Abstract
Light-induced retinal damage (LD) occurs after surgery or sun exposure. We previously showed that zinc (Zn2+) accumulated in photoreceptors and RPE cells after LD but prior to cell death, and pyruvate or nicotinamide attenuated the resultant death perhaps by restoring nicotinamide adenine dinucleotide (NAD+) levels. We first examined the levels of NAD+ and the efficacy of pyruvate or nicotinamide in oxidative toxicities using primary retinal cultures. We next manipulated NAD+ levels in vivo and tested the affect on LD to photoreceptors and RPE. NAD+ levels cycle with a 24-h rhythm in mammals, which is affected by the feeding schedule. Therefore, we tested the affect of increasing NAD+ levels on LD by giving nicotinamide, inverting the feeding schedule, or using transgenic mice which overexpress cytoplasmic nicotinamide mononucleotide adenyl-transferase-1 (cytNMNAT1), an NAD+ synthetic enzyme. Zn2+ accumulation was also assessed in culture and in retinal sections. Retinas of light damaged animals were examined by OCT and plastic sectioning, and retinal NAD levels were measured. Day fed, or nicotinamide treated rats showed less NAD+ loss, and LD compared to night fed rats or untreated rats without changing the Zn2+ staining pattern. CytNMNAT1 showed less Zn2+ staining, NAD+ loss, and cell death after LD. In conclusion, intense light, Zn2+ and oxidative toxicities caused an increase in Zn2+, NAD+ loss, and cell death which were attenuated by NAD+ restoration. Therefore, NAD+ levels play a protective role in LD-induced death of photoreceptors and RPE cells.
Keywords: rat, mouse, sirtuin, NMNAT1
Introduction
Light-induced retinal damage can be a problem after acute or chronic sun exposure and surgery (Fuller et al., 1978; Zigman et al., 1979; Kuhn et al., 1991; Thanos et al., 2001; Codenotti et al., 2002; Jain et al., 2009; Vojnikovic et al., 2009). Excessive light may accelerate the loss of vision and serves as a physiologic model for human retinal degenerative diseases such as age-related macular degeneration (AMD) (Marc et al., 2008; Organisciak and Vaughan, 2010). Intense light damage is preferentially confined to outer nuclear layer (ONL) in the superior central retina of albino rats and mice. This damage involves apoptotic rod cell death and necrotic cone cell death (reviewed in Gordon et al., 2002; Organisciak and Vaughan, 2010).
Zinc toxicity has been shown to be related to retinal ischemia, transient global ischemia, trophic deprivation-mediated neuronal death and hypoglycemia (Koh et al., 1996; Suh et al., 2004; Yoo et al., 2004; Choi et al., 2006; Suh et al., 2008; Sheline et al., 2010b). Excessive Zn2+ either from extracellular Zn2+ uptake through voltage gated calcium channels or intracellular release from Zn2+ binding proteins or organelles are neurotoxic (Sheline et al., 2000; Sheline et al., 2010b). We previously showed zinc toxicity in retinal degeneration as a result of pathologic light exposure. Excess Zn2+ triggers NAD+ loss, and NAD+ loss in turn inhibits glycolysis and ATP generation. Pyruvate and nicotinamide, which restore NAD+ levels in CNS neurons (Sheline et al., 2000; Cai et al., 2006), can attenuate these zinc neurotoxicities in retina (Sheline et al., 2010a). In this study, we further investigated the role of NAD+ in LD by inverting the feeding schedule and using transgenic mice overexpressing NMNAT1.
NAD+ has been shown to be an energetic sensor to allow metabolic pathway adaptation to nutrient fluctuations. Fasting or caloric restriction increases NAD+ levels (reviewed in (Naimi et al., 2010)). It is also been shown that PARP-1, which uses NAD+ to synthesize ADP-ribose polymers, oscillates in synchrony with the feeding-fasting cycle (Asher et al., 2010). Therefore, the feeding cycle entrains NAD+ levels in peripheral tissues. In this study, we manipulated NAD+ by changing the feeding cycle, injecting nicotinamide, or overexpressing the NAD+ synthetic enzyme NMNAT1 and tested sensitivity to LD.
1. Research Design And Methods
1.1. Rat light damage model
Seven wk old Sprague-Dawley albino rats (Charles River, Wilmington, MA) weighing 150–175g were acclimated under a cyclic, dim overhead fluorescent light (30 Lux) for 5d. Rats were dark adapted for 60h before light exposure. Pupils were dilated with 1% tropicamide ophthalmic solution USP in room light and exposed to bright cool white fluorescent light from 8× 20W-circular fluorescent bulbs (18 kLux) for 4h (start at 8:30am). (Gordon et al., 2002). The animal chamber was periodically gently rotated during the light exposure to ensure that the animals were awake with their eyes open. This was followed by recovery in the dark for 24 h. After this, they were returned to cyclic, dim overhead fluorescent light environment for six days. 500mg/kg nicotinamide (immediately before LD) was injected intraperitoneally into rats fed ad libitum for testing retinal NAD+ levels. All studies were conducted within guidelines established by the Institutional Animal Care and Use Committee (Louisiana State University Health Sciences Center, New Orleans), and were in accordance with the PHS Guide for the Care and Use of Laboratory Animals, USDA Regulations, and the AVMA Panel on Euthanasia guidelines.
1.2. Mouse light damage model
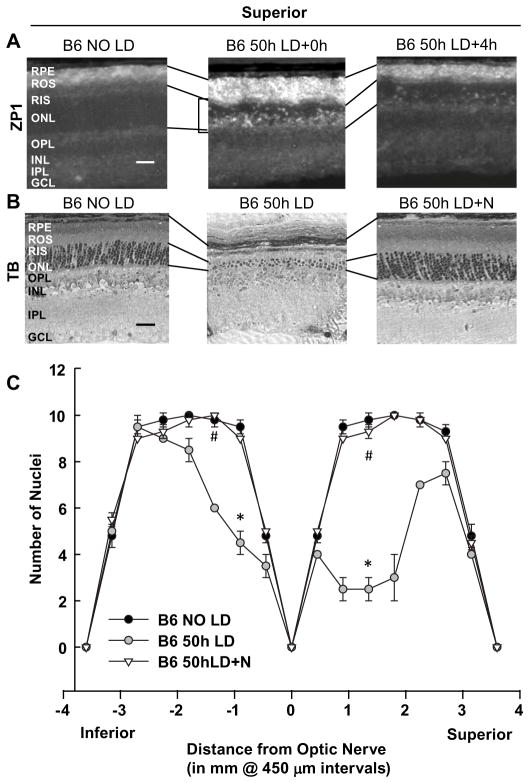
C57/BI6/J (Jackson Laboratories, Bar Harbor, ME, USA) and cytNMNAT1 mice which are on a C57/BI6/J background were acclimated for 5 days to a cyclic, dim overhead fluorescent light (30 Lux) followed by a 70h dark adaption. Pigmented mice contain melanin in their eyes which can protect the retina from irradiant absorption (Sanyal and Zeilmaker, 1988). In addition, the C57/BI6/J control and cytNMNAT1 transgenic mice in this study are on a RPE65Met450 background which are less susceptibility to light (Wenzel et al., 2001). Therefore, a longer exposure time starting in the evening was required to achieve the appropriate retinal damage in C57/BI6/J control mice. Pupils were dilated with 1% tropicamide ophthalmic solution USP under red light illumination every 12 hours, and then exposed to white fluorescent light from 8× 17W-straight fluorescent bulbs (70 kLux) for 50 h (start at 7:30pm). The animal chamber sits inside the tube of mirrored fluorescent bulbs, and was periodically gently rotated during the light exposure to ensure that the animals were awake with their eyes open. Purified diet gel (clear H2O, Portland, ME) was available during the light exposure to make sure the mice were well fed and hydrated. We showed previously that total dark maintenance potentiates light-induced Zn2+ accumulation and damage in rat photoreceptors (Sheline et al., 2010a). Therefore, after light exposure, mice were maintained totally in the dark for 7d until their retinas were examined. Mice were fed ad libitum, and food and water were changed daily under dim red light. LD in C57/BI6/J control mice showed similar ONL Zn2+ staining, death patterns, and efficacy of nicotinamide as in rats (Figure 3, and Sheline et al., 2010a).
Figure 3. Light induced Zn2+ accumulation and damage in C57/BL6/J mice were caused by 50h exposure to intense white light and prevented by nicotinamide.
A. 50h LD was performed on C57/Bl6 RPE65Met/Met mice and retinas were analyzed 0 and 4h after 50h of LD. Zn2+ accumulation (white regions) was assessed by ZP1 staining. Representative photomicrographs (n = 4) were taken of the mid superior regions of the retina at 0.2 second exposure. Notice the large increase in the number and intensity of Zn2+ stained cells in superior ONL (brackets), ROS, and RPE after LD exposure. B. Sister retinas were analyzed 7 days after LD by plastic sectioning, and staining with 0.1% Toluidine Blue. Photomicrographs were taken of the mid superior regions of the retina, and the lines show the alignment of retinal layers between panels. (N: Nicotinamide) C. ONL nuclei were counted at increasing distance from the optic nerve. * indicates a significant difference from control, and # indicates a significant difference from light damage at P < 0.05 by one-way ANOVA and a Student t-test. Layers are as marked. Bar represents 25 microns.
1.3. Day vs. night feeding
Day vs. night feeding started from the beginning of dark adaptation, and continued until euthanasia. For day fed rats, food was provided from 8:30am till 8:30pm. For night fed rats, food was provided from 8:30pm till 8:30am. Water was provided all the time for both of the two groups. After 60h dark adaptation, rats were exposed to intense light for 4h, followed by 24h total dark recovery. After this, rats were returned to cyclic, dim overhead fluorescent light environment for six days. Cyclic lights were on from 8:30am till 8:30pm. Food switches were under red dim light when dark adapted or under cyclic light and was continued until the retinas were examined on the seventh day post-exposure
1.4. Optical Coherence Tomography (OCT)
OCT is an optical signal acquisition and processing method providing extremely high-quality, micrometer-resolution, three-dimensional images from within optical scattering media (Spectralis, Heidelberg Engineering; Heidelberg, Germany) (Knott et al., 2011). On the seventh day after light damage, rats and mice were anesthetized with ketamine and xylazine and OCT was performed to measure the thickness of the mid superior outer nuclear layer (ONL). A real time eye tracker was used to couple confocal Scanning Laser Ophthalmoscopy (cSLO) and spectral domain-OCT (SD-OCT) scanners to position and stabilize the OCT scan on the retina. Scaling X was 3.24–3.31 μm/pixel; scaling Z was 3.87 μm/pixel. The built-in scale bar was used when performing OCT analysis. The thickness of the ONL was measured from the bottom edge of the outer plexiform layer to the top edge of the RIS at 3 points of the mid superior region and averaged.
1.5. Retinal Histology
On the seventh day, rats were sacrificed by CO2 asphyxiation. Eyes were fixed in 2% formaldehyde/2% glutaraldehyde, and cut in half along a superior-to-inferior meridian through the center of the optic nerve. After a one-hour fixation period in 1% osmium tetroxide and sequential dehydration in ethanol, eyes were embedded in plastic resin (Electron Microscopy Systems; Hatfield, PA). Retinal sections of 1.5 microns were cut, mounted on glass slides and stained with 0.1% toluidine blue for 2 mins. The number of ONL nuclei was counted on sections from 6 different retinas at increasing distances from the optic nerve on the superior-to-inferior meridian. Pictures were taken in the area of mid-superior hemispheres.
1.6. Retinal Zn2+ staining
Eyes of Sprague Dawley rats, cytNMNAT1 mice and C57/BI6/J control mice were collected 0–4h after light exposure under red light illumination. Fresh frozen cryostat sections (10 microns) were prepared, dried, and stained with the Zn2+ specific fluorescent dye, 5μM ZinPyr-1 (ZP1, TefLabs, Galveston, TX) for 2 min, washed with PBS, and photomicrographs were taken immediately using identical exposure times within each experiment as indicated (ex: 480nm; em: 530nm). There was no autofluorescence at this wavelength either basally or after light damage.
1.7. Primary retinal culture
Primary retinal cultures were generated from retinas (16 retinas/plate) of P2 mouse pups. Retinas were isolated and mechanically dissociated into single cells by trituration with fire-polished Pasteur pipettes. Triturated retinas were then plated in DMEM, 10% FBS, 1% glutamine, 0.1% P/S, 25mM KCL solution. Retinal cultures were grown in 5% CO2, 95% humidity in a 37°C incubator. Toxic exposures were initiated after 10 days of culturing. Cells were exposed to H2O2 in the presence of pyruvate, nicotinamide or NAD+. Cell viability was determined after 24h by adding propidium iodide (5 μg/ml) for 30 min at 37°C and fluorescence measured (ex 530/em 645).
1.8. Live-cell imaging
The Zn2+ specific dye FluoZin3 AM (5 μM; Invitrogen/Life Technologies, Carlsbad, CA) was loaded in the primary retinal culture for 30 min at 37°C and washed. PRC was exposed to 200 μM H2O2 for 1.5h. Photomicrographs of identical duration were then taken.
1.9. Determination of levels of NAD+
The effect that LD had on NAD+ levels in rat and mouse retinal tissues was determined at 0–24 h which was prior to the onset of cell death (Gordon et al., 2002). The effect that H2O2 had on NAD+ levels in PRC cultures was determined at 3hrs of H2O2 exposure. NAD+ measurements were made on retinal lysates from control and LD rats with or without nicotinamide treatment (intraperitoneal injection), and on control or cytNMNAT1 mice in 0.2N NaOH, 1mM EDTA lysates. This lysate was acidified followed by hydrolysis at 80°C for 20 min, neutralization and storage at −80°C. Acid hydrolysis destroys NADH allowing for determinations of NAD+ using the malate dehydrogenase/alcohol dehydrogenase cycling pair (Passonneau and Lowry, 1993). The NADH generated from malate was measured fluorimetrically (excitation at 365 nm, emission monitored at 460 nm) (Lin et al., 2001; Cai et al., 2006). The results obtained were compared with a calibration curve and normalized to protein content; replicate and duplicate reactions were highly reproducible.
1.10. Data analysis and statistics
Each experiment was performed with an n=6–12 from 2–3 experiments. Means ± SEM were plotted and analyzed for significance using a one-way ANOVA followed by a Student t-test with significance achieved by P< 0.05.
1.11. Reagents
All materials were purchased from Sigma Chemical Co. (Saint Louis, MO) unless otherwise stated.
2. Results
2.1. Oxidative stress (H2O2) induced [Zn2+]i increases in primary retinal cultures
Light induced damage has served as a human retinal degenerative disease model where light or oxidative injuries have been implicated. We therefore set parallel studies in vitro using H2O2-treated primary retinal cultures made from P2 CFW pups. After 10 days, cultures were preloaded with 5μM FluoZin3 AM for 30 mins and then washed out and exposed to 200 μM H2O2. There was an increase of [Zn2+]i after 1.5hrs as shown in figure 1A.. The specificity of the increase in [Zn2+]i was demonstrated using a zinc chelator, TPEN (data not shown). Pyruvate, nicotinamide or NAD+, which restored NAD+, do not affect [Zn2+]i in control or treated cultures, and do not bind Zn2+ (data not shown, and Sheline et al., 2000; Cai et al., 2006).
Figure 1. H2O2 exposure causes Zn2+ accumulation, toxicity, and NAD+ loss in PRC cultures.
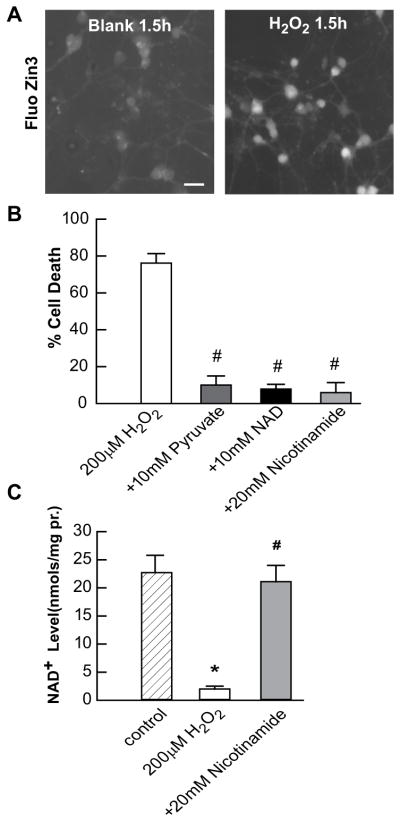
A. PRC cultures were pre-loaded with 5 μM FluoZin3-AM and exposed to 200 μM H2O2 as indicated. Photomicrographs were taken at equivalent exposures. B. Cell death was measured in sister cultures after exposure for 24 h as indicated using propidium iodide staining. C. NAD+ levels were measured in sister cultures after exposure for 3 h as indicated using enzyme-cycling reactions. * indicates difference from control, and # indicates difference from Zn2+ exposure at P < 0.05 by one-way ANOVA and a Student t-test, n>8.
2.2. Oxidative stress (H2O2) induced death of primary retinal cultures, which was attenuated by Zn2+ therapeutics
We have shown before that pyruvate, nicotinamide or NAD+ can protect 661W cone photoreceptors, Müller cells or ARPE-19 cells from H2O2 toxicity. Here we used primary retinal cultures. Similarly, pyruvate, nicotinamide or NAD+ attenuated primary cell death from oxidative stress as measured using 5μg/ml PI staining. The LD50 for H2O2 was 200 μM (data not shown). Chronic additions of 10mM pyruvate, 10mM NAD+ or 5mM nicotinamide were efficacious against 200 μM H2O2 (Figure 1B).
2.3. NAD+ levels in primary retinal cultures were reduced 3h after H2O2 toxicity, which could be restored by nicotinamide
Primary retinal cultures were stressed by 200 μM H2O2. Cells were collected after 3 hrs for NAD+ measurement, which is prior to cell death. NAD+ was reduced to 10% of control cells. Nicotinamide co-exposure restored NAD+ levels to 95% of control (Figure 1C).
2.4. Light-induced retinal damage was attenuated by feeding cycle inversion without changing the zinc staining pattern
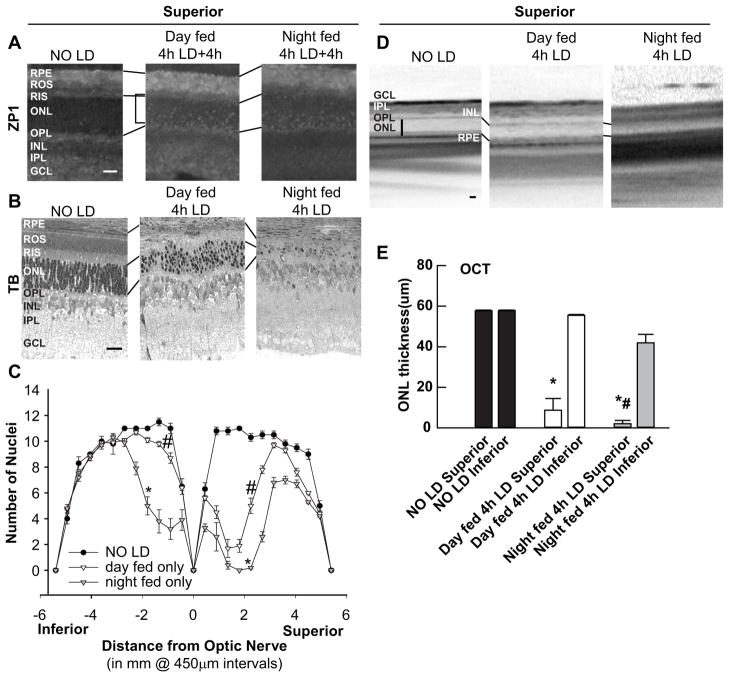
LD was performed on retinas from day fed vs. night fed albino rats and analyzed 4h (Zn2+, Figure 2A), or 7 days (death, Figure 2B–E) after 4h of intense light. Zn2+ accumulation (white regions) was assessed by ZP1 fluorescence in fresh frozen, dried cryostat sections. The number of photoreceptor nuclei was determined 7d after LD in littermate retinas and plotted as a spider graph. There was no significant change of zinc staining between day versus night fed animals. The retinal photoreceptor damage but not RPE cells, however, was attenuated in day fed rats. The mean thicknesses of mid-superior and inferior retinas determined by OCT were also plotted as bars.
Figure 2. Light-induced retinal damage was attenuated by feeding cycle inversion.
A. LD was performed on rats being fed a normal diet at the times indicated and analyzed 4 h after LD. Zn2+ accumulation was assessed by ZP1 staining (white regions). Photomicrographs of identical exposure were taken of the mid superior regions of the retina. B. Sister retinas were analyzed by plastic sectioning 7d after 4h of light damage. Eyes were cut along a superior to inferior meridian encompassing the optic nerve. Photomicrographs were taken of the mid superior regions of the retina. C. ONL nuclei were counted at increasing distance from the optic nerve, and were averaged and plotted as a function of distance from the optic nerve for each of the experimental conditions (n=6). D. Representative ONL images of central superior were taken from OCT. Vertical bar shows the distance measured. E. The mean thickness of OCT measurement in central superior and inferior hemispheres of the retina in microns. * indicates difference from control, and # indicates difference from light damage at P < 0.05 by one-way ANOVA and a Student t-test. Layers are as marked. Horizontal bar indicates 25 microns.
2.5. Light induced Zn2+ accumulation and damage in C57/BL6 mice were caused by a 50 h exposure to intense white light, and damage was attenuated by nicotinamide
LD was performed on retinas from C57/Bl6 RPE65Met/Met mice and analyzed 0 and 4 h (Zn2+, Figure 3A), or 7 days later (death, Figure 3B–C). Pigmented mice with RPE65Met/Met background are relatively resistant to light, therefore, a longer time with higher intensity (50h, 70klux) was required to get similar damage as in albino, light-sensitive animals. There was a substantial increase of zinc staining in specific layers of retina after 50h LD. Intraperitoneal injection of nicotinamide immediately before LD attenuated damage to both the RPE and photoreceptors as shown by visualization of plastic sections. The number of photoreceptor nuclei was determined 7d after LD and plotted as a spider graph.
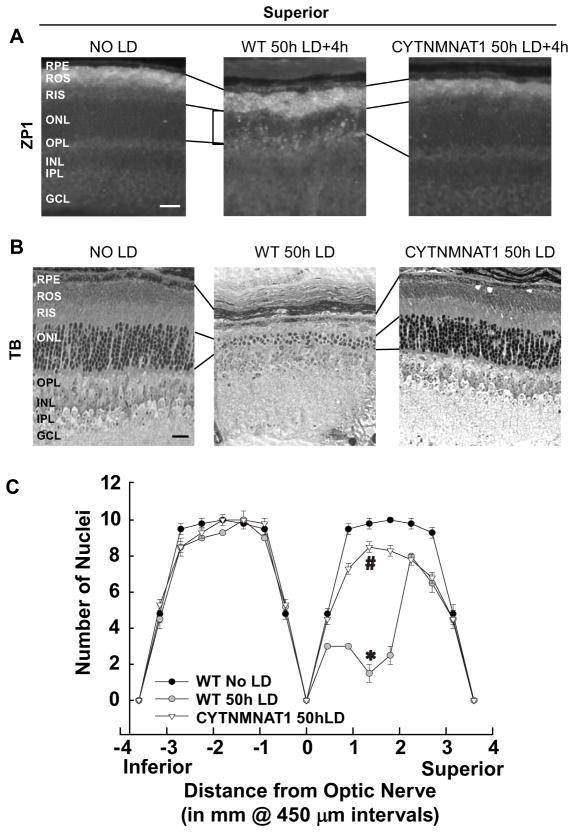
2.6. Light induced retinal damage was attenuated by cytoplasmic NMNAT1 overexpression
LD was performed on retinas from cytNMNAT1 mice in a C57/Bl6 RPE65Met/Met background. Zinc staining was examined 4h after 50h of light damage (Figure 4A). Cell death was analyzed 7 days after 50 h of light damage (Figure 4B–C). Overexpressing NMNAT1 attenuated retinal Zn2+ staining and cell death of RPE and photoreceptors from LD. The number of photoreceptor nuclei was determined 7d after LD and plotted as a spider graph.
Figure 4. Light-induced Retinal Damage was Attenuated by Cytoplasmic NMNAT1 Overexpression.
A. LD was performed on retinas from cytNMNAT1 mice in a C57/Bl6 RPE65Met/Met background and retinas analyzed for Zn2+ staining 4 h after LD. Zn2+ accumulation was assessed by ZP1 staining (white regions). Photomicrographs of identical exposure were taken of the mid superior regions of the retina. B. Sister retinas were analyzed 7 days (death) after 50 h of light damage. Photomicrographs were taken of mid superior regions of the retina. C. ONL nuclei were counted at increasing distance from the optic nerve. * indicates difference from control, and # indicates difference from light damage at P < 0.05 by one-way ANOVA and a Student t-test. Layers are as marked. Bar represents 25 microns.
2.7. NAD+ levels were reduced after LD, which could be attenuated by nicotinamide, day feeding, or cytNMNAT1 overexpression
Retinas from control and LD at different time points were isolated and lysates made for NAD+ determinations. There was a significant reduction of NAD+ in retinas which was maximal 4–20 h after LD in rat, and 12–24 h after LD in mouse, and this NAD+ reduction could be attenuated by nicotinamide. We showed previously that zinc accumulated most at 4h after LD in superior retinas of rat. The fluctuations of NAD+ levels and zinc staining after LD followed similar patterns. NAD+ was measured in retinas from day fed or night fed rats with or without LD. Although the basal level of NAD+ was lower in day fed rats, NAD+ was better preserved in day fed than in night fed rats. Retinas from wild type and cytNMNAT1 mice with or without LD were isolated and NAD+ measured. Retinas of cytNMNAT1 did not have significant NAD+ loss after LD, whereas WT mice had a significant reduction of NAD+ levels, similar to that seen in rat (Table 1).
Table 1.
LD induced a reduction of NAD+ which was attenuated by nicotinamide treatment, day feeding and NMNAT1 overexpression a.
| Conditions | NAD+ levels (nmols/mg pr.) |
|---|---|
| NO LD | 22.9±3.7 |
| 4h LD+0h | 17.2±3.4 |
| 4h LD+4h | 9.8±1.8 * |
| 4h LD+4h+Nicotinamide | 21.5±1.9 # |
| 4h LD+20h | 15.0±1.4 * |
| 4h LD+24h | 18.3±3.13 |
| 4h LD+24h+Nicotinamide | 43.5±5.9 *# |
| Day-fed NO LD (12:30pm) | 29.8±4.4 |
| Day-fed 4h LD+24h (12:30pm) | 25.2±2.9 |
| Night-fed NO LD (12:30pm) | 61.0±6.6 |
| Night-fed 4h LD+24h (12:30pm) | 33.9±8.4 * |
| WT-NO LD | 22.8±0.8 |
| WT-50h LD+24h | 12.4±3.4 * |
| CytNMNAT1-NO LD | 20.9±1.6 |
| CytNMNAT1-50h LD+24h | 25.4±3.6 |
Retinas from the indicated animals were used for NAD+ measurement (n=12).
indicates a significant difference from no LD control at P < 0.05, n=12.
indicates a significant difference from LD at P < 0.05, n=12.
3. Discussion
In this paper, we show that: 1) Oxidative stress (H2O2) induced an increase in Zn2+, a decrease in NAD+, and induced cell death in primary retinal cultures. Pyruvate, nicotinamide and NAD+ restored NAD+ levels and attenuated cell death, 2) NAD+ levels were reduced 4h after 4h LD in vivo, and nicotinamide injection restored NAD+ levels and attenuated LD to RPE and photoreceptors, 3) Light-induced retinal damage was attenuated by only feeding during the day, and 4) Overexpressing NMNAT1 attenuated Zn2+ staining and NAD+ loss and attenuated light damage to RPE and photoreceptors. We previously showed that intense light can induce early Zn2+ accumulation, specifically in severely damaged superior retinal layers, including RPE and photoreceptor cells, ROS, and RIS. This early (before cell death), preferential Zn2+ accumulation suggests that Zn2+ toxicity is involved in light-induced damage (Sheline et al., 2010a). Chronic or acute Zn2+ induces NAD+ loss resulting in glycolytic inhibition at GAPDH (increased levels of FBP/DHAP) in both neurons and glia, resulting in cell death. NAD+ restoration unblocked the inhibition of GAPDH, thereby allowing glycolytic flow from glyceraldehyde-3-phosphate to 1, 3-diphosphoglycerate and attenuation of cell death (Cai et al., 2006; Sheline et al., 2010b). [Zn2+]i increases and NAD+ decreases have also been shown in the rat global ischemia model, hypoglycemia, target deprivation, and other oxidative injuries (Koh et al., 1996; Suh et al., 2004; Suh et al., 2008; Sheline et al., 2010b; Sheline et al., 2013). Retina, especially photoreceptor neurons, requires high glucose-derived metabolic ATP production to survive (Winkler, 1981). Lack of ATP causes neuron degeneration and cell death (Nicholls, 2009; Chertov et al., 2011). The key glycolytic enzyme, GAPDH, has been shown to be a major protein associated with plasma membrane of photoreceptor outer segments (Hsu and Molday, 1990). Pyruvate, nicotinamide or NAD+ act as metabolic stimulants by maintaining NAD+, disinhibiting GAPDH and glycolysis, thereby attenuating retinal or neuronal cell death (Sheline et al., 2010a; Sheline et al., 2013). In this study, we have used different ways to reduce NAD+ loss, and thereby, attenuate retinal LD.
NAD+ levels were measured in primary retinal cultures and whole retinal lysates. We found that, NAD+ levels decreased 2–14h after oxidative stress or LD as the inverse pattern of the Zn2+ increase. Nicotinamide pretreatment restored retinal NAD+ levels, rescued downstream Zn2+ neurotoxicity and thereby attenuated retinal cell death both in vivo and in vitro. It has also been shown that oxidative stress can over activate PARP (Duan et al., 2007), causing NAD+ depletion and mitochondrial apoptosis-inducing factor release (Alano et al., 2010). Nicotinamide is not only a NAD+ precursor, it is also a PARP inhibitor, and it could restore mitochondrial functions (Klaidman et al., 2003). Direct administration of NAD+ has been shown to be beneficial in traumatic brain injury-induced neuron death (Won et al., 2012), brain ischemia (Zheng et al., 2012), and tumor necrosis factor-induced optic neuropathy (Kitaoka et al., 2009). The circulating blood supply of nicotinate crosses the blood-retinal barrier (BRB) through H+-monocarboxylate transporter (Tachikawa et al., 2011). These data make nicotinamide or NAD+ promising therapeutics in treating retinal or brain neurodegenerative diseases.
NAD+/NADH levels indicate cellular metabolic states. It is also an energetic sensor which induces certain metabolic pathways to fit the energetic demands. NAD+ levels are increased during fasting or caloric restriction (reviewed in (Naimi et al., 2010)). PARP-1 uses NAD+ to synthesize ADP-ribose polymers, and oscillations of PARP-1 activity are regulated by feeding signals (Asher et al., 2010). Light damage susceptibility is affected by circadian rhythm such that retinal damage was most severe when light exposure began during the dark (Vaughan et al., 2002). Dark-light circadian rhythm is the dominant cue for the central clock in the suprachiasmatic nucleus (SCN), but for peripheral tissues, the circadian clock is entrained by feeding and fasting, which is independent of SCN and the light cycle (Damiola et al., 2000; Stokkan et al., 2001). Therefore, the mechanisms modulating NAD+ levels during the feeding-fasting cycle which affect light damage susceptibility are different from the mechanisms that modulate the dark-light circadian rhythm which also affects light damage susceptibility. At the beginning of light exposure (8:30am) when the day-fed rats were fasting, NAD+ levels would be higher than that in night-fed rats. These higher levels of NAD+ at the start of LD protect day-fed rats from light damage. For our studies, NAD+ levels were measured at 12:30pm in all cases. By that time, NAD+ levels in no LD day-fed rats were lower than that in night-fed rats; but NAD+ levels were better preserved in day-fed rats. It should be noted that the Zn2+ staining patterns in these two groups after LD are similar, suggesting that the protective effect of day fed rats is not because of a reduction of Zn2+ accumulation but because of NAD+ preservation. The day time feeding cycle attenuated retinal cell death, and NAD+ loss suggesting NAD+ plays a role in LD.
It has been shown that mice overexpressing the NAD+ synthetic enzyme, nicotinamide mononucleotide adenyl transferase1 (NMNAT1), with a deletion of the nuclear localization signal resulted in cytoplasmic localization (cytNMNAT1) and increased efficacy against axonal degeneration (Sasaki et al., 2009). Compared to the wild type, the cytNMNAT1 transgenic mice preserved their NAD+ levels after LD and displayed a protective effect against intense light exposure. We noticed that cytNMNAT1 and nicotinamide not only protected photoreceptors from death, they also protected RPE from intense light exposure (Figures 3–4). But interestingly, cytNMNAT1 mice also showed less Zn2+ staining in RPE and ONL layers after light compared with wild type mice. A similar phenomenon was also observed in WLDs which overexpresses nuclear NMNAT1 and showed less Zn2+ staining and thalamic neuronal death after visual cortex ablation (Sheline et al., 2010b). NMNAT1 is also a pancreatic zinc binding protein, and is retained on zinc agarose columns from pancreatic lysates (in submission). Therefore, in addition to restoring NAD+ levels, NMNAT1 may also serve as a Zn2+ chaperone.
In conclusion, NAD+ plays an important role in Zn2+-mediated light induced retinal degeneration. Manipulations which restore NAD+ levels could protect retina from intense light exposure.
Oxidative stress (H2O2) induced an increase in Zn2+, a decrease in NAD+, and induced cell death in primary retinal cultures. Pyruvate, nicotinamide and NAD+ restored NAD+ levels and attenuated cell death.
Retinal NAD+ levels were reduced 4h after 4h LD, and nicotinamide injection restored NAD+ levels.
Light-induced retinal damage and NAD+ loss were attenuated by only feeding during the day.
Overexpressing NMNAT1 attenuated Zn2+ staining and NAD+ loss and attenuated light damage.
Acknowledgments
This work was supported by NIH grant DK 073446 (CTS), departmental grants from the Lions Eye Foundation and RPB, and by departmental funds.
We would like to thank Dr. Yongdong Zhou for help with the OCT measurements, and Dr. Jeffrey Milbrandt for providing the cytNMNAT1 mice, and helpful discussions.
ABBREVIATIONS
- TPEN
N,N,N′N′-tetrakis(−)[2-pyridylmethyl]-ethylenediamine
- NAD+
Nicotinamide adenine dinucleotide
- NMNAT1
Nicotinamide mononucleotide adenyl-transferase-1
- LD
light damage
- AMD
Age-related macular degeneration
- PARP1
Poly ADP-ribose polymerase 1
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- GCL
ganglion cell layer
- INL
inner nuclear layer
- ONL
outer nuclear layer
- IPL
inner plexiform layer
- OPL
outer plexiform layer
- ROS
rod outer segments
- RIS
rod inner segments
- [Zn2+]i
intracellular zinc concentration
- OS
oxidative stress
- ZP1
ZinPyr-1
Footnotes
Aspects of this paper were presented at the 2011 annual meeting of the Association for Research in Vision and Ophthalmology in Ft. Lauderdale, FL; and at the 2010 and 2011 Society for Neuroscience annual meetings in San Diego, CA, and Washington DC.
AUTHOR CONTRIBUTIONS
C.T.S. is the guarantor of this manuscript and had primary responsibility for research design and conduct, writing, and final content. S.B. designed and performed research, collected data, and revised and reviewed the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shi Bai, Email: sbai@lsuhsc.edu.
Christian T. Sheline, Email: csheli@lsuhsc.edu.
Literature Cited
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006;24:2169–2176. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- Chertov AO, Holzhausen L, Kuok IT, Couron D, Parker E, Linton JD, Sadilek M, Sweet IR, Hurley JB. Roles of glucose in photoreceptor survival. J Biol Chem. 2011;286:34700–34711. doi: 10.1074/jbc.M111.279752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim KA, Yoon YJ, Fujikado T, Joo CK. Inhibition of cyclooxygenase-2 expression by zinc-chelator in retinal ischemia. Vision Res. 2006;46:2721–2727. doi: 10.1016/j.visres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Codenotti M, Patelli F, Brancato R. OCT findings in patients with retinopathy after watching a solar eclipse. Ophthalmologica. 2002;216:463–466. doi: 10.1159/000067540. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–758. doi: 10.1113/jphysiol.2007.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D, Machemer R, Knighton RW. Retinal damage produced by intraocular fiber optic light. Am J Ophthalmol. 1978;85:519–537. doi: 10.1016/s0002-9394(14)75250-x. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2002;43:3511–3521. [PubMed] [Google Scholar]
- Hsu SC, Molday RS. Glyceraldehyde-3-phosphate dehydrogenase is a major protein associated with the plasma membrane of retinal photoreceptor outer segments. J Biol Chem. 1990;265:13308–13313. [PubMed] [Google Scholar]
- Jain A, Desai RU, Charalel RA, Quiram P, Yannuzzi L, Sarraf D. Solar retinopathy: comparison of optical coherence tomography (OCT) and fluorescein angiography (FA) Retina. 2009;29:1340–1345. doi: 10.1097/IAE.0b013e3181b0da88. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y, Hayashi Y, Kumai T, Takeda H, Munemasa Y, Fujino H, Ueno S, Sadun AA, Lam TT. Axonal and cell body protection by nicotinamide adenine dinucleotide in tumor necrosis factor-induced optic neuropathy. J Neuropathol Exp Neurol. 2009;68:915–927. doi: 10.1097/NEN.0b013e3181afecfa. [DOI] [PubMed] [Google Scholar]
- Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- Knott EJ, Sheets KG, Zhou Y, Gordon WC, Bazan NG. Exp Eye Res. England: 2010 Elsevier Ltd; 2011. Spatial correlation of mouse photoreceptor-RPE thickness between SD-OCT and histology; pp. 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kuhn F, Morris R, Massey M. Photic retinal injury from endoillumination during vitrectomy. Am J Ophthalmol. 1991;111:42–46. doi: 10.1016/s0002-9394(14)76894-1. [DOI] [PubMed] [Google Scholar]
- Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- Naimi M, Arous C, Van Obberghen E. Energetic cell sensors: a key to metabolic homeostasis. Trends Endocrinol Metab. 2010;21:75–82. doi: 10.1016/j.tem.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. Enzymatic Analysis A Practical Guide. Humana Press; NJ: 1993. [Google Scholar]
- Sanyal S, Zeilmaker GH. Retinal damage by constant light in chimaeric mice: implications for the protective role of melanin. Exp Eye Res. 1988;46:731–743. doi: 10.1016/s0014-4835(88)80059-9. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Zhou Y, Bai S. Light-induced photoreceptor and RPE degeneration involve zinc toxicity and are attenuated by pyruvate, nicotinamide, or cyclic light. Mol Vis. 2010a;16:2639–2652. [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Cai AL, Zhu J, Shi C. Serum or target deprivation-induced neuronal death causes oxidative neuronal accumulation of Zn2+ and loss of NAD+ Eur J Neurosci. 2010b;32:894–904. doi: 10.1111/j.1460-9568.2010.07372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Zhu J, Zhang W, Shi C, Cai AL. Mitochondrial Inhibitor Models of Huntington’s Disease and Parkinson’s Disease Induce Zinc Accumulation and Are Attenuated by Inhibition of Zinc Neurotoxicity in vitro or in vivo. Neurodegener Dis. 2013;11:49–58. doi: 10.1159/000336558. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Suh SW, Garnier P, Aoyama K, Chen Y, Swanson RA. Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol Dis. 2004;16:538–545. doi: 10.1016/j.nbd.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Murakami K, Martin PM, Hosoya K, Ganapathy V. Retinal transfer of nicotinate by H+-monocarboxylate transporter at the inner blood-retinal barrier. Microvasc Res. 2011;82:385–390. doi: 10.1016/j.mvr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S, Heiduschka P, Romann I. Exposure to a solar eclipse causes neuronal death in the retina. Graefes Arch Clin Exp Ophthalmol. 2001;239:794–800. doi: 10.1007/s004170100362. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–553. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vojnikovic B, Micovic V, Coklo M, Vojnikovic D. Sun exposure and visual field damage among children on the Adriatic Island Rab--possible initial risk factor in development of age-related macular degeneration. Coll Antropol. 2009;33:747–749. [PubMed] [Google Scholar]
- Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. J Neurosci. United States: 2001. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration; pp. 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Choi BY, Yoo BH, Sohn M, Ying W, Swanson RA, Suh SW. Prevention of traumatic brain injury-induced neuron death by intranasal delivery of nicotinamide adenine dinucleotide. J Neurotrauma. 2012;29:1401–1409. doi: 10.1089/neu.2011.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Lee JY, Lee SE, Koh JY, Yoon YH. Protection by pyruvate of rat retinal cells against zinc toxicity in vitro, and pressure-induced ischemia in vivo. Invest Ophthalmol Vis Sci. 2004;45:1523–1530. doi: 10.1167/iovs.03-1315. [DOI] [PubMed] [Google Scholar]
- Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012;512:67–71. doi: 10.1016/j.neulet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Zigman S, Datiles M, Torczynski E. Sunlight and human cataracts. Invest Ophthalmol Vis Sci. 1979;18:462–467. [PubMed] [Google Scholar]