Abstract
Background
The purinergic component of enteric inhibitory neurotransmission is important for normal motility in the gastrointestinal (GI) tract. Controversies exist about the purine(s) responsible for inhibitory responses in GI muscles: adenosine 5′-triphosphate (ATP) has been assumed to be the purinergic neurotransmitter released from enteric inhibitory motor neurons, however recent studies demonstrate that β-nicotinamide adenine dinucleotide (β-NAD+) and ADP-ribose mimic the inhibitory neurotransmitter better than ATP in primate and murine colons. The study was designed to clarify the sources of purines in colons of Cynomolgus monkeys and C57BL/6 mice.
Methods
HPLC with fluorescence detection was used to analyze purines released by stimulation of nicotinic acetylcholine receptors (nAChR) and serotonergic 5-HT3 receptors (5-HT3R), known to be present on cell bodies and dendrites of neurons within the myenteric plexus.
Key Results
nAChR or 5-HT3R agonists increased overflow of ATP and β-NAD+ from tunica muscularis of monkey and murine colon. The agonists did not release purines from circular muscles of monkey colon lacking myenteric ganglia. Agonist-evoked overflow of β-NAD+, but not ATP, was inhibited by tetrodotoxin (0.5 μM) or ω-conotoxin GVIA (50 nM), suggesting that β-NAD+ release requires nerve action potentials and junctional mechanisms known to be critical for neurotransmission. ATP was likely released from nerve cell bodies in myenteric ganglia and not from nerve terminals of motor neurons.
Conclusions & Inferences
These results support the conclusion that ATP is not a motor neurotransmitter in the colon and are consistent with the hypothesis that β-NAD+, or its metabolites, serve as the purinergic inhibitory neurotransmitter.
Keywords: enteric nervous system, purines, 5-HT3 receptors, nicotinic acetylcholine receptors, ATP, NAD, myenetric ganglia
INTRODUCTION
Gastrointestinal (GI) motility is regulated by enteric excitatory and inhibitory motor neurons which coordinate muscle contractions for propulsion of colonic contents.1 Muscles of the colon are under tonic neural inhibition2, 3, 4, and spontaneous inhibitory junction potentials (IJPs), mediated by release of neurogenic purines, contribute to tonic inhibition.5 Genetic deactivation of P2Y1 purine receptors delays transit through the colon.6 Therefore, purinergic inhibitory neurotransmission is an important component in regulating colonic motility.
The identity of the purine(s) responsible for enteric inhibitory responses is controversial. Adenosine 5′-triphosphate (ATP) has been assumed to be the purine neurotransmitter,7, 8, 9 but recent experiments on mouse10 and primate colons11 showed that another purine, β-nicotinamide adenine dinucleotide (β-NAD+) and its bioactive metabolite, adenosine 5′-diphosphate ribose (ADPR)12, mimic the endogenous purine neurotransmitter better than ATP. Measurements of purines released during stimulation of nerves by electrical field stimulation (EFS) demonstrated that both ATP and β-NAD+ are released in the colon, but these experiments were not capable of distinguishing the sites from which the two purines were released.11
Release of ATP and β-NAD+ from enteric neurons might occur from different sites by different mechanisms. For example, β-NAD+ (and ADPR) might be released from nerve varicosities at neuroeffector junctions, and ATP might be mainly released from extrajunctional sites, based on previous observations.11 We tested this hypothesis by measuring purines released in response to stimulation of nicotinic acetylcholine receptors (nAChR) and serotonin (5-hydroxytryptamine) 5-HT3 receptors (5-HT3R) known to be expressed on cell bodies and dendrites of myenteric interneurons and muscle motor neurons.5,13,14, 15, 16,17,18 For these stimuli to release neurotransmitters from nerve terminals of motor neurons, action potentials would need to develop and propagate to nerve varicosities, and Ca2+ entry via Cacna1 family Ca2+ channels would need to occur in “active zones” of varicosities to initiate vesicular fusion. Here we demonstrate that release of β-NAD+ in response to ganglionic stimulation is dependent upon these mechanisms, but release of ATP is not. These findings are not consistent with ATP serving as a motor neurotransmitter in colonic muscles. Targeting β-NAD+ synthesis and/or metabolism may provide a novel rational for treating problems of colonic transit.
MATERIALS AND METHODS
Tissue preparation
Proximal colons of Cynomolgus monkeys (Macaca fascicularis) were obtained from Charles River Laboratories Preclinical Services (Reno, NV). Monkeys, sedated with Ketamine (10 mg kg-1) and 0.7 ml Beuthanasia-D (Schering-Plough AH, Kenilworth, NJ), were exsanguinated (Charles River Laboratories Institutional Animal Care and Use Committee (IACUC)) for reasons unrelated to this project. C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were euthanized by sedation with isoflurane followed by cervical dislocation and exsanguination. The entire GI tract was removed and placed in oxygenated cold Krebs solution for further dissection. All experimental procedures were approved by the IACUC at University of Nevada.
Monkey proximal colon tunica muscularis (whole muscle, WM) tissues were prepared by dissecting away the mucosal layer. Monkey circular muscle (CM) tissues, containing only nerve terminals, were prepared by peeling away the longitudinal muscle with attached myenteric ganglia.11 C57BL/6 mouse colons were prepared by removing the mucosa and submucosa.
Purine overflow
Colonic segments (40-70 mg) were placed in 200-μl superfusion chambers10,11 and superfused with oxygenated Krebs (37°C; composition in mM): 118.5 NaCl, 4.2 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose, 1.8 CaCl2 (pH 7.4). L-NNA (100 μM) and atropine (1 μM) were present throughout. Superfusates were collected before and during stimulation of nAChR with epibatidine (500 μM, 30 s) or DMPP (500 μM, 30 s) or 5-HT3R with SR57227 (500 μM, 30 s) and etheno-derivatized as described.19 Experiments were also performed with the nAChR antagonist, hexamethonium (500 μM), or the 5-HT3R antagonist ondansetron (10 μM) for 30 minutes before stimulation with nAChR or 5-HT3R agonists, respectively. In some experiments, tissues were superfused with tetrodotoxin (TTX, 0.5 μM) or ω-conotoxin GVIA (ω-Ctx GVIA, 50 nM) for 30 min before stimulation with nAChR or 5-HT3R agonists.
HPLC assay of purines in tissue superfusates
A reverse-phased gradient Agilent Technologies 1200 liquid chromatography system equipped with a fluorescence detector (Agilent Technologies, Wilmington, DE) was used to detect 1,N6-etheno-derivatized nucleotides and nucleosides as described.10, 11 Etheno-ATP is approximately 10 fold more fluorescent than the compound generated by etheno-derivatization of β-NAD+.20 Areas of HPLC peaks were calculated and expressed in figures. Each peak was calibrated to individual etheno-derivatized purine standards. Results, normalized for sample volume and tissue weight, were expressed in femtomoles per milligram of tissue (fmol/mg).
HPLC fraction analysis
1,N6-etheno derivatization forms 1,N6-etheno-ADPR by either β-NAD+, ADPR or cyclic ADPR present in superfusates.21 The compounds forming eADPR during nAChR or 5-HT3R activation were determined by HPLC fraction analysis.10,11,21 Superfusates from 36 chambers were combined, concentrated and analyzed by HPLC. An Agilent Technologies 1200 Analytical Fraction Collector was employed to collect 400 μl fractions corresponding to retention times of cyclic ADPR (7.2-min fraction), ADPR (8.5-min fraction), and β-NAD+ (10.5-min fraction). Fractions were subjected to etheno-derivatization and reanalyzed by HPLC for eADPR content.
Statistics
Data presented are means ± SEM. Means are compared by a two-tailed, unpaired t test or by one-way ANOVA for comparison of more than two groups followed by a post hoc Bonferroni multiple comparison test (GraphPadPrism, v. 3, GraphPad Software, Inc., San Diego, CA). A probability value less than .05 was considered significant.
Drugs
(±)-exo-2-(6-Chloro-3-pyridinyl)-7-azabicyclo[2.2.1.]heptane (epibatidine) and 1-(6-Chloro-2-pyridinyl)-4-piperidinamine hydrochloride (SR57227) were purchased from Tocris Bioscience (Ellisville, MO). Atropine, carbenoxolone, dimethylphenylpiperazinium (DMPP), hexamethonium bromide, NG-nitro-L-arginine (L-NNA), ondansetron hydrochloride and ω-Ctx GVIA were purchased from Sigma-Aldrich (St. Louis, MO). TTX was purchased from Ascent Scientific (Cambridge, MA). All drugs were dissolved in deionized H2O, apart from epibatidine (dissolved in DMSO), and further diluted in perfusion solutions.
RESULTS
Release of ATP and β-NAD+ elicited by activation of nAChR
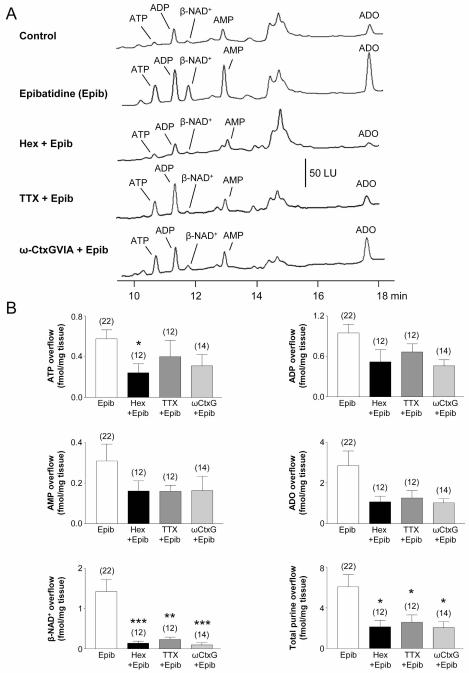
We tested whether stimulation of nAChR evoked release of purines in monkey whole tunica muscularis (WM). As reported previously11, monkey WM releases basal purines, including ATP, β-NAD+ and metabolites ADP, AMP, and adenosine (ADO) (Fig. 1A). Superfusion with nAChR agonist, epibatidine22 (500 μM for 30 s), enhanced purine release (Fig. 1A, B). HPLC fraction analysis determined that the 11.2-min peak is composed of ~92% β-NAD+, ~5% ADPR and ~3% cyclic ADPR; thus, β-NAD+ is the primary purine in the composite peaks. For simplicity, therefore, we refer to the purine eluted at 11.2 min as β-NAD+. Hexamethonium (500 μM) inhibited the epibatidine-evoked release of ATP (P < 0.05) and β-NAD+ (P < 0.001; Fig. 1B) verifying that the effect of epibatidine was mediated by nAChRs.
Fig. 1. Stimulation of nAChRs causes release of purines in monkey colon whole muscle preparations.
(A) Chromatograms of tissue superfusates collected before (control) and during stimulation of nAChRs with epibatidine (Epib, 500 μM, 30 s) in the absence and presence of hexamethonium (Hex, 500 μM for 30 min), tetrodotoxin (TTX, 0.5 μM for 30 min) or ω-conotoxin GVIA (β-CtxG, 50 nM for 30 min) in WM monkey colon. Small amounts of ATP, ADP, β-NAD+, AMP and ADO were present in superfusates before stimulation, likely to cause tonic purinergic inhibition in colon. Epibatidine-evoked release of purines was inhibited by hexamethonium. Epibatidine-evoked release of β-NAD+, but not of ATP, was reduced by the neurotoxins TTX and ω-CtxG. Scale applies to all chromatograms. LU, luminescence units. (B) Averaged data are means ± SEM and summarize release of ATP, ADP, AMP, ADO, β-NAD+ and total purines (calculated as ATP+ADP+AMP+ADO+β-NAD+) during activation of nAChRs with epibatidine (Epib). Overflow (femtomoles per milligram of tissue) is the overflow during nAChR activation less spontaneous overflow. All purines were evaluated simultaneously in the same samples. Each peak was calibrated to individual etheno-derivatized purine standards. Asterisks denote significant differences from epibatidine-evoked release (*P < 0.05, **P < 0.01, ***P < 0.001); number of experiments in parenthesis.
We tested whether release of purines in WM during nAChR activation is dependent upon nerve action potentials, which would be a requirement of release from motor neurons. The release of ATP in response to epibatidine was unaffected by TTX (P > 0.05), but the release of β-NAD+ was significantly inhibited by TTX (P < 0.001) (Fig. 1). These data suggest that release of β-NAD+ during activation of nAChR occurs at a site remote from the site of stimulation and requiring axonal action potentials for stimulus to couple to response. In contrast, the bulk of ATP released during activation of nAChR occurs from sites close to nicotinic receptors (e.g. nerve cell bodies).
We also tested the effects of an N-type voltage-dependent Ca2+ channel (VDCC) inhibitor, ω-Ctx GVIA, on purine release. ω-Ctx GVIA significantly depressed epibatidine-evoked release of β-NAD+ (P < 0.001), but did not affect the release of ATP (P > 0.05; Fig. 1). Therefore, release of β-NAD+ also required openings and influx of Ca2+ through N-type Ca2+ channels but this requirement was not present for release of ATP during nAChR activation. Overflow of metabolites, AMP and ADO, followed the general changes in β-NAD+ overflow and release of total purines was significantly reduced by both TTX (P < 0.05) and ω-Ctx GVIA (P < 0.05), suggesting that most of the AMP and ADO was due to metabolism of β-NAD+ (Fig. 1B) as previously suggested.11,12
DMPP, another nAChR agonist, also evoked release of ATP and β-NAD+ that was blocked by hexamethonium, but only the release of β-NAD+ was inhibited by TTX: release of β-NAD+ was reduced from 3.02 ± 1.1 fmol/mg (n=12) to 0.50 ± 0.28 (n=12, P < 0.05) and 0.54 ± 0.3 fmol/mg (n=14, P < 0.05) in the presence of hexamethonium and TTX, respectively. The DMPP-evoked release of ATP was reduced from 0.49 ± 0.16 (n=12) to 0.10 ± 0.06 fmol/mg in the presence of hexamethonium (n=12, P < 0.05).
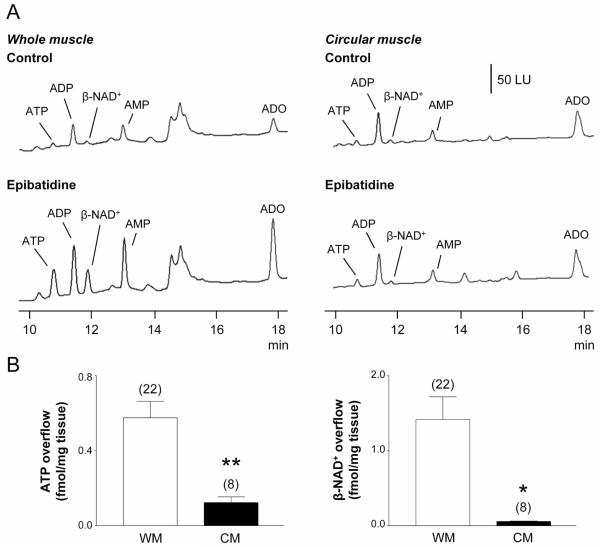
In the presence of TTX the DMPP-evoked release of ATP was 0.37 ± 0.09 (n=14) which was not significantly different from control release (P > 0.05). There have been reports of pre-synaptic nAChR at nerve terminals of motor neurons regulating neurokinin release.16,23 Activation of these receptors might enhance Ca2+ entry at nerve terminals and facilitate transmitter release independent of axonal action potentials.24 We tested the idea of pre-synaptic nAChR by stimulating CMs from monkey colon (i.e. muscles containing nerve terminals but no cell bodies; see11) with epibatidine. In contrast to WM (Fig. 1, Fig. 2) epibatidine evoked no additional release of purines in pure CMs (Fig. 2), demonstrating that activation of prejunctional nAChR does not contribute to the epibatidine-mediated purine release in WM.
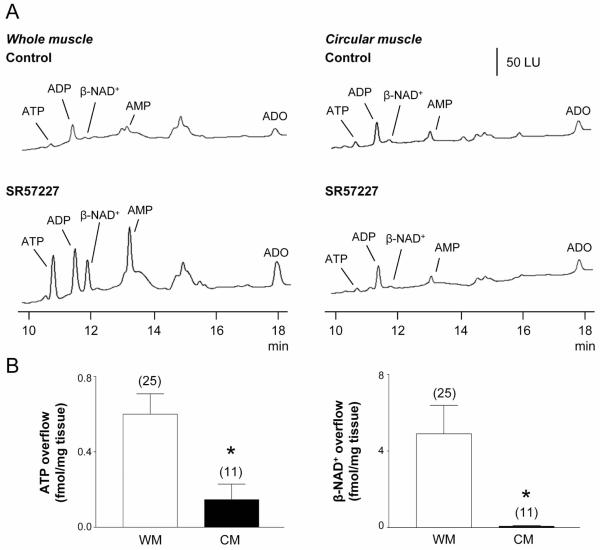
Fig. 2. Release of ATP and β-NAD+ during nAChR stimulation in whole muscle (WM) and circular muscle (CM) preparations of monkey colon.
(A) Chromatograms of tissue superfusates collected before (control) and during nAChR stimulation with epibatidine (500 μM, 30 s) in WM and CM preparations of monkey colon. Small amounts of ATP and β-NAD+ were present in WM and CM superfusates in the absence of agonist. Epibatidine evoked release of ATP and β-NAD+ in WM preparations, but not in CM preparations. Scale applies to all chromatograms. LU, luminescence units. (B) Averaged data are means ± SEM, showing epibatidine-evoked release of ATP (left) and β-NAD+ (right) from monkey WM and CM preparations. Overflow (femtomoles per milligram of tissue) is the overflow during nAChR activation less spontaneous overflow. Overflow of ATP and β-NAD+ was significantly less in CM preparations. Asterisks denote significant differences from WM release (*P < 0.05, **P < 0.01); number of experiments in parenthesis.
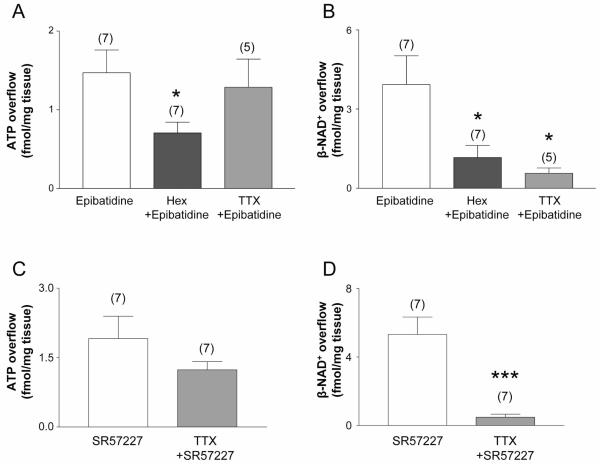
Finally, we examined the effects of epibatidine in mouse colon. Similar to monkey colon, epibatidine evoked release of ATP and β-NAD+ that was inhibited by hexamethonium (Fig. 3A,B). TTX significantly inhibited the epibatidine-evoked release of β-NAD+ (Fig. 3B), but did not affect ATP (Fig. 3A), suggesting that activation of nAChR in the mouse colon also evokes differential release of ATP and β-NAD+.
Fig. 3. ATP and β-NAD+ release during stimulation of nAChRs and 5-HT3Rs in mouse colon.
A and B show release of (A) ATP and (B) β-NAD+ during activation of nAChRs with epibatidine (500 μM, 30 s) in the absence and presence of hexamethonium (Hex, 500 μM for 30 min) or tetrodotoxin (TTX, 0.5 μM for 30 min) in mouse colon. Averaged data are means ± SEM. Overflow (femtomoles per milligram of tissue) is the overflow during nAChR activation less spontaneous overflow. Activation of nAChRs with epibatidine evoked release of ATP and β-NAD+ that was inhibited by the nAChR antagonist hexamethonium. The epibatidine-evoked release of β-NAD+, but not ATP, was reduced by TTX. Asterisks denote significant differences from epibatidine-evoked release (*P < 0.05); number of experiments in parenthesis. C and D show release of (C) ATP and (D) β-NAD+ during activation of 5-HT3 receptors with SR57227 (500 μM, 30 s) in the absence and presence of TTX (0.5 μM, 30 min) in mouse colon. Averaged data are means ± SEM. Overflow (femtomoles per milligram of tissue) is the overflow during 5-HT3 receptor activation less spontaneous overflow. The SR57227-evoked release of β-NAD+, but not ATP, was reduced by the neural blocker TTX (0.5 μM, 30-min perfusion). Asterisks denote significant differences from SR57227-evoked release (***P < 0.001); number of experiments in parenthesis.
Release of ATP and β-NAD+ elicited by activation of 5-HT3R
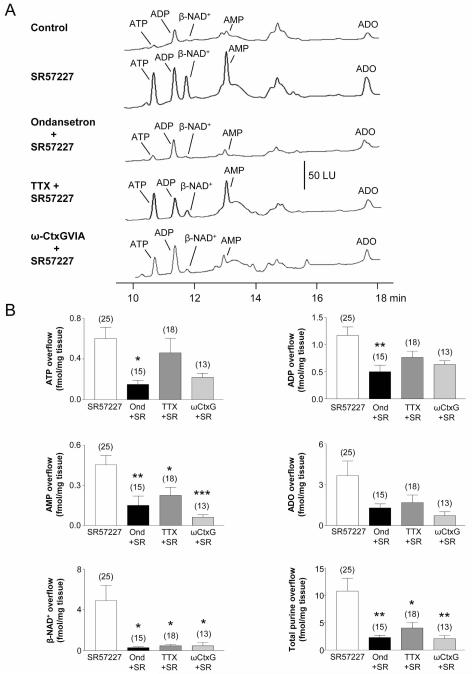
5-HT3Rs are also located on cell bodies of myenteric neurons and generate excitatory inputs to muscle motor neurons.18,25 Therefore, we also stimulated enteric neurons in monkey and murine colons with the selective 5-HT3R agonist, SR57227 (500 μM, 30 s).26 SR57227 stimulated release of ATP, β-NAD+, ADP, AMP and ADO from monkey WM preparations (Fig. 4A, B). The 5-HT3R antagonist ondansetron (10 μM) inhibited release of ATP and β-NAD+ evoked by SR57227 (P < 0.05; Fig. 4), confirming that release was mediated by 5-HT3R. HPLC fraction analysis of tissue superfusates demonstrated that β-NAD+ was the dominant substance forming the 11.2-min 1,N6-etheno-ADPR peak (11.2 min fraction contained ~83% β-NAD+, ~15.5% ADPR and ~1.5% cyclic ADPR). Release of β-NAD+ evoked by SR57227 was inhibited by TTX and ω-Ctx GVIA (P < 0.05 for both), but release of ATP was not affected (P > 0.05; Fig. 4). Therefore, 5-HT3R-mediated release of β-NAD+ required axonal action potentials to convey information between sites of stimulation and transmitter release. ATP release was unaffected by blocking Na+ channels and N-type VDCC. Release of AMP, ADO and total purines followed the same trend as β-NAD+, suggesting that most AMP and ADO in superfusates were derived from released β-NAD+ than from ATP (Fig. 4B).
Fig. 4. Purines release by stimulation of 5-HT3 receptors in monkey colon whole muscle (WM) preparations.
(A) Chromatograms of tissue superfusates collected before (control) and during activation of 5-HT3 receptors with SR57227 (500 μM for 30 s) in the absence and presence of ondansetron (10 μM, 30 min), tetrodotoxin (TTX, 0.5 μM, 30-min superfusion) or ω-conotoxin GVIA (β-CtxG, 50 nM, 30-min superfusion) in monkey WM. Small amounts of ATP, ADP, β-NAD+, AMP and ADO were present in superfusate samples in the absence of agonist. Stimulation of 5-HT3 receptors with SR57227 evoked additional release of purines that was inhibited by the 5-HT3 receptor antagonist ondansetron. SR57227-evoked release of β-NAD+, but not ATP, was reduced by the neural blockers TTX and ω-CtxG. Scale applies to all chromatograms. LU, luminescence units. (B) Averaged data are means ± SEM and show release of ATP, ADP, AMP, ADO, β-NAD+ and total purines (calculated as ATP+ADP+AMP+ADO+β-NAD+) during activation of 5-HT3 receptors with SR57227 (SR) and in the presence of ondansetron (Ond, 10 μM, 30 min), TTX (0.5 μM, 30 min), and β-CtxG (50 nM, 30 min). Overflow (femtomoles per milligram of tissue) is the overflow during 5-HT3 receptor activation less spontaneous overflow. Each peak was calibrated to individual etheno-derivatized purine standards. Asterisks denote significant differences from SR57227-evoked release (i.e. control release) (*P < 0.05, **P < 0.01, ***P<.001); number of experiments in parenthesis.
In contrast to WM, SR57227 failed to evoke release of purines from monkey CM (Fig. 5), indicating that SR57227-mediated release of ATP and β-NAD+ is not due to activation of receptors on nerve terminals within the CM.
Fig. 5. Release of ATP and β-NAD+ during 5-HT3 receptor stimulation in whole muscle (WM) and circular muscle (CM) preparations of monkey colon.
(A) Chromatograms of tissue superfusates collected before (control) and during 5-HT3 receptor activation with SR57227 (500 μM, 30 s) in WM and CM preparations of monkey colon. Small amounts of ATP and β-NAD+ were present in WM and CM superfusates in the absence of agonist. SR572227 evoked release of ATP and β-NAD+ in WM, but not in CM. Scale applies to all chromatograms. LU, luminescence units. (B) Averaged data are means ± SEM and show SR57227-evoked release of ATP (left) and β-NAD+ (right) from monkey WM and CM preparations. Overflow (femtomoles per milligram of tissue) is the overflow during 5-HT3 receptor activation less spontaneous overflow. Overflow of ATP and β-NAD+ was significantly less in CM preparations. Asterisks denote significant differences from WM release (*P < 0.05); number of experiments in parenthesis.
As in monkey colon, SR57227 evoked release of β-NAD+ and ATP from mouse colons (Fig. 3C,D). TTX inhibited the SR57227-evoked release of β-NAD+ (P < 0.001; Fig. 3B). The release of ATP showed a slight, but not significant, tendency of being reduced by TTX (P > 0.05; Fig. 3C).
We tested whether the release of ATP (or other purines) evoked by activation of 5-HT3R was dependent upon connexin or pannexin channels by exposing mouse colon muscles to carbenoxolone (100 μM for 35-50 minutes) prior to stimulation with SR57227. Carbenoxolone did not inhibit the release of ATP or β-NAD+; SR57227-evoked overflow of ATP was 1.91± 0.48 (n=7) and 1.82 ± 0.57 fmol/mg (n=3) before and in the presence of carbenoxolone, respectively (P > 0.05), and SR57227-evoked overflow of β-NAD+ was 5.32 ± 1.01 fmol/mg (n=7) and 4.54 ± 0.82 fmol/mg (n=3) before and in the presence of carbenoxolone, respectively (P > 0.05). In monkey colon epibatidine-evoked overflow of ATP was 0.57 ± 0.08 (n=22) and 0.46 ± 0.11 fmol/mg (n=8) before and in the presence of carbenoxolone, respectively (P > 0.05), and epibatidine-evoked overflow of β-NAD+ was 1.42 ± 0.3 (n=22) and 0.97 ± 0.16 fmol/mg (n=8) before and in the presence of carbenoxolone, respectively (P > 0.05).
DISCUSSION
We measured purines released in response to nAChR and 5-HT3R agonists. These receptors are localized on myenteric nerve cell bodies and dendritic projections of enteric neurons and mediate inputs to motor neurons from interneurons.16 We reasoned that stimulation of motor neurons from ganglion targets might provide an opportunity to clarify the purine(s) involved in motor neurotransmission. Activation of nAChRs and 5-HT3Rs evoked release of ATP and β-NAD+ in monkey and mouse colonic muscles, and release of β-NAD+, but not of ATP, was inhibited by the neurotoxins, TTX and β-Ctx GVIA. It has been previously reported that purinergic neurotransmission in colon is blocked by TTX and ω-Ctx GVIA.27,28,29,30,31 Thus, enteric inhibitory neurotransmission attributable to purines requires nerve action potentials and Ca2+ entry via N-type Ca2+ channels, and the release of β-NAD+ is consistent with these properties of motor neurotransmission. In contrast, ATP release in response to ganglionic stimulants was not affected by TTX and β-Ctx GVIA. Thus, it appears that ATP was released from ganglionic sources, possibly from the cell bodies of motor neurons. The source and mechanism of ATP was not investigated extensively because the goal of this study was not to elucidate the ganglionic sources of ATP, but to discriminate between sites of release for ATP and NAD+. Our findings make it very unlikely that ATP serves as a motor neurotransmitter in enteric inhibitory regulation of the colon and further clarify the role of NAD+10,11, or a metabolite12, as the purine motor neurotransmitter.
Previous studies have shown that stimulation of ganglionic nAChR and 5-HT3R elicits motor responses in GI muscles.32,33 nAChR and 5-HT3R are ligand-gated ion channels on cell bodies of myenteric interneurons and motor neurons that mediate fast postsynaptic excitatory potentials (fEPSPs) and activation of action potential.34,35 Binding of appropriate ligands increases open probabilities of nAChR and 5-HT3R channels, facilitating entry of cations (inward current) that depolarizes neurons and initiates action potentials. Action potentials propagate down axons, depolarize nerve terminals and activate N-type VDCC. Ca2+ entry into nerve terminals facilitates fusion of neuro-vesicles and transmitter release.22 Thus, neurotransmitter release initiated by somatodendritic ligand-gated ion channels would be expected to be inhibited by blockers of axonal action potentials and inhibitors of Ca2+ influx through VDCC at nerve terminals. Indeed we found that release of β-NAD+, but not ATP, satisfies these criteria for a motor neurotransmitter. Thus, our findings support previous studies probing post-junctional mechanisms of purinergic motor neurotransmission and suggest that β-NAD+ is a primary purinergic neurotransmitter in the colon (as depicted in Fig. 6).10,11
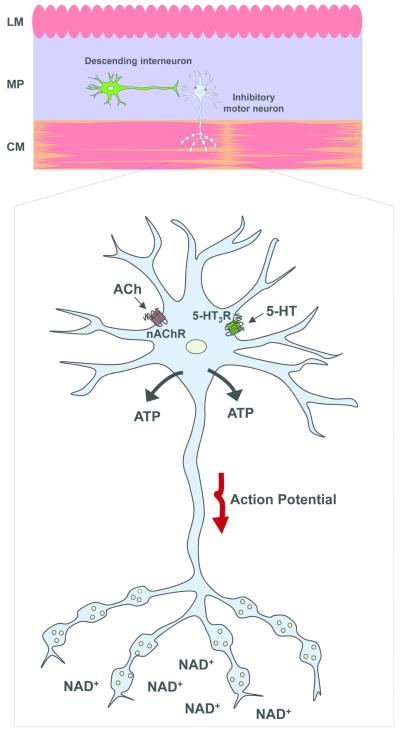
Fig. 6. Sites of release of ATP and β-NAD+ in response to stimulation of nAChR and 5-HT3R in colonic muscles.
ACh and 5HT released from descending interneurons in the myenteric plexus (MP) activate nAChR and 5-HT3R, respectively, on the cell bodies of inhibitory motor neurons and generates action potentials which propagate to nerve varicosities within muscle layers. β-NAD+ is released from nerve varicosities and serves as a primary enteric inhibitory motor neurotransmitter. ATP is released from ganglion sources (possibly nerve cell bodies) but not from nerve varicosities within muscle layers. LM, longitudinal muscle.
ACh binds to nAChRs on both excitatory and inhibitory motor neurons in the myenteric plexus. 34, 35 nAChR-mediated excitation of inhibitory motor neurons has also been demonstrated in human colon.36 We used epibatidine and DMPP in the present studies as ligands for nAChRs, and both agonists evoked release of ATP and β-NAD+. Epibatidine in particular, is a potent and highly selective nAChR agonist22 that activates neurons, but not glia, within myenteric ganglia in the colon.37
A subset of nAChRs has been reported at presynaptic nerve terminals where they modulate transmitter release.22,38,39 Direct stimulation of pre-synaptic nAChRs might cause transmitter release that is insensitive to TTX or ω-Ctx GVIA.38 Therefore, we tested the effects of epibatidine on pure CM preparations from which the myenteric plexus was removed.11 EFS has been shown to release β-NAD+ from CM,11 demonstrating that transmitter release mechanisms are intact in these muscles. Experiments on CM in the present study demonstrate that purine release is not coupled to pre-junctional nAChRs.
Serotonergic descending interneurons release both ACh and 5-HT16,40 and these interneurons appear to be important for exciting inhibitory motor neurons via nAChR and 5-HT3R and in producing tonic inhibition of CM.5 5-HT3Rs contribute to the inhibitory phase prior to propagation of a migrating motor complex. Antagonists of 5-HT3R and nAChR block spontaneous IJPs, suggesting that these receptors are expressed by inhibitory motor neurons that release purines.5 We examined this hypothesis directly in monkey and mouse colon by measuring purine release in response to a highly selective 5-HT3 receptor agonist, SR57227.26 SR57227 evoked release of β-NAD+, ATP, and their metabolites from monkey and mouse colonic WM, but failed to release purines from CM preparations. Therefore, 5-HT3Rs are likely localized to the nerve cell bodies as shown by immunohistochemistry studies.18
The assay techniques used in the present study cannot determine the exact population of enteric neurons that release purines in response to nAChR and 5-HT3R agonists. nAChR and 5-HT3R are present on cell bodies of descending interneurons and inhibitory motor neurons in rodent and human colon.5,17,18,36,39,41 Therefore, binding of these receptors could result in release of purines from either population of neurons. Interneurons release ATP as an excitatory neurotransmitter that can also mediate fEPSPs in motor neurons,42 but if interneurons were the main population of neurons activated by nAChR and 5-HT3R, then ATP release would have been inhibited by TTX.
nAChRs are also expressed by a variety of non-neuronal cells.43 Therefore, nicotinic agonists might cause ATP release from non-neuronal sources. A non-neuronal source is unlikely for β-NAD+, because neurotoxins abolished its release. Just as the source of ATP is unknown, so is the mechanism responsible for ATP release in colon muscles. ATP can be released into the extracellular space via several mechanisms in addition to vesicle exocytosis, including connexin hemichannels, pannexin channels, ABC transporters, P2X7 receptor pores, and volume-regulated channels.44, 45, 46, 47 We tested carbenoxolone, a blocker of connexin/pannexin channels, but this compound did not block release of ATP. Further studies are needed to clarify the mechanisms of ATP release from nerve cell bodies or other cells in the GI tract.
In summary, activation of ligand-gated ion channels (nAChRs or 5-HT3R), expressed by myenteric neurons in mouse and monkey colon, evokes release of purine nucleotides. β-NAD+ release, but not ATP release, was blocked by neurotoxins, supporting the role of β-NAD+ as a primary enteric inhibitory motor neurotransmitter. Our data suggest that ATP might function as a paracrine substance in ganglia but does not function as a motor neurotransmitter in the colon. We suggest that further studies of β-NAD+ synthesis, release and metabolism may provide unique opportunities to exploit this pathway for therapeutic regulation of colonic motility.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Deborah Russell and Charles River Preclinical Services for providing monkey tissue samples.
FUNDING
This work was supported by a NIH grant PO1 DK41315.
Glossary
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT3R
5-HT3 receptor
- ACh
acetylcholine
- ADP
adenosine 5′-diphosphate
- ADPR
ADP-ribose
- ADO
adenosine
- AMP
adenosine 5′-monophosphate
- ATP
adenosine 5′-triphosphate
- CM
circular muscle
- ω-Ctx GVIA
ω-conotoxin GVIA
- DMPP
dimethylphenylpiperazinum
- EFS
electrical field stimulation
- fEPSP
fast excitatory postsynaptic potential
- GI
gastrointestinal
- HPLC
high-performance liquid chromatography
- IJP
inhibitory junction potential
- L-NNA
NG-nitro-L-arginine
- β-NAD+
β-nicotinamide adenine dinucleotide
- nAChR
nicotinic acetylcholine receptor
- SK channels
small-conductance Ca2+-activated K+ channels
- TTX
tetrodotoxin
- VDCC
voltage-dependent Ca2+ channels
- WM
whole muscle
Footnotes
This project was presented previously at the Joint International Neurogastroenterology and Motility Meeting, 6-8 September 2012, Bologna, Italy (Mutafova-Yambolieva V, Durnin L, Sanders K. Release of β-NAD and ATP upon activation of myenteric 5-HT3 or nicotinic acetylcholine receptors in murine and primate colons. 2012; 24 (Suppl. 2):43:127).
DISCLOSURE
No competing interests declared.
AUTHOR CONTRIBUTION
LD performed experiments, analyzed results and participated in drafting the paper. KMS and VMY designed the experiments, interpreted results and wrote the paper. The entire work was performed at the Department of Physiology and Cell Biology of the University of Nevada School of Medicine.
Reference List
- 1.Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyster DJ, Bywater RA, Taylor GS. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology. 1995;108:1371–1378. doi: 10.1016/0016-5085(95)90684-3. [DOI] [PubMed] [Google Scholar]
- 3.Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst. 1998;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 4.Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK. The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G222–G232. doi: 10.1152/ajpgi.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2010;299:G144–G157. doi: 10.1152/ajpgi.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue L, Farrugia G, Sarr MG, Szurszewski JH. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- 10.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. PNAS. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. beta-Nicotinamide Adenine Dinucleotide Is an Enteric Inhibitory Neurotransmitter in Human and Nonhuman Primate Colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with beta-NAD(+) J Physiol. 2012;590:1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 14.Steele PA, Brookes SJ, Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45:227–239. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Galligan JJ. Synaptic activation and properties of 5-hydroxytryptamine(3) receptors in myenteric neurons of guinea pig intestine. J Pharmacol Exp Ther. 1999;290:803–810. [PubMed] [Google Scholar]
- 16.Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14:611–623. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 17.Mazzia C, Hicks GA, Clerc N. Neuronal location of 5-hydroxytryptamine3 receptor-like immunoreactivity in the rat colon. Neuroscience. 2003;116:1033–1041. doi: 10.1016/s0306-4522(02)00775-3. [DOI] [PubMed] [Google Scholar]
- 18.Kapeller J, Moller D, Lasitschka F, Autschbach F, Hovius R, Rappold G, Bruss M, Gershon MD, Niesler B. Serotonin receptor diversity in the human colon: Expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J Comp Neurol. 2011;519:420–432. doi: 10.1002/cne.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Analytical Biochemistry. 2002;305:269–276. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- 20.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. {beta}-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- 21.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of {beta}-Nicotinamide Adenine Dinucleotide upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. J Biol Chem. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- 22.Mandl P, Kiss JP. Inhibitory effect of hemicholinium-3 on presynaptic nicotinic acetylcholine receptors located on the terminal region of myenteric motoneurons. Neurochem Int. 2006;49:327–333. doi: 10.1016/j.neuint.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 23.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 24.Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]
- 25.Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 26.Bachy A, Heaulme M, Giudice A, Michaud JC, Lefevre IA, Souilhac J, Manara L, Emerit MB, Gozlan H, Hamon M. SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur J Pharmacol. 1993;237:299–309. doi: 10.1016/0014-2999(93)90282-m. [DOI] [PubMed] [Google Scholar]
- 27.Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979;282:415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- 28.Shuttleworth CW, Conlon SB, Sanders KM. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br J Pharmacol. 1997;120:707–713. doi: 10.1038/sj.bjp.0700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rae MG, Khoyi MA, Keef KD. Modulation of cholinergic neuromuscular transmission by nitric oxide in canine colonic circular smooth muscle. Am J Physiol. 1998;275:G1324–G1332. doi: 10.1152/ajpgi.1998.275.6.G1324. [DOI] [PubMed] [Google Scholar]
- 30.Gil V, gallego d, Grasa L, Martin MT, jimenez m. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G158–G169. doi: 10.1152/ajpgi.00448.2009. [DOI] [PubMed] [Google Scholar]
- 31.Bridgewater M, Cunnane TC, Brading AF. Characteristic features of inhibitory junction potentials evoked by single stimuli in the guinea-pig isolated taenia caeci. J Physiol. 1995;485:145–155. doi: 10.1113/jphysiol.1995.sp020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 1996;271:G849–G857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- 33.Borjesson L, Nordgren S, Delbro DS. DMPP causes relaxation of rat distal colon by a purinergic and a nitrergic mechanism. Eur J Pharmacol. 1997;334:223–231. doi: 10.1016/s0014-2999(97)01173-4. [DOI] [PubMed] [Google Scholar]
- 34.Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience. 1996;73:1029–1047. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- 35.Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol. 2005;208:2981–3001. doi: 10.1242/jeb.01732. [DOI] [PubMed] [Google Scholar]
- 36.Auli M, Martinez E, gallego d, Opazo A, Espin F, Marti-Gallostra M, jimenez m, Clave P. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol. 2008;155:1043–1055. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci. 2010;30:6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 39.Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390:497–514. [PubMed] [Google Scholar]
- 40.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Torocsik A, Oberfrank F, Sershen H, Lajtha A, Nemesy K, Vizi ES. Characterization of somatodendritic neuronal nicotinic receptors located on the myenteric plexus. Eur J Pharmacol. 1991;202:297–302. doi: 10.1016/0014-2999(91)90270-z. [DOI] [PubMed] [Google Scholar]
- 42.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 43.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 45.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8(3):359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutafova-Yambolieva VN. Neuronal and extraneuronal release of ATP and NAD(+) in smooth muscle. IUBMB Life. 2012;64:817–824. doi: 10.1002/iub.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]