Abstract
Background
According to clinical studies, depression and cerebrovascular disease influence each other. Despite this evidence, no studies have investigated the relationship between major depressive disorder (MDD) and cerebrovascular disease at the cellular level. Astrocytic processes are a crucial interface between blood vessels and neurons, and astrocyte density is reduced in MDD. This study investigated the coverage of vessels by astrocyte endfeet in the prefrontal cortex in MDD.
Methods
Thirteen pairs of MDD and non-psychiatric control subjects were used for double immunofluorescent staining and confocal image analysis. Frozen sections of gray matter from orbitofrontal area 47 and white matter from the ventro-medial prefrontal cortex were examined. Astrocytic processes (labeled with antibodies for aquaporin-4, AQP4 or glial fibrillary acidic protein, GFAP) were co-localized with blood vessels (labeled with an antibody to collagen IV) to measure the coverage of vessel walls by astrocyte processes.
Results
The coverage of blood vessels by endfeet of AQP4-immunoreactive (IR) astrocytes was significantly reduced by 50 percent in subjects with MDD as compared to controls (ANCOVA: F(1,23)=5.161, p=0.033). This difference was detected in orbitofrontal gray matter but not in white matter. Conversely, the coverage of vessels by GFAP-IR processes did not significantly differ between the groups.
Conclusions
A significant reduction in the coverage of gray matter vessels by AQP4-IR astrocyte processes in MDD suggests alterations in AQP4 functions such as regulation of water homeostasis, blood flow, glucose transport and metabolism, the blood brain barrier, glutamate turnover and synaptic plasticity.
Keywords: aquaporin-4, GFAP, glia, neurovascular unit, blood brain barrier, cerebrovascular disease
Introduction
According to clinical studies, depression and cerebrovascular disease influence each other (1–7). In major depressive disorder (MDD), neuroimaging studies demonstrate enlarged size and higher density of ischemic lesions in the frontal lobe (8–11). Other clinical studies postulate that the impairment of arterial endothelial function may mediate the effects of cardio- and cerebrovascular conditions in the development and progression of depressive symptoms (12–15). Despite this clinical evidence, no studies have investigated the cellular basis of the relationship between MDD and cerebrovascular disease.
One of the hallmarks of cellular pathology of MDD is a reduction in glial cells, and particularly in astrocytes. Several studies reported reduction in the density of glial fibrillary acidic protein (GFAP)-immunoreactive (IR) astrocytes, the area fraction occupied by GFAP-immunoreactivity and the level of GFAP protein in postmortem prefrontal cortex (PFC) of younger (<60) subjects with MDD as compared to non-psychiatric controls (16–19). Since astrocytes largely mediate the morphological and functional connection between the brain parenchyma and its vasculature, the astrocytic pathology reported in MDD could be related to abnormal vascular function.
Astrocytes in the central nervous system perform many important and diverse functions (20). One of these functions is the formation of the neuro-vascular unit which is composed of a neuron, an astrocyte and a blood vessel. This unit mediates the exchange of nutrients and other functional signals between these components (21, 22). Some processes within the neurovascular unit extend from the cell bodies of astrocytes and come in close association with pre-and post-synaptic structures, forming the so-called “tripartite synapses” (23). Other processes of astrocyte extend to and interact with blood vessels (25). Astrocytic endfeet are in intimate contact with the basal lamina that is a component of the vessel wall (25, 26). Astrocytic endfeet, tight junctions between endothelial cells and basal lamina together form the blood-brain barrier (BBB) (27).
Considering the central role of astrocytes in the neuro-vascular unit and the reduction of astrocyte and their markers in younger subjects with MDD (<60 years of age) (16, 17, 19), it is reasonable to hypothesize that coverage of cerebral blood vessels by astrocyte endfeet may be reduced in the PFC in MDD. To test this hypothesis, double fluorescent immunohistochemical staining was performed to visualize the co-localization of collagen IV, a well-known marker for blood vessels which labels the basal lamina around endothelial cells (28), together with glial fibrillary acidic protein (GFAP) and aquaporin-4 (AQP4), two different markers for astrocytes that label the processes of astrocytes, including the endfeet abutting the basal lamina. In contrast to GFAP, a cytoskeletal protein that is expressed in astrocyte processes and their cell bodies (25), AQP4 forms water channels that are localized predominantly in astrocytic endfeet contacting blood vessels (29, 30). Our analysis of co-localized astrocyte markers and a marker of basal lamina of blood vessels revealed reduced coverage of vessels by astrocyte processes labeled with AQP4 but not by those labeled with GFAP in subjects with MDD less than 60 years of age. Orbitofrontal cortex was examined in this study because previous work in postmortem tissue revealed astrocyte and neuronal pathology in this brain region in MDD (31, 32, 17). Moreover, neuroimaging studies report alterations in the volume (33, 34), blood flow (35) and functional activity (36, 37) in the orbitofrontal cortex in depressed patients.
Methods and Materials
Human Subjects
Post-mortem brain samples were collected at autopsy at the Cuyahoga County Coroner’s Office in Cleveland, OH, from 26 subjects (<60 years of age). Informed written consent was obtained from the legally defined next-of-kin of all subjects. Next-of-kin were interviewed and retrospective psychiatric assessments were conducted in accordance with Institutional Review Board policies (31). Thirteen subjects met clinical criteria for MDD in the last two weeks of life, and the other 13 subjects (termed normal controls) never met criteria for an Axis I diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, 38)(Table 1). A trained interviewer administered either the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) or the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) to knowledgeable next-of-kin of all subjects, as previously described (39, 40). Diagnoses for Axis I disorders were assessed independently by a clinical psychologist and a psychiatrist, and consensus diagnosis was reached in conference, using all available information from the knowledgeable informants, the coroner’s office, and any available hospitalizations and doctor’s records. Research on the psychological autopsy method has revealed that diagnoses from structured clinical interviews with family members are in good agreement with diagnoses based on reviewing the subject's medical records or the subject when living (41–43). In addition, strong inter-rater concurrence has been obtained when a structured clinical interview was used to collect information from depressed patients vs. information collected from next-of-kin (44). Responses from the subjects evaluated with the SADS-L were also recorded in the SCID, and these subjects met DSM-IV criteria for MDD using information collected with either structured diagnostic interview. Subjects were excluded from the study if symptoms based on clinical records or routine neuropathological staining revealed evidence of head trauma or neurologic disease. Toxicology assays were performed by the coroner’s office on blood and urine using gas chromatography with mass spectrometry or high performance liquid chromatography to detect the following classes of compounds: antidepressant or antipsychotic drugs, barbiturates, benzodiazepines, sympathomimetic amines, cocaine and its metabolites, opiates, phencyclidine, cannabinoids, antiepileptic drugs and ethanol (Table 1). Among 13 depressed subjects, 8 died by suicide and antidepressant drugs were detected in six subjects in toxicology screening (Table 1). The control subjects were matched with the depressed subjects for age, gender, post-mortem interval (PMI), time in freezer, and brain tissue pH (Table 1).
Table 1.
Demographic characteristics of control and MDD subjects
| Parameter | Controls (n=13) | MDD (n=13) |
|---|---|---|
| Age (years) (range) | 38.1 ± 3.1 (17–51) | 41.2 ± 2.6 (20–55) |
| PMI (hrs) (range) | 19.4 ± 1.7 (9.8–29) | 23.9 ± 2.4 (10–44) |
| pH (range) | 6.58 ± 0.06 (6.03–6.82) | 6.44 ± 0.08 (5.93–6.81) |
| TF (months) (range) |
118.8 ± 11.5 (67.5–171.5) | 122.0 ± 10.1 (70.5–172.5) |
| Gender (F:M) | 6:7 | 6:7 |
| Medication history* | none | n=7 |
| Toxicology: Clean Antidepressant drugs and other |
n=11 n=2 (CO, n=1 ; ethanol, n=1) |
n=4 n=9 (bupropion, venlafaxine, diphenhydramine, hydrocodone n=1; venlafaxine, n=2; sertraline, lidocaine, n=1; sertraline, morphine, n=1; paroxetine, cannabinoids, n=1; morphine, codeine, hydrocodone, diphenhydramine, n=1; ethanol, alprazolam, n=1; ethanol, n=1) |
| Cause of death | Cardiovascular disease, n=10; acute hemorrhagic pancreatitis, n=1; accident-house fire, n=1; acute pulmonary thromboembolism, n=1 | Suicide, n=8 (shot gun, n=3; CO poisoning, n=3; hanging, n=2); Other causes, n=5 (cardiovascular disease, n=3; accidental opiate intoxication, n=1; hyperkalemia, n=1) |
| Diagnosis | None, n=13 | MDD, n=11; MDD, alcohol abuse and Marijuana abuse, n=1; MDD and opiate dependence, n=1 |
| Duration of MDD (years) (range) |
not applicable | 9.0 ± 2.4 (0.16–25) |
| Onset of MDD (years) (range) |
not applicable | 32.1 ± 2.7 (14–48) |
| Smoking | Smokers, n=7 | Smokers, n=6 |
MDD - major depressive disorder; PMI - Post-mortem interval; TF - Time in freezer; CO - carbon monoxide.
Treatment with antidepressants within 4 weeks prior to death.
Data represent the mean ± SEM. The average age, PMI, TF and pH of MDD subjects were not statistically different from the control subjects (t-test).
Tissue Preparation
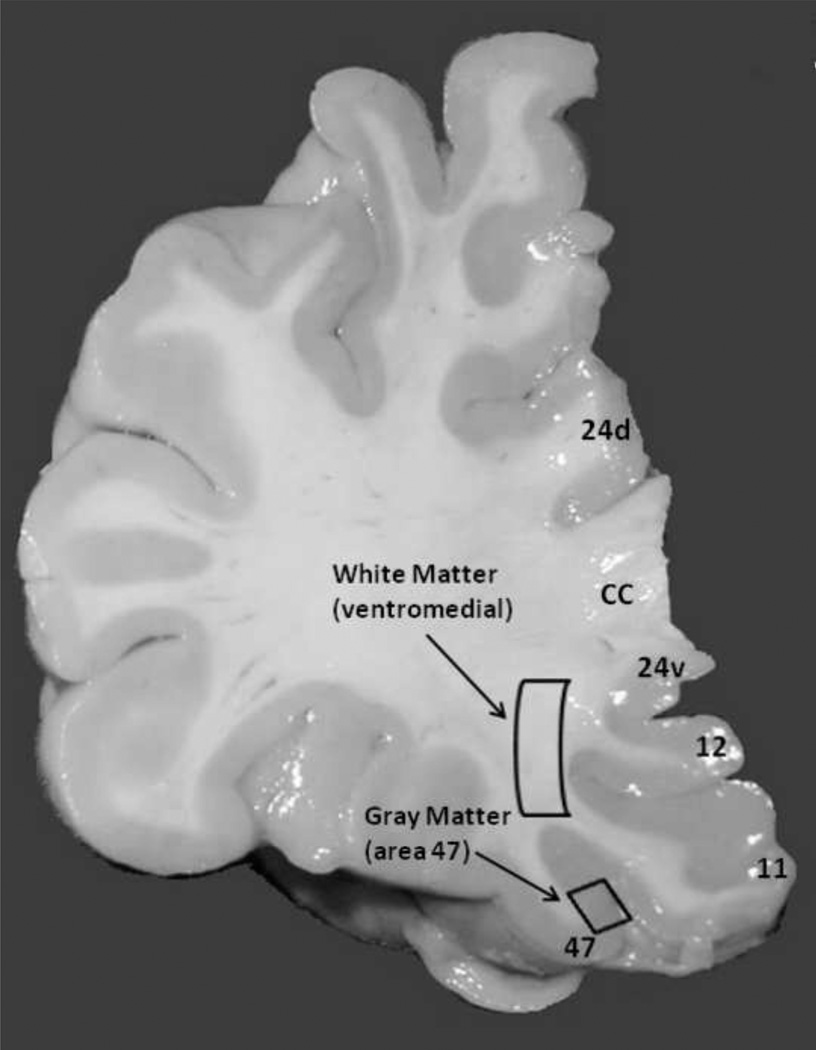
The frozen tissue blocks containing the ventral half of the left PFC (Fig. 1) were sectioned on a cryostat at 40 µm and stained for Nissl substance to identify cytoarchitectonic features of Brodmann area 47 of the orbitofrontal cortex (45) and underlying ventro-medial prefrontal white matter (see Supplementary Methods). Immediately adjacent sections were cut at 20 µm and used for double immunofluorescent staining.
Fig. 1.
Microphotograph of coronal section from the prefrontal cortex. The small rectangle indicates a part of gray matter of area 47 which was analyzed in this study. The second region examined in the study (larger rectangle) is located within the ventro-medial prefrontal white matter. Numbers indicate Brodmann areas. Cc, corpus callosum; d, dorsal, v, ventral.
Double Immunofluorescence
Frozen sections were fixed for 30 minutes in 4% paraformaldehyde in phosphate buffer saline (PBS, pH 7.4) and then washed in PBS followed by Tris-HCl buffered saline (TBS, pH 7.6). Subsequently, the sections were pre-incubated in normal 2% bovine serum in PBS for 30 min and incubated overnight in the same solution containing either a mixture of rabbit polyclonal anti-GFAP (1:1000, Sigma) and mouse monoclonal anti-Collagen IV (1:500, Sigma) antibody or with rabbit polyclonal anti-AQP4 (1:100, Sigma) and mouse monoclonal anti-Collagen IV (1:500, Sigma) antibody. After washing in TBS, sections were incubated for 90 min with a mixture of secondary anti-rabbit Cy5-conjugated and anti-mouse FITC-conjugated antibody (1:500). Sections were washed in TBS and incubated with an autofluorescence eliminator reagent (Millipore) for 5 min followed by washes in 75% ethanol. To minimize variability in the intensity of staining, sections from depressed and control subjects were processed in a yoked manner. Three coronal sections per subject were used to analyze coverage of vessels by astrocytic processes.
Coverage Analysis
Images of gray matter of area 47 and ventro-medial prefrontal white matter (Fig. 1) as characterized in Nissl stained sections were imposed on adjacent, immunostained sections to determine the borders for fluorescent image acquisition. Images (10–14 images/slide, through all layers of the gray matter of area 47 and an additional 10–14 images across underlying ventro-medial prefrontal white matter (see Supplementary Methods) were captured using a Nikon C1 confocal microscope with a 40× oil-immersion objective (1.0 NA) and with individual image frames measuring 175µm × 175µm. Images were converted from the existing proprietary file format (.ids) into tagged image file format (.tiff) files, for processing by Adobe Photoshop CS5 Extended (version 12.0.4, 64-bit, Adobe Systems, Inc.). Two (.tiff) files were produced for each image taken: one containing the appropriate color channel for identification of blood vessels (Collagen IV, green) and another containing the merge of the appropriate two channels for the identification of co-localization of astrocytic processes (AQP4 or GFAP, red) and blood vessels (green) (Fig. 2). In this merged file, green-only pixels corresponded to vessel portions immunolabeled only for collagen IV (Fig. 2A, D) and red-only pixels were considered to correspond to structures only labeled for AQP4 immunoreactivity (or with GFAP immunoreactivity) (Fig. 2B,E). Structures within a range of yellow and orange hue were deemed representing co-localization or very close apposition of AQP4 (or GFAP) and collagen IV immunolabeling Figs. 2C, F). Measurement of the area occupied by collagen IV-green immunolabeling (in the green-only file) and the area of co-localized immunolabeling (in the merged file) was performed as follows. For the identification of blood vessels, pixels green in hue (only detectable in the green channel) were isolated from the one-channel-only image whereas for the identification of areas of co-localization of the merged image, only pixels yellow to orange in hue were isolated. To ensure consistency between images, a reference color range was defined from a standardized color palette generated with the Photoshop software at the beginning of the project and used unchanged by the program during the processing of each image file. Once isolated, these processed images were converted to gray scale format and the area occupied by all the selected pixels was measured using ImageJ (version 1.45I, 64-bit, National Institutes of Health, USA). The final data are presented as a ratio of the area of yellow-orange pixels (representing co-localization and apposition of astrocytic GFAP or AQP4 and vessel collagen, Fig. 2, Supplemental Figures S1 and S2) divided by the total area of vessels, that is, area of co-localization/total area of vessels. “Coverage” is defined as this ratio.
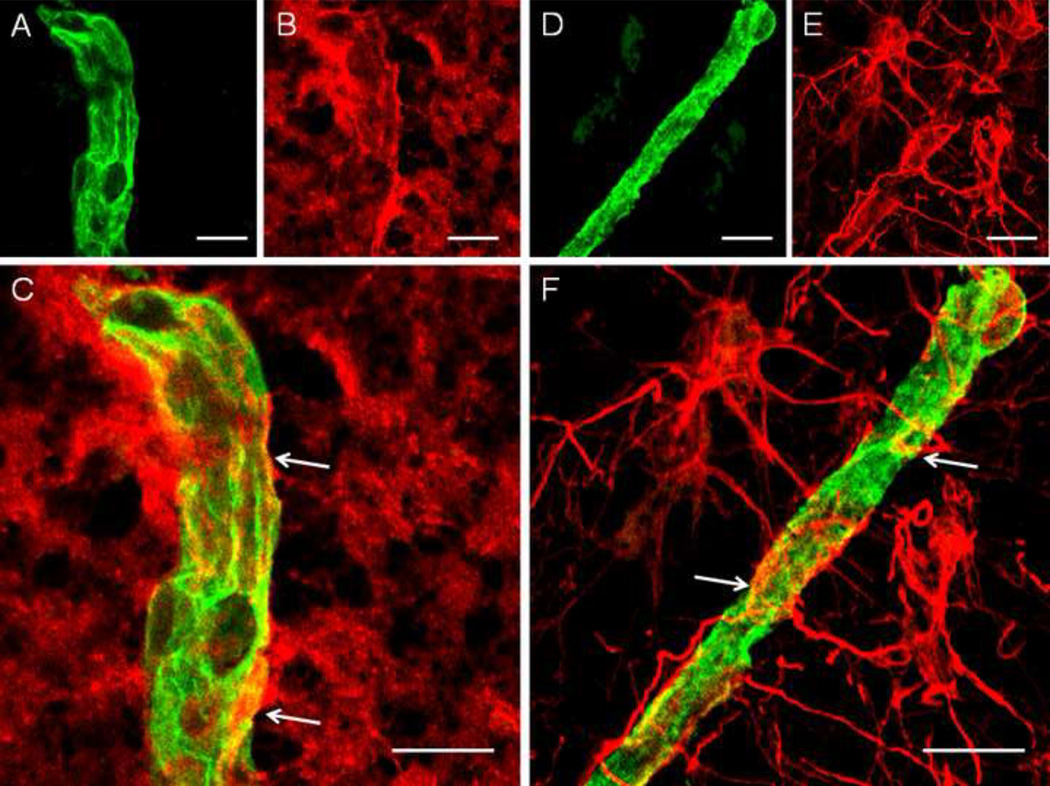
Fig. 2.
High power confocal images of immmunofluorescent staining of blood vessels with an antibody to collagen IV (green in A, D), astrocytes with an antibody to aquaporin-4 (red in B), and astrocytes with an antibody to GFAP (red in E) in a non-psychiatric control subject. Pictures (C) and (F) represent merged images of collagen IV (A) and AQP4 (B) immunoreactivity, whereas image (F) is a merged image of pictures of collagen IV (D) and GFAP (E) immunoreactivity. Yellow–to–orange colors on images (C) and (F) represent coverage of vessels by astrocytic AQP4 (C) or GFAP (F) immunoreactivity. White arrows on (C) and (F) indicate examples of contact between immunoreactive astrocytic processes and vessel collagen IV. Note that AQP4 immunoreactivity (red on pictures B, C) is more diffuse within astrocytic processes, whereas GFAP is more concentrated in astrocytic processes and these processes can be traced to GFAP-immunoreactive cell bodies (red on E, F). Scale bars on pictures (A), (B), (C), (D), (E) and (F) = 10µm. AQP4, aquaporin-4; GFAP, glial fibrillary acidic protein.
Statistical Analyses
Mean values for coverage were obtained from 10–14 images per area of interest, per section and from three sections per subject. Coverage values were then compared between the diagnostic groups using an unpaired t-test. Pearson correlation analysis was used to assess the influence on coverage of potentially confounding factors such as age, PMI, tissue pH, time in freezer, age at onset of depression and duration of depression. Analysis of covariance (ANCOVA) was performed (IBM SPSS, version 19.0) when significant correlations were noted between dependent variables and any potentially confounding variables.
Results
Gray Matter
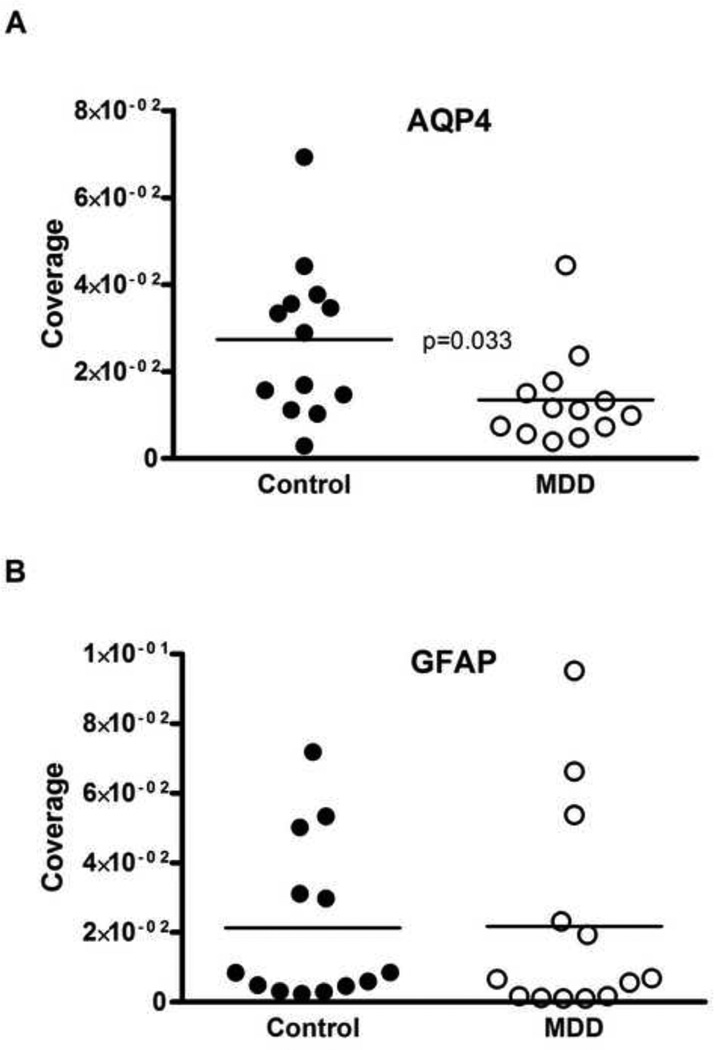
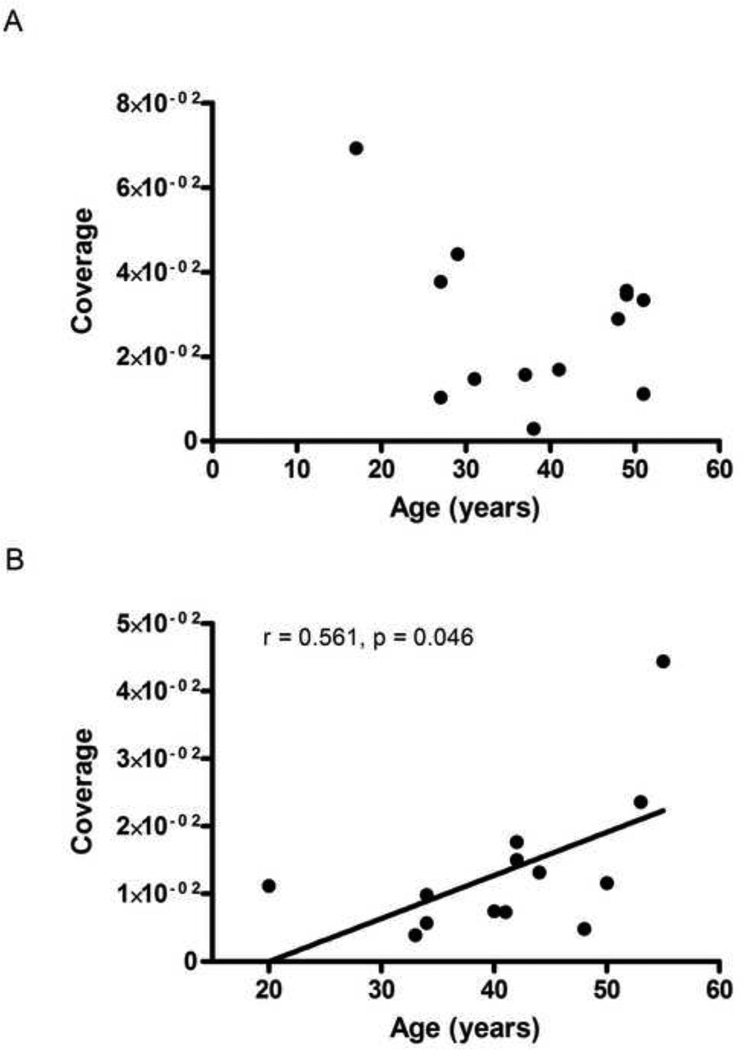
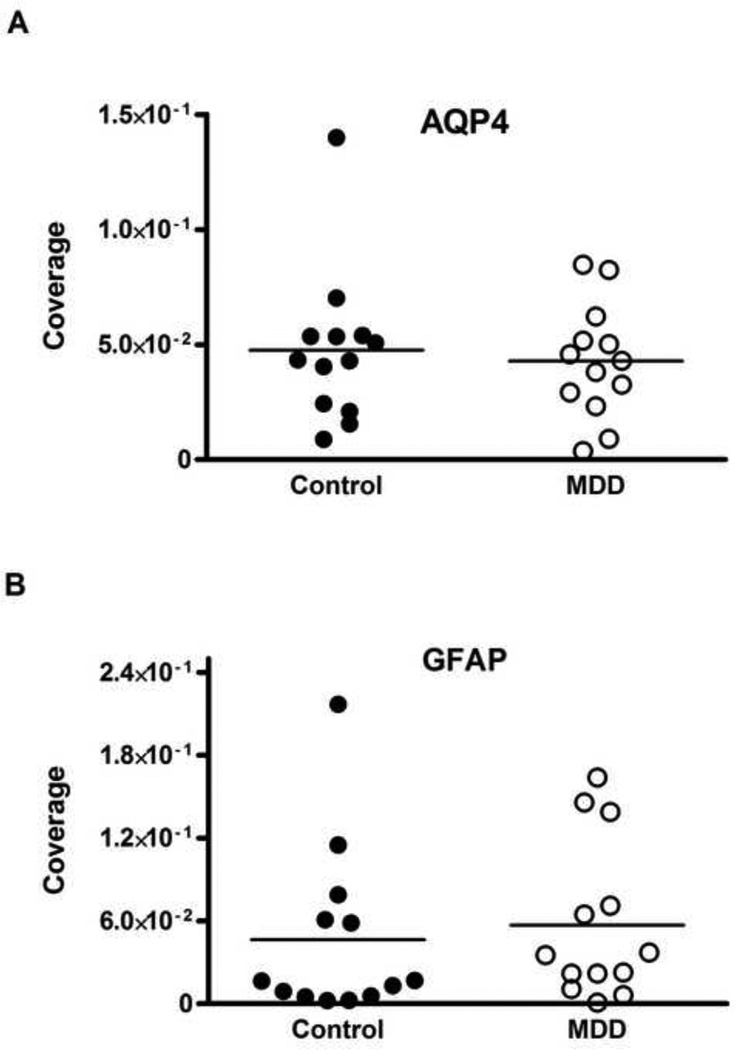
The coverage of collagen IV-IR vessels by AQP4-IR astrocyte processes was significantly reduced by 50 percent in MDD (1.35 ± 0.3 × 10−2) as compared to the controls (2.73 ± 0.49 × 10−2; t-test, t=2.378, df=24, p=0.026; Fig. 3A). Pearson correlation analyses revealed a significant, positive correlation between coverage and the age at the time of death but only in MDD group (r=0.561, p=0.046; Fig. 4). The analysis of covariance revealed that the results were not significantly influenced by age (ANCOVA with age as covariate, F(1,23)=5.161, p=0.033). However, no association between the coverage values and PMI, brain pH or storage time in freezer was detected. Similarly, there was no correlation between coverage and the age at onset and duration of depression. Moreover, there were no differences in coverage between MDD subjects with or without antidepressants in postmortem toxicology or between MDD subjects dying by suicide or not. Likewise, there was no difference between MDD females and MDD males (see Supplemental Table S1).
Fig. 3.
Scattered plots comparing coverage of vessels by AQP4 immunoreactivity (A) and GFAP immunoreactivity (B) measured in the gray matter of Brodmann area 47 (small rectangle on Fig. 1). There was a significant 50% reduction (t=2.378, df=24, p=0.026) in the coverage of vessels by AQP4 in subjects with MDD as compared to control subjects (A). The coverage of vessels measured with GFAP immunoreactivity did not significantly differ between the groups (B). “Coverage” represents the co-localization of immunoreactivity for astrocytic GFAP or AQP4 and vessel collagen IV (see Figs. 2C, F). AQP4, aquaporin-4; GFAP, glial fibrillary acidic protein; MDD, major depressive disorder.
Fig. 4.
The relationship between AQP4 coverage and age at the time of death in control (A) and MDD (B) subjects. There was a significant positive correlation (r=0.561, p=0.046) between coverage and age in the MDD group (B).
The coverage of collagen IV-IR vessels by GFAP-IR astrocyte processes in MDD (2.18 ± 0.85 × 10−2) was not significantly different from that in control subjects (2.13 ± 0.66 × 10−2; t-test, t=0.046, df=24, p=0.96; Fig. 3B). Correlation analysis revealed a significant negative association between GFAP-defined coverage and freezer storage time only in the control group (r = −0.623, p=0.023). Other variables such as age, pH, PMI, age at onset or duration of depression were not significantly correlated with GFAP coverage in either main cohort.
White Matter
Unlike the orbitofrontal cortex, there was no significant difference in white matter in the coverage of collagen IV-IR vessels by AQP4-IR astrocyte processes when comparing MDD (4.28±0.7 × 10−2) and control groups (4.76±0.9 × 10−2 ; t-test, t=0.54, df=12, p=0.595; Fig. 5A). There was also no significant correlation between the coverage values in control or MDD groups and PMI, brain pH or storage time in freezer.
Fig. 5.
Scatter plots comparing coverage of vessels by AQP4 (A) and GFAP (B) immunoreactivity measured in the ventromedial prefrontal white matter (larger rectangle on Fig. 1). In contrast to gray matter, in the white matter there were no significant differences in the coverage of vessels by (A) AQP4 (t=0.54, df=12, p=0.595) or (B) GFAP (t=1.13, df=12, p=0.279) immunoreactivity between subjects with MDD and control subjects. “Coverage” represents the co-localization of immunoreactivity for astrocytic GFAP or AQP4 and vessel collagen IV (see Fig. 2C, F). MDD, major depressive disorder.
The coverage of collagen IV-IR vessels by GFAP-IR astrocyte processes in MDD (5.71 ± 1.58 × 10−2) was not significantly different from that in controls (4.63 ± 1.73 × 10−2 ; t-test, t=1.13, df=12, p=0.279; Fig. 5B). A significant negative correlation in the control group was detected between the coverage values and storage time in freezer (r=−0.63, p=0.021).
Discussion
The coverage of blood vessels by astrocytic perivascular processes was significantly reduced by 50 percent in subjects with MDD as compared to non-psychiatric control subjects. This reduction was significant only in the case of astrocyte processes immunoreactive for AQP4 but not for GFAP. The difference between MDD and control subjects in AQP4 coverage was detected only in orbitofrontal gray matter and not in the underlying ventro-medial prefrontal white matter. The part of the orbitofrontal cortex where reduced coverage of vessels by AQP4 was observed corresponds to the region in which neuronal and glial pathology was reported in MDD (17, 31, 32). The coverage of vessels by AQP4 immunoreactive processes was not influenced by PMI, brain pH or storage time in freezer as there was no significant correlation between the coverage values and these potentially confounding variables. The present study although quantitative is not a stereological study as access to the entire orbitofrontal cortex was not available.
Aquaporin-4 is a key molecule for maintaining water and ion homeostasis associated with neuronal activity, and it is the predominant water channel in the CNS. This channel is expressed primarily in the endfeet of astrocytes (29, 30). GFAP, unlike AQP4, is very abundant along both thick and thin astrocytic processes and is also found in their endfeet and cell bodies (Figs. 2, 3). Thus, our observation of reduced coverage of vessels by AQP4-IR processes but not by GFAP-IR processes around vessels suggests a specific reduction of AQP4-containing astrocytic endfeet that are in direct contact with blood vessels. To our knowledge, this is the first study in depression showing a link between dysfunctional astrocytes and cortical blood vessels.
The absence of a significant difference between control and depressed groups in the GFAP coverage of vessels may be related to the relative ubiquity of GFAP-IR structures. Many astrocytic processes, and not just those forming functional endfeet, have a high likelihood of being in contact with or intruding into the basal lamina of vessels. With the analysis used in the present study, the coverage of vessels by these non-endfeet contacts was not distinguishable from actual endfeet coverage, and thus may have resulted in undetectable differences between the cohorts for GFAP processes surrounding vessels. In addition, while endfeet ending on relatively large blood vessels are both AQP4-IR and GFAP-IR, endfeet contacting capillaries are only AQP4-IR (46). The reduction of AQP4-IR coverage of vessels then would indicate a specific deficit on the ability of astrocytes to exchange metabolites or water in depressed subjects.
The present findings may have relevance to the role of stress and antidepressant treatment in the pathophysiology of depression. Animal studies reveal that stress decreases glial cell proliferation in the hippocampus and prefrontal cortex and the effects of stress are reversed by antidepressant treatment (47–49). In addition, AQP4 plays a role in regulating adult neurogenesis (50). A recent mice study reported that AQP4 knockout suppresses fluoxetine-induced enhancement of adult hippocampal neurogenesis (51) thus suggesting AQP4 and astrocytes as new cellular targets for the mechanism of action of antidepressant medications. Another line of evidence links glucocorticoids to decreased numbers of astrocytes in mouse hippocampus and prefrontal cortex (52). Glucocorticoids decreased the number of astrocytes by reducing expression of glucocorticoid receptors that are located on astrocytic nuclei. Human postmortem studies have shown reduction in the expression of glucocorticoid receptors (53) and markers of astrocytes (16, 17, 19) in the prefrontal cortex in subjects with MDD. Thus, one may speculate that an enhanced stress response (which is a risk factor for depression) may lead to a reduction of astrocytes and their AQP4-bearing processes around vessels.
Another study reports a decrease in the expression of mineralocorticoid receptors and their splice variants in the prefrontal cortex in MDD (53). This could be of relevance to the present study as mineralocorticoid receptors are expressed by astrocytes and contribute to stress-related HPA axis disturbances observed in depressed subjects 54, 55). Mineralocorticoids also maintain salt metabolism (56) thereby suggesting a link between a deficiency in AQP4 immunoreactivity and possible alterations in water and ion homeostasis in depression. As AQP4 is a key molecule for maintaining water homeostasis, one may speculate that alterations in water metabolism could underlie some of the volume changes and cortical thinning noted in the orbitofrontal cortex in depression (34, 31).
In the present study, there was a significant positive correlation between coverage of vessels by AQP4-IR processes and age in only MDD subjects. Thus, the older the subjects the more coverage is found on vasculature. Interestingly, our previous studies showed that even if the area fraction of GFAP-IR astrocyte processes and GFAP levels are low in relatively younger MDD subjects (<60 years old) as compared to controls, those parameters increase with age in depressed subjects to reach levels comparable to non-psychiatric control subjects (16, 19). Thus, it is possible that with increasing age there is an activation of astrocytes that results in a secondary increase in the expression of AQP4 within astrocytic endfeet. This activation could be a compensatory mechanism or a response in AQP4 coverage of capillaries to the decrease in neuronal density with advancing age in MDD (32). However, the exact functional significance of an age-dependent increase in astrocytic AQP4 remains to be elucidated.
In the ventro-medial prefrontal white matter, in contrast to orbitofrontal cortex, no significant differences were noted between MDD and control groups in the coverage of vessels by AQP4-IR endfeet or GFAP-IR endfeet. It is unclear why changes in AQP4-IR coverage of vessels in MDD are specific to the gray matter. However, gray matter, as opposed to white matter, may be more sensitive to synaptic and connectional plasticity. In contrast to gray matter, white matter has very few synapses (57, 58) and some functions of white matter astrocytes are also different from those of gray matter.
In the control group only, a negative correlation was noted between storage time of tissue in the freezer and coverage of vessels by GFAP immunoreactivity. Thus, it is possible that with longer time in freezer there is a reduction in GFAP immunoreactivity. On the other hand, the main result, that there are no differences in GFAP coverage between control and MDD groups remains the same whether or not co-varying for time in the freezer.
In light of the functions of astrocytic endfeet and the role of AQP4, a reduction in the coverage of blood vessels by endfeet and processes bearing AQP4 might have one or several functional consequences. A decrease in the number or extent of the endfeet per se might reduce the effective surface for exchange with the blood circulation and directly affect the regulation of cerebral blood flow during neuronal activity (21, 24, 60, 61). Astrocytes express receptors for many neurotransmitters and activation of these receptors can provoke oscillations in intracellular Ca+2. Altered Ca+2 flux in astrocytic endfeet contributes to vasodilation or vasoconstriction (21, 24). In mice, experimentally induced increases in the concentration of intracellular Ca+2 in astrocytic endfeet abutting the vessel wall were associated with an 18 percent increase in arterial cross-section area that also corresponded to a 37 percent increase in cerebral blood flow (26). Thus, a decrease in the cerebral blood flow observed in the PFC in depressed patients (62–65, 35) may be related to reductions in astrocyte density and markers reported in postmortem tissues in MDD (16, 17, 19) and to reduced coverage of vessels by astrocyte endfeet demonstrated here in MDD.
Astrocytic processes also make blood-born glucose and its high energy metabolites available to neurons (reviewed in 66). In astrocytes, glucose undergoes the process of glycolysis and oxidative phosphorylation, processes that are believed to provide the observed signal in functional magnetic resonance imaging and positron emission tomography (67–69). Thus, reduced coverage of vessels by astrocytic processes and their endfeet observed here in MDD maybe related to reductions in glucose metabolism reported by neuroimaging studies in the PFC of depressed patients (70–72). It remains to be determined if AQP4 is crucially involved in these live images as water transport through astrocytic AQP4 channels and glucose transport into the brain parenchyma are functionally connected (73).
Another consequence of reduced coverage of vessels by astrocytic endfeet in MDD may be impairment of the BBB in this disorder. The structures responsible for the interaction between astrocytes and endothelial cells are the astrocytic endfeet located in intimate contact with the basal lamina that is the part of the vessel wall (74, 26). The combination of astrocytic endfeet, tight junctions between endothelial cells and basal lamina together form the BBB (27). Since astrocyte endfeet are a major structural component of the BBB, decreased coverage of vessel walls by astrocytic endfeet labeled with AQP4 in MDD might be a result of or a contributor to morpho-functional impairment of the BBB. AQP4 helps maintain the BBB by distributing water, serving as an ion transporter, and assists in the maturation and integrity of the BBB (75, 76). Interestingly, in the AQP4 knockout mouse, there is increased permeability of the BBB and altered ultrastructure of tight junctions of brain microvessels and swollen perivascular astrocytic endfeet (77).
Finally, AQP4, a predominant water channel in the adult CNS (29), has other vital functions in addition to the well-known regulation of extravascular brain water and brain volume homeostasis (78). An AQP4 deficiency in AQP4 knockout mice alters basal amino acid and monoamine metabolism (79), glutamate turnover (80) and impairs synaptic plasticity (81).
There are several limitations to the present study. A relatively small number of depressed subjects were examined and only one prefrontal cortical region was examined. Only a subset of astrocytes with AQP4 localized to the endfeet wrapping blood vessels showed changes in MDD and therefore conclusions about a general diminished function of astrocyte must be interpreted with caution. The results presented in this paper only correlate depression with changes in coverage of blood vessels by astrocytic processes expressing AQP4. Further studies are needed to determine whether the observations regarding AQP4 are a cause or a consequences of depression. A potential influence of antidepressant medications on AQP4-IR coverage of blood vessels cannot be unequivocally ruled out as an antidepressant medication(s) was present in six of the 13 subjects with MDD. However, it is unlikely that these different medications would all have the same effect on the coverage of vessels by AQP4-IR in depression. In addition, two depressed subjects also met criteria for a psychoactive substance use disorder; although it is not likely that comorbidity had a significant effect on the main finding.
In summary, reduced coverage of blood vessels by AQP4-IR astrocyte processes in the orbitofrontal cortex in MDD suggests dysfunction of the neuro-vascular unit that may lead to dysregulation of cerebral blood flow, glucose transport and metabolism, impairment of the BBB, alterations in levels of monoamines, glutamate turnover and impairment in synaptic plasticity. Future studies in animal models related to depression and in cell culture should provide better insights into the functional consequences of reduced coverage of vessels by astrocytes. Nonetheless, the present study further supports the link between cerebrovascular pathology and MDD by providing the first evidence for such a link at the cellular level. It also reveals that AQP4 is involved in the pathology of MDD in addition to its well-known role in other pathological conditions such as brain edema, ischemia, hypoxia, multiple sclerosis and epilepsy.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Drs. James C. Overholser, George Jurjus, and Lisa Konick in establishing the psychiatric diagnoses and in collecting tissues. We thank the Cuyahoga County Coroner’s Office, Cleveland, OH, and the next-of-kin of our subjects for their participation and support. We acknowledge Gillian O’Dwyer, Yillianys Pride and Joan Dickerson for their excellent technical assistance. This study was supported by NIH grant RR17701 and MH67996.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Carney RM, Freedland KE, Jaffe AS. Depression as a risk factor for coronary heart disease mortality. Arch Gen Psychiatry. 2001;58:229–230. doi: 10.1001/archpsyc.58.3.229. [DOI] [PubMed] [Google Scholar]
- 2.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan KR. Comorbidity of late life depression: an opportunity for research on mechanisms and treatment. Biol Psychiatry. 2002;52:543–558. doi: 10.1016/s0006-3223(02)01468-3. [DOI] [PubMed] [Google Scholar]
- 4.Vataja R, Pohjasvaara T, Mäntylä R, Ylikoski R, Leskelä M, Kalska H, et al. Depression-executive dysfunction syndrome in stroke patients. Am J Geriatr Psychiatry. 2005;13:99–107. doi: 10.1176/appi.ajgp.13.2.99. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Treisman GJ, Lipsey JR, Morris PL, Robinson RG. Does stroke cause depression? J Neuropsychiatry Clin Neurosci. 1998;10:103–107. doi: 10.1176/jnp.10.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Wager-Smith K, Markou A. Depression: A repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci Biobehav Rev. 2011;35:742–764. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CQ, Dong BR, Lu ZC, Yue JR, Liu QX. Chronic diseases and risk for depression in old age: A meta-analysis of published literature. Ageing Res Rev. 2010;9:131–141. doi: 10.1016/j.arr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan KR. Depression as a contributing factor in cerebrovascular disease. Am Heart J. 2000;140:70–76. doi: 10.1067/mhj.2000.109980. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan K, Hays J, Blazer D. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–198. doi: 10.1016/s0002-9149(01)01623-x. 2001. [DOI] [PubMed] [Google Scholar]
- 12.Rybakowski JK, Wykretowicz A, Heymann-Szlachcinska A, Wysocki H. Impairment of Endothelial Function in Unipolar and Bipolar Depression. Biol Psychiatry. 2006;60:889–891. doi: 10.1016/j.biopsych.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 14.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–659. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 17.Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, Torrey EF, Yolken RH. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry. 2000;5:142–149. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 19.Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 22.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 23.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosc. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 27.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 28.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis research. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Pittman SD, Dilley G, Overholser J, Meltzer H, Stockmeier C. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 32.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan RR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol. Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 34.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 35.Nagafusa Y, Okamoto N, Sakamoto K, Yamashita F, Kawaguchi A, Higuchi T, Matsuda H. Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. J Affect Disord. 2012;140:296–299. doi: 10.1016/j.jad.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, Sugar CA, Altshuler LL. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res. 2010;183:209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, Matuskey D, Ruf B, Saricicek A, Wang F, Pearlson GD, Phillips ML, Blumberg HP. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202:30–37. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 39.Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia (SADS) 3rd ed. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- 40.First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatr Clin North Am. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- 41.Deep-Soboslay A, Akil M, Martin C, Bigelow L, Herman M, Hyde T, Kleinman J. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: A comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 43.DeJong TM, Overholser JC, Stockmeier CA. Apples to oranges?: a direct comparison between suicide attempters and suicide completers. J Affect Disord. 2010;124:90–97. doi: 10.1016/j.jad.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGirr A, Renaud J, Seguin M, Alda M, Benkelfat C, Lesage A, Turecki G. An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. J Affect Disord. 2007;97:203–209. doi: 10.1016/j.jad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Uylings HB, *, Sanz –Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G., * 3-D cytoarchitectonic parcellation of human orbitofrontal cortex. Correlation with postmortem MRI, *Both authors contributed equally. Psychiatry Research: Neuroimaging. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 48.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Zheng GQ, Li Y, Gu Y, Chen XM, Zhou Y, Zhao SZ, et al. Beyond water channel: aquaporin-4 in adult neurogenesis. Neurochem Int. 2010;56:651–654. doi: 10.1016/j.neuint.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G. Requirement AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263–1276. doi: 10.1038/npp.2008.185. [DOI] [PubMed] [Google Scholar]
- 52.Unemura K, Kume T, Kondo M, Maeda Y, Izumi Y, Akaike A. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J Pharmacol Sci. 2012;119:30–39. doi: 10.1254/jphs.12047fp. [DOI] [PubMed] [Google Scholar]
- 53.Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, et al. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 55.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch Gen Psychiatry. 2003;60:24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- 56.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 57.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 58.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh J, Tabunoki H, Yamamura T, Arima K, Konno H. Human astrocytes express aquaporin-1 and aquaporin-4 in vitro and in vivo. Neuropathology. 2007;27:245–256. doi: 10.1111/j.1440-1789.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 60.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 62.Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 63.Dotson VM, Beason-Held L, Kraut MA, Resnick SM. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int J Geriatr Psychiatry. 2009;24:809–819. doi: 10.1002/gps.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, Gangwani S, et al. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2011;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monkul ES, Silva LA, Narayana S, Peluso MA, Zamarripa F, Nery FG, et al. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a (15)O-H(2)O PET study. Hum Brain Mapp. 2012;33:272–279. doi: 10.1002/hbm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;35:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raichle ME. Cognitive neuroscience. Bold insights. Nature. 2001;412:128–130. doi: 10.1038/35084300. 2001. [DOI] [PubMed] [Google Scholar]
- 69.Rossi DJ. Another BOLD role for astrocytes: coupling blood flow to neural activity. Nat Neurosci. 2006;9:159–161. doi: 10.1038/nn0206-159. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 71.Hurwitz TA, Clark C, Murphy E, Klonoff H, Martin WR, Pate BD. Regional cerebral glucose metabolism in major depressive disorder. Can J Psychiatry. 1990;35:684–688. doi: 10.1177/070674379003500807. [DOI] [PubMed] [Google Scholar]
- 72.Martinot JL, Hardy P, Feline A, Huret JD, Mazoyer B, Attar-Levy D, et al. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry. 1990;147:1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- 73.Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129:851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 74.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meshorer E, Biton IE, Ben-Shaul Y, Ben-Ari S, Assaf Y, Soreq H, et al. Chronic cholinergic imbalances promote brain diffusion and transport abnormalities. FASEB J. 2005;19:910–922. doi: 10.1096/fj.04-2957com. [DOI] [PubMed] [Google Scholar]
- 76.Nico B, Frigeri A, Nicchia GP, Quondamatteo F, Herken R, Errede M, Ribatti D, et al. Role of aquaporin-4 water channel in the development and integrity of the blood-brain barrier. J Cell Sci. 2001;114:1297–1307. doi: 10.1242/jcs.114.7.1297. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Kong H, Hua X, Xiao M, Ding J, Hu G. Altered blood-brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport. 2008;19:1–5. doi: 10.1097/WNR.0b013e3282f2b4eb. [DOI] [PubMed] [Google Scholar]
- 78.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 79.Fan Y, Zhang J, Sun XL, Gao L, Zeng XN, Ding JH, et al. Sex- and region-specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin-4 knockout mice. J Neurosci Res. 2005;82:458–464. doi: 10.1002/jnr.20664. [DOI] [PubMed] [Google Scholar]
- 80.Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci. 2007;34:34–39. doi: 10.1016/j.mcn.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Li YK, Wang F, Wang W, Luo Y, Wu PF, Xiao JL, et al. Aquaporin-4 Deficiency Impairs Synaptic Plasticity and Associative Fear Memory in the Lateral Amygdala: Involvement of Downregulation of Glutamate Transporter-1 Expression. Neuropsychopharmacology. 2012;37:1867–1878. doi: 10.1038/npp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.