Abstract
The timing and developmental factors underlying the establishment of language dominance are poorly understood. We investigated the degree of lateralization of traditional frontotemporal and modulatory prefrontal‐cerebellar regions of the distributed language network in children (n = 57) ages 4 to 12—a critical period for language consolidation. We examined the relationship between the strength of language lateralization and neuropsychological measures and task performance. The fundamental language network is established by four with ongoing maturation of language functions as evidenced by strengthening of lateralization in the traditional frontotemporal language regions; temporal regions were strongly and consistently lateralized by age seven, while frontal regions had greater variability and were less strongly lateralized through age 10. In contrast, the modulatory prefrontal‐cerebellar regions were the least strongly lateralized and degree of lateralization was not associated with age. Stronger core language skills were significantly correlated with greater right lateralization in the cerebellum. Hum Brain Mapp 35:270–284, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: language, fMRI, pediatric, lateralization
INTRODUCTION
It is well established in adult studies, across a variety of tasks, that language is supported by a typically left lateralized, frontal‐temporal functional network, with approximately 95% of right‐handed adults having left dominant language representation [Bookheimer, 2002; Cabeza and Nyberg, 2000; Springer et al., 1999; Szaflarski et al., 2002]. Children activate the same frontal‐temporal network [Gaillard et al., 2001; Hertz‐Pannier et al., 1997; Holland et al., 2001]; however, developmental differences include a less lateralized pattern and more activation of areas beyond the basic network, including, but not limited to, right homologues, midline and subcortical regions (e.g. caudate, cingulate). Understanding the developmental trajectory of the lateralization of language may provide insights into the functional plasticity of the developing brain that coincides with critical or “sensitive” periods when the brain is still able to adapt (Johnson, Developmental Psychobiology, 2005). Plasticity of the developing language network is evident in populations that compensate for early insult by reorganizing language contralaterally [Bates and Roe, 2001; Boatman et al., 1999; Chugani et al., 1996; Curtiss et al., 2001; Muller et al., 1998; Staudt et al., 2001]; however, the developmental trajectory for language lateralization in typically developing children is not fully characterized. In addition, the relationship between strength of lateralization and task performance remains unclear.
Although some studies of typically developing children suggest that lateralization continues to increase with age, possibly into early adulthood [Everts et al., 2009; Gaillard, 2009; Holland et al., 2001; Szaflarski et al., 2006a], other studies contend that lateralization is comparable to adults by age six or seven [Ahmad et al., 2003; Balsamo et al., 2002; Gaillard et al., 2003a, 2001; Wood et al., 2004]. The only longitudinal study of lateralization found an increase in left inferior frontal gyrus (IFG) and angular gyrus (BA 39), but not in Wernicke's Area (BA 22) [Szaflarski et al., 2006b], indicating that regional differences are important to consider. Although language studies demonstrate that better performance or cognitive skills are associated with activation of specific regions [Balsamo et al., 2006; Blumenfeld et al., 2006; Cone et al., 2008; Schlaggar et al., 2002; Turkeltaub et al., 2003] and greater left lateralization [Everts et al., 2009; Gaillard et al., 2007], other studies have not found any correlations among performance and laterality variables [Holland et al., 2001; Wood et al., 2004]. Thus, previous studies are mixed.
The differing conclusions of previous studies may be due to methodological challenges. We outline these issues and how the present study addresses them. One issue is having a sample that captures the full scope of the developmental trajectory. Several studies are limited by relatively small age ranges or, if the age range is large, each age is not accounted for in the sample or is represented by one child [Ahmad et al., 2003; Balsamo et al., 2002; Everts et al., 2009; Gaillard et al., 2003a; Wood et al., 2004]. The caveat is that results from these studies may be unduly influenced by outliers when correlating laterality with performance and age. With a restricted age range, a relationship with degree of lateralization may go undetected because of little variance. In contrast, a series of studies with a large cohort of over 300 typical children over a wide age range from 5 to 17 years old found age‐related differences [Holland et al., 2007; Plante et al., 2006; Szaflarski et al., 2006a]. This series, however, lack measurement of scanner task performance and thus, have the methodological drawback of being unable to account for performance in the analysis of their age‐related differences in lateralization [Holland et al., 2001, 2007; Szaflarski et al., 2006b]. Our study includes a broad age range—age 4 to 12 years old with multiple children at each age—while also considering task performance.
Task design is a challenge of conducting developmental functional studies with each approach offering advantages and disadvantages [Berl et al., 2006; Luna et al., 2010; Schlaggar et al., 2002]. One approach is to use a common task design, but age‐related performance differences are the drawback. One way to address performance differences is to account for performance by subset analyses with groups that are matched on task performance [Schlaggar et al., 2002]. However, the two disadvantages of the matched approach are: (1) it presumes that the effort a child puts forth to perform similarly to an adult is comparable and (2) views the child that can achieve such a strong performance as “normal” when it is more likely they are of above average on that cognitive skill. Statistical methods may also be used to equalize performance mathematically with age as a covariate [Booth et al., 2004; Cone et al., 2008]; however, a pitfall of this approach is that depending on the relationship of performance to the other variables of interest, the model may reduce variance and/or the power to detect differences. Another strategy is to use a task that is performed easily across a wide age range [Luna et al., 2001; Poldrack, 2000; Turkeltaub et al., 2003]; however, this often limits the complexity of the task which is problematic when studying functions such as language. Language is inherently more complex than single words and to conduct an ecologically valid study of language, it would be important to have the stimuli used in tasks to be sentence based; however, this engenders the difficulty of equating complexity across age.
In contrast to these performance‐adjusted approaches described above, we take the approach of adjusting the difficulty level of the task for specific ages [Berl et al., 2010]. In this study, we manipulate word frequency within the same syntactic frame thereby adjusting one level of linguistic complexity but leaving other variables—grammatical and syntactic complexity, constant. By design, the overall word frequency level is well within a child's ability by using only the top 5,000 most common words in print. For perspective, even linguistically disadvantaged children know 5,000 words by first grade [Dickinson and Neuman, 2006]. Within this corpus of words, the difficulty level is stratified across three age groups. With our developmentally‐adjusted approach, we aim to control for developmental differences in task performance via the design of our task.
The different tasks used across studies may also account for discrepant results in age‐dependent changes in lateralization because depending on the task, the burden on the language network may be weighted differentially [Holland, 2001]. For example, listening to stories burdens the temporal regions of the language network to a greater extent than a verbal fluency task that burdens the frontal regions of the language network to a greater extent. We know from structural studies that different cortical regions have different trajectories for anatomical development [Shaw et al., 2008; Sowell et al., 1999]; thus there may be different trajectories for functional development. We address this issue by selecting a task that activates both frontal and temporal regions reliably on an individual basis.
Previous studies calculate laterality for traditional frontal and temporal language regions [Gaillard et al., 2004; Holland et al., 2007]; however, regions traditionally considered part of the language network have expanded to include the cerebellum. Usually considered for its primary role in motor control, recent imaging studies observe a strong link between the dominant frontal language area and contralateral cerebellum using connectivity analyses and demonstrating parallel laterality between regions [Ackermann et al., 2007; Booth et al., 2007; Jansen et al., 2005; Lidzba et al., 2008]. In addition, neuropsychological data suggest that disruption of the right cerebellum is associated with impairments in verbal fluency and verbal working memory [O'Hare et al., 2008; Richter et al., 2007]. In determining our regions of interest, we selected the cerebellum as well as the traditional frontotemporal language areas to examine lateralization of the cerebellum with a language task, which to our knowledge has not been examined in a large sample of typical children.
This study investigates language processing in typically developing children using fMRI while employing methods designed to expand findings and address limitations of previous studies. We aim to contribute to the understanding of the developmental timing and regional specificity of how lateralization of the language network typically develops including investigating if the degree of laterality is indicative of better cognitive skills. Understanding of normal development is essential for proper interpretation of studies of disordered populations and would thereby increase our understanding of the impact of neuropathological disease processes on cognition. Our word definition decision task—although slightly modified from a previous published task—strongly activates the dominant perisylvian region as well as regions that have a role in language processing but are less often discussed, including midline regions, subcortical structures, and the cerebellum [Balsamo et al., 2002]. Moreover, task and neuropsychological performance were incorporated in design and analyses to provide ecological validity to functional imaging findings and allow investigation of the relationship between them and changes in lateralization. Our comprehensive neuropsychological evaluation extends previous findings regarding the question of whether greater lateralization is related to better cognitive performance. Comparisons of laterality across several regions of interest—including the cerebellum—were conducted to examine differences across age and by region. We hypothesize that greater lateralization will be associated with older children, better task accuracy, and better neuropsychological performance. Based on neuroanatomical development, we expect lateralization of frontal regions to be different across age from temporal regions, but we expect lateralization of IFG and the cerebellum to be associated. We also expect atypical language dominance to be uncommon, on the order of 5%, for typical children.
MATERIAL AND METHODS
Participants
Seventy‐four healthy, right‐handed children participated in the protocol. Handedness was established by items similar to the Edinburgh Handedness Inventory (EHI) [Oldfield, 1971] but items are child‐friendly and require demonstration rather than verbal response [Harris, 1974]. To control for the influence of handedness on language dominance, only children who had strong right‐handed dominance were included. No child demonstrated more than one item as left. Of the 74 children, 17 children were excluded for the following reasons: seven for movement during scanning (see below for criteria), seven due to questionable task engagement (poor task accuracy and/or no significant clusters of activation), two due to aborted scans because of a technical difficulty, and one child due to request to be removed from the scanner. Thus, 57 children were included (31 boys), ranging in age from 4 years, 2 months to 12 years, 11 months old (mean age 8.9 years, distribution of age within Fig. 3). Participants underwent a neurological examination by a pediatric neurologist and had no history of developmental, learning, neurological, or psychiatric disorders. All participants were fluent English speakers. Participants were recruited from the community through flyers, a widely distributed hospital newsletter, and prerecorded “on‐hold” message for the hospital. The study was approved by Children's National Medical Center Institutional Review Board, with informed consent provided by the parents, and verbal and/or written assent provided by all children before any study procedure.
Figure 3.

Scatter plot of mean LI value for each participant by age for inferior frontal gyrus and Wernicke's Area (P < 0.05).
Neuropsychological Testing
Subjects' intelligence and language skills were assessed in a separate session before scanning. Intelligence was assessed by standardized administration with an age appropriate measure; either the Differential Scales of Ability (DAS, ages 4–5) [Elliot, 1990] or the Wechsler Abbreviated Scale of Intelligence (WASI, ages 6–12) [Wechsler, 1999]. The IQ measures contain multiple subtests, providing a full scale IQ (general conceptual ability for the DAS), verbal IQ (verbal cluster), and performance IQ (nonverbal cluster). A range of expressive and receptive language skills were assessed with subtests from the Clinical Evaluation of Language Fundamentals (Preschool Version (CELF‐P, age 4)) [Wiig and Secord, 2004] or Fourth Edition (CELF‐4, ages 5–12) [Semel et al., 2003] that comprise the Core Language composite. These subtests include repeating sentences verbatim, following directions, formulating sentences, and finding the similarity between two words. The NEPSY Verbal Fluency [Korkman et al., 1998] is a measure of a child's ability to generate words to semantic and phonemic cues.
Functional MRI Paradigm
Preparation for imaging used a mock scanner to ensure that the children felt comfortable about all aspects of the scanning experience, to emphasize the importance of staying still, and to master task instructions. Subjects performed an auditory description decision task (ADDT), a word definition decision, as part of a larger battery of language tasks that were performed during the same scanning session. The paradigm consisted of alternating 30‐s blocks of experimental and baseline conditions. The task consisted of 10 blocks (five of each condition), with total scan time being 5 min. Individual stimuli were presented every 3 s for a total of 10 stimuli per block.
The experimental condition consisted of prerecorded sentences that either accurately or inaccurately describe a common, concrete noun embedded in the same syntactic frame (e.g. something you sit on is a chair, something you wear on your head is a flamingo). Subjects were asked to press the button of the MR compatible response box if the description of each object was accurate. The task was designed with 70% of the items as targets requiring a button press to be correct, while 30% of items were foils. With the same syntactic frame, the linguistic variable that was manipulated was the vocabulary used in the sentences which was designed to be age‐appropriate based on word frequency normative data derived from children's reading materials [Carroll et al., 1971]. Three difficulty levels were developed: the easiest level contained only words that occur in the top 2,500 most common words of the corpus (U‐value >28.4) and was administered to children ages 4 to 6; the medium level contained words in the top 3,500 most common words of English (U‐value >17.8) and was administered to children ages 7 to 9, and the most difficult level contained words in the top 5,000 words in the corpus (U‐values >10) and was administered to children ages 10 to 12. Analyses are conducted using these three age groups (youngest (n = 13), middle (n = 20), oldest (n = 24)) as a categorical variable.
The baseline condition consisted of reverse speech created by reversing the sentences and appending some items with a tone. Subjects were asked to listen to the “silly talk” and indicate via button press when they heard a tone. Selection of a baseline task has methodological relevance when calculating a laterality index [see Seghier, 2008 for review]. The baseline condition was designed to match the experimental condition for primary audition, motor response, length of utterance, and volume of presentation. The two tasks differ on suprasegmental (i.e., prosodic) aspects, which may invoke differences in the right hemisphere, typically in the right temporal region [Bookheimer, 2002]; however, evidence suggests that prosodic differences are lessened when the linguistic information is not apparent [Behrens, 2004], which would apply in our design because the reverse speech is not intelligible. To ensure that the baseline condition was not more right lateralized than the experimental condition, we examined the comparison of our baseline > task, and the group map had no significant clusters in the ROIs used in the study—baseline has more activation in midline at the posterior cingulate.
Image Acquisition
Auditory stimuli were presented using Windows compatible E‐prime software version 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA). All stimuli were presented to subjects through MR compatible headphones which also facilitated communication between the subject and MR technician and reduced in‐scanner noise. Subjects' task performance (i.e., accuracy, reaction time) was monitored during the scan via a button press that was held in the left hand. Accuracy was computed using both correct hits and correct withholds, such that 100% was defined as always correctly pressing the button when required and not pressing the button when not required. Recorded reaction times were not absolute times, as the length of the cue was also included in the value; however, as cue length was a constant for each subject, any differences reflect true subject variability.
Functional data were acquired using a 3.0T Siemens Magnetom Trio equipped with a standard CP head coil. Blood oxygen level‐dependent (BOLD) changes were measured using a whole brain EPI sequence with parameters: TR = 3,000 ms, TE = 30 ms, FoV = 192 mm, and voxel size = 3.0 × 3.0 × 2.8 mm. Whole brain volumes consisted of 50 axial slices of 2.8 mm thickness and with 0.2 mm between slices. Following functional scans, anatomical images of participants were also collected using a sagittal T1 MPRAGE sequence, slice thickness of 1.0, TR of 1,600 ms and TE of 3.37. Axial images were collected parallel to the anterior commissure‐posterior commissure plane, which served as an origin of reference.
Functional MRI Data Analysis
Image data preprocessing and group analysis was performed using Statistical Parametric Mapping software (SPM8) (University College London, London) and the Statistical Analysis Toolbox through Matlab (The MathWorks, Inc; Natick, MA). Images were reconstructed and realigned, normalized to the MNI standard anatomical space, and then spatially smoothed using an 8 mm full width at half maximum Gaussian kernel and temporally filtered (high‐pass filter: 128 s). Regarding normalization, other groups find little difference in regional anatomic topography (several millimeters) in young children [Burgund et al., 2002]. In addition, our approach in this study is less sensitive to inaccuracies in spatial normalization than a voxel‐by‐voxel statistical analysis because a lateralization index (LI (see definition below)) is our dependent variable and we use large anatomic ROIs. Nonetheless, as a precaution, we utilized the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra Toolbox [DARTEL, Ashburner, 2007] to create a pediatric template based on our sample. Although cluster size was smaller and the coordinates for the peak magnitude was slightly different—but always within the same region—the results remained the same. Therefore, the normalization procedures do not account for the functional differences we see between age groups. We therefore report the results with normalization to the standard MNI space. Individual t‐maps were generated by comparing the experimental and baseline conditions on a voxel‐wise basis with movement parameters as covariates of noninterest. Individuals were removed from analysis if they moved more than a voxel (3 mm in any direction) on average during the scan. A group map was generated from individual activation maps using a random‐effects model to obtain a whole brain activation map and confirm that the expected network of brain regions was activated during the task.
Strength and Categorization of Language Dominance
Laterality index (LI) reflects the strength of laterality and is computed by totaling the number of active voxels in the right and left hemisphere and comparing the voxels active in each hemisphere using the basic formula:
Values range from 1 to −1, with higher positive values indicating left lateralization. Several methodological factors can influence LI calculations [for review see Seghier, 2008], and our approach addresses many of these issues. LI was calculated using the LI Toolbox bootstrap analysis option that yields an LI value by repeated resampling of the data, which yields a mean of all possible LI values [Wilke and Lidzba, 2007]. This option combats the effect of unstable LI values when a single threshold is chosen to determine if a voxel is activated [Wilke and Schmithorst, 2006]. In the majority of cases, we report the “weighted mean” of LI values, which is equal to the arithmetic mean of all possible LI values. In two cases, the range of possible LI values was greater than 1, suggesting a lack of homogeneity in the distribution. In those cases, we reported the “trimmed mean” of LI values because the possible LI values vary greatly [Wilke and Schmithorst, 2006]. The trimmed mean yields a mean for only the middle 50% of all possible LI values. LI was calculated for regions of interest (ROI) described in detail below. Cerebellum LI values were reversed given the expected contralateral activation to more accurately assess for differences in degree of lateralization.
Given that language lateralization is often a skewed population because the majority of any population is has left LI values, categorical distribution of language dominance was also used to examine developmental trends and for making comparisons with the distribution found in adults. We categorized regional language dominance as: left hemisphere (LH) dominance defined by LI values >0.2, right hemisphere dominance defined as LI values <−0.2, and bilateral representation as LI values between −0.2 and 0.2 [Binder et al., 1995; Gaillard et al., 2002; Pujol et al., 1999]. Given that the number of right and bilateral language in normal populations are often small, right and bilaterally dominant patterns were combined into a single category of “atypical” dominance for statistical analyses. We also examined whether individuals were consistent in their language dominance across regions.
Regions of Interest
We based our ROIs on anatomical areas that are well established as primary contributors to language processing and validated invasively using the intracarotid amytal procedure (IAP) and electrocortical stimulation [Gaillard et al., 2004; Hamberger et al., 2001]. We chose to use anatomical ROIs as there may be variability in location of activation related to development and eventually want to use this method with patient populations which we know have variability in activation that often lies at the margins of the normal group map [Rosenberger et al., 2009, Mbwana et al., 2009]. The disadvantage of functional ROIs is that they generally encompass a smaller area and may fail to capture this variability in activation that may lie outside the group map—thus impacting LI calculations. We first utilized an inclusive ROI as we are investigating the range of possible activation. ROIs were defined by anatomic designations using the Wake Forrest PickAtlas [Maldjian et al., 2003]. They included: (1) Anatomic Label for Inferior Frontal Gyrus (IFG), (2) Anatomic Label for Middle Frontal Gyrus (MFG), (3) Wernicke's Area (WA, as defined by Brodmann's areas 21, 22, and 39) Anatomic Label for Cerebellum.
Statistical Analyses
Descriptive statistics were used to characterize demographic, neuropsychological, task performance (task accuracy, baseline reaction time, experimental task reaction time), and LI variables. To examine the relationships among these variables the appropriate parametric and nonparametric two‐tailed correlation analyses (depending on the nature and distribution) were conducted. Partial correlations and regression analyses were also conducted to determine the independent contributions of age and performance in explaining variance. Age was examined both as a linear variable as well as a categorical variable of age group. Age group was based on the task level that was given comprising three groups: youngest: 4‐ to 6‐year olds, middle: 7‐ to 9‐year olds, and oldest: 10‐ to 12‐year olds. A 3 (age group) × 4 (ROI) repeated measures multivariate analysis of variance (MANOVA) was conducted to test for differences in LI values related to age and ROI with follow‐up post hoc analyses for pair‐wise comparisons. ROI and age were independent variables to match hypothesis driven analyses. Because cerebellum LI values were expected to be right lateralized, thus inverse values were utilized for the MANOVA. Given the strong correlation among neuropsychological scores (r = 0.51–0.59, P < 0.001), a composite verbal skills score was derived by transforming each verbal intelligence and language score to a z score and averaging them. This composite that by principal components factor analysis accounted for 61% of the variance, reduced the number of variables used to determine if verbal abilities accounted for variability in lateralization, allowing for a statistically conservative approach. Post hoc analyses examined the sensitivity of individual tests. χ2 analysis was utilized to test for differences in distribution of language dominance across age groups and compared with the expected adult rate of 5% atypical language representation in IFG and WA [Pujol et al., 1999; Springer et al., 1999; Szaflarski et al., 2002]. For the cerebellum, one study with adults reported that 58% of controls had right cerebellum dominance, which is the rate we used for that comparison [Szaflarski et al., 2002].
RESULTS
Laterality Index and Language Dominance
As expected, the group was on average left lateralized for cortical regions and right lateralized for the cerebellum (see Table 1). This was also true based on categorical language dominance (see Fig. 1) where the majority of children were left dominant (LI > 0.2) for IFG (80.7%), WA (87.7%), and MFG (68.4%), and right dominant (LI < −0.2) for the cerebellum (75.4%). The incidence of atypical language representation in children ages 4 to 12 was higher than typical adult rates (P < 0.001) across IFG (19.3% vs. 5%) and WA (12.3% vs. 5%), but lower comparable to the one published report for adults for the cerebellum (24.6% vs. 42%). Similar to previous work [Plante et al., 2006], no significant differences were identified based on gender for activation for any of the ROIs or lateralization. Gender was also not a factor for task performance or neuropsychological scores and thus was not entered as a variable on subsequent analyses. However, lateralization results were influenced by region and age.
Table 1.
Mean and standard deviations of LI and neuropsychological and task performance for the sample and by age group
| Measure | Total sample, mean (SD) (N = 57) | 4–6 y.o., youngest (N = 13) | 7–9 y.o., middle (N = 20) | 10–12 y.o., oldest, (N = 24) |
|---|---|---|---|---|
| Gender | 28 males (49.1%) | 5 males (38.5%) | 10 males (50%) | 13 males (54.2%) |
| IFG LI | 0.54 (0.44) | 0.34 (0.43)a | 0.48 (0.56) | 0.71 (0.25) |
| WA LI | 0.62 (0.29) | 0.45 (0.35)b | 0.65 (0.31) | 0.68 (0.21) |
| MFG LI | 0.38 (0.42) | 0.32 (0.39) | 0.39 (0.46) | 0.41 (0.42) |
| Cerebellum LI | −0.41 (0.47) | −0.30 (0.47) | −0.39 (0.47) | −0.50 (0.41) |
| Full Scale IQ (SS) | 116 (14) | 116 (10) | 121 (16) | 112 (13) |
| CELF Core Language (SS) | 114 (11) | 119 (12) | 112 (9) | 114 (12) |
| NEPSY verbal Fluency (ss) | 12 (3) | 11 (3) | 12 (3) | 13 (3) |
| Composite score (z) | 0.98 (0.75) | 0.94 (0.72) | 1.05 (0.76) | 0.94 (0.77) |
| Accuracy (%) | 83 (14) | 78 (16) | 82 (14) | 87 (14) |
| Response time (ms) | 964 (376) | 900 (507) | 973 (392) | 997 (262) |
| Baseline reaction time (ms) | 530 (434) | 704 (167)a | 581 (422) | 405 (503) |
SS = Standard score (normative mean = 100, SD = 15); ss = scaled score (normative mean = 10, SD = 3); z = z score (normative mean = 0, SD = 1).
P < 0.05, different from oldest group only.
P =0.06, different from oldest group only.
Figure 1.
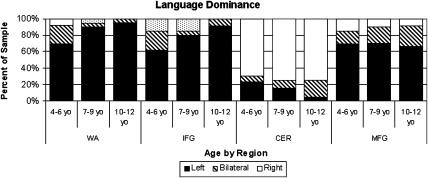
Distribution of categorical language dominance across age group and by region of interest. IFG = inferior frontal gyrus, WA = Wernicke's Area, CER = cerebellum, MFG = middle frontal gyrus.
Region
Repeated measures MANOVA revealed differences in lateralization by region (F = 8.39, P < 0.001; see Fig. 2). Post hoc comparison revealed that WA was the most left lateralized region with the least amount of variability among LI values (P < 0.05). IFG was the next strongest lateralized region IFG, although it was not significantly different from WA or the cerebellum but differed from MFG (P < 0.05). MFG was the least lateralized region, but did not differ significantly from the cerebellum.
Figure 2.
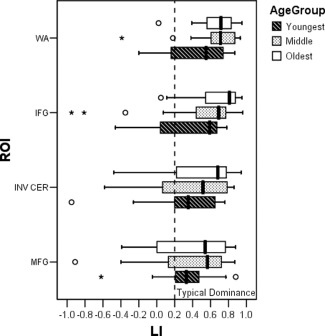
Clustered box plots of mean LI values for region of interest (ROI) (WA = Wernicke's Area, IFG = inferior frontal gyrus, INV CER = cerebellum with inversed LI values, MFG = middle frontal gyrus), clustered by Age group. Significant main effects of ROI and age group.
Age
Repeated measures MANOVA revealed a trend for differences in lateralization by age group (F = 3.07, P = 0.06). Post hoc testing revealed that the oldest children were more left lateralized than the youngest children (P < 0.05), while the middle age group was not significantly different from either age group.
Age by region
Although the interaction between age and region was not significant, post hoc testing suggested that lateralization differences related to age differed by region. Specifically, IFG and WA showed age related changes (P < 0.05 and P = 0.06, respectively), while no differences across age linearly or by age group were evident for MFG or the cerebellum (P > 0.35). There was also significantly more variability in LI values for the youngest age group for IFG and WA while variability was similar among all ages for MFG and the cerebellum. Linear regression showed that age accounted for similar amounts of variance in regional LI for IFG (R 2 = 0.10, P < 0.05, see Fig. 3) and WA (R 2 = 0.08, P < 0.05, see Fig. 3). Post hoc analyses revealed that for both WA and IFG, the age‐related changes were primarily driven by the youngest age group as there were no significant differences among the two older groups but there was a significant difference in lateralization between the oldest and youngest groups for IFG (P < 0.05) and a trend for WA (P = 0.06).
Similarly, there was a trend for differences in distribution of language dominance by age for IFG (see Fig. 1; P = 0.09) and WA (P = 0.06). For IFG, the youngest age group had the highest rate of atypical language dominance (38.5%) followed by the middle age group (20%) and the oldest children (8.3%). For WA, the youngest age group continued to have the highest rate of atypical language representation (30.8%), compared with the middle age group (10%), and oldest age groups (4.2%). Although across all ages, there was less atypical language for WA than for IFG. The distribution also revealed that there was more bilateral language representation at the younger ages for IFG and WA, but older groups were more likely to be definitively left or right dominant. In contrast, for MFG and CER, bilateral representation across ages was not significantly different but for MFG, the oldest group had more bilateral representation for the cerebellum.
Language dominance consistency
We examined individual differences by reporting percentages of children who had consistent language dominance across traditional language regions. Thirty‐five children (61.4%) had complete agreement among the IFG, WA, and cerebellum regions; all had left lateralized IFG, left lateralized WA, and right lateralized cerebellum. More children had agreement for two regions with 45 children (78.9%) showing agreement between WA and IFG; 41 children (71.9%) showing agreement between WA and the cerebellum; and 40 children (70.2%) showing agreement between IFG and the cerebellum. There were age group differences (P < 0.05). The majority of children in the older age groups (70% for both groups) were consistent in their lateralization across three regions while a minority of the youngest children showed consistency (31%); this pattern was most striking when looking at consistency between IFG and WA as 87.5% of the oldest and 85% of the middle age groups were consistent compared with 53.8% of the youngest group.
Of the 22 children who had inconsistency in their language dominance across regions, there were two instances of a diaschisis where IFG and WA had opposite lateralization. Ten children had consistency between IFG and WA, but the cerebellum was either bilateral or did not have the expected contralateral dominance. Nine children had consistency between one cortical region and the cerebellum and either IFG (n = 5) or WA (n = 4) was bilateral or had opposite laterality. The remaining case had right IFG, bilateral WA, and right cerebellum.
Neuropsychological Performance
As expected for a study of typical children, performance across all standardized neuropsychological measures was in the average range or better with comparable mean scores across measures (see Table 1). The range of abilities spanned from the low end of the average range to well above average (IQ range 88–156). There were no significant differences in neuropsychological performance across age for this cohort. Comparison of neuropsychological skills between children who were consistently lateralized across regions to those who were not revealed no significant differences and no differences in verbal skills were found for groups with atypical language representation in any area or across regions (P > 0.10). The composite measure of verbal skills was not significantly correlated with LI across any ROI for the whole group, but was sensitive in the youngest age group (see below). The only measure that had some specificity was that better core language skills on the CELF‐4 which was correlated with greater right lateralization in the cerebellum (r = −0.29, P < 0.05). There was also a trend for better object naming skills to be associated with greater left lateralization in WA (r = 0.25, P = 0.06) and right lateralization in the cerebellum (r = −0.26, P = 0.05).
Neuropsychological performance correlated with lateralization for the youngest children. There was a trend for better overall cognitive skills to be correlated with greater left lateralization in WA (r = 0.48, P = 0.09) and right lateralization of the cerebellum (r = −0.51, P = 0.08). The specific verbal skills that were correlated with stronger lateralization of WA and the cerebellum were object naming skills (r = 0.54 and −0.70, respectively) and core language skills on the CELF‐4 (r = 0.41 and −0.57, respectively) while higher verbal intelligence (r = 0.43) was also strongly correlated with left lateralization of WA.
Task Performance
The distribution for task performance was negatively skewed with subjects performing the fMRI task with adequate accuracy (median = 85%) and speed (see Table 1). Most errors were due to omissions (median = 90% of errors were omissions). Task accuracy was not correlated with LI across any ROI (P's > 0.20), despite a trend for a correlation with age (r = 0.26, P = 0.05). This is confirmed by hierarchical linear regression in that the significant relationship found between age and LI is unchanged when task accuracy is entered into the model. Response times were not correlated significantly with LI across any ROI or with age. The lack of age‐related differences in response time is likely due to the variability in reaction times being constrained by the maximum allowed time to respond and instead, age differences are reflected by the lower accuracy that are almost entirely due to omission errors.
Age and task performance
Accuracy was not associated with LI; however, we conducted further analyses given our premise of equating task performance through task design. Post hoc testing for age group differences in task accuracy revealed no differences among the three age groups (P > 0.10) indicating that our task manipulation to adjust for developmental level was successful. For analyses when age is kept as a linear variable, there is a correlation between age and task accuracy (r = 0.26, P = 0.05), and age and LI for IFG and WA. However, task accuracy was not correlated with LI of any region suggesting that age‐related differences in LI are not due to performance differences.
Whole Brain Activation
As a reference for the primary analyses that were conducted, activation for the single sample t‐test of all 64 participants contrasting experimental and baseline conditions is presented. A large area of robust left‐lateralized activation of classic language areas within frontal and temporal lobes was revealed (FWE, P < 0.05, Fig. 4). Peak activation was distributed throughout the left inferior frontal gyrus (IFG), encompassing Broca's Area (BA 44 and 45); left middle frontal gyrus (MFG; BA 9 and 46), and along the superior temporal sulcus, with peak temporal activation in Wernicke's Area (WA) (BA 22). Other areas of activation included right cerebellum, left superior frontal gyrus (BA 6 and 8), and left fusiform gyrus (BA 20). Right homologues of the classic language areas were active to a lesser degree and extent including right IFG (BA 45 and 47), right MFG (BA 9 and 46), and right superior temporal sulcus (BA 21 and 22). Additionally, left greater than right caudate, putamen, and thalamus were active during the task. Specific MNI coordinates of the foci shown in Figure 4 are listed in Table 2.
Figure 4.
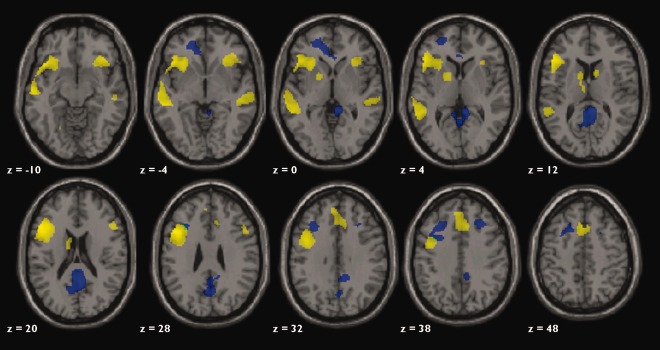
Axial slices of whole brain activation for ADDT task. Yellow represents the activation for all 57 children age 4 to 12 years old (FWE, P < 0.05). Blue represents activation unique to the youngest age group (P < 0.001, uncorrected). Green represents activation that is greater in the 4‐ to 6‐year‐old group and overlaps with the group map. Axial slices are in neurological convention (left is left hemisphere).
Table 2.
MNI coordinates and Brodmann Areas for regions displayed in Figure 4
| Group map activation across all ages 4–12 (yellow activation of Fig. 4) | ||||||
|---|---|---|---|---|---|---|
| MNI coordinates | Location | Brodmann area (BA) | Cluster size (voxels) | T | ||
| x | y | z | ||||
| −48 | 28 | 8 | Left inferior frontal gyrus | 45 | 15,170 | 10.64 |
| −52 | 34 | −6 | Left inferior frontal gyrus | 47 | 8.16 | |
| −44 | 16 | 26 | Left middle frontal gyrus | 46 | 7.99 | |
| −44 | 8 | 34 | Left middle frontal gyrus | 9 | 7.72 | |
| −52 | 14 | −4 | Left superior/middle temporal gyrus | 21/22 | 2,785 | 8.50 |
| −36 | −50 | −20 | Left fusiform gyrus | 37/20 | 5.81 | |
| 0 | 18 | 46 | Anterior cingulate | 32 | 880 | 7.72 |
| 32 | −62 | −24 | Right cerebellum | — | 81 | 6.49 |
| −4 | 14 | 52 | Superior frontal gyrus | 6/8 | 1,029 | 7.51 |
| 36 | 28 | −8 | Right inferior frontal gyrus | 45/47 | 674 | 6.16 |
| 54 | −26 | 4 | Right superior/middle temporal gyrus | 21 | 290 | 5.96 |
| 50 | 22 | 24 | Right middle frontal gyrus | 46 | 152 | 6.62 |
| −10 | 2 | 16 | Left caudate | — | 372 | 6.37 |
| −6 | −12 | 10 | Thalamus | — | 6.14 | |
| 10 | 8 | 12 | Right caudate | — | 47 | 6.47 |
| Activation unique to 4‐ to 6‐year olds (blue activation of Fig. 4) | ||||||
|---|---|---|---|---|---|---|
| 6 | −50 | 18 | Posterior cingulate/precuneus | 31/28/30 | 1,493 | 8.19 |
| −8 | 42 | −4 | Anterior cingulate/superior frontal gyrus | 32/10 | 57 | 5.48 |
| −18 | 52 | −4 | Left superior/middle frontal gyrus | 10 | 180 | 5.46 |
Age and activation
Whole brain analysis of age and activation was conducted to explore age‐related changes beyond the a priori language ROIs selected for the LI analyses. Younger age was correlated with greater activation in posterior cingulate, anterior cingulate, and right IFG (FDR, P < 0.05, >20 voxels in cluster). The age‐related findings were driven by the youngest group. Direct comparisons among the three age groups revealed that the two oldest groups were not significantly different from each other and did not activate any area greater than the youngest age group. The youngest group (Fig. 4, blue color), however, showed greater activation across several regions than both of the older groups (two‐sample t‐test, uncorrected, P = 0.001, >20 voxels in cluster). Similar to correlational analyses, posterior cingulate, anterior cingulate, and right IFG were engaged by the younger children to a greater degree than both older age groups.
DISCUSSION
In this sample of typical children who span a wide age range of childhood, the same fundamental language network as seen in adults was established by age four. However, developmental differences in laterality were observed and differed by region. Our study extends previous findings by showing that developmental changes that include strengthening of language lateralization with age for frontal and temporal regions are not associated with task performance differences. Temporal language was the most strongly lateralized region and the proportion of left language temporal dominance matched adults by 7 years of age. In contrast, middle frontal gyrus and the cerebellum were the least lateralized regions with a substantial proportion of children with bilateral representation. These patterns of lateralization in prefrontal and cerebellar regions did not differ by age. Right lateralization in the cerebellum appears beneficial as it was mildly associated with better core language skills and object naming, while stronger left lateralization of the temporal region was also modestly associated with object naming. More robust relationships between lateralization of language and neuropsychological skill were found for younger children.
Age‐related differences in lateralization were not due to performance differences, as neither task accuracy nor response time were associated with functional lateralization. These findings support the notion that the brain has a developmental propensity to lateralize language functions, but that there is a window of plasticity, before the age of seven, when language lateralization is less extreme and more variable for the entire frontotemporal network. In fact, bilateral or right dominance, as defined by LI values, occurred 30 to 40% of the time in young children. This window extends to age 10 for IFG.
The early and stable lateralization of Wernicke's area has been observed across other language tasks in large samples of children including listening to stories, syntactic prosody, word‐picture matching, and verb generation [Holland et al., 2007]. In studies of bilingual language representation, temporal regions are also noted to be the least variable [Kim et al., 1997]. Greater lateralization in Wernicke's Area was correlated with higher intelligence and better core language skills only for children under age seven. Thus, early specialization of temporal areas may be a marker of better language‐based cognitive functioning.
In contrast to temporal areas, anterior regions were less lateralized and showed greater variability in dominance across ages, which may be a reflection of their different roles in language performance. IFG demonstrated the greatest amount of change in lateralization across ages 4 to 12, suggesting that normal maturation of language functions, in the form of lateralization, has a protracted developmental trajectory for frontal compared with temporal regions of the language network.
The staggered timing in maturation between frontal and temporal lateralization may reflect general language acquisition whereby receptive skills typically lead expressive skills by roughly one productive stage as the demand of generating a response is a more advanced skill. Generation of responses requires executive control that is supported by the prefrontal cortex. Anterior regions are involved in decision‐making, working memory, task monitoring, and selective attention, which are under the cognitive domain of executive functions and known to have a protracted developmental course [Huttenlocher and Dabholkar, 1997; Owen, 1997; Thompson‐Schill et al., 2005]. A study examining timing of peak BOLD responses between temporal and frontal regions found that children had a longer delay between the time‐courses of the two regions than adults [Brauer et al., 2008], which they posited may reflect less automaticity of language processing. Thus, the timing of the lateralization of frontal networks may be tied to the ongoing integration of core language skills with other cognitive skills essential to language proficiency.
This observed functional pattern parallels the known maturational timing of underlying neuroanatomical structures. The trajectory of brain maturation varies across regions as studied post‐mortem, by dynamic MRI, and by diffusion tensor imaging (DTI); these methods show age dependent regional changes in white matter integrity and cortical thickness [Gogtay et al., 2004; Qiu et al., 2008; Shaw et al., 2008; Sowell et al., 1999; Su et al., 2008; Szaflarski et al., 2006b; Yakovlev and Lecours, 2004]. There is evidence for staggered timing between frontal and temporal regions in that frontal regions show a longer timeframe to reach peak cortical thickness and/or a plateau in development [Shaw et al., 2008; Sowell et al., 1999]. Specific changes associated with maturation include changes in cortical thickness within prefrontal cortex—suspected to be related to synaptic pruning and important for refining the neural network [Gogtay et al., 2004; Shaw et al., 2008]. Similarly, our study highlights middle childhood (between 6 and 10 years old) as the window for when changes in lateralization of IFG approach adult patterns, which maps directly to the timing of the onset of the slope of the developmental trajectories found in structural studies. Thus, our data support the link between functional hemispheric specialization and structural development. Moreover, the unique variance in lateralization accounted for by age may reflect the developmental timing of the underlying neuroanatomic structures, which as they mature, may permit the brain to make functional advances.
The developmental patterns of lateralization observed in MFG and the cerebellum had similarities. Both regions were the least lateralized regions, and no significant age effects were found across ages 4 to 12. This pattern may reflect that both MFG and the cerebellum have a role in working memory aspects of linguistic processing and may be invoked to the same extent across age. Although lateralization of the cerebellum was not found to significantly increase with age, it appeared to be a mirror image of the developmental pattern of IFG (see Figs. 1 and 2); however, age effects did not reach significance because of the greater degree of variability in LI values. It is posited that the cerebellum supports executive aspects of language functioning including fluency and verbal working memory [Chen and Desmond, 2005; Richter et al., 2007]. Our findings provide some support for this hypothesis as lateralization of the cerebellum was related to better composite language score which is comprised of tasks that require working memory including repeating sentences verbatim and following multistep directions. Although a button press was required for the task, the right cerebellar activation is not related to motor demands of the button press as the button box was placed in the left hand. Therefore, the cerebellum appears to have an important role in refining language functioning through better executive control. The developmental trajectory of the cerebellum may be unique in that there is ongoing specialization in the form of increased laterality across age like IFG, but because of its suspected role in modulating language performance like MFG, there is greater variability as individuals may engage the cerebellum bilaterally to a greater extent to compensate. This is reflected by the lower rate of typical language dominance found in the cerebellum compared with IFG or WA in adult studies [Szaflarski et al., 2002].
A limit of ROI analyses is that findings are restricted to these a priori regions; however, our whole brain analyses confirmed that developmental differences were largely limited to degree of activation within the traditional language network. The exception was the differences found for greater activation in the posterior and anterior cingulate in younger children. The posterior cingulate has a role in semantic and lexical retrieval [Burianova et al., 2010; Cavanna and Trimble, 2006; Koenig et al., 2005]. One study showed that greater activation of the posterior cingulate during encoding is associated with subsequent misses than hits on memory testing [Daselaar et al., 2009], which is consistent with the younger group who had the lowest task accuracy due to omission errors. Younger children's diminished accuracy may have been related to motor performance. It seems that they were slower during both baseline and experimental conditions and may have been unable to press the button fast enough before the next cue resulting in more missed items. Certainly, some missed answers may have been due to the child not knowing the answer to the question. Despite having designed the task to be easy for younger children in terms of word frequency, a 1‐s latency may ultimately have been too short of a response time for younger children, who are more variable in their response times. When designing future fMRI tasks for younger children it may be preferable to use longer response times or even a self‐paced task [Lee et al., 2007]. This consideration translates to patient populations as well, since they usually have more variability in their response times.
A possible limitation of our study is the assumption that there were no developmental differences in lateralization of our baseline. Previous studies were conducted with adults [Binder et al., 1999, 2000; Belin et al., 2002; Hickok, 2009; Perani et al., 1996], with the development of the functional lateralization of reverse speech being less clear. We are unable to determine lateralization of reverse speech in this study as we did not include another low level condition. Based on comprehensive reviews by Minagawa‐Kawai 2003 and Hickock 1997 as well as our own analyses, we believe that the LI differences related to our task‐baseline are not due to developmental differences in the baseline condition. First, if there are developmental differences, the literature suggests that the differences would likely be that the youngest group would be less left lateralized in perceiving fast temporal phonetic information. If this were the case, then if anything, the leftward LI differences we obtain in our forward speech analyses are an underestimate of the leftward developmental differences in lateralization. It is possible that developmental differences might be dependent on whether reverse speech is perceived as speech or not. If reverse speech is perceived as speech, then there likely would be a left lateralized pattern. Since the instructions in our study explicitly describe the baseline condition as “silly talk,” children are all primed to perceive it as speech. If language lateralization is a function of experience or learning [for review see Minagawa‐Kawai, et al., 2003], the older age group should in this case also be more left lateralized for the baseline task than the younger groups [Minagawa‐Kawai et al., 2011]. Furthermore, when we separate the contrast of baseline > task by age, the youngest age group has no areas of activation significantly different from any of the older groups even at more lenient thresholds (P < 0.001, uncorrected) and all groups had a high accuracy (>94%). The older group appears to be driving the group map results as they have greater activation in posterior cingulate. In summary, neither our additional analyses nor studies in the literature related to development of the lateralization of speech would suggest that our findings can be explained by developmental changes in the baseline task.
CONCLUSION
Theories of language development differ in explaining how asymmetry of function evolves. One theory proposes that lateralization is “fixed” by occurring early in development and following the same developmental sequence; alternatively, “progressive” lateralization contends that lateralization is an extended process [Lenneberg, 1967]. Both of these theories assume that greater lateralization is equated with better functioning. Alternatively, a recent proposal describes a complex set of variables including biological constraints, maturation, and experience to determine how performance and lateralization are related [Boles et al., 2008]. They contend that the common hypothesis that greater lateralization is associated with better performance is likely to be too simple. Rather, there will be individual differences in brain development combined with differences in the timing of the lateralization of different cognitive skills. Our findings support the notion that taking into consideration regional differences, the developmental window, and task demands may unravel some of the complexities of language development, while also confirming that there are aspects of language development that are robust across individual differences in that it is generally a lateralized network established early in development.
Nonetheless, even though we addressed some regional differences, a limitation of our study may be the large regions of interest that we used. An area of future study would be to conduct similar analyses within functional subregions of the frontal (e.g., BA 44, 45, 47) and temporal (e.g., BA 21, 22, 39, 40) language network as they have been identified as relating differently to age and performance [Berl et al., 2010; see Price, 2010 for review].
Regional differences in the timing of lateralization and its relationship to cognitive abilities have implications for understanding the impact of timing of developmental or acquired injuries as well as intervention. A recent study with four patients who had a diaschisis of language dominance on Wada language testing noted that all patients appeared to have expressive language supported by the hemisphere contralateral to the seizure focus, whereas receptive language remained in the hemisphere ipsilateral to the seizure focus [Lee et al., 2008]. These data combined with our findings reflect the possibility that the developmental window of plasticity for these patients had passed for temporal functions, but not for frontal aspects of the system. If it is assumed that targeting aspects of language that are more plastic will yield greater therapeutic gains, then language functions that rely on frontal areas should have a longer window for intervention, given their protracted development. On the other hand, our findings also argue for early intervention because other aspects of the network are near adult maturation by age seven, particularly temporally. Our findings are clinically relevant for understanding the risk of morbidity associated with surgical resection as a treatment for epilepsy and how that risk may change when assessed at different ages.
The elucidation of the developmental timing of lateralization provides understanding of how disease states perturb the normal development of language systems [Gaillard et al., 2007]. One application is within epilepsy populations where approximately 30% of adults have atypical language representation [Springer et al., 1999]. Similarly, 20 to 40%—depending on age group—of the children in our sample also showed atypical language. We also found that a third of our children were discordant in their laterality among cortical and cerebellar regions; this discordance was most likely in the youngest group. Taken together, these similar proportions between developmental and patient populations suggest that language can be sustained in the nondominant hemisphere because of the extended maturational window of the language system. Thus, profiles observed in our epilepsy group may reflect the persistence of a typical immature language profile rather than the possibility that language “reorganized” to the nondominant hemisphere due to seizure activity or underlying cause. The normal developmental pattern of a less lateralized network in children under age seven also provides an explanation for how the brain may permit compensation for early neurological injury and/or recovery from surgical intervention as large as a hemispherectomy.
ACKNOWLEDGMENTS
The contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The authors thank all the children and families who participated in our study.
REFERENCES
- Ackermann H, Mathiak K, Riecker A (2007): The contribution of the cerebellum to speech production and speech perception: Clinical and functional imaging data. Cerebellum 6:202–213. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD (2003): Auditory comprehension of language in young children: Neural networks identified with fMRI. Neurology 60:1598–1605. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD (2006): Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage 31:1306–1314. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, Gaillard WD (2002): A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol 59:1168–1174. [DOI] [PubMed] [Google Scholar]
- Bates E, Roe K (2001): Language development in children with unilateral brain injury In: Nelson CA, Luciano M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press. [Google Scholar]
- Behrens SJ (1985): The perception of stress and lateralization of prosody. Brain Lang 26:332–348. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD (2006): Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage 30:679–691. [DOI] [PubMed] [Google Scholar]
- Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, VanMeter J, Ratner NB, Vaidya CJ, Gaillard WD (2010): Functional anatomy of listening and reading comprehension during development. Brain Lang 114:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Rao S, Hammeke T, Frost JA, Bandettini P, Jesmanowicz A, Hyde J (1995): Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol 52:593–601. [DOI] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD (2006): Differential prefrontal‐temporal neural correlates of semantic processing in children. Brain Lang 99:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, Minahan R, Carson B, Brandt J, McKhann G (1999): Language recovery after left hemispherectomy in children with late‐onset seizures. Ann Neurol 46:579–586. [DOI] [PubMed] [Google Scholar]
- Boles DB, Barth JM, Merrill EC (2008): Asymmetry and performance: Toward a neurodevelopmental theory. Brain Cogn 66:124–139. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Trommer BL, Davenport ND, Parrish TB, Gitelman DR, Mesulam MM (2004): Brain‐behavior correlation in children depends on the neurocognitive network. Hum Brain Mapp 23:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T (2007): The role of the basal ganglia and cerebellum in language processing. Brain Res 1133:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Neumann J, Friederici AD (2008): Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage 41:1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Bucker RL, Snyder AZ, Petersen SE, Schlaggar BL (2002): The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage 17:184–200. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL (2010): A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 49:865–874. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B (1971): The American heritage word frequency book. Boston, MA: Houghton Mifflin. [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE (2005): Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24:332–338. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Muller RA, Chugani DC (1996): Functional brain reorganization in children. Brain Dev 18:347–356. [DOI] [PubMed] [Google Scholar]
- Cone NE, Burman DD, Bitan T, Bolger DJ, Booth JR (2008): Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. Neuroimage 41:623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss S, de Bode S, Mathern GW (2001): Spoken language outcomes after hemispherectomy: Factoring in etiology. Brain Lang 79:379–396. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R (2009): Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DK, Neuman SB, editors (2006): Early Literacy Research. New York: Guilford Press. [Google Scholar]
- Elliot C (1990): Differential Abilities Scale. San Antonio, TX: Psychological Corporations: Harcourt Brace and Company. [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, Perrig W, Steinlin M (2009): Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp 30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH (2002): Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 59(2):256–65. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH (2004): fMRI language task panel improves determination of language dominance. Neurology 63:1403–1408. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B (2003a): fMRI identifies regional specialization of neural networks for reading in young children. Neurology 60:94–100. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, Conry JA, Pearl PL, Ritter FF, Sato S, Vezina LG, Vaidya CJ, Wiggs E, Fratalli C, Risse G, Ratner NB, Gioia G, Theodore WH (2007): Atypical language in lesional and nonlesional complex partial epilepsy. Neurology 69:1761–1771. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz‐Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore W (2000): Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 54:108–105. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Xu B, Petrella JR, Balsamo L, Basso G (2001): Cortical localization of reading in normal children: An fMRI language study. Neurology 57:47–54. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB (2003b): Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp 18:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TFIII, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, Tamny T (2001): Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology 56:56–61. [DOI] [PubMed] [Google Scholar]
- Harris A (1974): Harris Tests of Lateral Dominance: Manual of Directions for Administration and Interpretation. New York: David McKay Co., Inc. [Google Scholar]
- Hertz‐Pannier L, Gaillard WD, Mott S, Cuenod CA, Bookheimer S, Weinstein S, Conry J, Papero PH, LeBihan D, Theodore WH (1997): Assessment of language hemispheric dominance in children with epilepsy using functional MRI. Neurology 48:1003–1012. [DOI] [PubMed] [Google Scholar]
- Hickok G (2009): The functional neuroanatomy of language. Phys Life Rev 6:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars A, Strawsburg RH, Schmithorst VJ, Ball WSJr (2001): Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14:837–843. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW (2007): Functional MRI of language lateralization during development in children. Int J Audiol 46:533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS (1997): Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178. [DOI] [PubMed] [Google Scholar]
- Jansen A, Floel A, Van Randenborgh J, Konrad C, Rotte M, Forster AF, Deppe M, Knecht S (2005): Crossed cerebro‐cerebellar language dominance. Hum Brain Mapp 24:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Relkin NR, Lee KM, Hirsch J (1997): Distinct cortical areas associated with native and second languages. Nature 388:171–174. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M (2005): The neural basis for novel semantic categorization. Neuroimage 24:369–383. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk O, Kemp S (1998): NEPSY: A Developmental Neuropsychological Assessment. USA: Psychological Corporations Harcourt Assessment, Inc. [Google Scholar]
- Lee D, Swanson SJ, Sabsevitz DS, Hammeke TA, Scott Winstanley F, Possing ET, Binder JR (2008): Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav 13:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Foss‐Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, Vaidya CJ (2007): Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage 38:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg EH (1967): Biological Foundations of Language. New York: Wiley. [Google Scholar]
- Lidzba K, Wilke M, Staudt M, Krageloh‐Mann I, Grodd W (2008): Reorganization of the cerebro‐cerebellar network of language production in patients with congenital left‐hemispheric brain lesions. Brain Lang 106:204–210. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (2001): Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13:786–793. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier CF (2010): Methodological approaches in developmental neuroimaging studies. Hum Brain Mapp 31:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Minagawa‐Kawai Y, Cristià A, Dupoux E (2011): Cerebral lateralization and early speech acquisition: A developmental scenario. Dev Cogn Neurosci 1:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chakraborty PK, Chugani HT (1998): Brain organization of language after early unilateral lesion: A PET study. Brain Lang 62:422–451. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER (2008): Neurodevelopmental changes in verbal working memory load‐dependency: An fMRI investigation. Neuroimage 42:1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM (1997): The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci 9:1329–1339. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW (2006): Sex differences in the activation of language cortex during childhood. Neuropsychologia 44:1210–1221. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2000): Imaging Brain plasticity: Conceptual and methodological issues—A theoretical review. Neuroimage 12:1–13. [DOI] [PubMed] [Google Scholar]
- Price C (2010): The anatomy of language: A review of 100 fMRI studies published in 2009. Ann NY Acad Sci 1191:62–88. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A (1999): Cerebral lateralization of language in normal left‐handed people studies by functional fMRI. Neurology 52:1038–1043. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL (2008): Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel‐wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232. [DOI] [PubMed] [Google Scholar]
- Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, Gizewski ER, Ziegler W, Karnath HO, Timmann D (2007): Cognitive functions in patients with MR‐defined chronic focal cerebellar lesions. J Neurol 254:1193–1203. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002): Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science 296:1476–1479. [DOI] [PubMed] [Google Scholar]
- Seghier ML (2008): Laterality index in functional MRI: methodological issues. Magn Reson Imaging 26:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W (2003): CELF 4: Clinical Evaluation of Language Fundamentals, 4th ed San Antonio, TX: Psychological Corporations Harcourt Assessment, Inc. [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP (2008): Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (1999): In vivo evidence for post‐adolescent brain maturation in frontal and striatal regions. Nat Neurosci 2:859–861. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122:2033–2046. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh‐Mann I (2001): Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology 57:122–125. [DOI] [PubMed] [Google Scholar]
- Su P, Kuan CC, Kaga K, Sano M, Mima K (2008): Myelination progression in language‐correlated regions in brain of normal children determined by quantitative MRI assessment. Int J Pediatr Otorhinolaryngol 72:1751–1763. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA (2002): Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology 59:238–244. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW (2006a): fMRI study of language lateralization in children and adults. Hum Brain Mapp 27:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK (2006b): A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol 59:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Bedny M, Goldberg RF (2005): The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15:219–224. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF (2003): Development of neural mechanisms for reading. Nat Neurosci 6:767–773. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999): Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wiig E, Secord W (2004): CELF P2: Clinical Evaluation of Language Fundamentals, Preschool, 2nd ed San Antonio, TX: Psychological Corporations Harcourt Assessment, Inc. [Google Scholar]
- Wilke M, Lidzba K (2007): LI‐tool: a new toolbox to assess lateralization in functional MR‐data. J Neurosci Methods 163:128–136. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ (2006): A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage 33:522–530. [DOI] [PubMed] [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, Saling MM, Jackson GD (2004): Language cortex activation in normal children. Neurology 63:1035–1044. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR (1967): The myelogenic cycles of regional maturation of the brain In: Minkowski, editor. Regional Development in Early Life. Oxford: Blackwell; pp 3–23. [Google Scholar]


