Abstract
Fundamentally understanding the suppressive mechanisms utilized by different subsets of tumor-infiltrating regulatory T (Treg) cells is critical for the development of effective strategies for anti-tumor immunotherapy. γδ Treg cells have recently been identified in human diseases including cancer. However, the suppressive mechanisms and functional regulations of this new subset of unconventional Treg cells are largely unknown. In the current studies, we explored the suppressive mechanism(s) utilized by breast tumor-derived γδ Treg cells on innate and adaptive immunity. We found that γδ Treg cells induced immunosenescence in the targeted naïve and effector T cells, as well as dendritic cells (DCs). Furthermore, senescent T cells and DCs induced by γδ Treg cells had altered phenotypes, impaired functions and developed potent suppressive activities, further amplifying the immunosuppression mediated by γδ Treg cells. In addition, we demonstrated that manipulation of TLR8 signaling in γδ Treg cells can block γδ Treg-induced conversion of T cells and DCs into senescent cells in vitro and in vivo. Our studies identify the novel suppressive mechanism mediated by tumor-derived γδ Treg cells on innate and adaptive immunity, which should be critical for the development of strong and innovative approaches to reverse the tumor suppressive microenvironment and improve effects of immunotherapy.
Keywords: γδ T cells, Regulatory T cells, Dendritic cells, Senescence, Immune suppression
Introduction
Manipulating the immune system to recognize and eradicate cancer cells is an important and highly attractive alternative approach to treat patients with malignant tumors. However, the tumor suppressive microenvironments created by tumor-associate regulatory T (Treg) cells are a major obstacle for effective anti-tumor immunity and successful tumor immunotherapy (1, 2). Recent studies have already identified several subsets of Treg cells, such as Tr1, Th3 and CD8+ Treg cells, in human cancers (3–5). Thus, a better understanding of the immunosuppressive mechanisms utilized by these tumor-derived Treg cells is critical for the success of immunotherapy for cancer.
Negative regulation mediated by conventional T cells in the tumor suppressive microenvironment has been extensively studied. However, little is known about the potential roles of γδ T cells in anti-tumor immune responses. The negative regulation of γδ1 T cells in mouse models of induced mucosal tolerance, ocular tolerance and self-tolerance has been well documented (6–9). Furthermore, studies from mouse tumor models have suggested that γδ T cells in the tumor microenvironment may be involved in the induction of tumor-specific immune tolerance (10–12). We discovered that enriched γδ1 Treg cell populations (7.2%–75.7%; mean 33.2%) in the tumor-infiltrating lymphocytes (TILs) obtained from breast cancer patients can suppress naïve and effector T cell responses and block the maturation and activities of dendritic cells (DCs) in vitro (13). We further showed that the high level of γδ T cells infiltrating in human breast cancer tissues was correlated with poor survival and high risk of relapse and could be used as a novel and independent prognostic factor in human breast cancer (14). These studies implicate the potential function of γδ Treg cells in the immunopathogenesis of human breast cancer. In addition, this new subset of Treg cells has also been identified in patients by more recent studies from other groups (15, 16).
Cellular senescence was initially described in human fibroblasts with limited passages in cell culture (17). There are two major categories of cellular senescence: (1) Replicative senescence, which occurs due to telomere shortening or dysfunction (18, 19); and (2) Premature senescence, which is induced by a variety of extrinsic forms of stress, such as oxidative stress, DNA damage, and activation of certain oncogenes (20–22). Recent studies suggest that replicative senescence also occurs within the human immune system. Accumulation of senescent CD8+ T cells has been found in persons during normal aging, younger persons with chronic viral infections, and patients with certain types of cancers (23–27). Furthermore, we more recently identified that naturally occurring human CD4+CD25+ Treg cells can induce responder T lymphocyte senescence (28). Senescent T cells develop significant phenotypic alterations, such as permanent loss of CD28 expression, cell cycle arrest, and up-regulation of the cell cycle-related genes p53, p21, and p16 (23, 28). In addition, senescent T cells have exhibited functional changes, including defective killing abilities and the development of potent negative regulatory functions (24, 27–31). However, the precise molecular mechanisms responsible for the induction of these senescent cells are still under investigation.
In the current studies, we further explored the suppressive mechanism(s) utilized by tumor-derived γδ Treg cells on innate and adaptive immunity. We found that γδ Treg cells can also induce both T cell and DC senescence, resulting in their impaired phenotypic and functional features. Importantly, these senescent T cells and DCs induced by γδ Treg cells became suppressive cells, further amplifying the immunosuppression mediated by Treg cells. In our efforts to identify the strategies to reverse γδ Treg cell suppression, we found that manipulation of TLR8 signaling in γδ Treg cells can block γδ Treg-induced conversion of T cells and DCs into senescent cells in vitro and in vivo in animal models. Our studies identify the novel suppressive mechanism mediated by tumor-derived γδ Treg cells on innate and adaptive immunity, which provide new insights relevant for the development of strong and innovative approaches for improved tumor immunotherapy.
Materials and Methods
T cell and other cell lines
Buffy coats from healthy donors were obtained from the Gulf Coast Regional Blood Center at Houston. These studies were approved by the Institutional Review Boards. Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats using Ficoll-Paque. Human naïve CD4+ and CD8+ T cells were purified from PBMCs of healthy donors by EasySep enrichment kits (StemCell Technologies). The purity of naïve T cells was >97%, as confirmed by flow cytometry. Human γδ Treg cells (primary or cell lines) were established from the primary breast cancer tissues in our laboratory and maintained in T cell medium containing 10% human AB serum and 50 u/ml IL-2 (13, 14).
Senescence associated β-Galactosidase (SA-β-Gal) staining
Senescence associated β-Galactosidase (SA-β-Gal) activity in senescent T cells was detected as previously described (28, 32). Naive CD4+ T cells, CD8+ T cells, or DCs were labeled with CFSE (4.5 µM), and co-cultured with or without γδ Treg or control T cells at different ratios of 10:1 to 1:1 in anti-CD3-coated 24-well plates for 3 or 5 days. In some experiments, naïve T cells and DCs were cultured in T cell supernatants from γδ Treg or control T cells. Naïve T cells or DCs were separated from co-cultures using FACS sorting gated on CFSE positive populations, and then washed in PBS (pH 7.2), fixed in 3% formaldehyde, and incubated overnight at 37°C with freshly prepared SA-β-Gal staining solution (1 mg/ml X-gal, 5 mM K3Fe[CN]6, 5 mM K4Fe[CN]6, 2 mM MgCl2 in PBS at pH 6.0). The stained cells were washed with H2O and examined microscopically.
For some experiments, the co-cultured naïve T cells were determined for SA-β-Gal expression in the presence of the following TLR ligands: Pam3CSK4 (200 ng/ml), Poly (I:C) (25 µg/ml), LPS (100 ng/ml), Flagellin (10 µg/ml), Loxoribine (500 µM), R837 (10 µg/ml), ssRNA40 (10 µg/ml) (Invivogen, San Diego, CA), and oligonucleotides CpG-B (3 µg/ml), Poly-T3 (3 µg/ml) and Poly-G3 (3 µg/ml) (synthesized by Invitrogen, Carlsbad, CA).
Cell cycle and apoptosis assays
Naïve CD4+ T cells were co-cultured with CFSE-labeled γδ Treg cells in the presence of plate-bound anti-CD3 antibody (2 µg/ml). After 72 hours of co-culture, apoptosis in naïve CD4+ T cells was analyzed after staining with PE-labeled Annexin V and 7-AAD (BD Biosciences, San Diego, CA) gating on CFSE negative cell populations. For cell cycle analysis, co-cultured naïve T cells were fixed with 70% ethanol overnight, washed with PBS and incubated with propidium iodide (10 µg/ml) and RNase A (100 µg/ml). Naïve CD4+ or CD8+ T cells co-cultured with or without the CD4-C1 effector T cells served as controls. All the stained cells were analyzed on a FACSCalibur (BD Bioscience) and data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Functional proliferation assays
Proliferation assays were performed by a [3H]-thymidine incorporation assay, as we previously described (13, 28, 33). Naïve CD4+ T cells (1 × 105) purified from healthy donors were co-cultured with γδ Treg cells, DCs or γδ Treg-treated T cells or DCs at different ratios in 200 µl of T cell assay medium containing 2% human AB. After 56 hours of culture, [3H]-thymidine was added at a final concentration of 1µCi/well, followed by an additional 16 hours of culture. The incorporation of [3H]-thymidine was measured with a liquid scintillation counter. In some experiments, a Transwell system was utilized to determine the suppressive mechanisms of γδ Treg cells, as well as senescent T cells and DCs, using 24-well Transwell plates (0.4 µm pore size; Corning Costar) (5, 34).
Western blot analysis
CFSE-labeled naïve CD4+ T cells were co-cultured with γδ Treg, or control T cells at a ratio of 5:1 in assay medium (5% human serum, no IL-2) in the presence of plate-bound anti-CD3 antibody (2 µg/ml) for 0, 1, 3, or 5 days. Treated CD4+ T cells were then sorted by FACS gating on CFSE positive T cells. Whole cell lysates of the purified CD4+ T cells were prepared for western blot analyses. Western blots were developed with Chemiluminescent Substrate (KPL, Maryland). The antibodies used in western blotting are as follows: anti-p53 goat polyclonal antibody, anti-p21 rabbit polyclonal antibody (C-19), and anti-p16 (Santa Cruz Biotechnology), and anti-actin rabbit polyclonal antibody (Cell Signaling Technology, Danvers, MA).
Flow cytometry analysis
The expression markers on T cells and DCs were determined by FACS analysis after surface or intracellular staining with anti-human specific antibodies conjugated with either PE or FITC. These human antibodies included: anti-CD4, anti-CD8, anti-CD27, anti-CD28, anti-CD80, anti-CD83, anti-CD86, anti-HLA-DR and anti-PD-L1, which were purchased from BD Biosciences. All stained cells were analyzed on a FACSCalibur flow cytometer (BD Bioscience) and data analyzed with FlowJo software (Tree Star).
Induction, phenotype and suppressive function of senescent DCs
Immature and mature DCs were derived from the monocytes of healthy donors in culture with IL-4 (100 ng/ml) and GM-CSF (100 ng/ml) with or without TNF-α (15 ng/ml) (R & D Systems). The immature or mature DCs were treated with γδ T cells as we described previously (13). In brief, 5 × 105 γδ T cells or control CD4-C1 effector T cells were co-cultured with 1 × 106 immature DCs in 24-well plates in medium containing IL-4 and GM-CSF with TNF-α. After 48 hours, the treated and untreated DCs were purified and divided into different groups. For senescence induction assay, DCs were performed SA-β-Gal staining. For phenotypic analysis, the surface markers of CD83, CD80, CD86, HLA-DR and PD-L1 of DCs were analyzed by flow cytometry. For cytokine secretion detection, the senescent and untreated DCs were stimulated with or without LPS (5 µg/ml, Invivogene) for 24 hours. Release of IL-1β, IL-6, IL-12 and TNF-α by DCs was measured by ELISA Kits (R & D Systems). The stimulatory and suppressive activities of normal and senescent DCs on the responding T cell proliferation were determined using [3H]-thymidine incorporation assays.
Antigen presentation function of DCs
The capacity of DCs to process and present M. tuberculosis purified protein derivative (PPD) (Statens Serum Institute) to T cells was determined. Immature and mature DCs were derived from the monocytes of healthy donors in culture with IL-4 (100 ng/ml) and GM-CSF (100 ng/ml) with or without TNF-α (15 ng/ml). Immature DCs were treated with medium, γδ Treg cells or CD4-C1 for 2 days, and then purified and pulsed with 20 µg/ml of PPD for 90 minutes. PPD-pulsed DCs (1×104) were cultured with autologous CD4+ T cells (1×105) for 14 days in the presence of 50 u/ml of IL-2 (at day 7, PPD-pulsed DCs were added into co-cultures again). PPD-specific IFN-γ-producing CD4+ T cells were determined by flow cytometry after re-stimulation with PPD-pulsed autologous mature DCs at a ratio of 10:1.
In vivo studies
Rag1−/− mice (lacking T and B cells) were originally purchased from NCI and maintained in the institutional animal facility. All animal studies have been approved by the Institutional Animal Care Committee. Naïve CD4+ T cells (5 × 106/mouse), γδ Treg (2 × 106/mouse) and CD8-C1 effector T cells (2 × 106/mouse) were pre-activated with anti-CD3 (2 µg/ml) and adoptively co-transferred into Rag1−/− mice through intravenous injection into the following groups: naïve CD4+ T cells alone, naïve CD4+ T cells plus γδ Treg cells, or naïve CD4+ T cells plus CD8-C1 effector T cells cells. Five to ten mice were included in each group. In a parallel experiment, γδ Treg cells were pretreated with TLR8 ligand (Poly-G3, 3 µg/ml) or control Poly-T3 (3 µg/ml) for 48 hours before injection. Blood, lymph nodes (LN) and spleens (SP) were harvested at 12 days post-injection and mononuclear cells were purified over Ficoll. The transferred human CD4+ and CD8+ T cells were isolated by FACS sorting for subsequent phenotypic and functional analyses in vitro. SA-β-Gal staining and 3H-thymidine incorporation assays were performed as described above.
Statistical Analysis
Unless indicated otherwise, data are expressed as mean ± standard deviation (SD). The significance of difference between groups was determined by a two-tailed Student's t-test or the one-way analysis of variance (ANOVA).
Results
Tumor-derived γδ Treg cells induce cell cycle arrest in responder T cells
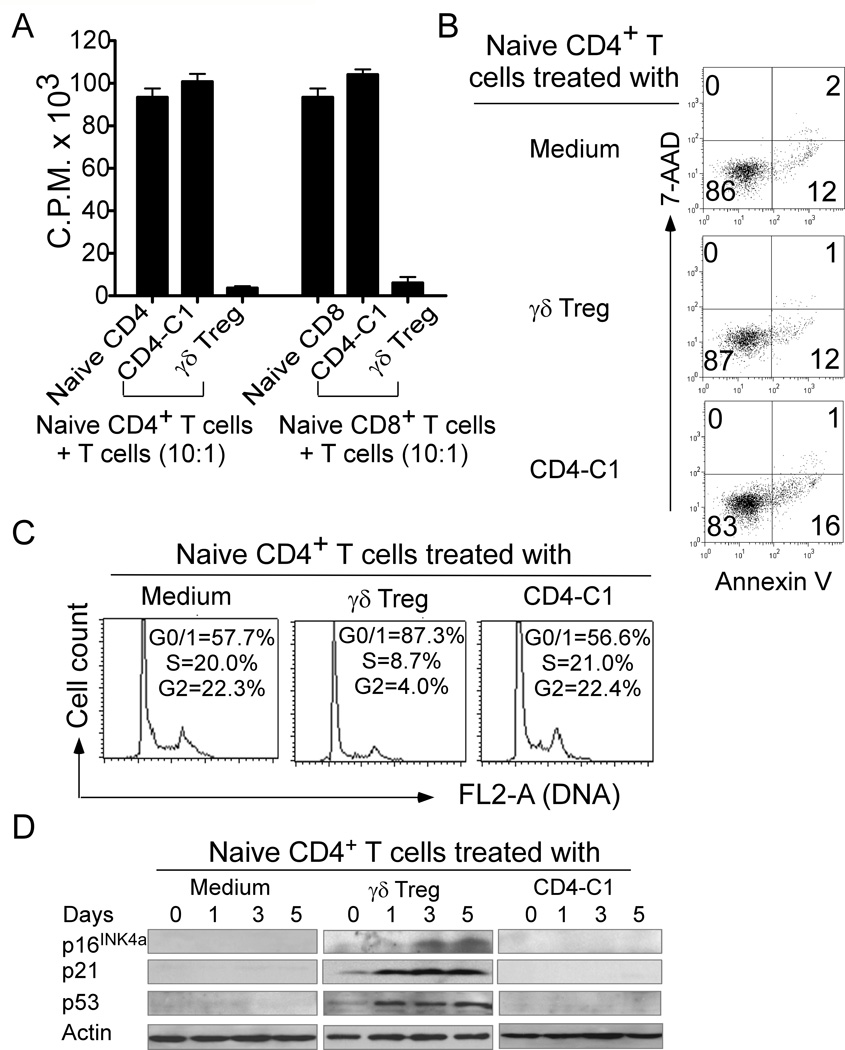
We previously demonstrated that high percentages of γδ Treg cells exist in the TILs of breast cancer patients (13, 14). To further explore the suppressive mechanisms utilized by tumor-derived γδ Treg cells (13, 14, 33), we first investigated the suppressive capacity of γδ Treg cells using functional proliferation assays. We observed that tumor-derived γδ Treg cells strongly inhibited the proliferation of naïve CD4+ and CD8+ T cells in the presence of anti-CD3 antibody using [3H]-thymidine incorporation assays; whereas control CD4-C1 effector cells, a Th1 cell line, increased the proliferation of naïve T cells (Figure 1A). Recent studies have shown that CD4+ Treg cells suppress naïve T cell proliferation through the induction of apoptosis or cytolysis in the Treg-treated cells (35). We then tested whether tumor-derived γδ Treg cells utilize the same mechanisms to inhibit T cell proliferation. Apoptosis and cell death in naïve CD4+ T cell populations co-cultured with γδ Treg cells were measured. As shown in Figure 1B, naïve CD4+ T cells in medium alone or treated with control CD4-C1 T cells contained 14–17% apoptotic T cells after stimulation with anti-CD3 antibody. However, γδ Treg cells did not induce increased apoptosis or cell death in CD4+ T cells, indicating that suppression in responder T cells induced by human γδ Treg cells is not through the induction of cell apoptosis and cytolysis. In parallel, we studied the cell cycle distribution of the naïve CD4+ T cells treated with γδ Treg cells. We found that 55%–60% of anti-CD3-activated naïve CD4+ T cells remained in G0/G1 in the medium alone and the control CD4-C1 treatment groups. In contrast, more than 85% of naïve CD4+ T cells treated with γδ Treg cells remained in G0/G1, indicating that γδ Treg cell treatment promotes the accumulation of naïve T cells in cell cycle arrest (Figure 1C). We thus determined whether cell cycle regulatory molecules p53, p21, and p16 are involved in γδ Treg-induced cell cycle arrest in responder T cells. As expected, significantly increased p53, p21, and p16 expressions were observed in naïve CD4+ T cells after treatment with γδ Treg cells (Figure 1D). These data clearly suggest that γδ Treg cells induce cell cycle arrest but not apoptosis or cytolysis of responder T cells.
Figure 1. The suppression of responder T cells mediated by human tumor-derived γδ Treg cells is due to the induction of cell G0/G1 cycle arrest.
(A) Suppression of naïve T cell proliferation by γδ Treg cells. CD4-C1 effector T cells served as a negative control displaying no suppressive activity. Naïve CD4+ or CD8+ T cells were co-cultured with γδ Treg or control T cells at a ratio of 10:1. The proliferation of naïve T cells in the presence of anti-CD3 antibody was determined by [3H]-thymidine incorporation assays. (B) The suppression of naïve CD4+ T cell proliferation mediated by γδ Treg cells is not due to the induction of apoptosis. (C) γδ Treg cells promoted the accumulation of naïve CD4+ T cells in G0/G1 cell cycle arrest. Naïve CD4+ T cells were co-cultured with CFSE-labeled γδ Treg or CD4-C1 cells in the presence of plate-bound anti-CD3 antibody. Apoptosis in naïve CD4+ T cells was analyzed after staining with PE-labeled Annexin V and 7-AAD gating on CFSE negative cell populations (in B). Cell cycle distribution in naïve CD4+ T cells was analyzed after incubation with 10 µg/ml propidium iodide and 100 µg/ml RNase A (in C). Naïve CD4+ T cells co-cultured with or without CD4-C1 T cells served as controls. (D) Cell cycle regulatory molecules p21, p16, and p53 were involved in human γδ Treg-induced T cell senescence. Cell treatment and procedure were the same as in (B) and (C). Co-cultured naïve CD4+ T cells were purified by FACS and then lysates prepared for Western blot analyses. Data shown in (A) to (D) are representative of three independent experiments with similar results.
γδ Treg cells suppress naïve and effector T cells through the induction of T cell senescence
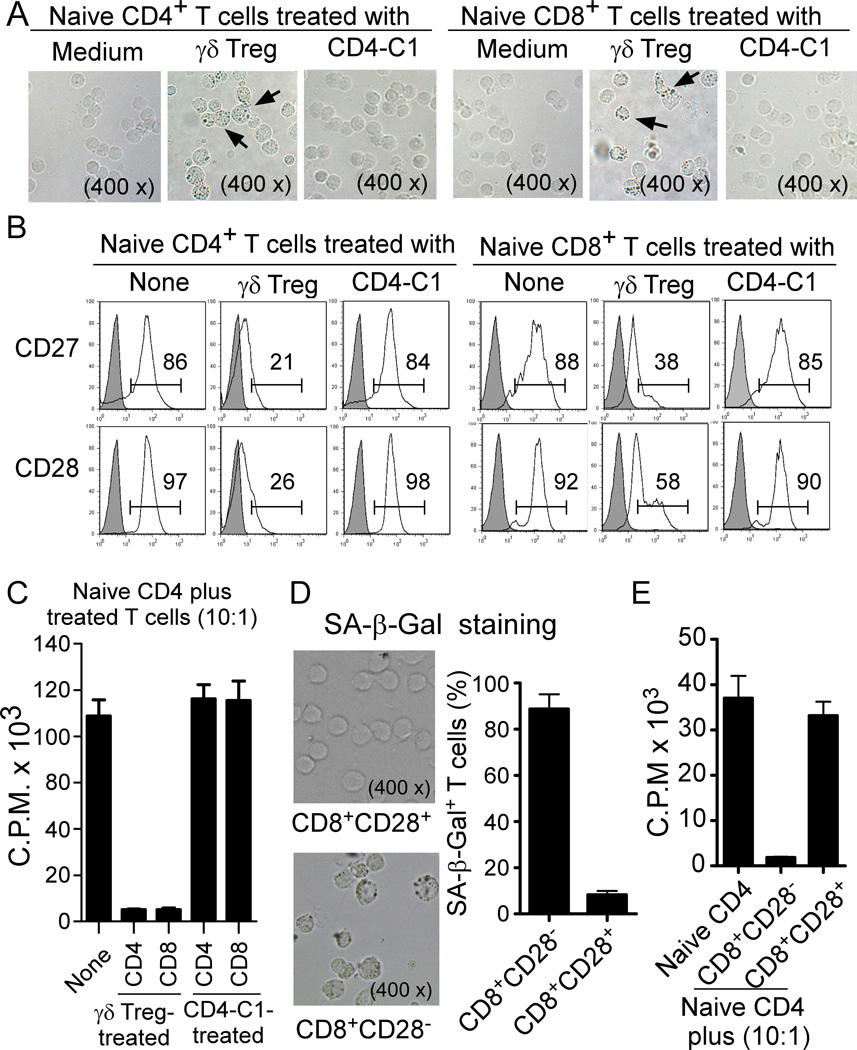
We have recently shown that human naturally occurring CD4+CD25+ Treg cells can induce responder T lymphocyte senescence (28). Given that our current studies showed that γδ Treg cell treatment resulted in the accumulation of naïve T cells in G0/G1 cell cycle phases as well as cell cycle arrest, we reasoned that γδ Treg cells performing their suppressive function on naïve T cells may utilize the same suppressive mechanism as that of CD4+CD25+ Treg cells. To test the possibility that γδ Treg cells may also induce responder T cell senescence, naïve CD4+ and CD8+ T cells were labeled with CFSE and then co-cultured with γδ Treg cells or control CD4-C1 effector T cells at different ratios in the presence of plate-bound anti-CD3 antibody (2 µg/ml) for different days. Co-cultured naïve T cells were then purified by FACS and senescence-associated-β-galactosidase (SA-β-Gal) expression, the first biomarker used to identify senescent human cells was determined (28, 32). As shown in Figure 2A, naïve CD4+ and CD8+ T cells treated with γδ Treg cells, significantly induced cell senescence resulting in SA-β-Gal expression. However, naïve CD4+ and CD8+ T cells cultured in medium only or co-cultured with CD4-C1 effector T cells did not induce SA-β-Gal expression. Furthermore, we observed that the percentages of the SA-β-Gal positive cell populations in naïve CD4+ and CD8+ T cells dramatically increased with longer times of co-culture with γδ Treg cells (Supplemental Figure 1). Accumulating evidence suggests that permanent loss of CD28 expression is the most consistent biological indicator of aging in senescent T cells in elderly people and in patients with chronic viral infections (23, 29). Thus, we examined whether human γδ Treg-induced senescent T cells can also down-regulate the expression of the co-stimulatory molecules CD27 and CD28. We found that naïve CD4+ and CD8+ T cells co-cultured with medium only or with CD4-C1 T cells expressed high levels of CD27 and CD28 co-stimulatory molecules. In contrast, naïve CD4+ and CD8+ T cells co-cultured with γδ Treg cells dramatically down-regulated co-stimulatory molecules CD27 and CD28 expression (Figure 2B). In addition, we observed that freshly purified γδ T cells from breast tumor tissues also significantly increased SA-β-Gal positive T cell populations and decreased CD27 and CD28 expression in the co-cultured naïve CD4+ T cells (Supplemental Figure 2A and 2B). To further identify the suppressive mechanisms mediated by tumor-derived γδ Treg cells, we found that the suppressive activity of tumor-derived γδ1 Treg cells relied on unknown soluble factors using Transwell assays (Data not shown). In addition, culture supernatants from γδ1 Treg cells strongly inhibited the proliferation of naïve CD4+ T cells and induced responder T cell senescence expressing SA-β-Gal (Supplemental Figure 2C and 2D). These results collectively suggest that both human naturally occurring CD4+CD25+ Treg and γδ Treg cells use the same mechanism to suppress naïve T cell proliferation through the induction of T cell senescence.
Figure 2. Human γδ Treg cells induce senescence in naïve CD4+ and CD8+ T cells.
(A) γδ Treg cell treatment significantly increased SA-β-Gal positive T cell populations in naïve CD4+ and CD8+ T cells. Naïve CD4+ or CD8+ T cells cultured in medium only or co-cultured with CD4-C1 effector T cells had little or no SA-β-Gal expression. CFSE-labeled naïve CD4+ or CD8+ T cells were incubated alone or co-cultured with γδ Treg or CD4-C1 T cells at a ratio of 5:1 in the presence of plate-bound anti-CD3 (2 µg/ml) for 5 days. The treated naïve CD4+ or CD8+ T cells were purified by FACS and SA-β-Gal expression determined. The SA-β-Gal positive T cells were identified with dark blue granules as indicated by the arrows. (B) Decreased expression of CD27 and CD28 in naïve CD4+ and CD8+ T cells treated with γδ Treg cells. Cell treatment and procedure were the same as in (A). CD27 and CD28 expression in treated naïve CD4+ and CD8+ T cells were analyzed by FACS gating on CFSE-positive populations. (C) Suppressive function of Treg-induced senescent T cells. Both senescent CD4+ and CD8+ T cells induced by γδ Treg cells strongly inhibited the proliferation of responding CD4+ T cells. In contrast, naïve T cells treated or untreated with control CD4-C1 effector T cells did not affect the proliferation of responding CD4+ T cells. Cell treatment and procedure were the same as in (A). Treated CD4+ and CD8+ T cells were purified and the suppressive activities on CD4+ T cell proliferation evaluated using [3H]-thymidine incorporation assays. (D) Senescent CD8+ T cells induced by γδ Treg cells dominantly existed in the CD8+CD28− cell populations. Cell treatment and procedure were the same as in (A). Treg-treated CD8+ T cells expressing CD28 were determined and sorted by FACS. SA-β-Gal expression in CD8+CD28+ and CD8+CD28− cell populations purified from Treg-treated CD8+ T cells was determined. Results shown in the right panel are mean ± SD from three independent experiments. (E) Suppressive function of CD8+CD28+ and CD8+CD28− cell populations purified from γδ Treg-treated CD8+ T cells. CD8+CD28− cell population strongly inhibited the proliferation of responding CD4+ T cells. In contrast, CD8+CD28+ cell population had minor suppressive activity on the proliferation of responding CD4+ T cells. Naïve CD4+ T cells were cocultured with purified CD8+CD28+ or CD8+CD28− cell populations for 3 days. The suppressive activities of these two populations on CD4+ T cell proliferation were evaluated using [3H]-thymidine incorporation assays. Results shown in (C) to (E) are mean ± SD from three independent experiments with similar results.
Since senescent T cells have exhibited functional changes, such as defective killing abilities and the development of potent negative regulatory functions (24, 27–31), we next investigated whether human γδ Treg-induced senescent T cells also have negative regulatory functions. We first evaluated cytokine profiles elaborated by the γδ Treg-induced senescent T cells stimulated with anti-CD3 antibody using an ELISA. Naïve CD4+ and CD8+ T cells treated with or without CD4-C1 effector T cells were included as controls. Both senescent CD4+ and CD8+ T cells secreted large amounts of proinflammatory cytokines IL-6, IFN-γ and TNF-α, but not other cytokines including IL-1β, IL-2 or IL-4. Furthermore, these Treg-induced senescent T cells secreted large amounts of IL-10 and moderate amounts of TGF-β1, whereas naïve CD4+ T cells treated with control CD4-C1 T cells did not secrete any IL-10 or TGF-β1 (Supplemental Figure 3), suggesting that these senescent T cells may have a negative regulatory function. We then evaluated the suppressive activities of the senescent T cells on the proliferation of additional responding naïve CD4+ T cells. We found that both senescent CD4+ and CD8+ T cells induced by tumor-derived γδ Treg cells strongly inhibited the proliferation of responding CD4+ T cells. In contrast, naïve CD4+ and CD8+ T cells treated with or without control CD4-C1 T cells had no suppressive effects on the proliferation of responding CD4+ T cells (Figure 2C). These results were consistent with our recent findings in the senescent T cells induced by CD4+CD25+ naturally occurring Treg cells (28), and further suggest that γδ Treg cells by inducing cell senescence in responder T cells can convert them into suppressive T cells. In addition, we purified CD28+ and CD28− populations from the γδ Treg-treated CD8+ T cells by FACS, and observed that senescent T cells (SA-β-Gal+) were mainly derived from CD28− responder T cell populations (Figure 2D). Interestingly, we also observed that only the CD8+CD28− populations, but not CD8+CD28+ populations, had potent suppressive effects on responding CD4+ T cell proliferation (Figure 2E). Collectively, these studies further confirm that tumor-derived γδ Treg cells utilize the induction of T cell senescence as the novel suppressive mechanism to inhibit T cell proliferation.
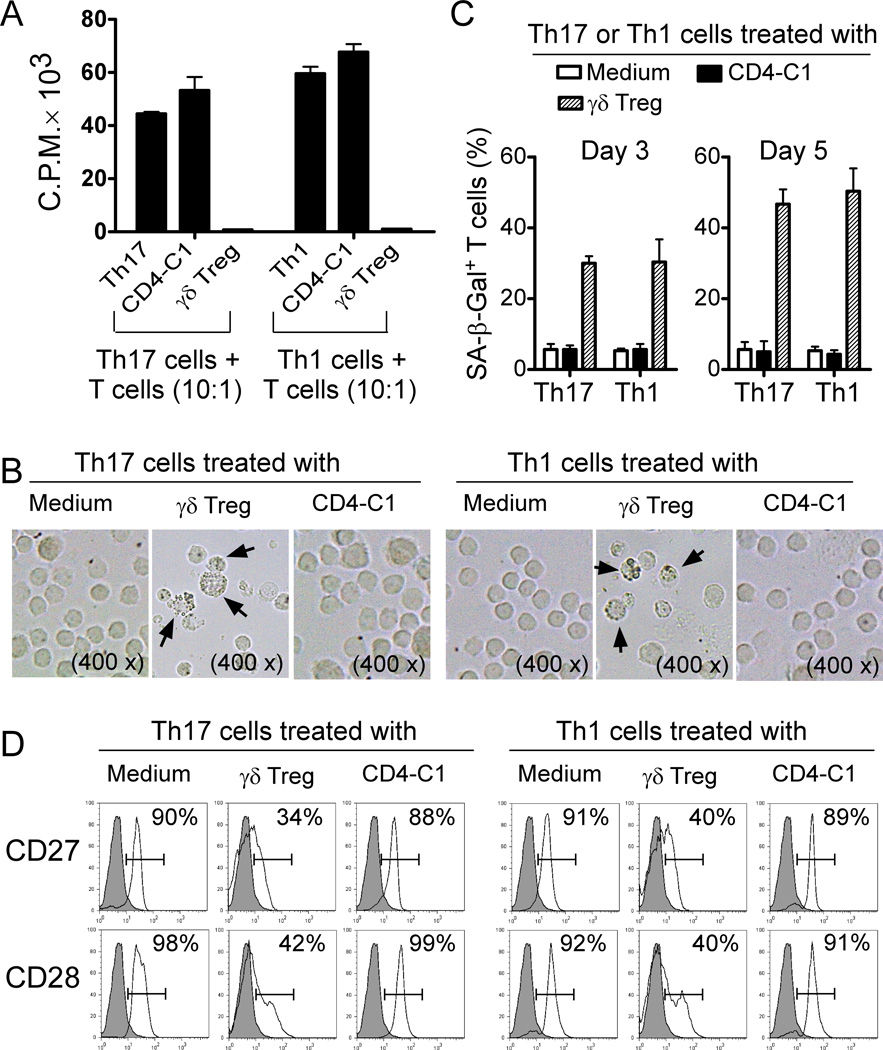
We next determined whether γδ Treg cells can also inhibit the proliferation of Th1 and Th17 effector T cell subsets, utilizing this novel suppressive mechanism of senescence induction. We included tumor-infiltrating Th1 and Th17 cell lines derived from breast cancer patients, as representative human Th1 and Th17 cells for our studies (36). We observed that γδ Treg cells also strongly inhibited the proliferation of Th1 and Th17 effector T cells (Figure 3A). Furthermore, Th1 and Th17 cells co-cultured with γδ Treg cells dramatically up-regulated SA-β-Gal expression and markedly down-regulated CD27 and CD28 costimulatory molecule expression (Figure 3B–3D). These results clearly indicate that the suppression of both naïve and effector T cells mediated by tumor-derived γδ Treg cells is due to the same suppressive mechanism that induces responder T cell senescence.
Figure 3. Human γδ Treg cells induce senescence in Th1 and Th17 cells.
(A) Suppression of Th1 and Th17 cell proliferation by γδ Treg cells. CD4-C1 effector T cells displayed no suppressive activity, and served as a negative control. Th1 or Th17 cells established from TILs were co-cultured with γδ Treg or control T cells at a ratio of 10:1. The proliferation of Th1 or Th17 cells in the presence of anti-CD3 antibody was determined by [3H]-thymidine incorporation assays. (B) and (C) γδ Treg cell treatment significantly increased SA-β-Gal positive T cell populations in Th1 or Th17 cells. Th1 or Th17 cells cultured in medium only or co-cultured with CD4-C1 effector T cells had little or no SA-β-Gal expression. CFSE-labeled Th1 or Th17 cells were incubated alone or co-cultured with γδ Treg or CD4-C1 T cells at a ratio of 5:1 in the presence of plate-bound anti-CD3 (2 µg/ml) for 3 or 5 days. The treated Th1 or Th17 cells were purified by FACS and stained with SA-β-Gal staining reagents after an additional 3 day culture. The SA-β-Gal positive T cells (5 day co-culture) were identified with dark blue granules as the arrows indicate in (B). (D) Decreased expression of CD27 and CD28 in Th1 and Th17 cells treated by γδ Treg cells. Cell treatment and procedure were the same as in (B). CD27 and CD28 expression in treated Th1 and Th17 cells (5 day treatment) were analyzed by FACS.
γδ Treg cells induce senescence in DCs resulting in their impaired phenotypic and functional features
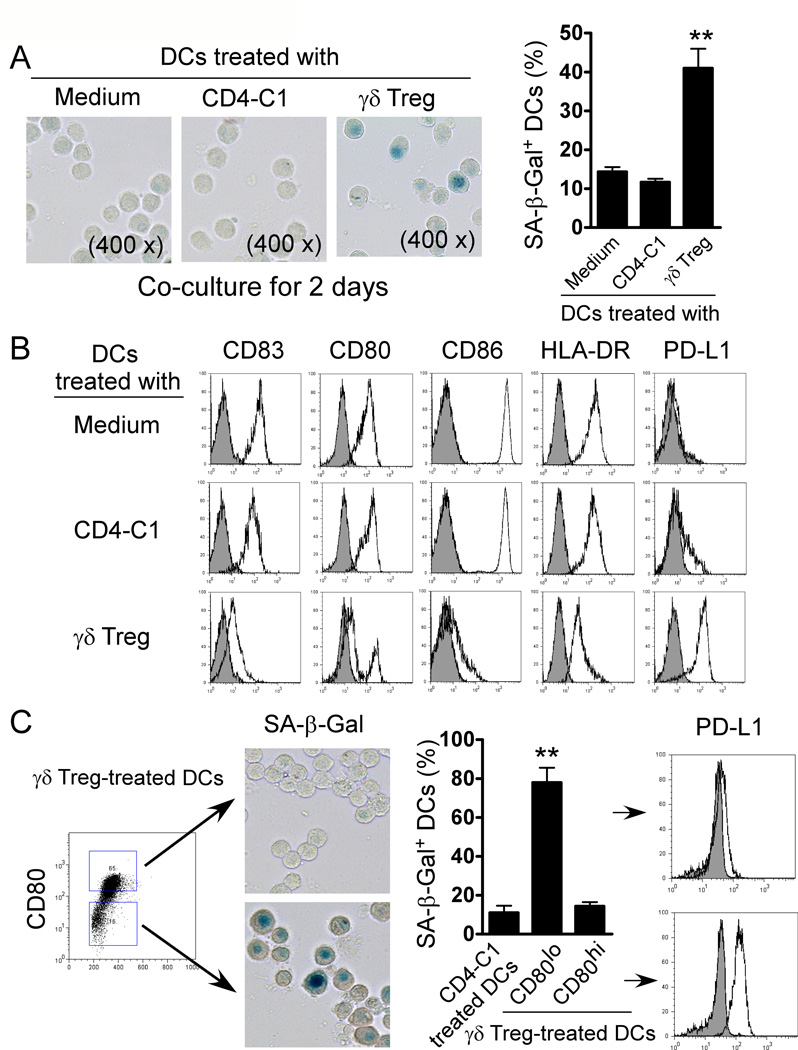
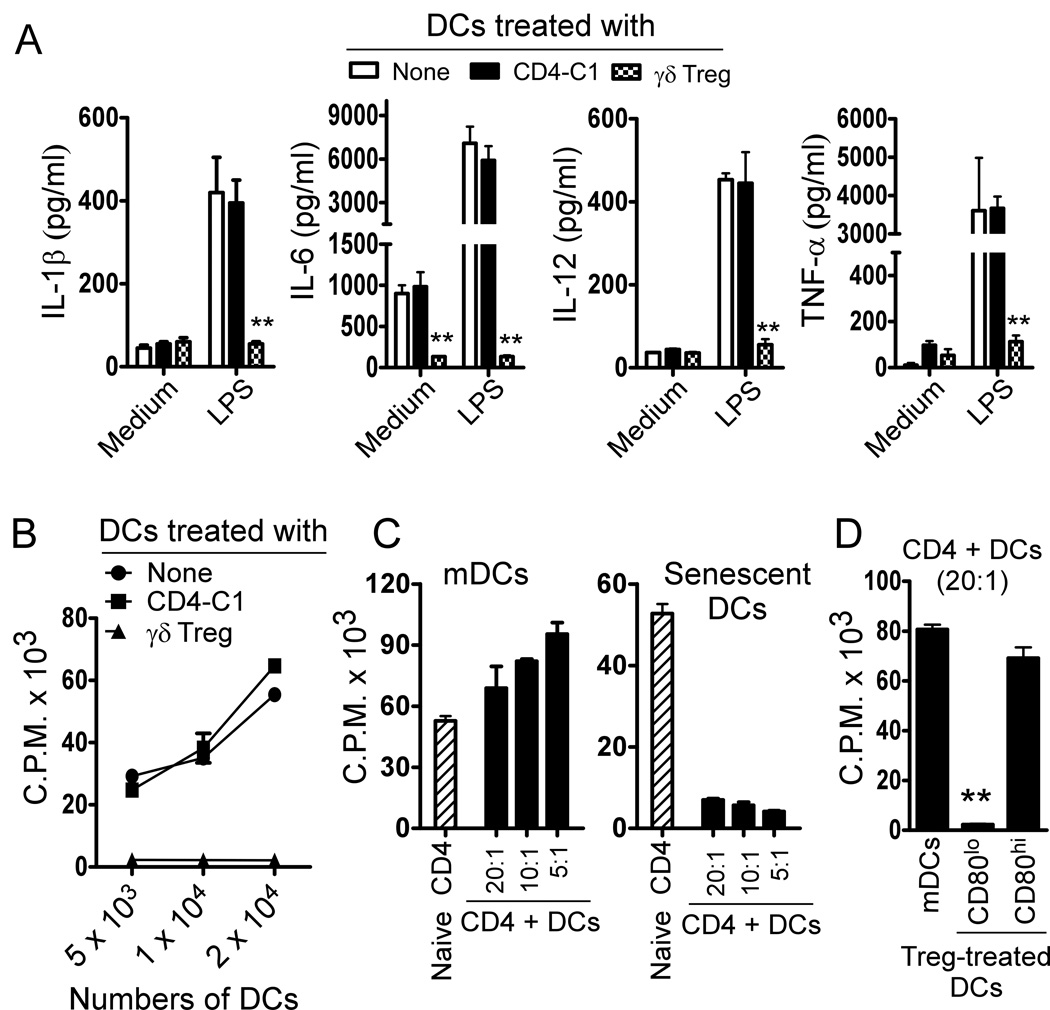
Our previous studies have shown that breast tumor-derived γδ Treg cells can block the maturation and function of DCs (13). We next investigated whether γδ Treg cells also utilized this novel suppressive mechanism of senescence induction to inhibit DC functions. Immature DCs were co-cultured with γδ Treg cells or control CD4-C1 effector T cells for 48 hours in medium containing IL-4, GM-CSF and TNF-α. Treated and untreated DCs were purified and SA-β-Gal expression determined. Surprisingly, significantly increased senescent DC populations (around 40%) were observed after co-culture with γδ Treg cells, whereas co-culture with CD4-C1 effector T cells or medium only induced minor SA-β-Gal expression in DCs (Figure 4A). In addition, we demonstrated that both freshly purified γδ T cells from breast tumor tissues and cell culture supernatants markedly up-regulated SA-β-Gal expression on DCs (Supplemental Figure 4A–4B). We further investigated whether senescent DCs induced by γδ Treg cells changed their maturation and functional markers. We observed that γδ Treg cell treatment not only induced senescence but also dramatically down-regulated CD83 expression in the treated DCs, suggesting that senescent DCs were deficient in maturation (Figure 4B). In addition, Senescent DCs induced by γδ Treg cells markedly down-regulated co-stimulatory molecules CD80, CD86 and HLA-DR expression, consistent with our previous finding (13). Notably, we found that γδ Treg-induced senescent DCs significantly up-regulated program death ligand1 (PD-L1) expression, a critical inhibitor molecule for the induction and maintenance of immune suppression (Figure 4B) (37). However, CD4-C1 effector T cell treatment did not affect DC maturation and co-stimulatory molecule expression (Figure 4B). These results suggest that γδ Treg-induced senescent DCs have a distinct deficiency in their maturation and co-stimulatory functions. Given that permanent loss of CD28 expression is the most consistent biological indicator of senescent T cells (23, 28, 29), and that senescent T cells (SA-β-Gal+) induced by Treg cells were mainly derived from CD28− responder T cell populations (Figure 2D), we thus reasoned that loss of co-stimulatory molecules, such as CD80 and CD86 might also be used as a biomarker for the senescent DCs induced by γδ Treg cells. CD80lo and CD80hi populations in the γδ Treg-treated DCs were purified by FACS. As expected, over 80% of DCs in CD80lo populations were SA-β-Gal+ DCs, whereas CD80hi DCs only had a minor senescent population, similar as those in CD4-C1 treated DCs. Furthermore, CD80lo DCs also highly expressed PD-L1 (Figure 4C). In addition, similar results as shown in CD80lo DCs were found in CD86lo DCs treated with γδ Treg cells (Data not shown). These data suggested that γδ Treg-induced senescent DCs were mainly derived from CD80lo and CD86lo populations, and that the loss of co-stimulatory molecules CD80 and CD86 was also a significant biomarker for the senescent DCs.
Figure 4. Human γδ Treg cells induce DC senescence.
(A) γδ Treg cell treatment markedly up-regulated SA-β-Gal expression on DCs. DCs cultured in medium only or co-cultured with CD4-C1 effector T cells had little SA-β-Gal expression. Immature DCs were incubated alone or co-cultured with γδ Treg or CD4-C1 T cells at a ratio of 5:1 in the presence of GM-CSF, IL-4 and TNF-α for 2 days. The treated DCs were purified and SA-β-Gal expression determined. Results shown in the right panel are mean ± SD from three independent experiments. **p<0.01, compared with the medium only and CD4-C1 treatment groups. (B) Decreased expression of CD83, CD80, CD86 and HLA-DR and upregulation of PD-L1 in DCs treated with γδ Treg cells. Cell treatment and procedure were the same as in (A). All these markers were analyzed by FACS. (C) Senescent DCs induced by γδ Treg cells dominantly existed in the CD80low cell populations. Furthermore, CD80low cell populations purified from Treg-treated DCs showed increased PD-L1 expression. Cell treatment and procedure were the same as in (A). γδ Treg-treated DCs expressing CD80 were sorted by FACS. SA-β-Gal and PD-L1 expression in CD80hi and CD80lo cell populations purified from γδ Treg-treated DCs were determined. Results shown in the right panel are mean ± SD from three independent experiments. **p<0.01, compared with the CD80hi group.
We then evaluated cytokine profiles elaborated by the γδ Treg-induced senescent DCs after stimulation with or without LPS. The untreated mature DCs secreted large amounts of proinflammatory cytokines IL-1β, IL-6, IL-12 and TNF-α. However, senescent DCs induced by γδ Treg cells dramatically decreased the release of these cytokines, whereas co-culture with control CD4-C1 T cells did not affect the cytokine secretion by DCs (Figure 5A). Furthermore, γδ Treg treatment did not promote the secretion of suppressive cytokines IL-10 and TGF-β by DCs (Data not shown). In addition to the identification of altered phenotypes and cytokine profile in Treg-induced senescent DCs, we also investigated whether senescent DCs induced by γδ Treg cells had impaired functions. We first tested whether γδ Treg-induced senescent DCs have a deficiency in the ability to stimulate the proliferation of naïve T cells. As shown in Figure 5B and Supplemental Figure 4C, senescent DCs induced by γδ Treg cells cannot stimulate both autologous and allogenic naïve CD4+ T cell proliferation. In contrast, DCs treated with or without CD4-C1 effector T cells strongly stimulated naïve CD4+ T cell proliferation. We then determined whether senescent DCs induced by γδ Treg cells also had negative regulatory function. We evaluated the suppressive activity of senescent DCs on the proliferation of responding CD4+ T cells stimulated by anti-CD3 antibody. We found that senescent DCs strongly inhibited the proliferation of responding CD4+ T cells. In contrast, mature DCs induced an increased proliferation of responding CD4+ T cells (Figure 5C). Furthermore, we found that the CD80lo DC population in γδ Treg-treated DCs had more potent suppressive activity, whereas CD80hi population had no suppressive effect on the proliferation of responding T cells (Figure 5D). In addition, we showed that the suppressive activity of these senescent DCs induced by γδ Treg cells was mediated through soluble factor(s) using Transwell assays (Supplemental Figure 4D). Taken together, our results indicate that human tumor-derived γδ Treg cells not only induce DCs into senescent DCs that have immature phenotypes, suppressed secretion of proinflammatory cytokines and impaired functions, but also convert them into suppressive DCs.
Figure 5. Characterization of human γδ Treg-induced senescent DCs.
(A) Significant decreases of cytokine secretion (IL-1β, IL-6, IL-12 and TNF-α) by γδ Treg–induced senescent DCs. DCs were incubated alone or co-cultured with γδ Treg or CD4-C1 T cells at a ratio of 1:1 in the presence of GM-CSF, IL-4 and TNF-α for 2 days. The treated DCs were purified and stimulated with medium or LPS for 24 hours. Releases of cytokines by DCs were determined by ELISA. Cytokine concentrations (pg/ml) are shown as mean ± SD from three independent experiments. **p<0.01, compared with the medium only and CD4-C1 treatment groups. (B) Decreased stimulation capacity on naïve T cell proliferation mediated by γδ Treg-induced senescent DCs. DCs treated with CD4-C1 T cells were included as a control, showing dose dependent stimulation activity. Cell treatment and procedure were the same as in (A). The treated DCs were purified and the stimulatory activities of DCs on the allogenic responding T cell proliferation determined using [3H]-thymidine incorporation assays. (C) Suppressive function of γδ Treg-induced senescent DCs. Senescent DCs induced by γδ Treg cells strongly inhibited the proliferation of responding CD4+ T cells. In contrast, mature DCs treated or untreated with control CD4-C1 effector T cells did not affect the proliferation of responding CD4+ T cells. Treated DCs were purified and the suppressive activities on CD4+ T cell proliferation were evaluated using [3H]-thymidine incorporation assays. Data shown in (B) and (C) are representative of three independent experiments with similar results. (D) Suppressive function of CD80hi and CD80lo cell populations purified from Treg-treated DCs. CD80low DC population strongly inhibited the responding CD4+ T cell proliferation. In contrast, CD80high DC population had minor suppressive activity on the proliferation of responding CD4+ T cells. The suppressive activities of these two populations on CD4+ T cell proliferation were evaluated using [3H]-thymidine incorporation assays. Results shown are mean ± SD from three independent experiments. **p<0.01, compared with the CD80hi group.
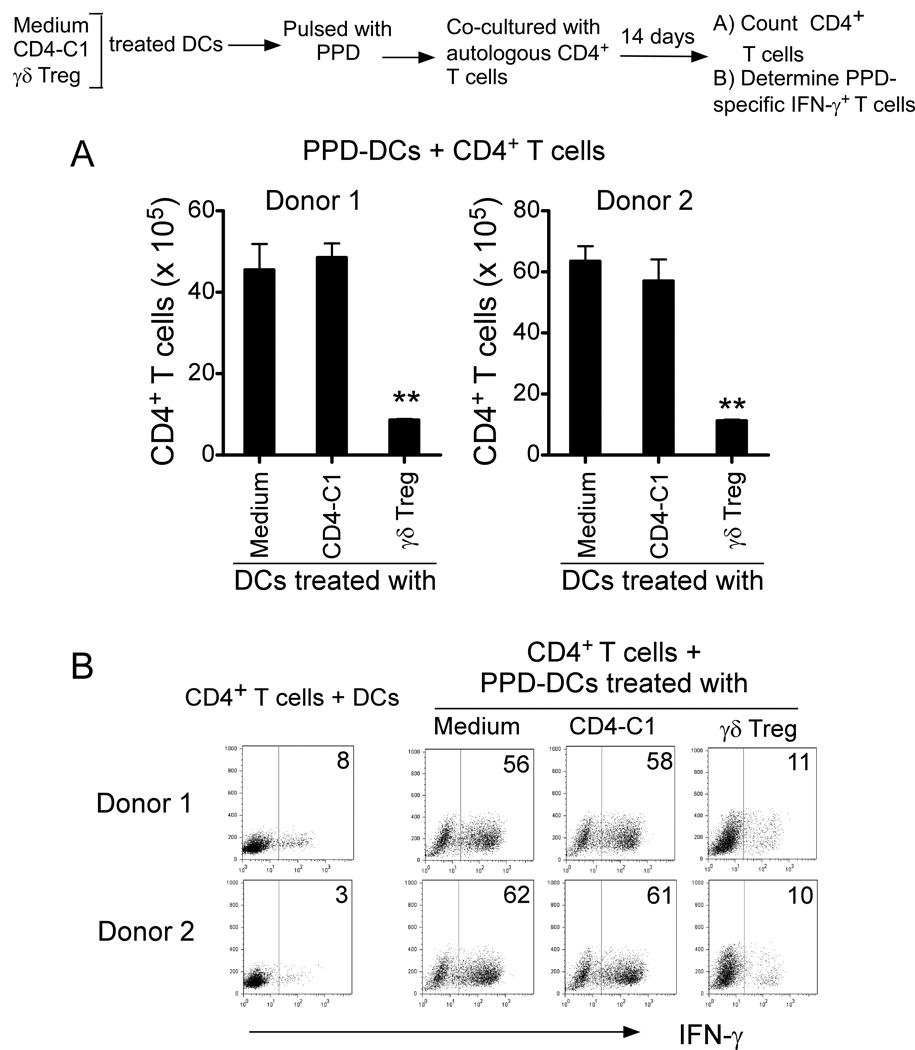
Senescent DCs induced by γδ Treg cells have impaired APC function to process and present antigen to T cells
Given that senescent DCs induced by γδ Treg cells have impaired functions to stimulate T cell proliferation, we next determined whether those senescent DCs still have the capacity to process and present a true antigen and induce antigen-specific T cell immune responses. To address this critical question, we used Mycobacterium tuberculosis PPD as a model antigen. CD4+ T cells purified from two healthy donors were co-cultured with PPD-pulsed autologous DCs, which were pre-treated with medium, CD4-C1 T or γδ Treg cells. As shown in Figure 6A, γδ Treg-induced senescent DCs pulsed with PPD had a weak ability to stimulate autologous T cell proliferation. However, PPD-pulsed DCs pre-treated with or without CD4-C1 effector T cells strongly stimulated autologous T cell proliferation, resulting in 40–60 fold number increases. In addition, PPD-pulsed DCs pre-treated with or without CD4-C1 T cells dramatically induced the increases of PPD-specific IFN-γ-producing T cell populations in the co-cultured T cells. In contrast, γδ Treg-induced senescent DCs as APCs only induced minor levels of PPD-specific IFN-γ-producing T cells (Figure 6B). These results suggested that senescent DCs induced by γδ Treg cells lost the capacity to process and present an antigen to T cells and induce antigen-specific T cell proliferation.
Figure 6. Senescent DCs induced by γδ Treg cells have impaired APC function to process and present antigen to T cells.
(A) γδ-Treg-induced senescent DCs pulsed with PPD had a weak ability to stimulate autologous T cell proliferation. However, PPD-pulsed DCs treated with or without CD4-C1 effector T cells strongly stimulated autologous T cell proliferation, resulting in up to 60-fold number increases. **p<0.01, compared with the DC groups treated with or without CD4-C1 T cells. (B) PPD-pulsed DCs treated with or without CD4-C1 T cells dramatically induced the increase of PPD-specific IFN-γ-producing T cell populations in the co-cultured T cells. In contrast, γδ Treg-induced senescent DCs as APCs only induced minor levels of PPD-specific IFN-γ-producing T cells. CD4+ T cells (1×105) purified from two healthy donors were co-cultured for 14 days with PPD-pulsed autologous DCs, which were pre-treated with medium, CD4-C1 or γδ Treg cells at ratio of 10:1. CD4+ T cell numbers were counted (in A). Furthermore, co-cultured CD4+ T cells were restimulated with PPD-pulsed autologous DCs for 2 days and IFN-γ-producing T cells determined by FACS analyses (in B).
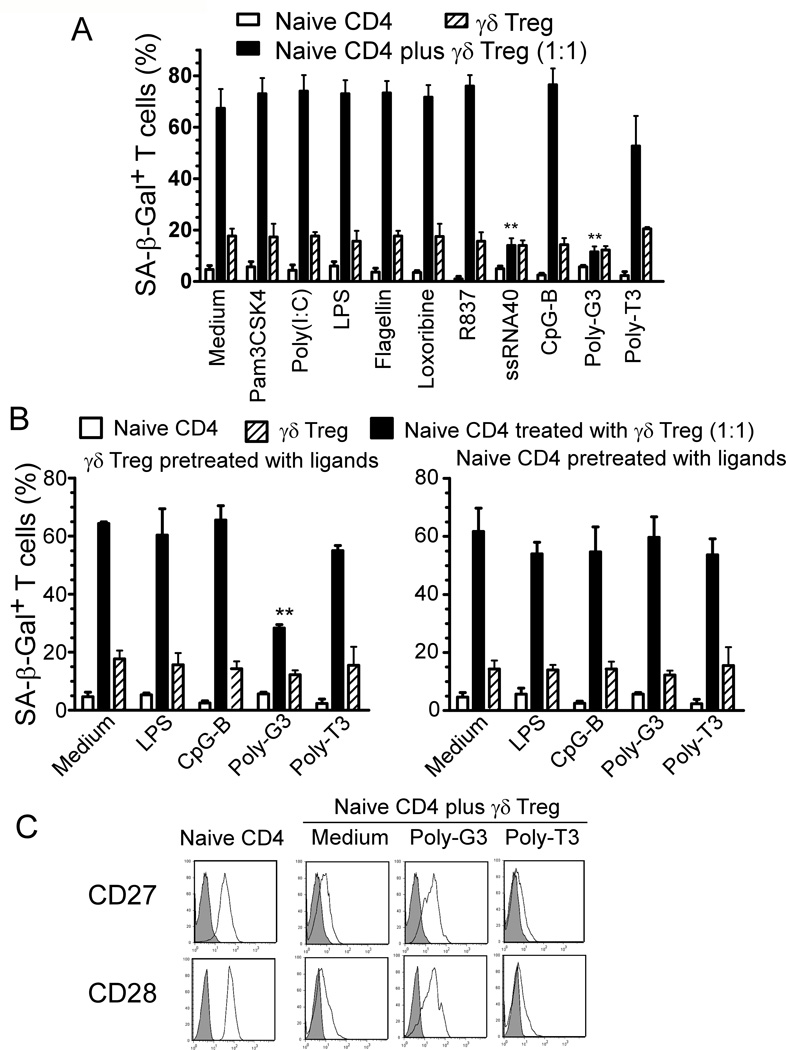
TLR8 signaling reverses γδ Treg cell-induced senescence in responder T cells and DCs
We have previously demonstrated that TLR8 signaling reversed suppressive functions mediated by different subsets of human Treg cells, including CD4+, CD8+ and γδ Treg cells (4, 13, 33). We further showed that TLR8 signaling can also prevent the induction of T cell senescence mediated by naturally occurring CD4+ Treg cells (28). We next tested whether TLR8 signaling can also reverse the process of γδ Treg-induced T cell senescence. We co-cultured naïve CD4+ T cells with γδ Treg cells in the presence or absence of a panel of TLR ligands and control Poly-T3, and tested for their ability to block the induction of T cell senescence. These TLR ligands included: Pam3CSK4 (TLR2), Poly (I:C) (TLR3), LPS (TLR4), Flagellin (TLR5), Loxoribine (TLR7), Imiquimod-R837 (TLR7), ssRNA40 (TLR8), Poly-G3 (TLR8) and CpG-B (TLR9) oligonucleotides. We found that only the TLR8 ligands Poly-G3 and ssRNA40 significantly blocked the induction of responder T cell senescence induced by γδ Treg cells identified by SA-β-Gal expression (Figure 7A). To exclude the possibility that TLR8 ligands prevented γδ Treg-induced senescence through direct effects on responder T cells, we pretreated γδ Treg cells or responder naïve CD4+ T cells with TLR ligands. After extensive washes, these pretreated cells were then co-cultured with untreated naïve CD4+ T cells or Treg cells, respectively, and senescent T cell populations in co-cultured naïve CD4+ T cells were determined. We observed that pretreatment of γδ Treg cells with Poly-G3 dramatically decreased the senescence induction in naïve CD4+ T cell populations; whereas pretreatment of naïve CD4+ T cells with TLR8 ligand Poly-G3 did not prevent Treg-induced T cell senescence (Figure 7B). Furthermore, we also tested whether TLR signaling can prevent the loss of co-stimulatory molecules, CD27 and CD28, in senescent T cells induced by γδ Treg cells. As expected, we found that the expression of CD27 and CD28 in γδ Treg-induced senescent T cells was significantly restored after treatment with TLR8 ligand Poly-G3 on γδ Treg cells (Figure 7C). Similarly, we further confirmed that treatment of γδ Treg cells with TLR8 ligands also blocked the senescence induction in DCs, consistent with our previous finding that TLR8 signaling can abrogate the suppressive effects of γδ Treg cells on the maturation and function of DCs (13) (Data not shown). These results clearly indicate that TLR8 signaling can prevent tumor-derived γδ Treg-mediated senescence induction in responder T cells and DCs.
Figure 7. TLR8 ligands reverse the γδ Treg-induced T cell senescence.
(A) TLR8 ligands Poly-G3 and ssRNA40, but not ligands for other TLRs, markedly reversed the ability of human γδ Treg cells to induce naïve CD4+ T cell senescence. CFSE-labeled naïve CD4+ T cells were incubated alone or co-cultured with γδ Treg cells in anti-CD3-coated plates in the presence of the indicated TLR ligands for 5 days. The treated naïve CD4+ T cells were purified by FACS sorting and SA-β-Gal positive T cell populations in different groups determined. Poly-T3 (3 µg/ml) served as a control. **p<0.01, compared with the groups treated with medium or other TLR ligands. Data shown are representative of three independent experiments with similar results. (B) TLR8 signaling-mediated reversal of Treg-induced T cell senescence was due to the effects on γδ Treg cells. Pretreatment with TLR8 ligand Poly-G3 in γδ Treg cells (left panel), but not in the naïve responder T cells (right panel), reversed Treg-induced T cell senescence. Naïve CD4+ T cells, or γδ Treg cells, were pretreated with different TLR ligands for 2 days, followed by co-culture with untreated γδ Treg cells or naïve CD4+ T cells, respectively, for 5 days. The numbers of SA-β-Gal positive cells in treated CD4+ T cells were then determined. **p<0.01, compared with the group not treated with TLR ligand. (C) Poly-G3 restored the expression of CD27 and CD28 in naïve CD4+ T cells induced by human γδ Treg cells. Naïve CD4+ T cells were cultured with γδ Treg at the ratio of 1:1 in the presence of Poly-G3 or control Poly-T3 for 5 days. Treated naïve CD4+ T cells were separated and CD27 or CD28 expression analyzed by FACS.
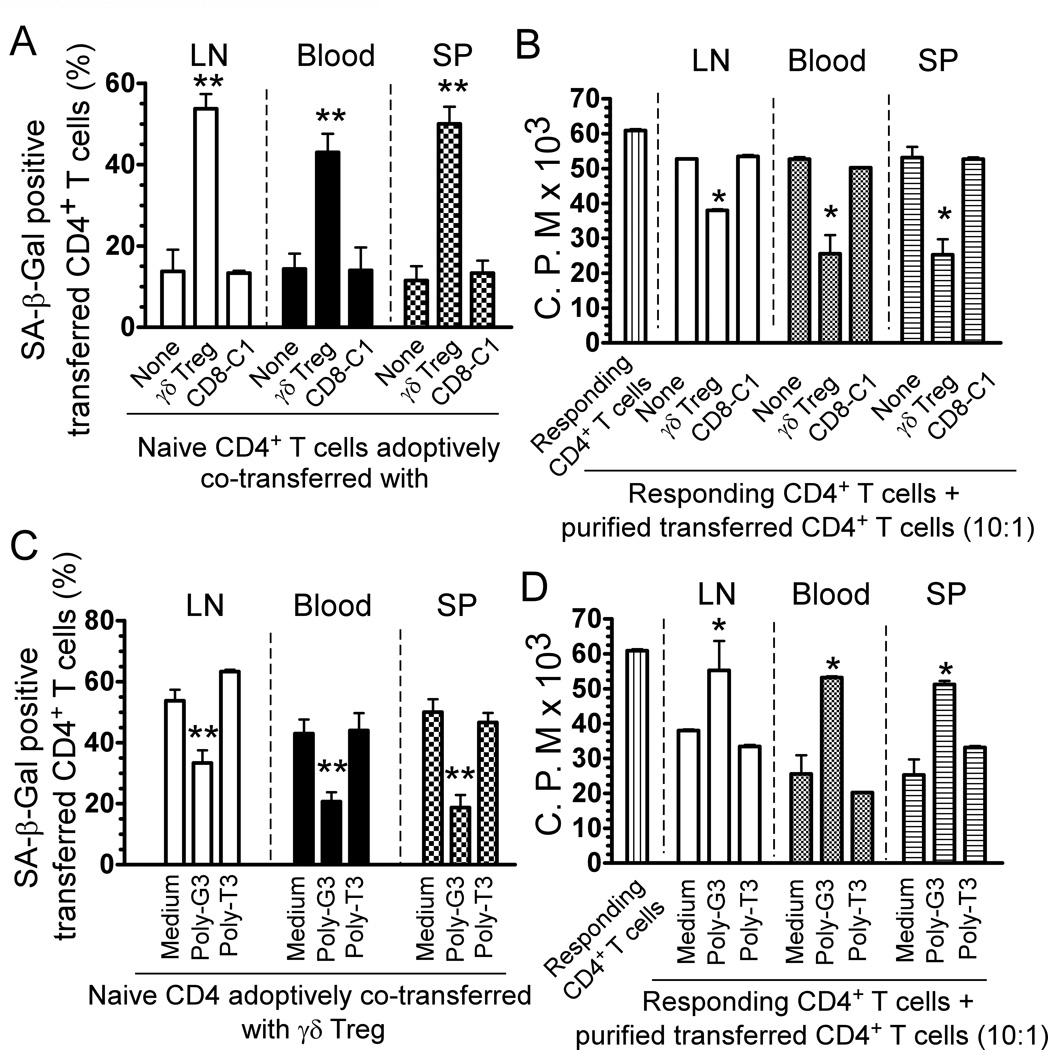
Prevention of T cell senescence induced by γδ Treg cells in vivo via TLR8 signaling
We next investigated whether tumor-derived γδ Treg cells can convert naive T cells into senescent T cells with potent suppressive activity in vivo (13, 28, 33). Naïve CD4+ T cells, γδ Treg cells and CD8-C1 effector T cells (a control) were pre-activated with anti-CD3 antibody, and adoptively co-transferred into Rag1−/− mice in different combinations, including pre-activated naïve CD4+ T cells alone, pre-activated CD4+ T cells plus γδ Treg cells, and pre-activated CD4+ T cells plus CD8-C1 effector T cells. Transferred human CD4+ T cells were isolated from blood, LNs, and spleens in Rag1−/− mice to determine their senescence and suppressive activity. As shown in Figure 8A, appropriately 10–15% of adoptively transferred pre-activated CD4+ T cells became senescent T cells in Rag1−/− mice at 12 days post injection. However, significantly increased senescent T cell populations were induced in pre-activated CD4+ T cells when co-transferred with γδ Treg cells (over 40%). In contrast, co-transfer with CD8-C1 T cells did not promote pre-activated T cell senescence. These results clearly indicate that human γδ Treg cells can induce responder T cell senescence in vivo. We then determined the suppressive activity of the recovered CD4+ T cells on the proliferation of responding T cells using 3H-thymidine incorporation assays (28). As expected, we found that the purified CD4+ T cells from different organs previously co-transferred with γδ Treg cells potently suppressed the proliferation of responding CD4+ T cells. However, purified CD4+ T cells previously co-transferred with or without control CD8-C1 effector T cells did not have any suppressive activity (Figure 8B).
Figure 8. Reversal of γδ Treg-induced senescence by TLR8 ligand in vivo.
(A) Increased SA-β-Gal positive cell populations were markedly induced in naïve CD4+ T cells after co-transfer with γδ Treg cells, whereas co-transfer with CD4-C1 T cells did not induce increased senescent CD4+ T cells. (B) The purified CD4+ T cells co-transferred with γδ Treg cells had potent suppressive activity on the proliferation of responding CD4+ T cells. Naïve CD4+ T cells (5 × 106/mouse), γδ Treg (2 × 106/mouse) and CD4-C1 T cells (2 × 106/mouse) were pre-activated with anti-CD3 antibody and adoptively co-transferred into Rag1−/− mice. Blood, LNs and SPs were harvested at 12 days post-injection. The transferred human CD4+ T cells were isolated for subsequent SA-β-Gal staining (A) and 3H-thymidine incorporation assays (B). * p<0.05 and **p<0.01, compared with the groups co-transferred with CD8-C1 T cells or alone. (C) and (D) Pretreatment of γδ Treg cells with Poly-G3 before co-injection, can significantly block the induction of senescence and reverse the suppressive activity in transferred naïve CD4+ T cells. γδ Treg were pre-treated with Poly-G3 or Poly-T3 (control) for 2 days, before co-transfer. The transferred human CD4+ T cells in different organs were isolated at 12 days post-injection for subsequent SA-β-Gal staining (C) and 3H-thymidine incorporation assays (D). *p<0.05 and **p<0.01, compared with the medium only and Poly-T3 groups.
Since we have shown that TLR8 signaling in Treg cells can control Treg-induced senescence in vitro, we next investigated whether we can prevent the induction of senescent T cells mediated by γδ Treg cells in vivo by the manipulation of TLR8 signaling in this adoptive transfer model. γδ Treg cells were pre-treated with TLR8 ligand (Poly-G3) or control Poly-T3 for 24 hours. The treated or untreated γδ Treg cells were co-transferred with anti-CD3-preactivated naïve CD4+ T cells into Rag1−/− mice following the same procedures described as above. The senescent cell populations and suppressive activity of the recovered CD4+ T cells were then investigated at 12 days post injection. As shown in Figure 8C, pretreatment of γδ Treg cells with Poly-G3 significantly blocked the induction of senescence in the transferred CD4+ T cells. Furthermore, we observed that pre-activated CD4+ T cells did not develop suppressive activity in vivo after being co-transferred with γδ Treg cells pretreated with Poly-G3, but not Poly-T3 (Figure 8D). These results collectively suggest that human tumor-derived γδ Treg cells can convert responder T cells into senescent T cells with suppressive function both in vitro and in vivo, and that manipulation of TLR8 signaling in Treg cells can also prevent γδ Treg-mediated induction of T cell senescence and subsequent immune suppression.
Discussion
Immunosuppressive microenvironments induced by different types of Treg cells present major barriers to successful anti-tumor immunotherapy. It is now widely acknowledged that the success of immunotherapy against cancer ultimately may depend on how well we understand the immunosuppressive mechanisms utilized by Treg cells, and whether or not we can modulate Treg function in the tumor microenvironment (1, 2). We recently discovered high percentages of γδ1 Treg cells existing in the human breast tumor microenvironment that suppressed CD4+, CD8+ and Vγ9Vδ2 T cells, and blocked the maturation and activity of DCs (13). In the current studies, we further explored the suppressive mechanism(s) utilized by tumor-derived γδ Treg cells on innate and adaptive immunity. We identified that human γδ Treg cells strongly suppressed naïve and effector T cell proliferation, as well as impaired DC functions through the induction of senescence in responder immune cells. In addition, we demonstrated that manipulation of TLR8 signaling in γδ Treg cells can prevent γδ Treg-induced conversion of T cells and DCs into senescent cells in vitro and in vivo. We have recently identified that human naturally occurring Treg cells also induced targeted T cell senescence (28). Therefore, our studies strongly suggest that although different types of Treg cells inhibit immune responses using different suppressive mediators at different levels, they may direct a similar fate in suppressed responder T cells. These studies should be critical for the development of strong and innovative approaches for improved tumor immunotherapy.
γδ T cells serve not only as sentinels in the innate immune system, but also act as a bridge between innate and adaptive immune responses, performing multiple functions (38–40). The roles of human Vγ9Vδ2 T cells in mediating immunity against microbial pathogens and tumors have been well described (38, 41). Several clinical trials focusing on the activation of Vγ9Vδ2 T cells as a cancer treatment in patients with renal cell carcinoma, non-Hodgkin lymphoma or multiple myeloma and prostate cancer, have shown promising results (42–44). Besides the important roles of γδ T cells in antimicrobial and antitumor immunity, recent studies in mice and humans suggested that γδ T cells may also have negative regulatory functions. The negative regulation of γδ T cells in mouse models of induced mucosal tolerance, ocular tolerance and self-tolerance, as well as prevention of food allergy has been well documented (6–9, 45). Furthermore, the immunomodulation role of IELs in epithelia tissues has been established (6, 46). In addition, studies from mouse tumor models have suggested that γδ T cells in the tumor microenvironment may be involved in the induction of tumor-specific immune tolerance (10–12). A high frequency of γδ1 T cells has been shown among TILs or circulating PBMCs from cancer patients (47, 48). However, little is known about negative regulatory function of these γδ T cells in anti-tumor immunity in cancer patients. We recently analyzed cell populations in TILs isolated from human breast tumors and were the first to identify high percentages of γδ1 Treg cells with potent suppressive function existing in the tumor microenvironment (13). We further explored the potential functions of γδ Treg cells in the immunopathogenesis of human breast cancer. We observed that patients with a high proportion of γδ T cells have advanced cancer stages and high lymph node metastasis. Importantly, high numbers of γδ T cells in breast cancer tissues identified poor survival rate and high risk of relapse patients (14). Dissecting the functional role of different subsets of regulatory TILs in the tumor suppressive microenvironment is critical for the development of effective strategies for anti-tumor immunotherapy.
Although significant progress has been made in delineating the molecules and mechanisms that Treg cells use to mediate suppression, the majority of these studies and mechanisms are performed and obtained from conventional Treg cells. However, the suppressive mechanisms induced by γδ Treg cells are still unclear. Recent studies suggested that the possible suppressive mechanisms utilized by mouse γδ T cells were through Fas and Fas ligand pathway, and/or secretion of suppressive cytokines TGF-β and IL-10 (7, 46). In our efforts to identify the suppressive mechanisms of human breast tumor-derived γδ Treg cells, we found that the suppressive effects mediated by human γδ Treg cells were through unknown soluble factors (Data not shown). Furthermore, our current studies clearly showed that human γδ Treg cells induced responder T cells and DCs into senescent immune cells, but not induction of their apoptosis or cytolysis. In addition, we characterized the Treg-induced senescent T cells and DCs, and showed that these cells had significant phenotypic and functional changes. γδ Treg-induced senescent T cells dramatically downregulated expression of co-stimulatory molecules CD27 and CD28 indicating their dysfunction. Senescent DCs also had immature phenotypes, suppressed secretion of effector cytokines and up-regulated suppressive molecule PD-L1, as well as impaired co-stimulation and APC functions. More importantly, besides the altered phenotypes, both senescent T cells and DCs converted into suppressive immune cells which had negative regulatory effects on immune responses. Future studies should be focused on the dissection of the suppressive molecules produced by tumor-derived γδ Treg cells responsible for suppressive effects and induction of immune cell senescence.
Given that senescent T cells and DCs induced by human breast tumor-derived γδ Treg cells possess potent suppressive function, the possibility of prevention of senescence induction and restoring the effector function of Treg-induced senescent T cells and DCs is also critical for anti-tumor immunity. TLRs have been recognized as critical components of the innate immune system and as very important for regulating Treg function (49, 50). We have demonstrated that human TLR8 signaling can reverse the suppressive functions of tumor-derived CD4+, CD8+ and γδ Treg cells (4, 13, 33). In the more recent and current studies, we further showed that manipulation of TLR8 signaling in naturally occurring CD4+ Treg and γδ Treg cells can also prevent senescence induction in responder immune cells by abrogating human Treg cell suppressive activity in vitro and in vivo (28). These studies provide a novel strategy capable of preventing human Treg cell suppressive function and augmenting immune responses directed against infectious diseases and cancer. Another challenge for the success of immunotherapy against cancer is how to identify the origin and mechanisms governing the increase of different types of Treg cells in cancer patients. Recent studies suggest that there are several potential sources of Treg cells that exist in tumor sites. One key mechanism responsible for accumulation of Treg cells within the tumor microenvironment is preferential recruitment of these Treg cells. Studies of Hodgkin’s lymphoma and ovarian cancer have shown that tumor microenvironmental CCL22 derived from cancer cells specifically recruits CCR4 positive CD4+ Treg cells to tumor sites (51, 52). Our current and previous studies have shown that increased numbers of γδ T cells were only observed in breast tumor tissues but not in normal breast tissues, suggesting the recruitment and expansion of γδ T cells by the tumor microenvironment (13). Our future studies will also focus on the identification of mechanisms responsible for the accumulation of γδ T cells in the tumor microenvironments.
In summary, our current study provides the critical evidence that human tumor-derived γδ Treg cells directly suppress both innate (DCs) and adaptive immune cells (naïve and effector T cells) through the induction of senescence in the responder immune cells. Importantly, γδ Treg-induced senescent T cells and DCs altered their phenotypes and functions. Both senescent DCs and T cells had potent suppressive activities which may result in an amplified immune suppression induced indirectly by γδ Treg cells in the tumor suppressive microenvironment. Furthermore, we demonstrated that γδ Treg-induced T cell senescence can be prevented by the manipulation of TLR8 signaling in γδ Treg cells. These studies provide new insights relevant for the development of novel strategies capable of augmenting anti-tumor immune responses by eliminating or reversing the suppressive functions of γδ Treg cells.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Richard Di Paolo for providing Rag1−/− mice, and Joy Eslick and Sherri Koehm for FACS sorting and analyses.
Financial support
This work was partially supported by grants from the American Cancer Society (RSG-10-160-01-LIB, to G. P), the Melanoma Research Alliance (to G. P), and the NIH (to G. P).
Footnotes
Conflict-of interest disclosure
The authors declare no financial or commercial conflict of interest.
References
- 1.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 2.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 4.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 6.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapp JA, Kapp LM, McKenna KC. Gammadelta T cells play an essential role in several forms of tolerance. Immunol Res. 2004;29:93–102. doi: 10.1385/IR:29:1-3:093. [DOI] [PubMed] [Google Scholar]
- 8.Komori HK, Meehan TF, Havran WL. Epithelial and mucosal gamma delta T cells. Current opinion in immunology. 2006;18:534–538. doi: 10.1016/j.coi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 10.Kapp JA, Kapp LM, McKenna KC, Lake JP. gammadelta T-cell clones from intestinal intraepithelial lymphocytes inhibit development of CTL responses ex vivo. Immunology. 2004;111:155–164. doi: 10.1111/j.0019-2805.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke Y, Kapp LM, Kapp JA. Inhibition of tumor rejection by gammadelta T cells and IL-10. Cellular immunology. 2003;221:107–114. doi: 10.1016/s0008-8749(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Seo N, Tokura Y, Takigawa M, Egawa K. Depletion of IL-10- and TGF-beta-producing regulatory gamma delta T cells by administering a daunomycin-conjugated specific monoclonal antibody in early tumor lesions augments the activity of CTLs and NK cells. J Immunol. 1999;163:242–249. [PubMed] [Google Scholar]
- 13.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, Schwartz T, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, Peng G. Tumor-Infiltrating gammadelta T Lymphocytes Predict Clinical Outcome in Human Breast Cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Kang N, Zhang X, Dong X, Wei W, Cui L, Ba D, He W. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:6693–6700. doi: 10.4049/jimmunol.1002776. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 18.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 19.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 20.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 21.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, Fietta AM. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ, Rowland-Jones SL. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 30.Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+CD28− T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 31.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 32.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 34.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–2670. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 35.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 36.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 37.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 39.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 40.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science (New York, N.Y. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 41.Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive gamma9delta2 T cells mediate protective tuberculosis immunity. J Immunol. 2008;181:4471–4484. doi: 10.4049/jimmunol.181.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 44.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer research. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bol-Schoenmakers M, Marcondes Rezende M, Bleumink R, Boon L, Man S, Hassing I, Fiechter D, Pieters RH, Smit JJ. Regulation by intestinal gammadelta T cells during establishment of food allergic sensitization in mice. Allergy. 2011;66:331–340. doi: 10.1111/j.1398-9995.2010.02479.x. [DOI] [PubMed] [Google Scholar]
- 46.Pennington DJ, Vermijlen D, Wise EL, Clarke SL, Tigelaar RE, Hayday AC. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv Immunol. 2005;87:27–59. doi: 10.1016/S0065-2776(05)87002-6. [DOI] [PubMed] [Google Scholar]
- 47.Bas M, Bier H, Schirlau K, Friebe-Hoffmann U, Scheckenbach K, Balz V, Whiteside TL, Hoffmann TK. Gamma-delta T-cells in patients with squamous cell carcinoma of the head and neck. Oral oncology. 2006;42:691–697. doi: 10.1016/j.oraloncology.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Kowalczyk D, Skorupski W, Kwias Z, Nowak J. Flow cytometric analysis of tumour-infiltrating lymphocytes in patients with renal cell carcinoma. British journal of urology. 1997;80:543–547. doi: 10.1046/j.1464-410x.1997.00408.x. [DOI] [PubMed] [Google Scholar]
- 49.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. The Journal of experimental medicine. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer research. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 52.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.