Abstract
In human epithelial cancers, the microRNA (miRNA) mir-30d is amplified with high frequency and serves as a critical oncomir by regulating metastasis, apoptosis, proliferation, and differentiation. Autophagy, a degradation pathway for long-lived protein and organelles, regulates the survival and death of many cell types. Increasing evidence suggests that autophagy plays an important function in epithelial tumor initiation and progression. Using a combined bioinformatics approach, gene set enrichment analysis and miRNA target prediction, we found that mir-30d might regulate multiple genes in the autophagy pathway including BECN1, BNIP3L, ATG12, ATG5, ATG2. Our further functional experiments demonstrated that the expression of these core proteins in the autophagy pathway was directly suppressed by mir-30d in cancer cells. Finally, we showed that mir-30d regulated the autophagy process by inhibiting autophagosome formation and LC3B-I conversion to LC3B-II. Taken together, our results provide evidence that the oncomir mir-30d impairs the autophagy process by targeting multiple genes in the autophagy pathway. This result will contribute to understanding the molecular mechanism of mir-30d in tumorigenesis and developing novel cancer therapy strategy.
Keywords: mir-30d, autophagy, cancer, microRNA
INTRODUCTION
Autophagy is an evolutionarily conserved process for delivering cellular materials and organelles to lysosome for degradation, and releasing them to the cytoplasm for recycle use [1,2]. This process is initiated by enclosing materials destined for degradation within double-membraned vacuoles, called autophagosome, and followed by fusion with lysosomes for promoting degradation of the luminal content. Autophagy is present in cells at a low basal level and can be induced when cells are undergoing environmental stress. This process helps to maintain cellular homeostasis and prevents organism from damaging and diseases. The function of autophagy in cancer development is believed to be cell context-dependent. It has been reported that in vivo disrupting the expression of autophagy genes, e.g. BECN1 and ATG5, leaded to tumor initiation [3,4,5,6], suggesting autophagy pathway has tumor suppression function. Meanwhile autophagy can promote tumor cell survival, especially in oncogenic RAS-driven cancers [7,8,9].
mir-30d is one of the members in the mir-30 microRNA (miRNA) family. The mir-30 family regulates a wide range of physiological processes in normal tissues and cancers, including development [10,11,12,13,14], metastasis [15,16,17], apoptosis [18,19,20,21], senescence [22], proliferation [23,24,25] and differentiation [26,27]. Recently it has been reported that mir-30d was amplified in more than 30% of multiple types of human epithelial tumors [28,29]. It suggests that mir-30d is a novel oncogene involved in tumor development and homeostasis, serving as a potential biomarker or drug target in human cancers.
MATERIALS AND METHODS
Cell Culture
Cancer cells were cultured in RPMI1640 (Cellgro) supplemented with 10% fetal bovine serum (FBS; Invitrogen). For autophagy induction, cells were either treated with 200nM rapamycin (Sigma) supplemented in complete medium or serum starved with Hank’s buffer (Stemcell Technologies), both at 37°C for 4h. Lysosomal protease inhibitors E64d (1mg/ml; Sigma) and Pepstatin A (1mg/ml; Sigma) were added when necessary.
Plasmids
The GFP-LC3 and the psiCHECK2 reporter vectors were purchased from Addgene and Promega, respectively. The target sequences of the mir-30d in the 3′ UTR of its target genes were listed in Table S1.
Quantitative Real-time PCR (qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) and reverse-transcribed using a high capacity RNA-to-cDNA kit (Applied Biosystems). cDNA was quantified on an ABI Prism 7900 sequence detection system (Applied Biosystems). PCR was performed using Power SYBR Green PCR master mix (Applied Biosystems).
Western Blotting (WB)
Cells were lysed in mammalian protein extraction reagent (Pierce) with protease inhibitor cocktail (Sigma). 15 μg of total protein was separated by 10% SDS-PAGE under denaturing conditions and transferred to PVDF membranes (Millipore). Membranes were blocked in 5% nonfat milk (Bio-Rad) and then incubated with the following primary antibodies: anti-ATG5, anti-ATG12, anti-BECN1, anti-BNIP3L, and anti-LC3B (Table S2). After incubation with a secondary antibody conjugated with HRP (Amersham Biosciences) together with an HRP-conjugated primary antibody for β-actin (Sigma), immunoreactive proteins were visualized using the LumiGLO chemiluminescent substrate (Cell Signaling).
Luciferase Reporter Assay
Cells were plated on a 24-well plate 24 h before transfection at 50% confluence. 30 nM miRNA mimics (Ambion) were transfected using Lipofectamine RNAiMAX (Invitrogen). 24 h post-transfection, 0.125 μg of reporter vector was transfected using FuGENE6 transfection reagent (Roche). 48 h after reporter vector transfection, cells were harvested, and reporter assays were performed using a dual luciferase reporter assay system (Promega).
Bioinformatic analysis
miRNA and mRNA expression microarray data were retrieved from a public accessible database, Cell Miner. http://discover.nci.nih.gov/cellminer/. Gene set enrichment analysis (GSEA) algorithm was used to identify the pathways that were significantly enriched between mir-30d low and high tumor cells. http://www.broadinstitute.org/gsea/index.jsp. The autophagy gene set was listed in Table S3. TargetScan algorithm was used to predict mir-30d targets. http://www.targetscan.org.
RESULTS
The autophagy pathway was bioinformatically predicted to be regulated by mir-30d
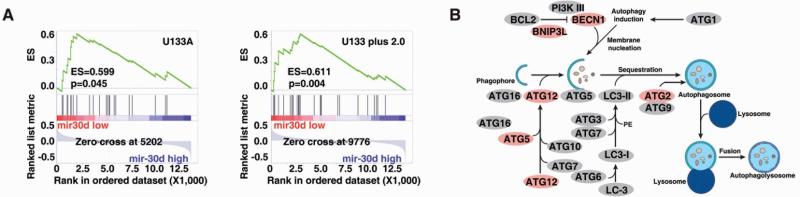
We have reported that mir-30d is frequently amplified in human cancers, suggesting that mir-30d is a potential oncomir. To further characterize the function of mir-30d in human cancers, we performed the gene set enrichment analysis (GSEA) in sixty human cancer cell lines (NCI60). Both miRNA and mRNA profiling microarray data of NCI60 cell lines were retrieved from the Cell Miner (http://discover.nci.nih.gov/cellminer/). GSEA is a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between two biological states. Based on the expression level of mir-30d, NCI60 cell lines were divided into mir-30d high and mir-30d low groups. A ranked gene list was generated by comparing the mRNA microarray data of mir-30d low group with mir-30d high group (mir-30d low v.s. high). Then enrichment of different pathway gene sets in this ranked gene list were evaluated using GSEA. The GSEA analysis indicated that the autophagy pathway was significantly enriched based on mir-30d expression in two Affymetrix gene expression microarray datasets analyzed in the present study (U133A and U133 plus 2.0) (Fig. 1A). The inverse correlation between the expression levels of mir-30d and multiple genes in the autophagy pathway indicated that mir-30d may negatively regulate this pathway. Then, we asked whether mir-30d directly targeted these genes in the autophagy pathway. To test this hypothesis, we also identified mir-30d potential targets in silico using TagetScan, an algorithm for miRNA target prediction. Five core proteins in the autophagy pathway were predicted to be mir-30d targets, i.e. containing miRNA binding sites in the 3′UTRs of their mRNA sequences, including BECN1, BNIP3L, ATG12, ATG5 and ATG2 (Fig. 1B). The functions of these genes in the autophagy process have been well characterized. For example, BECN1 localizes to the trans-Golgi network and participates in autophagosome formation [30]. BNIP3L indirectly activates phagophore formation by recruiting autophagy proteins or by releasing BECN1 from Bcl-xL [31]. ATG12 is covalently bound to ATG5 and their ubiquitination is required to form autophagosome [32,33]. Thus we hypothesized mir-30d may inhibit the autophagy process by targeting these autophagy-related genes and suppressing their expression.
Figure 1. The autophagy pathway was bioinformatically predicted to be regulated by mir-30d.
(A) Two mRNA expression profiling datasets of NCI60 were retrieved from Cell Miner database (left panel: U133A) and (right panel: U133 plus 2.0). Using GSEA algorithm, the molecular pathways that were significantly enriched based on mir-30d expression were predicted. The autophagy pathway was identified. Enrichment score (ES) and p value are shown as indicated. (B) Using TargetScan algorithm, multiple genes in the autophagy pathway was predicted to be regulated by mir-30d (indicated as pink).
mir-30d represses the expression levels of multiple core proteins in the autophagy pathway in cancer cells
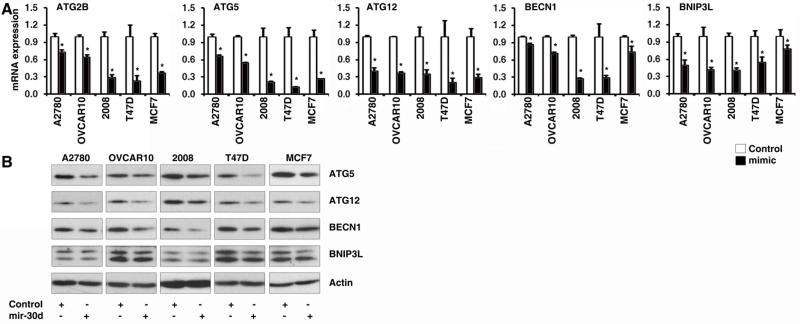
miRNA post-transcriptionally regulates gene expression through either degradation of mRNA transcripts or inhibition of protein translation. To experimentally validate mir-30d represses genes in the autophagy pathway, we enforced expression of mir-30d by transfecting chemically synthesized mir-30d mimics to cancer cells, followed by detection of the mRNA levels of the target genes by qRT-PCR. As shown in Fig. 2A, in ovarian cancer cell lines A2780, OVCAR10 and 2008, as well as in breast cancer cell lines T47D and MCF7, the mRNA levels of ATG2B, ATG5, ATG12, BECN1 and BNIP3L was remarkably suppressed by mir-30d mimic compared to mimic control. The same results were found when we performed Western Blots to detect the protein levels of these mir-30d potential targets in the above cancer cell lines that were transfected with mir-30d mimic or control mimic. ATG5, ATG12, BECN1 and BNIP3L were downregulated by mir-30d at protein level (Fig. 2B). These results demonstrated that mir-30d inhibited the expression of these genes at both mRNA and protein levels.
Figure 2. mir-30d represses the expression levels of multiple core proteins in the autophagy pathway in cancer cells.
The mir-30d or control mimic was transfected to A2780, OVCAR10, 2008, T47D and MCF7 cells cultured in nutrient rich medium. The mRNA levels and protein levels of the mir-30d predicted targets were detected by qRT-PCR (A) and Western Blots (B). qPCR results are expressed as mean ± S.D. and with significance at p < 0.05.
Multiple core proteins in the autophagy pathway are direct targets of mir-30d in cancer cells
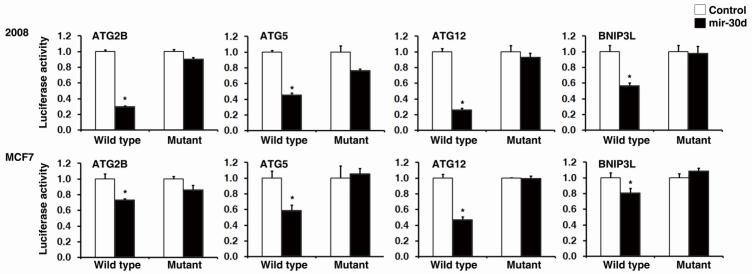
miRNAs are known to regulate gene expression in a sequence-specific manner via binding to the recognition sites in the 3′UTR of the targeted mRNAs. Functional mature miRNA strand is selectively incorporated into RNA-induced silencing complex (RISC) and guides the complex specifically to its mRNA targets through base-pairing interactions. To test whether mir-30d regulation to autophagy pathway genes depends on its binding sites on 3′UTR sequences of the target genes, we constructed reporter plasmids with wild type or mutant mir-30d binding sites from 3′UTR of target genes (ATG2B, ATG5, ATG12 and BNIP3L) inserted to the 3′ downstream sequence of the luciferase gene. Reporter assay was performed by co-transfecting 2008 and MCF7 cells with wild type or mutant reporter plasmids and mir-30d expressing vector or control vector. We found that mir-30d potently decreased the luciferase activity of wild type reporter plasmids representing all five target genes (ATG2B, ATG5, ATG12, BECN1 and BNIP3L) examined in this study, whereas it had no effect on the mutant forms (Fig. 3). We concluded that mir-30d suppresses autophagy pathway gene expression in a sequence-specific manner and the suppression depends on mir-30d binding sites within 3′UTR sequences of the target genes.
Figure 3. Multiple core proteins in the autophagy pathway are direct targets of mir-30d in cancer cells.
2008 and MCF7 cell lines cultured in nutrient rich medium were co-transfected with mir-30d mimic and reporter plasmids with wild type or mutant 3′UTR binding sites of mir-30d from its predicted target genes. Reporter assay was performed and luciferase activity of mir-30d mimic transfected cells was normalized to control mimic transfected cells. Results are shown as mean ± S.D.
mir-30d functionally impairs the autophagy process in cancer cells
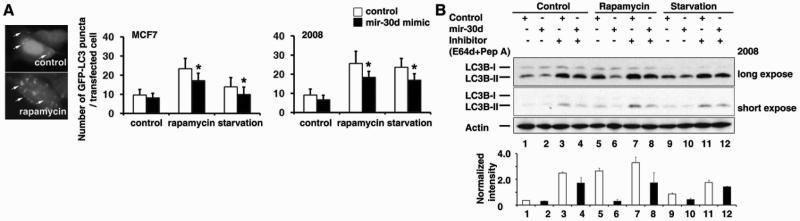
Our above results indicated that mir-30d negatively regulates the expression of multiple core proteins in the autophagy pathway. We next asked whether mir-30d functionally inhibits the autophagy process. To address this question, a GFP-LC3 fusion protein expression reporter was first examined. Upon autophagy induction, one form of the microtubule-associated protein light chain 3 (LC3), named LC3-I, turns into another form LC3-II. LC3-II is accumulated and resides in the autophagosomal membranes, serving as a marker for autophagosome formation. By quantifying the GFP-LC3 puncta in each cell under fluorescence microscopy, the effectiveness of autophagy induction can be determined (Fig. 4A, left panel). MCF7 and 2008 cells were transfected with a GFP-LC3 fusion protein expression vector and treated with rapamycin or serum starvation, both of which are strong inducer of autophagy. Enforced expression of mir-30d in both MCF7 and 2008 cells significantly reduced the GFP-LC3 puncta (mir-30d mimic transfected cells compared to the control transfection) after treatment with either rapamycin or serum starvation (Fig. 4A, middle and right panel). This indicated that mir-30d may block LC3-II production and autophagosome formation. To confirm this phenomenon, we also performed Western Blots for LC3B protein to detect the conversion of LC3B-I to LC3B-II (Fig. 4B). In cells overexpressing mir-30d, protein levels of LC3B-II were lower than in empty vector-transfected cells (lanes 1 v.s. 2, 5 v.s. 6, 9 v.s. 10), under either autophagy induction or control treatment (representing basal level of autophagy). When the above cells were further treated with lysosomal protease inhibitors E64d and Pepstatin A to stabilize LC3B protein level, a similar pattern was still observed, i.e. the protein level of LC3B-II was lower in mir-30d overexpressing cells (lanes 3 v.s. 4, 7 v.s. 8, 11 v.s. 12). Taken together, we demonstrated that overexpressing mir-30d resulted in less efficient conversion of LC3B-I to LC3B-II. The lower protein level of LC3B-II in mir-30d overexpressing groups was not due to a more rapid lysosomal turnover of LC3B-II, because the protein level was not rescued when blocking lysosome function by inhibitors. Thus the inefficient production of LC3B-II (hence fewer autophagosome formation) caused by mir-30d overexpression may explain the inhibitory effect of mir-30d on autophagy process.
Figure 4. mir-30d functionally impairs the autophagy process in cancer cells.
(A) GFP-LC3 puncta were observed by fluorescence microscopy (left panel) after 200nM rapamycin treatment for 4h. MCF7 (middle panel) and 2008 cells (right panel) transfected with control or mir-30d mimic were subjected to rapamycin or serum starvation treatment. Quantification result of the number of GFP-LC3 puncta in each transfected cell was shown as mean ± S.D. *, p< 0.05. (B) 2008 cells transfected with control vector or mir-30d overexpression vector were treated with rapamycin or serum starvation, and with or without lysosomal protease inhibitors (E64d+Pep A). Western blot was performed to detect the protein levels of LC3B-I and LC3B-II, and the quantification data were shown.
DISCUSSION
Autophagy is a conserved physiological process helps to maintain homeostasis in organism, either on a long-term basis to prevent tissue damage and diseases, or in acute response to environmental stress. The function of autophagy in tumor development is context-dependent. It plays the role of tumor suppression by preventing tumor initiation. In vivo disrupting the expression of autophagy genes, such as BECN1 and ATG5, leaded to liver cancer onset [3,4,5,6]. Loss of BECN1 gene has also been reported in some human cancers [3,34], suggesting its tumor suppressive function. Meanwhile, autophagy limits tumor development by participating antioxidant defense and eliminating dysfunctional mitochondria [35,36], which is critical for reducing ROS and oxidative stress. However, autophagy also promotes cancer by enabling survival of cancer cells under starvation condition [37,38,39]. Many cancer cell lines were reported to display very high basal level of autophagy, especially in cancers expressing oncogenic RAS [7,8,9]. These cancer cells are usually autophagy addictive and depend on autophagy to maintain survival under normal, as well as stress, conditions. So identifying the mechanism and function of autophagy process under different cell context will be necessary for developing therapeutic strategies targeting autophagy pathway in human diseases.
The mir-30 microRNA family is widely expressed in multiple tissues and cell types [40,41]. It has been reported that mir-30 was amplified in more than 30% of multiple types of human epithelial tumors [28,29]. The expression pattern of mir-30 in normal and cancerous cells implicated the fundamental role of this miRNA family in sustaining normal physiological function as well as its involvement in diseases. Consistent with this conclusion, mir-30 was recently found to participate in a wide range of physiological processes in normal tissues and cancers, including development [10,11,12,13,14], metastasis [15,16,17], apoptosis [18,19,20,21], senescence [22], proliferation [23,24,25] and differentiation [26,27]. More and more evidence suggested that mir-30 was a novel oncomir. It is intriguing to identify the direct mRNA targets of this miRNA family, from which the function of mir-30 can be further explained and explored. Elucidating the mechanism of how mir-30 is involved in tumorigenesis should open a new field to targeted cancer therapy against this miRNA family.
In present study, we identified mir-30d regulated autophagy process by directly targeting multiple genes of the autophagy pathway. Consistent with our finding, another mir-30 family member, mir-30a, has been reported to regulate autophagy via repressing BECN1 expression in tumor cells [42,43]. So besides the previously known function of the mir-30 family in cancer cell metastasis, proliferation, apoptosis, and senescence [15,16,18,19,22,28], we now added a new piece of evidence that mir-30d also suppressed the autophagy process, which is critical to cancer cell metabolism and homeostasis. These findings place the mir-30 family to the stage of drug development for human cancers as a therapeutic target.
A great amount of studies suggested there is a subtle and context-dependent relationship between autophagy and apoptotic cell death. Autophagy can either promote apoptosis or sustain cell viability in different situations [37,44,45,46]. Our previous results found mir-30d played an important role in cancer cell apoptosis [28]. Now we demonstrated that mir-30d is also a critical regulator to autophagy. So it will be of interest to investigate whether the two pathways regulated by mir-30d have crosstalk, and whether their interaction is mir-30d dependent. Thus by linking the autophagy and apoptosis pathways together, and probably associating autophagy with senescence and migration as well, mir-30d acts as a key player in the network concerting cancer onset, survival, progression and metastasis.
Supplementary Material
Highlights.
Gene set enrichment analysis indicated mir-30d might regulate the autophagy pathway;
mir-30d represses the expression of BECN1, BNIP3L, ATG12, ATG5 and ATG2;
BECN1, BNIP3L, ATG12, ATG5 and ATG2 are direct targets of mir-30d;
mir-30d inhibits autophagosome formation and LC3B-I conversion to LC3B-II;
mir-30d regulates the autophagy process.
ACKNOWLEDGMENTS
This work was supported, in whole or in part, by DoD W81XWH-10-1-0082 (LZ), NIH R01-CA142776 (LZ), P50-CA83638-7951 (LZ), and the Ovarian Cancer Research Fund (LZ). J. Tanyi was supported by NIH 5K12HD000849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- [4].Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, Debnath J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2010;22:165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012 doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- [11].Le Guillou S, Sdassi N, Laubier J, Passet B, Vilotte M, Castille J, Laloe D, Polyte J, Bouet S, Jaffrezic F, Cribiu EP, Vilotte JL, Le Provost F. Overexpression of miR-30b in the Developing Mouse Mammary Gland Causes a Lactation Defect and Delays Involution. PLoS One. 2012;7:e45727. doi: 10.1371/journal.pone.0045727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marchand A, Proust C, Morange PE, Lompre AM, Tregouet DA. miR-421 and miR-30c Inhibit SERPINE 1 Gene Expression in Human Endothelial Cells. PLoS One. 2012;7:e44532. doi: 10.1371/journal.pone.0044532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nigro A, Menon R, Bergamaschi A, Clovis YM, Baldi A, Ehrmann M, Comi G, De Pietri Tonelli D, Farina C, Martino G, Muzio L. MiR-30e and miR-181d control radial glia cell proliferation via HtrA1 modulation. Cell Death Dis. 2012;3:e360. doi: 10.1038/cddis.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao X, Mohan R, Ozcan S, Tang X. MicroRNA-30d Induces Insulin Transcription Factor MafA and Insulin Production by Targeting Mitogen-activated Protein 4 Kinase 4 (MAP4K4) in Pancreatic beta-Cells. J Biol Chem. 2012;287:31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jiang L, Lin C, Song L, Wu J, Chen B, Ying Z, Fang L, Yan X, He M, Li J, Li M. MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-kappaB/IkappaBalpha negative feedback loop. J Clin Invest. 2012;122:33–47. doi: 10.1172/JCI58849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Franzetti GA, Laud-Duval K, Bellanger D, Stern MH, Sastre-Garau X, Delattre O. MiR-30a-5p connects EWS-FLI1 and CD99, two major therapeutic targets in Ewing tumor. Oncogene. 2012 doi: 10.1038/onc.2012.403. [DOI] [PubMed] [Google Scholar]
- [18].Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS, Young KH, Li Y. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30:843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Su SF, Chang YW, Andreu-Vieyra C, Fang JY, Yang Z, Han B, Lee AS, Liang G. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene. 2012 doi: 10.1038/onc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, Fiore D, De Marinis P, Croce CM, Condorelli G. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2012 doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- [22].Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci U S A. 2011;108:522–527. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tanic M, Yanowsky K, Rodriguez-Antona C, Andres R, Marquez-Rodas I, Osorio A, Benitez J, Martinez-Delgado B. Deregulated miRNAs in hereditary breast cancer revealed a role for miR-30c in regulating KRAS oncogene. PLoS One. 2012;7:e38847. doi: 10.1371/journal.pone.0038847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baraniskin A, Birkenkamp-Demtroder K, Maghnouj A, Zollner H, Munding J, Klein-Scory S, Reinacher-Schick A, Schwarte-Waldhoff I, Schmiegel W, Hahn SA. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis. 2012;33:732–739. doi: 10.1093/carcin/bgs020. [DOI] [PubMed] [Google Scholar]
- [25].Ichikawa T, Sato F, Terasawa K, Tsuchiya S, Toi M, Tsujimoto G, Shimizu K. Trastuzumab produces therapeutic actions by upregulating miR-26a and miR-30b in breast cancer cells. PLoS One. 2012;7:e31422. doi: 10.1371/journal.pone.0031422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG, Scheideler M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol. 2011;8 doi: 10.4161/rna.8.5.16153. [DOI] [PubMed] [Google Scholar]
- [27].Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, Dani C, Barbry P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12:R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li N, Kaur S, Greshock J, Lassus H, Zhong X, Wang Y, Leminen A, Shao Z, Hu X, Liang S, Katsaros D, Huang Q, Butzow R, Weber BL, Coukos G, Zhang L. A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer. Cancer Res. 2012;72:154–164. doi: 10.1158/0008-5472.CAN-11-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lu Y, Ryan SL, Elliott DJ, Bignell GR, Futreal PA, Ellison DW, Bailey S, Clifford SC. Amplification and overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at 8q24.22-q24.23 in medulloblastoma. PLoS One. 2009;4:e6159. doi: 10.1371/journal.pone.0006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- [33].Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem. 1998;273:33889–33892. doi: 10.1074/jbc.273.51.33889. [DOI] [PubMed] [Google Scholar]
- [34].Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- [35].Kim JH, Kim HY, Lee YK, Yoon YS, Xu WG, Yoon JK, Choi SE, Ko YG, Kim MJ, Lee SJ, Wang HJ, Yoon G. Involvement of mitophagy in oncogenic K-Ras-induced transformation: overcoming a cellular energy deficit from glucose deficiency. Autophagy. 2011;7:1187–1198. doi: 10.4161/auto.7.10.16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2011;7:531–532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA, Debnath J, Shokat KM, Weiss WA. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3:ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bar M, Wyman SK, Fritz BR, Qi J, Garg KS, Parkin RK, Kroh EM, Bendoraite A, Mitchell PS, Nelson AM, Ruzzo WL, Ware C, Radich JP, Gentleman R, Ruohola-Baker H, Tewari M. MicroRNA discovery and profiling in human embryonic stem cells by sequencing of small RNA libraries. Stem Cells. 2008;26:2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stark MS, Tyagi S, Nancarrow DJ, Boyle GM, Cook AL, Whiteman DC, Parsons PG, Schmidt C, Sturm RA, Hayward NK. Characterization of the Melanoma miRNAome by Deep Sequencing. PLoS One. 2010;5:e9685. doi: 10.1371/journal.pone.0009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, Liu CG, Yang JM. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5:816–823. doi: 10.4161/auto.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen X, Zhang CY, Zhang Q, Zen K. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J Biol Chem. 2012;287:4148–4156. doi: 10.1074/jbc.M111.307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- [46].Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.