Abstract
Effects of concomitant inhibition of the PI3K/AKT/mTOR pathway and Bcl-2/Bcl-xL (BCL2L1) were examined in human myeloid leukemia cells. Tetracycline-inducible Bcl-2 and Bcl-xL dual knockdown sharply increased PI3K/AKT/mTOR inhibitor lethality. Conversely, inducible knockdown or dominant-negative AKT increased whereas constitutively active AKT reduced lethality of the Bcl-2/Bcl-xL inhibitor ABT-737. Furthermore, PI3K/mTOR inhibitors (e.g., BEZ235, PI-103) synergistically increased ABT-737-mediated cell death in multiple leukemia cell lines and reduced colony-formation in leukemic but not normal CD34+ cells. Notably, increased lethality was observed in 4/6 primary AML specimens. Responding, but not non-responding, samples exhibited basal AKT phosphorylation. PI3K/mTOR inhibitors markedly down-regulated Mcl-1 but increased Bim binding to Bcl-2/Bcl-xL; the latter effect was abrogated by ABT-737. Combined treatment also markedly diminished Bax/Bak binding to Mcl-1, Bcl-2 or Bcl-xL. Bax, Bak, or Bim (BCL2L11) knockdown, or Mcl-1 over-expression significantly diminished regimen-induced apoptosis. Interestingly, pharmacologic inhibition or shRNA knockdown of GSK3α/β significantly attenuated Mcl-1 down-regulation and decreased apoptosis. In a systemic AML xenograft model, dual tet-inducible knockdown of Bcl-2/Bcl-xL sharply increased BEZ235 anti-leukemic effects. In a subcutaneous xenograft model, BEZ235 and ABT-737 co-administration significantly diminished tumor growth, down-regulated Mcl-1, activated caspases, and prolonged survival. Together, these findings suggest that anti-leukemic synergism between PI3K/AKT/mTOR inhibitors and BH3 mimetics involves multiple mechanisms, including Mcl-1 down-regulation, release of Bim from Bcl-2/Bcl-xL as well as Bak and Bax from Mcl-1/Bcl-2/Bcl-xL, and GSK3α/β, culminating in Bax/Bak activation and apoptosis. They also argue that combining PI3K/AKT/mTOR inhibitors with BH3-mimetics warrants attention in AML, particularly in the setting of basal AKT activation and/or addiction.
Keywords: PI3K, Bcl-2, Bcl-xL, apoptosis, leukemia
INTRODUCTION
PI3K/AKT/mTOR is one of the most frequently dysregulated survival signaling pathways in cancer due to multiple genetic aberrations (1). In acute myelogenous leukemia (AML), this pathway is activated in 50–80% of patients (2) and is frequently associated with FLT3, Ras, and c-KIT mutations (2), PI3K delta isoform amplification (3), or autocrine IGF-1/IGF-1R signaling induction (4). Activation of PI3K leads to AKT activation, which signals to various downstream substrates including GSK-3, FOXO, mTOR, Bad, MDM2 and NF-κB, and modulates diverse cell processes including survival, proliferation, apoptosis, and autophagy, among others (5). Recently, multiple inhibitors of this pathway have been developed, several of which (e.g., BEZ235, GDC0981) are currently undergoing clinical evaluation in various tumor types including AML (6).
Over-expression of anti-apoptotic Bcl-2 members such as Bcl-2, Bcl-xL, and Mcl-1, occurs frequently in cancers, particularly hematological malignancies such as AML, resulting in defective apoptosis leading to enhanced cell survival and drug resistance (7). Several agents have been developed to target these proteins directly e.g., ABT-737, a BH3 mimetic that binds with high affinity to and antagonizes the functions of Bcl-2 and Bcl-xL but not Mcl-1 (8). Preclinical studies demonstrated that ABT-737 induces apoptosis and potentiates the anti-tumor activity of multiple agents in various cancers, including leukemia (8). ABT-263, a clinical derivative of ABT-737 is currently undergoing phase I and II clinical evaluation in various tumor types including leukemia (9).
Recent studies indicate that PI3K inhibitors efficiently down-regulate Mcl-1, an event that plays an important role in transformed cell lethality (10–12). Furthermore, data from several laboratories, including our own, indicate that Mcl-1, as well as Bim, which is also tightly regulated by the PI3K pathway (13, 14), play important roles in determining ABT-737 sensitivity (15–18). These considerations, together with evidence of a contribution of Bcl-2 and Bcl-xL dysregulation in leukemogenesis (7), raise the possibility that interference with Bcl-2 and Bcl-xL function might potentiate PI3 kinase inhibitor activity in this disease. The purpose of the present studies was to determine whether, and by what mechanisms, dual inhibition of Bcl-2/Bcl-xL might cooperate with PI3K/mTOR inhibition to induce cell death in AML cells.
METHODS
Cells
Human leukemia U937, KG1, and MV4-11 cells were purchased from American Type Culture Collection (ATCC) and cultured as before (11). These cells were authenticated by ATCC (Basic STR Profiling) at the end of the studies. Various stable or inducible cell lines used in these studies were described in Supplementary Methods.
Isolation of patient-derived leukemic blasts and normal CD34+ cells
Bone marrow or peripheral blood were collected from patients with acute myeloblastic leukemia (AML), FAB subtype M2, with a preponderance of blasts, and further enrichment of mononuclear cell populations achieved by Ficoll-Hypaque gradient separation as we have previously described (19). Normal bone marrow CD34+ cells were obtained with informed consent from patients undergoing routine diagnostic procedures for non-myeloid hematopoiethc disorders as before (20). These studies have been sanctioned by Virginia Commonwealth University Investigational Review Board.
Mutation analysis
Mutation analysis was performed on genomic DNA extracted from primary blasts as previously described (11).
Reagents
ABT-737 was provided by Abbott laboratories (Abbott Park, IL). BEZ235 was purchased from Chemie Tek (Indianapolis, IN). PI-103, LY294002, GSK3 inhibitor IX (2'Z,3'E)-6-bromoindirubin-3'-oxime (BIO), and its inactive analogue MeBIO were purchased from Calbiochem. CHIR-98014 was purchased from Sellek chemicals. MK-2206 was provided by Merck.
Assessment of apoptosis
Apoptosis was routinely assessed by Annexin V/PI analysis as previously described (21). TUNEL analysis was also employed in some experiments as before (22), and visualized by confocal microscopy.
Cell growth and viability
Cell growth and viability were assessed by CellTiter-Glo Luminescent Assay (Promega).
Clonogenicity
Colony-formation assays were performed in methylcellulose as previously described (11).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as previously described (19, 21). Antibodies are listed in Supplementary Materials.
Bax and Bak conformational change
Bax and Bak conformational change was assessed as previously described (11).
Subcellular fractionation
Cytosolic and membrane fractions were separated as previously described (19).
In vivo studies
Animal studies were conducted under an approved protocol by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Two murine models were used: 1) Systemic xenograft model: female NOD/SCID-gamma (Jackson laboratories) were injected intravenously via tail vein with 5×106 luciferase-expressing U937 cells in which double knockdown of Bcl-2 and Bcl-xL is achieved by doxycycline. Mice were monitored for tumor growth using the IVIS 200 imaging system (Xenogen Corporation, Alameda, CA), separated into 2 groups, one of which was fed with doxycycline-supplemented pellet (200 mg/kg, Bio-Serv, Frenchtown, NJ). Both groups were treated every 24 h 6 days a week with BEZ235 administered by gavage. 2) Subcutaneous model: female athymic nude mice (Charles River laboratories) were inoculated subcutaneously in the flank with 2.5 × 106 U937 cells. Once tumors became apparent, mice were treated twice daily 5 days per week with BEZ235 ± ABT-737 administered intraperitoneally. Tumor volumes were calculated using the formula (length × width2)/2, and when tumor length reached 2 cm, mice were euthanized.
Statistical analysis
The significance of differences between experimental conditions was determined using the Student's t test for unpaired observations. Survival rates were analyzed by Kaplan–Meyer and comparisons of survival curves and median survival were analyzed by logrank test.
RESULTS
Ectopic expression of Bcl-2 or Bcl-xL increases leukemia cell resistance to apoptosis induced by inhibitors of the PI3K/AKT pathway
To test the hypothesis that Bcl-2 and Bcl-xL confer resistance to PI3K/AKT pathway inhibition in leukemia cells, U937 cells ectopically overexpressing Bcl-2 or Bcl-xL were employed. These cells displayed significant resistance to the dual PI3K/mTOR inhibitors BEZ235 and PI-103, and the AKT inhibitor perifosine (Supplementary Figure 1A), as well as the PI3K inhibitor LY294002 (data not shown), raising the possibility that Bcl-2 and Bcl-xL inhibition may potentiate leukemia cell apoptosis induced by PI3K/AKT/mTOR pathway inhibitors.
Dual knockdown of Bcl-2 and Bcl-xL strikingly potentiates PI3K inhibitor-mediated apoptosis in U937 cells
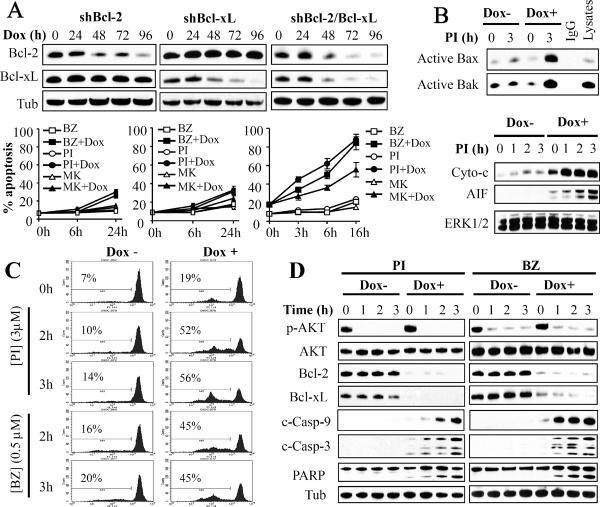
To determine whether Bcl-2 and Bcl-xL disruption in U937 cells enhances PI3K-mediated apoptosis, inducible knockdown of Bcl-2, Bcl-xL, or both was carried out using a tetinducible shRNA lentiviral system. Time course analysis (Figure 1A, upper panel) revealed that doxycycline induced a sharp decline in levels of Bcl-2, Bcl-xL, or both in the corresponding cells which was observed after 48 h exposure and persisted over the ensuing 48 h. Interestingly, while individual knockdown of Bcl-2 or Bcl-xL modestly but significantly increased BEZ235, PI-103, or MK-2206 lethality following 6–24 h exposure (Figure 1A; lower panel), rapid and striking (e.g., 3 h) apoptosis was observed in cells in which both Bcl-2 and Bcl-xL were knocked down (Figure 1A; lower panel). Similar results were obtained with LY294002 (data not shown) and when cell growth and viability were monitored (Supplementary Figure 1B). These events were associated with pronounced Bax and Bak conformational changes (e.g., by PI-103; Figure 1B, upper panel), reflecting activation, rapid and profound cytosolic release of cytochrome c and AIF (Figure 1B, lower panel), loss of mitochondrial membrane potential (by PI-103 and BEZ235; Figure 1C), and apoptosis, reflected by caspase-3,-9, and PARP cleavage (Figure 1D). In contrast, agents exhibited minimal effects in the absence of doxycycline (Figures 1A–D). Notably, AKT was rapidly dephosphorylated/inactivated by PI3K/mTOR inhibitors (e.g., BEZ235 or PI-103) with or without doxycycline (Figure 1D). Conversely, inducible expression of DN-AKT or shAKT knockdown significantly increased whereas constitutively active AKT-DD significantly decreased ABT-737 lethality, reflected by caspase-3/PARP cleavage and Annexin V/PI positivity (Supplementary Figures 2A–C). Collectively, these findings indicate that the PI3K/AKT/mTOR pathway and Bcl-2/Bcl-xL interact coordinately to promote cell survival, suggesting that simultaneous targeting of these pathways may represent an effective antileukemic strategy.
Figure 1. Dual Bcl-2/Bcl-xL knockdown strikingly potentiates PI3K inhibitor-mediated apoptosis in U937 cells.
(A, upper panels) Western blot (WB) analysis of U937 cells displaying inducible knockdown of Bcl-2 ± Bcl-xL following exposure to 1 μg/ml doxycycline (Dox). Alternatively, cells were left untreated or treated with doxycycline for 72 h, then exposed to BEZ235 (BZ; 0.5 μM), PI-103 (PI; 3 μM), or MK-2206 (MK; 3 μM) for the designated intervals, after which Annexin V/PI (A, lower panels), Bax/Bak conformational change (active Bax/Bak; B, upper panel), cytochrome c/AIF release (B, lower panel), loss of Δψm (C), or AKT phosphorylation and caspase activation (D) were monitored. Error Bars: S.D of 4 independent experiments.
Co-treatment with PI3K/mTOR inhibitors and ABT-737 strikingly induces apoptosis in human leukemia cells
Dose response analysis of U937 cells revealed that while 0.5 μM ABT-737 was minimally toxic by itself, it substantially increased cell death induced by BEZ235 (e.g., 50–1000 nM; Figure 2A) or PI-103 (e.g., 1–4 μM, Figure 2B). Conversely, marginally toxic concentrations of BEZ235 (0.5 μM) or PI-103 (3 μM) sharply increased ABT-737 lethality (e.g., 0.25 – 2 μM; Figure 2C). Time course analysis of cells exposed simultaneously to BEZ235 (0.5 μM) and ABT-737 (0.5 μM) revealed approximately 20% cell death at 8 h, and substantially more pronounced lethality after 18 h (70 %, Supplementary Figure 3). Similar results were obtained in multiple other leukemia cell lines, including FLT3-ITD-dependent MV4-11 and KG1 cells (Figure 2D). Treatment of U937 cells with BEZ235 and ABT-737 induced pronounced cytosolic release of cytochrome c and AIF, membrane translocation of Bax (Figure 2E), and cleavage/activation of caspases-3, and -9, and PARP (Figure 2F). In contrast, BEZ235 or ABT-737 alone had only minimal effects (Figures 2E–F).
Figure 2. PI3K/mTOR inhibitors and ABT-737 strikingly induce apoptosis in human leukemia cells.

Annexin V/PI analysis of U937 cells following 18 h exposure to 0.5 μM ABT-737 and the designated concentrations of BEZ235 (A) or PI-103 (B), the designated concentrations of ABT-737 and 0.5 μM BEZ235 or 3 μM PI-103 (C). Error Bars: S.D of 3 independent experiments. (D) Annexin V/PI analysis in KG1 and MV4-11 cells following 24 h exposure to BEZ235 (500 nM and 300 nM respectively) ± ABT-737 (200 nM and 10 nM respectively). (E–F) Western Blot (WB) analysis.
Co-treatment with BEZ235 and ABT-737 sharply increases lethality and reduces colony formation of primary AML blasts while largely sparing normal CD34+ cells
TUNEL assays of primary AML blasts revealed that simultaneous, but not individual, administration of BEZ235 and ABT-737 markedly increased apoptosis (Figure 3A), associated with marked AKT dephosphorylation and pronounced caspase-3 and PARP cleavage (Figure 3B), similar to results obtained in U937 cells. Moreover, BEZ235/ABT-737 co-exposure enhanced cell death in 4 out of 6 AML specimens assayed (Figure 3C). In contrast, comparable BEZ235 and ABT-737 exposures, alone or in combination, exerted only minimal toxicity toward normal CD34+ cells (Figure 3D). Interestingly, all responding AML samples exhibited discernible basal AKT phosphorylation/activation (Figure 3E). In contrast, non-responding specimens (#1 and #6), like normal CD34+ cells, displayed no detectable phospho-AKT (Figure 3E). Protein levels of Bcl-2, Bcl-xL, Mcl-1, and Bim exhibited considerable variability among the samples analyzed. Importantly, 2 responding samples exhibited high Mcl-1 protein levels. Mutation analysis revealed that 2 of the responding specimens harbored FLT3 mutations, including a point mutation (FLT3/D835) in patient #2 and internal tandem duplication (FLT3/ITD) in patient #3. However, no mutations in PI3Kα, AKT1, Kras, Nras, or MEK1 were detected (Supplementary Table 1).
Figure 3. BEZ235/ABT-737 induces apoptosis in and reduces colony formation of primary AML blasts while sparing normal CD34+ cells.

TUNEL assay using confocal microscopy (A) and WB analysis (B) in primary AML cells (AML #2) exposed to BEZ235 (200 nM) ± ABT-737 (25 nM) for 24 h. (C) Annexin V/PI following treatment (24 h) as in (A) of six primary AML (peripheral blood: 1, 2, and 5; bone marrow: 3, 4, and 6; blast percentage of unseparated white cells were 70, 80, 74, 70, 90, and 56% respectively). Samples were further enriched for mononuclear cells by Ficoll separation. (D) Annexin V/PI analysis of two normal CD34+ specimens following 24 h treatment. Results are presented as the percentage of dead cells for each treatment using the formula ([treatment – control]/[100 – control]) × 100. (E) WB of six primary AML (left panel), three normal CD34+ specimens (N#1 and N#2 as in Figure 3D and an additional sample N#3, right panel). Colony-forming units (CFUs) for two primary AML samples AML#2 and an additional sample AML#7 exhibiting AKT phosphorylation as monitored by Western blot (F and inset) and the three normal CD34+ specimens shown in Figure 3E (G) treated with ABT-737 (25 nM) ± BEZ235 (100 nM) or LY294002 (LY; 3 μM) expressed as percentage relative to untreated cells. Error Bars: S.D of 3 replicates; *, p < 0.01.
Parallel colony-forming assays revealed that combined, but not individual, treatment with BEZ235 or LY294002 and ABT-737 strikingly reduced leukemic cell colony formation (Figure 3F). In contrast, the colony forming capacity of normal CD34+ cells was not significantly reduced by agents alone or in combination (Figure 3G).
Mcl-1 downregulation contributes to BEZ235/ABT-737 lethality
BEZ235 significantly reduced Mcl-1 protein levels in U937 cells (Figure 4A) as well as primary AML blasts (Figure 4B, upper panel) and KG1 cells (data not shown). Moreover, Mcl-1 down-regulation by BEZ235 persisted in cells co-treated with ABT-737, which by itself increased Mcl-1 protein levels (Figure 4A), consistent with reports involving other cells (23). Notably, ABT-737 significantly increased Noxa protein levels, but, expression was sharply diminished by treatment with BEZ235 administered alone or in combination with ABT-737 (Figure 4A). A modest increase in Bax expression was observed following exposure to agents alone or in combination (Figure 4A). In contrast, no major changes occurred in expression of other anti-apoptotic family members i.e., Bcl-2 or Bcl-xL or pro-apoptotic members Bim, Bad, Bak, or NBK (Figure 4A). Notably, increased Mcl-1 protein levels induced by the Bcl-2/Bcl-xL antagonist ABT-737 were recapitulated genetically by dual Bcl-2/Bcl-xL knockdown (Figure 4B, lower panel).
Figure 4. Mcl-1 downregulation plays a functional role in BEZ235/ABT-737 lethality and involves Bax and Bak.
WB of U937 cells (A), primary AML blasts (B, upper panel), and tet-inducible Bcl-2/Bcl-xL dual knockdown (B, lower panel) following exposure to BEZ235 (U937: 0.5 μM; AML: 200 nM) ± ABT-737 (U937: 0.5 μM; AML: 25 nM). (C) Annexin V/PI in U937 cells ectopically expressing Mcl-1 following treatment (18 h) with BEZ235 ± ABT-737 (0.5 μM each). Error Bars: S.D of 3 independent experiments; *, p < 0.01. (D) Bax/Bak conformational change in U937 cells following 16 h exposure to 0.5 μM ABT-737 ± 0.5 μM BEZ235 or 3 μM PI-103. WB of Bak (E) or Bax (F) immunoprecipitates or input lysates prepared from U937 cells exposed to BEZ235 ± ABT-737 (0.5 μM each). (G) Apoptosis in Bax- or Bak-knockdown U937 cells treated for 18 h as in (D). Error Bars: S.D of 3 independent experiments; *, p < 0.02 for shBak, and p < 0.01 for shBax.
Finally, U937 cells ectopically expressing Mcl-1 were significantly more resistant than control cells to combined treatment with BEZ235/ABT-737 (Figure 4C), arguing for a significant functional role for Mcl-1 down-regulation in PI3K/mTOR inhibitor/ABT-737 lethality.
Bax and Bak play important functional roles in BEZ235/ABT-737 anti-leukemic activity
Immunoprecipitation studies revealed that combined, but not individual, treatment with BEZ235 and ABT-737 induced pronounced Bax and Bak conformational change (Figure 4D. Furthermore, the amount of Bak or Bax bound to Mcl-1 sharply declined following 4-8 h treatment with BEZ235 (Figures 4E and 4F respectively). Decreased Bak/Bcl-xL or Bax/Bcl-2 binding was also observed at 8 h of treatment. In addition, ABT-737 markedly diminished binding of Bak to Bcl-xL and of Bax to both Bcl-2/Bcl-xL, but not to Mcl-1. However, combined treatment with BEZ235/ABT-737 sharply reduced Bak and Bax binding to all anti-apoptotic members Bcl-2, Bcl-xL, and Mcl-1 (Figures 4E, and 4F).
Finally, Bax or Bak knockdown significantly protected cells from BEZ235/ABT-737 lethality, reflected by diminished caspase-3 and PARP cleavage (Supplemental Figure 4A) and Annexin V/PI positivity (Figure 4G). Together, these findings demonstrate that BEZ235 and ABT-737 exposure releases Bak and Bax from the major neutralizing molecules Bcl-2, Bcl-xL, and Mcl-1, and that Bax and Bak play significant functional roles in regimen anti-leukemic activity.
BEZ235/ABT-737-mediated apoptosis involves Bim but not Bad
Immunoprecipitation studies revealed that while BEZ235 reduced the amount of Mcl-1 bound to Bim presumably reflecting Mcl-1 downregulation, it markedly increased Bim binding to Bcl-2 and Bcl-xL (Figure 5A). Significantly, these effects were essentially abrogated by ABT-737 co-treatment. Similar events occurred with LY294002 and PI-103 (Supplementary Figure 4B). Notably, U937 Bim knockdown cells displayed marked resistance to combined treatment with ABT-737 and BEZ235 or PI-103 compared to controls (Figures 5B–C). Similar results were obtained in Bim knockdown MV4-11 cells (Supplementary Figure 4C). In contrast, Bad knockdown cells were fully sensitive to these treatments (Figure 5C). Comparable results were observed when ABT-737 was combined with LY294002 (data not shown). In accord with evidence of a significant role for Bim and Bax/Bak in BEZ235/ABT-737 lethality, ectopic expression of Bcl-2 or Bcl-xL, which, like Mcl-1 sequester Bim, and Bax/Bak (24, 25), significantly protected cells against ABT-737 and BEZ235 or PI-103 lethality (Supplementary Figure 5).
Figure 5. Bim, but not Bad, plays a functional role in BEZ235/ABT-737 lethality.

(A) WB of Bim immunoprecipitates or input lysates of U937 cells treated with BEZ235 ± ABT-737 (0.5 μM each). (B–C) WB and Annexin V/PI in Bim or Bad knockdown, or negative control (NC; scramble shRNA) U937 cells following 18 h exposure to ABT-737/BEZ235 (0.5 μM each) or ABT-737/PI-103 (3 μM). Error Bars: S.D of 3 independent experiments; *, p < 0.01.
GSK3α/β plays a functional role in BEZ/ABT lethality
To determine whether GSK3α/β, a major downstream target of AKT, contributed to the lethality of concomitant PI3K pathway and Bcl-2/Bcl-xL inhibition, multiple approaches were employed. Exposure to BEZ235 alone or with ABT-737 sharply decreased β-catenin and c-Myc, two well established GSK3α/β substrates, indicating GSK3α/β activation (26, 27) (Figure 6A). A modest decrease in GSK3 phosphorylation also occurred after BEZ235/ABT-737 treatment. Notably, the GSK3 inhibitors CHIR-98014 or BIO, but not the inactive analogue MeBIO, significantly attenuated BEZ235 anti-leukemic activity in Bcl-2/Bcl-xL doxycycline knockdown cells (Figure 6B). Furthermore, BEZ235/ABT-737 lethality was also significantly reduced by CHIR-98014 or BIO, but not MeBIO (Figure 6C). Of note, these GSK3 inhibitors alone exhibited no lethal effects (Supplemental Figure 6A). Similar results were obtained when cell growth and viability were assessed (Supplemental Figure 6B). Interestingly, effects of GSK3 inhibitors on BEZ235/ABT-737 lethality correlated with significant attenuation of Mcl-1 down-regulation, complete reversal of β-catenin and c-Myc down-regulation, and substantially decreased caspase-3 activation by CHIR-98014 or BIO, but not by MeBIO (Figure 6D).
Figure 6. Role of GSK3α/β in BEZ235/ABT-737 lethality.
(A) WB in U937 cells following exposure (24 h) to BEZ235 ± ABT-737 (0.5 μM each). Viability assay (B) in tet-inducible Bcl-2/Bcl-xL dual knockdown U937 cells following 24 h treatment with BEZ235 ± 2 μM BIO, MeBIO, or CHIR-98014 (CHIR) in the presence or absence of doxycycline (1 μg/ml, added 72 h prior to treatment). Error Bars: S.D of 3 independent experiments; *, p < 0.01 in each case. Annexin V/PI (C) and WB (D) in U937 cells following 16 h exposure to BEZ235/ABT-737 (0.5 μM each) in the presence or absence of BIO, MeBIO, or CHIR-98014 (2 μM each). Error Bars: S.D of 3 independent experiments; * p < 0.05. WB (E) and Annexin V/PI (F) in GSK3α or GSK3β knockdown U937 cells following 16 h exposure to BEZ235/ABT-737 (0.5 μM each). Error Bars: S.D of 3 independent experiments; *, p < 0.05 for shGSK3α and p < 0.02 for shGSK3β.
Parallel studies revealed that GSK3α or GSK3β shRNA knockdown also significantly reduced BEZ235/ABT-737-mediated caspase-3 and PARP cleavage (Figure 6E), and cell death (Figure 6F). Notably, these effects were associated with attenuation of Mcl-1 down-regulation (Figure 6E). Together, these findings argue for a functional role for GSK3α/β activation in lethality induced by concurrent Bcl-2/Bcl-xL and PI3K pathway inhibition, and implicate Mcl-1 down-regulation/degradation in this phenomenon.
Concomitant PI3K and Bcl-2/Bcl-xL inhibition increases apoptosis, inhibits, tumor growth, and enhances survival in in vivo leukemia xenograft models
To determine whether antagonizing Bcl-2 and Bcl-xL functions enhances PI3K inhibition lethality in vivo, a systemic xenograft mouse model employing luciferase-labeled U937 cells expressing dox-inducible shRNAs against both Bcl-2 and Bcl-xL was employed. Interestingly, BEZ235 significantly reduced in vivo tumor growth following dual Bcl-2/Bcl-xL knockdown compared to control tumors with intact Bcl-2/Bcl-xL (Figure 7A), associated with prolonged median survival i.e., from 13 to 23 days (Supplementary Figure 7A). Survival curves also differed significantly; p < 0.03 by logrank test. No effects of doxycycline alone on tumor growth or survival were observed (not shown).
Figure 7. In vivo anti-leukemic activity of combined BEZ235 and ABT-737 treatment.
(A) NOD/SCID/gamma mice were tail-vein inoculated with tet-inducible Bcl-2/Bcl-xL dual knockdown U937 cells expressing luciferase, treated with BEZ235 (45 mg/Kg) ± doxycycline, and imaged using the IVIS 200 system. (B) Tumor growth in nude mice bearing subcutaneous U937 xenografts and treated with BEZ235 (35 mg/kg) ± ABT-737 (100 mg/kg) twice a day (at hour 0 and at hour 18). Error bars: SD of 3 independent experiments involving 5 mice/condition each. *, p < 0.02 versus either agent alone. (C) Photographs of 2 representative tumors for each group following 8 days of treatment. (D) Two sets of xenograft-bearing mice were treated as in (B) twice over a 24 h interval after which tumors were excised, lysed, and subjected to WB. (E) Kaplan-Meyer survival plot involving 12–13 mice/condition treated as in (B). Mice that accidentally died during the treatment procedure or were sacrificed before the maximal tumor size was reached (e.g. due to tumor bleeding) were not included in this analysis. The survival curves were significantly different for combined treatment compared to either BEZ235 or ABT-737 alone; p < 0.01; logrank test.
Parallel studies employing a subcutaneous U937 xenograft mouse model revealed that ABT-737/BEZ235 co-treatment significantly reduced tumor growth (Figure 7B–C, and Supplementary Figure 7B), In contrast, effects were less pronounced with BEZ235 and no effect was observed with ABT-737. Western blot analysis performed on excised tumor tissue from treated animals revealed that BEZ235 alone or with ABT-737 markedly decreased Mcl-1 protein levels and AKT phosphorylation, analogous to in vitro observations (Figure 7D). Interestingly, combined but not individual treatment sharply potentiated apoptosis manifested by a pronounced increase in caspase-3 processing and PARP cleavage (Figure 7D). Finally Kaplan-Meier analysis (Figure 7E) revealed that combined treatment significantly prolonged survival (p < .01 versus single agent treatment; logrank test) whereas no effect was observed with ABT-737, and only a modest prolongation with BEZ235. Notably, mouse weights did not exhibit major changes (e.g., > 10%) in mice treated with agents alone or in combination (Supplementary Figure 7C). Together, these findings indicate that combined treatment with BEZ235 and ABT-737 significantly inhibits tumor growth in vivo in association with diminished AKT phosphorylation, Mcl-1 down-regulation, apoptosis induction, and prolonged survival in leukemia-bearing mice.
DISCUSSION
Pre-clinical studies have shown that AML cells are susceptible to PI3K inhibitors (28, 29). However, as in the case of other targeted agents, interruption of a single signaling pathway may be insufficient to induce cell death (30). Strategies to enhance the anti-tumor activity of PI3K inhibitors include simultaneously interrupting the MEK/ERK pathway (11), inhibiting histone deacetylases (31, 32), or cyclin-dependant kinases (CDKs) (33) among others. Here, dual inhibition of Bcl-2 and Bcl-xL by the BH3 mimetic ABT-737 was employed to potentiate PI3K inhibitor anti-leukemic activity based on several considerations. First Bcl-2 family members are frequently dysregulated in transformed cells e.g, over-expression of Bcl-2, Bcl-xL, or Mcl-1 (7), and/or diminished expression/loss of Bim, Bax, Noxa, and Bik (34–36). Second, PI3K inhibitors disrupt the balance between the pro- and anti-apoptotic Bcl-2 members via down-regulation of Mcl-1 and up-regulation/activation of Bak, Bax, Bim, and Bad (10). Third, these events, particularly Mcl-1 down-regulation, play important roles in determining sensitivity to Bcl-2/Bcl-xL inhibitors (e.g., ABT-737) (15–17).
The observation that tet-inducible dual knockdown of Bcl-2 and Bcl-xL sharply increases, whereas ectopic expression significantly diminishes PI3K inhibitor lethality indicate that these anti-apoptotic proteins play critical functional roles in protecting leukemic cells from lethality triggered by PI3K pathway interruption. Conversely, evidence that expression of tet-responsive dominant-negative or shRNA constructs against AKT substantially increase whereas constitutively active AKT decrease ABT-737 lethality argues that the PI3K/AKT pathway plays an important functional role in protecting leukemia cells from BH3 mimetic-induced cell death. Notably, individual Bcl-2 or Bcl-xL knockdown also potentiated PI3K/AKT inhibitor lethality, but enhancement was significantly less than for dual Bcl-2 and Bcl-xL knockdown. Such findings suggest that Bcl-2 and Bcl-xL cooperate to prevent apoptosis induced by PI3K/AKT pathway interruption. Consistent with this notion, simultaneous pharmacologic inhibition of the PI3K/AKT pathway (e.g., by BEZ235) and Bcl-2/Bcl-xL (e.g., by ABT-737) recapitulated the lethality of genetic interventions, and markedly reduced colony formation of multiple AML cell lines and primary blast specimens. Importantly, this regimen exerted only modest toxicity toward normal hematopoietic progenitors (CD34+). This may reflect the preferential toxicity of BH3 mimetics such as ABT-737 toward transformed cells (8, 15), as well as the dependence of leukemic cells on PI3K/AKT activation for survival (2).
Between 50–80% of patient-derived AML cells display phosphorylation of AKT (2). Analysis of AKT phosphorylation patterns in primary blasts raises the possibility of a correlation between AKT activation and responses to combined treatment with PI3K inhibitors and ABT-737. Specifically, 4 out of 6 specimens analyzed responded to the treatment and all exhibited basal AKT phosphorylation. In contrast, basal AKT phosphorylation was not detected in non-responding specimens or in normal CD34+ cells. While leukemic cell AKT activation may be multi-factorial e.g. secondary to c-Kit or FLT3 mutations, IGF-R activation etc., or infrequently, mutations in PI3K or AKT (2), in many instances, mechanisms of AKT activation are unknown. Of note, only two of the four specimens displaying AKT activation had identifiable mutations (i.e. FLT3). Regardless of the cause, a subset of leukemia cells may be addicted to this pathway, manifested by basal AKT activation, and thus particularly sensitive to PI3K/AKT inhibitor/BH3 mimetic regimens. However, analysis of a substantially larger number of specimens, taking into account expression of other relevant proteins (e.g., Mcl-1 and Bcl-2/Bcl-xL), and the possible confounding effects of cell sample heterogeneity, will be required to assess potential correlations between basal AKT status and responses more definitely. Similar considerations apply to comparisons between the relative abilities of AKT-associated mutations versus basal activation status in predicting sensitivity. Such studies are currently in progress. Of note, some of the responding specimen (e.g., #2 and #4) exhibited high basal levels of Mcl-1, an anti-apoptotic protein implicated in leukemogenesis (37, 38), raising the possibility that this strategy may be effective in AML characterized by high Mcl-1 expression.
The ability of dual PI3K/mTOR inhibitors such as BEZ235 or PI-103 to down-regulate Mcl-1 in leukemia cells is consistent with results involving other cell types (39). Classically, this effect has been attributed to multiple mechanisms: a) transcriptional, via inhibition of the CREB transcription factor (40), b) translational, via inhibition of the mTOR/p70S6K/4EBP axis (41), and c) post-translational, through enhanced Mcl-1 proteasomal degradation (42, 43). The finding that ectopic Mcl-1 expression significantly attenuated PI3K/mTOR inhibitor/ABT-737 lethality argues that Mcl-1 down-regulation contributes functionally to cell death. This is supported by the observation that ABT-737 administration or Bcl-2/Bcl-xL knockdown, increased Mcl-1 protein levels, a phenomenon shown to confer a survival advantage to leukemia cells (17, 23). Interestingly, while BEZ235 alone down-regulated Mcl-1, it did not significantly increase lethality. It is likely that Mcl-1 down-regulation cooperates with disruption of Bcl-2 and Bcl-xL function, and potentially Mcl-1-independent effects of BEZ235, to trigger an increase in apoptosis (15–17).
PI3K/AKT pathway inhibition leads to GSK3 activation, which may exert anti-tumor effects through multiple downstream targets, including Mcl-1, Bim, FOXO-3, β-catenin, and c-Myc, among others (44, 45). The finding that direct inhibition of GSK3 by BIO or CHIR-98014 significantly diminished BEZ235 lethality in cells in which Bcl-2 and Bcl-xL were knocked down, along with diminished BEZ235/ABT-737 lethality in cells in which GSK3 was disabled (e.g., by BIO or CHIR-98014 or by shRNA-knockdown) argue that GSK3α/β plays a significant functional role in lethality. Because GSK3 promotes Mcl-1 proteasomal degradation (42, 43), GSK3 knockdown or inhibition may protect cells from BEZ235/ABT-737 lethality by preventing this process. In support of this concept, Mcl-1 down-regulation in BEZ235/ABT-737-treated cells was attenuated when GSK3 was inhibited pharmacologically or by shRNA. However, Mcl-1 down-regulation was not completely prevented, suggesting alternative mechanism(s) of Mcl-1 regulation. It is recognized that Mcl-1 down-regulation may not be the sole mechanism by which GSK3 promotes BEZ235/ABT737 lethality, and that alternative or additional GSK3 downstream events may contribute to this process. Nevertheless, these data are compatible with the notion that GSK3 contributes functionally to BEZ235/ABT737 lethality through a mechanism involving Mcl-1 downregulation.
PI3K/mTOR inhibitors and ABT-737 co-administration induced multiple perturbations in the expression of and interactions between Bcl-2 family members, which collectively may have contributed to cell death. Recent studies have highlighted the importance of interactions involving proteins such as Bim, rather than simply expression patterns (e.g., of Mcl-1) in determining cell fate (46). In the present study, PI3K/mTOR inhibitors profoundly modulated Bim distribution manifested by a marked increase of Bim binding to Bcl-2 and Bcl-xL. This phenomenon, which to the best of our knowledge, has not previously been described, could have contributed to cell death attenuation. Significantly, ABT-737 completely abrogated Bim binding to Bcl-2/Bcl-xL, which may have allowed Bim to activate Bax and Bak, thereby exerting pro-apoptotic actions. This notion is supported by the observed release of Bak and Bax from all major antiapoptotic Bcl-2 members. Specifically, ABT-737 exposure abrogated binding of Bak to Bcl-xL, and Bax to Bcl-2 and Bcl-xL. Moreover, binding of both Bak and Bax to Mcl-1 was also markedly reduced by PI3K inhibitors, presumably a consequence of Mcl-1 down-regulation. In this context, Bak and Bax are normally maintained in an inactive state in part by tethering to Bcl-2, Bcl-xL, and Mcl-1, and simultaneous inhibition of these processes dramatically increases cell death (47). It should be noted that while many studies have described a physical binding between Mcl-1 and Bax in diverse cell types (46, 48), other studies reported weak or no binding between these proteins (47, 49). A possible explanation for these discrepancies may reflect the stoichiometric abundance of other Mcl-1 partners (e.g., Noxa, Bak) as well as Bax partners (e.g., Bcl-2 and Bcl-xL) in various cells. Collectively, these findings support a model wherein combined PI3K inhibitor and ABT-737 exposure leads to multiple events promoting cell death including a) Bim release from Bcl-2/Bcl-xL (by ABT-737) or from Mcl-1 (by PI3K/mTOR inhibitor); b) Bak and Bax release from Bcl-2/BclxL/Mcl-1; and c) Bak/Bax activation and apoptosis. This interpretation is supported by evidence that Bim, Bax, or Bak shRNA knockdown, or ectopic expression of Mcl-1, Bcl-2, or Bcl-xL significantly diminished PI3K/mTOR inhibitor/ABT-737 mediated lethality. Finally, previous studies have implicated BAD in PI3K inhibitor lethality (50). However, Bad shRNA knockdown, in sharp contrast to Bim, failed to protect leukemia cells from BEZ235/ABT-737 lethality, suggesting that Bad does not play a major role in this setting.
Finally, in vivo studies demonstrated that simultaneous inhibition of Bcl-2/Bcl-xL and the PI3K/AKT pathway markedly inhibited leukemia growth and increased survival in an AML xenograft model in association with several events observed in vitro i.e., AKT dephosphorylation/inactivation, Mcl-1 down-regulation, and caspase activation, suggesting that sufficiently high BEZ235 and ABT-737 concentrations can be achieved in vivo to recapitulate in vitro actions. Of note, little toxicity was observed in animals with the doses and schedules used in these studies. The present findings also suggest that multiple factors contribute to these interactions, including Mcl-1 down-regulation, perturbations in associations between pro- (e.g., Bim, Bak, and Bax) and anti-(e.g., Bcl-2/Bcl-xL/Mcl-1) proteins, and raise the possibility that basal AKT activation status may predict susceptibility to this approach. Collectively, these studies argue that strategies employing PI3K/mTOR inhibitors to enhance the anti-leukemic activity of BH3-mimetics such as ABT-737 warrants attention in AML, particularly in the setting of disease in which cells may be addicted to AKT. Accordingly, further efforts to test this concept are currently underway.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by awards CA93738, CA100866-01, CA130805, CA142509, CA148431, and awards from the Leukemia and Lymphoma Society of America (LSA # 6181-10) and from the Multiple Myeloma Research Foundation.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
REFERENCE LIST
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18:1333–49. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 3.Sujobert P, Bardet V, Cornillet-Lefebvre P, Hayflick JS, Prie N, Verdier F, et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood. 2005;106:1063–6. doi: 10.1182/blood-2004-08-3225. [DOI] [PubMed] [Google Scholar]
- 4.Chapuis N, Tamburini J, Cornillet-Lefebvre P, Gillot L, Bardet V, Willems L, et al. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica. 2010;95:415–23. doi: 10.3324/haematol.2009.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical trial. www.clinicaltrials.gov.
- 7.Shangary S, Johnson DE. Recent advances in the development of anticancer agents targeting cell death inhibitors in the Bcl-2 protein family. Leukemia. 2003;17:1470–81. doi: 10.1038/sj.leu.2403029. [DOI] [PubMed] [Google Scholar]
- 8.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 9.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender A, Opel D, Naumann I, Kappler R, Friedman L, von SD, et al. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene. 2011;30:494–503. doi: 10.1038/onc.2010.429. [DOI] [PubMed] [Google Scholar]
- 11.Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, et al. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood. 2009;114:4507–16. doi: 10.1182/blood-2008-09-177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood. 2000;96:1756–63. [PubMed] [Google Scholar]
- 13.Essafi A, Fernandez de MS, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 4.Sunters A, Fernandez de MS, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 15.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 18.Del GM, V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–27. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 20.Rahmani M, Mayo M, Dash R, Sokhi UK, Dmitriev IP, Sarkar D, et al. Melanoma differentiation associated gene-7/interleukin-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by endoplasmic reticulum stress. Mol Pharmacol. 2010;78:1096–104. doi: 10.1124/mol.110.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmani M, Dai Y, Grant S. The histone deacetylase inhibitor sodium butyrate interacts synergistically with phorbol myristate acetate (PMA) to induce mitochondrial damage and apoptosis in human myeloid leukemia cells through a tumor necrosis factor-alpha-mediated process. Exp Cell Res. 2002;277:31–47. doi: 10.1006/excr.2002.5548. [DOI] [PubMed] [Google Scholar]
- 22.Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, et al. Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res. 2003;63:8420–7. [PubMed] [Google Scholar]
- 23.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26:778–87. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Gomez-Bougie P, Bataille R, Amiot M. Endogenous association of Bim BH3-only protein with Mcl-1, Bcl-xL and Bcl-2 on mitochondria in human B cells. Eur J Immunol. 2005;35:971–6. doi: 10.1002/eji.200425878. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 26.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 27.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101:9085–90. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A, et al. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res. 2010;16:5424–35. doi: 10.1158/1078-0432.CCR-10-1102. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, et al. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia. 2008;22:1698–706. doi: 10.1038/leu.2008.144. [DOI] [PubMed] [Google Scholar]
- 30.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 31.Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, et al. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003;22:6231–42. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- 32.Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–32. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Rahmani M, Dai Y, Conrad D, Krystal G, Dent P, et al. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent process. Cancer Res. 2003;63:1822–33. [PubMed] [Google Scholar]
- 34.Sturm I, Stephan C, Gillissen B, Siebert R, Janz M, Radetzki S, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13:619–27. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- 35.Zantl N, Weirich G, Zall H, Seiffert BM, Fischer SF, Kirschnek S, et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;26:7038–48. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]
- 36.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–9. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–43. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–5. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–61. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24:1136–50. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–14. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 46.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–39. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–67. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–31. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.