Abstract
Hip fracture risk rises exponentially with age, but there is little knowledge about how fracture-related alterations in hip structure differ from those of aging. We employed computed tomography (CT) imaging to visualize the three-dimensional (3D) spatial distribution of bone mineral density (BMD) in the hip in relation to age- and incident hip fracture. We used inter-subject image registration to integrate 3D hip CT images into a statistical atlas comprising women aged 21-97 years (n=349) and a group of women with (n=74) and without (n=148) incident hip fracture 4-7 years after their imaging session. Voxel-based morphometry was used to generate Student’s t-test statistical maps from the atlas, which indicated regions that were significantly associated with age or with incident hip fracture. Scaling factors derived from inter-subject image registration were employed as measures of bone size. BMD comparisons of young, middle-aged, and older American women showed preservation of load-bearing cortical and trabecular structures with aging, whereas extensive bone loss was observed in other trabecular and cortical regions. In contrast, comparisons of older Icelandic fracture women with age-matched controls showed that hip fracture was associated with a global cortical bone deficit, including both the superior cortical margin and the load-bearing inferior cortex. Bone size comparisons showed larger dimensions in older compared to younger American women and in older Icelandic fracture women compared to controls. The results indicate that older Icelandic women who sustain incident hip fracture have a structural phenotype that cannot be described as an accelerated pattern of normal age-related loss. The fracture-related cortical deficit noted in this study may provide a biomarker of increased hip fracture risk that may be translatable to DXA and other clinical images.
Keywords: Osteoporosis, proximal femur, statistical parametric mapping, age, fracture
Introduction
Hip fracture in the elderly is a devastating outcome of decades of bone loss. Fracture incidence is expected to double in the next ten years with the increasing proportion of elderly people in North America, Europe and other industrialized regions,(1) and this fact underlies the need to understand how age-related changes in hip structure predispose towards hip fracture. Age-related bone loss at the hip occurs through a series of structural changes in bone that tend to preserve mechanically loaded elements, while less mechanically stimulated structures such as the superior femoral neck cortex are attenuated with age.(2) These age-weakened structures are highly implicated in hip fracture pathophysiology because they sustain the primary impact forces from the posterolateral falls that precede most hip fractures. However, recent findings seem to indicate that hip fracture involves a complex set of structural deficits, including regions that show considerable age-related bone loss(3) as well as regions such as the inferior cortex that are relatively protected with age.(4) However, thus far, studies that have examined age- and fracture-related variations of hip structure have analyzed narrow cross-sections of the femoral neck(3, 5-7) or have focused on the differences in the cortical bone compartment between fracture subjects and controls.(8) At this point, there has been no direct evaluation of how fracture-related deficits of proximal femoral structure overlie the structural alterations associated with normal aging.
Our study goal was to compare the spatial distribution of age-related bone loss in women with incident hip fracture to that of normal elderly women. To visualize the shared features of volumetric bone mineral density (vBMD) distribution and bone size, and to understand how these features correspond to normal physiologic load bearing, we employed inter-subject image registration and voxel-based morphometry (VBM)(9) (Figure 1) to integrate volumetric quantitative computed tomography (vQCT) images from two population-based cohorts. One was comprised of healthy American women across a wide age range (Aging Study),(10) and the other was comprised of elderly Icelandic women who sustained an incident hip fracture and age-matched controls (Fracture Study).(11)
Figure 1.
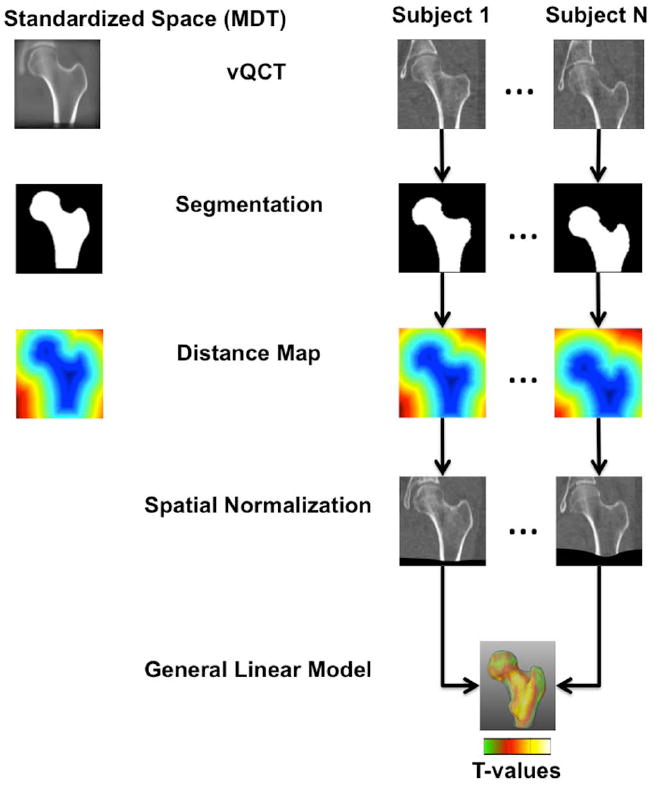
Flow diagram illustrating the steps of the adapted VBM technique used in this study. All images were segmented and femoral shapes were represented as distance maps. Using the distance maps, all scans in the study were spatially normalized to a standardized space (MDT). Aligned vBMD images were smoothed and groups were compared using a general linear model approach generating T-maps.
Materials and Methods
Human subjects
Aging Study
A total of 373 women enrolled between 2001 and 2002 in an age-stratified, random sample of Rochester, MN, residents(10) were included in the current study. For this study, subjects were divided into young (age<45 years), middle-age (45≤age<60 years), and older American women (age≥60 years). Informed consent was obtained from all participants in the study, which was approved by Mayo Clinic’s Institutional Review Board and the Committee on Human Research at the University of California, San Francisco. The Icelandic National Bioethics Committee and the Icelandic Data Protection Authority also approved the study.
Fracture Study
A subset of women enrolled in the AGES Reykjavik cohort, an ongoing population-based study of Icelandic men and women,(11, 12) was included in the current study. Baseline CT scans of 5,500 subjects from this cohort were obtained between 2002 and 2006. Subjects were followed for four to seven years (mean five years), and those with incident hip fractures during this period (but without documented hip fracture prior to baseline) were identified through the AGES-Reykjavik Fracture Registry.(13) Approximately two sex- and age-matched control subjects for each hip fracture woman were randomly selected(14) for a total of 222 women (74 fracture cases). For this study, subjects were divided into older Icelandic control (no hip fracture) and older Icelandic fracture (incident hip fracture) women. Informed consent was obtained from all participants in the study, which was approved (VSN 00-063) by the National Bioethics Committee in Iceland, the Institutional Review Board of the Intramural Research Program of the National Institute on Aging, and the Committee on Human Research at the University of California, San Francisco.
Image acquisition
Aging Study
For each subject, single-energy CT scans of the left and right hip joints were obtained with a multi-detector CT scanner (Light Speed QX-I; GE Medical Systems, Wakesha, WI, USA) with in-plane voxel size 0.74 × 0.74 mm2 and 2.5-mm slice thickness. A QCT calibration phantom (Mindways Inc., Austin, TX, USA) was scanned along with each subject for individual conversion of Hounsfield Units (HU) to equivalent reference concentrations of aqueous K2HPO4.
Fracture Study
For each subject at baseline, images of the left and right hip joints were obtained using a four-detector CT system (Sensation, Siemens Medical Systems, Erlangen, Germany) and reconstructed to an in-plane voxel size of 0.98 × 0.98 mm2 and a slice thickness of 1 mm. Each subject was scanned along with a solid QCT calibration phantom (Image Analysis, Inc., Columbia, KY, USA) containing cells of 0, 75, and 150 mg/cm3 equivalent concentration of calcium hydroxyapatite.
Image preprocessing
Based on the calibration phantoms, CT HU were converted to equivalent K2HPO4 density and equivalent concentration of calcium hydroxyapatite in anonymized scans from the Aging and Fracture Studies, respectively. Phantom-based cross-calibration between the Aging and Fracture Studies was not possible, thus a cross-calibration was performed based on peak vBMD values of shaft cortical bone of 20 age-matched subjects, 10 older American women, and 10 older Icelandic control women. This cross-calibration was based on the assumption that in the absence of cortical porosities, cortical bone of normal age-matched women exhibits similar vBMD.
Femoral contours of the left hips were semi-automatically delineated on a slice-by-slice basis in each scan(14, 15) to generate 3D representations of bone shape. Since the scans had lower spatial resolution along the slice direction, images and contours were upsampled to isotropic voxel sizes matching the in-plane spatial resolutions.
VBM
We adapted VBM to study vBMD of the proximal femur using vQCT images as shown in Figure 1. Tissue contrast can be assumed to be constant within a particular tissue class in adult brains,(16) but the proximal femur exhibits spatially varying contrast as the result of its internal structure, making gray-level based (HU or vBMD) spatial normalization a suboptimal choice for VBM. Given the assumption that all proximal femora have similar outer shapes, spatial normalization was done based on level-set representations of femoral surfaces using distance maps.(17, 18) In order to make the standardized space less dependent on the selection of the reference subject, a minimum deformation template (MDT)(19) representing the average size and shape of young American women was constructed using multi-resolution affine (nine-parameter) and non-linear(20) transformations. All calibrated images in both studies (Aging and Fracture) were then spatially normalized to the MDT concatenating affine and non-linear registrations to reduce the number of interpolation steps, and using distance maps to minimize the distortion of the spatial distribution of vBMD values. Spatially-normalized calibrated images were smoothed with an isotropic Gaussian kernel to ensure that 1) each voxel contained the average amount of vBMD from its local neighborhood; 2) that the data were more normally distributed by the central limit theorem, thus increasing the validity of parametric statistical tests; and 3) to compensate for the inexact nature of spatial normalization.(9, 21)
Scale analysis
The MDT was transformed to an anatomic coordinate system where the long axis of the femoral neck (mediolateral (ML) direction) was along the z-axis of the anatomic coordinate system, and the x- and y-axes of the anatomic coordinate system were along the height (inferosuperior (IS) direction) and width (anteroposterior (AP) dimension) of the femoral neck, respectively (Figure 2). The global amount of contraction or expansion that was needed in each dimension for each subject in both studies (Aging and Fracture) to match the MDT in the anatomic coordinate system was computed based on multi-resolution affine transformations (nine-parameter) using distance maps.
Figure 2.
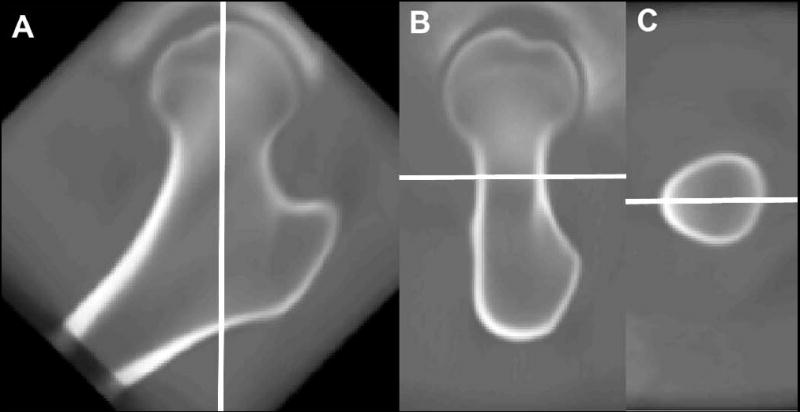
Anatomic coordinate system used for scale analysis. The z-axis passes through the long axis of the femoral neck (A; ML direction), and the y- and x-axes are along the width (B; AP direction) and height (C; IS direction) of the femoral neck, respectively.
Statistical analysis
Subject characteristics
Statistical significance of differences in subject characteristics (age, height, weight, and total femoral areal BMD (aBMD) by vQCT (Fracture Study only)) between subgroups in the Aging and Fracture Studies was established using analysis of variance. In the Aging Study, the Bonferroni correction for multiple-comparison correction was used. Differences were considered significant at P<0.05.
VBM
Older Icelandic women with incident hip fracture combined age-related bone loss with specific features or structural changes that caused them to have increased fracture risk compared to age-matched controls. Our study design, in comparing age- and fracture-related structural variations from two different studies, assumes that older Icelandic controls experienced a pattern of bone loss similar to that of older American women. To test this assumption, we generated vBMD difference maps comparing young American women with older American women and older Icelandic control women.
In addition to the vBMD difference maps generated above, vBMD difference maps comparing young and middle-age American women, older Icelandic control and fracture women, and young American and older Icelandic fracture women were generated. Student’s t-test statistical maps, referred to as T-maps, of the voxel-wise vBMD differences previously mentioned, were then obtained using a general linear model approach. The vBMD values at each voxel location of the spatially normalized and smoothed images were used as the dependent variable, with group membership (young or middle-age, young or older, control or fracture, young or control, young or fracture) as the independent variable. Height and weight were included as covariates in comparisons involving women of the Aging Study, while age, height, and weight were used in the comparison of the Fracture Study (control versus fracture).(22-24) Significance corrections for multiple comparisons over the whole proximal femur were done using false discovery rate (FDR) correction (q=0.05).(25)
Scale analysis
The scale factors that were needed along each dimension to match each subject to the MDT in the anatomic coordinate system were compared between subgroups in each study using analysis of variance. The Bonferroni correction for multiple-comparison correction was used in the Aging Study. Scale differences were considered significant at P<0.05. General linear models with the scale factors along each dimension as the dependent variable and age or fracture status as the independent variable were also evaluated. Covariates included height and weight in the Aging Study, and age, height, weight, and aBMD in the Fracture Study.
Finite element analysis
In order to relate spatial patterns of vBMD changes associated with aging to patterns of mechanical stress and strain, we used finite element analysis methods described previously to compute distributions of von Mises stress and principal strain from vQCT scans of a representative control and a representative fracture subject from the Fracture Study.(14) These calculations were carried out in loading conditions simulating single-legged stance, which represents the loads on the hip associated with normal ambulation as well as in a condition simulating a posterolateral fall. The von Mises stress represents the distribution of loading force on a per element basis whereas the principal strain maps depict the deformation of the elements in relation to the loading. The representative control and fracture women were the older Icelandic control and older Icelandic fracture women whose aBMD, age, height, and weight were closest to the mean parameters of their corresponding subgroups.
Results
Subject characteristics
Table 1 summarizes the subject characteristics for the different subgroups of the Aging and Fracture Studies included in this work. A total of 24 women of the Aging Study were excluded from this study for a final group of 349 women. Reasons for exclusion included insufficient image quality, presence of pathologies in the proximal femur, as well as femoral segmentation problems. In the Aging Study, older women were shorter (P<0.0001) than young and middle-age women, and weighed less (P<0.05) than middle-age women. In the Fracture Study, aBMD was lower in older women with incident hip fracture than in their age-matched controls (P<0.0001).
Table 1.
Descriptive Statistics for Women of the Aging and Fracture Studies.
| Measure | Na | Min | Max | Mean | SD | P |
|---|---|---|---|---|---|---|
| Young American | ||||||
| Age (years) | 94 | 21 | 44 | 34.1 | 6.6 | 0.0001b |
| Height (cm) | 94 | 151 | 178.1 | 165.4 | 6.0 | 0.0001c |
| Weight (kg) | 94 | 44.9 | 113.5 | 72.1 | 17.0 | |
| Middle-Age American | ||||||
| Age (years) | 98 | 45 | 59 | 51.9 | 4.0 | 0.0001b |
| Height (cm) | 98 | 153.7 | 179.7 | 163.9 | 5.8 | 0.0001c |
| Weight (kg) | 98 | 50.6 | 151.2 | 77.3 | 18.4 | 0.05d |
| Older American | ||||||
| Age (years) | 157 | 60 | 97 | 72.5 | 8.8 | 0.0001b |
| Height (cm) | 157 | 143 | 179.5 | 160.3 | 6.4 | 0.0001c |
| Weight (kg) | 157 | 40.5 | 119.9 | 71.8 | 13.7 | 0.05d |
|
| ||||||
| Older Icelandic Controls | ||||||
| Age (years) | 148 | 67 | 92 | 79.2 | 5.7 | |
| Height (cm) | 147 | 139.2 | 172.9 | 159.2 | 5.6 | |
| Weight (kg) | 147 | 37.2 | 112 | 68.4 | 13.7 | |
| aBMD (g/cm2) | 148 | 0.337 | 1.239 | 0.694 | 0.155 | 0.0001 |
| Older Icelandic Fracture | ||||||
| Age (years) | 74 | 67 | 93 | 79.4 | 5.9 | |
| Height (cm) | 73 | 145.1 | 173.4 | 159.6 | 6.1 | |
| Weight (kg) | 73 | 39.2 | 111.2 | 64.5 | 15.1 | |
| aBMD (g/cm2) | 74 | 0.392 | 0.960 | 0.604 | 0.123 | 0.0001 |
SD = Standard deviation.
Differences in the number of data points for each measure within each subcohort are due to missing data.
Significantly different between all groups in the Aging Study.
Significantly different from the older group in the Aging Study.
Significantly different between the middle-age and older groups in the Aging Study.
VBM
The 3D location, size and direction of vBMD changes in the proximal femur associated with aging are shown in Figures 3A-3C. In these figures, the spatial patterns of bone loss observed in middle-age American women (Figure 3A) were accentuated in older American women (Figure 3B), and similar spatial patterns of bone loss were observed in the Aging and Fracture Studies (Figure 3C). In these figures, vBMD differences smaller than 15 mg/cm3 were rendered semi-transparent. Mid-coronal cross-sections depicting voxels where the vBMD differences were significant between young and middle-age American women, and between young and older American women are shown in Figures 3D and 3E, respectively. These figures represent mid-coronal cross-sections of T-maps generated using VBM, where non-significant voxels after FDR correction for multiple comparisons have been rendered transparent. These T-maps have been adjusted for inter-group differences in weight and height.
Figure 3.
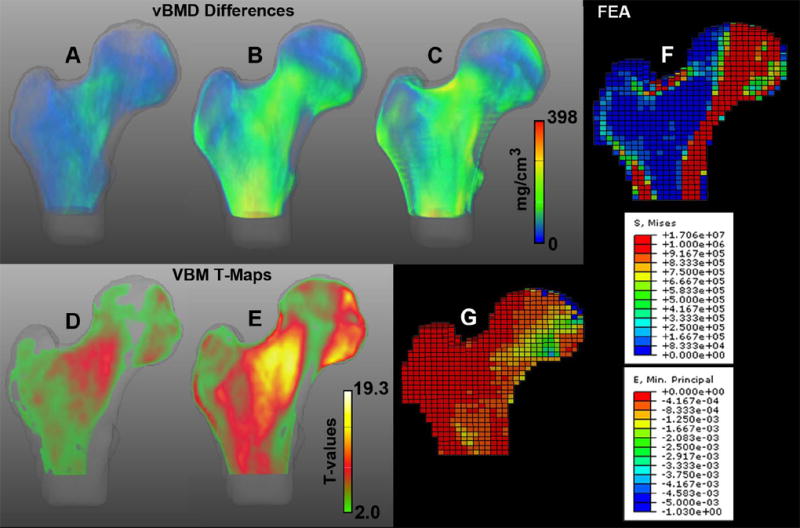
Spatial distribution of vBMD differences associated with aging. Anterior views of volumetric representations of voxel-wise vBMD differences between young (n=94) and middle-age (n=98) American women (A; 0-177 mg/cm3), young (n=94) and older (n=157) American women (B; 0-398 mg/cm3), and between young American (n=94) and older Icelandic control (n=148) women (C; 0-393 mg/cm3). Voxels were assigned transparency based on their difference values, from full transparency for zero vBMD differences, to full opacity for vBMD differences=398 mg/cm3. Mid-coronal cross-sections of the T-maps of the voxel-wise vBMD differences between young (n=94) and middle-age (n=98) American women (D; 2.1-9.5 T-values), and young (n=94) and older (n=157) American women (E; 2.0-19.3 T-values). Voxels were assigned transparency based on their T-values, from full transparency for non-significant voxels (T<2·1 in D; T<2·0 in E), to full opacity for T-values=19.3. FEM stance loading von Mises stress map (F) and principal strain map (G) of a representative control women. FEA = Finite Element Analysis.
The 3D vBMD differences and the mid-coronal cross-sections of the T-maps shown in Figure 3 depict large and significant vBMD changes associated with aging in the inner region of the femoral neck, medial aspect of the femoral head, and in regions at the level of the quadrate tubercle (yellow-red regions in the vBMD difference maps, and yellow-white regions in the T-maps). The inferior aspect of the femoral neck was the site of major vBMD preservation with aging (semi-transparent and transparent regions), followed by medial and lateral regions at the level of the lesser trochanter, the superolateral aspect of the femoral head, and superior regions of the greater trochanter (blue areas in the vBMD difference maps, and green regions in the T-maps).
The 3D location, size and direction of vBMD changes in the proximal femur associated with incident hip fracture are shown in Figure 4A, while a mid-coronal cross-section of the T-map showing voxels where the vBMD differences were significant between older Icelandic control and fracture women is shown in Figure 4B. Similar to Figure 3, vBMD differences smaller than 15 mg/cm3 were rendered semi-transparent, and non-significant voxels after FDR correction for multiple comparisons were rendered transparent. The T-Map in Figure 4B has been adjusted for age, height, and weight.
Figure 4.
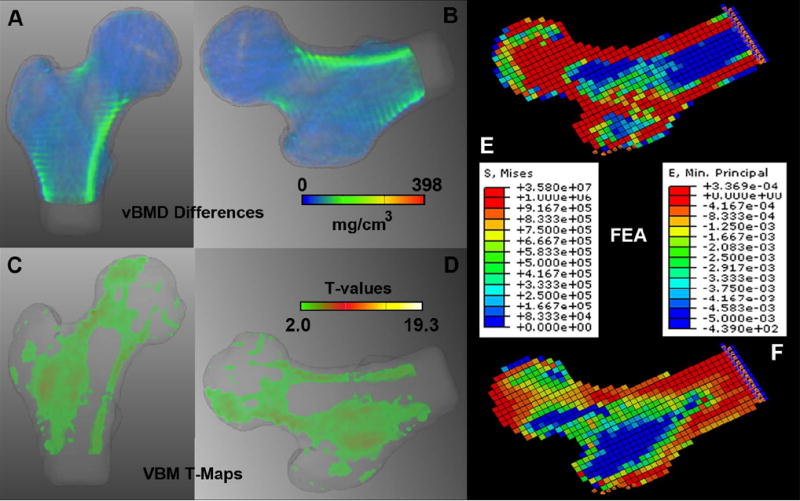
Spatial distribution of vBMD changes associated with incident hip fracture. (A) Anterior view of a volumetric representation of voxel-wise vBMD differences between older Icelandic control (n=148) and fracture (n=74) women (0-223 mg/cm3). (B) Rotated version of A simulating a posterolateral fall. Color-coding and transparency were standardized to a dynamic range of 0-398 mg/cm3 as in Figures 3A-C. (C) Mid-coronal cross-section of the T-map of the voxel-wise vBMD differences between older Icelandic control (n=148) and fracture (n=74) women (2.3-6.9 T-values). (D) Rotated version of C simulating a posterolateral fall. Voxels were assigned transparency based on their T-values, from full transparency for non-significant voxels (T<2·3), to full opacity for T-values=19.3 as in Figures 3D-E. (E) FEM posterolateral fall loading von Mises stress map of a representative woman with incident hip fracture. (F) FEM posterolateral fall loading principal strain map of the same representative woman with incident hip fracture. FEA = Finite Element Analysis.
The 3D vBMD differences and the mid-coronal cross-section of the T-map shown in Figure 4 depict large and significant vBMD changes associated with incident hip fracture in the superior and inferior aspects of the femoral neck, and in the intertrochanteric region (yellow-red regions in the vBMD difference map, and yellow-white regions in the T-map).
Figure 5 shows mid-coronal and neck-axial cross-sections of the T-maps of the comparison of young American women with older Icelandic control (Figure 5A) and fracture women (Figure 5B), indicating that normal older women experience similar bone loss in both studies, and that fracture-related changes overlay those due to aging.
Figure 5.
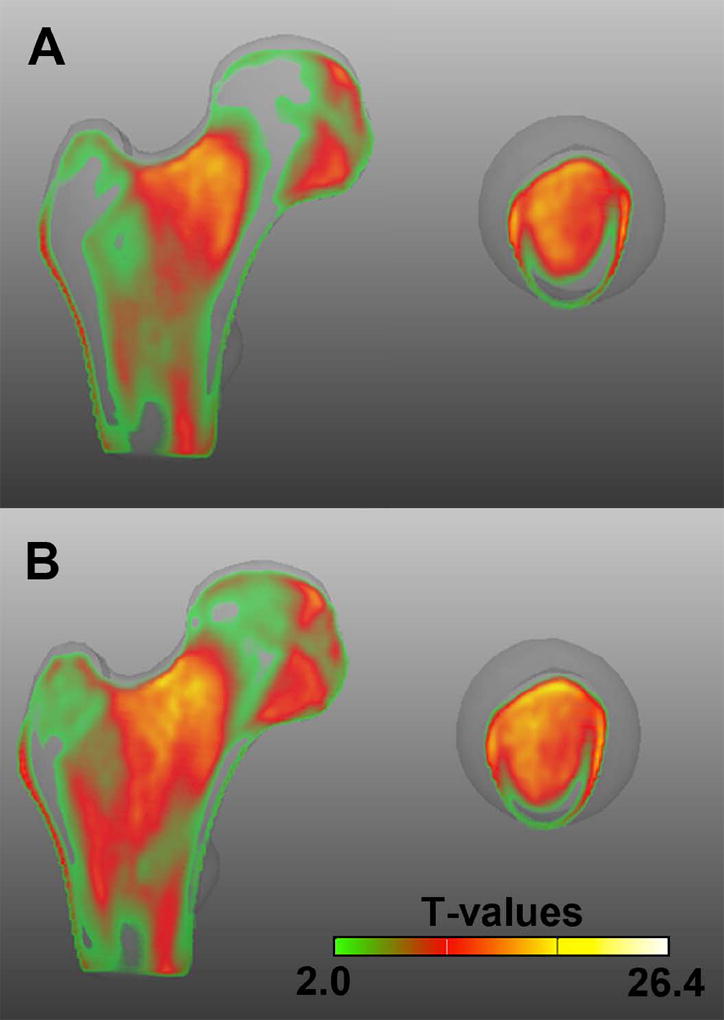
Comparison of the Aging and Fracture Studies. Mid-coronal and neck-axial cross-sections of the T-maps of the voxel-wise vBMD differences between young American (n=94) and older Icelandic control (n=148) women (A; 2.1-26.4 T-values), and between young American (n=94) and older Icelandic fracture (n=74) women (B; 2.0-25.8 T-values). Voxels were assigned transparency based on their T-values, from full transparency for non-significant voxels (T<2·1 in A; T<2·0 in B), to full opacity for T-values=26.4.
Scale analysis
In the Aging Study, proximal femora of older women had larger AP dimensions than proximal femora of middle-age (P<0.05) and young (P<0.05) women. Proximal femora of older women also had larger IS dimensions than proximal femora of young women (P<0.0001). No significant scale differences were found along the ML axis of the neck between groups (P>0.05). With respect to the Fracture Study, proximal femora of older women with incident hip fracture had larger AP and IS dimensions than those of the controls (P<0.05). No significant scale differences were found along the ML axis of the neck between groups (P>0.05).
The age coefficients of the general linear models in which scale was considered the dependent variable were significantly different than zero for all three dimensions in the Aging Study (P<0.0001). The fracture coefficients of the general linear models in which scale was considered the dependent variable were significantly different than zero for the AP (P=0.0261) and IS (P=0.0483) dimensions in the Fracture Study. The general trend was for larger bone sizes for older women in the Aging Study, and larger bone sizes for women with incident hip fracture in the Fracture Study. In both studies, taller women had significantly larger bone sizes in the three dimensions (P<0.0001). Greater weights were significantly associated with smaller bone sizes in the three dimensions in the Aging Study (P<0.0001) but with larger AP (P=0.0178) and IS (P=0.0030) dimensions in the Fracture Study. In the Fracture Study, age was significantly associated with a larger AP dimension (P=0.0204), while aBMD was significantly associated with smaller AP (P=0.0346) and IS (P=0.0035) dimensions. Table 2 summarizes the least squares mean scaling factors for each subgroup and dimension.
Table 2.
General linear model analysis of scaling factors for the Aging and Fracture Studies.
| Subgroup | Least squares mean scaling factors ± standard deviation
|
||
|---|---|---|---|
| Anteroposteriora,b | Inferosuperiora,c | Mediolaterala | |
| Young American | 1.0066±0.0369 | 0.9950±0.0396 | 1.0005±0.0394 |
| Middle-age American | 0.9912±0.0356 | 0.9754±0.0362 | 0.9974±0.0389 |
| Older American | 0.9718±0.0301 | 0.9577±0.0339 | 0.9945±0.0341 |
|
| |||
| Older Icelandic Controls | 0.9091±0.0266 | 0.9309±0.0252 | 0.9494±0.0214 |
| Older Icelandic Fracture | 0.8893±0.0282 | 0.9104±0.0267 | 0.9414±0.0234 |
A smaller scaling factor indicates overall larger proximal femoral size in the given dimension.
American women were adjusted for height and weight.
Older Icelandic women were adjusted for age, height, weight, and aBMD.
Age coefficient was significantly different than zero in the Aging Study (P<0.0001).
Fracture coefficient was significantly different than zero in the Fracture Study (P=0.0261).
Fracture coefficient was significantly different than zero in the Fracture Study (P=0.0483).
Finite element analysis
In order to relate spatial patterns of vBMD changes associated with aging to patterns of mechanical stress and strain, Figures 3F and 3G were included to respectively depict mid-coronal cross-sections of von Mises stress and principal strain distributions computed from finite element models simulating a single-legged stance. These models were calculated from a vQCT scan of a representative control woman using methods described previously.(14) High stress can be observed in the compressive trabecular bands and inferior cortex (Figure 3F), while high strain can be observed in the inferomedial aspect of the femoral head (Figure 3G). The representative control woman had aBMD=0.697 g/cm2, age=82 years, height=155.15 cm, and weight=48 kg.
In order to compare the spatial patterns of fracture-related differences to the stresses and strains exerted on the hip during a fall, bones in Figures 4A-4B were rotated to match Figures 4C and 4D, which respectively depict the distribution of von Mises stress and principal strain computed by vQCT-derived finite element models simulating a posterolateral fall. These models were calculated from a vQCT scan of a representative fracture woman using methods described previously.(14) High stress can be observed in the cortical bone, most of the femoral head, and inter-trochanteric region (Figure 4C), while high strain can be observed along the neck and in the inter-trochanteric region (Figure 4D). The representative fracture woman had aBMD=0.604 g/cm2, age=84 years, height=166.7 cm, and weight=63.5 kg.
Discussion
At load-bearing skeletal sites such as the proximal femur, aging entails a structural rearrangement of bone tissue that reduces loss of bone function in the context of increased bone resorption. Adaptive processes are activated to preserve whole bone stiffness in physiologic loading by increasing overall bone size and offsetting the effects of endosteal resorption at mechanically loaded sites such as the inferomedial femoral neck cortex, and by protecting load bearing trabecular structures such as the principal compressive band. In this work, by employing inter-subject image registration in conjunction with VBM to analyze image data from two population-based cohort studies, we were able to quantify 3D differences in proximal femoral density distribution and size between women across a large age range, and anatomically relate these changes to the structural deficits associated with incident hip fracture.
The statistical atlas analyses comparing young American women with middle-age American, older American, and older Icelandic control women depicted a relative preservation of load bearing sub-regions of the proximal femur with age. The spatial patterns of vBMD changes associated with aging displayed in the difference maps and T-maps (Figure 3) were in agreement with the stress distribution computed for single-legged stance loading by finite element modeling (FEM). Regions of maximum stress in stance FEM coincided with regions in the difference maps and T-maps that showed smaller and less statistically significant differences with age. In addition to illustrating minimal age-related deficits of the inferior cortex and the principal compressive bands, Figure 3 showed age variations that were consistent with a sharp progression of bone loss in the superior cortex and in the trabecular bone of the femoral neck and trochanteric regions. These results were consistent with those of previous studies, which demonstrated relative preservation of the inferior cortex(2-4, 7) and endosteal thinning of the superior cortex.(3, 4, 7) The age-related variations of BMD distribution demonstrated by the statistical maps was consistent with the bone size information derived from the analyses of the scaling factors computed in the inter-subject image registrations. Older age was found to predict greater bone size independently of height and weight, and results agreed with previous aging studies: Bones of older women were significantly larger along directions parallel to the width and height of the femoral neck than bones of young women,(10, 26-28) consistent with the presence of periosteal apposition.
Applying inter-subject image registration and VBM to images from the Aging and Fracture Studies allowed us merge the spatial variation of age-related changes and the fracture-related structural deficits into the same anatomic coordinate system. Because older Icelandic women who sustained incident fracture were healthy and ambulatory at the time they were imaged, we expected that they would show structural characteristics of an adaptation to greater bone loss (as previously confirmed in this cohort). Such characteristics of an accentuated aging process would include larger bone dimensions,(29-31) relative preservation of load-bearing structures,(2) and greater attenuation of less mechanically stimulated regions of the proximal femur.(2) The comparison of scaling factors between older Icelandic women with incident hip fracture and their age-matched controls supported this idea by showing that fracture women had larger overall bone dimensions than controls independently of differences in age, height, weight, and aBMD. In keeping with this, VBM analysis also showed (Figure 4) that older Icelandic women who fractured their hips had relative deficits in the superior cortex and in the medullary trabecular bone regions that coincided with locations of peak strain in fall loading and that were lower with age in Figure 3. These observations of diminished superior femoral neck and trochanteric vBMD were generally consistent with the locations of diminished cortical thickness reported in hip fracture subjects in a cross-sectional case-control study reported by Poole and colleagues.(8) However, the difference map and T-map of Figure 4 depicted that, compared to controls, older Icelandic women with hip fracture had a pronounced deficit of vBMD at the inferomedial proximal femoral cortex, a primary load-bearing region that evidenced minimal differences between young and older American women. This finding, which was not observed by Poole et al., is consistent with previous studies in our cohort, which showed that subjects with incident hip fracture had diminished inferoanterior femoral neck cortical thickness(4) and reduced whole bone strength in the single legged stance loading condition,(14) in which load is primarily applied to the inferior cortex. This deficit may be an inherited characteristic of hip architecture present in subjects at high risk for hip fracture. However, it is also possible that hip fracture subjects may have impairments in calcium metabolism requiring extensive bone resorption to maintain calcium homeostasis. In these cases, cortical sites such as the thick proximal femoral cortices would provide a potential calcium reservoir, resulting in an overall rate of bone resorption that is too high to be counteracted by compensatory mechanisms such as periosteal apposition.(32) While a thinner inferomedial cortex would reduce the overall stiffness and strength of the proximal femur in physiologic loading, what effect would it have in withstanding fall forces? Although Figure 4 shows that the inferomedial cortex is highly stressed in falls, the strain distribution shows that it is a site of minimal deformation, and it not known to be a site of fracture initiation. It is possible, however that the inferomedial cortex modulates the distribution of strain in the proximal femur in a fall. Thus, a thinner cortex may change the distribution of strain in the proximal femur, and add to the peak strains in the sub-regions known to fail structurally in falls.
The comparison of T-maps computed between older Icelandic fracture and control women, and young American women (Figure 5), demonstrate how fracture-related patterns overlie the structural differences between young American and older Icelandic control women. In the fracture group, the zone of trabecular bone loss expanded laterally into the inter-trochanteric region, and into the load bearing cortical and trabecular structures (principal compressive band) that were minimally different in the control group. The specific deficit in the inferior cortex was observed even though our scaling analysis indicated that the fracture group had larger bone size than the age-matched controls. Thus, subjects who sustain incident hip fracture may have an overall higher level of bone resorption that even affects bone compartments that are heavily loaded in routine mechanical usage as well as those that are less stimulated. A higher level of periosteal apposition, which would be consistent with the larger bone size, may be insufficient to compensate for this higher resorption level, leading to a net deficit in vBMD.
This study had strengths and limitations. Strengths included the use of data from two population-based cohorts, the prospective design of the Fracture Study, the spatial normalization strategy minimizing distortion of the internal distribution of vBMD values in the hip, and the spatial integration into a single atlas of two of the most relevant aspects in the study of osteoporosis: aging and hip fracture. The first limitation of this study was the exclusive analysis of Caucasian women, which may not generalize to men and to other ethnicities. A second limitation was that the fracture study did not differentiate between hip fracture types, such as subcapital, neck, and trochanteric fractures, which may have structural differences, a limitation that we plan to address as additional fracture cases occur. The third limitation was that there was no opportunity to scan the same phantom on the scanners used for the two studies, because the scanner used for the Aging Study was no longer available. Therefore, in order to represent the vBMD values for the two studies on the same scale, we carried out a cross-calibration using standardized diaphyseal sections through the upper thigh on the two sets of images, using as a reference value the peak vBMD of bone in the thick cortex of the femoral shaft, which in the absence of cortical porosity is equivalent for adults (~1.05 g/cm3). The estimate of cortical vBMD in these diaphyseal regions may differ from that obtained in the proximal femur due to differences in beam hardening associated with differences in osseous and soft tissue composition. Unfortunately, in the proximal femoral region, the lower cortical thickness introduces partial volume averaging effects that would introduce variability into the cross-calibration, and thus we used the upper thigh, where this value could be robustly estimated. Another limitation is the effect of partial volume averaging on quantification of proximal femoral cortical bone. Partial volume averaging errors affect measurement of structures that are small compared with the spatial resolution of the imaging system, which comprised the CT scanner (non-isotropic voxel dimensions of ~1 mm in-plane and 1-3 mm out of plane) and the interpolation errors associated with the inter-subject registration process. For thin cortices such as the superomedial aspect of the femoral neck (thickness of less than 500 microns), this results in underestimation of BMD and overestimation of volume or thickness measurements, with smaller errors for estimation of mass. As intrinsic cortical vBMD is generally considered to be constant, the differences we observed in the superior cortex between younger and older subjects and between fractures and controls could represent contributions of both cortical porosity and cortical thickness. These errors are less significant for the inferomedial cortex, which has thicknesses ranging from 3-4 mm in humans.(33) This study also has a final limitation inherent to most imaging analysis techniques based on image registration, i.e. intensity interpolation. To minimize this effect, the affine and non-linear transformations were concatenated to reduce the number of interpolation steps from two to one.
In conclusion, the presented analytic approach has allowed us to identify for the first time the overall structural features of the proximal femur that distinguish women at high risk for hip fracture from those that are associated with normal aging. In this study, compared to normal women of the same age and without hip fracture, women with incident hip fracture were characterized by increased loss of bone along the endocortical margin, both at the superior cortex as reported previously in the literature, but also at the load-bearing inferior cortex, a structure that is largely preserved in normally aging women. Furthermore, the analysis of scaling factors derived from the inter-subject image registration process showed that women who sustained hip fracture appeared to have larger outer femoral neck dimensions than non-fractured women, consistent with an increased compensatory response to greater bone deficit at the load-bearing inferior cortex. Our findings also have direct clinical implications. The observed deficit at a prominent structure such as the inferior cortex may provide a biomarker of increased fracture risk that could be detected in diagnostic images. Furthermore, evidence from pre-clinical studies indicates that the newer generation of anabolic and anti-resorptive medications may have enhanced effects on cortical bone that could potentially reverse the structural deficits observed in this study.(34-37) Thus, by showing that women who sustain incident hip fracture have a structural phenotype fundamentally distinct from that of normal aging, this report has provided new information on the pathophysiology of hip fracture and has illuminated skeletal features that may improve the ability to find and treat individuals at risk for this most devastating outcome of osteoporosis.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIA R01AG028832, NIH/NIAMS R01AR46197, NIH/NIA Professional Services Contract HHSN311200900345P, NIH/NIAMS R01AR027065, and M01-RR00585/UL1-RR024150 (Center for Translational Science Activities). The Age, Gene/Environment Susceptibility Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study was approved by the Icelandic National Bioethics Committee, (VSN: 00–063) and the Data Protection Authority.
Authors’ roles: JCG implemented the image registration tools to perform VBM, analyzed data, interpreted results, and wrote the first draft of the report. RH, IS, and TS provided the femoral segmentations of the Aging Study. SS provided the femoral segmentations of the Fracture Study. SA, EJA, and TMT provided advise on the statistics. KS provided support with the selection of fracture subjects. XC provided the Chinese fracture dataset. LJM-III interpreted results. JK provided the FEM analyses and interpreted results. VG provided the images of the Fracture Study. SK provided the images of the Aging Study, designed the study, interpreted results, and co-led the study. TBH designed the study, interpreted results and co-led the study. TFL designed the study, interpreted results, led the study and edited the report.
Footnotes
Supplemental material has been included with the submission.
Disclosure
All authors state that they have no conflicts of interest.
References
- 1.Bureau UC. US Census Bureau: International Database. Bureau UC; 2006. Table 94. [Google Scholar]
- 2.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366(9480):129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CD, Mayhew PM, Power J, Poole KE, Loveridge N, Clement JG, et al. Femoral neck trabecular bone: loss with aging and role in preventing fracture. Journal of Bone and Mineral Research. 2009;24(11):1808–18. doi: 10.1359/jbmr.090504. [DOI] [PubMed] [Google Scholar]
- 4.Johannesdottir F, Poole KE, Reeve J, Siggeirsdottir K, Aspelund T, Mogensen B, et al. Distribution of cortical bone in the femoral neck and hip fracture: a prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone. 2011;48(6):1268–76. doi: 10.1016/j.bone.2011.03.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell KL, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M, et al. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. Journal of Bone and Mineral Research. 1999;14(1):111–9. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree NJ, Kroger H, Martin A, Pols HA, Lorenc R, Nijs J, et al. Improving risk assessment: hip geometry, bone mineral distribution and bone strength in hip fracture cases and controls. The EPOS study. European Prospective Osteoporosis Study. Osteoporos Int. 2002;13(1):48–54. doi: 10.1007/s198-002-8337-y. [DOI] [PubMed] [Google Scholar]
- 7.Poole KE, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N, et al. Changing structure of the femoral neck across the adult female lifespan. Journal of Bone and Mineral Research. 2010;25(3):482–91. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 8.Poole KE, Treece GM, Mayhew PM, Vaculik J, Dungl P, Horak M, et al. Cortical thickness mapping to identify focal osteoporosis in patients with hip fracture. PloS one. 2012;7(6):e38466. doi: 10.1371/journal.pone.0038466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 10.Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. Journal of Bone and Mineral Research. 2004;19(12):1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 11.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, et al. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39(3):644–51. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siggeirsdottir K, Aspelund T, Sigurdsson G, Mogensen B, Chang M, Jonsdottir B, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol. 2007;22(9):631–9. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
- 14.Keyak JH, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Zhao S, et al. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone. 2011;48(6):1239–45. doi: 10.1016/j.bone.2011.03.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyak JH, Rossi SA, Jones KA, Skinner HB. Prediction of femoral fracture load using automated finite element modeling. J Biomech. 1998;31(2):125–33. doi: 10.1016/s0021-9290(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 16.Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, et al. A unified statistical approach to deformation-based morphometry. Neuroimage. 2001;14(3):595–606. doi: 10.1006/nimg.2001.0862. [DOI] [PubMed] [Google Scholar]
- 17.Reinertsen I, Descoteaux M, Drouin S, Siddiqi K, Collins DL. Vessel driven correction of brain shift. Lect Notes Comput Sc. 2004;3217:208–16. [Google Scholar]
- 18.Suh JW, Wyatt CL. Deformable registration of prone and supine colons for CT colonography. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1997–2000. doi: 10.1109/IEMBS.2006.260249. [DOI] [PubMed] [Google Scholar]
- 19.Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30(1):209–19. doi: 10.1002/hbm.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vercauteren T, Pennec X, Perchant A, Ayache N. Non-parametric diffeomorphic image registration with the demons algorithm. Med Image Comput Comput Assist Interv. 2007;10(Pt 2):319–26. doi: 10.1007/978-3-540-75759-7_39. [DOI] [PubMed] [Google Scholar]
- 21.Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev. 2005;1(2):105–13. [Google Scholar]
- 22.Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23(1):242–51. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 23.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129(Pt 5):1218–28. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- 24.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 26.Kaptoge S, Dalzell N, Jakes RW, Wareham N, Day NE, Khaw KT, et al. Hip section modulus, a measure of bending resistance, is more strongly related to reported physical activity than BMD. Osteoporosis Int. 2003;14(11):941–9. doi: 10.1007/s00198-003-1484-2. [DOI] [PubMed] [Google Scholar]
- 27.Power J, Loveridge N, Lyon A, Rushton N, Parker M, Reeve J. Osteoclastic cortical erosion as a determinant of subperiosteal osteoblastic bone formation in the femoral neck’s response to BMU imbalance. Effects of stance-related loading and hip fracture. Osteoporosis Int. 2005;16(9):1049–56. doi: 10.1007/s00198-004-1803-2. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter RD, Sigurdsson S, Zhao S, Lu Y, Eiriksdottir G, Sigurdsson G, et al. Effects of age and sex on the strength and cortical thickness of the femoral neck. Bone. 2011;48(4):741–7. doi: 10.1016/j.bone.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40(1):169–74. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Ito M, Wakao N, Hida T, Matsui Y, Abe Y, Aoyagi K, et al. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone. 2010;46(2):453–7. doi: 10.1016/j.bone.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 31.Bousson VD, Adams J, Engelke K, Aout M, Cohen-Solal M, Bergot C, et al. In vivo discrimination of hip fracture with quantitative computed tomography: results from the prospective European Femur Fracture Study (EFFECT) Journal of Bone and Mineral Research. 2011;26(4):881–93. doi: 10.1002/jbmr.270. [DOI] [PubMed] [Google Scholar]
- 32.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 33.Bagi CM, Wilkie D, Georgelos K, Williams D, Bertolini D. Morphological and structural characteristics of the proximal femur in human and rat. Bone. 1997;21(3):261–7. doi: 10.1016/s8756-3282(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 34.Ominsky MS, Stouch B, Schroeder J, Pyrah I, Stolina M, Smith SY, et al. Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone. 2011;49(2):162–73. doi: 10.1016/j.bone.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. Journal of Bone and Mineral Research. 2011;26(5):1012–21. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 36.Cusick T, Chen CM, Pennypacker BL, Pickarski M, Kimmel DB, Scott BB, et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. Journal of Bone and Mineral Research. 2012;27(3):524–37. doi: 10.1002/jbmr.1477. [DOI] [PubMed] [Google Scholar]
- 37.Jayakar RY, Cabal A, Szumiloski J, Sardesai S, Phillips EA, Laib A, et al. Evaluation of high-resolution peripheral quantitative computed tomography, finite element analysis and biomechanical testing in a pre-clinical model of osteoporosis: a study with odanacatib treatment in the ovariectomized adult rhesus monkey. Bone. 2012;50(6):1379–88. doi: 10.1016/j.bone.2012.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


