Abstract
Objective
Pancreatic diseases pose significant diagnostic challenge as signs and symptoms often overlap. We investigated the potential of pancreatic juice neutrophil gelatinase–associated lipocalin (NGAL), macrophage inhibitory cytokine-1 (MIC-1), and carbohydrate antigen 19-9 (CA19-9) to aid in the diagnosis of patients with symptoms suggestive of pancreatic diseases.
Methods
105 chronic pancreatitis (CP), pancreatic cancer (PC), and non-pancreatic non-healthy (NPNH, patients with symptoms mimicking pancreatic disease but found to be free of pancreatic pathology) patients underwent endoscopic pancreatic juice collection following secretin-stimulation. NGAL and MIC-1 levels were measured by enzyme-linked immunosorbent assay while CA19-9 was measured by radioimmunoassay.
Results
NGAL, MIC-1, and CA19-9 were significantly elevated in the pancreatic juice of CP and PC patients as compared to NPNH controls (p<0.034). NGAL appeared most promising in differentiating diseased versus non-diseased pancreata (AUCs=0.88–0.91) while MIC-1 was found to be higher in PC than CP patients (p=0.043). Interestingly, MIC-1 levels in diabetic PC patients were higher than in non-diabetic PC (p=0.030) and diabetic CP patients (p=0.087). CA19-9 showed the least ability to distinguish patient groups (AUCs=0.61–0.76).
Conclusions
Pancreatic juice NGAL shows potential utility in establishing pancreatic etiology in the context of non-specific symptoms while MIC-1 may aid in differentiating PC from CP.
Keywords: Pancreatic Disease, NGAL, MIC-1, CA19-9, Pancreatic Juice
INTRODUCTION
Pancreatic disease is highly prevalent in Western countries, with a combined annual incidence of more than 2 million people in the United States alone [1–3]. Pancreatic diseases are clinically difficult to conclusively diagnose, as signs and symptoms consistent with a particular pancreatic etiology frequently overlap with multiple others, as is the case with chronic pancreatitis (CP) and pancreatic cancer (PC), to name a few. There are also myriad instances where one pancreatic disease is a risk factor for and potential driving force behind another pancreatic disease, with symptoms of both diseases often difficult or impossible to differentiate. In addition, many diseases that are not of a pancreatic etiology strongly mimic those that are, thereby increasing the time, expense, and risk of test-associated morbidity in ruling out pancreatic pathology and establishing a definitive diagnosis. This diagnostic complexity warrants the development of novel, minimally invasive methods of differentiating disease etiologies in the context of nonspecific symptoms potentially arising from one or more pancreatic pathologies. To date, serum-, plasma-, bile-, and urine-based biomarkers, as well as increasingly complex clinical evaluation criteria, have all proven disappointing in this arena.
One promising method for obtaining such a distinction is through evaluation of biomarkers in pancreatic juice. Pancreatic juice consists of biomolecules secreted by pancreatic exocrine cells as well as sloughed ductal cells and cell components that, after traveling through the ductal network of the pancreas, combine with bile in the common bile duct and are emptied into the duodenum through the sphincter of Oddi [4]. Owing to this intimate relationship with pancreatic chemical production and ductal cell status, pancreatic juice has the potential to act as a surrogate window into the cellular processes and pathologies occurring within the pancreas, allowing direct interpretability of the pancreatic environment that is surpassed only by core biopsies. Further, because it enables one to investigate disease within the entire ductal system of the pancreas, pancreatic juice analysis theoretically has more diagnostic applications than biopsy. Additionally, unlike other diagnostic methods such as bile collection via endoscopic retrograde cholangiopancreatography (ERCP), pancreatic juice collection by routine upper endoscopy is mildly invasive and carries a negligible risk of complication.
Several clinical biomarkers are under investigation or are currently in clinical use for the diagnosis, prognostic prediction, or progression evaluation of various pancreatic diseases [3, 5–13]. Two biomarkers that are proving of increasing importance in benign and malignant pancreatic diseases, and have thus-far remained unexplored in pancreatic juice are NGAL and MIC-1. NGAL is a secreted protein whose expression is elevated in multiple human cancers, including pancreatic cancer [9, 14–17]. In a previous study, we found that NGAL is a significant predictor of disease severity and outcome in patients with acute pancreatitis [1]. In a small pilot study, we also found that plasma NGAL levels were significantly higher in PC patients than in healthy controls. Another pancreatic juice biomarker, MIC-1, a member of the transforming growth factor beta superfamily, was originally identified as a gene expressed in the context of macrophage activation [18]. MIC-1is aberrantly expressed in PC tissues, and serum MIC-1 levels have been reported to be significantly higher in patients with PC than in healthy controls and patients with chronic pancreatitis [8]. A third biomarker, CA19-9, is the only serum PC biomarker currently approved by the U.S. Food and Drug Administration; however, its utility in pancreatic juice biomarker has undergone little investigation.
The purpose of the current study was to determine whether pancreatic juice NGAL, MIC-1, and/or CA19-9 could be used to distinguish pancreatic diseases from each other as well as to differentiate patients with pancreatic diseases from those free of pancreatic pathology.
MATERIALS AND METHODS
Study Population
This prospective study was approved by the Mayo Clinic Institutional Review Board (#07-0000099). Written informed consent was obtained from all patients prior to study enrollment. 111 patients presenting with epigastric pain/discomfort warranting EUS-FNA evaluation and originally diagnosed with CP, PC, or found to have no pancreatic disease (non-pancreatic non-healthy controls, NPNH) were enrolled in the study. After initial evaluation and pancreatic juice collection, definitive final diagnoses were made.
Age, gender, body mass index (BMI), race, DM2 status, alcohol history, and smoking history were collected from each patient (Supplementary Table 1). All Samples were blinded prior to analysis.
Collection of Secretin-Stimulated Exocrine Pancreatic Secretions (SSEPS)
All patients underwent upper endoscopy after an overnight fast. Patients were moderately sedated with midazolam and meperidine before the procedure. Synthetic human secretin (ChiRhoStim, ChiRhoClinInc, Burtonsville, MD) at a dose of 0.2 μg/kg (or a total dose of 16 μg) was administered intravenously for 1 minute immediately before endoscopy intubation. Gastric fluid was aspirated before intubation of the pylorus to minimize contamination. With the endoscope positioned in the second portion of the duodenum, opposite the papilla of Vater (without cannulation), a 2.3-mm plastic aspiration catheter (Hobbs Medical, Stafford Springs, CT) was passed through the biopsy channel of the endoscope until visible on screen in the endoscopic monitor. Pancreatic juice exiting the papilla was then suctioned through the catheter for 10 minutes into a dry 20-mL tube. Approximately, 5–8 ml of fluid was collected from each patient. The collected fluid was immediately aliquoted in 2-mL vials, snap-frozen in liquid nitrogen, and stored at −80°C until assays were performed. A pan-protease inhibitor cocktail pill (Roche Diagnostic Corporation, Indianapolis, IN) was added to the collected pancreatic juice to inhibit proteolytic degradation.
Determination of Final Diagnosis
Three gastroenterologists (MR, TAW and MBW) who were blinded to the results of the assays determined the final diagnosis of the patients based on standard clinical practice. Patients with no personal history of pancreatic disease and normal pancreatic tests (ERCP, EUS, computed tomography, or magnetic resonance imaging) were deemed to have normal pancreata and thus considered to be non-pancreatic non-healthy (NPNH) controls.
NGAL Measurement
NGAL levels were measured quantitatively using the DuoSet enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) for human NGAL according to the manufacturer’s instructions. The pancreatic juice samples were aliquoted and stored at −70°C immediately following receipt to avoid repeated freeze-thaw cycles. Standard curves were produced from standards provided with the kit and serially (log2) diluted from 4 ng/mL to 15.6 pg/mL. All samples were plated in triplicate. Samples with readings greater than that of the highest standard were diluted appropriately and the assay was repeated. ELISA plates were read at 450 nm with an absorbance correction at 540 nm. The collected data were analyzed using SOFTMAX PRO software (Molecular Devices Corp., Sunnyvale, CA).
MIC-1 Measurement
MIC-1 concentrations in pancreatic juice were measured at UNMC. MIC-1 levels were measured quantitatively using the DuoSet ELISA kit (R&D Systems) for human MIC-1 according to the manufacturer’s instructions using the same methods employed for NGAL as measured at UNMC.
CA19-9 Measurement
Concentrations of CA19-9 in pancreatic juice were measured at UNMC by a solid phase radioimmunoassay (Centocor, Malvern, PA or Fujiribio), according to the manufacturer’s instructions. All samples were analyzed in duplicate and expressed in arbitrary units (U/mL) with 1 unit of activity corresponding to approximately 0.8 ng of purified antigen [19].
Statistical Analysis
Continuous variables were summarized using sample medians, 25th percentiles, and 75th percentiles. Mann-Whitney tests were used to make pair-wise comparisons in biomarker expression between CP patients, PC patients, and NPNH patients. Receiver-operating characteristic (ROC) curves were constructed and AUCs and 95% confidence intervals (CIs) were estimated to assess each biomarker’s ability to differentiate between the three patient groups. Sensitivity, specificity, and corresponding 95% CIs were estimated for each biomarker. For ease of presentation, these quantities are presented only at the marker concentration at which both sensitivity and specificity were simultaneously maximized. P-values < 0.05 were considered statistically significant.
A secondary analysis to explore patient demographic and clinicopathologic information as potentially confounding factors was performed comparing biomarker expression between the patient subgroups. The focus of this exploratory analysis was to descriptively summarize biomarker expression in the various patient subgroups; however, p-values resulting from Mann-Whitney tests are presented in the tables. No adjustment for multiple testing was made owing to the exploratory nature of this analysis. Statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC) and S-Plus software version 8.0.1 (Insightful Corporation, Seattle, WA).
RESULTS
One hundred eleven patients presenting with epigastric pain/discomfort warranting EUS-FNA evaluation and diagnosed with CP, PC, or being free of pancreatic disease (non-pancreatic non-healthy controls, NPNH) were enrolled in the study. Six patients were lost to screening failure and thus were excluded from the analysis, leaving 105 patients for analysis: CP (n=24), PC (n=58) and NPNH (n=23).
Differentiating Non-Pancreatic Non-Healthy Patients from Chronic Pancreatitis Patients
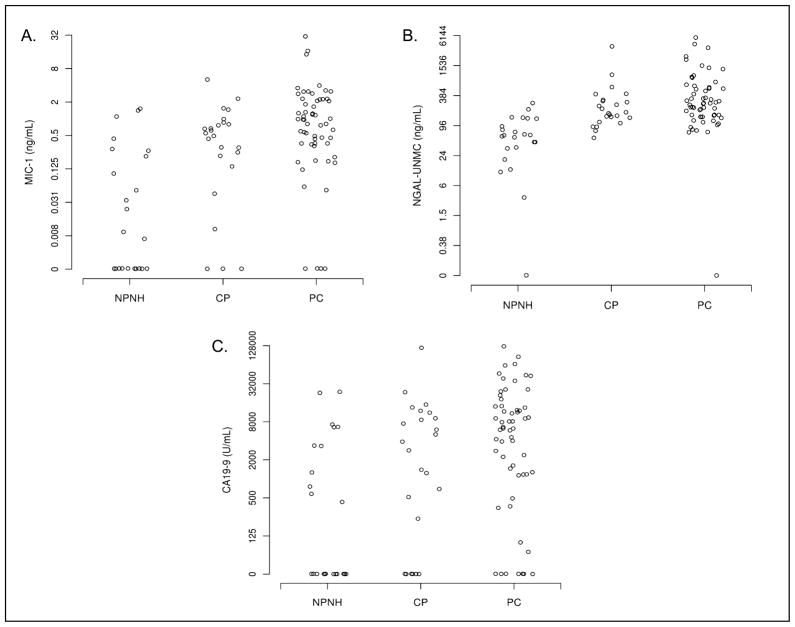
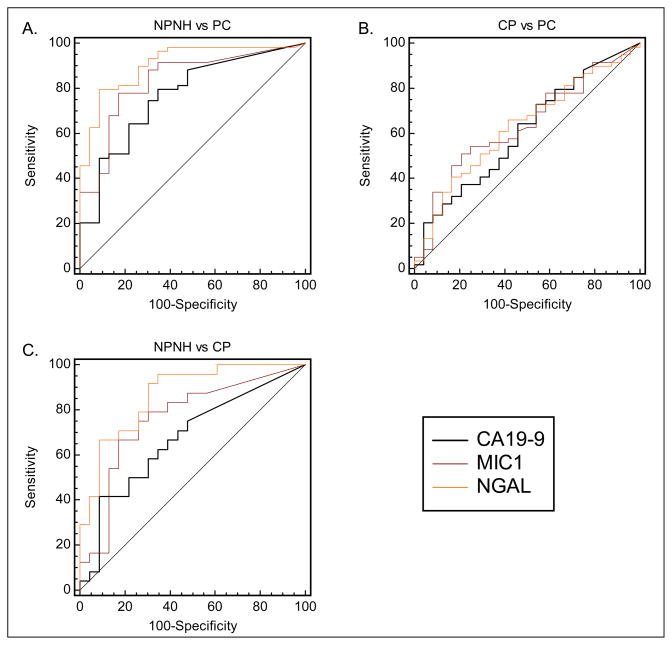
All marker concentrations were significantly higher in CP patients than in NPNH controls (P ≤ 0.034, Table 1, Figure 1). ROC curves for each marker reveal that NGAL (AUC (95% CI) = 0.88 (0.78–0.98)) differentiated NPNH patients from CP patients more effectively than MIC-1 or CA19-9 (AUC (95% CI) = 0.76 (0.62–0.90) and 0.67 (0.51–0.82) respectively) (Figure 2, Table 1). NGAL also showed the greatest sensitivity (75%, 95% CI = 53–90%) and specificity (74%, 95% CI = 52–96%) in differentiating CP from NPNH patients (Table 1). In contrast, the sensitivity and specificity (95% CI) of MIC-1 and CA19-9 were 71% (49–87%) and 74% (52–90%), and 63% (41–81%) and 65% (43–84%) respectively.
Table 1.
Biomarker Concentrations and Comparisons in NPNH, CP, and PC Patients
| Median marker concentration (25th percentile, 75th percentile) | ||||||||
| Marker |
NPNH (n=23)
|
CP (n=24)
|
PC (n=58)
|
|||||
| MIC-1 (ng/mL) | 0.0 (0.0, 0.3) | 0.5 (0.2, 0.8) | 0.9 (0.3, 2.2) | |||||
| NGAL (ng/mL) | 61 (34, 133) | 173 (126, 307) | 266 (144, 557) | |||||
| CA19-9 (U/mL) | 0 (0, 3363) | 3340 (116, 10111) | 6471 (1171, 14207) | |||||
| Group Comparisons (ROC Curves) | ||||||||
| Groups | Marker | AUC (95% CI) | P valuea | Cutoffb |
Sensitivity
|
Specificity
|
||
| % (Fraction) | 95% CI | % (Fraction) | 95% CI | |||||
| CP vs. NPNH | MIC-1 (ng/mL) | 0.76 (0.62–0.90) | <0.001 | 0.21 | 71% (17/24) | 49%–87% | 74% (17/23) | 52%–90% |
| NGAL (ng/mL) | 0.88 (0.78–0.98) | <0.001 | 132.8 | 75% (18/24) | 53%–90% | 74% (17/23) | 52%–90% | |
| CA19-9 (U/mL) | 0.67 (0.51–0.82) | 0.034 | 758.8 | 63% (15/24) | 41%–81% | 65% (15/23) | 43%–84% | |
| PC vs. NPNH | MIC-1 (ng/mL) | 0.83 (0.73–0.93) | <0.001 | 0.29 | 78% (45/58) | 65%–87% | 78% (18/23) | 56%–93% |
| NGAL (ng/mL) | 0.91 (0.84–0.98) | <0.001 | 138 | 79% (46/58) | 67%–89% | 83% (19/23) | 61%–95% | |
| CA19-9 (U/mL) | 0.76 (0.65–0.88) | <0.001 | 2221.7 | 84% (49/58) | 73%–93% | 52% (12/23) | 31%–73% | |
| PC vs. CP | MIC-1 (ng/mL) | 0.63 (0.50–0.76) | 0.043 | 0.63 | 57% (33/58) | 43%–70% | 58% (14/24) | 37%–78% |
| NGAL (ng/mL) | 0.61 (0.48–0.74) | 0.110 | 220.49 | 59% (34/58) | 45%–71% | 58% (14/24) | 37%–78% | |
| CA19-9 (U/mL) | 0.61 (0.48–0.74) | 0.110 | 5868.2 | 55% (32/58) | 42%–68% | 58% (14/24) | 37%–78% | |
AUC, area under the curve; CI, confidence interval; MIC-1, macrophage inhibitor cytokine-1; NGAL, neutrophil gelatinase–associated lipocalin; CA19-9, carbohydrate antigen 19-9; NPNH, non-pancreatic non-healthy; CP, chronic pancreatitis; PC, pancreatic cancer.
Mann-Whitney U test.
Cut-off value is the marker level that maximized the sensitivity and specificity averages.
NOTE. For estimation of sensitivity, chronic pancreatitis patients were considered to have disease and non-pancreatic non-healthy patients were considered to have no disease.
Figure 1. Distribution of biomarkers analyzed in the pancreatic juice of non-pancreatic non-healthy, chronic pancreatitis, and pancreatic cancer patients.
Distribution of pancreatic juice macrophage inhibitor cytokine-1 (MIC-1; A); neutrophil gelatinase–associated lipocalin (NGAL; B); and carbohydrate antigen 19-9 (CA19-9; C) of non-pancreatic non-healthy (NPNH) patients, chronic pancreatitis (CP) patients, and pancreatic cancer (PC) patients. NGAL and MIC-1 were measured by enzyme-linked immunosorbent assay (ELISA), while CA19-9 was measured by radioimmunoassay. MIC-1 and NGAL values are given in ng/mL, CA19-9 values are given in U/mL.
Figure 2. Comparison ROC curves examining the ability of tested biomarkers to discriminate between chronic pancreatitis, pancreatic cancer, and non-pancreatic non-healthy patients.
Receiver-operating characteristic curves examining the ability of macrophage inhibitor cytokine-1 (MIC-1); neutrophil gelatinase–associated lipocalin (NGAL); and carbohydrate antigen 19-9 (CA19-9) to differentiate A) non-pancreatic non-healthy (NPNH) patients from pancreatic cancer (PC) patients; B) chronic pancreatitis (CP) patients from PC patients; and C) CP patients from NPNH patients. Both NGAL and MIC-1 were found to be superior to CA19-9 in differentiating patients with pancreatic disease (PC and CP) from their NPNH counterparts.
Differentiating Non-Pancreatic Non-Healthy Patients from Pancreatic Cancer Patients
All marker concentrations were significantly higher in PC patients than in the NPNH group (all P ≤ 0.001, Table 1, Figure 1). ROC curves for each marker indicate that NGAL (AUC (95% CI) = 0.91 (0.84–0.98)), and MIC-1 (AUC (95% CI) = 0.83 (0.73–0.93)) differentiated NPNH from PC patients more effectively than CA19-9 (AUC (95% CI)= 0.76 (0.65–0.88)) (Figure 2, Table 1). NGAL showed the greatest sensitivity (79%, 95% CI = 67–89%) and specificity (83%, 95% CI = 61–95%) in differentiating PC from NPNH patients (Table 1). In contrast, the sensitivity and specificity (95% CI) was 78% (65–87%) and 78% (56–93%), and 84% (73–93%) and 52% (31–73%) for MIC-1 and CA19-9 respectively.
Differentiating Chronic Pancreatitis Patients from Pancreatic Cancer Patients
MIC-1 was the only marker for which there was a statistically significant difference in concentration between PC and CP patients (P = 0.043, Table 1, Figure 1). No noticeable differences were observed in NGAL or CA19-9 (P = 0.110) (Figure 2).
Further, we investigated whether combining MIC-1 with either NGAL or CA19-9 would enhance the ability to distinguish the two groups. MIC-1 levels were evaluated with each of the other biomarkers to identify direct or inverse relationships between them that might improve its diagnostic utility but no obvious trends were observed (Supplementary Figure 1).
Exploratory Correlation of Pancreatic Juice NGAL, MIC-1, and CA19-9 with Patient Demographics
Exploratory analyses of pancreatic juice biomarker concentrations based on patient demographics are shown in Table 2. No difference in pancreatic juice biomarker levels were observed based on patient age, BMI, gender, smoking history, or current alcohol use for NPNH or CP patients. While no differences between CA19-9 or NGAL levels based on demographic information were observed in PC patients, we found that PC patients aged ≥68 years had elevated MIC-1 levels as compared to those <68 years old (median level (IQR) = 1.26 (0.59–2.30) ng/mL and 0.49 (0.20–1.16) ng/mL respectively, P = 0.03). MIC-1 levels were also higher in men than in women with PC (median level (IQR) = 1.26 (0.36–2.86) ng/mL and 0.57 (0.17–0.98) ng/mL respectively, P = 0.03). However, BMI, smoking history, and current alcohol use did not correlate with pancreatic juice MIC-1 levels in PC patients.
Table 2.
Biomarker Concentrations in Pancreatic Juice Based on Patient Demographics
| Characteristic | CA19-9 (U/mL)
|
NGAL-UNMC (ng/mL)
|
MIC-1 (ng/mL)
|
|||
|---|---|---|---|---|---|---|
| Median (IQR) | P value | Median (IQR) | P value | Median (IQR) | P value | |
| NPNH patients (n = 23) | ||||||
| Age, yearsa (median: 65) | 0.26 | 0.30 | 0.66 | |||
| < Median (n = 11) | 581 (0–6621) | 61 (34–78) | 0.00 (0.00–0.29) | |||
| ≥ Median (n = 12) | 0 (0–3328) | 54 (27–136) | 0.03 (0.00–0.24) | |||
| BMIa (median: 27) | 0.18 | 0.76 | 0.72 | |||
| < Median (n = 12) | 0 (0–596) | 50 (27–82) | 0.03 (0.00–0.24) | |||
| ≥ Median (n = 10) | 918 (0–6549) | 62 (45–134) | 0.00 (0.00–0.05) | |||
| Sexb | 0.44 | 0.29 | 0.30 | |||
| Male (n = 6) | 918 (0–6549) | 71 (59–134) | 0.00 (0.00–0.05) | |||
| Female (n = 17) | 0 (0–3293) | 56 (20–93) | 0.02 (0.00–0.27) | |||
| History of smokingb | 0.58 | 0.77 | 0.57 | |||
| No (n = 15) | 0 (0–3363) | 62 (34–133) | 0.00 (0.00–0.27) | |||
| Yes (n = 8) | 506 (0–4921) | 57 (24–102) | 0.02 (0.00–0.24) | |||
| Current alcohol useb | 0.55 | 0.56 | 0.97 | |||
| No (n = 9) | 0 (0–6549) | 63 (45–133) | 0.02 (0.00–0.21) | |||
| Yes (n = 14) | 581 (0–1256) | 56 (20–78) | 0.01 (0.00–0.29) | |||
| CP patients (n = 24) | ||||||
| Age, yearsa(median: 64) | 0.28 | 0.49 | 0.67 | |||
| < Median (n = 12) | 5144 (0–10111) | 168 (111–269) | 0.52 (0.19–0.72) | |||
| ≥ Median (n = 12) | 2634 (462–8975) | 210 (141–367) | 0.55 (0.11–0.92) | |||
| BMIa(median: 23) | 0.65 | 0.95 | 0.65 | |||
| < Median (n = 11) | 1387 (0–9070) | 178 (137–250) | 0.61 (0.25–0.76) | |||
| ≥ Median (n = 10) | 6266 (0–11153) | 242 (90–419) | 0.49 (0.14–1.54) | |||
| Sexb | 0.64 | 0.35 | 0.88 | |||
| Male (n = 16) | 3923 (603–8828) | 208 (126–367) | 0.49 (0.18–0.83) | |||
| Female (n = 8) | 1940 (0–13416) | 153 (118–229) | 0.58 (0.15–1.11) | |||
| History of smokingb | 0.88 | 0.35 | 0.98 | |||
| No (n = 8) | 2634 (257–8501) | 276 (118–716) | 0.67 (0.01–0.92) | |||
| Yes (n = 16) | 4398 (116–10111) | 164 (126–266) | 0.46 (0.23–0.78) | |||
| Current alcohol useb | 0.98 | 0.34 | 0.47 | |||
| No (n = 14) | 2634 (514–7487) | 223 (137–413) | 0.61 (0.22–0.86) | |||
| Yes (n = 10) | 5693 (0–11955) | 153 (114–250) | 0.37 (0.04–0.76) | |||
| PC patients (n = 58) | ||||||
| Age, yearsa(median: 68) | 0.86 | 0.11 | 0.03 | |||
| < Median (n = 29) | 6230 (1453–11921) | 226 (143–477) | 0.49 (0.20–1.16) | |||
| ≥ Median (n = 29) | 6627 (368–14207) | 304 (157–876) | 1.26 (0.59–2.30) | |||
| BMIa(median: 26) | 0.36 | 0.90 | 0.07 | |||
| < Median (n = 30) | 7418 (1163–26086) | 266 (143–535) | 1.11 (0.36–2.82) | |||
| ≥Median (n = 24) | 6429 (1447–11577) | 266 (173–642) | 0.52 (0.17–1.18) | |||
| Sexb | 0.40 | 0.53 | 0.03 | |||
| Male (n = 35) | 8100 (1453–14059) | 282 (144–557) | 1.26 (0.36–2.86) | |||
| Female (n = 23) | 4557 (70–18248) | 214 (111–600) | 0.57 (0.17–0.98) | |||
| History of smokingb | 0.71 | 0.24 | 0.76 | |||
| No (n = 27) | 8111 (1139–18248) | 331 (119–1310) | 0.76 (0.18–2.30) | |||
| Yes (n = 29) | 6314 (1626–11234) | 225 (144–337) | 0.98 (0.36–1.81) | |||
| Current alcohol useb | 0.76 | 0.16 | 0.37 | |||
| No (n = 30) | 7247 (1171–13423) | 306 (122–1310) | 0.68 (0.17–2.08) | |||
| Yes (n = 26) | 7207 (1453–21033) | 223 (144–337) | 0.98 (0.43–2.1) | |||
CA19-9, carbohydrate antigen 19-9; NGAL, neutrophil gelatinase associated lipocalin; MIC-1, macrophage inhibitor cytokine-1; IQR, interquartile range;
BMI, body mass index; NPNH, non-pancreatic non-healthy; CP, chronic pancreatitis; PC, pancreatic cancer.
P values obtained using Kendall’s correlation test.
P values obtained using Wilcoxon rank sum test.
Exploratory Correlation of Pancreatic Juice NGAL, MIC-1, and CA19-9 with Clinicopathologic Characteristics
Patient history of type-2 diabetes (DM2) was singled out for additional analysis due to its strong correlation with PC diagnosis as well as the mounting evidence that it may be an early, non-specific symptom of PC development [20]. Due to the fact that only three NPNH patients used in this study had a positive history of DM2, this group was omitted from this analysis. In patients with CP, no difference was observed in any of the measured biomarkers based on DM2 status (P ≤ 0.23, Table 3). In PC patients, however, MIC-1 was found to significantly correlate with a past diagnosis of DM2 (median value for patients with positive DM2 status was 1.81 ng/mL while it was 0.61 ng/mL for those with a negative DM2 history, P = 0.03), while no significant differences were found in NGAL or CA19-9 levels (P ≤ 0.17, Table 3). Further, in comparing CP patients with a history of DM2 to their PC counterparts, it was found that CA19-9, NGAL-UNMC, and MIC-1 were all elevated in the PC group with moderate significance (P = 0.067–0.082, Table 4). NGAL-MDACC was also found to be higher in PC patients with a history of DM2 as compared to DM2-positive CP patients (189 ng/mL vs. 86 ng/mL), though this difference was found to be non-significant (P = 0.16, Table 4).
Table 3.
Pancreatic Juice Biomarker Levels in CP and PC Patients with a History of Diabetes Mellitus Type 2 (DM2)
| DM2 status | CA19-9 (U/mL)
|
NGAL (ng/mL)
|
MIC-1 (ng/mL)
|
|||
|---|---|---|---|---|---|---|
| Median (IQR) | P valuea | Median (IQR) | P valuea | Median (IQR) | P valuea | |
| CP patients (n = 24) | 0.23 | 0.67 | 0.97 | |||
| No (n = 18) | 4937 (692–11153) | 193 (114–314) | 0.52 (0.14–0.86) | |||
| Yes (n = 6) | 373 (0–5046) | 158 (144–238) | 0.55 (0.22–0.76) | |||
| PC patients (n = 58) | 0.17 | 0.25 | 0.03 | |||
| No (n = 47) | 5898 (1171–14059) | 256 (138–557) | 0.61 (0.18–1.64) | |||
| Yes (n = 11) | 11234 (6314–26086) | 297 (219–910) | 1.81 (0.76–3.08) | |||
Table 4.
Pancreatic Juice Biomarker Levels in CP and PC Patients with a History of Diabetes Mellitus Type 2 (DM2)
| Marker | CP (n = 6) | PC (n = 11) | P valuea |
|---|---|---|---|
|
| |||
| CA19-9 (U/mL) | 373 (0, 0, 5047, 11955) | 11234 (0, 6314, 26086, 85573) | 0.067 |
| NGAL-UNMC (ng/mL) | 158 (90, 144, 238, 419) | 297 (143, 219, 910, 3495) | 0.082 |
| MIC-1 (ng/mL) | 0.55 (0.00, 0.22, 0.76, 2.31) | 1.81 (0.32, 0.76, 3.08, 16.64) | 0.082 |
NOTE. Data are presented as the sample median (minimum, 25th percentile, 75th percentile, maximum
CA19-9, carbohydrate antigen 19-9; NGAL, neutrophil gelatinase associated; MIC-1, macrophage inhibitor cytokine-1.
Wilcoxon rank test.
In determining whether measurement of NGAL, MIC-1, or CA19-9 in pancreatic juice has potential utility in the staging of newly diagnosed PC, initial staging by EUS was used to stratify PC patients as having resectable or non-resectable tumors. Pathological staging was evaluated to confirm true tumor resectability in patients with tumors deemed to be resectable by EUS. Fourteen patients had EUS-determined resectable tumors and forty-four had unresectable tumors. Statistical analysis revealed no difference in pancreatic juice NGAL, MIC-1, or CA19-9 levels with regard to tumor resectability (P = 0.12–0.93, Supplementary Table 2).
Pancreatic juice marker levels were also analyzed for correlation with overall survival in PC patients to determine their prognostic potential. None of the markers was correlated with survival time (Supplementary Table 3); however, this result is not conclusive as the number of PC patients was insufficient, precluding optimal analysis.
DISCUSSION
We found that NGAL, MIC-1, and CA19-9 levels in pancreatic juice were significantly higher in CP and PC patients than in their NPNH counterparts (P< 0.034). Of these biomarkers, NGAL appears to hold the most individual promise in differentiating patients with pancreatic disease from those without, giving sensitivities of 79% and 75%, specificities of 83% and 74%, and areas under the curve (AUCs) of 0.91 and 0.88 when comparing PC and CP, respectively, to NPNH patients. MIC-1, however, also shows clinical potential when comparing PC to NPNH patients (sensitivity 78%, specificity 78%, AUC: 0.83). Furthermore, MIC-1 showed a significant difference in levels between PC and CP patients (P = 0.043), with no noticeable difference observed for NGAL or CA19-9 (P>0.10). Interestingly, these results are similar to available data from previously published studies evaluating these markers in patient sera (Supplementary Table 4) [8, 9, 21–23].
Few studies have investigated the role of pancreatic juice in diagnosing pancreatic disease. One study found that the levels of certain heavy metals, such as chromium, were significantly elevated in the pancreatic juice of PC patients as compared to healthy controls and may have diagnostic significance [24]. Another study yielded a set of genes from the cells present in pancreatic juice that accurately differentiated healthy participants from those with PC [25]. A comparison of the proteome of pancreatic juice from PC patients to that of patients with other pancreatic diseases yielded pancreatitis-associated protein-1 (HIP/PAP-1) that, when validated by ELISA, showed significant elevation in PC, but not in non-malignant pancreatic diseases, as compared to healthy controls [26]. Another recent study identified 20 proteins that are significantly elevated (p< 0.05) at least 2-fold or greater in pancreatic juice collected from three patients with preneoplastic PanIN-3 lesions compared to a control sample comprising pooled pancreatic juice from five patients with benign pancreatic diseases (sphincter of Oddi dysfunction and CP), some of which have also been previously shown to be elevated in PanIN tissues [27]. Human telomerase reverse transcriptase (hTERT) expression analysis in pancreatic juice from patients with PC and IPMN revealed that a positive staining for hTERT was nearly 85% sensitive and over 80% accurate in distinguishing benign from malignant cells, results far superior to those allowed by cytology [28]. One study comparing the proteome of pooled pancreatic juice from 9 PC samples and 9 control samples (including CP, gallstone induced pancreatitis, benign cystic neoplasm, and cystic fibrosis) found 3 proteins—matrix metalloproteinase-9, oncogene DJ-1, and α-1B-glycoprotein precursor—to be differentially upregulated in PC patients [29]. Additionally, several studies have analyzed the diagnostic abilities of pancreatic juice CA19-9 in the context of PC and CP as compared to healthy controls with results that are in general highly similar to those reported in this study, showing that pancreatic juice CA19-9 values are higher in PC than in CP and give a sensitivity for PC ranging between 70–90% [25, 30, 31]. One study in particular indicated that the sensitivity of elevated CA19-9 in pancreatic juice was 90% and 66% for PC and CP respectively, comparable to our values of 84% and 63% [31]. Interestingly however, data regarding pancreatic juice CA19-9 levels in the context of chronic pancreatitis has been inconsistent, with values ranging from normal to elevated and indistinguishable from PC [25, 30, 31].
Despite the general promise of these studies, several factors limit their clinical interpretability. Use of pooled samples prevents the direct comparison of individual groups and unblinded sample and/or biomarker studies could lead to bias. Small sample sizes and failure to investigate the consistency of biomarker level measurements across various medical centers and testing methods also curbs interpretability. Further, some platforms used in previous studies are not easily converted to the clinical setting which is problematic as protein elevation detectability is often inconsistent between different platforms.
In comparison, the current study has several strengths. This investigation recruited a sufficient number of patients to enable in-depth and powerful statistical analyses. Further, ample pertinent clinicopathologic and demographic patient data were obtained, allowing us to evaluate potential confounders while providing the ability to better determine the true clinical relevance of these tests.
Importantly, no difference in pancreatic juice NGAL, MIC-1, or CA19-9 levels was found in NPNH or CP patients based on our exploratory analysis of patient demographics (Table 2). In PC patients, however, pancreatic juice MIC-1 levels correlated with patient age and gender. Consequently, though no multivariate analysis could be performed due to lack of sufficient patient numbers in each group, we believe the biomarker differences found between NPNH and CP patients are indeed due to the respective disease states. We are less confident, however, that data skewing due to confounders was not a factor in comparisons of MIC-1 levels in PC patients to those in other groups, though the matched patient age to the NPNH and CP patients and the matched gender distribution to the CP patients reduce this likelihood.
In our exploratory analyses, we also found that MIC-1 is higher in DM2-positive PC patients than in DM2-positive CP patients which approached statistical significance (P = 0.082) (Table 4). This point is of particular importance as MIC-1 serum levels have been previously found to be elevated in DM2 patients as compared to healthy controls, a comparison that could not be evaluated in pancreatic juice through this study [32]. Additionally, DM2 and PC appear to have a potential synergistic effect on pancreatic juice MIC-1 levels as DM2-positive PC patients were found to have significantly higher levels than DM2-negative PC patients (Table 3). These results suggest that pancreatic juice MIC-1 levels might be useful in differentiating DM2 patients with PC from DM2 patients without PC (P = 0.030). The ability to make such a differentiation is of increasing importance as the link between DM2 and PC becomes better established.
Based on the results of our study, NGAL, MIC-1 and CA19-9 remain situationally promising markers in pancreatic juice as they may help narrow the potential etiologies of diseases with non-specific gastroenterological symptoms to being of pancreatic origin. Such utility carries high clinical relevance as it could allow patients to be more effectively stratified according to their risk of pancreatic and non-pancreatic diseases, thereby aiding in diagnostic decision making regarding employment of invasive tests for definitive diagnoses. However, it is unlikely that these biomarkers could be used to specifically distinguish between pancreatic diseases.
Despite the strengths of this study design, several weaknesses prevail. While the patient population considered as NPNH was clinically relevant in that all NPNH patients had symptoms resulting in pancreatic pathology being considered on their respective differential diagnoses, it is doubtless that not all diseases presenting with symptomatology mimicking pancreatic disease were represented in this group. As such, the true clinical specificity of pancreatic juice NGAL, MIC-1, and CA19-9 in diagnosing pancreatic disease cannot be determined. Additionally, any definitive conclusions regarding biomarker levels with respect to DM2 patients are hindered by the lack of ability to include the NPNH patient group in this analysis. Further, patient sera were not available for comparatory analysis of the diagnostic utility of these markers across the two body fluids.
Per our present findings, future study using larger patient groups is warranted for the development of pancreatic juice NGAL, MIC-1 and CA19-9 testing in the differentiation of pancreatic disease and to further determine the existence of any correlation with patient diabetic status. Additionally, efforts should be made to compare the levels of these biomarkers in pancreatic juice to their respective levels in patient serum. In this manner, pancreatic juice may be evaluated for its superiority to serum as a source for biomarkers in the context of pancreatic disease and it can be determined if serum levels of the biomarkers reflect their levels in juice. Further, the results of this study should be combined with those of future studies assessing additional pancreatic juice biomarkers that are more closely associated with PC cells, such as mucins, in an attempt to improve the specificity of pancreatic juice biomarker testing in the context of pancreatic disease.
Supplementary Material
Acknowledgments
Grant Support: Surinder K Batra, Subhankar Chakraborty, Sukhwinder Kaur, Kavita Mallya, Michael Baine Maneesh Jain and Aaron R. Sasson are supported, in part, by grants from the NIH (U01EDRN CA111294, R01 CA131944, and P50 SPORE CA127297). Sushovan Guha is supported by grants from MDACC McNair Foundation Scholar Award and the Cyrus Scholar Award. Massimo Raimondo is supported by Mayo Clinic Institutional Research Grant Award.
The authors on this manuscript are thankful to the Core Facility and NCI SPORE program at UNMC for providing technical help and support for the work presented here.
Footnotes
Disclosures: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing Assistance: No writing assistance was utilized in the production of this manuscript.
Author Contributions: Sukhwinder Kaur was involved in performing biomarker assays at UNMC and writing the manuscript. Michael J. Baine was involved in assay interpretation, study design, and writing of the manuscript. Sushovan Guha was involved in study design, and performing biomarker assays at MDACC. Nobou Ochi was involved in performing NGAL assays at MDACC. Subhankar Chakraborty was involved in study design and writing of the manuscript. Kavita Mallya was involved in performance of biomarker assays at UNMC. Colleen Thomas and Julia Crook were involved in statistical analysis of the data. Michael B. Wallace was involved in collection of SSEPS samples for the study. Timothy A. Woodward was involved in collection of SSEPS samples for the study. Maneesh Jain was involved in interpretation of UNMC assays and the writing of the manuscript. Verna Skinner acted as the Clinical Study Coordinator for this work. Massimo Raimondo was involved in study design and collection of SSEPS samples for the study. Surinder K. Batra was involved in study design and writing of the manuscript.
References
- 1.American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes In the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.Chakraborty S, Kaur S, Muddana V, et al. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol. 2010;105:2050–2059. doi: 10.1038/ajg.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty S, Baine MJ, Sasson AR, et al. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815:44–64. doi: 10.1016/j.bbcan.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumour markers in gastrointestinal cancers–EGTM recommendations. European Group on Tumour Markers. Anticancer Res. 1999;19:2811–2815. [PubMed] [Google Scholar]
- 6.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464–1484. doi: 10.1016/s0016-5085(99)70298-2. [DOI] [PubMed] [Google Scholar]
- 7.Goggins M. Molecular markers of early pancreatic cancer. J Clin Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 8.Koopmann J, Buckhaults P, Brown DA, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 9.Moniaux N, Chakraborty S, Yalniz M, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540–1547. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni XG, Bai XF, Mao YL, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Pleskow DK, Berger HJ, Gyves J, et al. Evaluation of a serologic marker, CA19-9, in the diagnosis of pancreatic cancer. Ann Intern Med. 1989;110:704–709. doi: 10.7326/0003-4819-110-9-704. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 13.Welsh JB, Sapinoso LM, Kern SG, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer M, Eickhoff JC, Gould MN, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108:389–397. doi: 10.1007/s10549-007-9619-3. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen BS, Borregaard N, Bundgaard JR, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim R, Ahmed N, Borregaard N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer. 2007;120:2426–2434. doi: 10.1002/ijc.22352. [DOI] [PubMed] [Google Scholar]
- 17.Monier F, Surla A, Guillot M, et al. Gelatinase isoforms in urine from bladder cancer patients. Clin Chim Acta. 2000;299:11–23. doi: 10.1016/s0009-8981(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 18.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Villano BC, Brennan S, Brock P, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29:549–552. [PubMed] [Google Scholar]
- 20.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buxbaum JL, Eloubeidi MA. Molecular and clinical markers of pancreas cancer. JOP. 2010;11:536–544. [PubMed] [Google Scholar]
- 22.Furuya N, Kawa S, Hasebe O, et al. Comparative study of CA242 and CA19-9 in chronic pancreatitis. Br J Cancer. 1996;73:372–376. doi: 10.1038/bjc.1996.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talar-Wojnarowska R, Gasiorowska A, Olakowski M, et al. Clinical value of serum neopterin, tissue polypeptide-specific antigen and CA19-9 levels in differential diagnosis between pancreatic cancer and chronic pancreatitis. Pancreatology. 2010;10:689–694. doi: 10.1159/000320693. [DOI] [PubMed] [Google Scholar]
- 24.Rosty C, Christa L, Kuzdzal S, et al. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002;62:1868–1875. [PubMed] [Google Scholar]
- 25.Chen R, Pan S, Duan X, et al. Elevated level of anterior gradient-2 in pancreatic juice from patients with pre-malignant pancreatic neoplasia. Mol Cancer. 2010;9:149. doi: 10.1186/1476-4598-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers CD, Fukushima N, Sato N, et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biol Ther. 2006;5:1383–1389. doi: 10.4161/cbt.5.10.3323. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima A, Murakami Y, Uemura K, et al. Usefulness of human telomerase reverse transcriptase in pancreatic juice as a biomarker of pancreatic malignancy. Pancreas. 2009;38:527–533. doi: 10.1097/MPA.0b013e3181a16d28. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, Lu Z, Yang A, et al. Comparative proteomic analysis of human pancreatic juice: methodological study. Proteomics. 2007;7:1345–1355. doi: 10.1002/pmic.200600086. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, Tonack S, Smith R, et al. Confounding effect of obstructive jaundice in the interpretation of proteomic plasma profiling data for pancreatic cancer. J Proteome Res. 2009;8:142–148. doi: 10.1021/pr800451h. [DOI] [PubMed] [Google Scholar]
- 30.Nishida K, Tasaki N, Miyagawa H, et al. Estimation of carbohydrate antigen (CA) 19-9 levels in pure pancreatic juice of patients with pancreatic cancer. Am J Gastroenterol. 1988;83:126–129. [PubMed] [Google Scholar]
- 31.Wakabayashi T, Sawabu N, Takemori Y, et al. Diagnostic significance of cancer-associated carbohydrate antigen (CA19-9) concentrations in pancreatic juice: analysis in pure pancreatic juice collected by endoscopic aspiration and immunohistochemical study in chronic pancreatitis. Pancreas. 1993;8:151–159. doi: 10.1097/00006676-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Dostalova I, Roubicek T, Bartlova M, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397–404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.