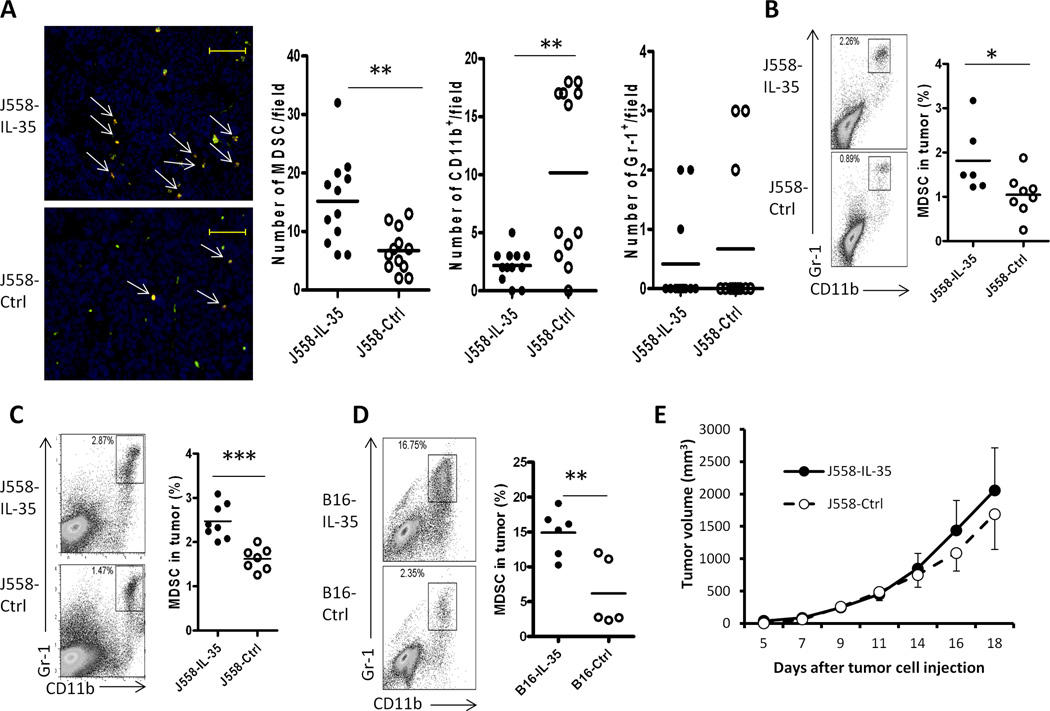
IL-35-positive tumors also contained increased numbers of myeloid cells in comparison to J558-ctrl tumors. Fluorescence staining of tumor sections from J558-IL-35 tumor grown in Rag2−/− mice revealed accumulation of more myeloid derived suppressor cells (MDSC, CD11b+Gr1+) (Fig.5A). In contrast, numbers of CD11b single positive cells were lower in J558-IL-35 tumors when compared to control tumors (Fig.5A). However, the numbers of Gr1 single positive cells were low and did not differ between the two types of tumors (Fig.5A). Flow cytometry analysis of disassociated tumor cells verified the fluorescence staining results obtained from tumor sections (Fig.5B). Furthermore, increased numbers of CD11b+Gr1+ cells were also found in J558-IL-35 tumors grown in BALB/c mice (Fig.5C) and B16-IL-35 tumors from C57BL/6 mice (Fig.5D) in comparison to their controls. To determine if increased MDSC accumulation was responsible for enhanced tumor growth, we injected three doses of anti-Gr1 mAb (250 µg per dose) into J558-IL-35 or J558-Ctrl cell-inoculated Rag2−/−BALB/c mice. We have previously shown that this regimen could completely deplete Gr1+ cells (22). We found that anti-Gr1 mAb treatment largely eliminated tumor growth difference between the two groups of mice (Fig.5E). Thus, MDSC accumulation is largely responsible for the enhanced growth of J558-IL-35 tumors in Rag2−/−BALB/c mice.
Fig.5. Increased numbers of CD11b+Gr1+ myeloid cells in the tumor microenvironment of IL-35-positive tumors.
J558-IL-35 and J558-Ctrl tumors from Rag2−/− mice were analyzed for the infiltration of myeloid cells by immunofluorescence staining and microscopy (A). Frozen tissue sections were co-labeled for CD11b (Alexa488) and Gr1 (Texas Red) and images were analyzed and quantified using the ImageJ software. Scale bars, 200µm. Three random fields from each slide/tumor were analyzed, and each dot represents values from one microscope field. **P<.001 by student’s t test. Flow cytometry was also used for the analysis of myeloid cells in IL-35-positive and IL-35-negative tumors (B–D). Single cell suspensions were prepared from tumors grown in Rag2−/− (B), BALB/c (C) and C57BL6 (D) mice and stained for CD11b and Gr-1 followed by flow cytometry analysis. Each circle represents data from a single tumor. *P<.05; **P<.01 by student’s t test. (E) 5 × 106 J558-IL-35 or J558-Ctrl cells were injected into each BALB/c mouse s.c. followed by treatment with 250 µg/mouse of anti-Gr1 mAb (RB6-8C5, BioXcell) i.p. on day 0, 5 and 10. Mice were observed for tumor growth over time. Five mice per group were used for this experiment and data shown represent two experiments with similar results.
4. IL-35 does not enhance migration of myeloid cells in vitro
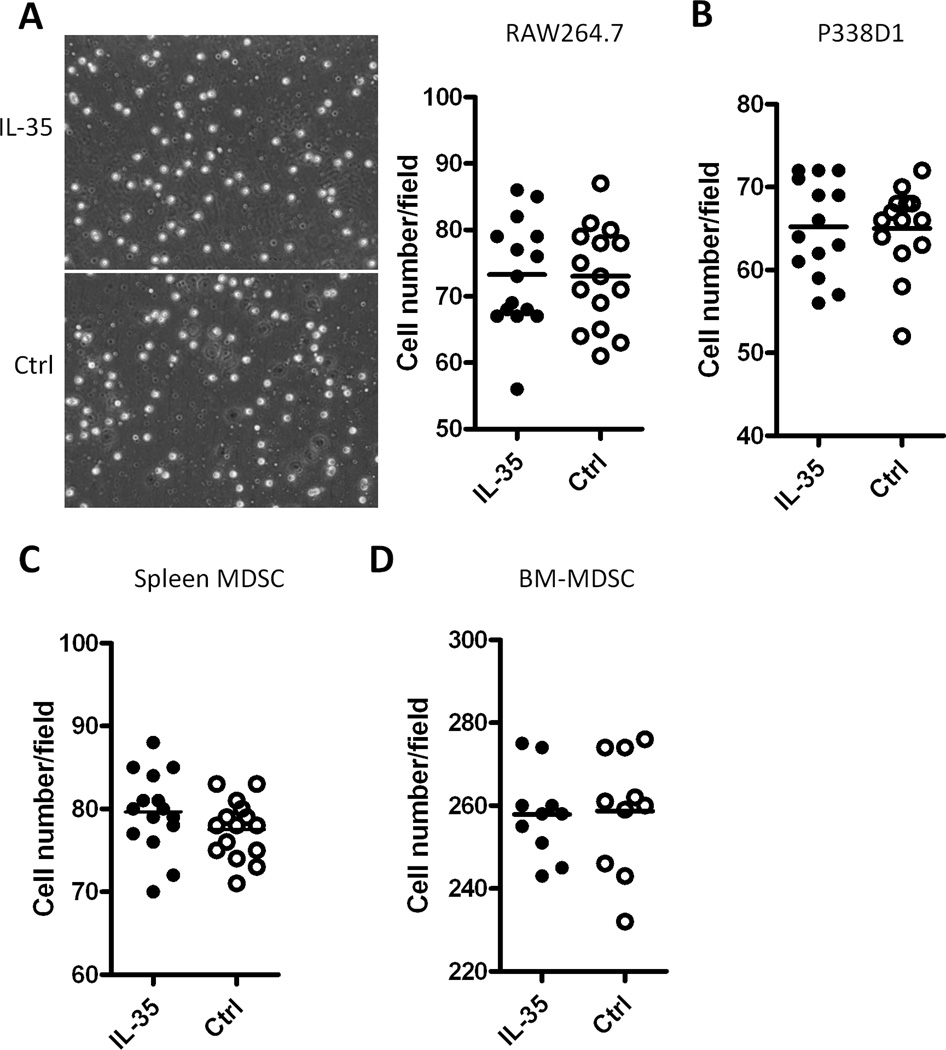
The increased accumulation of myeloid cells in IL-35-positive tumors suggests that IL-35 may play a chemotaxis effect on myeloid cells. To test this possibility, we performed transmigration assay using a trans-well system in the presence or absence of IL-35. Different lineages of myeloid cells, including macrophage cell lines Raw264.7 (H-2b), P338D1 (H-2d), MDSC purified from the spleen of J558-tumor bearing mice and bone marrow-derived CD11b+Gr1+ cells were used in the transmigration assay. As shown in Fig.6, similar numbers of Raw264.7 cells (Fig.6A), P338D1 cells (Fig.6B), spleen MDSC (Fig.6C) and bone marrow CD11b+Gr1+ cells (Fig.6D) migrated to chambers containing IL-35 or the control medium. Thus, IL-35 does not directly increase migration of myeloid cells.
Fig.6. IL-35 does not increase migratory activity of myeloid cells.
Migration assay using a trans-well system with or without IL-35 was performed to test the migration capacity of Raw264.7 cells (Fig.6A), P338D1 cells (Fig.6B), spleen MDSC (Fig.6C) and bone marrow Gr1+ cells (Fig.6D). Cells that migrated to the bottom side of the trans-well membrane were stained with Dapi, and random fields from each well were photographed under fluorescence microscope. Numbers of cells in each field were quantified using the ImageJ software (NIH). Each dot represents values from one microscopic field and data shown represents three experiments with similar results.
5. IL-35 production in the tumor microenvironment suppresses CTL responses
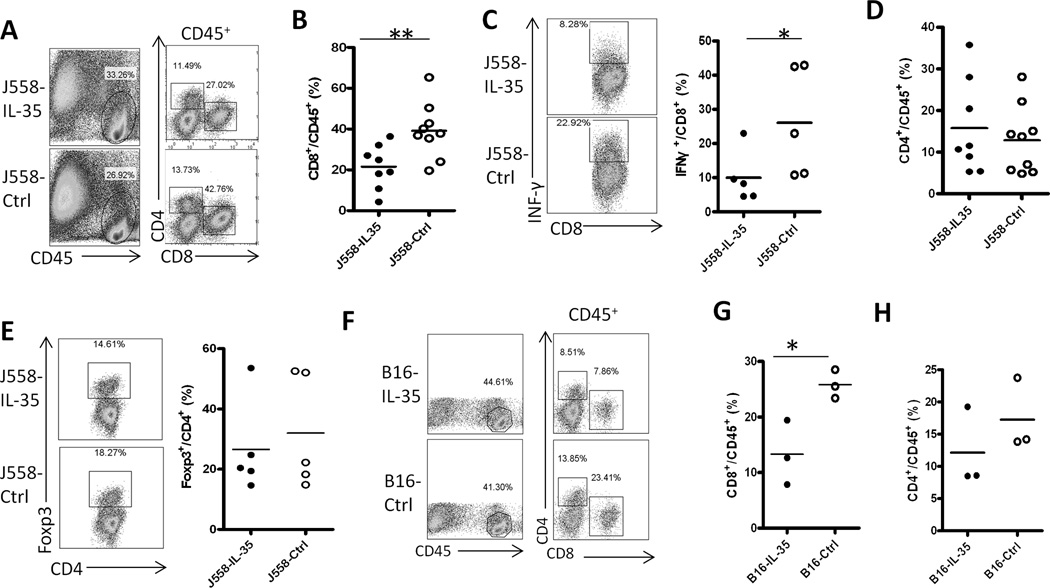
Since the IL-35-positive tumors contained higher numbers of myeloid suppressor cells, which are known to suppress CTL responses in tumors (23–24), we therefore hypothesized that IL-35 production in the tumor microenvironment inhibits CTL responses. To test this hypothesis, we compared T cell responses in the J558-IL-35 and J558-ctrl tumors grown in normal BALB/c mice. As shown in Fig.7A, we found that total leukocyte numbers (CD45+) were increased in J558-IL-35 tumors. However, among CD45+ leukocyte population, numbers of CD8+ T cells were greatly reduced in J558-IL-35 tumors (Fig.7A and Fig.7B). CD8+ T cells from J558-IL-35 tumors were also less capable of producing IFN-γ in comparison to CD8+ T cells collected from J558-ctrl tumors (Fig.7C). However, the percentages of CD4+ T cells (Fig.7D) and CD4+Foxp3+ Treg cells (Fig.7E) were not significantly affected. Similar to the J558 tumors, we also observed that B16-IL-35 tumors grown in C57BL6 mice contained significantly reduced numbers of CD8+ T cells (Fig.7F and Fig.7G). On the contrary, the numbers of CD4+ T cells were not significantly affected (Fig.7F and Fig.7H).
Fig.7. Expression of IL-35 contributes to an immune suppressive microenvironment.
J558 cells or B16 cells with or without IL-35 expression were injected into each BALB/c or C57BL6 mouse s.c., when tumors were fully established (about 1 cm in length), mice were sacrificed and T cell responses in tumors were evaluated by flow cytometry. A–E: T cell responses in IL-35 positive and negative J558 tumors from BALB/c mice. F–H: T cell responses in IL-35 positive and negative B16 tumors from C57BL6 mice. Each circle represents data from a single mouse/tumor. *P<.05; **P<.01 by student’s t test.
6. IL-35 does not directly inhibit tumor antigen-specific CTL proliferation and effector functions
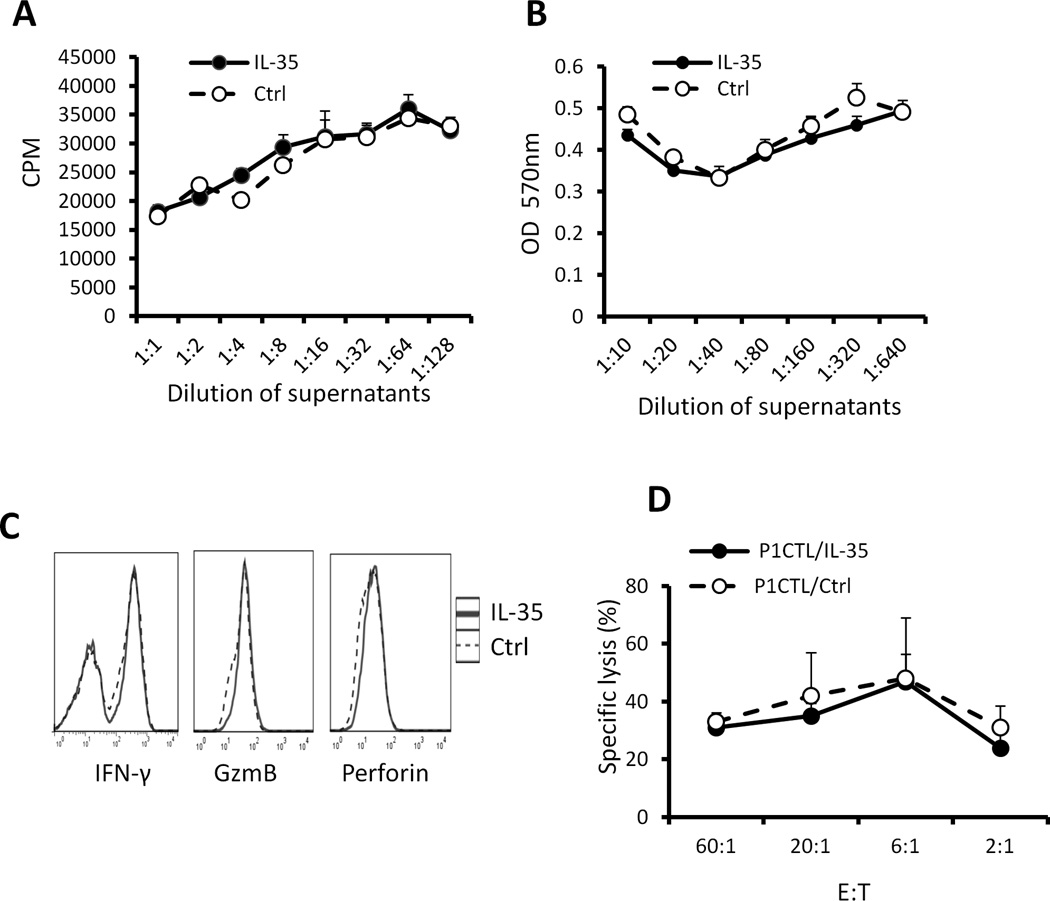
To determine whether IL-35 production directly inhibits tumor antigen specific CD8+ T cell proliferation and effector function, we cultured P1CTL transgenic T cells that recognize tumor antigen P1A in the presence of IL-35 produced by J558 cells. There were no differences in the proliferation and growth of P1CTL cells either in the presence or absence of IL-35 as determined by 3H-thymidine incorporation assay (Fig.8A) and MTT assay (Fig.8B). IL-35-stimulated P1CTL cells also expressed similar levels of IFN-γ, Granzyme B (Fig.8C) and exhibited similar cytotoxicity to P1A-positive P815 target cells (Fig.8D).
Fig.8. IL-35 does not directly affect differentiation of tumor antigen specific CTL.
Splenocytes from P1CTL transgenic mice were activated with P1A peptide (0.2 µg/ml) in the presence or absence of IL-35. 3H-Tritium incorporation assay (A) and MTT assay (B) was used to determine cell proliferation and survival. Intracellular staining and flow cytometry were used to determine IFN-γ and Granzyme B expression in activated P1CTL cells (C). 51Cr-release assay was used to determine cytotoxicity of activated P1CTL cells to P815 target cells (D).*P<.05, paired student’s t test. Data shown represent at least three experiments with similar results.
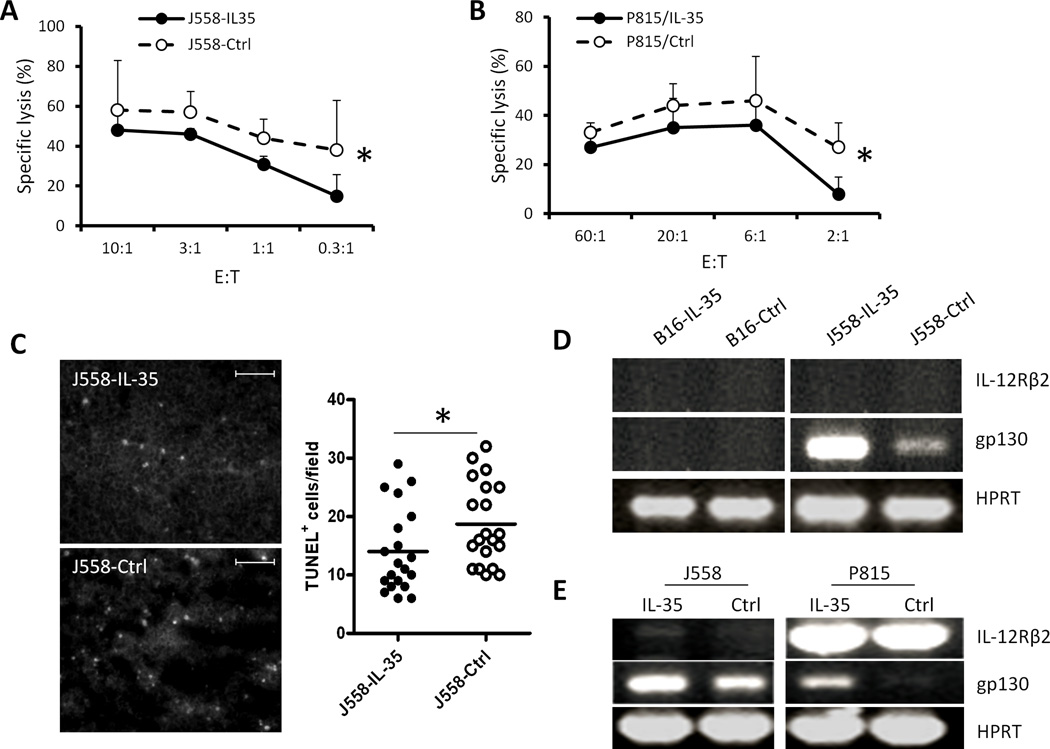
7. IL-35 induces resistance of cancer cells to destruction by tumor antigen-specific CTL
Despite expressing similar levels of P1A antigen and MHC class I (Fig.2), J558-IL-35 cells were more resistant to P1CTL destruction compared to J558-Ctrl cells (Fig.9A). Similarly, IL-35-stimulated P815 cells were also found to be more resistant to P1CTL lysis (Fig.9B) compared to control P815 cells. Consistent with this observation, established J558-IL-35 tumors in BALB/c mice contained less apoptotic tumor cells compared to J558-Ctrl tumors (Fig.9C). Thus, IL-35 renders tumor target cells more resistant to CTL destruction. To determine if cancer cell resistance to CTL destruction was due to IL-35 receptor signaling, we determined the expression of IL-35R subunits (IL-12Rβ2 and gp130) in IL-35-positive and IL-35-negative tumor cells by RT-PCR. As shown in Fig.9D, while expression of IL-12Rβ2 and gp130 were barely detectable in B16 cells, increased gp130 expression was detected in J558-IL-35 cells compared to J558-Ctrl cells. To determine if induction of gp130 was IL-35-specific, we cultured J558 and P815 cells with or without IL-35 for 24 h, we then determined gp130 expression by RT-PCR. As shown in Fig.9E, IL-35 increased gp130 expression in both J558 and P815 cells.
Fig.9. IL-35 induces tumor cell resistance to CTL destruction.
P1CTL cells were activated with P1A peptide (0.2µg/ml) for 5 days. 51Cr-release assay was used to determine cytotoxicity of activated P1CTL cells to J558-IL-35/J558-Ctrl cells (A) and P815 cells treated with or without IL-35 (B). Data shown represent three experiments with similar results. Frozen tissue sections from J558-IL-35 or J558-Ctrl tumors grown in BALB/c mice were labeled for TUNEL and images were photographed under a fluorescent microscope and quantified using the ImageJ software (C). Scale bars, 200µm. Three random fields from each slide/tumor were analyzed, and each dot represents values from one microscope field. *P<.05 by student’s t test. RT-PCR was used to detect IL-35 receptor subunits in B16-IL-35/B16-Ctrl and J558-IL-35/J558-Ctrl cells (D) or in J558 and P815 cells treated with or without IL-35 (E). Data shown in D and E represent three experiments with similar results.
Discussion
Our study validates the hypothesis that IL-35 is produced in human cancer tissues, and tumor-derived IL-35 plays important roles in tumor progression and tumor immune surveillance.
We at first demonstrated that IL-35 is produced in human cancer tissues. Among the three types of human cancer tissues examined, IL-35 is mainly found to be expressed in tumor stromal cells. It will be thus interesting to determine the types of cells in cancer stromas which produce IL-35. Previous studies have revealed that tumor infiltrating dendritic cells (TIDC) (12, 15) express EBI3, and therefore TIDC could be a source of IL-35. Additionally, tumor infiltrating Foxp3+ Treg cells could be another source of IL-35. EBI3 was known to be expressed in Epstein–Barr virus (EBV)-associated Hodgkin lymphoma (HL), diffuse large B lymphoma, nasopharyngeal carcinoma (12, 25) and also in EBV-negative malignant tumors (14). In this study we observed that some diffuse large B lymphoma cells are also positive for IL-35. Therefore IL-35 is abundantly produced in many human cancer tissues and multiple cell types including some cancer cells.
We next observed that tumor-derived IL-35 considerably increased tumorigenesis in both immune competent and immune deficient mice. The pro-tumor effect is very rapid, which necessitates sacrifice of mice injected with IL-35-positive cancer cells within two to three weeks after tumor cell injection. This pro-tumor effect of IL-35 has been confirmed in two different tumor models, plasmacytoma J558 and B16.F10 melanoma. Previous studies have reported that IL-35-producing Tr35 cells can inhibit tumor growth via suppression of anti-tumor immune responses (7). Since in this study the pro-tumor effect of IL-35 is also observed in Rag1/2-deficient mice, the rapid tumor growth of IL-35 producing tumors is therefore not solely due to suppression of adaptive immunity. The IL-35 effect in tumors also differs from another EBI3-containing cytokine, IL-27, which has potent tumor inhibiting effects (26–31). Expression of EBI3 in human lung cancer cells has been reported to promote lung cancer cell growth in vitro (14). However, in this study we have shown that IL-35 expression does not affect tumor growth and proliferation in vitro. It is not known if the discrepancy of the results is due to lack of IL-35 heterodimer formation in lung cancer cells. Thus, for the first time we have shown that tumor-derived IL-35 has a pro-tumor effect in vivo.
We demonstrated that IL-35 production in the tumor microenvironment increased CD11b+Gr1+ myeloid cell accumulation and tumor angiogenesis (reflected by increased density of CD31+ blood vessels and VEGF production in tumor bed). Since IL-35 production by cancer cells does not affect their growth and proliferation in vitro, it is likely that IL-35 producing cancer cells are capable of inducing host cells to promote tumor growth. In this regard, increased numbers of CD11b+Gr1+ MDSC were present in the tumors of IL-35-positive tumors. MDSC is a known cell type that can promote tumor angiogenesis via production of VEGF and other pro-angiogenesis factors (32–34). Thus, it is likely that increased angiogenesis and tumor growth in IL-35-positive tumors is due to increased accumulation of CD11b+Gr1+ myeloid cells. Indeed, depletion of Gr1+ myeloid cells abrogated tumor growth enhancement by IL-35. Our in vitro migration assay revealed that tumor produced IL-35 does not have a direct role in MDSC chemotaxis. Quantitative RT-PCR analysis also revealed that VEGF expression was not altered in J558-IL-35 and B16-IL-35 cells in comparison to their relative control cells (not shown). Thus, at this stage it remains to be determined what signal pathways are activated in tumor cells by IL-35, which are responsible for initially accumulating CD11b+Gr1+ myeloid cells into tumors and thereby, inducing tumor angiogenesis.
Finally, in this study we have demonstrated that tumor-derived IL-35 induces a suppressive tumor microenvironment, which in turn inhibits tumor immunity. MDSC induces immune suppression and induction of CTL responses (23–24). Consistent with this observation, we have found that in immune competent mice, spontaneous CTL responses in IL-35-positive tumors are inhibited. However, IL-35 does not directly inhibit CTL proliferation, differentiation and effector functions in in vitro assays and this is in contrast to other IL-12 family cytokines such as IL-12 and IL-27, which can significantly affect CTL differentiation (35–37). Thus, it is likely that the inhibition of CTL responses in IL-35-positive tumors is due to accumulation of MDSC in tumors, which subsequently inhibits CTL responses.
Although IL-35 does not have direct effects on CTL activation and effector functions, we have shown that IL-35-treated target cells are less susceptible to CTL mediated destruction. Consistent with this effect, we found that less apoptotic tumor cells were observed in IL-35-positive tumors than in IL-35-negative tumors grown in immune competent mice. This effect is unlikely mediated by down-regulation of MHC class I or antigen expression, as IL-35 expressing tumor cells has similar levels of MHC class I and antigen. Our RT-PCR results suggest that IL-35 up-regulates gp130 expression in tumor cells. Interestingly, gp130 signaling has been shown to mediate cancer cell resistance to chemotherapy (38). Since IL-35 is known to signal through gp130 dimmer (4), it is highly suggestive that IL-35 signaling via gp130 induces cancer cell resistance to CTL destruction. Thus, IL-35 induced cancer cell-resistance to CTL destruction could be one mechanism by which cancer cells escape CTL destruction in tumors.
Taken together, our investigation determining the role of tumor-derived IL-35 on tumor growth and immunity has revealed novel functions of IL-35 in promoting tumor growth and inhibition of anti-tumor CTL responses. Because IL-35 can be produced by both cancer cells and tumor infiltrating stromal cells, therefore further investigation elucidating the cross-talk between cancer cells, tumor infiltrating stromal cells and MDSC via IL-35 may help to better understand tumor progression and immune evasion. Accordingly, targeting IL-35 mediated cross-talk may be a novel immunotherapeutic approach for the treatment of cancer patients.
Acknowledgments
Funding support: This study is supported by grants from the National Cancer Institute (R01CA138427 to XFB) and American Cancer Society (RSG-09-188-01-LIB to XFB). ZW is supported by a pre-doctoral fellowship (2010616034) from the China Scholarship Council.
References
- 1.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 3.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 4.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol. 2011;187:462–471. doi: 10.4049/jimmunol.1100259. [DOI] [PubMed] [Google Scholar]
- 9.Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of Autoimmune Diabetes by Ectopic Pancreatic beta-Cell Expression of Interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devergne O, Coulomb-L'Hermine A, Capel F, Moussa M, Capron F. Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedobitek G, Pazolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198:310–316. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- 13.Poleganov MA, Bachmann M, Pfeilschifter J, Muhl H. Genome-wide analysis displays marked induction of EBI3/IL-27B in IL-18-activated AML-derived KG1 cells: critical role of two kappaB binding sites in the human EBI3 promotor. Mol Immunol. 2008;45:2869–2880. doi: 10.1016/j.molimm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Nishino R, Takano A, Oshita H, Ishikawa N, Akiyama H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y. Identification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer. Clin Cancer Res. 2011;17:6272–6286. doi: 10.1158/1078-0432.CCR-11-0060. [DOI] [PubMed] [Google Scholar]
- 15.Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Bregeaud L, Perennec M, Brousse N, Kastelein R, Devergne O. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217–1228. doi: 10.1016/S0002-9440(10)62340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 17.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Liu JQ, Talebian F, El-Omrani HY, Khattabi M, Yu L, Bai XF. Tumor expression of CD200 inhibits IL-10 production by tumor-associated myeloid cells and prevents tumor immune evasion of CTL therapy. Eur J Immunol. 2010;40:2569–2579. doi: 10.1002/eji.201040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JQ, Joshi PS, Wang C, El-Omrani HY, Xiao Y, Liu X, Hagan JP, Liu CG, Wu LC, Bai XF. Targeting activation-induced cytidine deaminase overcome tumor evasion of immunotherapy by CTLs. J Immunol. 2010;184:5435–5443. doi: 10.4049/jimmunol.0903322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 21.Wang LX, Talebian F, Liu JQ, Khattabi M, Yu L, Bai XF. IL-10 contributes to the suppressive function of tumour-associated myeloid cells and enhances myeloid cell accumulation in tumours. Scand J Immunol. 2012;75:273–281. doi: 10.1111/j.1365-3083.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talebian F, Liu JQ, Liu Z, Khattabi M, He Y, Ganju R, Bai XF. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS One. 2012;7:e31442. doi: 10.1371/journal.pone.0031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonin J, Larousserie F, Bastard C, Picquenot JM, Couturier J, Radford-Weiss I, Dietrich C, Brousse N, Vacher-Lavenu MC, Devergne O. Epstein-Barr virus-induced gene 3 (EBI3): a novel diagnosis marker in Burkitt lymphoma and diffuse large B-cell lymphoma. PLoS One. 2011;6:e24617. doi: 10.1371/journal.pone.0024617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 27.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 28.Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004;24:3763–3767. [PubMed] [Google Scholar]
- 29.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 30.Salcedo R, Hixon JA, Stauffer JK, Jalah R, Brooks AD, Khan T, Dai RM, Scheetz L, Lincoln E, Back TC, Powell D, Hurwitz AA, Sayers TJ, Kastelein R, Pavlakis GN, Felber BK, Trinchieri G, Wigginton JM. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J Immunol. 2010;184:2348–2354. doi: 10.4049/jimmunol.0902371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 33.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 37.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 38.Borsellino N, Bonavida B, Ciliberto G, Toniatti C, Travali S, D'Alessandro N. Blocking signaling through the Gp130 receptor chain by interleukin-6 and oncostatin M inhibits PC-3 cell growth and sensitizes the tumor cells to etoposide and cisplatin-mediated cytotoxicity. Cancer. 1999;85:134–144. [PubMed] [Google Scholar]