Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the third cause of cancer-related death worldwide, and its incidence is increasing. Despite the significant improvement in HCC management in the last 30 years, there are no effective chemoprevention strategies, and only 1 systemic therapy has been approved for patients with advanced tumors. This drug, sorafenib, acts on tumor cells and the stroma. HCC develops from chronically damaged tissue that contains large amounts of inflammation and fibrosis, which also promote tumor progression and resistance to therapy. Increasing our understanding of how stromal components interact with cancer cells and the signaling pathways involved could identify new therapeutic and chemopreventive targets.
Keywords: Liver Cancer, extracellular matrix, angiogenesis, chemoprevention
HCC is the most frequent primary form of liver cancer and the third most deadly tumor globally, following lung and stomach cancers1. With more than 750,000 new cases diagnosed every year worldwide, HCC is the sixth most common neoplasm 2. Unlike other carcinomas, its incidence is steeply increasing, mainly due to the increasing prevalence of advanced hepatitis C virus (HCV) infection. HCC commonly arises in the setting of cirrhosis (>80% of cases), appearing 20-30 years following the initial insult to the liver. The use of antivirals and vaccination has successfully diminished the incidence of hepatitis B (HBV)-related HCC, although there are no effective chemopreventive strategies to attenuate the development of cancer once cirrhosis is established3. Most HCC patients are diagnosed at advanced/symptomatic stages when limited therapeutic options are available. The results of the randomized Phase-III SHARP (Sorafenib HCC Assessment Randomized Protocol) trial demonstrated that the multikinase inhibitor sorafenib improved overall survival of patients with advanced HCC4, representing a breakthrough in the clinical management of this cancer.
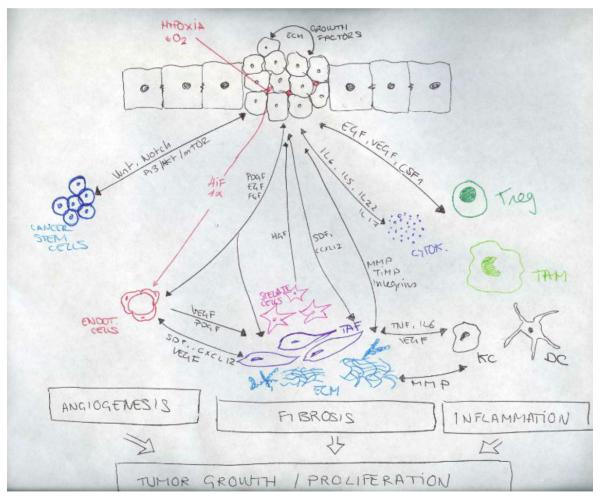
The liver tumor microenvironment (TME) is a complex mixture of tumoral cells within the ECM, combined with a complex mix of stromal cells and the proteins they secrete. Together, these elements contribute to the carcinogenic process. Cancer cells do not manifest the disease alone and the stroma is inappropriately activated in cancer to contribute to malignant characteristics of tumor cells. The TME and the tumor cells create a complex cellular system with reciprocal signalling (Figure 1).
Figure 1.
Cellular components of the microenvironment and molecular mechanism influencing tumor growth and progression. Stromal, inflammatory and cancer cells interact among them to create a complex interaction network that origin a permissive microenvironment and favor tumor progression. TAFs, tumor associated fibroblasts; CSF-1, colony stimulating factor 1; EC, endothelial cells; KC, Kupffer cells; VEGF, vascular endothelial growth factor; FGF, Fibroblast growth factor PDGF, platelet-derived endothelial cell growth factor: Tregs, regulatory T cells; HGF, Hepatocyte Growth Factor; EGFR, Epidermal Growth Factor Receptor, MMPs, metaloproteinases; TIMP, Tissue Inhibitor of Metalloproteinases; HIF-1, HIF-1, hypoxia-inducible factor 1; TAM, Tumor associated macrophages; SDF-1, stromal cell-derived factor 1; CSC, Cancer stem cells; DC, Dendritic cells; TNF, Tumor Necrosis Factor.
Stromal components of the ME can be divided into three subclasses: angiogenic cells, immune cells and cancer associated fibroblastic cells. There is growing evidence of the contribution of stromal cells to the hallmarks of cancer: sustaining proliferative signalling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, reprogramming energy metabolism and evading immune destruction5. Alterations within the microenvironment, in particular in stromal fibroblasts, may influence tumor initiation in adjacent epithelia and promote progression 6, 7. Moreover, the ME also plays an important role in chemoresistance 8,9 and drug delivery 10. Targeting stromal cells to abrogate their tumor-supporting role represents an attractive therapeutic strategy.
The role of microenvironment in tumor initiation and progression in HCC is critical. For instance, the status of non-tumoral tissue has an important role predicting tumor recurrence, which affects 70% of patients after resection or local ablation 11. Typically, there are two patterns of HCC recurrence: true metastasis of the primary tumor (generally within 2 years following resection/transplantation, defined as ‘early recurrence’) and de novo tumor (after 2 years from treatment or ‘late recurrence’) 12, 13. Among these features, late recurrence is generally dictated by the persistence of pro-tumorigenic signals within the damaged milieu of the fibrotic and cirrhotic liver 14; distinct molecular subgroups of HCC have been identified and linked to poor prognosis 15-20. In another context, the information encoded within the surrounding adjacent non-tumoral tissue is essential to predicting the outcome of patients at very early stages (i.e., tumors less than 2 cm without vascular invasion or extrahepatic spread), and has been suggested to be even more relevant than the genomic profile of the tumor itself13. These findings highlight the profound involvement of a dynamic network of non-tumoral cells, molecules and soluble factors in the generation of a supportive and permissive environment for HCC initiation and progression.
In this review, we provide an overview of current knowledge on the role of the tumor microenvironment in HCC and highlight potential prognostic and therapeutic implications.
The importance of the tumor microenvironment
The development and progression of HCC is a multistage process. A chronic insult (e.g. HCV, HBV and alcohol) induces liver injury through oxygen species (ROS) production, cellular DNA damage, endoplasmic reticulum (ER) stress, and necrosis of damaged hepatocytes. Most HCCs arise in the setting of chronic hepatitis induced by HCV or HBV infection. HCV is a single-stranded RNA virus that cannot integrate into the host genome, but triggers an immune-mediated inflammatory response that promotes neoplastic transformation of damaged hepatocytes. Conversely, HBV can integrate into the genome of infected hepatocytes and promotes hepatocarcinogenesis through sustained inflammatory damage, hepatocyte regeneration and direct oncogenic transformation following integration of the viral genome into host genes, and the transactivating potential of several viral oncoproteins, especially HBx. The sustained dysregulation of the liver cell by HBV infection can ultimately affect DNA repair mechanisms and promote mutational events, which contribute to malignant transformation of hepatocytes.
The hepatic response involves the activation of hepatic stellate cells and macrophages, which produce components of the extracellular matrix and growth factors that promote migration of endothelial cells, neo-angiogenesis and fibrosis. This process is associated with distortion of the parenchyma and vascular architecture characterized by progressive capillarization, with reduction of endothelial cell fenestrae size, and deposition of basement membrane components including collagen type IV and laminin within the space of Disse. This process, in the context of inflammation and oxidative DNA damage, favors the accumulation of mutations and epigenetic aberrations in pre-neoplastic hepatocytes or liver stem cells, thereby promoting the development of dysplastic nodules and their malignant transformation to early HCC 21. Therefore, HCC is not just a mixture of cells and extracellular matrix (ECM); it contains several cell types that interact with each other and the surrounding tissue, creating a complex interaction network within a permissive microenvironment. The stromal components support tumor growth and promote invasion through the stimulation of cancer cell proliferation, migration and invasion, and activation of angiogenesis, which together determine the phenotype of the tumor.
Relevance of microenvironment in other malignancies
The link between inflammation and generation of a pre-neoplastic milieu has been reported in many diseases, such as in the development of colorectal and pancreatic carcinomas in the context of inflammatory bowel disease and chronic pancreatitis, respectively 22. Once the cancer has been established, the contribution of the microenvironment to the regulation of tumor behaviour has been well recognized for other malignancies, including breast, lung and pancreatic carcinomas 23.
Abnormal ECM production and altered physical propertise are frequently reported in malignancies. In breast carcinoma, for example, the tumor stroma is 10 times stiffer than normal breast, partially due to excess activity of lysyl oxidase and accumulation of collagen and other ECM components 24. Similarly, in pancreatic ductal adenocarcinoma, the large amounts of ECM proteins, activated fibroblasts, stellate cells and inflammatory cells has been described as a ‘fortress-like’ hypovascular barrier that impairs the delivery of chemotherapeutics and promotes aggressive neoplastic cell behavior 25.
Different aspects of tumor biology, including development, progression and response to therapy can be affected by components of the tumor microenvironment. In mice, the recapitulation of human breast tumor orthotopic xenografts is largely determined by the presence of human tumor-derived stromal fibroblasts. Accordingly, studies in human tissues showed that tumor associated fibroblasts (TAFs) isolated from breast carcinomas promoted the growth of breast cancer cells through the production of soluble factors, such as colony stimulating factor 1 (CSF-1) 26. In line with these findings, gene expression studies from the tumor microenvironment of human breast carcinomas reported up-regulation of several factors, including chemokines with pro-tumorigenic function 27.
The findings that the degree of activation of the stroma affects tumor growth and progression has led to the concept of ‘stromal staging’, which has potential clinical utility. For example, the increased production of ECM proteins including fibronectin, collagen IV, and tenascin C is associated with poor prognosis in patients with small cell lung carcinoma 28. This is related to the activation of pro-survival and anti-apoptotic pathways in neoplastic cells following their adhesion to components of the ECM, for example by the stimulation of the PI3K/Akt pathway downstream of β1-integrins 29. Furthermore, the abundance of specific stromal cells correlates with patient outcome. In breast cancer, the density of tumor-associated macrophages is associated with poor survival and reduced response to chemotherapy 30. Furthermore, recent findings indicate that the detection of p53 mutations in the stromal component increases the likelihood of nodal metastasis, suggesting that mutation-bearing stromal cells can provide a favorable setting for tumor spread 31. Interestingly, the stroma may also mediate resistance to molecular therapies by secreting growth factors (e.g. HGF/Met) that can stimulate survival responses and prevent apoptosis. This indicates that the tumor microenvironment actively favors the selection and expansion of cellular clones that are more likely to survive and adapt to the changes induced by stromal cells 32.
Indeed, chemotherapy induces the production of CSF1, a chemo-attractant for macrophages, which exacerbates tumor progression by promoting angiogenesis, invasion and metastasis of neoplastic cells 33. In one study, the pharmacological blockade of macrophage recruitment markedly improved the ability of the chemotherapeutic agent paclitaxel to slow the growth of both primary and metastatic tumors 30. Finally, a stromal gene signature predicts resistance to neo-adjuvant chemotherapy in estrogen receptor-negative breast cancer patients 34.
Biological processes involved in tumor microenvironment
The precancerous milieu of chronic liver disease is characterized by neoangiogenesis, including several vascular abnormalities such as arterialization and sinusoidal capilarization, as well as inflammation and fibrosis. These biological processes become more pronounced with progression of liver failure, where the incidence of cancer increases exponentially (Figure 2). Synchronous events occurring in this setting also include hypoxia, oxidative stress and autophagy.
Figure 2.
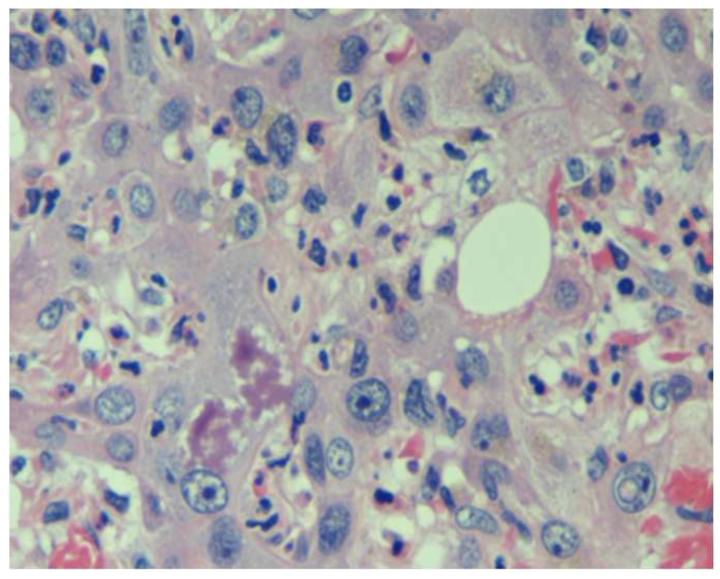
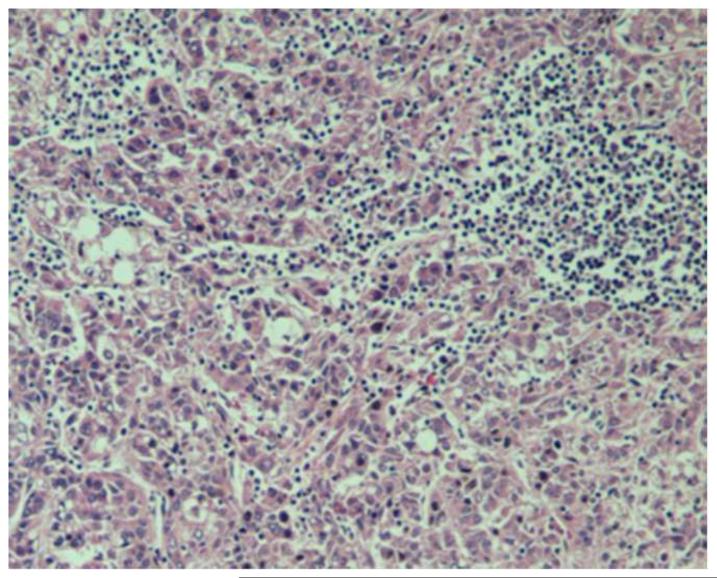
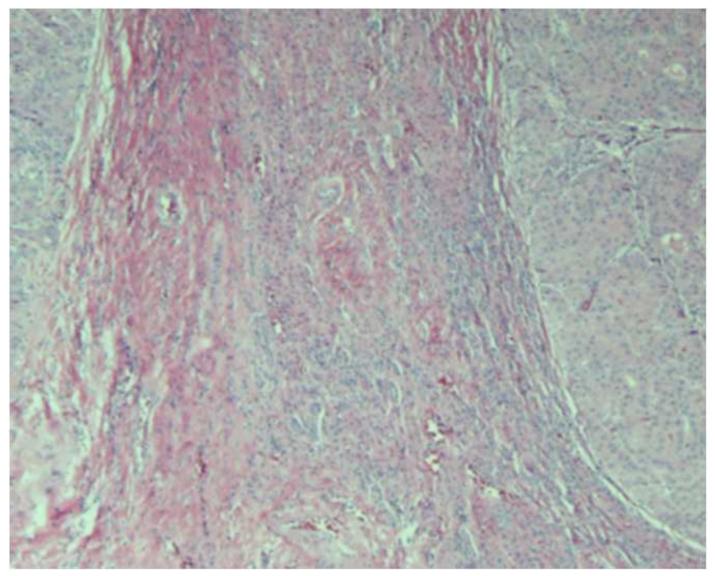
Pathological features that may be present in hepatocellular carcinoma (HCC). (a) Poorly-differentiated HCC. Tumor cells have marked pleomorphic nuclei and an inflammatory infiltrate consisting of neutrophils. Ballooning degeneration and production of Mallory’s hyalines are also noted. H&E original magnification 400X. (b) Poorly-differentiated HCC with tumor cells arranged in a solid pattern. A focus of lymphocytic inflammatory infiltrate is present. H&E, original magnification 100X. (c) Well-differentiated multinodular HCC with dense fibrosis forming a wide septum that separate two HCC nodules. H&E, original magnification 40X. (d ) Increased vascularization in HCC. Vessels are highlighted by CD34 immunostaining. These vessels are nourishing the tumor. Original magnification 100X. Images courtesy of Dr. M. Isabel Fiel, Mount Sinai School of Medicine.
Angiogenesis
Angiogenesis plays an important role in hepatocarcinogenesis from its early stages 37. HCC is a highly vascularized tumor; indeed, pathological angiogenesis is one of the main contributors to chronic liver diseases. The hepatic wound healing response due to chronic liver injury leads to fibrogenesis, a process that entails secretion of several pro-angiogenic factors by the stromal cells, especially MMP, PDGF, TGFβ1, FGF and VEGF. Moreover ECM deposition and anatomical alterations during the fibrogenic process provoke resistance to blood flow that reduces metabolic exchange of oxygen, favoring hypoxia.
Indeed, gene expression of vascular endothelial growth factor (VEGF), the most critical pro-angiogenic factor, is already induced in dysplastic nodules and further increases according to the progression of HCC development 37. Once the tumor is established, the survival of neoplastic cells requires the formation of a new vascular network to provide nutrients and oxygen. The angiogenic process in HCC is complex and tightly regulated, resulting from the balance between multiple angiogenic and anti-angiogenic factors from the tumor and the host cells. Growth of the tumor mass creates a nutrient and oxygen-deprived environment, which induces the activation and proliferation of endothelial cells (EC) to sprout new vessels from pre-existing ones35. EC become proliferative and liberate enzymes to disrupt the basement membrane, and they eventually migrate to their final location where they assemble to form a new vessel together with the ECM36.
The expression of VEGF correlates with HCC aggressiveness37. The effects of VEGF are transduced following binding to its receptors, VEGFR1 and VEGFR2 to activate several signaling pathways involved in proliferation, migration and invasion of EC38. In addition, VEGF can function as a cytokine that directly affects hepatic stellate cells, Kupffer cells and hepatocytes39, 40, and mediates the dissolution of the vascular basement membrane and the interstitial matrix37. VEGF and angiopoietin-2 (Ang-2) plasma levels have been identified as independent prognostic biomarkers in patients with advanced HCC41. Ang-2, is frequently up-regulated in HCC and boosts the effect of VEGF on EC42. Moreover the Tie-2 receptor is expressed by both EC and stellate cells, further emphasizing the complex orchestration of angiogenic regulation in liver tumors.
Fibroblast growth factor (FGF) is a member of the heparin-binding growth factors that acts synergistically with VEGF to induce angiogenesis, while platelet-derived endothelial cell growth factor (PDGF) is involved in cell migration and new vessel maturation. Cancer cells secrete PDGF, which acts through a paracrine mechanism that involve other cells types as EC and fibroblasts, and correlates with cancer progression43. Other significant mediators in tumor neoagiogenesis are integrins and cadherins, which mediate cell-matrix and cell-cell interactions respectively to establish contacts required for new vascular tube formation36.
Inflammation
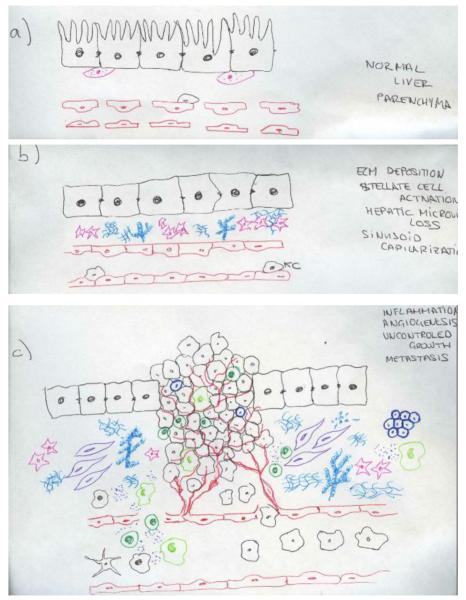
HCCs usually arise in a diseased liver with a dynamic inflammatory environment that predisposes to cancer initiation. Inflammation, an essential part of the liver’s wound healing response and undoubtedly beneficial in a short term, perpetuates chronic injury. Chronic inflammation drives a maladaptive reparative reaction and stimulates liver cell death and regeneration, eventually associated with the development of dysplastic nodules and cancer (Figure 3).
Figure 3.
Anatomical and cellular alterations leading to HCC development. (a) Normal liver parenchyma. Hepatocytes with microvilli and fenestrated sinusoidal cells that favours the metabolic exchange. Space of Disse with few quiescence stellate cells containing lipid droplets. (b) Fibrotic liver. Upon chronic liver injury, hepatocytes loose the microvilli and sinusoids their fenestration, stellate cells become activated, loosing the lipid droplets and secreting ECM. (c) Hepaocellular carcinoma. Malignant transformation of hepatocytes with uncontrolled growth. Infiltration of inflammatory cells and cytokines. Development of new vessels (neoangiogenesis) and extense fibrosis with recruitment of tumor associated fibroblasts and cancer stem cells.
Several inflammatory mediators have been implicated in sustained inflammation and immunosuppression associated with HCC development. Carcinogenesis is associated with persistent cytokine production than can stimulate many liver cell types with a variety of unique as well as redundant interactions. Altered cytokine profiles have been described in HCC not only in tumor cells, but also in the surrounding tissue, however the full portrait of their mechanistic role remains uncover. A predominant role of the Th2-like (IL-4, IL-8, IL-10 and IL-5) cytokine compared to Th1-like (IL-1α, IL-1β, IL-2, TNFα) in the microenvironment has been associated with a more aggressive and metastatic HCC phenotype44, 45. IL-6 is an abundant cytokine in cirrhotic livers and produced by KC in response to hepatocyte damage and potent activator of STAT3, and its elevation in serum is associated with risk of HCC and poor prognosis 13, 46. Moreover, modulation of the inflammatory microenvironment by suppression of HGF and IL-6 production by estrogens, represses HCC metastasis47. High IL-22 levels have been detected in the HCC microenvironment, leading to tumor growth, inhibition of apoptosis, and promotion of metastasis due to STAT3 activation48. IL-10 up-regulation is also present in HCC tumors49, 50 as well as in their microenvironment42 and confers a high risk of progression after resection. However, its specific risk in HCC development remains unknown. Higher IL-2 and IL-15 in the peri-tumoral liver tissue is also associated with a decreased rate of intrahepatic tumor recurrence and prolonged overall survival51.
Chemokines (e.g. CXCL12, CX3CL1, CCL20) are cytokine-like molecules with chemotactic properties critical to cell trafficking into and out of the tumor microenvironment. They orchestrate the inflammatory response though their binding to four families of receptors (CCR, CXCR, CX3CR and XCR) found mainly in inflammatory, endothelial and epithelial cells. Chemokines have been implicated in many key steps of cancer development including evasion of the immune system, angiogenesis, invasion and dissemination52. The CXCL12-CXCR4 axis is especially important in angiogenesis regulation, and is highly expressed in HCC compared to cirrhosis53. CXCL12 binds CXCR4 in endothelial cells and promotes migration, proliferation and development of new vessels, acting synergistically with VEGF51. It has also been implicated in HCC growth, invasion and metastasis55, 56.
Another important axis in HCC regulation is the CCL20-CCR6, which mediates the recruitment of circulating regulatory T cells (Tregs) into the tumor microenvironment. Its up-regulation is associated with tumor growth promotion, a low level of differentiation and the presence of intrahepatic metastasis57. NF-kB and STAT3 are signalling pathways involved in the hepatic inflammatory response to injury that is critical for liver regeneration with overlapping target genes. NF-kB plays a role in hepatocarcinogenesis, however its function varies depending on the mouse model and type of injury applied. In humans, pro-inflammatory stimuli such as hepatitis viruses58 and free fatty acids59 activate NF-kB, which might initiate and promote HCC in the inflamed liver. STAT3 remains inactive in non-stimulated cells and becomes rapidly activated through phosphorylation by cytokines and growth factors produced within the tumor microenvironment. Active STAT3 has been detected in HCC specimens, and is associated with a more aggressive phenotype and poor prognosis60.
The gut microbiota also plays a role in HCC pathogenesis. Chronic liver disease is often associated with translocation of the intestinal bacteria and gut derived LPS via TLR4 can amplify the tumorigenic response of the liver to promote HCC 61-63.
Several growth factors regulate the immune and inflammatory response in the HCC microenvironment, in particular TGF-β, HGF and EGF. Transforming growth factor β (TGFβ), a tumor suppressor in normal and premalignant cells acts as an oncogenic growth factor in cancer cells64. It is expressed mostly in stromal cells rather than malignant epithelial cells and is markedly increased in HCC65. Reduced expression of TGFβ receptor II has been correlated with a poor prognosis in HCC, as defined by larger tumor size, poor differentiation, intrahepatic metastasis and shorter recurrence-free survival65. FGF and Hepatocyte Growth Factor (HGF) control proliferation and invasion of HCC cells66, 67. Overexpression of the HGF receptor c-Met has been detected in several human tumors68 including HCC, where it is associated with a poor outcome. Similarly, a c-Met-regulated expression signature defines a subgroup of HCC with a poor prognosis and aggressive phenotype69. Epidermal growth factor receptor (EGFR) also plays an important role in tumor progression and tumor-associated angiogenesis via regulation of several angiogenic factors, with a direct effect on tumor and endothelial cells70.
Fibrosis
The ECM is essential to support the liver’s architecture and constantly interacts with the environment, allowing signal transduction and changes in gene expression71. In disease, the activity of the ECM remodeling enzymes is deregulated, leading to a fibrotic microenvironment characterized by increased stiffness and abundance of growth factors that contribute to tumorigenesis71. An excess of ECM production together with a reduced ECM turnover characterizes liver fibrosis. Deregulation of collagen cross-linking and ECM stiffness plays a causative role in cancer pathogenesis by enhancing integrin-signalling72. This situation leads to an excessive deposition of fibrillar collagen types I and II and fibronectin in the liver. There is also enhanced growth, survival and proliferation of tumoral cells through regulation of the integrin family. Integrins α1β1 and α2β1 have also been implicated in progression and cell invasion73, as their inhibition reduces migration of liver cancer cells induced by several growth factor (TGFβ1, EGF or bFGF).
Deregulation of ECM homeostasis directly affects epithelial cells and leads to cellular transformation and metastasis. Tumor growth requires the breakdown of pre-existing boundaries and rearrangement of liver tissue, a process mainly regulated by metaloproteinases (MMPs) and Tissue Inhibitor of Metalloproteinases (TIMPs). Over-expression of MMPs can compromise the basement membrane barrier and facilitate tissue invasion by cancer cells. HCC is associated with a higher proteolytic activity, high MMP2 levels. Moreover, an imbalance between MMP2 and TIMP2 correlates with the occurrence of metastasis, leading to a poor outcome74. Linear and thick collagen fibers are often found in areas with active tissue invasion75 and vascularization76, and several studies have shown that tumor cells migrate on collagen fibers76.
ECM is also essential for tumor angiogenesis. To initiate vascular branching, the basement membrane must be removed mainly by MMPs. ECM is also involved in vessel lumen formation, tubulogenesis and deposition of a supportive basement membrane. Notably, tumor new vasculature is more porous and leaky than normal77, 78, facilitating immune cell infiltration, metastasis and tumor progression. ECM also modulates activation of immune cells and can regulate T cell activation79 and immune cell differentiation, for example by impairing the normal maturation of T helper cells71, 80.
ECM stiffness also plays an important role in HCC development. Lysyl oxidase 2, an enzyme able to modify ECM stiffness via promoting cross-linking of fibrilar collagen 1 is involved in the creation of a pathologic stroma able to promote tumor growth and metastasis81.
Other biological processes
Hypoxia
Although HCC is a highly vascularized tumor, neoplasic vessels are functionally abnormal and areas of hypoxia are common. Reduced oxygen availability induces expression of hypoxia-inducible factor 1 (HIF-1), a major transcription factor that regulates the expression of several genes with critical roles in angiogenesis, immune evasion, invasion and metastasis82. Hypoxia stimulates growth and blocks apoptosis of HCC, and levels of HIF-1 correlate with a worse prognosis83.
Oxidative stress
Cancer cells, besides generating oxidative stress intrinsically, are also exposed to a pro-oxidant environment generated by several stromal components84. Overproduction of ROS provokes nitrosative and oxidative stress through interaction with DNA, RNA, lipid and proteins, leading to an increase in mutations, genomic instability, epigenetic changes, and protein dysfunction. Fibroblast activation is profoundly affected by oxidative stress and produces several mediators implicated in tumor progression. Tumor associated macrophages (TAM) can generate ROS due to activation NOX2 and iNOS that promote tumor progression, invasion and metastasis. Moreover, tumoral conditions such as hypoxia produce oxidant species that promote DNA mutations in neoplastic cells. Recently, mutations in specific genes (RPS6KA3-AXIN1 and NFE2L2-CTNNB1) that alter Wnt/β-catenin signaling have been associated with oxidative stress and metabolism that cooperate in liver carcinogenesis85. Moreover, altered oxidative stress pathways in noncancerous human liver tissue can predict hepatocellular carcinoma recurrence. High levels of ROS promotes invasiveness of hepatic tumor cells86 and contribute to tumor invasion via MMP production.
Autophagy
Autophagy, a catabolic process up-regulated under metabolic stress conditions, is induced in tumor microenvironment. Stromal components are exposed to oxidative stress conditions induced by cancer cells that together with hypoxia induce autophagy87. Autophagy in the tumor stroma acts as a pro-survival mechanism that generates energy able to fuel cancer cells alleviating the metabolic imbalance and promoting survival88. It has been postulated as one of the escape mechanism for cancer cells during antiangiogenic treatment89. Although the role of autophagy in the setting of HCC is still under development, defects in autophagic genes (BECN1, ATG7) have been described in HCC cells 90, 91. Autophagy modulation has been identified as s promising therapeutic strategy in combination with molecular target therapy92.
Cellular components of the HCC microenvironment
HCC usually arises in a severely perturbed microenvironment that hastens dysfunction of epithelial cells and malignant transformation. Targeting the components of the microenvironment therefore emerges as a rationale preventive strategy (Figure 1). Here we describe the main cellular components in the microenvironment and identify potential molecular targets for therapies.
Immune cells
HCC is rich in immune cells. Tumor infiltrating lymphocytes (TIL) are the primary immune component in solid tumors and are comprise a host antitumor reaction93. Most TIL cells are CD4+ (helper or Treg cells). Treg cells have a detrimental impact in cancer development, as they promote immune tolerance to neoplastic cells. Tregs usually infiltrate HCC, and a predominance of Treg over TCD8+ is associated with a worse prognosis94; additionally, Treg levels have been correlated with HCC stages95. Myeloid-derived suppressive cells (MDSC) also play a role in T cell regulation and induction, favoring a suppressive immune response within the microenvironment96. Increased secretion of IL-17 by CD4+ lymphocytes in HCC also correlates with increased postoperative recurrence following resection97. MDSC and Treg are both important in the establishment and promotion of immune suppression. Dendritic cells (DC) are decreased and dysfunctional in patients with HCC, and contribute to the insufficient immune antitumoral response. DC vaccination has even been proposed as an antitumor therapy in HCC, but would first require a better understanding of the hepatic microenvironment for its full development 98.
Fibroblasts and macrophages
TAFs are the major source of collagen in the HCC stroma however their origin is still a matter of debate. They differ from normal fibroblast in their ability to secrete high levels of stromal cell-derived factor 1 (SDF-1) and CXCL12, and promote tumor growth and angiogenesis99. There is a complex crosstalk between TAF and tumor cells. For instance, both can secrete PDGF and TGFβ that leads to stellate cell activation and consequently ECM deposition, but they also enhance growth and migration of cancer cells100. TAF also interact with the microvasculature by secreting VEGF and MMPS as well as several hepatocyte proliferation factors such as HGF101. TAF also secrete immune-modulatory cytokines (IFN-γ, IL-6 and TNF) that can mobilize cytotoxic T lymphocytes, natural killer cells and TAM102. TAM, the most abundant cell component, represents a subset of myeloid CD11b+ tumor-infiltrating cells characterized by the expression of the Tie-2 angiopoietin receptor103. TAMs suppress antitumor immunity in HCC, and their density has been correlated with poor prognosis104. TAM also release EGF, chemokines, MMP and VEGF that regulate tumor growth, ECM remodeling and angiogenesis, invasion and metastasis105. Recently, it has been shown that c-Myc controls the activation 106 of TAM, as well as impairing VEGF signalling and infiltrating inflammatory cells107. There is a bi-directional crosstalk, and environmental conditions such as hypoxia can also affect Myc signalling108.
Cancer stem cells (CSC)
defined by their self-renewing and differentiation capacity have been proposed as the clonogenic core of several tumors. In HCC, liver CSCs can be isolated based on their expression of several cell markers (EpCAM, CD133, CD90, CD44, CD24, CD13, and OV6)109. Additionally, signaling pathways identified in liver cancer are active in isolated liver CSCs (e.g., Wnt, Notch, TGF-β, Hedgehog and PI3K/AKT/mTOR), supporting the idea that CSCs contribute to the molecular heterogeneity of HCC110, 111.
Although the clinical relevance of CSCs remains elusive, there is growing evidence supporting a role in initiating and sustaining primary tumors and facilitating metastasis112-115. Recent data support a strong association between a hepatic progenitor cell origin of the tumor and prognosis in HCC 22. One therapeutic approach may be to target not only the signaling pathways involved in stem cell fate (self-renewal and multilineage differentiation potential), reproduction and proliferation (Notch, Wnt, Hedghog)116, but also the stem cell niche117. This approach may undermine CSC self-renewal and reproduction. However, they are a complicated target because of their chemo- and radio-resistance, and their ability to stimulate angiogenesis 118.
Animal models to study the tumor microenvironment
Liver carcinogenesis is a multi-step process with several cellular and mechanical deregulations that eventually lead to malignant transformation of hepatocytes. Numerous mouse models successfully produce HCC, however not all of them mimic the pathogenic sequence of human HCC that starts with fibrosis, cirrhosis, angiogenesis and preneoplastic nodules before HCC develops. There are four main categories of murine HCC models: chemically induced, oncogene driven, xenograft and genetically modified. Chemically induced models (N-nitrosodiethylamine (DEN)) are among the most common used in HCC research, 119, 120. When associated with CCl4, the DEN model mimics the sequence of injury-fibrosis-malignant transformation that occurs in humans61. Conditional over-expression of the oncogenenic protein Myc in which the expression of human Myc can be regulated in murine liver will induce HCC, whereas Myc inactivation results in tumor regression121. Additionally, depletion of AMPK-related kinase 5 in mice with deregulated expression of MYC and HCC prolongs survival122.
In xenograft models, human cancer cells are injected into immune-deficient mice. Orthotropic implantation of tumor cells in the liver is preferable to subcutaneous xenograft models, as it better replicates the tumor microenvironment123. To improve the reproducibility further, tumor cells can be injected after fibrosis is established by either CCl4 or thiocetamide injection.
Genetically modified mice (GMM) are engineered to mimic pathophysiological and molecular features of HCC124. There are a huge number of GMM (over-expression of myc, β- catenin and HRAS, TGFα, deficiency of PTEN among others), which have been reviewed elsewhere123, 124, 126. Several animal models can reproduce the human stepwise development of HCC. Platelet-derived growth factor C transgenic mice start developing steatosis and activation of stellate cells that progresses into bridging fibrosis, angiogenesis and tumorigenesis127.
Multi-drug resistance gene 2 (Mdr2) knockout mice are a well-established model of inflammation-associated HCC. These mice lack a liver-specific P-glycoprotein and mimic human intrahepatic cholestasis very well 128, 129. HCC development is also preceded by chronic inflammation in mice over-expressing lymphotoxins α and β in hepatocytes 130.
To date, the exact role NF-kB signaling in hepatocarcinogenesis is not totally understood and may depend on the mouse model and injury used. Several components of the NF-kB canonical pathway have been manipulated in a range of models, often yielding conflicting results. Deletion in hepatocytes of either NEMO, a regulatory inhibitor if the iKK pathway, as well as TAK1, leads to spontaneous steatohepatitis and HCC 131. Conditional liver specific deletion of IKK2 increases liver tumor formation in DEN treated mice 120, whereas inhibition of the NF-kB signal in the Mdr2-KO mice 132, and transgenic over-expression in hepatocytes of lymphotoxins α and β 130 developed chronic hepatitis at 9 months and HCC at 12 months. High-throughput technology has made possible the characterization of tumors at the gene expression level and has revolutionized our understanding of HCC. Gene expression signatures obtained from experimental animals and hepatic cells can be integrated into the gene expression patterns for human HCC, thus identifying the best-fit mouse models to study human cancer127.
The prognostic relevance of the non-tumor adjacent tissue in patients with HCC
Genomic studies have demonstrated the relevance of the tumor microenvironment to predicting outcome in patients with HCC (Table 1). A 36-gene signature originating from the surrounding non-neoplastic liver tumor was reported to predict multicentric occurrence or late recurrence in patients with HCV-related HCC 133. We identified a poor prognosis signature driven by late recurrence originating from the adjacent cirrhotic tissue in patients with early HCCs using two different patient cohorts13. The signature reflected the presence of a pro-tumorigenic milieu (‘field effect’) with promoting effects on the development of metachronous tumors independent from the primary resected HCC. Interestingly, the same gene signature was able to predict the development of HCC in 216 patients with HCV-associated cirrhosis who were followed up for about 10 years 134. This signature included genes involved in inflammation (IL-6, Nf-kB signalling) and EGF. The role of the EGF pathway is certainly important in the molecular pathogenesis of HCC due to its known oncogenic activity and the availability of molecular inhibitors targeting this cascade. Recently, the presence of a specific polymorphism within the EGF gene (EGF 61*G) was correlated with a high risk of developing HCC in patients with chronic hepatitis C, advanced fibrosis and cirrhosis 135, 136. The presence of this polymorphism is linked to a prolonged half-life of EGF mRNA, which promotes sustained EGF signalling in the damaged pre-neoplastic tissue thereby promoting hepatocarcinogenesis.
Table 1.
Molecular and cellular markers from the tumor microenvironment with clinical significance in HCC
| Molecular Marker | Cohort of patients | Etiology | Clinical Significance | Reference |
|---|---|---|---|---|
| 186-gene signature | 82 (training set) + 224 (validation set) |
HCV (73%,training; 48% validation) |
Poor survival, late recurrence | Hoshida et al13 |
| 17-gene signature | 115 | HBV (96%) | Venous metastases | Budhu et al45 |
| 36-gene signature | 40 | HCV (100%) | Multicentric occurrence, late recurrence | Okamoto et al133 |
| 19-miRNA signature | 28 | Other* (18.5%) | Poor survival | Jiang et al137 |
| 14-immune gene | 57 (training set) + 98 (validation set) |
Virus-related (75% training; 67% validation) |
Good prognosis | Chew et al138 |
| High levels IL-2 and IL-15 | 453 | HBV (91%) | Decreased intrahepatic tumor recurrence, prolonged overall survival |
Zhou et al51 |
| Increased Treg | 123 | HBV (100%) | Tumor size and poor prognosis | Fu et al94 |
| Increased TAM | 137 | HBV (90%) | Poor prognosis | Ding et al104 |
Abbreviations: HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; HCV: Hepatitis C virus; Treg: Regulatory T cells; TAM: Tumor associated macrophages.
Other includes etiologies not related to alcohol, hemochromatosis, HBV and HCV infection.
In addition to gene expression studies, a signature constituted by 19 microRNAs (miRNAs) derived from the adjacent non-tumoral tissue of HCC patients with different etiologies was proposed to accurately identify patients with poor prognosis 137. Similar to other studies based on transcriptomic data 13, miRNA profiling from the tumor failed to predict patient outcomes, however.
Interaction between tumor cells with stromal and endothelial cells can also have profound effects on the capability of tumor cells to migrate and invade the extracellular matrix and the newly-formed vascular vases, thereby promoting the development of metastasis. Expression profiles from livers bearing metastatic HCC were different from livers without metastatic tumors 42. These authors generated a 17-gene signature predictive of metastasis development using surrounding non-tumoral tissue from HBV-related HCC samples. The signature was enriched in Th2-dominant cytokines and differed considerably from the signature of the primary tumor. Importantly, both the poor prognosis signature from the adjacent tissue and the metastatic-related signature were independent of the global inflammation status of the liver, suggesting that specific changes within the microenvironment affected HCC progression 13, 45. Furthermore, both signatures highlighted the involvement of inflammatory and immune components in the pathogenesis of this disease. Conversely, the presence of a 14-immune gene signature including CXCL10, CCL5 and CCL2, which attract CD8+ T and NK cells, was associated with a better prognosis in patients with early HCC 138. Importantly, CD8+ T and NK cells display anti-tumor activity, reflected in the enhanced activated caspase-3 expression in cancer cells.
The crosstalk between tumor cells and stroma is mutual. Indeed, HCC cells might promote the recruitment and activation of immune cells to the tumor niche. Oncogenic β-catenin signalling was found to promote an inflammatory program in hepatocytes that involved direct transcriptional control by β-catenin and activation of the NF-κB pathway, which exacerbated HCC aggressiveness and metastasis 139. In addition, HCC cells can produce IL-8, whose levels have been associated to poor survival140, through activation of p38 MAPK, ERK and PI3K/Akt signalling pathway 141.
Targeting the tumor stroma: a promising challenge for new therapies
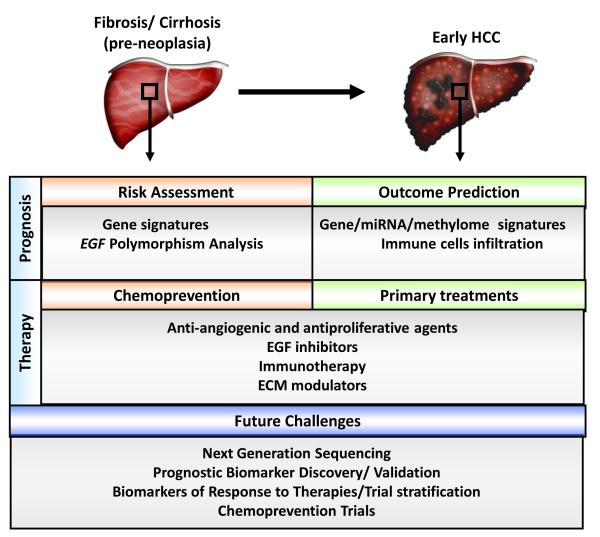
In recent years the tumor stroma has emerged as a critical target for therapy in patients with pre-neoplastic conditions or established HCC (Figure 4). Modulators of different biological processes including inflammation, fibrosis, angiogenesis and signals of proliferation and survival might be effective in the prevention and primary treatment of early HCC. Due to the implications of inflammatory pathways (e.g. IL-6, Nf-kB) and EGF signalling in cirrhotic patients at high risk of developing HCC and in subjects with early HCC, strategies interfering with these networks might be effective in chemoprevention and primary treatment. The pro-tumorigenic role of EGF pathway at pre-neoplastic stages is further strengthened by the evidence that cirrhotic subjects with the 61*G polymorphism within the EGF gene have an increased risk of developing HCC compared to other cirrhotic patients. Therefore, inhibitors of the EGF pathway such as tyrosine kinase inhibitors of the intracellular domain of EGFR receptor (e.g. erlotinib, gefitinib,) are promising agents to explore in the chemoprevention mode. One of the key problems of chemoprevention studies is that the targeted population is so broad that the studies would require thousands of patients and long follow-up to demonstrate any clinical benefit. These issues can be overcome by targeting patients at high risk of HCC development, for instance by selecting patients presenting with the poor prognosis signature which is present in ~20% of cases 13, 121 or in those harboring the G/G phenotype within the EGF gene. Certainly, all these approaches have not yet reached early clinical studies, and thus are far from being tested in pivotal trials for regulatory approval. Beyond inhibitors of the EGF cascade, pre-clinical studies have shown that sorafenib might be effective as a chemopreventive agent. Sorafenib reduced liver fibrosis in rats treated with thioacetamide (TAA) and decreased portal pressure, as well as yielding a remarkable improvement in liver damage, intrahepatic inflammation and angiogenesis of cirrhotic rats142.
Figure 4.
Schematic representation of therapeutic opportunities and application of prognostic biomarkers in the management of patients with HCC and pre-neoplastic conditions.
Since the tumor microenvironment plays a pivotal role in the natural history of HCC, there is a strong rationale for modulating the dynamic crosstalk between the tumor and the stroma as primary treatment of this disease. An important advantage of altering the tumor microenvironment is underscored by the fact that the target cells are genetically stable and therefore less likely to develop resistance 143. Since angiogenesis is a hallmark of HCC, therapies blocking the growth of new vessels or normalizing the tumor vasculature system represent key strategies to block tumor dissemination. In this setting, sorafenib simultaneously acts on the tumor vasculature (by targeting VEGFR2, VEGFR3 and PDGFR-β) and tumor cells (by inhibiting the Ras/MEK/ERK pathway), thereby blocking angiogenesis and tumor proliferation 144. In particular, anti-angiogenic agents might be beneficial in patients subjected to Transcatheter Arterial Chemoembolization (TACE) treatment, where high levels of VEGF have been reported after this procedure due to the high hypoxic conditions induced by the interruption of the blood flow into the tumor 145. Several anti-angiogenic agents are currently under investigation in phase II/III clinical trials with patients with HCC (Table 2). Most of them are small molecules inhibitors targeting molecular mediators of angiogenesis and growth factor receptors (e.g. VEGFR, PFGFR, FGFR). Others are specific monoclonal antibodies, such as bevacizumab, which targets VEGF and has been approved by the Food and Drug Administration (FDA) for the treatment of several cancers, including metastatic colon cancer and ramucirumab, a monoclonal antibody against VEGFR2 146. Despite the initial excitement about the use of antiangiogenic therapies for HCC treatment, several concerns about their adverse effects (e.g. gastro-intestinal bleeding, thromboembolic events, hypertension) have emerged. A phase III trial evaluating the efficacy of sunitinib compared with sorafenib has been prematurely halted due to severe adverse events and futility related to the administration of sunitinib 147. Furthermore, the acquisition of resistance to antiangiogenic agents through activation of alternative pathways represents a threat that undermines the clinical management of HCC patients37.Alternative strategies targeting the tumor microenvironment are currently under investigation. A considerable number of clinical trials based on immunotherapy have been performed in HCC patients. Nevertheless, the conclusions of these studies have been unsatisfactory and there is not enough positive clinical data supporting their efficacy in HCC. This lack of efficacy can be partly explained by the redundancy of the immune components in the tumor microenvironment. Nevertheless, new strategies need to be designed given the importance of inflammatory pathways and immune regulation to HCC. Early positive data has been reported with STAT3 inhibitor in preclinical models with altered TGF-β signalling. Similarly, a monoclonal antibody designed to boost anti-tumor immune response by binding and stimulating T lymphocytes (anti-CTLA4, tremelimumab) is under investigation in patients with HCV-related HCC (ClinicalTrials.gov Identifier: NCT01008358)148. These and other approaches targeting the HCC microenvironment will be tested in advanced clinical stages in the near future. Currently, a few agents modulating inflammatory pathways are under clinical evaluation in HCC. Among these, a phase I/II evaluation of OPB-31121, an orally administered STAT3 inhibitor, in patients with progressive HCC, is ongoing (Table 2).
Table 2.
Molecular therapies assessed or under investigation targeting the tumor microenvironment in HCC
| Biological Target | Drug | Molecular Targets | Stage of Development |
|---|---|---|---|
| Angiogenesis | Sorafenib | VEGFR1, VEGFR2, VEGFR3, PDGFRα, PDGFRβ, Raf1, CKIT, RET |
Approved for the treatment of advanced HCC |
| Sunitinib | VEGFR1, VEGFR2, VEGFR3, PDGFRα, PDGFRβ, CKIT, RET |
Phase III- failure (1st line) | |
| Brivanib | VEGFR2, FGFR1 | Phase III- failure (1st and 2nd line) | |
| Linifanib | VEGFR, PDGFR | Phase III-halted (1st line) | |
| Ramucirumab | VEGFR2 | Phase III (2nd line) | |
| TSU-68 | VEGFR, PDGFR, FGFR | Phase II/III | |
| Apatinib | VEGFR2 | Phase II | |
| AMG386 | Ang1, Ang2 | Phase II | |
| Axitinib | VEGFR, PDFGR,CKIT | Phase II | |
| BIBF1120 | VEGFR, PDFGR, FGFR | Phase II | |
| Cediranib | VEGFR1, VEGFR2, VEGFR3 | Phase II | |
| Foretinib | VEGFR, c-Met | Phase II | |
| IMC-1121B | VEGFR2 | Phase II | |
| NGR-hTNF | CD13 | Phase II | |
| Pazopanib | VEGFR, PDGFR CKIT | Phase II | |
| Regorafenib | VEGFR, TIE2 | Phase II | |
| TRC105 | CD105 | Phase II | |
| Vandetanib | VEGFR, EGFR | Phase II | |
| Bevacizumab | VEGF | Phase I/II | |
| E7080 | VEGFR, FGFR, SCFR | Phase I/II | |
| Lenvatinib | VEGFR2, VEGFR3 | Phase I/II | |
| Vatalanib | VEGFR1, VEGFR2, VEGFR3, PDGFR, CKIT | Phase I/II | |
| Pazopanib | VEGFR, PDGFR CKIT | Phase I | |
| Lenalidomide | VEGF | Phase I | |
| Growth factor signaling | Everolimus | mTOR | Phase III (2nd line) |
| Erotinib | EGFR | Phase III- failure in combination to sorafenib (1st line) |
|
| Cetuximab | EGFR | Phase II | |
| Lapatinib | EGFR,Her2/Neu | Phase II | |
| ARQ197 | Met | Phase II | |
| Tremelimumab | CTLA4 | Phase II | |
| Inflammation/ Immune system | OPB-31121 | STAT3 | Phase I/II |
| Licartin | HAb18G/CD147 | Phase II/IV | |
|
| |||
| Invasion/metastasis | PI-88 | Endo-β-glucoronidase heparinase | Phase II/III |
Modulators of signalling that control ECM remodelling or inhibitors of metastasis (e.g. TGF-β, HGF/c-Met, MMPs) might be used as an alternative approach for targeting the tumor microenvironment. Nevertheless, clinical trials with MMP inhibitors have shown no efficacy in patients with advanced stages of cancer and produced some intolerable side effects 149. Of note, the TGF-β inhibitor LY2109761 showed promising pre-clinical results in a xenograft model of HCC 150.
Recently, a randomized controlled phase II trial in patients with unresectable HCC who did not respond or were intolerant to first-line therapy demonstrated that tivantinib (ARQ197), a specific inhibitor of Met, increases overall survival and time to progression of patients whose tumors expressed high levels of Met151. Furthermore, cabozantinib (XL184), a dual c-Met/VEGFR2 inhibitor, has demonstrated early evidence of anti-tumor activity in a randomized discontinuation phase II study 152.
Finally, a successful strategy might require a combination of therapies targeting both the microenvironment and the tumor itself. In this context, depletion of TAM using zoledronic acid significantly improved response to sorafenib in a xenograft model of HCC 153.
Conclusion and Future Prospects
HCC commonly arises in a damaged organ featured by extensive inflammation and fibrosis. Different players including immune cells, hepatic stellate cells and macrophages react to liver injury by producing cytokines and components of the extracellular matrix, which promote angiogenesis, and survival of damaged hepatocytes or cancer stem cells. This regenerative response favours the accumulation of mutations and epigenetic aberrations which lead to malignant transformation of pre-neoplastic nodules. The interaction between stromal and tumor cells is dynamic and dramatically alters the behavior and aggressiveness of HCC, particularly at early stages of disease. Recent studies have highlighted the role of EGF and inflammatory pathways in the development of HCC in cirrhotic patients as well as in the likelihood of recurrence in patients with early HCC undergoing surgical resection. These findings point out new targets for chemoprevention and primary treatment. Although several anti-angiogenic and anti-proliferative agents are currently under investigations in phase II/III clinical trials, there is still a significant lack of studies on modulators of the ECM components and inhibitors of inflammatory pathways. Indeed, considering the pivotal implication of immune cells and signalling in HCC, a therapeutic reprogramming of the immune microenvironment in tumors might represent a promising strategy for improving the efficacy of standard anticancer treatments (e.g. sorafenib). These strategies should aim to bolster antitumor immunity, for example, by decreasing the number of Treg and reversing the imbalance of both immune/inflammatory cytokines and immune cells. In this context, the development of animal models mimicking the natural changes of HCC microenvironment represents an unmet need for the pre-clinical evaluation of such combinations.
Although there has been much progress in understanding the alterations within the tumor microenvironment in HCC, validated biomarkers of poor prognosis and response to therapy from the tumor stroma are still lacking. Nonetheless, the recent advent of next-generation sequencing technology represents a powerful and promising technology to uncover novel alterations with potential clinical relevance for the treatment of cirrhosis and early HCC.
Acknowledgments
Grant support: JML is supported by grants from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (J.M.L: 1R01DK076986), European Commission Framework Programme 7 (HEPTROMIC, Proposal No: 259744), The Samuel Waxman Cancer Research Foundation, the Spanish National Health Institute (J.M.L: SAF-2010-16055), and the Asociación Española Contra el Cáncer. SLF is supported by NIH Grants RO1DK56621, K05AA01840, R01AA020709 and P20AA017067.
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- ECM
extracellular matrix
- TAFs
tumor associated fibroblasts
- CSF-1
colony stimulating factor 1
- EC
endothelial cells
- KC
Kupffer cells
- Ang-2
angiopoietin-2
- VEGF
vascular endothelial growth factor
- FGF
Fibroblast growth factor PDGF, platelet-derived endothelial cell growth factor: Tregs, regulatory T cells
- TGFβ
Transforming growth factor β
- HGF
Hepatocyte Growth Factor
- EGFR
Epidermal Growth Factor Receptor, MMPs, metaloproteinases
- TIMP
Tissue Inhibitor of Metalloproteinases
- HIF-1
HIF-1, hypoxia-inducible factor 1
- TAM
Tumor associated macrophages: TIL, Tumor infiltrating lymphocytes
- MDSC
Myeloid-derived suppressive cells
- SDF-1
stromal cell-derived factor 1
- CSC
Cancer stem cells
- DC
Dendritic cells
- DEN
N-nitrosodiethylamine
- GMM
genetically modified mice
- TACE
Transcatheter Arterial Chemoembolization
- FDA
Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 140:1410–26. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Trimboli AJ, Cantemir-Stone CZ, Li F, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signalling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 8.McMillin DW, Delmore J, Weisberg E, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–9. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsiades CS, Mitsiades N, Munshi NC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–44. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Di. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 12.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Me. 2008;359:2045–7. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 13.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Me. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao Y, Ruan J, Yeh SH, et al. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci U S A. 108:12042–7. doi: 10.1073/pnas.1108715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Re. 2008;68:6779–88. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyault S, Rickman DS, de Reynies A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatolog. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 17.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Re. 2009;69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Re. 2008;68:1451–61. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Me. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva A, Hoshida Y, Toffanin S, et al. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 16:4688–94. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Che. 2006;387:349–60. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 22.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Onco. 2002;10:153–69. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Bio. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signalling. Cel. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cel. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cel. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Me. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signa. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 30.Denardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Me. 2007;357:2543–51. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 32.Yates LR, Campbell PJ. Evolution of the cancer genome. Nat Rev Genet. 13:795–806. doi: 10.1038/nrg3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer P, Bonnefoi H, Anderle P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 35.North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Coulon S, Heindryckx F, Geerts A, et al. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146–62. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Poon RT, Li Q, et al. Both antiangiogenesis- and angiogenesis-independent effects are responsible for hepatocellular carcinoma growth arrest by tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2005;65:3691–9. doi: 10.1158/0008-5472.CAN-04-3462. [DOI] [PubMed] [Google Scholar]
- 39.LeCouter J, Moritz DR, Li B, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–3. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenberger BM, Tan PK, Niederleithner H, et al. Autocrine VEGF signalling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–79. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Pena CE, Lathia CD, et al. Plasma Biomarkers as Predictors of Outcome in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshiji H, Kuriyama S, Noguchi R, et al. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut. 2005;54:1768–75. doi: 10.1136/gut.2005.067900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronzert DA, Pantazis P, Antoniades HN, et al. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci U S A. 1987;84:5763–7. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 45.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Tilg H, Wilmer A, Vogel W, et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–74. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 47.Wang YC, Xu GL, Jia WD, et al. Estrogen suppresses metastasis in rat hepatocellular carcinoma through decreasing interleukin-6 and hepatocyte growth factor expression. Inflammation. 2012;35:143–9. doi: 10.1007/s10753-011-9299-3. [DOI] [PubMed] [Google Scholar]
- 48.Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–9. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 49.Chia CS, Ban K, Ithnin H, et al. Expression of interleukin-18, interferon-gamma and interleukin-10 in hepatocellular carcinoma. Immunol Lett. 2002;84:163–72. doi: 10.1016/s0165-2478(02)00176-1. [DOI] [PubMed] [Google Scholar]
- 50.Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–9. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H, Huang H, Shi J, et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut. 2010;59:1699–708. doi: 10.1136/gut.2010.218404. [DOI] [PubMed] [Google Scholar]
- 52.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–33. [PubMed] [Google Scholar]
- 54.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–72. [PubMed] [Google Scholar]
- 55.Sutton A, Friand V, Brule-Donneger S, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Pan Z, Li A, et al. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–8. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida H, Iwashita Y, Sasaki A, et al. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:161–8. doi: 10.1111/j.1440-1746.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim HR, Lee SH, Jung G. The hepatitis B viral X protein activates NF-kappaB signalling pathway through the up-regulation of TBK1. FEBS Lett. 2010;584:525–30. doi: 10.1016/j.febslet.2009.11.091. [DOI] [PubMed] [Google Scholar]
- 59.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–97. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–33. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 63.Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803–12. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abou-Shady M, Baer HU, Friess H, et al. Transforming growth factor betas and their signalling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209–15. doi: 10.1016/s0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 66.Amann T, Bataille F, Spruss T, et al. Reduced expression of fibroblast growth factor receptor 2IIIb in hepatocellular carcinoma induces a more aggressive growth. Am J Pathol. 2010;176:1433–42. doi: 10.2353/ajpath.2010.090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neaud V, Faouzi S, Guirouilh J, et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458–66. doi: 10.1053/jhep.1997.v26.pm0009397985. [DOI] [PubMed] [Google Scholar]
- 68.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 69.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, et al. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Luca A, Carotenuto A, Rachiglio A, et al. The role of the EGFR signalling in tumor microenvironment. J Cell Physiol. 2008;214:559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 71.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang C, Zeisberg M, Lively JC, et al. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–7. [PubMed] [Google Scholar]
- 74.Giannelli G, Bergamini C, Marinosci F, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. 2002;97:425–31. doi: 10.1002/ijc.1635. [DOI] [PubMed] [Google Scholar]
- 75.Provenzano PP, Eliceiri KW, Campbell JM, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 77.Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hewitt RE, Powe DG, Morrell K, et al. Laminin and collagen IV subunit distribution in normal and neoplastic tissues of colorectum and breast. Br J Cancer. 1997;75:221–9. doi: 10.1038/bjc.1997.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adler B, Ashkar S, Cantor H, et al. Costimulation by extracellular matrix proteins determines the response to TCR ligation. Cell Immunol. 2001;210:30–40. doi: 10.1006/cimm.2001.1800. [DOI] [PubMed] [Google Scholar]
- 80.Bollyky PL, Wu RP, Falk BA, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–43. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 82.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–47. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 83.Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1178–82. doi: 10.1111/j.1440-1746.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 84.Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–8. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung JS, Park S, Park SH, et al. Overexpression of romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012;143:1084–1094. e7. doi: 10.1053/j.gastro.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 87.Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, et al. Understanding the “lethal” drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10:537–42. doi: 10.4161/cbt.10.6.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eng CH, Abraham RT. The autophagy conundrum in cancer: influence of tumorigenic metabolic reprogramming. Oncogene. 2011;30:4687–96. doi: 10.1038/onc.2011.220. [DOI] [PubMed] [Google Scholar]
- 89.Migneco G, Whitaker-Menezes D, Chiavarina B, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: evidence for stromal-epithelial metabolic coupling. Cell Cycle. 2010;9:2412–22. doi: 10.4161/cc.9.12.11989. [DOI] [PubMed] [Google Scholar]
- 90.Gong K, Chen C, Zhan Y, et al. Autophagy-related Gene 7 (ATG7) and Reactive Oxygen Species/Extracellular Signal-regulated Kinase Regulate Tetrandrine-induced Autophagy in Human Hepatocellular Carcinoma. J Biol Chem. 2012;287:35576–88. doi: 10.1074/jbc.M112.370585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding ZB, Shi YH, Zhou J, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–75. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 92.Shi YH, Ding ZB, Zhou J, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–72. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 93.Qin LX. Inflammatory Immune Responses in Tumor Microenvironment and Metastasis of Hepatocellular Carcinoma. Cancer Microenviron. 2012 doi: 10.1007/s12307-012-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 95.Shen X, Li N, Li H, et al. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745–54. doi: 10.1007/s00432-010-0833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–8. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 98.Chen S, Akbar SM, Tanimoto K, et al. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148:49–57. doi: 10.1016/s0304-3835(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 99.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 100.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012 doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 103.Lucas T, Abraham D, Aharinejad S. Modulation of tumor associated macrophages in solid tumors. Front Biosci. 2008;13:5580–8. doi: 10.2741/3101. [DOI] [PubMed] [Google Scholar]
- 104.Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40:381–9. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Wu SD, Ma YS, Fang Y, et al. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218–25. doi: 10.1016/j.ctrv.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Pello OM, De Pizzol M, Mirolo M, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–21. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 107.Sodir NM, Swigart LB, Karnezis AN, et al. Endogenous Myc maintains the tumor microenvironment. Genes Dev. 2011;25:907–16. doi: 10.1101/gad.2038411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–13. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461–72. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–29. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marquardt JU, Thorgeirsson SS. Stem cells in hepatocarcinogenesis: evidence from genomic data. Semin Liver Dis. 2010;30:26–34. doi: 10.1055/s-0030-1247130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee TK, Castilho A, Cheung VC, et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 113.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 115.Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–56. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 116.Baek HJ, Lim SC, Kitisin K, et al. Hepatocellular cancer arises from loss of transforming growth factor beta signalling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128–37. doi: 10.1002/hep.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–43. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 118.Yao Z, Mishra L. Cancer stem cells and hepatocellular carcinoma. Cancer Biol Ther. 2009;8:1691–8. doi: 10.4161/cbt.8.18.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schiffer E, Housset C, Cacheux W, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–14. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 120.Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 121.Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 122.Liu L, Ulbrich J, Müller J, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, Tang ZY, Hou JX. Hepatocellular carcinoma: insight from animal models. Nat Rev Gastroenterol Hepatol. 2012;9:32–43. doi: 10.1038/nrgastro.2011.196. [DOI] [PubMed] [Google Scholar]
- 124.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–58. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 125.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–86. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 127.Campbell JS, Hughes SD, Gilbertson DG, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–94. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katzenellenbogen M, Mizrahi L, Pappo O, et al. Molecular mechanisms of liver carcinogenesis in the mdr2-knockout mice. Mol Cancer Res. 2007;5:1159–70. doi: 10.1158/1541-7786.MCR-07-0172. [DOI] [PubMed] [Google Scholar]
- 129.Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- 130.Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 133.Okamoto M, Utsunomiya T, Wakiyama S, et al. Specific gene-expression profiles of noncancerous liver tissue predict the risk for multicentric occurrence of hepatocellular carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol. 2006;13:947–54. doi: 10.1245/ASO.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 134.Hoshida Y, Sangiovanni A, et al. Gene expression signature predicts outcome of liver cirrhosis. Hepatology. 2009;50:312A. [Google Scholar]
- 135.Abu Dayyeh BK, Yang M, Fuchs BC, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology. 141:141–9. doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tanabe KK, Lemoine A, Finkelstein DM, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53–60. doi: 10.1001/jama.2007.65. [DOI] [PubMed] [Google Scholar]
- 137.Jiang J, Gusev Y, Aderca I, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–27. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 61:427–38. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anson M, Crain-Denoyelle AM, Baud V, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 122:586–99. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ren Y, Poon RT, Tsui HT, et al. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996–6001. [PubMed] [Google Scholar]
- 141.Wang Y, Wang W, Wang L, et al. Regulatory mechanisms of interleukin-8 production induced by tumour necrosis factor-alpha in human hepatocellular carcinoma cells. J Cell Mol Med. 16:496–506. doi: 10.1111/j.1582-4934.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mejias M, Garcia-Pras E, Tiani C, et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–56. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 143.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 144.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signalling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 146.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 147.Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin Cancer Res. 18:1824–6. doi: 10.1158/1078-0432.CCR-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melero I Sangro BR-BJ, Iñarrairaegui M, Lasarte JJ, Sarobe P, Larrea E, Prieto J. Antiviral and antitumoral effects of the anti-CTLA4 agent tremelimumab in patients with hepatocellular carcinoma (HCC) and chronic hepatitis C virus (HCV) infection: Results from a phase II clinical trial. Annual Meeting of the American Association for Cancer Research. 2012 Abstract nr 4387. [Google Scholar]
- 149.Xu J, Xu HY, Zhang Q, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res. 2007;5:605–14. doi: 10.1158/1541-7786.MCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 150.Mazzocca A, Fransvea E, Lavezzari G, et al. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140–51. doi: 10.1002/hep.23118. [DOI] [PubMed] [Google Scholar]
- 151.Borbath IPC, Rimassa L, Daniele B, Salvagni S, Van Laethem JL, Van Vlieberghe H, Trojan J, Kolligs F, Weiss A, Barahona N, Gasbarrini A, Lencioni M, Von Roemeling RW, Schwartz B, Abbadessa G, Lamar M, Santoro A. Tivantinib in Met+ pretreated hepatocellular carcinoma (HCC): a randomized controlled phase 2 trial (RTC) International Liver Cancer Association (ILCA) Annual Meeting. 2012 Abstract # 0-023. 2012;Berlin, Germany. [Google Scholar]
- 152.Zhu AX. Molecularly targeted therapy for advanced hepatocellular carcinoma in 2012: current status and future perspectives. Semin Oncol. 39:493–502. doi: 10.1053/j.seminoncol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 16:3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]