Abstract
Intermediate filaments are assembled from a diverse group of evolutionary conserved proteins and are specified in a tissue-, cell type-, and context-dependent fashion in the body. Genetically-determined mutations in intermediate filament proteins account for a large number of diseases, ranging from skin fragility conditions to cardiomyopathies and premature aging. Keratins, the epithelial-specific intermediate filaments, are now recognized as multi-faceted effectors in their native context. In this review, we emphasize the recent progress made in defining the role of keratins towards the regulation of cytoarchitecture, cell growth and proliferation, apoptosis, and cell motility during embryonic development, in normal adult tissues, and in select diseases such as cancer.
Keratins are the epithelial-specific members of the superfamily of intermediate filament (IF) genes and proteins. As many as 28 type I and 26 type II keratin genes are tightly regulated in a pairwise fashion, reflecting the heteromeric nature of the 10 nm filaments they form, as well as in a tissue-specific and differentiation-dependent manner in body epithelia [1–3] (Box1). Rapid pace progress in recent years has set forth the notion that keratin IFs fulfill two fundamental roles in epithelial cells: 1) structural support, without which incident physical trauma exposes an inherent fragility and leads to loss of integrity, and 2) regulation of metabolic processes and of pathways governing their growth, proliferation, migration and apoptosis. These two roles involve regulated interactions with a diverse group of cellular proteins [4, 5].
Box 1 Introduction to keratin intermediate filament genes and proteins (Refs. [1–3]).
There are ~70 genes that code for intermediate filament (IF)-forming proteins in the human genome, with an astounding 54 of them coding for keratin proteins that are expressed in epithelia.
IF proteins represent a very heterogeneous group, with sizes ranging from 40 kDa (keratin 19) to 240 kDa (nestin). This said, they all share a common tripartite domain structure, consisting of a central α-helical rod domain featuring long range, coiled-coil forming heptad repeats and variable end domains located at their N- and C-termini.
IF genes can be partitioned into six major subtypes based on either gene substructure or sequence homology over the defining rod domain existing in all IF proteins. Keratins comprise the type I (28 members) and type II (26 members) IF genes.
All 28 type I keratin genes but one are organized as a cluster on the long arm of human chromosome 17 (mouse chromosome 11), whereas all 26 type II keratin genes along with the type I K18 gene are clustered on the long arm of human chromosome 12 (mouse chromosome 15)
Mature, 10-nm keratin filaments are obligatory heteropolymers, with type I and type II proteins occurring in a 1:1 molar ratio. This requirement explains the coordinated transcription of a least one of each of the two subtype of keratin genes in epithelial cells. Remarkably, many type I genes are co-regulated with specific type II “partner” genes, leading to the notion of pairwise expression of keratin genes.
The transcriptional regulation of keratin genes and the primary structure of their protein products are highly conserved across mammals, from mice to man, pointing to an intimate relationship between the complement of keratin proteins and the epithelial phenotype.
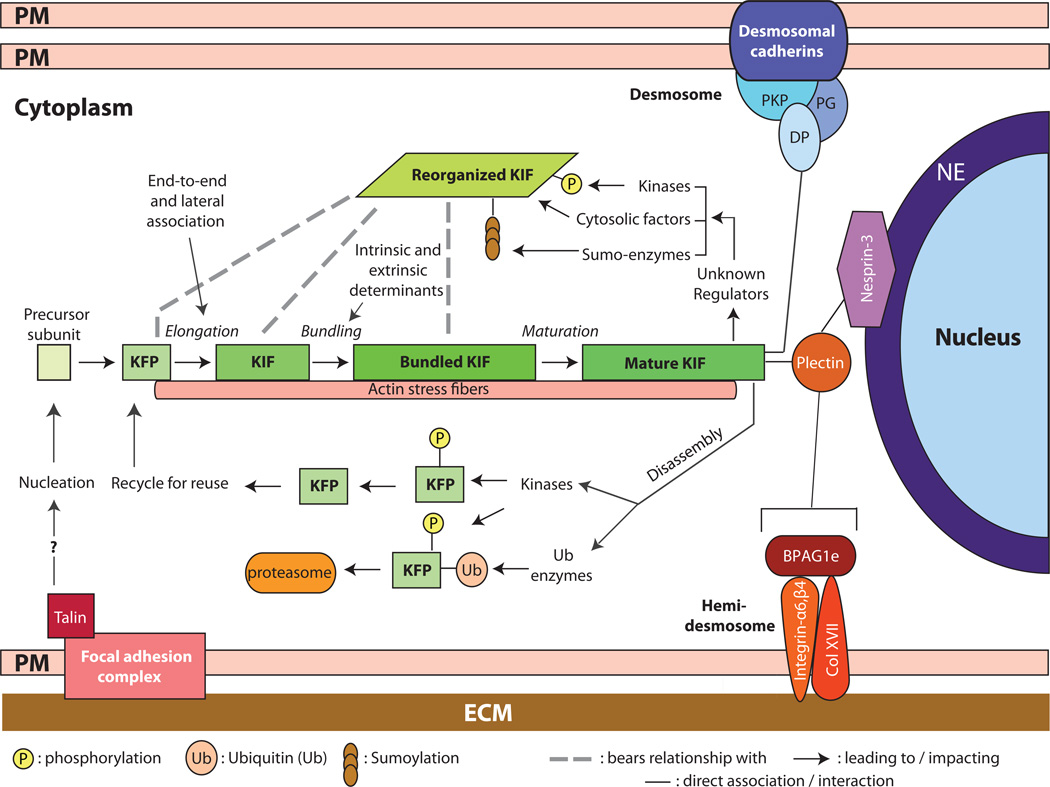
Substantive progress has been achieved in spatially mapping, in living epithelial cells, the initiation of keratin assembly, and the growth, maturation and turnover of the keratin IF network. These advances (Figure 1) have been covered in recent reviews [6, 7]. Likewise, the mechanisms responsible for attachment of keratin filaments at sites of cell-cell and cell-matrix adhesion, and at the nuclear surface, are better understood and have been recently reviewed (Figure 1; see [8–10]). The role of keratin mutation as causative agents in inherited epithelial disorders continues to receive much attention, and have been commented upon as well [11–14]. For this review, we chose to focus on recent progress made in characterizing the role of keratin proteins in regulating cytoarchitecture, protein synthesis and growth, apoptosis, and epithelial cell motility in a myriad of contexts including embryonic development, normal adult tissues, and in select diseases such as cancer.
Figure 1.
Assembly, organization, and regulation of keratin intermediate filaments (KIFs). Live imaging studies in epithelial cells in culture show that keratin filament assembly is initiated at the periphery of the cell, near focal adhesions, and that newly formed filaments and their maturation into an organized network takes place in the context of a continuous centripetal flow with disassembly and turnover steps taking place near the nuclear envelope. The resulting “keratin cycle” is highly dependent on interactions with F-actin, with additional proteins, and on several types of post-translational modifications including phosphorylation, ubiquitination, sumoylation and (though not shown here) O-glycosylation. Most likely, the biological context dictates the rate of flow through this cycle. The figure also conveys that KIFs are attached at the surface of the nucleus (via a plectin/Nesprin-3 complex), at desmosome cell-cell adhesion sites, (via desmoplakin (DP), among other proteins), and at hemidemosome cell-matrix adhesions (via plectin and BPAG1e). Not shown here are the interactions with F-actin and microtubules. The structural support role of KIFs depends upon their organization as a crosslinked network that is fully integrated with other structural elements within and between epithelial cells. NE: nuclear envelope; PM: plasma membrane; ECM: extracellular matrix; DP: desmoplakin; PKP: plakophilin; KFP: keratin filament precursor; KIF: keratin intermediate filament.
BUILDING UPON THE ALREADY KNOWN: STRUCTURAL SUPPORT, RESPONSE TO STRESS, AND CYTOARCHITECTURE
Body surfaces and several internal organs are lined by polarized epithelial sheets. Among their multiple roles, these tissues protect us from environmental stresses that encompass many forms, including mechanical, cytotoxic, oxidative, and metabolic insults. Interference with these protective roles underlies many diseases [11, 15, 16]. Accordingly, significant efforts continue to be devoted to determining when, where and how the protective roles of keratin are manifested.
Structural support
The structural support function of keratins is brought to the fore in skin fragility disorders involving mutations in epidermal keratins [11, 15, 16]. Epidermolysis bullosa simplex (EBS) and epidermolytic hyperkeratosis (EHK) are examples of genetic conditions caused by mutations in K5/K14 and K1/K10, the keratin pairs expressed in the basal and suprabasal layers of epidermis, respectively, and are characterized by cytolysis of keratinocytes and loss of structural integrity in the relevant epidermal layers [11, 13, 14]. Several key aspects of the human EBS phenotype are manifested in transgenic mice expressing dominantly-acting deletion mutants in K14 [17–19] and in mice null for K14 20] or K5 21]. Likewise, transgenic mice expressing a truncated, dominant negative K10 mutant protein [22] or the K10 R154C mutant [23] exhibit lesions that resemble EHK. By contrast, inactivating K10 triggers hyperproliferation as is seen in EHK but no obvious cell fragility in the epidermis [24, 25]. This difference is likely related, at least in part, to the upregulation of K5 and K14 proteins, and their co-polymerization with K1, in differentiating suprabasal keratinocytes of K10 null mouse epidermis [24]. A recent study supports that notion, as it reports that mice doubly null for K10 and K1 exhibit a lethal neonatal phenotype along with extensive skin lesions and cytolysis of suprabasal epidermal keratinocytes [26]. The K1/K10 double-null mouse phenotype also hints at the involvement of keratins in regulating the integrity of the nucleus as well as desmosome-based adhesion, as further discussed below.
Keratin mutation-based fragility phenotypes closely correlate with alteration in the micromechanical properties of the cytoskeleton [11, 12, 27, 28]. A role for keratin filaments in providing structural support [28–32] is promoted by their unique intrinsic properties – for instance, their ability to self-organize into crosslinked networks [33, 34] – as well as by their linkages at cell-cell (desmosomal) and cell-matrix (hemidesmosomal) junctions, and to F-actin and microtubules [8–10] (Figure 1). While the ultrastructural features associated with various keratin deficiencies in situ clearly point to their importance for cell and tissue mechanics, understanding this role at a deeper level is a challenging proposition given limitations inherent to the ex vivo cellular models and assays being used [30, 31, 35–38]. Disruption of keratin IF anchorage at adhesive junctions via inherited mutations can also single-handedly result in disorders accompanied by keratinocyte and tissue fragility [39–41]. In addition to fostering mechanical integration at a cellular and supracellular levels, attachment of keratin IFs can also impacts the formation, size and molecular composition of desmosomes [42, 43].
Response to stresses
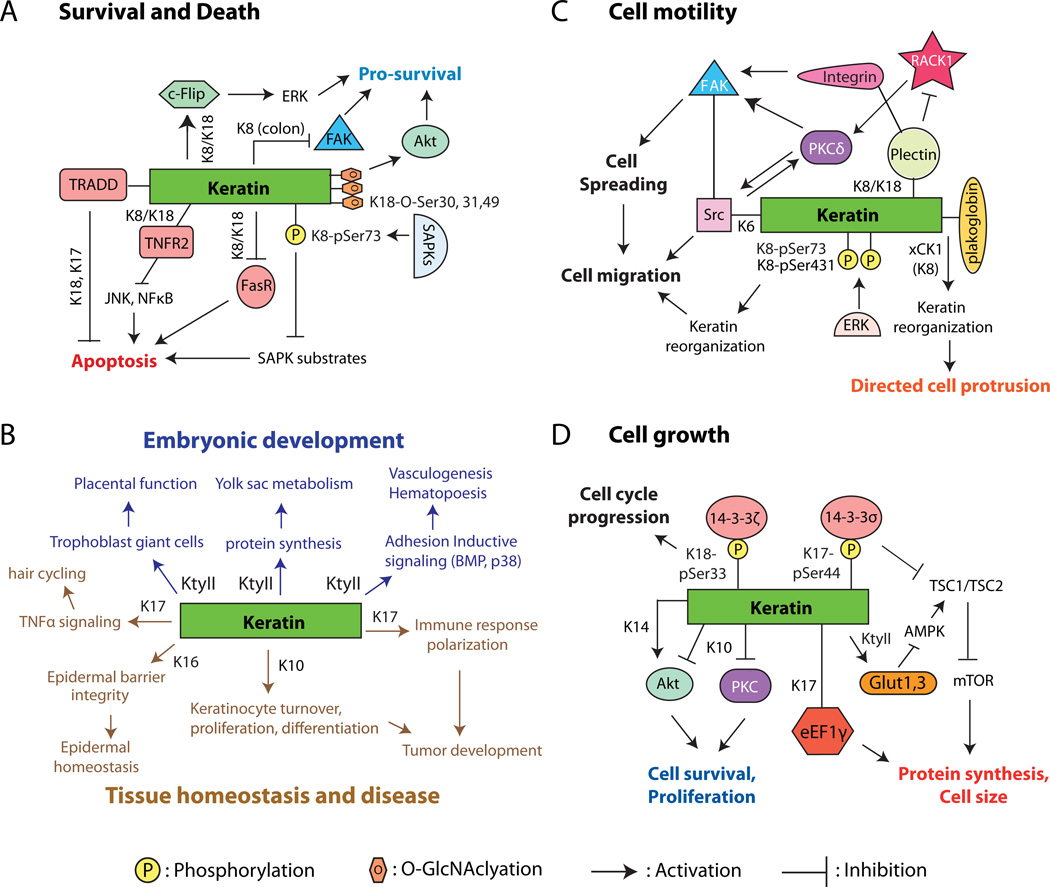
Keratin IFs afford crucial protection to internal organs, even though these are unlikely to experience mechanical stress to the extent that skin and other surface epithelia do. The liver, for instance, is a metabolism-intensive organ that functions in part to detoxify foreign substances. Previous efforts have clearly established that, in part through site-specific phosphorylation in cis, K8 and K18 are instrumental in the ability of liver hepatocytes to cope with a broad variety of metabolic, oxidative, and chemical stresses [44–46]. A recent study revealed that, in addition to phosphorylation, covalent O-linked N-acetylglucosamine (O-GlcNAc) modification (at serine residues 30/31/49) on K18 enhances the stress-buffering function of liver keratins [47]. Indeed, relative to controls mice that overexpress the human K18 S30/31/49A mutant (which cannot undergo O-GlcNAc modification) show multi-organ failure and increased lethality when treated with streptozotocin, PUGNAc (1, 5-hydroximolactone), or antibodies to Fas. The authors, Ku et al. [47], propose that under normal conditions the site-specific O-glycosylation of K18 serves to positively regulate the activity of the pro-survival Akt and PKC kinases, thereby protecting cells against apoptosis and promoting their adaptation to stresses (Figure 2A).
Figure 2.
Examples of cellular and physiological processes regulated by keratin proteins. A) Cell survival and cell death. B) Embryonic development (top; blue lettering) and tissue homeostasis and disease (bottom; brown lettering). C) Cell motility and related processes. D) Cell growth. In all cases, lines connecting keratins and the molecule(s) of interest convey a physical interaction. Abbreviations: Akt, Protein kinase B; AMPK, AMP-activated protein kinase; eEF1γ, Eukaryotic translation elongation factor 1 gamma; ERK, Extracellular signal-regulated kinase; FAK, Focaladhesion kinase-1; FasR, Fas-ligand receptor; Glut1, 3, glucose transporters 1, 3; K, mammalian keratins; KtyII, Type II mammalian keratins; mTOR, Mammalian target of rapamycin; PKC, protein kinase C; RACK1, Receptor for activated protein kinase C; SAPK, stress activated protein kinases; Src, Src kinase; TNFR2, Tumor necrosis factor receptor 2; TRADD, Tumor necrosis factor receptor type I-associated Death domain protein; TSC1/2, Tuberous Sclerosis Complex (Hamartin/Tuberin); xCK, Xenopus cytokeratin (ortholog to mammalian K18).
A role for keratins in regulating the nucleus?
In addition to their roles at cell junctions, the keratin IF network may be important for regulating the size and shape of the nucleus. Plectin, a well-known IF-binding protein [9, 48] was recently found to bind the outer nuclear envelope protein nesprin-3 [49] (Figure 1), providing the long-awaited rationale for the attachment of cytoplasmic IFs to the nucleus [50]. Consistent with this, targeted deletion of nesprin-3 in Zebrafish impairs the perinuclear concentration of keratin filaments [51].
Lee et al. [52] succeeded in crystallizing and solving the first atomic resolution structure of a keratin heterotypic complex consisting of the heptad repeat-containing 2B subdomains of K5 and K14. The interface of the coiled-coil heterodimer features several asymmetric (i.e., unidirectional) bonds that likely guide the intrinsic process of type I-type II heterodimer formation. Surprisingly, however, a symmetry-related contact in the crystal lattice has coiled-coil heterodimers forming a rather astonishing X-shaped object, owing to a trans-dimer homotypic disulfide bond involving cysteine 367 in K14. This disulfide bond is enriched in the perinuclear region of basal keratinocytes of epidermis, where it is poised to stabilize a cage of keratin IFs that may impact the size and shape of the nucleus at an early stage of differentiation [52]. This cysteine residue is conserved in K14 and related type I keratins including K10. Given this, it is intringuing that the K1/K10 double-null mice show premature loss of nuclei in the suprabasal layers of their epidermis, along with reduced levels of emerin, lamin A/C, and Sun-1, despite a relatively normal differentiation program [26]. While caution is warranted at this time, such findings suggest that as is the case for nuclear lamins [53], the perinuclear cytoplasmic network of keratin IFs might also contribute to define nuclear morphology and participate in nuclear physiology.
KERATINS MODULATE CELLULAR GROWTH, APOPTOSIS AND MOTILITY: IMPLICATIONS FOR EMBRYONIC, ADULT, AND DISEASE SETTINGS
Embryonic development
The role of keratins in embryonic development has been difficult to define because of complicating factors including functional redundancy and genetic background effects [54]. Early mouse models with K8 or combined K18/K19 deficiency nevertheless have clearly pointed to a role for keratins in influencing embryonic development by impacting trophoblast giant cells and placental functions [55–57] (Figure 2B). To get around functional redundancy, Magin and colleagues boldly deleted the entire type II keratin gene cluster on mouse chromosome 15, thereby generating the KtyII−/− model [58]. This deletion proves lethal at E9.5 in mouse embryos (i.e., mid-way through gestation), but their epithelial tissues appear largely intact, with no sign of cell lysis or tissue fragility. Instead, death correlates with other factors including mislocalization of the GLUT-1 and GLUT-3 transporters, increased AMP kinase activity, depressed mTORC1 activity and reduced protein synthesis in the yolk sac [58]. Additionally, KtyII−/− embryos exhibit defective adhesion between the endoderm and mesoderm layers, possibly accounting for severe defects in vasculogenesis and hematopoiesis, along with a placental defect (Figure 2B) related to the altered distribution of secondary trophoblast giant cells. The latter could result in hyperoxia in the decidual tissue owing to impaired vasculogenesis and insufficient gas exchange between maternal and embryonic blood [59].
Cells are called upon to migrate during embryogenesis, often as cohesive assemblies, in order to form new structures and tissues. A recent study examining the mechanically-induced collective migration of Xenopus cells sheds new light on the regulation of embryonic migration events by keratins [60]. Weber et. al. observed that polarized protrusion and migration of single mesendoderm cells occurs in the direction opposite from where a pulling force is applied to surface-exposed C-cadherins. Remarkably, this manipulation triggers a reorganization of keratin IFs including their recruitment to cadherin sites, and requires xCK1 (hK8) expression. Silencing the junctional protein plakoglobin (PG) produces similar protrusive defects and affects keratin network reorganization after pulling. Likewise, deleting either xCK1 or xPG randomizes the protrusive activity of mesendodermal cells in actual embryos, presenting compelling evidence for participation of keratins and PG in collective cell migration in vivo (Figure 2C). Whether these findings relate to the involvement of K6, PG and other keratin associated proteins in the regulation of keratinocyte motility during adult setting (see next Section) is worth pursuing.
Adult tissue homeostasis: Enacting the proper balance between competing processes
Much has been learned about keratin’s role in cellular growth through studies showing the ability of select keratins to positively regulate cell cycle progression, entry into mitosis, and protein synthesis [45, 61] via interaction with key effectors such as 14-3-3 proteins (Figure 2D). Docking sites enabling 14-3-3 binding are generated via site-specific phosphorylation of keratins by growth-promoting kinases [45, 62, 63]. Depending on cell type and context, such keratin-14-3- 3 complexes can subsequently impact cell cycle-associated regulators like Cdc25, as in the case of Xenopus eggs [64], and mTOR (Target Of Rapamycin), as in epidermal keratinocytes [65]. The kinase Akt represents another powerful effector that is strongly (and differentially) impacted by various keratins. Akt activity is increased when K8/K18 are genetically depleted in liver hepatocytes [66] but is decreased in K17 null mouse keratinocytes [65] or when K14 is silenced in the HaCaT (human) keratinocyte cell line or in an oral squamous carcinoma cell line [67]. Further, K10 has been reported to bind to and negatively impact Akt activity and cellular proliferation [68] though this has been disputed [69] (Figure 2D). The keratin-Akt relationship, whether in physical or functional terms [25, 69, 70], is worth a thorough re-examination given its ramifications.
Regulation of apoptosis represents an additional mechanism through which keratin IFs are able to coordinate cell and tissue growth [5]. Keratins can accomplish this by “sequestering” death-promoting effector molecules such as tumor necrosis factor receptor 2 (TNFR2) [71] and TNF receptor-associated death domain-containing protein (TRADD) [72–74], by influencing the targeting and density of relevant cell surface death receptors [75, 76], or by acting as “sponges” to absorb excessive stress and pro-apoptotic signals [77, 78] (Figure 2A). A recent study revealed that, in contrast to the evidence accumulated to date [44], K8 can be pro-apoptotic in the colonic epithelia, a phenomenon that depends upon the microflora. Transcriptional profiling in FVB/N K8−/− colons, which show hyperplasia and colitis, revealed an unexpected upregulation of various pro-survival factors (e.g. survivin) and pathways [79] (Figure 2A). Such findings point (yet again) to the context-dependence of keratin function, and to the impact of partners and processes susceptible to genetic background effects.
The response to injury elicits changes in homeostasis and triggers significant alterations in IF gene expression and/or IF protein regulation in surviving wound-proximal cells in a broad array of tissues and organs, ranging from the central nervous system to skin [80]. The importance of these changes is conveyed by the altered outcomes observed whenever the “IF response to injury” is genetically manipulated. Somewhat paradoxically, genetic ablation of the two K6 isoforms (K6a and K6b), which are normally induced in wound proximal keratinocytes soon after injury to the skin [81], results in enhanced migration of keratinocytes so long as they are not subjected to a mechanically challenging setting, which exposes their inherent fragility [82]. This enhanced migration, it turns out, involves a robust stimulation of Src activity in K6a/K6b null keratinocytes [83]. In addition to binding to and regulating Src directly[83], K6a/K6b and associated proteins (e.g., plectin) may partner to sequester signaling molecules such as RACK1 and negatively impact upstream effectors, like protein kinase C (PKC) and integrin and focal adhesion kinase (FAK), to regulate cell migration [84, 85] (Figure 2C). Like K6a/K6b null cells, Plakophilin1-null, Plectin-null, Plakoglobin-null, as well as Epiplakin-null keratinocytes also migrate faster than their wild-type counterparts, with Plectin- and Plakoglobin-null keratinocytes exhibiting enhanced Src activity [86–89]. The possibility arises, therefore, that a larger complex comprised of keratins and associated proteins is able to interact with and regulate Src activity [83] (Figure 2C). Whether the relevant molecular events take place in detergent-resistant membrane (lipid rafts), as proposed [83], and how they are regulated in actively migrating cells, awaits further investigation.
Like the K6 paralogs, K16 and K17 exhibit a dual mode of transcriptional regulation, with constitutive expression in specific cellular compartments within epithelial appendages (e.g., hair follicle, nail, various glands, oral papillae, etc.) and inducible expression after tissue injury and in diseases such as psoriasis and cancer [90]. K6a/K6b and K17 null mice have been available for more than a decade, and a fair amount has been learned from their study [91–93]. By contrast the consequences of K16 ablation, a long-awaited development, were only recently brought to light [94]. As previously seen in K6a/K6b null animals, K16 null mice exhibit oral lesions early after birth, reflecting cell fragility in the filiform papillae of dorsal tongue epithelium [94]. These lesions are considerably less severe, however, likely due to the presence of K17), and a third of the K16 null mice survive to adulthood. At that stage, the mice develop a severe palmoplantar keratoderma phenotype that exceeds and overshadows the limited amount of cytolysis occurring in footpad epidermis [94]. The K16 null mice thus provide an opportunity to acquire insight into the genesis of the palmoplantar keratoderma lesions that arise in individuals with pachyonychia congenita, a group of disorders caused by mutations in K6a, K6b K16 or K17 95].
Keratins and cancer: More than mere biomarkers…
Carcinogenesis involves a departure from normal cell differentiation pathways, and is typically accompanied by alterations in the regulation of keratin genes and proteins. Keratin profiling provides biomarkers that are useful for the diagnosis and, increasingly, the prognosis or associated risk for many types of epithelial-based tumors [96, 97]. The issue arises, therefore, as to whether such alterations in keratin expression or sequence impact any aspect of tumor biology.
Basal cell carcinoma is the most frequent tumor in the human population [98] and invariably features an induction of K17 in the context of what is, otherwise, a relatively simple keratin profile [99, 100]. DePianto et al. [101] reported a delay in the inception of Gli2-induced basal cell carcinoma-like tumors in the absence of K17, correlating with marked reductions in keratinocyte proliferation and skin inflammation. In such Gli2-induced mouse ear tumors, remarkably, the genetic loss of K17 polarizes the immune response and production of cytokines from a Th1/Th17- to a Th2-dominated profile. In particular, the K17 status was shown to impact the expression of chemokines shown by others [102] to participate in the pathogenesis of basal cell carcinoma in humans, e.g., CxCl5, CxCl9, CxCl10, and CxCl11, in a keratinocyteautonomous fashion [101]. Interestingly, K5 deficiency is associated with the misregulation of a distinct group of chemokines, in particular Ccl2, Ccl19, Ccl20, correlating with an increased density of Langerhans (dendritic) cells in epidermis [103]. The emerging notion of an immune modulatory role for keratins is certainly worth paying attention to, going forward. In addition to these findings, a recent genome-wide association study identified SNPs (single nucleotide polymorphisms) in the human K5 locus that confer an increased lifetime risk of developing basal cell carcinoma [104]. Of note, K5 is the main type II partner for K17 in this tumor [101].
Cancer and metastasis provide yet another very important setting involving altered cell motility and migration. As shown a decade ago by Beil et al. [30], treating human pancreatic cancer cells with SPC (sphingosylphosphorylcholine, a bioactive lipid found in high levels in ovarian cancer patients) induces a perinuclear collapse of keratin filaments coinciding with phosphorylation of K18 at Ser52 and K8 at Ser431, a softening of the cytoplasm, and enhanced cell migration. Transglutaminase-2 and mitogen-activated protein kinase family members (JNK and ERK) have been recently shown to stimulate K8-Ser431 phosphorylation, promote the perinuclear reorganization of keratin IFs, and enhance tumor cell migration [105, 106] (Figure 2C). In the distinct settings of cell culture and oral squamous cell carcinoma, however, overexpression of shRNA-resistant K8 phospho-mutants of S73 or S431 in K8-knockdown cells is associated with increases in cell motility and in tumorigenicity [107]. This apparent incongruity may arise from differences in cell types utilized and methods used to induce cell migration, highlighting yet again the importance of context when studying keratin regulation and function.
Conclusion
Who would have thought, a decade ago, that keratins and associated proteins would be found to regulate protein synthesis, act as immune modulators, and regulate individual as well as collective cell migration in settings ranging from embryonic development to cancer? Because of their diversity, abundance, mode of polymerization, and various other properties, keratin proteins are poised to function in ways that extend beyond a conventional role as intracellular cytoskeletal proteins. Areas of greatest need in this field include an understanding of IF structure with atomic resolution; a better appreciation of the mechanisms regulating the assembly, dynamics and turnover of keratin IFs in various epithelial settings; gaining a sense for the significance and role(s) of the soluble pool of IF subunits within cells; and understanding how keratin IF function may be partitioned to specific subcellular domains, and is otherwise manifested in a context-dependent fashion.
ACKNOWLEDGMENTS
We thank members of our laboratory for support. We apologize to those authors whose recent work could not be included in this review. This effort was made possible by grants AR44232, AR42047, and CA160255 (to P.A.C.) and T32CA009110 (to R.P.H.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E. Keratins and the skin. Ann. Rev. Cell Dev. Biol. 1995;11:123–153. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- 3.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 4.Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010;20:79–91. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 6.Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol. 2011;194:669–678. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leube RE, Moch M, Kolsch A, Windoffer R. "Panta rhei": Perpetual cycling of the keratin cytoskeleton. Bioarchitecture. 2011;1:39–44. doi: 10.4161/bioa.1.1.14815. Refs. 6 and 7 are excellent reviews covering recent developments in our understanding of keratin dynamics in cultured epithelial cells.
- 8.Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suozzi KC, Wu X, Fuchs E. Spectraplakins: Master orchestrators of cytoskeletal dynamics. J Cell Biol. 2012;197:465–475. doi: 10.1083/jcb.201112034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuruta D, Hashimoto T, Hamill KJ, Jones JC. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J Dermatol Sci. 2011;62:1–7. doi: 10.1016/j.jdermsci.2011.01.005. Refs. 8,9–10 offer distinct and complementary takes on the role various types of associated proteins (binding partners) in organizing and regulating keratin and other types of intermediate filaments.
- 11.McLean WH, Moore CB. Keratin disorders: from gene to therapy. Hum Mol Genet. 2011;20:R189–R197. doi: 10.1093/hmg/ddr379. [DOI] [PubMed] [Google Scholar]
- 12.Coulombe PA, Lee CH. Defining keratin protein function in skin epithelia: epidermolysis bullosa simplex and its aftermath. J Invest Dermatol. 2012;132:763–775. doi: 10.1038/jid.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamcheu JC, Siddiqui IA, Syed DN, Adhami VM, Liovic M, Mukhtar H. Keratin gene mutations in disorders of human skin and its appendages. Arch Biochem Biophys. 2011;508:123–137. doi: 10.1016/j.abb.2010.12.019. Refs. 11, 12, and 13 review the recent progress made in the area of keratin -based genodermatoses.
- 14.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, Sim SY, Goh WL, Ng KW, et al. : The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 16.Omary MB, Coulombe PA, McLean WHI. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 17.Coulombe PA, Hutton ME, Letai A, Hebert A, Paller AS, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 18.Cao T, Longley MA, Wang XJ, Roop DR. An inducible mouse model for epidermolysis bullosa simplex: implications for gene therapy. J Cell Biol. 2001;152:651–656. doi: 10.1083/jcb.152.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991;64:365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E. The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol. 1995;129:1329–1344. doi: 10.1083/jcb.129.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters B, Kirfel J, Bussow H, Vidal M, Magin TM. Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell. 2001;12:1775–1789. doi: 10.1091/mbc.12.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter RM, Leitgeb S, Melton DM, Swensson O, Eady RAJ, Magin TM. Gene targeting at the mouse keratin 10 locus: Severe skin fragility and changes in cytokeratin expression in the epidermis. J. Cell Biol. 1996;132:925–936. doi: 10.1083/jcb.132.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arin MJ, Longley MA, Wang XJ, Roop DR. Focal activation of a mutant allele defines the role of stem cells in mosaic skin disorders. J Cell Biol. 2001;152:645–649. doi: 10.1083/jcb.152.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichelt J, Bussow H, Grund C, Magin TM. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol Biol Cell. 2001;12:1557–1568. doi: 10.1091/mbc.12.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci. 2002;115:2639–2650. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- 26. Wallace L, Roberts-Thompson L, Reichelt J. Deletion of K1/K10 does not impair epidermal stratification but affects desmosomal structure and nuclear integrity. J Cell Sci. 2012;125:1750–1758. doi: 10.1242/jcs.097139. Ref. 26 represents the third instance in which a specific, natural keratin pairing has been genetically deleted in the mouse genome (the precedents are K8/K19 and K6/K17). The phenotype of the K1/K10 double null epidermis points to a role of keratin filaments in "regulating" the nucleus.
- 27.Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Yamada S, Wirtz D, Coulombe PA. A 'hot-spot' mutation alters the mechanical properties of keratin filament networks. Nat Cell Biol. 2001;3:503–506. doi: 10.1038/35074576. [DOI] [PubMed] [Google Scholar]
- 29.Fudge DS, Gardner KH, Forsyth VT, Riekel C, Gosline JM. The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads. Biophys J. 2003;85:2015–2027. doi: 10.1016/S0006-3495(03)74629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Van Veldhoven PP, Gern U, Wolff-Hieber E, Eggermann J, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- 31. Lulevich V, Yang HY, Isseroff RR, Liu GY. Single cell mechanics of keratinocyte cells. Ultramicroscopy. 2010;110:1435–1442. doi: 10.1016/j.ultramic.2010.07.009. This study is important in that it assesses the contribution of each of F-actin, microtubules, and keratin intermediate filaments to the mechanical properties of normal human skin keratinocytes in primary culture (and they are different, with a dominant influence by keratin).
- 32.Yamada S, Wirtz D, Coulombe PA. Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol Biol Cell. 2002;13:382–391. doi: 10.1091/mbc.01-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH, Coulombe PA. Self-organization of keratin intermediate filaments into cross-linked networks. J Cell Biol. 2009;186:409–421. doi: 10.1083/jcb.200810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JS, Lee CH, Coulombe PA. Modeling the self-organization property of keratin intermediate filaments. Biophys J. 2010;99:2748–2756. doi: 10.1016/j.bpj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci U S A. 2008;105:889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter N, Busch T, Seufferlein T, Spatz JP. Elastic moduli of living epithelial pancreatic cancer cells and their skeletonized keratin intermediate filament network. Biointerphases. 2011;6:79–85. doi: 10.1116/1.3601755. [DOI] [PubMed] [Google Scholar]
- 37.Flitney EW, Kuczmarski ER, Adam SA, Goldman RD. Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments. FASEB J. 2009;23:2110–2119. doi: 10.1096/fj.08-124453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beriault DR, Haddad O, McCuaig JV, Robinson ZJ, Russell D, Lane EB, Fudge DS. The mechanical behavior of mutant K14-R125P keratin bundles and networks in NEB-1 keratinocytes. PLoS One. 2012;7:e31320. doi: 10.1371/journal.pone.0031320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonkman MF, Pasmooij AM, Pasmans SG, van den Berg MP, Ter Horst HJ, Timmer A, Pas HH. Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am J Hum Genet. 2005;77:653–660. doi: 10.1086/496901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 41.Uitto J, Pulkkinen L, McLean WH. Epidermolysis bullosa: a spectrum of clinical phenotypes explained by molecular heterogeneity. Mol Med Today. 1997;3:457–465. doi: 10.1016/s1357-4310(97)01112-x. [DOI] [PubMed] [Google Scholar]
- 42.Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, Thorne ME, Gaudry CA, Park JK, Myung K, et al. Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J Cell Biol. 2005;171:1045–1059. doi: 10.1083/jcb.200510038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toivola DM, Nieminen MI, Hesse M, He T, Baribault H, Magin TM, Omary MB, Eriksson JE. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology. 2001;34:1174–1183. doi: 10.1053/jhep.2001.29374. [DOI] [PubMed] [Google Scholar]
- 44.Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–1649. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- 45.Toivola DM, Ku NO, Resurreccion EZ, Nelson DR, Wright TL, Omary MB. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology. 2004;40:459–466. doi: 10.1002/hep.20277. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Q, Ji X, Chen L, Greenberg HB, Lu SC, Omary MB. Keratin mutation primes mouse liver to oxidative injury. Hepatology. 2005;41:517–525. doi: 10.1002/hep.20578. [DOI] [PubMed] [Google Scholar]
- 47. Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol. 2010;12:876–885. doi: 10.1038/ncb2091. This is an outstanding study with major implications on a number of fronts. To name two it provides a long-awaited significance for modification of Ser/Thr residues with single O-GlNaC sugar groups, and refine our understanding of the basis for the cytoprotective influence of K8/K18 in liver hepatocytes.
- 48.Wiche G, Winter L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture. 2011;1:14–20. doi: 10.4161/bioa.1.1.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupin I, Sakamoto Y, Etienne-Manneville S. Cytoplasmic intermediate filaments mediate actindriven positioning of the nucleus. J Cell Sci. 2011;124:865–872. doi: 10.1242/jcs.076356. [DOI] [PubMed] [Google Scholar]
- 51. Postel R, Ketema M, Kuikman I, de Pereda JM, Sonnenberg A. Nesprin-3 augments peripheral nuclear localization of intermediate filaments in zebrafish. J Cell Sci. 2011;124:755–764. doi: 10.1242/jcs.081174. With this study the authors add to a previous and important report (ref. 49) identifying nesprin-3 as a plectin-dependent mediator of the attachment of cytoplasmic intermediate filaments at the surface of the nucleus.
- 52. Lee CH, Kim MS, Chung BM, Leahy DJ, Coulombe PA. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat Struct Mol Biol. 2012;19:707–715. doi: 10.1038/nsmb.2330. This is the first structure, with atomic resolution, for a heteropolymeric intermediate filament system (namely, keratins 5 and 14). The authors also describe the surprising occurrence of a disulfide bond in the crystal structure, which does exist in vivo where it reportedly promotes a specific organization of keratin filaments at the surface of the nucleus.
- 53. Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012 doi: 10.1016/j.tig.2012.06.001. One of many excellent recent reviews on the topic of nuclear lamins.
- 54.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 55.Jaquemar D, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, Oshima RG. Keratin 8 protection of placental barrier function. J Cell Biol. 2003;161:749–756. doi: 10.1083/jcb.200210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamai Y, Ishikawa T, Bosl MR, Mori M, Nozaki M, Baribault H, Oshima RG, Taketo MM. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol. 2000;151:563–572. doi: 10.1083/jcb.151.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and-3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kroger C, Vijayaraj P, Reuter U, Windoffer R, Simmons D, Heukamp L, Leube R, Magin TM. Placental vasculogenesis is regulated by keratin-mediated hyperoxia in murine decidual tissues. Am J Pathol. 2011;178:1578–1590. doi: 10.1016/j.ajpath.2010.12.055. Refs. 58 and 59 report on some of the most obvious consequences of deleting the entire type II keratin gene cluster on mouse chromosome 15. This is a technical tour-de-force, and the resulting model will provide complementary information to efforts involving keratin gene manupulations, one or two at a time.
- 60. Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–115. doi: 10.1016/j.devcel.2011.10.013. This is an exceptional study reporting that keratin filaments and plakoglobin each contribute to define polarization and directional migration in cells that are mechanically-induced to migrate. Surprisingly, these two elements are seemingly acting at the cellular pole opposite the direction of migration.
- 61.Kim S, Kellner J, Lee CH, Coulombe PA. Interaction between the keratin cytoskeleton and eEF1Bgamma affects protein synthesis in epithelial cells. Nat Struct Mol Biol. 2007;14:982–983. doi: 10.1038/nsmb1301. [DOI] [PubMed] [Google Scholar]
- 62. Pan X, Kane LA, Van Eyk JE, Coulombe PA. Type I keratin 17 protein is phosphorylated on serine 44 by p90 ribosomal protein S6 kinase 1 (RSK1) in a growth- and stress-dependent fashion. J Biol Chem. 2011;286:42403–42413. doi: 10.1074/jbc.M111.302042. This study examines, for the first time, the regulation and significance of site-specific phosphorylation on a keratin that is expressed prominently in the skin.
- 63.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol. 2010;11:75–81. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 64.Margolis SS, Perry JA, Forester CM, Nutt LK, Guo Y, Jardim MJ, Thomenius MJ, Freel CD, Darbandi R, Ahn JH, et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759–773. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 66.Galarneau L, Loranger A, Gilbert S, Marceau N. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp Cell Res. 2007;313:179–194. doi: 10.1016/j.yexcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 67. Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell. 2011;22:4068–4078. doi: 10.1091/mbc.E10-08-0703. These authors provide evidence, from RNAi-induced silencing, that K14 participates in the regulation of keratinocyte growth and proliferation. This defect was unanticipated given that it has not ben seen in mouse and human models of K14 deficiency.
- 68.Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paramio JM, Santos M, Jorcano JL. The ends of a conundrum? J Cell Sci. 2007;120:1145–1147. doi: 10.1242/jcs.005348. author reply 1147–1148. [DOI] [PubMed] [Google Scholar]
- 70.Vijayaraj P, Sohl G, Magin TM. Keratin transgenic and knockout mice: functional analysis and validation of disease-causing mutations. Methods Mol Biol. 2007;360:203–251. doi: 10.1385/1-59745-165-7:203. [DOI] [PubMed] [Google Scholar]
- 71.Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inada H, Izawa I, Nishizawa M, Fujita E, Kiyono T, Takahashi T, Momoi T, Inagaki M. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J Cell Biol. 2001;155:415–426. doi: 10.1083/jcb.200103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev. 2006;20:1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoneda K, Furukawa T, Zheng YJ, Momoi T, Izawa I, Inagaki M, Manabe M, Inagaki N. An autocrine/paracrine loop linking keratin 14 aggregates to tumor necrosis factor alpha-mediated cytotoxicity in a keratinocyte model of epidermolysis bullosa simplex. J Biol Chem. 2004;279:7296–7303. doi: 10.1074/jbc.M307242200. [DOI] [PubMed] [Google Scholar]
- 75.Gilbert S, Loranger A, Daigle N, Marceau N. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol. 2001;154:763–773. doi: 10.1083/jcb.200102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gilbert S, Loranger A, Lavoie JN, Marceau N. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis. 2012;17:880–894. doi: 10.1007/s10495-012-0733-2. This study builds on a previous one (ref. 75) and provides a more refined understanding of how K8/K18 impact the display and function of Fas receptors at the surface of hepatocytes. It also reports a physical association and functional link between keratins and lipid rafts (see ref. 83 for a similar finding).
- 77.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3-proteins. EMBO J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Habtezion A, Toivola DM, Asghar MN, Kronmal GS, Brooks JD, Butcher EC, Omary MB. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc Natl Acad Sci U S A. 2010;108:1445–1450. doi: 10.1073/pnas.1010833108. Until this study, K8/K18 had been shown to exert an anti-apoptotic, pro-surviving influence. This is one of many examples highlighted in this text whereby biological context and/or environmental or genetic modifiers contribute to define the nature of keratin function in vivo.
- 80.DePianto D, Coulombe PA. Intermediate filaments and tissue repair. Exp Cell Res. 2004;301:68–76. doi: 10.1016/j.yexcr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi K, Yan B, Yamanishi K, Imamura S, Coulombe PA. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics. 1998;53:170–183. doi: 10.1006/geno.1998.5476. [DOI] [PubMed] [Google Scholar]
- 82.Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol. 2012;197:381–389. doi: 10.1083/jcb.201107078. This study reports that select keratins, and K6 in particular, directly bind the SH2 domain of Src (in a phosphorylation-independent fashion) and that this interaction negatively regulates Src activity and keratinocyte migration. Along with refs. 86–89 and ref. 60, this points to a potential role for the keratin IF system in promoting the orderly (directional) migration of multicellular epithelial assemblies.
- 84.Osmanagic-Myers S, Wiche G. Plectin-RACK1 (receptor for activated C kinase 1) scaffolding: a novel mechanism to regulate protein kinase C activity. J Biol Chem. 2004;279:18701–18710. doi: 10.1074/jbc.M312382200. [DOI] [PubMed] [Google Scholar]
- 85.Bordeleau F, Galarneau L, Gilbert S, Loranger A, Marceau N. Keratin 8/18 modulation of protein kinase C-mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol Biol Cell. 2010;21:1698–1713. doi: 10.1091/mbc.E09-05-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174:557–568. doi: 10.1083/jcb.200605172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.South AP, Wan H, Stone MG, Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA, Hart IR, McGrath JA. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J Cell Sci. 2003;116:3303–3314. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- 88.Goto M, Sumiyoshi H, Sakai T, Fassler R, Ohashi S, Adachi E, Yoshioka H, Fujiwara S. Elimination of epiplakin by gene targeting results in acceleration of keratinocyte migration in mice. Mol Cell Biol. 2006;26:548–558. doi: 10.1128/MCB.26.2.548-558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin T, Getsios S, Caldelari R, Kowalczyk AP, Muller EJ, Jones JC, Green KJ. Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2005;102:5420–5425. doi: 10.1073/pnas.0501676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGowan K, Coulombe PA. The wound repair-associated keratins 6-16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem. 1998;31:173–204. [PubMed] [Google Scholar]
- 91.Wojcik SM, Longley MA, Roop DR. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol. 2001;154:619–630. doi: 10.1083/jcb.200102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong P, Colucci-Guyon E, Takahashi K, Gu C, Babinet C, Coulombe PA. Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. J Cell Biol. 2000;150:921–928. doi: 10.1083/jcb.150.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGowan KM, Tong X, Colucci-Guyon E, Langa F, Babinet C, Coulombe PA. Keratin 17 null mice exhibit age- and strain-dependent alopecia. Genes Dev. 2002;16:1412–1422. doi: 10.1101/gad.979502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lessard JC, Coulombe PA. Keratin 16-null mice develop palmoplantar keratoderma, a hallmark feature of pachyonychia congenita and related disorders. J Invest Dermatol. 2012;132:1384–1391. doi: 10.1038/jid.2012.6. This is the first instance of a human-like palmoplantar keratoderma (PPK) phenotype in a mouse model in which a keratin gene was manipulated. The findings show that, against expectations, PPK-like lesions can arise as a loss-of-function phenotype in mouse. The study has direct implications for the pathogenesis of pachyonychia congenita, a genodermatose with complex clinical and pathological features.
- 95.Leachman SA, Kaspar RL, Fleckman P, Florell SR, Smith FJ, McLean WH, Lunny DP, Milstone LM, van Steensel MA, Munro CS, et al. Clinical and pathological features of pachyonychia congenita. J Investig Dermatol Symp Proc. 2005;10:3–17. doi: 10.1111/j.1087-0024.2005.10202.x. [DOI] [PubMed] [Google Scholar]
- 96. Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–138. doi: 10.1038/onc.2010.456. A comprehensive and much needed review of the intimate link between keratins and various tumors.
- 97.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 100.Markey AC, Lane EB, Macdonald DM, Leigh IM. Keratin expression in basal cell carcinomas. Br J Dermatol. 1992;126:154–160. doi: 10.1111/j.1365-2133.1992.tb07813.x. [DOI] [PubMed] [Google Scholar]
- 101. Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42:910–914. doi: 10.1038/ng.665. This study establishes that a tumor-associated keratin has an impact on skin tumorigenesis through an unexpected and profound influence on the types of chemokines and cytokines that are synthesized and secreted by tumor keratinocytes (and, presumably, other cell types in the tumor milieu).
- 102.Lo BK, Yu M, Zloty D, Cowan B, Shapiro J, McElwee KJ. CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am J Pathol. 2010;176:2435–2446. doi: 10.2353/ajpath.2010.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth W, Reuter U, Wohlenberg C, Bruckner-Tuderman L, Magin TM. Cytokines as genetic modifiers in K5−/− mice and in human epidermolysis bullosa simplex. Hum Mutat. 2009;30:832–841. doi: 10.1002/humu.20981. [DOI] [PubMed] [Google Scholar]
- 104.Stacey SN, Sulem P, Masson, et al. New common variants affecting susceptibility to basal cell carcinoma. Nature Genetics. 2009;41:909–914. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park MK, Lee HJ, Shin J, Noh M, Kim SY, Lee CH. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim Biophys Acta. 2011;1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Busch T, Armacki M, Eiseler T, Joodi G, Temme C, Jansen J, von Wichert G, Omary MB, Spatz J, Seufferlein T. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 2012;125:2148–2159. doi: 10.1242/jcs.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Alam H, Gangadaran P, Bhate AV, Chaukar DA, Sawant SS, Tiwari R, Bobade J, Kannan S, D'Cruz AK, Kane S, et al. Loss of keratin 8 phosphorylation leads to increased tumor progression and correlates with clinico-pathological parameters of OSCC patients. PLoS One. 2011;6:e27767. doi: 10.1371/journal.pone.0027767. Refs. 105–107 provide distinct takes on the important role of postranslational modifications in regulating the organization of keratin filaments and key properties of tumor cells, such as growth and migration.