Abstract
The abrupt discontinuation of prolonged benzodiazepine treatment elicits a withdrawal syndrome with increased anxiety as a major symptom. The neural mechanisms underlying benzodiazepine physical dependence are still insufficiently understood. Flumazenil, the non-selective antagonist of the benzodiazepine binding site of GABAA receptors was capable of preventing and reversing the increased anxiety during benzodiazepine withdrawal in animals and humans in some, but not all studies. On the other hand, a number of data suggest that GABAA receptors containing α1 subunits are critically involved in processes developing during prolonged use of benzodiazepines, such are tolerance to sedative effects, liability to physical dependence and addiction. Hence, we investigated in the elevated plus maze the level of anxiety 24 h following 21 days of diazepam treatment and the influence of flumazenil or a preferential α1-subunit selective antagonist βCCt on diazepam withdrawal syndrome in rats. Abrupt cessation of protracted once-daily intraperitoneal administration of 2 mg/kg diazepam induced a withdrawal syndrome, measured by increased anxiety-like behavior in the elevated plus maze 24 h after treatment cessation. Acute challenge with either flumazenil (10 mg/kg) or βCCt (1.25, 5 and 20 mg/kg) alleviated the diazepam withdrawal-induced anxiety. Moreover, both antagonists induced an anxiolytic-like response close, though not identical, to that seen with acute administration of diazepam. These findings imply that the mechanism by which antagonism at GABAA receptors may reverse the withdrawal-induced anxiety involves the α1 subunit and prompt further studies aimed at linking the changes in behavior with possible adaptive changes in subunit expression and function of GABAA receptors.
Keywords: elevated plus maze, βCCt, antagonism, benzodiazepines, physical dependence
1 Introduction
Benzodiazepines are reasonably safe and effective drugs in short-term treatment of different psychiatric and neurological disorders (anxiety, sleep disturbances, muscle spasms and seizure disorders). However, their protracted use remains debatable because it is associated with development of tolerance to some of their effects, liability for physical dependence and abuse potential. Abrupt cessation of prolonged benzodiazepine treatment is followed by a withdrawal syndrome, the main indicator of physical dependence. Benzodiazepine withdrawal has been characterized by many signs (eg, anxiety, insomnia and in more severe cases seizures) that are opposite to their expected therapeutic effects. Despite the half-century long clinical and experimental experience, the molecular and neurobiological mechanisms underlying the development of benzodiazepine dependence are still insufficiently understood (Dell’osso and Lader, 2012; Licata and Rowlett, 2008).
Non-selective benzodiazepines, such as diazepam, bind to GABAA receptors containing α1, α2, α3 or α5 subunits in addition to the γ2 subunit and allosterically modulate their activity. Therefore, it could be expected that alterations in GABAA receptor function provide possible mechanisms for the development of tolerance and physical dependence (reviewed in Bateson, 2002). A number of in vitro and ex vivo studies have suggested that abrupt withdrawal from the prolonged exposure to benzodiazepines results in expression changes of distinct GABAA receptor subunits (reviewed in Uusi-Oukari and Korpi, 2010). These changes could be associated with a decrease of postsynaptic GABA sensitivity (Gallager et al., 1984), or “functional uncoupling” between the recognition sites of benzodiazepines and GABA (Ali and Olsen, 2001; Hu and Ticku, 1994). More recently, it has been proposed that GABAA receptors containing α1 subunits (α1GABAA receptors) are critically involved in processes that underlie tolerance to the sedative effect, liability to physical dependence and addiction (Mirza and Nielsen, 2005; Tan et al., 2010; van Rijnsoever et al., 2004). However, the phenomena such as withdrawal and dependence liability are hardly explainable on the basis of the neuronal adaptations at the level of GABAA receptors only, and involvement of other neurotransmitters, such as glutamate and dopamine, has been also implicated (Allison and Pratt, 2003; Diaz et al., 2011).
Flumazenil is a non-selective antagonist of the benzodiazepine binding site of GABAA receptors. In previous studies, the administration of flumazenil after prolonged benzodiazepine treatment tended to exert an ambiguous influence on the anxiety level. Namely, while some experiments showed that flumazenil can precipitate withdrawal symptoms, there was also evidence that flumazenil is capable of preventing and reversing the increased anxiety during benzodiazepine withdrawal in animals and humans (File and Hitchcott, 1990; Hood et al., 2009; Licata and Rowlett, 2008). File and Hitchcott (1990) have proposed that the anxiety level of the subject determines the direction of the flumazenil’s influence on anxiety: when this is high, such as during benzodiazepine withdrawal, flumazenil would become anxiolytic; when this is low, flumazenil would increase anxiety. However, the receptor mechanism by which flumazenil affects anxiety during benzodiazepine withdrawal is still not established.
In laboratory animals, physical dependence to benzodiazepines may be observed as the emergence of characteristic symptoms, such as withdrawal anxiety, 12, 24 or 48 h upon abrupt cessation of the prolonged treatment (dos Santos et al., 2010; Licata and Rowlett, 2008). An anxiogenic response to benzodiazepine withdrawal in rodents is most commonly assessed using the elevated plus maze (EPM), and quantified by a decrease in the percentage of open arm entries and the percentage of time spent in open arms (dos Santos et al., 2010; File et al., 1987). Based on the proposed role of α1-containing GABAA receptors in alterations in GABA-ergic neurotransmission following prolonged exposure to benzodiazepines, and also the observed bidirectional effects of flumazenil, it is worth to investigate in parallel the influence of a non-selective antagonist (flumazenil) and a preferential α1-subunit selective antagonist (βCCt) on diazepam withdrawal syndrome. In the present study, we assessed in the EPM the level of anxiety 24 h following 21 days of diazepam treatment and examined the influence of flumazenil and βCCt on rat behavior during diazepam withdrawal. The doses of antagonists were chosen in accordance with our previous studies, which demonstrated that flumazenil applied in doses up to 20 mg/kg and βCCt applied in doses up to 30 mg/kg were behaviorally inactive on their own in the EPM test (Savić et al., 2004).
2 Materials and methods
2.1 Animals
Experiments were carried out on seventy four male Wistar rats (Military Farm, Belgrade, Serbia), weighing 180–200 g at the beginning of experiments. All procedures in the study conformed to EEC Directive 86/609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade. The rats were group-housed (six per cage) in transparent plastic cages with tap water and food pellets available ad libitum and kept in standard laboratory conditions. The temperature of the animal room was 22±1 °C, the relative humidity 40–70%, the illumination 120 lx, and 12/12 h light/dark period (light on at 6:00 h). All handling, daily administration of treatment and testing took place during the light period of the cycle, between 9:00 and 13:00 h.
2.2 Drugs
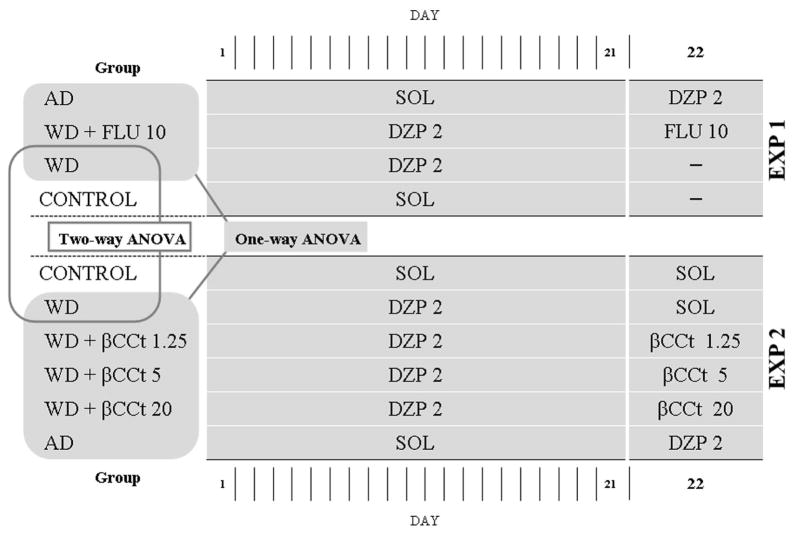
The compounds used in this study were diazepam (Galenika, Serbia), the non-selective GABAA antagonist flumazenil (Feicheng BoYuan Fine Chemicals Co., Ltd, China) and the preferential α1-subunit affinity selective GABAA antagonist βCCt (t-butyl-β-carboline-3-carboxylate), synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, USA. The compounds were dissolved/suspended in a solvent containing 85% distilled water, 14% propylene glycol, and 1% Tween-80 and were administered intraperitoneally (IP) in a volume of 1 ml/kg. 2.3 Experimental procedure Two separate experiments were performed, as illustrated in Fig. 1. In Experiment 1, the rats were randomly divided into four pre-treatment groups: two groups were repeatedly treated once daily with diazepam (2 mg/kg/day) and the other two groups received solvent IP, during 21 days. On the testing day (22nd day), 24 h after the last pre-treatment injection, the animals repeatedly treated with diazepam were acutely challenged with flumazenil 10 mg/kg or did not receive any treatment. The rats repeatedly treated with solvent during 21 days, received 2 mg/kg diazepam or did not receive any treatment on the testing day.
Figure 1.
The schematic presentation of experimental design and statistical analysis. AD –acute administration of diazepam; WD – diazepam withdrawal; FLU – flumazenil; SOL –solvent; DZP – diazepam; EXP – experiment.
In Experiment 2, another set of experimentally naïve animals was randomly distributed among six pre-treatment groups: four groups were repeatedly treated once daily with diazepam (2 mg/kg/day), and the other two groups received treatment with solvent during 21 days. On the testing day, 24 h after the last pre-treatment injection, animals repeatedly treated with diazepam were acutely challenged with either solvent or βCCt at a dose of 1.25 mg/kg, 5 mg/kg or 20 mg/kg. On the other hand, the animals repeatedly treated with solvent received either 2 mg/kg diazepam or solvent as a treatment.
2.4 Behavior in the EPM
The EPM apparatus was constructed of sheet metal, with a black rubber floor. It consisted of two opposed open arms (50 × 10 cm) with ledges (0.3 cm high) and two opposed enclosed arms (50 × 10 × 40 cm), connected by the junction area (10 × 10 cm). The whole apparatus was elevated 50 cm above the floor. Illumination in the experimental room was provided with one red neon tube fixed on the ceiling above the maze. Light intensity was 10 lx on the surface of the closed arms. Twenty minutes after administration of the appropriate treatment on the testing day, single rats were placed in the centre of the maze, facing one of the enclosed arms and were allowed to freely explore the apparatus during 5 minutes. The standard spatiotemporal variables were recorded and analyzed using the ANY-maze Video Tracking System software (Stoelting Co., Wood Dale, IL, USA) in accordance with our previous studies (Savić et al., 2004; 2010). The primary indices of anxiety were the percentage of open arm entries, the percentage of time spent on the open arms and time spent in the distal parts of the open arms. The parameters closely related to motor activity were the total distance travelled, number of total entries and closed arm entries. An entry into an open arm, closed arm, or the distal part of the open arm was scored when 90% of animal crossed the virtual line separating the neighboring zones, whereas an exit occurred when more than 90% of animal left the respective zone. The distal part of open arms was defined as the area of the most distant 30% of open arms. Additionally, time spent in risk assessment behavior, defined as exiting a closed arm with forepaws and head only, and investigating the surroundings, was scored by an observer blind to treatment assignments. The risk assessment behavior represents a behavioral dimension closely related to certain aspects of anxiety (avoidance of danger, decision making, approach/avoid conflict) (Cruz et al., 1994; Rodgers and Johnson, 1995).
2.5 Statistical analysis
All numerical data presented in the figures were given as the mean ± SEM. In order to validate the experimental model of withdrawal-induced anxiety in our laboratory settings, a two-way ANOVA with treatment (withdrawal groups and control groups) and experimental condition (Experiment 1 and Experiment 2) as factors were applied. The effects of the antagonists, flumazenil and βCCt, on diazepam withdrawal-induced anxiety were analyzed by two separate one-way ANOVAs, in which withdrawal groups (WD) and acute diazepam groups (AD) served as reference groups for anxiogenic and anxiolytic response, respectively. Groups included in each statistical analysis were depicted in the Figure 1. If the ANOVA was significant (P<0.05), post hoc Student-Newman-Keuls test (SNK’s test) was performed. The animals that fell from the EPM were excluded from data analysis. Statistical analyses were performed with ANY-maze software, where applicable, while SigmaPlot 11.0 (Systat Software Inc., Richmond, CA, USA) was used elsewhere.
3 Results
3.1 The effects of diazepam withdrawal on behavior in the EPM
Table 1 shows the effects of diazepam withdrawal on the anxiety-related and motor activity-related parameters in the EPM. The two-way ANOVA, with treatment group and experimental condition as factors, revealed significant effect of treatment on the percentage of open time (P=0.023). At the same time, decrease in percentage of open arm entries and the time spent in the distal parts of the open arms for groups withdrawn from diazepam compared to control groups was close to significant (P=0.085 and P=0.052, respectively). Time spent in risk assessment behavior tended to be greater in groups withdrawn from diazepam (P=0.075). The influence of treatment on the motor activity-related parameters (total distance travelled, total entries and closed arm entries) was not statistically significant. Neither experimental condition as factor nor interactions between factors were statistically significant.
Table 1.
The effects of diazepam withdrawal on behavior in the EPM and the accompanying two-way ANOVAs with treatment groups and experimental conditions as factors. Treatment groups were control (SOL) and diazepam-withdrawn (WD) groups, whereas experimental conditions were Experiment 1 and Experiment 2. Data are mean ± SEM; number of animals per treatment group was 6–8.
| Parameter | Experiment 1 | Experiment 2 | Two-way ANOVA
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group (SOL vs. WD) | Experimental condition (experiment 1 vs. experiment 2) | Interaction | ||||||||
|
| ||||||||||
| SOL | WD | SOL | WD | F(1,25) | P | F(1,25) | P | F(1,25) | P | |
| % open time | 43.50±3.34 | 28.86±6.48 | 46.67±8.16 | 31.25±6.70 | 5.85 | 0.023 | 0.20 | 0.659 | 0.01 | 0.951 |
| %open entries | 41.63±2.58 | 33.71±4.47 | 37.00±5.20 | 34.25±3.56 | 1.86 | 0.085 | 0.27 | 0.606 | 0.44 | 0.516 |
| Time in distal part of open arms (s) | 36.49±3.24 | 20.67±8.00 | 38.43±8.75 | 24.60±8.26 | 4.18 | 0.052 | 0.16 | 0.689 | 0.02 | 0.899 |
| Risk assessment behavior (s) | 33.50±6.70 | 49.91±7.43 | 39.78±13.70 | 62.05±12.61 | 3.45 | 0.075 | 0.78 | 0.385 | 0.08 | 0.781 |
| Total distance travelled (m) | 10.31±0.86 | 9.56±0.51 | 9.65±0.65 | 9.26±0.52 | 0.71 | 0.407 | 0.51 | 0.480 | 0.07 | 0.781 |
| Total entries | 12.88±0.95 | 11.43±0.43 | 11.50±0.85 | 10.88±0.72 | 1.75 | 0.197 | 1.52 | 0.228 | 0.28 | 0.604 |
| Closed entries | 7.50±0.63 | 7.57±0.57 | 7.17±0.60 | 7.25±0.80 | 0.01 | 0.910 | 0.23 | 0.633 | 0.01 | 0.933 |
3.2 Experiment 1
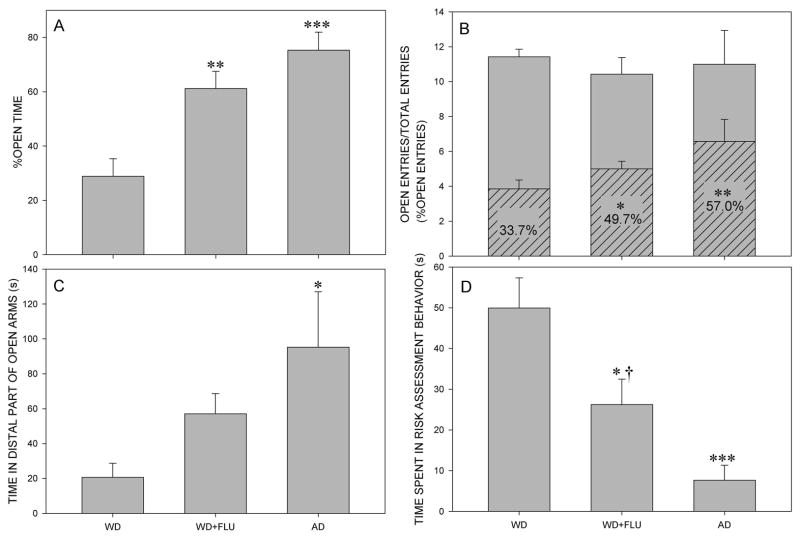
In regard to percentage of open time, the analysis showed a significant main effect of factor treatment (F(2,18)=13.34, P<0.001). Post hoc SNK’s test revealed that the percentage of open time was increased for groups WD + FLU and AD, in comparison with WD group (P=0.003 and P<0.001, respectively), as shown in Fig. 2A. The one-way ANOVA also revealed a significant main effect of treatment on the percentage of open arm entries (F(2,18)=6.38, P=0.008). Animals from WD + FLU and AD groups showed significantly higher percentage of open arm entries than animals from WD group (P=0.028 and P=0.007, respectively), as shown in Fig. 2B. The influence of treatment on the time spent in the distal part of open arms was nearly significant (F(2,18)=3,47, P=0.053) and post hoc SNK’s test showed a significant difference between animals from AD and WD group (P=0.018; Fig. 2C). Overall, there was a significant effect of treatment on the time spent in risk assessment behavior (F(2,18)=12.48, P<0.001). Post hoc comparison revealed a significant decrease in this parameter for WD + FLU and AD groups compared to WD group (P=0.012 and P<0.001, respectively). Moreover, animals from WD + FLU group spent significantly more time in risk assessment behavior than animals from AD group (P=0.042; Fig. 2D). In regard to motor activity-related parameters, one-way ANOVA did not reveal a significant influence of treatment on the total distance travelled (F(2,18)=0.32, P=0.729) (data not shown) and total entries (F(2,18)=0.16, P=0.857) (Fig. 2B). However, the influence of treatment on closed arm entries was significant, according to one-way ANOVA (F(2,18)=4.84, P=0.021). Post hoc test revealed that animals from AD group exerted significantly fewer closed arm entries compared to animals from WD group (P=0.018); this parameter could be observed in the Fig. 2B, as differences in total entries (gray bars) and open arm entries (hatched bars).
Figure 2.
The effects of diazepam withdrawal (WD), administration of 10 mg/kg flumazenil to diazepam withdrawn animals (WD+FLU) and acute administration of diazepam (AD) on the anxiety-related parameters. Graphs A, C and D represent the influence of treatment on the percentage of open time, the time spent in the distal parts of open arms and the time spent in risk assessment behavior, respectively. Graph B shows the effects of treatment on the percentage of open arm entries, indicated as the ratio of open arm entries (hatched bars) and total entries (open gray bars). * P<0.05, ** P<0.01, *** P<0.001 compared to the diazepam-withdrawn group (WD group); † P<0.05 compared to the acute diazepam group (AD group). The number of animals per treatment group was 7.
3.3 Experiment 2
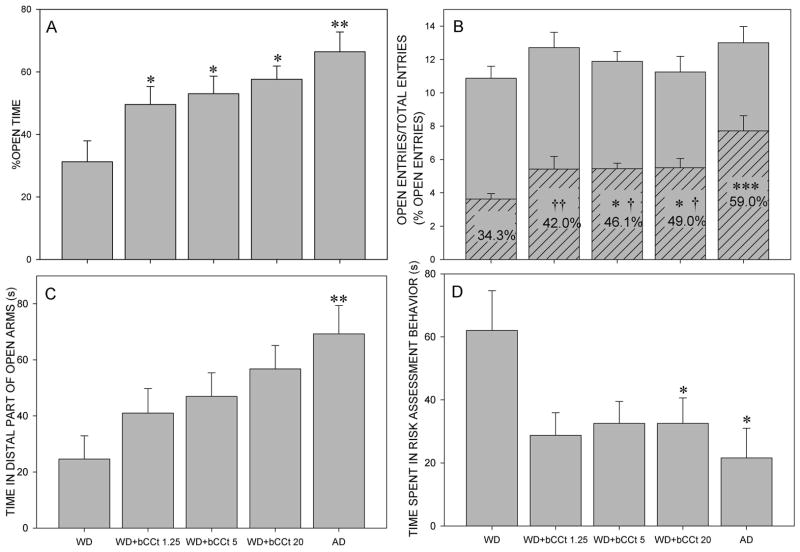
Considering the percentage of open time, the one-way ANOVA demonstrated a significant influence of treatment (F(4,34)=5.01, P=0.003). Post hoc test showed that for all the three groups withdrawn from diazepam and challenged with βCCt (WD + 1.25 βCCt, WD + 5 βCCt, WD + 20 βCCt), as well as for AD group, the percentage of open time was increased compared to WD group (P=0.032, P=0.031, P=0.014 and P=0.001, respectively; Fig. 3A). The influence of treatment on the percentage of open arm entries was also significant (F(4,34)=6.88, P<0.001). SNK’s test revealed that groups WD + 5 βCCt, WD + 20 βCCt and AD showed significantly higher percentage of open arm entries compared to WD group (P=0.049, P=0.021 and P<0.001, respectively). Simultaneously, the percentage of open arm entries was lower for all the three groups challenged with βCCt (WD + 1.25 βCCt, WD + 5 βCCt, WD + 20 βCCt) compared to AD group (P=0.007, P=0.03, P=0.046, respectively), as presented in Fig. 3B. The influence of treatment on the time spent in the distal part of open arms was significant (F(4,34)=3.53, P=0.016) and the AD group was different compared to WD group (P=0.009; Fig. 3C). In regard to the time spent in risk assessment behavior, there was also a significant effect of treatment (F(4,34)=2.88, P=0.037). Animals from the groups WD + 1.25 βCCt and WD + 5 βCCt tended to have the decreased time spent in risk assessment behavior in comparison with WD group (P=0.067 and P=0.072, respectively), while decreases of this parameter reached statistical significance for the groups WD + 20 βCCt and AD (P=0.028 and P=0.029 in comparison with WD group, respectively; shown in Fig. 3D). There was no influence of treatment on the analyzed motor activity-related parameters, namely total distance travelled (F(4,34)=0.76, P=0.561), total entries (F(4,34)=1,18, P=0.335) and closed arm entries (F(4,34)=2.02, P=0.113) (data not shown).
Figure 3.
The effects of diazepam withdrawal (WD), administration of 1.25 mg/kg, 5 mg/kg or 20 mg/kg βCCt to the diazepam withdrawn animals (WD+ βCCt 1.25, WD+ βCCt 5, WD+ βCCt 20) and acute administration of diazepam (AD) on the anxiety-related parameters. Graphs A, C and D represent the effects of treatment on the percentage of open time, the time spent in the distal parts of open arms and the time spent in risk assessment behavior. Graph B presents the effects of treatment on the percentage of open arm entries, indicated as the ratio of open arm entries (hatched bars) and total entries (open gray bars). * P<0.05, ** P<0.01, *** P<0.001 compared to the diazepam-withdrawn group (WD group); † P<0.05, †† P<0.01 compared to the acute diazepam group (AD group). The number of animals per treatment groups was 8, 7, 9, 8 and 7, for WD, WD+ βCCt 1.25, WD+ βCCt 5, WD+ βCCt 20 and AD group respectively.
4 Discussion
The withdrawal syndrome that occurs after abrupt discontinuation of chronic drug use is usually characterized by the emergence of a negative emotional state involving anxiety (Koob and Volkow, 2010). The EPM is a valuable animal model to study anxiety-like behavior that occurs during benzodiazepine withdrawal in rodents (File and Hitchcott, 1990). Using this paradigm, File and Andrews (1991) showed that repeated IP administration of 2 mg/kg diazepam, once daily during 21 days, could induce an anxiogenic response, measured 24 hours after the last dose of diazepam. In the present study, the similar pattern of anxiety-like responses in rats after diazepam withdrawal was observed in both experiments. Increased anxiety was indicated by the decrease in open arm exploration and nearly significant increase in the time spent in risk assessment behavior.
In order to validate the experimental model of withdrawal-induced anxiety, we opted to use a proper vehicle control in one, but not in the other experiment (Experiment 2 vs. Experiment 1). Namely, it is known that intraperitoneal saline injection in rodents affects core temperature and heart rate, indicating an acute stress response (Dilsaver and Majchrzak, 1990; Meijer et al., 2006), which may bias the outcome in an anxiety test. However, subtle differences between two experimental designs did not influence the animals’ behavior in our study, as there were no significant differences for factor experimental condition in the two-way ANOVA analysis for any of the observed parameters.
It has been postulated that benzodiazepine withdrawal affects several neuronal circuits. A close interrelationship between glutamatergic, dopaminergic and GABAergic neurons in key brain regions involved in the development of dependence and expression of anxiety is exemplified by findings that activation of α1GABAA receptors triggers synaptic plasticity in the mesolimbic dopamine pathway and mediates inhibition of glutamatergic projection neurons in basolateral amygdala (Heikkinen et al., 2009; Mcdonald and Mascagni 2004; Tan et al., 2010). Moreover, withdrawal after protracted benzodiazepine treatment leads to down-regulation of α1GABAA receptors and functional uncoupling between GABA- and benzodiazepine- binding sites (Hu and Ticku, 1994; Tietz et al., 1999). These data suggest that prolonged diazepam treatment and subsequent withdrawal may modulate neuronal circuits by adaptations at α1GABAA receptors. A major role of these receptors in processes underlying dependence liability has been supported by findings from pharmacological studies. Namely, SL651498, L-838,417 and TPA 023, the compounds which bind to and modulate the benzodiazepine-sensitive GABAA receptors, but are devoid of efficacy at the α1 subtype, are less prone to induce dependency (Ator et al., 2010; Griebel et al., 2003).
The results from Experiment 1 are in agreement with the finding that flumazenil is able to reverse the anxiety induced by withdrawal of diazepam in rats (File and Hitchcott, 1990). In the present study, acute challenge with flumazenil resulted in a significant increase of the percentages of open arm time and entries, as well as decrease of the time spent in risk assessment, compared to the rats subjected to withdrawal, but without challenge. Moreover, acute challenge with flumazenil induced an anxiolytic-like effect close, though not identical, to that seen with acute administration of diazepam. On the other hand, some previous reports indicated that flumazenil may precipitate a withdrawal syndrome in animals and humans (File and Hitchcott 1990; Kaminski et al., 2003; Mintzer et al., 1999). File and Hitchcott (1990) suggested that such precipitation of withdrawal with flumazenil may be expected when tolerance to the anxiolytic effects of benzodiazepines has not yet developed and when subsequent anxiogenic response to drug withdrawal on its own does not ensue. Although the tolerance to the anxiolytic effect has not been investigated in the present study, the observed anxiogenic response during withdrawal could be seen as predictive of flumazenil’s anxiolytic effect. Having in mind the data on adaptive changes at α1GABAA receptors in key brain regions during withdrawal (reviewed in Uusi-Oukari and Korpi, 2010), and the propensity of flumazenil to rapidly reverse the GABA subsensitivity and the decreased α1-subunit protein expression (Gallager et al., 1984; Tietz et al, 1999), we hypothesize that the antagonist’s ability to act through α1GABAA receptors may be responsible for the observed anxiolytic-like effect.
In Experiment 2, βCCt, a 20-fold selective antagonist at α1GABAA receptors (Cox et al., 1995), was employed to further investigate the contribution of these receptors to the processes underlying the emergence of the increased anxiety during diazepam withdrawal. The interpretation of the data is critically dependent on the degree to which the in vitro selectivity of βCCt is reflected in vivo. In mice dosed with 30 mg/kg of βCCt, the binding reduction of radiolabeled flumazenil followed the relative distribution of the α1-subunit, which revealed retained selectivity of βCCt binding (Griebel et al., 1999). Nevertheless, there was a study in mice suggesting that βCCt was able to antagonize the anxiolytic-like effects of chlordiazepoxide (Belzung et al., 2000), which are thought to be dominantly mediated by non-α1GABAA receptors (Smith and Rudolph, 2012). However, chlordiazepoxide significantly increased motor activity in the given experimental conditions in the EPM (Belzung et al., 2000), and such a finding is known to have a potential to confound the data interpretation, sometimes leading to ‘false positive’ results (Dawson and Tricklebank, 1995). On the other hand, in an experiment in rats, βCCt failed to antagonize the anxiolytic effect of chlordiazepoxide (Carroll et al., 2001). This suggests existence of species differences between rats and mice, which may contribute to explanation of discrepancies between the reported (Belzung et al., 2000) and present results and indirectly support that βCCt was selective for α1GABAA receptors in vivo in rats.
Acute challenge with βCCt, 24 hours after the last diazepam injection, led to a significant reduction of diazepam withdrawal-induced anxiety. The influence of βCCt on the anxiety-related parameters in the EPM was partly affected by the dose administered. Namely, while acute challenge with any of the three doses of βCCt (1.25, 5 and 20 mg/kg) significantly increased the percentage of open time compared to the diazepam-withdrawn group, only administration of the two higher doses significantly increased the percentage of open arm entries, whereas only the dose of 20 mg/kg induced a significant decrease in the time spent in risk assessment behavior. While the influence of acute challenge with βCCt on anxiety-related parameters during withdrawal was comparable, with subtle differences between parameters, to the effects of the nonselective antagonist flumazenil, it could be observed that the anxiolytic-like effects of acute administration of diazepam tended in both experiments to be somewhat more pronounced than after acute challenge with two antagonists. On the whole, these data imply that GABAA receptors in brain were altered 24 hr after termination of the protracted diazepam treatment, becoming susceptible to modulation by βCCt and flumazenil in a manner akin to the action of diazepam in experimentally naive rats. This hypothesis could be connected with findings of a decrease of α1-containing GABAA receptors in certain rodent brain regions, 24 hr after diazepam withdrawal (Impagnatiello et al., 1996).
In summary, the present study confirmed that abrupt cessation of protracted once-daily IP administration of 2 mg/kg diazepam could induce a withdrawal syndrome in rats, measured by increased anxiety-like behavior in the EPM paradigm 24 h after treatment cessation. Additionally, the study provided evidence that acute challenge with both, a non-selective antagonist flumazenil and a preferential α1-subunit selective antagonist βCCt, alleviates the diazepam withdrawal-induced anxiety, and even induces anxiolytic-like response in rats withdrawn from diazepam. These findings suggest a common mechanism by which flumazenil and βCCt may reverse the withdrawal-induced anxiety. In order to further elucidate the mechanism of alleviation of the benzodiazepine withdrawal syndrome, future research should be directed to the downstream changes induced by long-term activation and acute antagonism at GABAA receptors containing the α1 subunit. Finally, a closer examination of dose-response relationships with βCCt would be one of prerequisites needed for possible clinical testing of this selective antagonist following the withdrawal of benzodiazepines.
Highlights.
Flumazenil can either precipitate or prevent benzodiazepine withdrawal anxiety.
We found that it prevented anxiety-like behavior in diazepam-withdrawn rats.
The α1-subunit selective antagonist βCCt mimicked the effect of flumazenil.
Both ligands even induced anxiolytic-like response in rats withdrawn from diazepam.
βCCt may be beneficial in prevention/treatment of benzodiazepine dependence.
Acknowledgments
This work was supported in part by The Ministry of Science, R. Serbia – Grant No. 175076 (MMS) and by NIMH 46851 (JMC).
We acknowledge the support of this work by the Research Growth Initiative of the University of Wisconsin-Milwaukee and the Lynde and Harry Bradley Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2001;79:1100–8. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- Allison C, Pratt JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacology & Therapeutics. 2003;98:171–195. doi: 10.1016/s0163-7258(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8:5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Griebel G. Beta-CCT, a selective BZ-omega1 receptor antagonist, blocks the anti-anxiety but not the amnesic action of chlordiazepoxide in mice. Behav Pharmacol. 2000;11:125–31. doi: 10.1097/00008877-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Carroll M, Woods JE, II, Seyoum RA, June HL. The role of the GABA A1 subunit in mediating the sedative and anxiolytic properties of benzodiazepines. Alcohol Clin Exp Res. 2001;25:12A. [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Cox E, Hagen T, McKernan R, Cook JM. Bz1 receptor subtype specific ligands: synthesis and biological properties of βCCt, a Bz1 receptor subtype specific antagonist. Med Chem Res. 1995;5:710–8. [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilsaver SC, Majchrzak MJ. Effects of placebo (saline) injections on core temperature in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:417–22. doi: 10.1016/0278-5846(90)90029-g. [DOI] [PubMed] [Google Scholar]
- dos Santos L, de Andrade TG, Graeff FG. Social separation and diazepam withdrawal increase anxiety in the elevated plus-maze and serotonin turnover in the median raphe and hippocampus. J Psychopharmacol. 2010;24:725–31. doi: 10.1177/0269881109106954. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308:74–7. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- File SE, Andrews N. Low but not high doses of buspirone reduce the anxiogenic effects of diazepam withdrawal. Psychopharmacology (Berl) 1991;105:578–582. doi: 10.1007/BF02244384. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Aranko K. Anxiogenic effects in benzodiazepine withdrawal are linked to the development of tolerance. Brain Res Bull. 1987;19:607–10. doi: 10.1016/0361-9230(87)90079-7. [DOI] [PubMed] [Google Scholar]
- File SE, Hitchcott PK. A theory of benzodiazepine dependence that can explain whether flumazenil will enhance or reverse the phenomena. Psychopharmacology (Berl) 1990;101:525–32. doi: 10.1007/BF02244232. [DOI] [PubMed] [Google Scholar]
- Heikkinen AE, Möykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34:290–8. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (omega) receptor subtypes. Psychopharmacology (Berl) 1999;146:205–13. doi: 10.1007/s002130051108. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Depoortere H, Françon D, Avenet P, Schoemaker H, Evanno Y, et al. SL651498, a GABAA receptor agonist with subtype-selective efficacy, as a potential treatment for generalized anxiety disorder and muscle spasms. CNS Drug Rev. 2003;9:3–20. doi: 10.1111/j.1527-3458.2003.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S, O’Neil G, Hulse G. The role of flumazenil in the treatment of benzodiazepine dependence: physiological and psychological profiles. J Psychopharmacol. 2009;23:401–9. doi: 10.1177/0269881108100322. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Chronic benzodiazepine agonist treatment produces functional uncoupling of the gamma-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol. 1994;45:618–25. [PubMed] [Google Scholar]
- Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, Guidotti A. Modifications of gamma-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol. 1996;49:822–31. [PubMed] [Google Scholar]
- Kaminski BJ, Sannerud CA, Weerts EM, Lamb RJ, Griffiths RR. Physical dependence in baboons chronically treated with low and high doses of diazepam. Behav Pharmacol. 2003;14:331–42. doi: 10.1097/01.fbp.0000082131.08343.0e. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–46. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- Meijer MK, Spruijt BM, van Zutphen LF, Baumans V. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim. 2006;40:382–91. doi: 10.1258/002367706778476370. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stoller KB, Griffiths RR. A controlled study of flumazenil-precipitated withdrawal in chronic low-dose benzodiazepine users. Psychopharmacology. 1999;147:200–9. doi: 10.1007/s002130051161. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EØ. Do subtype-selective gamma-aminobutyric acid A receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther. 2006;316:1378–85. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Savić MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, Joksimović S, Clayton T, Sr, Ramerstorfer J, Milinković MM, Roth BL, et al. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–86. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugresić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav. 2004;79:279–90. doi: 10.1016/j.pbb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–74. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- van Rijnsoever C, Täuber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–90. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]