Abstract
The dietary acid load created by the typical Western diet may adversely impact the skeleton by disrupting calcium metabolism. Whether neutralizing dietary acid with alkaline potassium salts results in sustained improvements in calcium balance remains controversial. In this randomized, double blind, placebo controlled study, 52 men and women (mean age 65.2 + 6.2 years) were randomly assigned to potassium citrate 60 mmol, 90 mmol or placebo daily with measurements of bone turnover markers, net acid excretion, and calcium metabolism including intestinal fractional calcium absorption and calcium balance obtained at baseline and six months. At six months, net acid excretion was significantly lower in both treatment groups compared to placebo and negative, meaning subjects’ dietary acid was completely neutralized (−11.3 mmol/day, 60 mmol/day; −29.5 mmol/day, 90 mmol/day, P < 0.001 compared to placebo). At 6-months, 24-hour urine calcium was significantly reduced in persons taking potassium citrate 60 mmol (−46 ± 15.9 mg/day) and 90 mmol (−59 ± 31.6 mg/day) daily compared with placebo (p<0.01). Fractional calcium absorption was not changed by potassium citrate supplementation. Net calcium balance was significantly improved in participants taking potassium citrate 90 mmol/day compared to placebo (142 ± 80 mg/day, 90 mmol vs. −80 ± 54 mg/day, placebo; p = 0.02). Calcium balance was also improved on potassium citrate 60 mmol/day, but this did not reach statistical significance (p=0.18). Serum C-telopeptide decreased significantly in both potassium citrate groups compared to placebo (−34.6 ± 39.1 ng/L, 90 mmol/d, p=0.05; −71.6 ± 40.7 ng/L, 60 mmol/day, p=0.02) while bone specific alkaline phosphatase did not change. Intact parathyroid hormone was significantly decreased in the 90 mmol/day group (p=0.01). Readily available, safe, and easily administered in an oral form, potassium citrate has the potential to improve skeletal health. Longer term trials with definitive outcomes such as bone density and fracture are needed.
INTRODUCTION
As the population ages, osteoporosis imposes an ever-increasing health risk. Bone loss leads to fracture, morbidity, and mortality, making osteoporosis prevention and treatment imperative. In addition to calcium and vitamin D which have been shown to be important nutritional approaches to maximizing skeletal health, there may be other important nutritional contributors to the pathophysiology of bone loss. It has long been postulated that the low grade metabolic acidosis generated by the metabolism of typical Western diets causes release of alkaline salts from the mineral phase of the skeleton, a homeostatic response that mitigates the degree of acidosis.
Once based primarily on the fruits and vegetables that serve as rich sources of alkaline potassium salts, modern diets now consist of greater levels of acid precursors from disproportionately higher protein and cereal grain intake and disproportionately lower fruit and vegetable intake. The limited fruit and vegetable intake leads to a chronic, systemic state of low-grade metabolic acidosis that progressively worsens in the setting of age-related declines in renal function and consequent diminished renal acid-base regulation (1). Partially compensating for the acidogenic diet and resultant downward trajectory of systemic pH, the skeleton serves as a base reservoir. To maintain systemic pH homeostasis, alkaline salts of calcium (phosphates, carbonates, hydroxides) are liberated from the skeleton, calcium and phosphorus are lost permanently in the urine, and bone density declines (2).
Given that metabolic acidosis may have a direct and unfavorable effect on bone, investigators have studied whether provision of exogenous sources of base can restore the acid-base status and improve calcium metabolism. Those studies, though limited by small numbers of participants and short duration, have yielded encouraging results. When administered to study participants, both potassium bicarbonate and potassium citrate reduce the hypercalciuria associated with an acidogenic diet (3;4). Intake of base in the short term has been shown to reduce serum and urine markers of bone resorption, improve calcium balance, and increase markers of bone formation (3–7). Bone density data are limited to two studies with mixed results. One study suggests that supplementation with potassium citrate may improve bone mineral density (BMD) in older subjects, although that study lacked a placebo control (8). The other study showed no effect on bone density; however, this study also showed no effect on urine calcium excretion, an effect otherwise uniformly demonstrated throughout the literature (9). Additionally, some research suggests that, though calcium excretion in the urine declines with higher potassium intakes, intestinal absorption of calcium may concomitantly decrease, resulting in no net benefit (10). Until now, these findings have yet to be challenged by a randomized, long-term study designed specifically to look at the relative changes in the balance of calcium absorption, excretion, and net calcium balance during exogenous base administration.
In this trial, we present the first data in humans to determine whether base, in the form of oral potassium citrate, has persistent and beneficial effects on net calcium balance, bone turnover, and gastrointestinal fractional calcium absorption. We hypothesized that 6-months of potassium citrate would have a net positive effect on the calcium economy compared to placebo, by lowering urine calcium excretion with no effect on intestinal calcium absorption, resulting in a net improvement in calcium balance.
MATERIALS AND METHODS
Subjects
Healthy men and women greater than 55 years of age were recruited through population based direct mailings and advertisements in the San Francisco Bay area. Women were greater than five years past menopause to avoid the rapid bone turnover state that occurs following menopause. As alkaline potassium salts decreases urine calcium excretion and individuals with low urine calcium excretion may be less responsive to treatment, we included men and women with a 24-hour urine calcium excretion at or above the average median value observed in a similar population in previous studies (120 mg/day women, 140 mg/day men). Potential study participants were excluded if they had medical conditions that would predispose them to gastrointestinal intolerance of oral potassium (delayed gastric emptying, esophageal stricture). Individuals with conditions that may predispose to a higher risk for hyperkalemia were also excluded including those with type 1 diabetes, serum bicarbonate < 17mmol/L, baseline serum potassium ≥ 5.0 mmol/L, and GFR < 50 ml/min by Modification of Diet in Renal Disease (MDRD) equation. Current or past use of medication altering potassium handling (e.g. angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, potassium sparing diuretic) or current use of potassium supplementation were also exclusion criteria. Men and women with known metabolic bone disease (Paget’s, multiple myeloma, hyperparathyroidism, uncontrolled thyroid disease) were also excluded. Additional criteria for exclusion included the following: unwillingness to discontinue home doses of calcium and vitamin D supplementation in exchange for study doses, current or use within the past 6 months of estrogen, selective estrogen receptor modulators, or calcitonin; current or past use of parathyroid hormone; use of bisphosphonates for more than six months; poorly controlled diabetes mellitus (HbA1c > 8%) or proteinuria > 1+ by urine dipstick; bilateral hip replacements; malignancy in five years prior to study start excluding ductal carcinoma in situ and non-melanoma skin cancer.
Potential study participants who responded to advertisements were pre-screened over the phone. Those who were eligible and willing to come for additional evaluation were scheduled for screening visits at the CTSI Clinical Research Services (CRS) facility at the University of California San Francisco. Of the 2353 subjects screened via telephone call, 373 were evaluated in the clinic. The primary reasons for not being evaluated in the clinic were subjects who met exclusion criteria on the telephone screen (n=928) or declined to participate because of the intensive calcium balance procedures (n=791). Baseline screening labs included 24 hour urine calcium, serum electrolytes and minerals, thyroid stimulating hormone (TSH), 25-hydroxyvitamin D, and parathyroid hormone (PTH). Participants were screened while consuming their self selected diet and supplements, averaging a total calcium intake of 1401 ± 640 mg/day as estimated by validated dietary questionnaire (11); 71% of participants were consuming at least some calcium from supplements during screening. By design, a little more than half of screened subjects did not meet the 24 hour urine calcium inclusion criterion of having a urine calcium excretion above the median value. Following the screening visits and run-in procedures described below, 52 individuals were ultimately eligible to participate in the study and randomized to treatment. All study questionnaires and protocols were approved by the UCSF Institutional Review Board. Written informed consent was provided by each study participant. The study was registered on www.clinicaltrials.gov (NCT00282126).
Study design
Eligible study participants were counseled by the study dietitian to achieve approximately 600 mg of calcium per day in their diet. They also were started on calcium citrate (630 mg elemental calcium) and vitamin D3 400 IU, taken once daily in the morning to achieve daily recommended intakes (12); they were asked to discontinue other vitamin and mineral supplementation. In addition, participants began a two week run-in period with placebo potassium citrate pills to ensure they would be able to consume the required 11 pills per day (nine potassium citrate or placebo, one calcium, one vitamin D). Subjects who successfully completed the two week run-in were randomized to one of three groups: 1) potassium citrate 60 mmol/day, 2) potassium citrate 90 mmol/day, or 3) placebo. The randomization scheme was stratified by gender. After the two week run-in with placebo tablets as well as study-prescribed calcium and vitamin D, baseline laboratory studies were drawn including a 24-hour urine collection for calcium and net acid excretion (NAE) as well as serum and urine for markers of bone turnover (C-telopeptides (CTX)), bone specific alkaline phosphatase (bsALP)), PTH, and 1,25 dihydroxyvitamin D. After four weeks total of standardized calcium and vitamin D intake, subjects completed a baseline calcium balance study, described below.
After completion of all baseline measurements including calcium balance studies, subjects began their randomly assigned study medication (potassium citrate 90 mmol/day, potassium citrate 60 mmol/day, or placebo). To allow for time-dependent up-regulation of renal potassium handling and to monitor serum potassium levels, participants were started on one tablet per day of study medication (potassium citrate 10 mmol or placebo) with a dose increase of one additional tablet per day until reaching nine tablets per day in the ninth week of dose escalation. Blind was maintained by all subjects consuming nine tablets per day; for subjects randomized to 60 mmol/day of potassium citrate, three of the nine tablets were placebo. Once reaching target dose, the supplement was continued for six months. For participant safety, during the 9-week dose-escalation phase of the study, serum potassium was measured weekly and then at the final 6-month study visit to screen for hyperkalemia. Also collected at 6-months were serum electrolytes, 24-hour urine calcium and net acid excretion as well as bone turnover markers, PTH, and 1,25-dihydroxyvitamin D. The calcium balance studies were repeated at the six month time point.
Measurements
Anthropometrics and questionnaires
Study participant height and weight were measured with a stadiometer and balance beam scale, respectively. To assess the tolerability of potassium citrate supplementation, a validated gastrointestinal symptom questionnaire was administered at the second screening visit and at each study visit (13). At each study visit, a study dietitian met with subjects and performed a 24 hour dietary recall and continued counseling to ensure adherence with the desired dietary calcium intake of 600 mg per day during the portion of the study where subjects were on a self selected diet.
Calcium balance and intestinal fractional calcium absorption studies
After randomization, but prior to starting potassium citrate or placebo supplementation, study participants completed a 12 day calcium balance study and underwent measurement of intestinal fractional calcium absorption using stable calcium isotopes. Calcium balance and absorption studies were repeated at the end of the 6-month potassium citrate intervention. For the balance studies, participants consumed a controlled metabolic diet for a 12-days, keeping pertinent nutrients including calcium, sodium, potassium, and phosphorus constant (table 1) and within recommended dietary intakes. As previous data have shown steady state calcium balance is achieved after six days, from days seven to twelve of the balance period, participants collected all of their urine in 24 hour increments and collected all stools to determine calcium balance as has been described previously (14). Additionally, participants were admitted to the CRS on either the eighth or ninth day of the metabolic period for measurement of intestinal fractional calcium absorption. Oral Ca44 and intravenous Ca43 isotopes were administered, followed by measurement of isotope ratios in serum 24 hours after the dose and fractional calcium absorption calculated as described previously (15;16). Completeness of collections was monitored with urine creatinine measures and the use of polyethylene glycol as a marker for calcium excretion in the stool.
Table 1.
Nutrient content of controlled diet consumed by the study subjects during the baseline and end of study calcium balance studies.
| Energy (kJ/day) | Protein (g/day) | Fat (g/day) | Sodium (mg/day) | Calcium (mg/day) | Phosphorus (mg/day) | Potassium (mmol/day) | |
|---|---|---|---|---|---|---|---|
| Women | 8105 ± 79 | 66 ± 1 | 49 ± 7 | 2103 ± 17 | 556 ± 47 | 1138 ± 199 | 3058 ± 244 |
| Men | 10,694 ± 218 | 82 ± 1 | 79 ± 7 | 2806 ± 63 | 618 ± 47 | 1410 ± 156 | 3342 ± 316 |
Values are average intake for the rotating three day menu ± SD.
Calcium isotope ratios were measured on an ELEMENT-2 High Resolution Inductively Coupled Plasma Mass Spectrometer (ThermoFinnigan Corp, Bremen, Germany) using an Aridus Desolvating Sample Introduction system with a T1H nevulizer (Cetac Technologies, Omaha, NE). Serum, urine, and fecal calcium were measured using atomic absorption spectrophotometry (5100 PC; Perkin-Elmer, Norwalk, CT).
Net Acid Excretion
Urine bicarbonate and titratable acid were determined by titration according to published methods (17;18). The 24-hour urine collection containers were prepared prior to distribution to the participants with a few grains of thymol (preservative) and 70 mls of mineral oil. Mineral oil was added to prevent diffusion of carbon dioxide into the sample from the air which would affect the net acid excretion measurement. For each urine sample, pH was measured, and an excess of HCl was added to the sample; the carbonic acid formed from the reaction of the urine bicarbonate and acid was driven off by boiling. The sample was then back titrated to the original sample pH with a NaOH standard, allowing for quantification of bicarbonate in the original sample. The titration was then continued to pH 7.4, allowing for determination of titratable acid. Urine ammonium concentration was determined by the phenol method. Net acid excretion was calculated as the sum of the excretion rates of titratable acid and ammonium minus that of bicarbonate.
Serum measures: bone turnover markers, calcitropic hormones
Bone-specific alkaline phosphatase, a marker of bone formation, was measured in serum by the DiaSorin LIAISON® BAP OSTASE® assay (DiaSorin, Stilwater, MN). C-telopeptide of type I collagen was measured using the Serum CrossLaps® ELISA from Immunodiagnostic Systems Inc (IDS Inc), Fountain Hills, AZ. Parathyroid hormone was measured at Heartland Assays using DiaSorin N-TACT® PTH SP assay (DiaSorin Stilwater, MN). The 1,25 dihydroxyvitamin D measurements were also performed at Heartland Assays using the method described by Hollis et al. (19). 25-hydroxyvitamin D was measured by high pressure liquid chromatography in the UCSF clinical laboratory.
Statistical analysis
Analyses for the primary research questions of the difference in urine calcium excretion and fractional calcium absorption between treatment groups were addressed by comparing the change in calcium excretion among the treatment groups by ANOVA. For outcomes with significance differences among the three groups, we subsequently tested for differences between the 90 mmol/day and placebo group, followed by testing for significant differences between the 60 mmol/day and placebo group, and finally between the 90 mmol/day vs. 60 mmol/day groups. Mean changes in the endpoints were compared using a two-sample t-test comparing the change from baseline to the 6 month measurement. The outcomes of net calcium balance, net acid excretion, and calcitropic hormones were conducted in a parallel fashion. Bone turnover marker data were not normally distributed and were analyzed using Kruskal Wallis and Wilcoxon-Mann-Whitney tests.
Sample Size and Power Calculations
Sample size was calculated based on primary outcomes of change in urine calcium excretion and change in fractional calcium absorption. A sample size of 15 participants per group with two sided alpha=0.05, provided 80% power to detect differences in change in urine calcium excretion as small as 58 mg/day and differences in change in fractional calcium absorption as small as 5.1% using standard deviations from previous studies and published values in the literature (20;21). This sample size also had 80% power to detect differences in change in calcium balance as small as 78 mg/day with two sided alpha=0.05 (3). Seven additional subjects were recruited and randomized to allow for attrition.
RESULTS
Characteristics of study participants are presented in table 2. Of the 52 subjects, 18 participants were randomized to placebo, 17 participants to potassium citrate 60 mmol/day, and 17 participants to potassium citrate 90 mmol/day. There were no significant differences in age or body mass index (BMI) amongst study groups. The majority of participants were Caucasian females. There were no significant differences in baseline measures of 24-hour urine calcium or potassium, net acid excretion, serum potassium, 25-hydroxyvitamin D, 1,25 dihydroxyvitamin D or PTH amongst participants in the study groups. Markers of bone formation (bsALP) and bone resorption (CTX) were not significantly different amongst treatment groups at baseline.
Table 2.
Baseline characteristics for the 52 men and women over age 55 years randomized to placebo (n=18), potassium citrate 60 mmol/day (n=17), or potassium citrate 90 mmol/day (n=17).
| Placebo (n=18) | Potassium Citrate 60 mmol/day (n=17) | Potassium Citrate 90 mmol/day (n=17) | |
|---|---|---|---|
| Age (years) | 65.2 ± 6.2 | 64.7 ± 5.9 | 63.8 ± 6.4 |
| Gender (% female) | 67% | 67% | 73% |
| Race (% Caucasian) | 83% | 100% | 78% |
| BMI (g/m2) | 26.0 ± 4.0 | 25.1 ± 2.7 | 24.3 ± 3.3 |
| Urine calcium (mg/day) | 184 ± 59 | 206 ± 94 | 189 ± 82 |
| 25 hydroxyvitamin D (nmol/L) | 87.5 ± 25.2 | 78.9 ± 26.3 | 80.0 ± 26.8 |
| Weight (kg) | 68.9 ± 12.9 | 69.4 ± 13.6 | 67.6 ± 11.7 |
| Self selected pre-study total calcium intake (mg/day) | 1473 ± 704 | 1415 ± 614 | 1486 ± 656 |
| Using calcium supplements prior to enrollment (%) | 77% | 60% | 73% |
Values are mean ± SD or percent. Baseline values were not significantly different among treatment groups. BMI=body mass index.
Subject adherence, supplement tolerance and safety
A total of six participants withdrew from the study after randomization. Primary reasons for study withdrawal included dislike of the controlled diet and personal issues. No participant left the study due to gastrointestinal intolerance of the potassium citrate. One participant developed hyperkalemia requiring study withdrawal. This participant began the study with a baseline serum potassium of 4.5 mmol/L which rose to 5.4 mmol/L (4.9 upper limit of normal) with dose escalation of potassium citrate to 40 mmol/day. Serum potassium was 3.9 mmol/L one week following supplement discontinuation. The participant’s GFR was 58 by MDRD calculation. All collected data on study participants were used until the time of study discontinuation with the exception of one participant in the placebo group. In this case, stool collection for calcium balance studies was inadequate both at baseline and 6-months as determined by less than 15% recovery of the co-administered non-absorbable stool marker polyethelyne glycol, necessitating elimination of these data from calcium balance analyses.
Potassium and net acid excretion
At the end of the study, urine potassium did not change in participants on placebo, but significantly increased in subjects on potassium citrate 60 mmol and 90 mmol/day (Table 3). Urine potassium was significantly greater in those on potassium citrate 90 mmol/day compared with those on 60 mmol/day (138.6 ± 13.6 mmol/day vs. 100.6 ± 9.6 mmol/day, respectively; p = 0.05). With the exception of the participant noted above who was removed with the study for hyperkalemia, serum potassium did not increase significantly in any group at any dose of potassium citrate or placebo at six months. At the six month timepoint, serum potassium averaged 4.2 ± 0.1 mmol/L in the placebo group, 4.3 ± 0.1 mmol/L in the 60 mmol/day group, and 4.3 ± 0.1 mmol/L in the 90 mmol/day group. Net acid excretion decreased in parallel with higher doses of potassium citrate in study groups. Participants on both doses of potassium citrate showed lower and sustained six month net acid excretion when compared to participants on placebo (NAE = −29.5 ± 4.5 mmol/day in the 90 mmol/day treatment group; −11.3 ± 4.9 mmol/day in the 60 mmol/day treatment group vs. 16.1 ± 4.5 in the placebo group; p < 0.001 for each potassium group vs. placebo). The difference in net acid excretion between the 60 mmol/day and 90 mmol/day groups was of borderline statistical significance (p=0.09).
Table 3.
Net acid excretion, potassium parameters, and bone turnover markers for the 52 men and women over age 55 years randomized to placebo (n=18), potassium citrate 60 mmol/day (n=17), or potassium citrate 90 mmol/day (n=17).
| Placebo | K Citrate 60 mmol/day | K Citrate 90 mmol/day | |
|---|---|---|---|
|
| |||
| Net Acid Excretion (mmol/day) | |||
| Baseline | 22.4 ± 4.0 | 22.9 ± 7.8 | 17.0 ± 4.3 |
| Six months | 16.1 ± 4.5 | −11.3 ± 4.9a | −29.5 ± 4.5a |
| Change | −5.6 ± 3.3 | −31.9 ± 7.8b | −39.8 ± 7.6a |
|
| |||
| Serum Potassium (mmol/L) | |||
| Baseline | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 |
| Six months | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.3 ± 0.1 |
| Change | −0.02 ± 0.1 | 0.15 ± 0.1 | 0.26 ± 0.1 |
|
| |||
| Urine Potassium (mmol/day) | |||
| Baseline | 85.2 ± 8.4 | 75.3 ± 10.4 | 73.4 ± 8.8 |
| Six months | 67.3 ± 4.7 | 100.6 ± 9.6a | 138.6 ± 13.6a,c |
| Change | −13.1 ± 8.0 | 42.0 ± 14.9b | 67.3 ± 14.0b |
|
| |||
| Bone specific alkaline phosphatase (μg/L) | |||
| Baseline | 11.8 ± 1.3 | 11.8 ± 1.3 | 11.3 ± 1.1 |
| Six months | 10.8 ± 1.1 | 10.6 ± 1.2 | 9.0 ± 1.1 |
| Change | −0.95 ± 0.8 | −1.1 ± 0.9 | −1.8 ± 0.8 |
|
| |||
| Serum C-telopeptide (ng/L) | |||
| Baseline | 186.1 ± 35.3 | 253.7 ± 27.2 | 256.8 ± 37.9 |
| Six months | 231.1 ± 18.0 | 174.6 ± 26.6 | 222.9 ± 38.5 |
| Change | 44.0 ± 27.6 | −71.6 ± 40.7d | −34.6 ± 39.1d |
Values are mean ± SE. Statistical significance indicated by superscripted letters:
p<0.001 vs. placebo;
p=0.002 vs. placebo;
p<0.05 vs potassium citrate 60 mmol/day;
p<0.05 vs. placebo.
Calcium metabolism
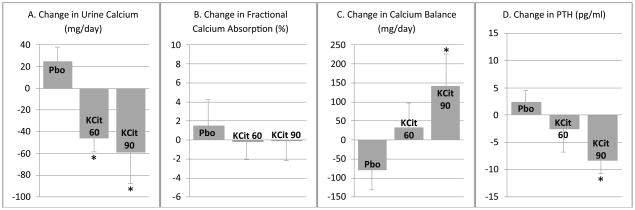
There was a significant reduction in 24-hour urine calcium in those participants taking potassium citrate 60 mmol/day (−46 ± 15.9 mg/day) and 90 mmol/day (−59 ± 31.6 mg/day) when compared to placebo (p < 0.005 for both comparisons). The difference in urine calcium reduction in participants taking potassium citrate 60 versus 90 mmol/day was not statistically significant (Figure 1, panel A). Potassium citrate did not affect intestinal absorption of calcium, as there were no significant differences in the change in fractional calcium absorption with either potassium citrate 60 or 90 mmol/day when compared with placebo (Figure 1, panel B). Calcium balance showed that participants taking 90 mmol/day of potassium citrate for six months had a significant improvement in calcium balance when compared with participants on placebo (142 ± 80 mg/day in the 90 mmol/day treatment group vs. −80 ± 54 mg/day in the placebo group; p < 0.05). Participants on potassium citrate 60 mmol/day also showed an improvement in calcium balance (33 ± 66 mg/day), after 6 months when compared with placebo but this did not reach statistical significance (p = 0.18). There was no significant difference in change in calcium balance between potassium citrate 60 mmol/day and potassium citrate 90 mmol/day (Figure 1, panel C). Intact PTH decreased in both potassium citrate treatment groups, reaching statistical significance in the 90 mmol/day group while there were no significant differences in 1,25-dihydroxy vitamin D levels during the study period (Figure 1, panel D).
Figure 1.
Change in calcium parameters and parathyroid hormone after six months of potassium citrate supplementation. Panel A. Change in urine calcium excretion from baseline to end of study, Panel B. Change in fractional calcium absorption from baseline to end of study, Panel C. Change in calcium balance from baseline to end of study, Panel D. Change in intact parathyroid hormone (PTH) from baseline to end of study. *p<0.05 vs. placebo. Values are mean ± SE.
Bone turnover markers
In both groups of participants taking potassium citrate at 60 and 90 mmol/day, CTX, the serum marker of bone resorption, decreased significantly during the six months of therapy compared to participants on placebo alone (Table 3). There was no significant change in serum bsALP, the marker of bone formation, in any group during the six month study. There were no significant differences in bone turnover markers between the 60 mmol/day and 90 mmol/day potassium citrate groups.
DISCUSSION
In this trial, we provide the first evidence that oral administration of potassium citrate results in a long-term, positive effect on calcium balance by reducing urine calcium and having a neutral effect on gastrointestinal absorption of calcium. Supplementation with either 60 mmol or 90 mmol of daily potassium citrate neutralized the dietary acid load while having no significant effect on serum potassium levels in all but one participant and caused no gastrointestinal side effects. The decrease in markers of bone resorption in participants taking potassium citrate compared to placebo also suggests that exogenous alkalinizing potassium salts may directly benefit the skeleton, though the bone turnover data should be interpreted cautiously as the study was not powered on this outcome. Nonetheless, this finding is consistent with previous studies showing lower bone resorption during treatment with increased dietary or supplemental potassium (3;4;6;20). Overall, our data support the hypothesis that potassium citrate can ameliorate the bone loss that occurs with age.
The relationship between nutrition and the skeleton is complex, particularly with respect to the components of the diet that contribute to the dietary acid load. Acid precursors in the diet consist of sulfur containing amino acids present in proteins and cereal grains while base precursors consist of the alkaline potassium salts present in fruit and vegetables. While metabolized protein does generate acid, adequate protein is critically important for achieving peak bone mass and maintaining skeletal health. Epidemiologic evidence suggests that the relationship between dietary protein and the skeleton is multifaceted. Diets with high animal to vegetable protein intake ratios are associated with an increased rate of femoral neck bone loss and hip fracture (22). However, hunter-gatherer diets containing two to three times as much protein than typically consumed in Western diets are net base producing due to the high consumption of vegetable foods and lack of cereal grains in these diets (23). Thus, it is the balance between the acid and base generating components of the diet that potentially impacts calcium balance.
Given this importance of adequate protein consumption to bone accrual and maintenance, the optimal approach to reducing the acid:base ratio in the diet would be through increased consumption of base rather than reduction in protein intake. Indeed, over the last several decades, evidence has accrued demonstrating that exogenous base administration has beneficial effects on the calcium economy and measures of skeletal metabolism. Two previous short term balance studies have examined calcium metabolism during administration of alkaline potassium salts (3;5). While neither measured intestinal fractional calcium absorption directly, both demonstrated a decrease in urine calcium excretion with no impact on stool calcium excretion, leading to a net improvement in calcium balance. When measured directly using stable calcium isotopes, fractional calcium absorption was not affected by potassium citrate supplementation (24). Despite these data, controversy remains as to the net effect of potassium-containing foods (fruits, vegetables, dairy) on calcium metabolism, particularly in the long term. Specifically, in one study, women consuming diets designed to match their habitual self selected diet, dietary potassium intake was associated with lower urine calcium excretion, but also lower intestinal calcium absorption (10). The authors speculated that adaptation to dietary potassium intake occurs with time, leading to no appreciable net influence on the calcium economy in the long term. In this study, however, higher potassium intakes in participants came from more meat and milk than from fruits and vegetables, the former having fewer bicarbonate-generating organic anions (like citrate) compared to the latter. Thus potassium intake in this study may not have been a good surrogate for base intake, explaining the discrepant findings compared to the balance studies utilizing alkaline potassium salts.
Our data show that potassium alkali administration at doses of both 60 and 90 mmol/day decrease serum CTX, a marker of bone resorption. Other groups have noted reductions in other serum and urine markers of bone resorption (C- and N-telopeptides, P1NP, urine hydroxyproline) with potassium alkali treatment (3;4;7). Although our study did not find an increase in serum bsALP, serum osteocalcin levels, another marker of bone formation, increased with potassium bicarbonate treatment in postmenopausal women in a previous study (3). The high fruit and vegetable Dietary Approaches to Stop Hypertension (DASH) diet also resulted in a decrease in markers of bone resorption (6). Note, however, the diet included in this study was also high in dairy, thus providing calcium in addition to potassium and magnesium, which may have influenced outcomes.
It remains unknown whether dietary supplementation with alkaline salts can improve long-term bone density or fracture outcomes in persons at risk for ongoing bone loss, osteoporosis and fracture. One previous study of longer-term potassium salt administration in free living individuals demonstrated persistent urine calcium lowering effects over three years. In this study, men and women were given potassium bicarbonate at doses of 30, 60 and 90 mmol/day for 3 years. Though balance studies were not performed, subjects on potassium supplements maintained persistently lower urine calcium excretion (25), consistent with our data showing that longer term alkaline potassium supplementation has a persistent effect on the calcium economy.
The effects of potassium citrate administration on bone density are not clear, as there have been only two previous trials, one positive and one negative, looking at bone density as an outcome. However, both studies had significant limitations. The first and positive study involved the administration of low dose potassium citrate (30 mmol/day) versus potassium chloride (30 mmol/day) to postmenopausal women with low bone density (8). In this study, women receiving potassium citrate had higher bone density than those in the potassium chloride group at the end of 12 month. However, this study did not include a placebo; thus, it is difficult to know if the observed difference in bone density represented a beneficial effect of potassium citrate or an adverse effect of potassium chloride. The second and negative study showed no difference in bone density in healthy postmenopausal women after 24 months of potassium citrate or fruit and vegetable supplementation compared to women on placebo (9). However, this study utilized doses of potassium citrate (18.5 mmol/day and 55.5 mmol/day) and amounts of fruit/vegetable supplementation that may have been too low to impact calcium balance. Additionally, urine calcium was significantly lower only in the higher dose potassium citrate group and only at the six month timepoint in the 24 month trial, raising questions about adherence to or effectiveness of the potassium preparation used in the trial.
While both potassium groups in our study showed an improvement in calcium balance, this reached statistical significance only in the 90 mmol/day group. Additionally, net acid excretion was significantly lower in participants taking potassium citrate 90 mmol/day compared to those taking 60 mmol/day. We would argue that these findings imply potassium citrate 90 mmol/day has the greatest potential for preventing bone loss and fracture in the long-term.
Potassium citrate was well-tolerated at doses as high as 90 mmol daily with demonstrably positive effects on the calcium economy. Potassium citrate is a base found naturally in food products which is then metabolized to potassium bicarbonate and other agents. However, unlike potassium bicarbonate, it is available in oral preparations that are inexpensive and available in a slow release formula that minimizes gastrointestinal intolerance. In our study, there was only one study participant who became mildly hyperkalemic on a dose of potassium citrate 40 mmol/day. That result was surprising given that an extensive literature review and studies performed by our group have never before demonstrated even mild hyperkalemia with escalating doses of potassium salt administration. In future studies, participants’ serum potassium will require monitoring with alkali salt dose escalation.
There are limitations to our study. Though powered sufficiently for the calcium balance studies, the study was small and thus not generalizable to men and women of a wider age range or ethnicity. Power was limited for other outcomes such as the bone turnover markers. Similar to what is reported in the US population, our study participants had a positive mean net acid excretion at baseline, suggestive of a net acid-producing diet. However, because we selected men and women for the study with higher baseline urine calcium values, the effects of potassium citrate on calcium balance in those with lower baseline urine calcium cannot be determined.
The implications of our study are potentially far-reaching. Bringing together years of speculation and clinical studies, we show that potassium citrate positively and definitively benefits the calcium economy, relieving the skeleton of its role as a source of base reservoir. If the demonstrated improvement in calcium balance and decreased makers of bone resorption over 6 months continues, potassium citrate administration has the potential to mitigate age-related declines in bone density and strength associated with the Western diet. Readily available, safe, and easily administered in an oral form, potassium citrate also has the potential to reduce fracture in at-risk populations. Future studies are needed to determine the role that alkalinizing potassium salts will play in prevention and treatment of osteoporosis and the looming threat it poses to aging men and women in the Western world.
Acknowledgments
This work was funded by a contract from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (N01-AR-5-2275) and supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Potassium citrate and placebo were provided by Mission Pharmacal Company, San Antonio, TX. Mission Pharmacal had no role in study design, conduct of the study, data analysis, or the manuscript.
Disclosures
KM has nothing to disclose.
CW serves on the Advisory Board for Pharmavite.
LA has nothing to disclose.
AS has nothing to disclose.
DS has nothing to disclose.
Authors’ roles: Study design: DS, CW, LA. Study conduct: DS. Data collection: DS, CW. Data analysis: DS, KM. Data interpretation: DS, KM, AS, LA. Drafting manuscript: DS, KM. Approving final version of manuscript: All authors. DS takes responsibility for the integrity of the data analysis.
References
- 1.Frassetto L, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: Role of age-related renal functional decline. Am J Physiol. 1996;271:1114–22. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 2.Lemann JJ, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol. 2003 Nov;285(5):F811–32. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 3.Sebastian A, Morris RC., Jr Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;331(4):279. doi: 10.1056/NEJM199407283310421. [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B, Harris SS, Palermo NJ, et al. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009 Jan;94(1):96–102. doi: 10.1210/jc.2008-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemann J, Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int. 1989;35:688–95. doi: 10.1038/ki.1989.40. [DOI] [PubMed] [Google Scholar]
- 6.Lin PH, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003 Oct;133(10):3130–6. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 7.Marangella M, Di Stefano M, Casalis S, et al. Effects of Potassium Citrate Supplementation on Bone Metabolism. Calcif Tissue Int. 2004;74:330–5. doi: 10.1007/s00223-003-0091-8. [DOI] [PubMed] [Google Scholar]
- 8.Jehle S, Zanetti A, Muser J, et al. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006 Nov;17(11):3213–22. doi: 10.1681/ASN.2006030233. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald HM, Black AJ, Aucott L, et al. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr. 2008 Aug;88(2):465–74. doi: 10.1093/ajcn/88.2.465. [DOI] [PubMed] [Google Scholar]
- 10.Rafferty K, Davies KM, Heaney RP. Potassium intake and the calcium economy. J Am Coll Nutr. 2005 Apr;24(2):99–106. doi: 10.1080/07315724.2005.10719450. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 12.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011 Jan;96(1):53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Brit J Surg. 1995;82:216–22. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 14.Spence LA, Lipscomb ER, Cadogan J, et al. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr. 2005 Apr;81(4):916–22. doi: 10.1093/ajcn/81.4.916. [DOI] [PubMed] [Google Scholar]
- 15.Yergey AL, Abrams SA, Vieira NE, et al. Determination of fractional absorption of dietary calcium in humans. J Nutr. 1994 May;124:674–82. doi: 10.1093/jn/124.5.674. [DOI] [PubMed] [Google Scholar]
- 16.Lee W, McCabe GP, Martin BR, Weaver CM. Validation of a simple isotope method for estimating true calcium fractional absorption in adolescents. Osteoporos Int. 2011 Jan;22(1):159–66. doi: 10.1007/s00198-010-1203-8. [DOI] [PubMed] [Google Scholar]
- 17.Gyory AZ, Edwards KDG. Simultaneous titrimetric determination of bicarbonate and titratable acid of urine. Aust J Exp Biol Med Sci. 1967;45:141–7. doi: 10.1038/icb.1967.11. [DOI] [PubMed] [Google Scholar]
- 18.Lin SL, Chan JC. Urinary bicarbonate: a titrimetric method for determination. Clin Biochem. 1973 Sep;6:207–10. doi: 10.1016/s0009-9120(73)80028-1. [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW, Kamerud JQ, Kurkowski A, et al. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996 Apr;42(4):586–92. [PubMed] [Google Scholar]
- 20.Sellmeyer DE, Schloetter M, Sebastian A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J Clin Endocrinol Metab. 2002;87:2008–12. doi: 10.1210/jcem.87.5.8470. [DOI] [PubMed] [Google Scholar]
- 21.Spence LA, Lipscomb ER, Cadogan J, et al. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr. 2005 Apr;81(4):916–22. doi: 10.1093/ajcn/81.4.916. [DOI] [PubMed] [Google Scholar]
- 22.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr. 2001 Jan;73(1):118–22. doi: 10.1093/ajcn/73.1.118. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian A, Frassetto LA, Sellmeyer DE, et al. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002 Dec;76(6):1308–16. doi: 10.1093/ajcn/76.6.1308. [DOI] [PubMed] [Google Scholar]
- 24.Sakhaee K, Maalouf NM, Abrams SA, Pak CY. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2005 Jun;90(6):3528–33. doi: 10.1210/jc.2004-2451. [DOI] [PubMed] [Google Scholar]
- 25.Frassetto L, Morris RC, Jr, Sebastian A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. J Clin Endocrinol Metab. 2005 Feb;90(2):831–4. doi: 10.1210/jc.2004-1350. [DOI] [PubMed] [Google Scholar]