Abstract
Neurosteroids are a class of endogenous steroids synthesized in the brain that are believed to be involved in the pathogenesis of neuropsychiatric disorders and memory impairment. Ammonia impairs long-term potentiation (LTP), a synaptic model of learning, in the hippocampus, a brain region involved in memory acquisition. Although mechanisms underlying ammonia-mediated LTP inhibition are not fully understood, we previously found that activation of N-methyl-D-aspartate receptors (NMDARs) is important. Based on this, we hypothesize that metabolic stressors, including hyperammonemia, promote untimely NMDAR activation and result in neural adaptations that include the synthesis of allopregnanolone (alloP) and other γ-aminobutyric acid (GABA)-potentiating neurosteroids that dampen neuronal activity and impair LTP and memory formation. Using an antibody against 5α-reduced neurosteroids, we found that 100 μM ammonia acutely enhanced neurosteroid immunostaining in pyramidal neurons in the CA1 region of rat hippocampal slices. The enhanced staining was blocked by finasteride, a selective inhibitor of 5α-reductase, a key enzyme required for alloP synthesis. Finasteride also overcame LTP inhibition by 100 μM ammonia, as did picrotoxin, an inhibitor of GABA-A receptors. These results indicate that GABA enhancing neurosteroids, synthesized locally within pyramidal neurons, contribute significantly to ammonia-mediated synaptic dysfunction. These results suggest that manipulation of neurosteroid synthesis could provide a strategy to improve cognitive function in individuals with hyperammonemia.
Keywords: Hepatic encephalopathy, GABA receptors, finasteride, allopregnanolone, tetrahydrodeoxycorticosterone, ammonia, LTP, neurosteroid
Introduction
Neurosteroids are a class of endogenous molecules synthesized from cholesterol that modulate both glutamatergic and GABAergic systems in the brain (Baulien 1981, 1997). Neurosteroids are believed to be involved in the pathogenesis of several neuropsychiatric disorders, including mood disorders, epilepsy, and alcoholism (Zorumski et al., 2000, 2013; Gunn et al., 2011). In the hippocampus, pyramidal neurons are the primary cells that express the molecular machinery for cholesterol trafficking (Valdez et al., 2010) and the key transporters and enzymes required for steroid synthesis, including steroidogenic acute regulatory protein (StAR) (Kimoto et al., 2001; King et al., 2002; Kim et al., 1996; Lavaque et al, 2006), translocator protein 18kDa (TSPO) (Tokuda et al., 2010), P450 side chain cleavage enzyme (Kimoto et al, 2001; Shibuya et al., 2003) and 5alpha-reductase (Agis-Balboa et al., 2006). TSPO is the rate limiting step in neurosteroid synthesis and promotes the movement of cholesterol to the inner mitochondrial membrane where it is converted to pregnenolone. TSPO was previously known as the peripheral (mitochondrial) benzodiazepine receptor and is the site of action by which benzodiazepines promote neurosteroidogenesis (for review: Zorumski et al., 2013). In addition to expressing steroidogenic enzymes and transporters, excitatory neurons are the primary cells that are immunopositive for neurosteroids under basal condition in the brain (Saalman et al., 2007, Tokuda et al., 2010, 2011).
Because we previously found that low concentrations of N-methyl-D-aspartate (NMDA) inhibit hippocampal long-term potentiation (LTP), a cellular model of memory and learning (Izumi et al., 1992a,b) via neurosteroid synthesis (Tokuda et al., 2011), we hypothesized that stressors that trigger NMDA receptor (NMDAR) activation impair neuronal function through local brain steroidogenesis (Zorumski and Izumi, 2012). In the CNS, calcium influx through NMDARs enhances pregnenolone formation and synthesis of neurosteroids in the hippocampus (Kimoto et al., 2001), and recent studies have found that low level tonic NMDAR activation is sufficient to promote neurosteroid synthesis in hippocampal pyramidal neurons (Tokuda et al., 2011). Neurosteroid production in the hippocampus by NMDAR activation negatively modulates the induction of LTP by initiating a form of neurosteroid-dependent metaplasticity (Tokuda et al., 2011). Thus, untimely activation of NMDARs, triggered by various stressful events, may result in LTP inhibition and memory impairment via neurosteroid production. Ethanol intoxication is an example of such an event. Ethanol acutely inhibits LTP induction only at high concentrations (Izumi et al., 2005b) via a mechanism that includes NMDAR activation and neurosteroid synthesis (Izumi et al., 2007; Tokuda et al., 2011).
Somewhat akin to ethanol, ammonia inhibits LTP induction by a mechanism involving untimely NMDAR activation (Izumi et al., 2005a). We have shown that the inhibition of LTP induction by 100 μM ammonia is overcome by 2-amino-5-phosphonovalerate (APV), an NMDAR antagonist. In the present study, we hypothesized that exposure to ammonia is a metabolic stressor to pyramidal neurons (Izumi et al., 2005a) and inhibits synaptic plasticity and memory acquisition through NMDAR activation and neurosteroid production. To test this, we examined whether the inhibition of LTP induction by ammonia is overcome by pharmacological blockage of neurosteroid production and whether ammonia facilitates neurosteroid production in the hippocampus.
Materials and Methods
Animals
Protocols for animal use were approved by the Washington University Animal Studies Committee in accordance with the NIH guidelines for care and use of laboratory animals.
Hippocampal slice preparation
Hippocampal slices were prepared as descried previously (Zorumski et al., 1996) from postnatal day 30–32 male Sprague-Dawley rats purchased from Harlan (Indianapolis, IN). Rats were anesthetized with isoflurane and decapitated. Slices were cut transversely into 500 μm slices using a rotary slicer in artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2/5% CO2 at 4–6 °C. Acutely prepared slices were placed on nylon mesh in 10 ml beakers containing gassed ACSF and maintained for at least 1 h at 30 °C before experiments.
Immunohistochemistry
Hippocampal slices used for immunohistochemistry were initially screened by electrophysiology to diminish slice-to-slice variability in staining for 5α-reduced neurosteroids (Tokuda et al., 2010, 2011). Immunostaining was performed as previously described (Tokuda et al., 2010). Slices were incubated with various reagents in separate 10 ml beakers. Following drug treatment, slices were fixed in fresh 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min. Samples were then washed with PBS and incubated in blocking solution (1% donkey serum/PBS) for 2 h at 25°C. Slices were incubated with a primary antibody raised in sheep against 5α-reduced neurosteroids diluted 1:2500 in blocking solution for 48h at 4°C. The polyclonal antibody against 5α-steroids primarily recognizes alloP and has minimal cross-reactivity with other neurosteroids in rats (Bernardi et al., 1998). This antibody has also been previously characterized in immunostaining studies in rat brain tissue (Saalman et al, 2007, Tokuda et al., 2010).
After incubation with primary antibody, slices were rinsed with PBS and incubated with a secondary antibody, Alexa Flour 488 donkey anti-sheep IgG (diluted 1:500), for 2 h at 25°C. After staining, slices were washed with PBS and mounted onto microscope slides with Fluoromount-G (Southern Biotech, Birmingham, AL).
Confocal images were obtained using a 60X objective (1.4 N.A.), a C1 laser scanning confocal microscope and Z-C1 software (Nikon Instruments, Melville, NY). All parameters were kept constant within an experiment. Digital images were analyzed and the average intensity of the tissue was measured using MetaMorph software (Universal Imaging Corporation, Downingtown, PA).
Extracellular field potential recording
For electrophysiology, slices were incubated in a submerged recording chamber with continuous bath perfusion of oxygenated ACSF at 2 ml/ min at 30°C. Extracellular recordings were obtained from the apical dendritic layer of the CA1 region elicited with 0.1 ms constant current pulses through a bipolar stimulating electrode (Rhodes Medical Instruments Inc., Summerland, CA) in stratum radiatum. Long-term potentiation (LTP) was induced by applying a single 100 Hz × 1 s high frequency stimulation (HFS) using a 50% maximal stimulus. To determine the 50% maximal stimulus, 6 different strength stimuli were delivered prior to monitoring. The same 6 stimuli were repeated 60 min following HFS for statistical comparisons of changes in excitatory postsynaptic potential (EPSP) slopes at the half-maximal point. Signals were digitized and analyzed using PCLAMP software (Axon Instruments, Union City, CA). Isolated NMDAR-mediated synaptic responses were studied in an extracellular solution containing 2 mM calcium and 0.1 mM magnesium. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was added to this solution at 30 μM to inhibit non NMDAR-mediated EPSPs.
Statistical Analysis
All data are expressed as mean ± s.e.m. Student’s t-test was used for comparisons between two groups. If an equal variance test failed, the non-parametric Mann–Whitney rank sum test was applied. For multiple comparisons, analysis of variance followed by post hoc Holm-Sidak test was employed. Statistical analyses were performed using commercial software (SigmaStat 3.11; Systat Software Inc., Richmond, CA). P-values of less than 0.05 were considered statistically significant.
Materials
Finasteride and neurosteroids were obtained from Steraloids (Newport, RI). PK11195 was purchased from Tocris (St. Louis, MO). All other chemicals were purchased from Sigma (St. Louis, MO). The antibody against 5α-reduced neurosteroids was purchased from Dr. Robert Purdy, University of California-San Diego. Alexa Flour 488 was purchased from Invitrogen (Carlsbad, CA).
Results
Inhibition of LTP by ammonia and effects of finasteride
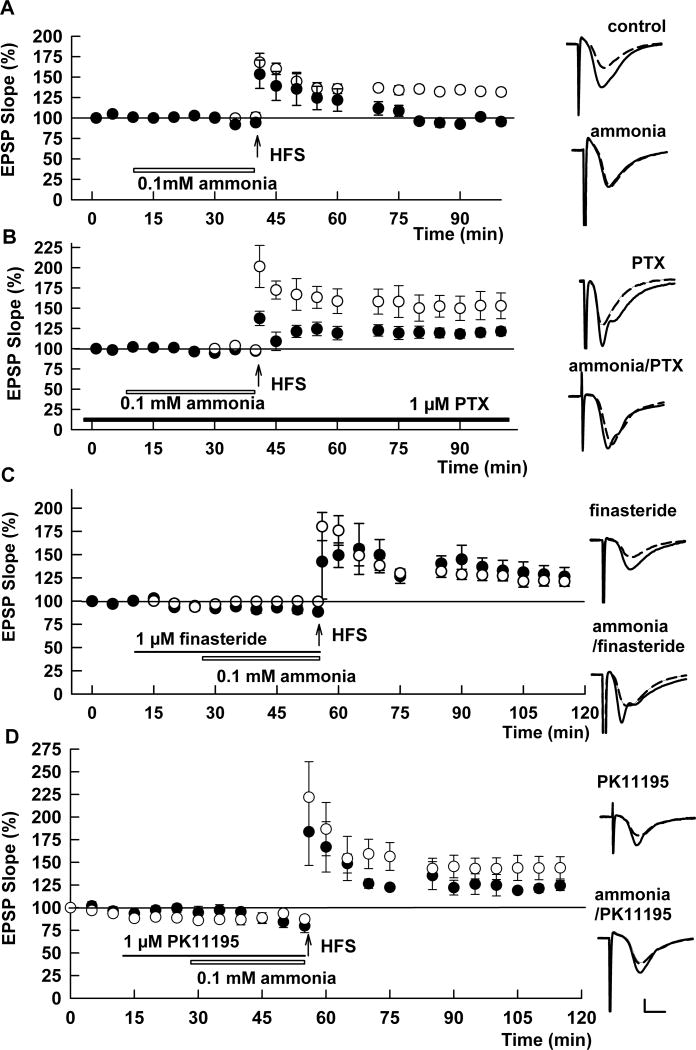
Confirming our previous results (Izumi et al., 2005a), we found that LTP was reliably induced in naïve hippocampal slices from 30 day old rats, but was significantly reduced when 100 μM ammonia was administered for 30 min prior to high frequency stimulation (HFS) (EPSP slopes: 131.7 ± 4.3 % and 95.5 ± 4.4 % of baseline measured 60 min following HFS, respectively; N=5 each; p < 0.001; Fig. 1A). Because a lower concentration of ammonia (30 μM) did not significantly alter LTP compared to controls (121.0 ± 6.1% of baseline, N=5; p = 0.189), we focused on mechanisms contributing to the effects of 100 μM ammonia in subsequent experiments. Based on a proposed role for GABA receptors in the CNS effects of hyperammonemia (Schafer and Jones, 1982; Basile and Jones, 1997), we examined whether GABA-A receptors contribute to ammonia-mediated LTP inhibition. In the presence of 1 μM picrotoxin (PTX), an inhibitor of GABA-A receptors, 100 μM ammonia failed to inhibit LTP induction (EPSP slope: 121.3 ± 5.3%, N=5, Fig. 1B). This degree of LTP did not differ significantly from LTP induced in the presence of PTX alone (143.1 ± 11.0, N=6, p = 0.112, Fig. 1B).
Fig. 1.
Ammonia-mediated LTP inhibition involves GABA-A receptors and neurosteroidogenesis. A. In control slices, delivery of a100 Hz × 1 s high frequency stimulus (HFS, arrow) induced robust LTP in the CA1 region as measured by changes in EPSP slopes (open circles). Administration of 100 μM ammonia for 30 min (open bar) prior to and during delivery of HFS attenuated the potentiation of EPSPs (closed circles). B. Ammonia failed to inhibit LTP induction when HFS was administered in the presence of 1 μM PTX, a GABA-A receptor antagonist (black bar). Open circles show effects of PTX alone whereas closed circles show effects of PTX plus ammonia. C. The 5α-reductase inhibitor, finasteride (1μM, closed bar) also overcame the effects of ammonia on LTP. Open circles show effects of finasteride alone whereas closed circles show effects of finasteride plus ammonia. D. Effects of PK11195, a TSPO ligand, on ammonia-mediated LTP inhibition. Because we previously observed that PK11195 has both agonistic and antagonistic effects on TSPO (see supplemental data of Tokuda et al., 2010), we used PK11195 at 1 μM, a concentration at which the agonistic effects are minimal and at which antagonistic effects are partial. In spite of its complex actions, PK11195 overcame the inhibitory effects of ammonia and allowed LTP induction. Open circles show effects of PK11195 alone whereas closed circles show effects of PK11195 plus ammonia. Traces depict EPSPs before (dashed lines) and 60 min after HFS (solid lines). Scale; 1mV, 5 msec.
In prior studies, we found that LTP can be impaired by activation of GABA-A receptors triggered by stressful conditions that promote the synthesis of GABA-enhancing neurosteroids (Zorumski and Izumi, 2012). We thus tested whether neurosteroidogenesis contributes to ammonia-mediated LTP inhibition. In the presence of 1 μM finasteride, a selective inhibitor of 5α-reductase, a key enzyme required for neurosteroid synthesis, LTP was successfully induced in the presence of 100 μM ammonia (EPSP slope: 126.3 ± 9.8%, N=5, p < 0.05 vs. ammonia; Fig. 1C). Finasteride alone did not alter LTP induction compared to controls (128.9 ± 7.2 %, N=5, Fig. 1C). LTP inhibition by ammonia was also overcome by PK11195, an inhibitor of TSPO (EPSP slope: 128.4 ± 7.5%, N=5, p < 0.05 vs. ammonia; Fig. 1D). PK11195 alone did not alter LTP induction compared to controls (136.7 ± 6.1 %, N=5, Fig. 1D).
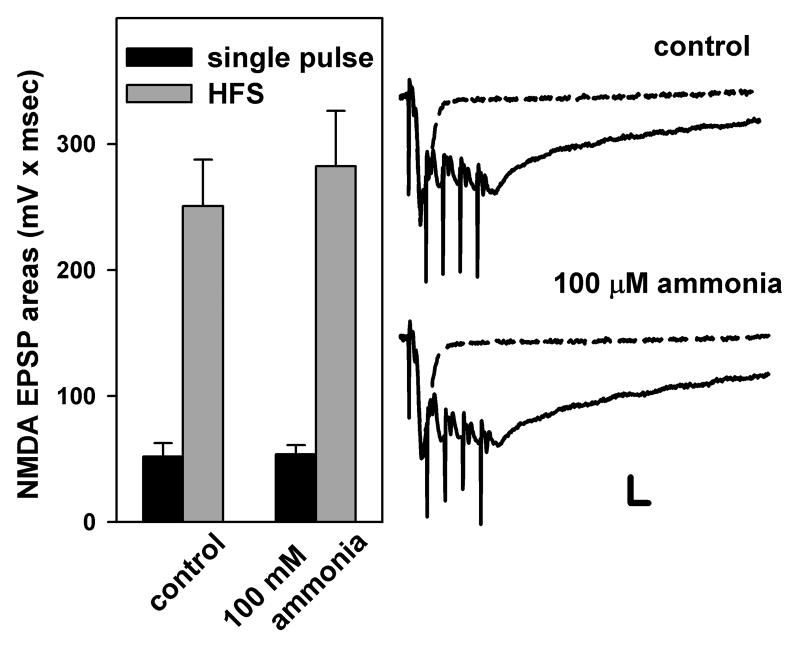
It is possible that ammonia impairs LTP induction by reducing NMDAR-mediated synaptic responses. To test this, we examined isolated NMDAR-mediated EPSPs evoked by single stimuli and by trains of 100 Hz HFS in the presence of low magnesium and CNQX. To avoid toxic effects of HFS in low magnesium, we examined trains of 5 pulses at 100 Hz. The total areas of NMDAR-mediated EPSPs evoked by both single pulses (52.0 ± 10.5 mV × msec) and by 5 pulse trains of HFS (250.8 ± 36.8 mV × msec) were not significantly altered by 100 μM ammonia (53.8 ± 7.2 and 382. 4 mV × msec, respectively, n=6, Fig. 2).
Fig. 2.
NMDAR-mediated EPSPs evoked by single pulse and HFS. NMDAR-mediated EPSPs were elicited by single pulses (black bars in histogram and dotted traces) or by trains of 5 pulses at 100 Hz (grey bars in histogram and solid traces). EPSP areas were measured before and after administration of 100 μM ammonia. Dashed traces depict NMDAR-mediated EPSPs elicited by single pulses, while solid traces show NMDAR EPSPs activated by brief trains of stimulation at 100 Hz. Scale; 1mV, 10 msec.
Neurosteroids and ammonia-mediated LTP inhibition
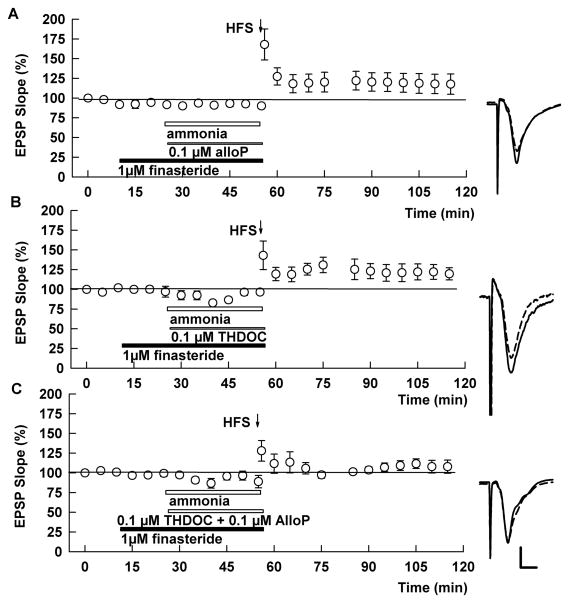
To determine whether finasteride’s ability to overcome the effects of ammonia results from dampening of neurosteroid synthesis, we examined whether administration of exogenous neurosteroids reverse LTP induction in the presence of ammonia plus finasteride (Tokuda et al, 2010, 2011). Against our expectation, however, we found that LTP was still induced in the presence of 0.1 μM alloP plus finasteride (EPSP slope; 117.7 ± 12.8%, N=6, p = 0.365 vs. control LTP; Fig. 3A). LTP was also induced when finasteride was combined with 0.1 μM tetrahydrodeoxycorticosterone (THDOC), another GABAergic neurosteroid (EPSP slope; 119.7 ± 7.2%, N=6, p=0.208; Fig. 3B). In contrast, LTP was blocked when these two neurosteroids were simultaneously administered with ammonia and finasteride (EPSP slope; 107.9 ± 8.4%, N=6, p < 0.05 vs. control LTP; Fig. 2C), but not when 0.2 μM alloP or THDOC was administered alone (EPSP slope; 119.0 7.8%, 125.7 8.3%, N=5, respectively).
Fig. 3.
Effects of exogenous neurosteroids on ammonia-mediated LTP inhibition. A. When 100 μM ammonia (white bar) was co-applied with 1 μM finasteride (black bar) in the presence of 0.1μM alloP (thin bar), HFS successfully induced LTP. B. The effects of 1 μM finasteride on ammonia-mediated LTP inhibition was also not overcome by co-administration of 0.1 μM THDOC alone. C. In the presence of both 0.1 μM AlloP and 0.1 μM THDOC, ammonia inhibited LTP induction in the presence of finasteride. Traces depict EPSPs before (dashed lines) and 60 min after HFS (solid lines). Scale; 1mV, 5 msec.
Ammonia enhances neurosteroid staining
These results indicate that ammonia impairs LTP induction by promoting neurosteroid synthesis locally in the hippocampus. We examined this directly by determining the effects of ammonia on neurosteroid immunostaining in the CA1 region using an antibody that recognizes alloP and other 5α-reduced steroids. Consistent with earlier reports (Saalman et al., 2007; Tokuda et al., 2010, 2011), steroid immunostaining was largely confined to CA1 pyramidal neurons. Fifteen min administration of 100 μM ammonia increased neurosteroid staining (Fluorescence Intensity: 182.9 ± 20.2% vs. control, n = 6, Fig. 4A,B,D, p < 0.01). The enhanced staining in the presence of ammonia was blocked completely by 1 μM finasteride (Fluorescence Intensity: 94.6 ± 21.9%, n = 6, Fig. 4C,E). Similar applications of finasteride alone did not alter basal staining (Fluorescence Intensity: 94.5 ± 15.7%, n=4, Fig. 4D,E). Consistent with our prior finding that APV, an NMDAR antagonist, overcomes the effect of ammonia on LTP (Izumi et al., 2005a), we found that the enhanced neurosteroid immunostaining observed with ammonia was also significantly suppressed by APV (Fluorescence Intensity: 257.6 ± 20.8 % vs. 135.4±13.9 %, respectively, p<0.05 Fig. 5).
Fig. 4.
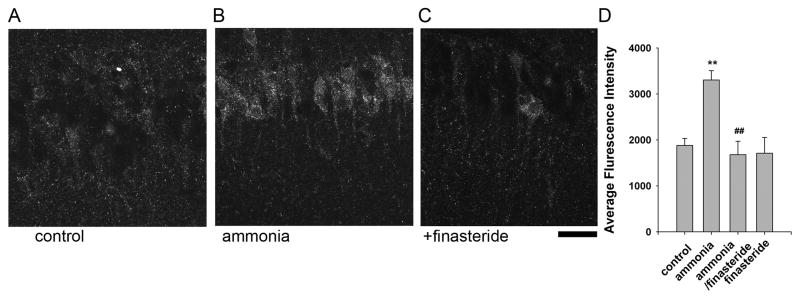
Ammonia-mediated neurosteroidogenesis results from activation of 5α-reductase. A. The photomicrograph shows low level immunostaining against 5α-reduced neurosteroids in cell bodies of CA1 pyramidal neurons in naïve hippocampal slices. B. Following 30 min incubation with 100μM ammonia, enhanced immunostaining is observed in CA1 pyramidal neurons. C. The enhanced staining in the presence of ammonia was blocked by 1μM finasteride. Calibration bar: 25 μm. D. Summary of immunostaining studies showing fluorescence intensity (arbitrary units) as mean ± s.e.m. P-values are calculated by Student t test (n = 6; ** P < 0.01 vs. control, and ## P < 0.01 vs. ammonia alone)
Fig. 5.
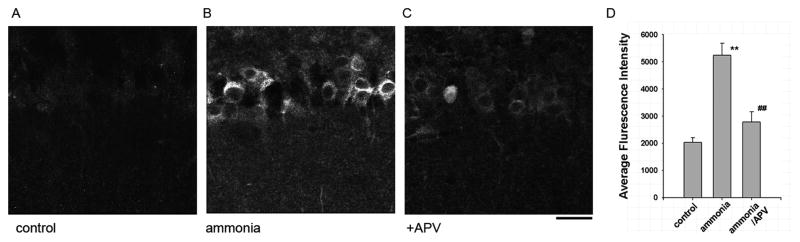
Ammonia-mediated neurosteroidogenesis results from activation of NMDARs. A. The photomicrograph shows low level immunostaining against 5α-reduced neurosteroids in cell bodies of CA1 pyramidal neurons in naïve hippocampal slices. B. Neurosteroid immunostaining was enhanced by 30 min incubation with 100 μM ammonia. C. The enhanced staining was blocked by 50 μM D-2-amino-5-phosphonovalerate (APV), an NMDAR antagonist. Calibration bar: 25 μm. D, Summary of immunostaining studies showing fluorescence intensity (arbitrary units) as mean ± s.e.m. P-values are calculated by Student t- test (n = 4; ** P < 0.01 vs. control. ##P < 0.01 vs. ammonia alone.)
Discussion
In the hippocampus, neurosteroid synthesis can be triggered by multiple mechanisms including ethanol (Sanna et al., 2004; Tokuda et al., 2011), certain benzodiazepines (Bernardi et al., 1998; Tokuda et al., 2010) and NMDAR activation (Kimoto et al., 2001; Tokuda et al., 2011). In the present study, we also showed that in the hippocampus ammonia exposure triggers neurosteroid production to inhibit LTP induction.
Although LTP induction in the CA1 region is critically dependent upon activation of NMDARs, these receptors also trigger neurosteroid synthesis (Kimoto et al., 2001; Tokuda et al., 2011). It has long been known that excessive activation of NMDARs plays a pivotal role in neuronal damage and excitotoxicity. Furthermore, it has been shown that ammonia can promote activation of NMDARs (Elmlili et al., 2010) and that the neuronal damage acutely induced by exposure to ammonia in experimental animals is mediated by NMDARs (Zielinska et al., 2003; Rodrigo et al., 2009). Based on prior reports indicating that NMDAR activation promotes neurosteroid production in the hippocampus (Kimoto et al., 2001; Tokuda et al., 2011), and our previous finding that ammonia inhibits LTP through NMDAR activation (Izumi et al., 2005a), our results suggest that ammonia triggers the activation of NMDARs and drives the synthesis of GABA potentiating neurosteroids. In contrast, we found that inactivation of NMDARs during HFS is unlikely to be involved in ammonia-mediated LTP inhibition (Fig. 2). The effects of ammonia result in synaptic dysfunction and likely contribute to cognitive impairment in patients exposed to high levels of ammonia. How ammonia triggers untimely NMDAR activation is uncertain but could include effects on extracellular glutamate levels (Elmlili et al., 2010) and/or postsynaptic/receptor effects (Hermenegildo et al., 2000; Rodrigo et al., 2009).
Because most ammonia exists in an ionized form, it cannot diffuse through blood brain barriers. Thus, ammonia is not typically detected in the CSF of healthy adults at rest (Nybo et al., 2005) and only modest levels are found in neurological patients not suffering from liver diseases (with levels ranging from 8 to 26 μM, Huizenga et al., 1998) or in healthy adults following exercise without glucose supplementation (up to 30 μM, Nybo et al., 2005). Accumulation of ammonia in the CNS, however, has long been thought to be a major factor contributing to hepatic encephalopathy (HE) (Seegmiller et al., 1954), and it has been shown that ammonia levels in the CSF correlate with the severity of HE (with levels ranging from 70 to 233 μM with hepatic coma, Vergara et al., 1974). Ammonia has multiple actions in the CNS that could contribute to psychiatric disturbances, including major depression, that often accompany HE. These include adverse effects on energy metabolism, modulation of GABAergic neurotransmission, and activation of NMDARs (Jalan and Hayes 1997). Consistent with the finding that hepatic cirrhosis disrupts memory acquisition in experimental animals (Méndez et al., 2008), ammonia has been shown to impair LTP induction in the hippocampus (Munoz et al., 2000; Monfort et al., 2004; Izumi et al., 2005; Chepkova et al., 2006). In the present ex vivo study, we show that neurosteroid synthesis in hippocampal pyramidal neurons contributes significantly to ammonia’s ability to modulate LTP. Prior studies have shown that neurosteroids accumulate in the CNS of cirrhotic patients (Ahboucha et al., 2006), and in the brains of experimental animals with liver damage and hyperammonemia (Ahboucha et al., 2012).
In addition to ammonia, other studies suggest the involvement of γ-aminobutyric acid (GABA) type-A receptor potentiating neurosteroids in HE. In autopsied brains from individuals with hepatic coma, expression of TSPO, previously known as the peripheral (mitochondrial) benzodiazepine receptor, is up-regulated (Lavoie et al., 1990). Similar changes have been shown by positron emission tomography (PET) (Cagnin et al., 2006), and in rats subjected to portacaval anastomosis (Leong et al., 1994). The up-regulation of TSPO suggests a role for GABAergic neurosteroids based on the fact that TSPO regulates neurosteroid synthesis by controlling the movement of cholesterol to the inner mitochondrial membrane for conversion to pregnenolone.
A major pathway involves the conversion of pregnenolone to alloP by the sequential actions of 5α-reductatse and 3α-hydroxysteroid dehydrogenase. Pregnenolone is also converted by 21β hydroxylase to corticosterone and then to THDOC (Belelli and Lambert, 2005; Gunn et al., 2011). Thus, the ability of finasteride, a specific 5α reductase inhibitor, to overcome the effects of ammonia on LTP indicates that activation of these pathways and accumulation of GABAergic neurosteroids are likely to be involved in the synaptic and perhaps cognitive dysfunction associated with elevated ammonia levels. Elevated levels of two major neurosteroids (alloP and THDOC) are reported in autopsied brain tissue from patients with hepatic coma (Ahboucha et al., 2006), and in rodents subjected to acute liver failure (Ahboucha et al., 2012). Although the present study indicates an important role for neurosteroids in ammonia’s acute effects in the hippocampus, it does not appear that either alloP or THDOC alone is responsible for the adverse effects on synaptic function. In our studies both of these major GABAergic neurosteroids were required to overcome the effects of finasteride on LTP, suggesting that they may make distinct contributions to ammonia’s actions on neuronal function.
Acute production of neurosteroids can be a homeostatic response to metabolic and other stressors. Because alloP and THDOC markedly enhance the function of GABA-A receptors (Akk et al., 2007; Chisari et al., 2010), this is also consistent with the idea that GABAergic mechanisms help to dampen excessive neural activity under stressful conditions, serving as a type of neuroprotective mechanism. Thus, while inhibiting either GABA-A receptors or neurosteroid synthesis can overcome the acute effects of ammonia on hippocampal synaptic plasticity and would be expected to have cognitive enhancing effects in the presence of elevated ammonia, efforts to block neurosteroid production could have other deleterious effects in the face of ongoing metabolic insults. Of note, finasteride and a related 5α-reductase inhibitor, dutasteride, are currently used clinically for patients with prostatic hyperplasia and for individuals with alopecia (Aggarwal et al., 2010). Depression and altered cognition has sometimes been observed as a side effect in patients treated with these 5α-reductase inhibitors and this likely reflects, in part, changes in endogenous neurosteroid production (Altomare and Capella, 2002; Rahimi-Ardabili et al., 2006; Traish et al., 2011; Römer and Gass 2010; Irwig, 2012).
In summary, we found that acute exposure to ammonia facilitates neurosteroid production through activation of NMDARs and inhibits LTP induction through a GABA-A receptor-mediated effect. Further study is required to determine the effects of more chronic exposure of ammonia on neurosteroid metabolism and cognitive function.
Highlights.
Ammonia inhibits LTP, a cellular model of memory, via neurosteroid production.
Ammonia enhances neurosteroid immunostaining in pyramidal neurons.
Finasteride is used to antagonize 5α-reductase, an enzyme for neurosteroidgenesis.
Finasteride overcomes ammonia-mediated LTP inhibition.
Enhanced neurosteroid immunostaininig by ammonia is blocked by finasteride.
Acknowledgments
This work was supported by NIH grants MH077791, GM47969 and AA017413 and the Bantly Foundation. We thank Dr. Barry A. Hong, Department of Psychiatry of Washington University of School of Medicine for thoughtful suggestions to initiate this study. We also thank the late Robert Purdy, a pioneer in neurosteroid research, for the neurosteroid antibody and helpful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5alpha-reductase inhibitors. Steroids. 2010;75:109–153. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahboucha S, Gamrani H, Baker G. GABAergic neurosteroids: The “endogenous benzodiazepines” of acute liver failure. Neurochem Int. 2012;60:707–714. doi: 10.1016/j.neuint.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Pomier-Layrargues G, Mamer O, Butterworth RF. Increased levels of pregnenolone and its neuroactive metabolite allopregnanolone in autopsied brain tissue from cirrhotic patients who died in hepatic coma. Neurochem Int. 2006;49:372–378. doi: 10.1016/j.neuint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Therap. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol. 2002;29:665–669. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Basile AS, Jones EA. Ammonia and GABAergic neurotransmission: interrelated factors in the pathogenesis of hepatic encephalopathy. Hepatology. 1997;25:1303–1305. doi: 10.1002/hep.510250636. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormones in the brain: several mechanisms. In: Fuxe K, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Pergamon Press; Oxford: 1981. pp. 3–14. [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;138:316–321. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Taylor-Robinson SD, Forton DM, Banati RB. In vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathy. Gut. 2006;55:547–553. doi: 10.1136/gut.2005.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis. 2006;23:512–521. doi: 10.1016/j.nbd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlili N, Boix J, Ahabrach H, Rodrigo R, Errami M, Felipo V. Chronic hyperammonemia induces tonic activation of NMDA receptors in cerebellum. J Neurochem. 2010;112:1005–1014. doi: 10.1111/j.1471-4159.2009.06520.x. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA-A receptor interactions: a focus on stress. Front Neurosci. 2011;5:1–20. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermenegildo C, Monfort P, Felipo V. Activation of N-methyl-Daspartate receptors in rat brain in vivo following acute ammonia intoxication: Characterization by in vivo brain microdialysis. Hepatology. 2000;31:709–715. doi: 10.1002/hep.510310322. [DOI] [PubMed] [Google Scholar]
- Huizenga JR, Teelken AW, Tangerman A, de Jager AE, Gips CH, Jansen PL. Determination of ammonia in cerebrospinal fluid using the indophenol direct method. Mol Chem Neuropathol. 1998;34:169–177. doi: 10.1007/BF02815078. [DOI] [PubMed] [Google Scholar]
- Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012;73:1220–1223. doi: 10.4088/JCP.12m07887. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Low concentrations of N-methyl-D-aspartate inhibit the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992a;137:245–248. doi: 10.1016/0304-3940(92)90414-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992b;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Izumi M, Matsukawa M, Funatsu M, Zorumski CF. Ammonia-mediated LTP inhibition: effects of NMDA receptor antagonists and L-carnitine. Neurobiol Dis. 2005a;20:615–624. doi: 10.1016/j.nbd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal LTP and LTD are mediated by different mechanisms. Neuroscience. 2005b;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jalan R, Hayes PC. Hepatic encephalopathy and ascites. Lancet. 1997;350:1309–1315. doi: 10.1016/S0140-6736(97)07503-X. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. Steroidogenic acute regulatory protein in the brain. Neuroscience. 2006;138:741–747. doi: 10.1016/j.neuroscience.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Lavoie J, Layrargues GP, Butterworth RF. Increased densities of peripheral-type benzodiazepine receptors in brain autopsy samples from cirrhotic patients with hepatic encephalopathy. Hepatology. 1990;11:874–878. doi: 10.1002/hep.1840110524. [DOI] [PubMed] [Google Scholar]
- Leong DK, Therrien G, Swain MS, Butterworth RF. Densities of binding sites for the “peripheral-type” benzodiazepine receptor ligand 3H-PK11195 are increased in brain 24 hours following portacaval anastomosis. Metab Brain Dis. 1994;9:267–273. doi: 10.1007/BF01991200. [DOI] [PubMed] [Google Scholar]
- Méndez M, Méndez-López M, López L, Aller MA, Arias J, Arias JL. Working memory impairment and reduced hippocampal and prefrontal cortex c-Fos expression in a rat model of cirrhosis. Physiol Behav. 2008;95:302–307. doi: 10.1016/j.physbeh.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Monfort P, Muñoz MD, Felipo V. Hyperammonemia impairs long-term potentiation in hippocampus by altering the modulation of cGMP-degrading phosphodiesterase by protein kinase G. Neurobiol Dis. 2004;15:1–10. doi: 10.1016/j.nbd.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Muñoz MD, Monfort P, Gaztelu JM, Felipo V. Hyperammonemia impairs NMDA receptor-dependent long-term potentiation in the CA1 of rat hippocampus in vitro. Neurochem Res. 2000;25:437–441. doi: 10.1023/a:1007547622844. [DOI] [PubMed] [Google Scholar]
- Nybo L, Dalsgaard MK, Steensberg A, Moller K, Secher NH. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J Physiol. 2005;563:285–290. doi: 10.1113/jphysiol.2004.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6:7. doi: 10.1186/1472-6904-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo R, Cauli O, Boix J, Elmli N, Agusti A, Felipo V. R ole of NMDA receptors in acute liver failure and ammonia toxicity: therapeutical implications. Neurochem Int. 2009;55:113–118. doi: 10.1016/j.neuint.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Römer B, Gass P. Finasteride-induced depression: new insights into possible pathomechanisms. J Cosmet Dermatol. 2010;9:331–332. doi: 10.1111/j.1473-2165.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- Saalman YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3α-hydrox, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DF, Jones EA. Hepatic encephalopathy and the gamma-aminobutyric-acid neurotransmitter system. Lancet. 1982;1:18–20. doi: 10.1016/s0140-6736(82)92559-4. [DOI] [PubMed] [Google Scholar]
- Seegmiller JE, Schwartz R, Davidson CS. The plasma ammonia and glutamine content in patients with hepatic coma. J Clin Invest. 1954;33:984–988. doi: 10.1172/JCI102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori T, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872–884. doi: 10.1111/j.1743-6109.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- Valdez CM, Smith MA, Perry G, Phelix CF, Santamaria F. Cholesterol homeostasis markers are localized to mouse hippocampal pyramidal and granule layers. Hippocampus. 2010;20:902–905. doi: 10.1002/hipo.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara F, Plum F, Duffy TE. Alpha-ketoglutaramate: increased concentrations in the cerebrospinal fluid of patients in hepatic coma. Science. 1974;183:81–83. doi: 10.1126/science.183.4120.81. [DOI] [PubMed] [Google Scholar]
- Zielińska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Assessment of synaptic effects of nitric oxide in hippocampal neurons. Methods Neurosci. 1996;31:282–299. [Google Scholar]
- Zorumski CF, Mennerick S, Isenberg KE, Covey DF. Potential clinical uses of neuroactive steroids. Curr Op Invest Drugs. 2000;1:360–369. [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress & depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]