Abstract
B cells are critical in the initiation and maintenance of lupus. Autoreactive B cells clonally expand, isotype switch and mutate—properties associated with memory B cells (MBC), which are typically generated via germinal centers (GCs). The development and functions of autoreactive MBC in lupus are poorly understood. Moreover, mounting evidence implicates the extrafollicular (EF) response in the generation of switched and mutated autoantibodies that are driven by BCR and TLR co-recognition, raising the question of whether MBC are generated in this context. Here we investigated autoreactive MBC generation associated with this type of response. We transferred B cells from AM14 site directed BCR transgenic mice into non-transgenic normal recipients and elicited an EF response with anti-chromatin Ab, as in prior studies. By following the fate of the stimulated cells at late time points we found that AM14 B cells persisted at increased frequency for up to 7 weeks. Furthermore, these cells had divided in response to Ag, but were subsequently quiescent, with a subset expressing the memory marker CD73. These cells engendered rapid, isotype switched secondary plamablast responses upon restimulation. Both memory and rapid secondary responses required T cell help to develop, emphasizing the need for T-B collaboration for long-term self-reactivity. Thus, using this model system, we show that the EF response generated persistent and functional MBC that share some but not all of the characteristics of traditional MBC. Such cells could play a role in chronic or flaring autoimmune disease.
Introduction
The kinetics of autoreactive B cell activation and persistence are under active investigation. A number of types of early autoreactive primary responses are extrafollicular (EF), TLR driven, and result in massive bursts of short-lived antibody forming cells (AFCs) (1–5). While isotype switch and somatic hypermutation in such anti-self B cells can occur at the EF site (1, 6), under some conditions GCs may be the preferred site for generation of autoantibodies (7). The later stages of disease—evolution and maintenance—are likely to be more reliant on persistent, matured, or “memory” type autoreactive responses. These are less well understood, but of critical importance, as it is during ongoing or later disease that patients require therapeutic intervention.
There are two possible sources for anti-self Ab found in chronic autoimmune disease: bona fide long-lived plasma cells or short-lived AFC that are chronically replenished. However, neither of these sources explain all observed characteristics of disease progression, in which there is affinity maturation of autoantibodies as well as waxing and waning, or “flares” associated with Systemic Lupus Erythematosus (SLE). In particular if long-lived plasma cells were the only source of autoantibody this would not be consistent with the lupus flare, Nor would an exclusive source of autoantibodies deriving from long-lived plasma cells be consistent with the drop in titer of certain autoantibodies, such as anti-DNA, after B cell depletion with anti-CD20 treatment in patients (8). Conversely, it is not obvious how a short-lived AFC response would allow for progressive increases in affinity. One possible resolution to these seemingly inconsistent facts would rely on autoreactive MBC generation; such cells, if they were formed, could be a critical intermediate population. They could allow for both waxing and waning following reactivation and be the source of affinity maturation. Though MBC have been characterized in SLE patients (9, 10), the origins and generation of autoreactive MBC have been relatively little-explored in humans or mouse models of SLE.
Memory is the long-term outcome of adaptive immunity. Classically-defined MBC differentiate following an acute T-cell dependent stimulus and a GC reaction (11). The classical MBC population comprises diverse cell types and functions. MBC can be IgM+ or class-switched (12–16) and in mice can express the surface markers CD73, PD-L2, and/or CD80 (16, 17). B cell memory is sometimes associated with affinity maturation driven by somatic hypermutation (13, 17). However, a more important quality of MBC is the ability to respond faster than their naïve counterparts (18). One definitive quality shared by all MBC is to be in a resting state; it is thought that memory cells cannot develop unless they have been separated from Ag, as has been demonstrated for CD8+ memory T cells (19, 20).
While it was originally thought that the GC is the only site for generation of MBC, numerous studies have shown MBC can develop in the context of impaired GCs (15, 21–26) or in their complete absence (27). Furthermore, MBC can develop in response to T-independent Ag (28–32). Thus, the most inclusive definition of memory requires only Ag exposure with subsequent longevity and quiescence, but does not necessarily require a GC or T cell help.
As noted, in several mouse models of lupus, anti-nuclear and rheumatoid factor (RF) B cell activation is largely TLR-driven, GC-independent, and EF-localized (3–5). In such models, T cells certainly play a role, but they are not essential for isotype switch, mutation or differentiation to AFCs (5, 33). This type of activation is found in autoimmune-prone mice including MRL.Faslpr, B cell-activating factor of the TNF family transgenic (Tg), and NZB/W (1, 2, 4, 34, 35). Although short-lived plasmablasts are the primary cells thought to arise from an EF response (36), it is unclear whether this response could generate MBC. Thus, with respect to autoimmune disease pathogenesis, the questions remain what contribution naïve or MBC make in giving rise to short-lived plasmablasts, and/or to GCs.
Since memory formation is typically thought to require resolution of the response and separation of specific cells from stimulatory Ag, whether or how MBC form in autoimmunity remains unclear, as autoimmunity is a chronic disease with a continuous supply of self-Ag. However, SLE can be relapsing and remitting (37), thus potentially providing intervals of lower self-Ag and a less inflammatory environment, allowing for B cells to rest prior to reactivation. Indeed, there is evidence of autoreactive MBC in patients with autoimmune disease. Identified in humans by the widely-used marker CD27, MBC have been found with autoreactive specificity by virtue of carrying the 9G4 idiotype in SLE patients (9, 10). Furthermore, populations of CD27+ MBC that have V region somatic mutations have been identified in SLE patients (38), albeit at a similar frequency to normal controls and independent of disease activity. Likewise, in RA, CD27+ MBC have been identified in the synovium and synovial B cells also harbor V region mutations and evidence of clonal expansion (39–41). Furthermore, in both RA and SLE, the return of MBC following Rituximab depletion of B cells correlates with earlier relapse (42–45). In almost all cases, the actual specificity of MBC in patients with systemic autoimmune diseases has not been determined. In particular, the contribution of classical autoantibody specificities, like anti-nuclear antibodies, has not been established in memory compartments of diseased vs healthy individuals. However, in one interesting study, in a single patient the memory compartment did contain mutated B cells with Ro and La specificity (46). This suggests that under some circumstances specific recruitment of self-reactive B cells with authentic disease-related specificities can be recruited into the memory compartment.
Although we know that CD27+ autoreactive B cells can be found in patients, we cannot determine their history or determine cellular and molecular mechanisms in vivo. This can be approached in mice; however, we are not aware of characterization of MBC in mouse models of autoimmunity. Nonetheless, there is reason to believe that autoreactive MBC do exist in lupus prone mice. Numerous examples of mutated and expanded clones in MRL.Faslpr, MRL/+, and NZB/W have been characterized (47–54). While these may have directly differentiated from chronic EF or GC responses, the extent of expansion and mutation is consistent with memory development. In particular, in some clones, highly mutated members are found at the same time as other cells with few or no mutations, indicating asynchronous development (55). These features are consistent with a memory cell derivation of some autoreactive B cells.
To directly investigate development of memory in autoreactive B cell immune responses, we have been focusing on RF B cells, a specificity found in both SLE and RA (56, 57). To track a population of RF B cells, others and we have used the AM14 BCR H chain Tg and site directed (sd)-Tg mice, in which B cells, paired with an endogenously derived Vκ8 light chain, are specific for IgG2aa (58). This HL pairing can be detected by the anti-idiotype antibody 4–44, allowing specific tracking of RF B cells. 4–44+ AM14 B cells spontaneously differentiate to plasmablasts that mutate and switch at the EF site, only on autoimmune prone genetic backgrounds and only in the presence of the self Ag IgG2aa (1, 2, 59, 60). However, similar to the situation in human SLE patients, the history of spontaneously activated 4–44+ AM14 B cells cannot be assessed in aged diseased mice due to chronic presence of the autoantigen.
An advantage of AM14 B cells is that they remain ignorant of their self-Ag in non-autoimmune prone mice (61). However, when non-autoimmune prone AM14 mice are challenged with TLR ligand-containing anti-chromatin antibodies, B cell-intrinsic MyD88 drives differentiation of AM14 B cells to plasmablasts at the histologically identical EF site, but does not drive detectable GC responses (6). Interestingly, this response proceeds in the absence of T cells although T cells can support the response (5, 33). Furthermore, AM14 B cells transferred into non-Tg recipients undergo a similar response, which allows us to define the history of a finite 4–44+ population in vivo following acute activation with anti-chromatin Ab (33). Here we have used this system to test whether autoreactive MBC persist following an EF response and if so, whether their development and function require T cells.
Materials and Methods
Mice
AM14 sd-Tg mice were generated as previously described and backcrossed at least 8 generations to the BALB/cJ strain (59). BALB/cJ recipients were purchased from Jackson Laboratories. DO11.10+/+ Tcra−/− BALB/c mice were obtained from Dr. Kim Bottomly (Yale University, New Haven, CT, USA) (62).
B Cell Isolation and Adoptive Transfer
AM14 sd-Tg B cells were isolated from splenocytes using the EasySep Mouse B Cell Enrichment Kit (StemCell Technologies), following manufacturer’s instructions, yielding 95% purity or better as determined by flow cytometry. 3 million AM14 sd-Tg B cells per mouse were injected i.v. in sterile PBS.
Ascites preparation and immunization
Rag−/− BALB/c mice were injected with pristane (Sigma) prior to i.p. injection of 10 million PL2-3 hybridoma cells (63) in sterile PBS. Ascites was collected after one week, sterile filtered, and quantitated using ELISA. 0.5 mg PL2-3 ascites was injected on days 0, 2, and 4 following AM14 sd-Tg B cell transfer for a full primary response to generate memory and/or 2.5 days prior to sacrifice for a secondary or early primary control response.
CFSE Labeling
B cells were labeled in 0.1% BSA in sterile PBS at a concentration of 50 million cells per ml in 10 µm CFSE (Invitrogen) for 10 minutes at 37°C protected from light.
Flow Cytometry
Antibodies were purified in the laboratory as previously described (58) unless purchased as indicated. Single cell suspensions of splenocytes were blocked using clone 24G2 and dead cells were excluded using ethidium monoazide (Invitrogen). Cells were fixed using 1% paraformaldehyde and permeabilized using 1× Permwash (BD) with rat serum (US Biological). The following antibodies were used: 4–44 biotin, 4–44 Alexa 647, CD73 PE (TY/23, BD), and Ki67 FITC (SP6, Abcam). Data were collected using an LSRII (BD) and analyzed using FlowJo software (Tree Star). Doublets were excluded during analysis.
ELISpot Assay
Immulon 4 plates were coated with polyclonal goat anti-mouse anti-IgG2a or anti-IgM (Southern Biotech). Splenocytes were incubated for 5–6 hours at 37°C as previously described (6). AFCs were detected using 4–44 biotin, SA-AP (Invitrogen), and BCIP (Amresco) in agarose. AFCs were counted using a dissecting microscope.
Sequencing
Fixed and permeabilized naïve 4–44+ and memory 4–44+ CD73+ cells were sorted using a FACS Aria (BD). Sequences were obtained as previously decribed (1). Briefly, genomic DNA was obtained using proteinase K digestion. Following inactivation at 95°C, nested PCRs were performed using PFU Ultra II (Stratagene). Sequences were isolated using the Zero Blunt Topo Cloning Kit for Sequencing (Invitrogen) and were analyzed by the Keck Facility at Yale. Sequences were aligned using MegAlign software (Lasergene).
Results
Anti-chromatin Abs drive resting long-lived RF B cells
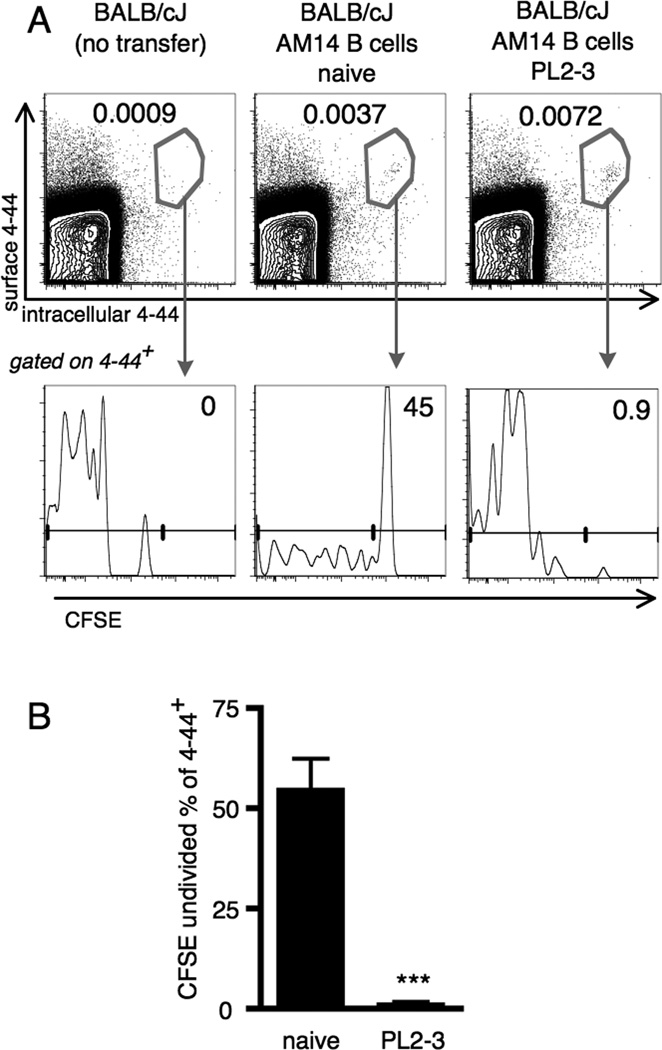
To investigate whether the EF AM14 B cell response to PL2-3 anti-chromatin Abs could be long-lived, persist, and provide functional memory, we transferred AM14 B cells to BALB/c recipients. In this way, no new naïve AM14 B cells could emerge to potentially confound the analysis. Additionally, CFSE labeling was used to track divisions that had occurred following Ag-specific activation with PL2-3. We identified the transferred cells of interest and distinguished authentic rare B cells (4–44+, Fig. 1A, upper panels, center and right) from background staining (Fig. 1A, upper panels, left) by staining both surface and intracellular compartments using 4–44 labeled with different fluorochromes (64). Intracellular as well as surface staining was used to gain more specificity and to reduce background of cells with surface staining of cyotphilic Ab, as only authentic AM14 B cells have both surface and intracellular 4–44+ Ig. CFSE-labeled 4–44+ cells transferred into hosts given PL2-3 were uniformly CFSE negative at week 4 after activation by PL2-3 (Fig. 1B), in agreement with earlier findings that 99% of 4–44+ cells had fully diluted CFSE by day 6 (33). Of note, CFSE-bright 4–44+ cells in unimmunized mice were detectable, indicating that they had not divided (Fig. 1A, lower panels). Just 0.002% of live cells were 4–44+ and CFSE negative in the naïve mice, 4 weeks after transfer, similar to our previous findings at day 6 (33). Background from recipient cells is impossible to exclude completely when analyzing rare transferred cell populations and these are very likely the source of this population, though we cannot exclude that some homeostatic proliferation may have occurred. Regardless, there is a striking difference between PL2-3 treated and non-treated recipients in CFSE dilution (Fig. 1B) Virutally all 4–44+ cells exposed to PL2-3 underwent at least 7 divisions.
Figure 1. Residual RF B cells have divided extensively four weeks after transfer and PL2-3 challenge.
Splenocytes were analyzed by flow cytometry from BALB/cJ mice at week 4 after transfer of CFSE labeled AM14 sd-Tg B cells and activation by PL2-3 as described in Materials and Methods. (A) Representative flow cytometry plots showing gating of 4–44+ (upper panels, showing live events) and CFSE dilution (lower panels, showing 4–44+ events). (B) Frequency of undivided cells within the 4–44+ population compiled from at least 9 mice per group and 3 independent experiments. Bars represent the mean + SEM. *** p < 0.001 by Mann-Whitney two-tailed test.
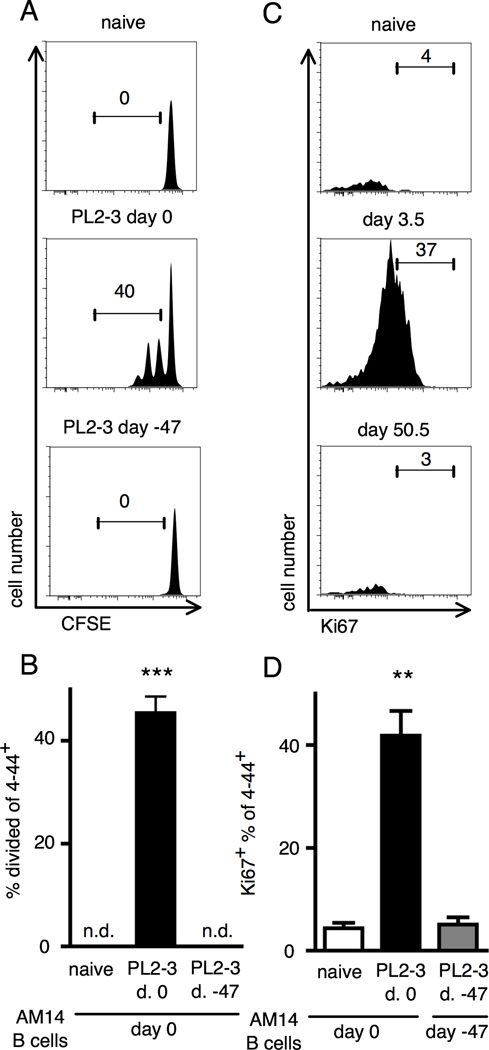
If the Ag-exposed cells remaining at 4 weeks post-transfer were bona fide memory cells, they should be in a resting state. However, some residual plasmablasts were still present at 4 weeks post-transfer (data not shown). As we sought to analyze a population of pure MBC, we increased the resting period to 7 weeks. To determine if this time point was most appropriate for assessing resting memory, we transferred CFSE labeled AM14 B cells into mice that were either naïve, that were given PL2-3 7 weeks prior to transfer, or that were given PL2-3 on the day of the cell transfer. As expected, transferred cells proliferated in response to PL2-3 given at the time of transfer. However, 7 weeks following PL2-3 immunization there was not sufficient Ag remaining to induce any detectable proliferation (Fig. 2A and B). Thus, although 4–44+ cells were detectable 4 weeks post-transfer and challenge, we determined that 7 weeks after PL2-3 administration would be a more appropriate time point to study the putative memory and recall responses in subsequent experiments, as we could document that there was no physiologically stimulatory Ag remaining from primary immunization by this time.
Figure 2. Analysis of residual Ag and proliferative state of residual RF B cells 47 days after initial challenge.
(A) Splenocytes were analyzed by flow cytometry from BALB/cJ mice at day 1.5 after transfer of CFSE labeled B cells. Mice were given either nothing, one injection of PL2-3 at day 0, or 3 injections of PL2-3 starting at day -47. Representative plots of 4–44+ cells are shown with CFSE staining on the x-axis. Gates and numbers show percentages of the parent gate (live 4–44+ B cells). (B) Compiled data from at least 6 mice per group and three independent experiments. n.d. = no division observed. (C) Splenocytes were analyzed by flow cytometry from BALB/cJ mice at day 3.5 or day 50.5 after transfer of AM14 sd-Tg B cells and activation by PL2-3 as described in Materials and Methods. Representative flow cytometry plots showing live 4–44+ B cells with Ki67+ staining on the x-axis. (D) Compiled data from at least 5 mice per group and two independent experiments. Bars represent the mean + SEM. **p < 0.01 and *** p < 0.001 by Mann-Whitney two-tailed test.
To determine if AM14 B cells immunized 7 weeks earlier were indeed resting, we stained for the cell cycle marker Ki67. Nearly all the 4–44+ cells were not Ki67+ and thus not transiting the cell cycle (Fig. 2C and D). This was in contrast to the positive control mice, sacrificed during an early and active response to PL2-3, wherein more than 40% of the 4–44+ cells were Ki67+. Thus, 7 weeks after immunization, 4–44+ AM14 B cells were both Ag-experienced and non-cycling.
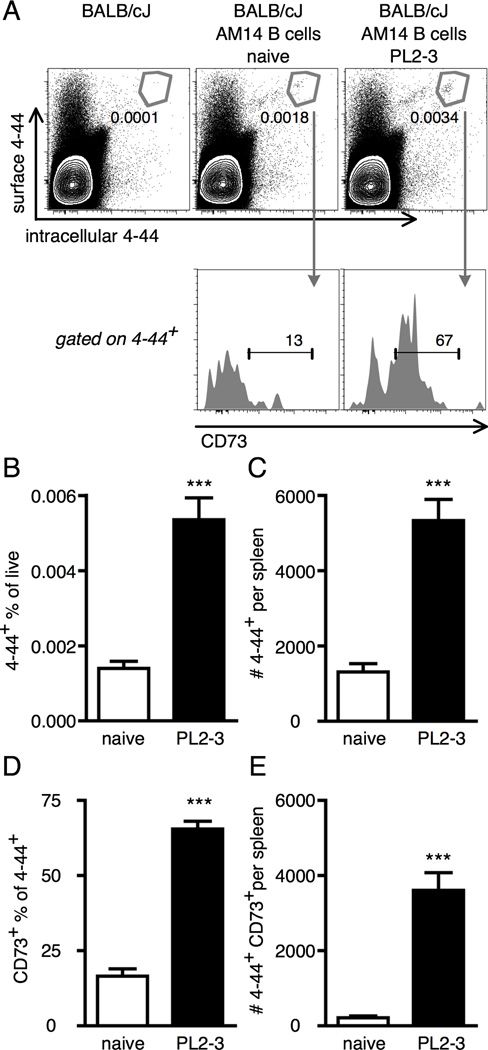
We therefore further investigated AM14 B cell persistence after at least 7 weeks following activation by PL2-3. In mice given AM14 B cells and PL2-3 8 weeks earlier, the 4–44+ population was 4-fold expanded compared with naïve controls. Both groups were above the very low background levels found in the no cell transfer controls (Fig 3A, upper panels, B and C). CD73 has been identified as a marker of MBC in mice (17). CD73 was found on the majority of Ag-exposed 4–44+ B cells in PL2-3 treated mice in contrast to background levels within the naïve 4–44+ population (Fig 3A, lower panels, and D). There was greater than 10-fold more 4–44+ CD73+ cells in PL2-3 immunized mice compared to naïve mice (Fig 3E). Thus, Ag-exposed but non-cycling AM14 B cells persist for many weeks following activation by PL2-3 and express a marker typically found on classical MBC.
Figure 3. Anti-chromatin antibodies induce long-lived CD73+ RF B cells.
Splenocytes were analyzed by flow cytometry from BALB/cJ mice at week 8 after transfer of AM14 sd-Tg B cells and activation by PL2-3 as described in Materials and Methods. (A) Representative flow cytometry plots showing gating of 4–44+ (upper panels, showing live events) and CD73+ (lower panels, showing 4–44+ events). Numbers are percentages of the parent gates (total live cells for upper panels and 4–44+ cells for lower panels). (B and C) Quantitation of 4–44+ frequencies and numbers. (D and E) Quantitation of CD73+ frequencies within the 4–44+ population and numbers of 4–44+ CD73+ cells. (B–E) At least 6 mice per group and two independent experiments were compiled. Bars represent the mean + SEM. *** p < 0.001 by two-tailed Mann-Whitney test.
One hallmark of B cell activation is affinity maturation, as reflected by somatic hypermutations of the B cell receptor. However, it should be noted that mutation is largely absent in some subsets of GC-dependent MBC (13, 17, 65) as well as in MBC generated in mice that could not form GCs (27). However, SHM has been found at the EF site in aged AM14 MRL.Faslpr mice during ongoing autoimmunity (1) as well as during the acute PL2-3 induced response (6). To test whether long-lived AM14 B cells gained mutations during activation, we sorted 4–44+ cells from naïve mice and 4–44+ CD73+ cells from PL2-3 immunized mice, 8 weeks post-immunization. The results reflect two independent sorts with sequences obtained from 3 naïve mice and 6 memory mice total. Sequencing the Vκ8 light chain from these sorted cells revealed only a very low level of mutation that did not differ significantly between naïve 4–44+ B cells and Ag exposed 4–44+ CD73+ B cells. In both naïve and memory 4–44+ cells, 12.5% of sequences were mutated. In naïve cells, 3 sequences were mutated out of 24 analyzed, and all were bearing only one mutation. In memory cells, 6 sequences were mutated out of 48 analyzed. Of these, 5 sequences had one mutation while the remaining sequence had 4 mutations.
RF B cell memory and rapid secondary responses are T-dependent
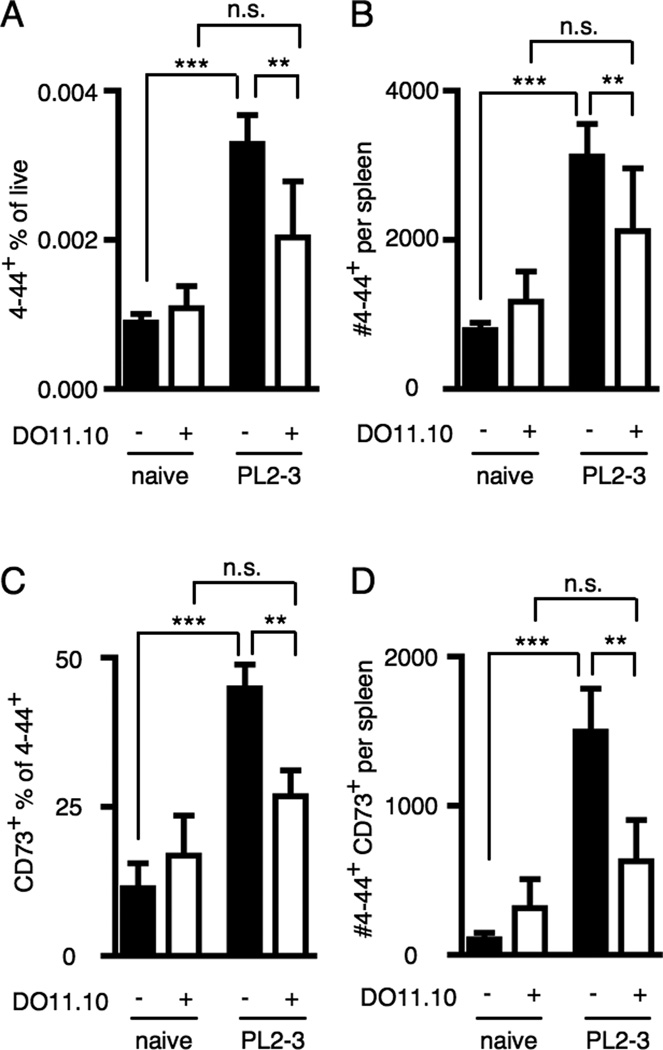
The primary response of transferred AM14 B cells to anti-chromatin Abs is present but reduced in magnitude when T cells are restricted to an irrelevant monoclonal population such as is found in the DO11.10+/+ Tcra−/− (DO11.10) mouse (33). To test whether persistence of Ag-experienced AM14 B cells is affected by the quality of T cell help, we transferred AM14 sd-Tg B cells to either wild type BALB/cJ (WT) mice or DO11.10 BALB/c mice, gave PL2-3, and analyzed splenocytes 7 wk later.
In DO11.10 recipients, there was no significant expansion of 4–44+ cells (Fig. 4A and B) nor was there a significant increase in CD73+ 4–44+ cells (Fig. 4C and D). WT recipients did show such increases upon immunization as expected, and the numbers and frequencies of 4–44+ and CD73+ 4–44+ cells were significantly higher than those in DO11.10 recipients. Thus, Ag-specific T cell help is required to support full development of an expanded long-lived 4–44+ population following activation by anti-chromatin Ab.
Figure 4. Anti-chromatin Ab-driven RF B cell persistence requires T cell help.
Splenocytes were analyzed from BALB/cJ or DO11.10+/+ Tcra−/− BALB/cJ mice at week 7 after transfer of AM14 sd-Tg B cells and activation with PL2-3 as described in Materials and Methods. (A–D) Populations were analyzed by flow cytometry as in Fig. 3. (A and B) Quantitation of 4–44+ frequencies and numbers. (C and D) Quantitation of CD73+ frequencies within the 4–44+ population and numbers of 4–44+ CD73+ cells. At least 6 mice per group and 4 independent experiments were compiled. Bars represent the mean + SEM. **p < 0.01 and ***p < 0.001 by Mann-Whitney two tailed test.
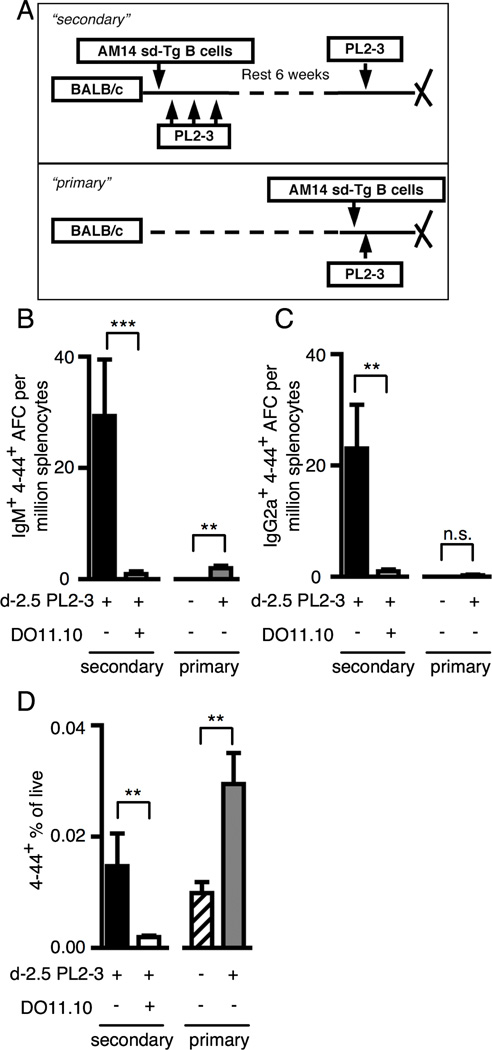
In addition to persistence, functional specific B cell memory implies a capacity for rapid secondary responses. Hence, it was possible that even in the absence of significant expansion, residual AM14 B cells in immune DO11.10 mice were qualitatively altered. To test this, we re-challenged DO11.10 or WT mice that had received AM14 B cells and PL2-3 7 weeks prior, and analyzed responses at day 2.5 (Fig 5A). Critically, this design also allowed us to determine if AM14 MBC formed in a WT background could mount accelerated and qualitatively different secondary responses. Strikingly, after only 2.5 days following secondary PL2-3 immunization, a 4–44+ AFC response had emerged in WT recipients, which consisted of both IgM and IgG2a AFC (Fig. 5B and C). This was not found in DO11.10 recipients. The rapid emergence of AFCs at day 2.5 is remarkable as primary EF plasmablast responses are expected no earlier than day 3.5 (66) (and unpublished observations). To establish a direct comparison between memory and naïve responses, we freshly transferred AM14 B cells to WT recipients such that the precursor frequency was 2-fold greater than that in the memory mice. However, at the time point analyzed (day 2.5), these “new primary” control animals were only starting to make a small 4–44+ IgM AFC response and barely made a 4–44+ IgG2a AFC response. In both cases, the mean number of AFCs detected per million splenocytes was less than 2. Notably, the secondary 4–44+ IgM AFC response was 30-fold greater compared with the new primary response after normalization for precursor frequency.
Figure 5. RF Ag-experienced MBC give rise to rapid T-dependent secondary responses.
(A) Experimental design. “Secondary” responses were generated in BALB/cJ or DO11.10+/+ TCRα−/− BALB/cJ mice at week 7 after transfer of AM14 sd-Tg B cells and activation with PL2-3 as described in Materials and Methods. “Primary” responses were analyzed at day 2.5 following cell transfer. Both “secondary” and “primary” mice received one injection with PL2-3 at 2.5 days prior to sacrifice. (B–C) Numbers of IgM (B) and IgG2a (C) 4–44+ AFC per million splenocytes. (D) Quantitation of 4–44+ frequencies analyzed by flow cytometry as in Fig. 3. At least 6 mice per group and 3 independent experiments were compiled. Bars represent the mean + SEM. **p<0.01 and ***p<0.001 by Mann-Whitney two tailed test.
The day 2.5 secondary response in WT mice was also associated with a 4-fold increase in the frequency of the 4–44+ population (comparing Fig. 4A “PL2-3” black bar which shows the frequency of 4–44+ cells in immune mice, prior to reimmunization, and Fig. 5D “secondary” black bar). Similarly, expansion in the WT new primary was 3-fold (Fig. 5D, hatched vs. gray bars); therefore, proliferative capacity during the primary response and secondary response were comparable. This expansion of the 4–44+ population in a recall response was T cell-dependent, as expansion in DO11.10 mice following the secondary treatment with PL2-3 was 10-fold less than that found in WT mice (Fig 5D). Thus, we find that autoreactive B cell memory persistence and function is T-dependent; further, autoreactive MBC that developed in this system have been reprogrammed compared to their naïve counterpart to differentiate rapidly to both IgM and IgG2a plasmablasts.
Discussion
In this paper we have defined the long-term kinetics of an EF Rheumatoid Factor response, taking advantage of the well-characterized AM14 system (1, 2, 33, 58, 59). RF B cells in the natural setting of murine lupus (67–69) as well as in this experimental system (5), are stimulated by immune complexes that contain nucleic acids; these responses are TLR7/TLR9-dependent. As such, the RF system serves as a model for anti-nuclear responses that has the experimental advantage that self-Ag can be controlled and introduced. Indeed the RF system was the first to call attention to the TLR-dependent nature of anti-nuclear responses (70), and since this initial demonstration multiple lines of evidence have pointed to the role of TLR/BCR co-ligation in stimulation of both RF and anti-DNA/RNA B cells (3, 67, 71). Hence, the study of RF B cells provides insight into ANA clones through its activation by anti-chromatin Ab.
Despite the prior notion that such responses were inherently short-lived, we found that in response to anti-chromatin Ab, AM14 RF B cells can persist beyond the primary response, and are functional at 7 weeks post-immunization. Thus, the EF response is temporally and functionally more complex than previously recognized. The most striking and important finding is that AM14 RF B cells that persist after an initial EF response provide bona fide functional memory, as they are able to differentiate to both IgM and IgG AFCs more rapidly and robustly than their counterparts in a primary response. Additionally, they show qualities of memory in that they are marked by CD73 and are resting as determined by lack of Ki67 staining. However, it is important to point out that these MBC do differ from classic GC-derived MBC in lacking substantial V region somatic mutation. Thus, the EF response does generate a form of memory. We further suggest that in situations where GC B cells also generate more classical MBC, whether in normal immunity or autoimmunity, the EF response is also likely creating MBC, thus contributing a different quality of MBC to the total memory compartment; this notion is supported by studies of the NP immune response (23).
In the absence of Ag-specific T cells, AM14 B cells can make both IgM and isotype-switched primary AFC responses, albeit of a somewhat reduced magnitude (33). In contrast, absence of Ag-specific T cells leads to an essentially complete loss of AM14 MBC formation and response, in contrast to certain types of B cell memory that can be generated in a T-independent fashion (28), This requirement of T cells for development and function of EF-derived memory is evocative of previously published work showing primary switched Ab secretion but deficiency in memory responses if GCs or T cell help was absent (21, 24, 27). Thus, in determining whether MBC will persist following activation, the site of activation may not be as critical as the availability of T cell help.
These findings suggest that autoreactive B cell memory and rapid AFC differentiation could contribute to SLE progression, as autoreactive MBC differentiate to secrete antibody faster than their naïve counterparts. Since disease can be relapsing and remitting, flares could be initiated by reactivation of memory and recall AFC responses, instead of, or in addition to activation of naïve precursors. Indeed, autoreactive CD27+ MBC have been characterized in SLE and RA patients (9, 10) and their presence is correlated with relapse (42–45).
Although both switched and unswitched MBC populations have been studied by several groups (12, 13, 15–17, 72, 73), it is unclear which classes of MBC would contribute to autoimmunity. The MBC we identified from AM14 B cells in response to anti-chromatin Ab are largely unswitched (data not shown) and unmutated whereas MBC studied in patients can be switched and mutated. Three lines of reasoning suggest that both of these populations are relevant to disease. First, the presence of switched, mutated, MBC in human patients does not rule out the possibility of unswitched, unmutated memory. In fact, unswitched MBC have been characterized in SLE patients (10).
Second, the MBC we have studied resulted from a single acute challenge on a non-autoimmune prone strain. In contrast, MBC generated during autoimmunity are either the result of a chronic response or serial acute responses. This concept is supported by the finding that in the first week, on a genuine autoimmune prone strain, MRL.Faslpr, AM14 B cells accumulated more mutations compared with their counterparts in BALB/c mice. It is possible and even likely that multiple rounds of stimulation of the unswitched population we observed would result in persistent switched, mutated MBC. Supporting this notion, upon re-challenge there was a very rapid switch to IgG2a of a large proportion of putative IgM+ precursors, in that the secondary response at day 2.5 was about 40% IgG2a. In this vein, the switched memory response seen in BALB/c mice, as would also be expected in autoimmune mice, suggests that a major difference between the two environments is the exposure to Ag and possibly other inflammatory signals in the latter, rather than any intrinsic differences in the B cells, though such differences of course cannot be ruled out.
Third, the specificity of MBC identified so far has not been well-defined. In one pioneering study, the frequency of polyreactive or HEp2 ELISA-reactive IgG MBC did not differ between four patients and controls (46). This suggests that the IgG memory compartment may not include some classic autoreactive specificities—these autoreactive specificities could instead be limited to the IgM memory compartment or may be rare among MBC. However, in one patient anti-Ro52/La type specificities were detected, suggesting that more classical IgG memory can be generated at least occasionally and for some specificities (46). This study did not examine the prevalence of RF. It will be interesting to learn if unswitched, unmutated MBC clones can be identified in patients or even murine lupus models, particularly early in disease.
Initiation and primary differentiation of AM14 B cells in response to anti-chromatin Ab can occur in the absence of T cell help (5, 33). Similarly, autoimmunity driven by B cell-activating factor of the TNF family overexpression does not require T cells (4), nor are T cells needed to drive loss of self tolerance by anti-DNA B cells in B6 mice (74). TLR7-dependent activation and differentiation of RNA-specific B cells in B6 mice also does not require T cells, as it occurs unimpeded on a RAG-deficient background (3). Nonetheless, T cells were still critical for the generation and function of AM14 MBC, even though these B cells were largely unswitched and unmutated. The T-dependence of autoreactive MBC formation is yet another dimension of the cooperative B–T interactions that take place at many steps of lupus pathogenesis (75–86). What emerges from these and other studies is a model in which TLR and BCR signals drive initial autoreactive B cell activation independent of T cells, while T cells and T-B interaction subsequently become critical for optimal B cell expansion (33). Whether qualitatively different MBC responses depend on intrinsic changes in such cells, or are programmed by memory T cells, or both, remains unknown. Here we have extended prior knowledge by showing that T cells are required as well for development and reactivation of long-lived autoreactive MBC that are generated via an EF pathway.
The investigation of different immunization scenarios has revealed plasticity in roles of both GCs and T cells in B cell memory formation, in contrast to the orthodox view that B cell memory is strictly T cell and GC-dependent. In our case, B cell memory elicited by chromatin-containing immune complexes was GC-independent but T cell-dependent. In what ways might this type of immunogen be distinct from others? Most likely the defining feature of nucleic-acid-containing self-Ags that dominate in lupus is the ability to co-stimulate endosomally-expressed TLRs, provide strong BCR crosslinking, and associate with proteins that could contain T cell epitopes. This type of Ag thus combines features of TI-1, TI-2 and TD Ags. Taken together, this work and prior studies of the AM14 response (5, 33, 59) suggest that this type of Ag functions as TI for initiation but as TD for full development of the B cell response, without eliciting a GC response. In a similar vein, Foote et al. demonstrated that peritoneal B1b cells can stably expand following E. cloacae immunization, but whether this was GC-dependent or required T cells was not formally tested, even if the nominal Ag dextran is considered a TI Ag (29).
Our findings are also in keeping with data from an elegant system devised by Eckl-Dorna and Batista in which they coupled beads with both protein Ag and CpG DNA, simulating an Ag similar to DNA-containing immune complexes; such Ags drove an exclusively EF plasmablast response, though in these studies memory formation was not investigated (87). By using a defined model system with direct relevance to the activation of autoreactive B cells in lupus, our studies connect basic insights into the pathways of MBC formation with the pathogenesis of autoantibody generation and disease. These findings should form a basis for further investigation of how autoreactive B cell memory progresses during chronic disease.
Acknowledgements
We would like to acknowledge expert technical assistance from Cuiling Zhang and the highest standards in animal care from Joanne Fonck and the Yale Animal Resource Center. We would like to thank Kim Good-Jacobson, Kevin Nickerson, and Shinu John for critical reading of the manuscript.
Sources of support: This work was supported by NIH grant R01-AI073722 to M.J.S. and NIH Kirschstein National Research Service Award Predoctoral Fellowship 1F31-AI071694 to R.A.S.
Abbreviations
- AFC
Antibody Forming Cell
- EF
Extrafollicular
- GC
Germinal Center
- MBC
Memory B Cell
- RF
Rheumatoid Factor
- SLE
Systemic Lupus Erythematosus
- sd-Tg
site directed Transgenic
- Tg
Transgenic
- WT
Wild Type
References
- 1.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 2.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 3.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 8.Ferraro AJ, Drayson MT, Savage CO, MacLennan IC. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 9.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 11.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 12.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 13.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or "memory" B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 19.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 22.Hikida M, Casola S, Takahashi N, Kaji T, Takemori T, Rajewsky K, Kurosaki T. PLC-gamma2 is essential for formation and maintenance of memory B cells. J Exp Med. 2009;206:681–689. doi: 10.1084/jem.20082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inamine A, Takahashi Y, Baba N, Miyake K, Tokuhisa T, Takemori T, Abe R. Two waves of memory B-cell generation in the primary immune response. Int Immunol. 2005;17:581–589. doi: 10.1093/intimm/dxh241. [DOI] [PubMed] [Google Scholar]
- 24.Karrer U, Lopez-Macias C, Oxenius A, Odermatt B, Bachmann MF, Kalinke U, Bluethmann H, Hengartner H, Zinkernagel RM. Antiviral B cell memory in the absence of mature follicular dendritic cell networks and classical germinal centers in TNFR1−/− mice. J Immunol. 2000;164:768–778. doi: 10.4049/jimmunol.164.2.768. [DOI] [PubMed] [Google Scholar]
- 25.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 28.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Ghosn EE, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, Herzenberg LA. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A. 2012;109:5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Ghosn EE, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, Herzenberg LA. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc Natl Acad Sci U S A. 2012;109:5382–5387. doi: 10.1073/pnas.1121631109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proc Natl Acad Sci U S A. 2011;108:7932–7937. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 37.Barr SG, Zonana-Nacach A, Magder LS, Petri M. Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2682–2688. doi: 10.1002/1529-0131(199912)42:12<2682::AID-ANR26>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, Lipsky PE, Radbruch A, Dorner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 39.Gause A, Gundlach K, Carbon G, Daus H, Trumper L, Pfreundschuh M. Analysis of VH gene rearrangements from synovial B cells of patients with rheumatoid arthritis reveals infiltration of the synovial membrane by memory B cells. Rheumatol Int. 1997;17:145–150. doi: 10.1007/s002960050026. [DOI] [PubMed] [Google Scholar]
- 40.Scheel T, Gursche A, Zacher J, Haupl T, Berek C. V-region gene analysis of locally defined synovial B and plasma cells reveals selected B cell expansion and accumulation of plasma cell clones in rheumatoid arthritis. Arthritis Rheum. 2011;63:63–72. doi: 10.1002/art.27767. [DOI] [PubMed] [Google Scholar]
- 41.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 43.Moller B, Aeberli D, Eggli S, Fuhrer M, Vajtai I, Vogelin E, Ziswiler HR, Dahinden CA, Villiger PM. Class-switched B cells display response to therapeutic B-cell depletion in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R62. doi: 10.1186/ar2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roll P, Dorner T, Tony HP. Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum. 2008;58:1566–1575. doi: 10.1002/art.23473. [DOI] [PubMed] [Google Scholar]
- 45.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 46.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 48.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tillman DM, Jou NT, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB × NZW)F1 mice. J Exp Med. 1992;176:761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marion TN, Tillman DM, Jou NT. Interclonal and intraclonal diversity among anti-DNA antibodies from an (NZB × NZW)F1 mouse. J Immunol. 1990;145:2322–2332. [PubMed] [Google Scholar]
- 51.Behar SM, Lustgarten DL, Corbet S, Scharff MD. Characterization of somatically mutated S107 VH11-encoded anti-DNA autoantibodies derived from autoimmune (NZB × NZW)F1 mice. J Exp Med. 1991;173:731–741. doi: 10.1084/jem.173.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behar SM, Scharff MD. Somatic diversification of the S107 (T15) VH11 germ-line gene that encodes the heavy-chain variable region of antibodies to double-stranded DNA in (NZB × NZW)F1 mice. Proc Natl Acad Sci U S A. 1988;85:3970–3974. doi: 10.1073/pnas.85.11.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp-+/+ mouse: oligoclonality of the antibody response and recognition of a determinant composed for histones H2A, H2B, and DNA. J. Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 55.Shan H, Shlomchik MJ, Marshak-Rothstein A, Pisetsky DS, Litwin S, Weigert MG. The mechanism of autoantibody production in an autoimmune MRL/lpr mouse. J. Immunol. 1994;153:5104–5120. [PubMed] [Google Scholar]
- 56.Dorner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr Opin Rheumatol. 2004;16:246–253. doi: 10.1097/00002281-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. Rheumatoid factors in systemic lupus erythematosus: association with clinical and laboratory parameters. SLE study group. Rheumatol Int. 2000;19:107–111. doi: 10.1007/s002960050112. [DOI] [PubMed] [Google Scholar]
- 58.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 59.Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–618. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.William J, Euler C, Leadbetter E, Marshak-Rothstein A, Shlomchik MJ. Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J Immunol. 2005;174:6872–6878. doi: 10.4049/jimmunol.174.11.6872. [DOI] [PubMed] [Google Scholar]
- 61.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related RF autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J. Exp. Med. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dittrich AM, Chen HC, Xu L, Ranney P, Connolly S, Yarovinsky TO, Bottomly HK. A new mechanism for inhalational priming: IL-4 bypasses innate immune signals. J Immunol. 2008;181:7307–7315. doi: 10.4049/jimmunol.181.10.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 64.Townsend SE, Goodnow CC, Cornall RJ. Single epitope multiple staining to detect ultralow frequency B cells. J Immunol Methods. 2001;249:137–146. doi: 10.1016/s0022-1759(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 66.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 67.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, Arnold CN, Baccala R, Silverman GJ, Beutler BA, Theofilopoulos AN. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 71.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson SM, Tomayko MM, Shlomchik MJ. Intrinsic properties of human and murine memory B cells. Immunol Rev. 2006;211:280–294. doi: 10.1111/j.0105-2896.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 73.Tomayko MM, Anderson SM, Brayton CE, Sadanand S, Steinel NC, Behrens TW, Shlomchik MJ. Systematic comparison of gene expression between murine memory and naive B cells demonstrates that memory B cells have unique signaling capabilities. J Immunol. 2008;181:27–38. doi: 10.4049/jimmunol.181.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7777. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 75.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 76.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma J, Xu J, Madaio MP, Peng Q, Zhang J, Grewal IS, Flavell RA, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- 78.Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by gammadelta T cells. J Immunol. 1996;157:5689–5698. [PubMed] [Google Scholar]
- 79.Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 80.Peng SL, McNiff JM, Madaio MP, Ma J, Owen MJ, Flavell RA, Hayday AC, Craft J. alpha beta T cell regulation and CD40 ligand dependence in murine systemic autoimmunity. J Immunol. 1997;158:2464–2470. [PubMed] [Google Scholar]
- 81.Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- 82.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 83.Iwai H, Abe M, Hirose S, Tsushima F, Tezuka K, Akiba H, Yagita H, Okumura K, Kohsaka H, Miyasaka N, Azuma M. Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. J Immunol. 2003;171:2848–2854. doi: 10.4049/jimmunol.171.6.2848. [DOI] [PubMed] [Google Scholar]
- 84.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinstein JS, Hernandez SG, Craft J. T cells that promote B-Cell maturation in systemic autoimmunity. Immunol Rev. 2012;247:160–171. doi: 10.1111/j.1600-065X.2012.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]