Abstract
Background & Aims
In intestinal inflammation the gut microbiota induces an innate immune response by activating epithelial and immune cells that initiate or maintain inflammation. We investigated whether the microbiota can also activate local microvascular cells and induce angiogenesis.
Methods
Human intestinal microvascular endothelial cells (HIMEC) and intestinal fibroblasts (HIF) were exposed to bacterial ligands specific for TLR2/6 and 4, and NOD1 and NOD2, and cell proliferation, migration, transmigration, tube formation and production of pro-angiogenic factors were measured. The ability of the ligands to induce ex vivo vessel sprouting in an aortic ring assay and in vivo angiogenesis using a collagen gel assay were also assessed.
Results
Bacterial ligands induced proliferation, migration, transmigration, tube formation of HIMEC, vessel sprouting and in vivo angiogenesis; they also stimulated production of angiogenic factors from HIMEC and HIF, and HIF-derived angiogenic factors promoted HIMEC proliferation. To various degrees, all ligands induced angiogenic responses, but these were ligand- and cell type-dependent. Responses were mediated through RIP2-and TRAF6-dependent signaling, involved the MAPK and NF-κB pathways and the upregulation of VEGF-R2 and FAK. Knockdown of RIP2 and TRAF6 by RNA interference and neutralization of IL-8, bFGF and VEGF inhibited TLR/NLR-induced HIMEC angiogenesis.
Conclusions
The gut microbiota can selectively activate mucosal endothelial and mesenchymal cells to promote specific angiogenic responses in a TLR- and NLR-dependent fashion. This innate immunity-mediated response may expand the mucosal microvascular network, foster immune cell recruitment, and contribute to chronic intestinal inflammation.
Keywords: angiogenesis, IBD, TLR, NLR
INTRODUCTION
Health depends on a mutually beneficial interaction between the host and its microbiota. It is increasingly evident that the commensal gut microbiota is involved not only in the development of systemic immunity1, but also the pathogenesis of various immune-mediated conditions like type 1 diabetes, cardiovascular, neurologic and liver diseases2–5. This is also true in inflammatory bowel disease (IBD), where microbial products play a role in initiation or maintenance of inflammation by modulating the function of epithelial and immune cells6. Non-immune cells, however, also contribute to IBD pathogenesis, such as endothelial and mesenchymal cells7, but whether and how microbial factors modulate their function is poorly understood.
Microbes are recognized by Toll-like (TLR) and NOD-like (NLR) receptors widely expressed by multiple cell types, leading to physiologic or pathological responses. In the intestine commensal bacteria can trigger inflammation in a host with a disrupted epithelial barrier8,9, resulting in translocation and activation of cells in the mucosa. While the consequences of TLR and NLR activation on epithelial and immune cells have been extensively investigated10,11, little information is available on the effect of microbial products on non-immune cells, particularly mucosal endothelial cells.
Microbial products may contribute to disease pathogenesis by affecting endothelial cell function in conditions such as atherosclerosis and liver diseases12,13. This effect is mediated by specific ligands for TLRs or NLRs on endothelial cells14,15, and subsequent triggering of angiogenesis, as reported in rheumatoid arthritis16. During postnatal development, intestinal angiogenesis accompanies the acquisition of gut microbiota17, but there is no information on whether activation of mature mucosal endothelial cells through TLRs/NLRs promotes angiogenesis, a component of human and experimental IBD18,19.
Angiogenesis is a complex process involving not only endothelial cells but also mesenchymal cells, which are in close proximity of the microvasculature20. Fibroblasts release various pro-angiogenic factors upon cytokine stimulation18 and express functional TLRs and NLRs21, but whether activation of these microbial receptors also induces release of such factors is unknown.
We investigated whether bacterial ligands induce angiogenesis by activating intestinal mucosa microvascular and mesenchymal cells. The results show that specific ligand-mediated activation of TLR2/6, TLR4, NOD1 and NOD2, which recognize a broad range of Gram-positive and -negative bacteria, promotes an angiogenic response mediated by a direct stimulatory effect on endothelial cells and an indirect effect on mesenchymal cells leading to production of pro-angiogenic factors.
MATERIALS AND METHODS
Transfections
HIMEC were transfected with RIP2 or TRAF6 siRNA and their respective scrambled siRNA (both from Thermo Scientific, Lafayette, CO) using the Amaxa system (Amaxa Biosystems, Köln, Germany). HIMEC (0.5×106) were suspended in 100 μl nucleofector solution for endothelial cells (Lonza, Walkersville, MD), mixed with different volumes of 20 nmol RIP2, TRAF6 or scrambled siRNA, transferred to a cuvette and nucleofected according the manufacturer's instructions. Cells were then transferred into 6-well plates containing pre-warmed culture medium. Some cells were left in nucleofector solution without applying electroporation to serve as negative control; 48 h after electroporation cells were used for the transmigration and Matrigel™ assays. Optimal doses and time points for optimal RIP2 and TRAF6 knockdown were pre-determined by immunoblot using antibodies against RIP2 (Alexis, San Diego, CA) and TRAF6 (Santa Cruz, Santa Cruz, CA), respectively (see supplemental materials).
HEK293T cells were transfected using Polyfect (Qiagen, Valencia, CA) according to the manufacturer's instructions. Briefly, a transfection mix comprised of 0.25 μg pκB-EGFP (gift of Xin Lin, University of Texas)22,15 ng pcDNA3-HA-Nod1 (gift of Gabriel Nuñez, University of Michigan23, 1.75 μg pcDNA3 and 12 μL Polyfect was added to 2×105 HEK293T cells in a 6 well plate. Medium was changed to fresh DMEM with 10% fetal bovine serum (FBS) after 5h and cells used for in vitro Matrigel™ assays 48 h post-transfection.
Mouse aortic ring assay
Rings of mouse aorta were cultured in three-dimensional collagen gels as described24. Tie2-green fluorescent protein (GFP) mice (Jackson Laboratories, Bar Harbor, ME) expressing GFP exclusively in endothelial cells were sacrificed by CO2 inhalation, and aortas dissected and transferred to ice-cold minimum essential medium (MEM, Sigma-Aldrich, St. Louis, MO) containing 1% pen/strep/fungizone (PSF, Lonza). The peri-aortic fibroadipose tissue was carefully removed and 1 mm long aortic rings were sectioned and rinsed in MEM. Ring-shaped explants of mouse aorta were then embedded in 300 μl of a mixture of growth factor-depleted Matrigel™ (BD Biosciences, Bedford, MA) and 199 medium containing 1% PSF and 2.5% mouse serum (Innovative Research, Novi, MI) in 48-well plates. After 20 min polymerization at 37°C 500 μl 199 medium supplemented with 2.5% mouse serum, 1% PSF, with and without bacterial ligands or VEGF, were added. The cultures were kept at 37°C for two weeks, medium was changed every second day, and gels were examined every other day by phase microscopy and harvested at predetermined time points.
Harvested aortic rings in Matrigel™ were incubated overnight in Histochoice (Amresco, Solon, OH) prior to embedding in paraffin. Slide sections were deparaffinized and re-hydrated by processing through Clear-rite 3 (2×3 minutes), Flex-100 (2 min, then 1 min) and Flex-95 (2 min, then 1min) (Richard-Allan Scientific, Kalamazoo, MI) followed by tap water rinse. Tissue sections were blocked in HBSS with 2% FBS for 1 hr at 25 C°. Primary antibodies, (rabbit anti-CD31 and goat anti-GFP 1:100 dilution; Abcam 28365 and 6673, respectively; Abcam, Cambridage, MA) were applied in the same blocking buffer overnight at 4 C°. Slides were washed twice in HBSS and once in blocking buffer prior to applying donkey anti-rabbit Alexa-Fluor 488 (Life Technologies, Grand Island, NY) diluted 1:1000 (Alexa A-21206). Slides were washed 3 times prior to the addition of rabbit anti-goat Biotin (Jackson Immunological Res 305-066-045) diluted1:100 and Streptavidin-568 (Alexa S-11226) diluted 1:500 in blocking buffer. Slides were mounted in Vectashield plus DAPI (Vector Labs, Burlingame, CA) and examined with a fluorescence microscope at 40× magnification.
In vivo collagen gel assay
Collagen gels were prepared as described25. A solution comprising 1.5 mg/ml rat tail collagen Type 1 (BD Biosciences), 25 mM HEPES (Lonza), 1.5 mg/ml sodium bicarbonate, 10% FBS, 30% EGM-2 medium (Lonza) in EBM-2 medium, at pH 7.4 was prepared on ice. After gentle mixing 1 ml of the suspension was placed into 12-well plates, allowed to polymerize for 30 min at 37°C, and covered with complete EGM-2 overnight. Gels were bisected and implanted into a preformed subcutaneous pocket lateral of the midventral line of mice.
Gels contained medium alone, VEGF (200 ng/ml; R&D Systems, Minneapolis, MN) or one of the following TLR4 or NOD1 ligands: ultrapure lipopolysaccharide (LPS) from 0111:B4 E.coli (100 ng/ml; InvivoGen, San Diego CA), crude LPS from 0111:B4 E.coli (1 μg/ml; Sigma L2630) or iE-DAP (5 μg/ml; AnaSpec, Inc., San Jose, CA). Seven days after gel implantation mice were sacrificed by CO2 inhalation and gels were excised, fixed in 10% formaldehyde for 4 hours followed by 70% ethanol overnight, paraffin embedded, stained for H&E and anti-mouse CD31 (Abcam).
RESULTS
NLR and TLR activation induce HIMEC migration, transmigration and proliferation
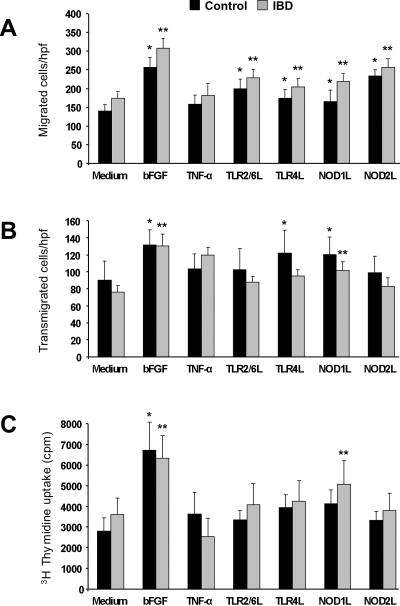
Angiogenesis involves a series of cellular events among which growth and movement are essential to new vessel formation26. We tested whether endothelial cell migration, transmigration and proliferation were induced by TLR or NLR activation. Using the wound migration assay, ligands for TLR2/6, TLR4, NOD1 and NOD2 significantly (p<0.04–0.001) enhanced migration of HIMEC regardless of whether control or IBD HIMEC were used (Fig.1A and supplemental Fig. 1A and 1B). Exposure to basic fibroblast growth factor (bFGF), but not TNF-α, both potent non-microbial stimuli, significantly increased migration (Fig.1A). In contrast, transmigration was significantly increased only by TLR4 and NOD1 activation (p<0.03–0.01) (Fig. 1B), and only IBD HIMEC significantly increased proliferation in response to NOD1 activation (p<0.01) (Fig. 1C). As seen for migration, TNF-α failed to significantly enhance transmigration and proliferation. IBD cells tended to migrate more than control, but this difference was not significant; the same was true in regard to transmigration and proliferation.
Figure 1.
A) TLR and NLR activation-induced HIMEC migration
Wounded control and IBD HIMEC monolayers were exposed to optimal stimulatory doses of bFGF, TNF-α and TLR2/6L, TLR4L, NOD1 and NOD2 ligands, and migrated cells counted after 24 h. *p<0.04–0.01 for stimulated vs unstimulated (medium) control HIMEC; **p<0.02–0.002 for stimulated vs unstimulated (medium) IBD HIMEC (n=10 and 14 for control and IBD HIMEC, respectively); L: ligand; hpf: high power field.
B) TLR and NLR activation-induced HIMEC transmigration
Control and IBD HIMEC were place in the upper compartment of a Boyden chamber, optimal stimulatory doses of bFGF, TNF-α and TLR2/6L, TLR4L, NOD1 and NOD2 ligands were placed in the lower compartment, and the number of transmigrated cells counted after 8 h. *p<0.03–0.01 for stimulated vs unstimulated (medium) control HIMEC; **p<0.01 for stimulated vs unstimulated (medium) IBD HIMEC (n=8 for both control and IBD HIMEC).
C) TLR and NLR activation-induced HIMEC proliferation
Control and IBD HIMEC monolayers were exposed to optimal stimulatory doses of bFGF, TNF-α and TLR2/6L, TLR4L, NOD1 and NOD2 ligands, and proliferation assessed by 3H thymidine uptake after 24 h. *p<0.001 for stimulated vs unstimulated (medium) control HIMEC; **p<0.01–0.001 for stimulated vs unstimulated IBD HIMEC (n=10 and 11 for control and IBD HIMEC, respectively).
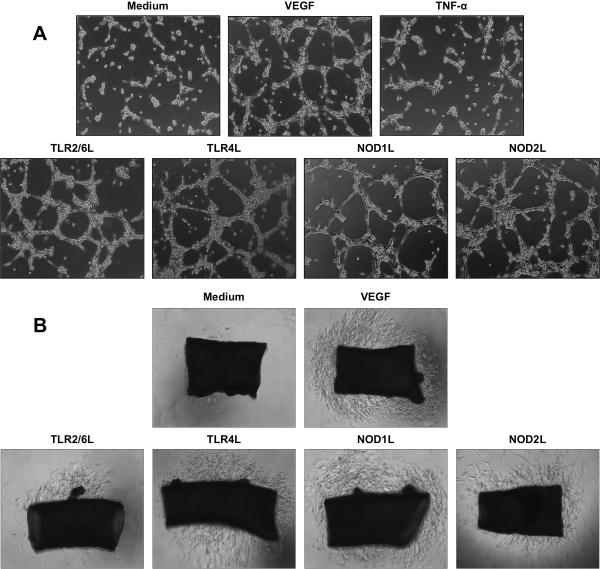
Induction of HIMEC tube formation and vessel sprouting by NLR and TLR activation
To determine whether TLRs and NLRs could mediate angiogenesis under conditions representative of what may occur in tissue, we performed assays involving tube formation and vessel sprouting ex vivo and in vivo.
Initially we tested the ability of TLRs and NLRs to mediate tube formation in Matrigel™, a more physiological response than that resulting from monolayer culture27. Compared to medium alone, HIMEC stimulated with TLR2/6, TLR4, NOD1 or NOD2 ligands readily formed tubules with thinner and longer bridges; this effect was comparable to or faster than that induced by VEGF, while TNF-α failed to induce tube formation (Fig. 2A and supplemental Fig. 2).
Figure 2.
A) TLR and NLR activation-induced HIMEC tube formation
Control and IBD HIMEC were seeded on Matrigel™ in the presence and absence of optimal stimulatory doses of VEGF, TNF-α and TLR2/6L, TLR4L, NOD1 and NOD2 ligands, and tube formation visually assessed up to 24 h. Figure is representative of 9 and 8 experiments for control and IBD HIMEC, respectively, at 6 h.
B) TLR- and NLR-induced HIMEC vessel sprouting in murine aortic ring assay
Murine aortic rings were placed in Matrigel™ in the presence and absence of optimal stimulatory doses of VEGF, TNF-α and TLR2/6L, TLR4L, NOD1 and NOD2 ligands, and vessel sprouting visually assessed after 6 days. Figure is representative of 5 and 5 experiments for control and IBD HIMEC, respectively.
Second, we performed a murine aortic ring assay, which combines the advantages of both in vivo and in vitro assays and is based on induction of endothelial cell branching28. Similarly to tube formation, all ligands stimulated vessel sprouting (Fig. 2B), activation of TLR4 and NOD1 being consistently more effective than TLR2/6 and NOD2 activation, and as effective as VEGF. The endothelial nature of the sprouting cells was indicated by typical microvascular structures with multiple subsequent branching (Supplemental Fig. 3A), and confirmed by CD31 staining of aortic rings from Tie2 GFP mice (Supplemental Fig. 3B and 3C). No differences were noted between control and IBD HIMEC in regard to tube formation or cell sprouting.
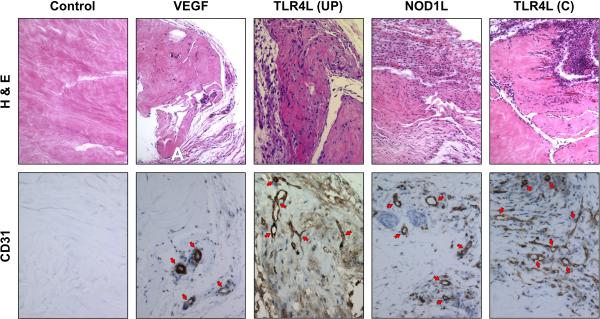
Next, the capacity of TLR4 and NOD1 ligands to induce angiogenesis in vivo was tested using a collagen gel system25. Seven days after implantation, control gels were essentially devoid of cells or vessels, and VEGF-containing gels only contained scattered cells. In contrast, gels containing ultrapure LPS or NOD1L exhibited various degrees of cell infiltration and microvascular formation compared to control gels; when crude LPS, which contains various other bacterial products29, was used gels displayed heavy cellular infiltration and the highest degree of in vivo angiogenesis (Fig. 3).
Figure 3.
VEGF-, TLR4L- and NLRL-induced cell infiltration and vessel formation in vivo
Collagen gels individually containing medium alone (control), VEGF, TLR4L, or NOD1L were surgically implanted in the abdominal subcutaneous tissue of mice, harvested after 7 days, and stained for H&E and CD31 to visualize vessel growth. Upper panels were taken at 10× and lower panels at 40× magnification. Figure is representative of 5 experiments (TLR4L-UP: ultrapure LPS; TLR4L-C: crude LPS).
Induction of HIMEC proliferation by TLR/NLR-activated HIF conditioned medium
Angiogenesis involves the interaction of endothelial cells with other cell types26. Since mesenchymal cells are in close proximity of endothelial cells and produce pro-angiogenic factors30–32, we investigated whether mucosal fibroblasts contribute to mucosal angiogenesis. HIF were exposed to the same TLR and NLR ligands and the ability of HIF-conditioned medium to induce HIMEC angiogenesis was tested.
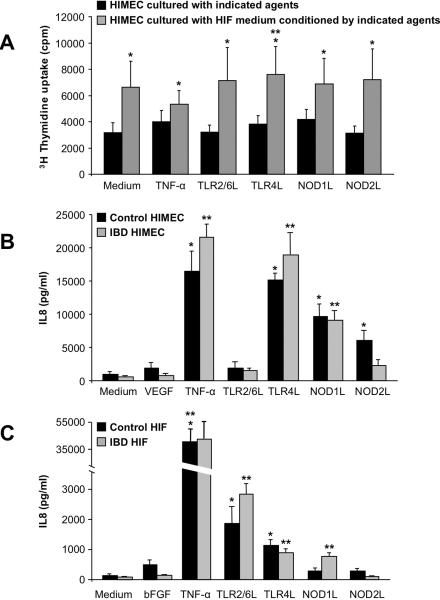
We first cultured HIF alone, in the presence of TNF-α, or individual TLR2/6, TLR4, NOD1 and NOD2 ligands; after 24 hours medium was removed, cells washed to remove any residual stimuli, and cultured alone for an additional 48 h to generate HIF-conditioned medium. Conditioned medium from both unstimulated and TNF-α-stimulated HIF dose-dependently increased HIMEC proliferation with maximal effect at 50% dilution (Supplemental Fig. 4A). At this dilution unstimulated and ligand-activated HIF-conditioned medium induced a significant (p<0.05–0.03) increase in HIMEC proliferation compared to the direct effect of the medium or ligands alone (Fig. 4A). HIF medium conditioned by bFGF or IL-1β induced a similar response (not shown). This effect was not induced by FBS, as HIMEC exposed to various concentrations of FBS for variable periods of time failed to proliferate more than HIMEC cultured in the absence of FBS (legend of Fig. 4A). In view of the potent stimulatory activity of HIF-conditioned medium even in the absence of added stimuli, only TLR4 activation induced a significant (p<0.03) further increase of HIMEC proliferation compared to medium of HIF cultured alone (Fig. 4A). Results were independent of the control or IBD origin of HIMEC or HIF.
Figure 4.
A) HIMEC proliferation in response to HIF-conditioned medium
HIMEC were cultured alone (medium), in the presence of TNF-α or optimal stimulatory doses of TLR and NLR ligands (black columns), or in HIF medium conditioned alone (medium), by TNF-α, or the same doses of TLR and NLR ligands (grey columns); after 24 h proliferation was assessed by 3H thymidine uptake. *p<0.05–0.03 for TLR/NLR activation-conditioned HIF medium compared to direct stimulation with TLR and NLR ligands; **p<0.03 for TLR4 activation-conditioned HIF medium compared to unstimulated HIF medium (n=10 for both control and IBD HIMEC). HIF (n=3) were also cultured in the presence of 0%, 2%, 5%, 10% and 20% FBS for 24, 48 and 72 hs, respectively, and proliferation assessed as described above. None of the different concentrations of FBS induced a significant increase in HIMEC proliferation compared to HIMEC cultured alone (0% FBS) at any of the 3 times points (not shown).
B) IL-8 production by TLR- and NLR-activated HIMEC
Control and IBD HIMEC monolayers were exposed to optimal stimulatory doses of VEGF, TNF-α, TLR2/6, TLR4, NOD1 and NOD2 ligands for 24 h and levels of IL-8 measured by ELISA in the culture supernatants. *p<0.03–0.001 for stimulated vs unstimulated (medium) control HIMEC; **p<0.004–0.001 for stimulated vs unstimulated (medium) IBD HIMEC (n=5 for both control and IBD HIMEC).
C) IL-8 production by TLR- and NLR-activated HIF
Control and IBD HIF monolayers were exposed to optimal stimulatory doses of bFGF, TNF-α, TLR2/6, TLR4, NOD1 and NOD2 ligands for 24 h and levels of IL-8 measured by ELISA in the culture supernatants. *p<0.05–0.004 for stimulated vs unstimulated (medium) control HIF; **p<0.03–0.004 for stimulated vs unstimulated IBD HIF (n=3 and 4 for control and IBD HIF, respectively).
Induction of HIMEC and HIF pro-angiogenic factors by TLR/NLR activation
HIMEC produce autocrine IL-8 to promote angiogenesis18, but induction of HIMEC proliferation by HIF-conditioned medium suggested the presence of pro-angiogenic factors in this medium30–32. Therefore, we investigated the production of IL-8, bFGF and VEGF by HIMEC and HIF in response to TLR/NLR activation as well as TNF-α, bFGF and VEGF. Bacterial ligands stimulated production of angiogenic factors, but this response was ligand- or cell type-dependent.
HIMEC production of IL-8 increased significantly in response to TLR4, NOD1 and NOD2 but not TLR2/6 ligands (Fig. 4B); bFGF increased significantly only in IBD HIMEC responding to NOD1 and NOD2 ligands (Supplemental Fig. 4B), while VEGF was not modulated (not shown). HIF produced significantly more IL-8 in response to TLR2/6, TLR4 and NOD1 but not NOD2 ligands (Fig. 4C); HIF spontaneously produced VEGF, but its level did not increase in response to TLR/NLR activation (not shown). When compared to bacterial ligands, TNF-α induced HIMEC to produce IL-8 to a degree comparable to that induced by TLR4 activation, but higher than NOD1 and NOD2 activation (Fig. 4B), and TNF-α was far more potent than TLR/NLR ligands in stimulating IL-8 production by HIF (Fig. 4C). bFGF and VEGF exhibited no or low capacity to induce angiogenic factor production by HIMEC or HIF. Significant differences were not noted between control and IBD HIMEC.
In additional experiments the following angiogenic factors were assessed in HIF supernatants: insulin-like growth (IGF-1), IL-6, placenta growth factor-1 (PIGF-1), stem cell factor (SCF), TGF-β (transforming growth factor-1), epidermal growth factor (EGF), nerve growth factor (NGF), leptin, PDGF-BB (platelet-derived growth factor-BB), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1α (MIP-1α); of these, variable amounts (from 16 to 2500 pg/ml) of IGF-1, IL-6, PDGF-BB, MCP-1 and MIP-1α were present in the supernatants; PIGF-1, SCF, TGF-β, EGF, NGF and leptin were either absent or present at borderline detection levels.
Inhibition of angiogenesis by blockade of TLR/NLR-induced angiogenic factors
We next investigated to what extent the TLR/NLR-induced angiogenic response was a direct effect of the bacterial ligands or an indirect effect dependent on production of angiogenic factors. IL-8 was blocked together with bFGF or VEGF and HIMEC migration and proliferation were measured as indicators of angiogenesis (Fig. 1A, 1C).
The combined blockade of bFGF and IL-8, both of which are produced by HIMEC (see above), significantly (p<0.05–0.001) reduced HIMEC migration directly induced by TLR/NLR activation (Supplemental Fig. 5A); bFGF-, but not TNF-α-, dependent migration was also reduced. In these experiments proliferation was unaffected (not shown).
We then cultured HIMEC in HIF-conditioned medium with and without blockade of IL-8 and VEGF and measured cell proliferation. A significant (p<0.05–0.01) reduction of proliferation was observed in response to TLR2/6-, TLR4- and NOD1- but not NOD2-conditioned medium (supplemental results and Supplemental Fig. 5B). Since TNF-α induced abundant IL-8 production by HIF (Fig. 4C), neutralization also significantly decreased the proliferation induced by TNF-α-conditioned medium; the effect of control bFGF was not significantly decreased. Results were independent of the control or IBD origin of the HIMEC.
Dependency on RIP2 for NLR-induced angiogenesis
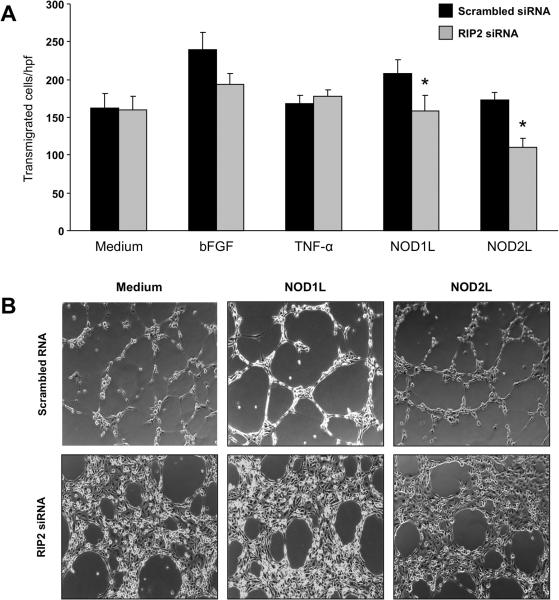
RIP2 is an adaptor molecule essential for NLR signaling33, and we investigated its involvement in NOD1- and NOD2-mediated angiogenesis by RNA interference. After determining optimal knockdown conditions (Supplemental Fig. 6A), 0.1 nmol/ml and 48 h were selected and applied to transmigration and tube formation assays. Compared to scrambled siRNA, RIP2 siRNA significantly (p<0.01) reduced transmigration of HIMEC stimulated by NOD1 and NOD2 ligands, but not by bFGF or TNF-α (Fig. 5A). Comparable results were observed in the tube formation assay, with marked inhibition seen when NOD1 or NOD2 ligands were used (Fig. 5B). A strong inhibition of tube formation was unexpectedly observed in HIMEC transfected with RIP2 siRNA even in the absence of NLR ligands (Fig. 5B). Therefore, we investigated whether NOD1 or NOD2 ligands were inadvertently present in the Matrigel™ itself, but this was not the case as shown by negative fluorescence-based NF-κB reporter gene assays (supplemental Results and Supplemental Fig. 7).
Figure 5.
A) Effect of RIP2 knockdown on NLR activation-induced HIMEC transmigration
Transmigration was performed as described above in the legend of Figure 1B, using cells transfected with scrambled or RIP2 siRNA and then exposed to medium alone, bFGF, TNF-α, or NOD1 and NOD2 ligands for 8 h. *p<0.01 for siRIP2 RNA vs scrambled siRNA (n=3 and 3 for control and IBD HIMEC, respectively); hpf: high power field.
B) Effect of RIP2 knockdown on NLR-induced HIMEC tube formation
Tube formation assay was performed as described above in the legend of Figure 2A, using cells transfected with scrambled or RIP2 siRNA and then exposed to medium alone, NOD1L or NOD2L. Figure is representative of 3 and 2 experiments with control and IBD HIMEC, respectively, at 4 h.
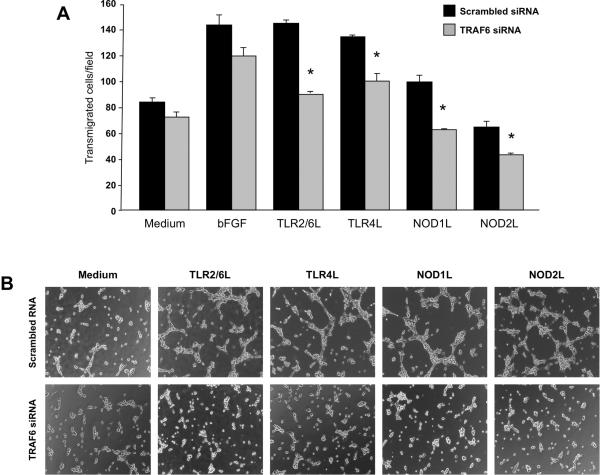
Involvement of TRAF6 in TLR/NLR-induced angiogenesis
Since TRAF6 is an adaptor molecule essential for both NLR and TLR signaling34, we blocked its activity with TRAF6 siRNA to investigate its involvement in TLR2/6-, TLR4-, NOD1- and NOD2-mediated angiogenesis. After establishing optimal inhibitory conditions (0.4 nmol/ml and 48 h) (supplemental Results and Supplemental Fig. 6B), transmigration and tube formation assays were performed. Knockdown of TRAF6 mRNA significantly (p<0.04–0.006) inhibited all TLR/NLR-induced HIMEC transmigration compared to cells transfected with scrambled siRNA, with no significant effect on bFGF-induced transmigration (Fig. 6A). Using TRAF6 siRNA tube formation was inhibited in TLR- and NLR-activated HIMEC, but not in HIMEC cultured in medium alone (Fig. 6B).
Figure 6.
A) Effect of TRAF6 knockdown on TLR and NLR activation-induced HIMEC transmigration
Transmigration was performed as described above in the legend of Figure 1B, using cells transfected with scrambled or TRAF6 siRNA and then exposed to medium alone, bFGF or TLR2/6, TLR4, NOD1 and NOD2 ligands for 8 h. *p<0.04–0.006 for siTRAF6 RNA vs scrambled RNA. (n=3 and 3 for control and IBD HIMEC, respectively); hpf: high power field.
B) Effect of TRAF6 knockdown on TLR- and NLR-induced HIMEC tube formation
Tube formation assay was performed as described above in the legend of Figure 2A, using cells transfected with scrambled or TRAF6 siRNA and then exposed to medium alone, TLR2/6, TLRL4, Nod1L or Nod2L. Figure is representative of 3 and 2 experiments with control and IBD HIMEC, respectively, at 5 h.
Involvement of the MAPK and NF-κB pathways, VEGF-R2 and FAK in TLR/NLR-induced angiogenesis
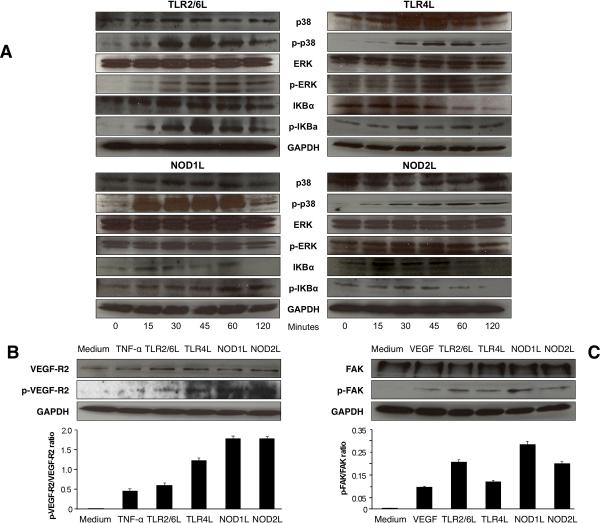
Multiple pathways are involved in signaling downstream of TLR and NLR35,36, and we investigated the activation of MAPK and NF-κB pathways in HIMEC by assessing the phosphorylation of IKBa, p38 and ERK. As shown in Fig. 7A, activation of TLR2/6 and TLR4 as well as of NOD1 and NOD2 by their respective ligands led to a strong and time-dependent phosphorylation of all the above signaling molecules.
Figure 7.
A) TLR- and NLR-mediated MAPK and NF-κB activation
HIMEC monolayers were exposed to optimal stimulatory doses of TLR2/6, TLR4, NOD1 or NOD2 ligands for the indicated time points, harvested, lysed, and protein extracted for immunoblotting using antibodies against total and phosphorylated (p) p38, ERK, IKBa and GAPDH. Figure is representative of 3 control and 3 IBD HIMEC, respectively.
B) TLR and NLR activation-induced VEGF-R2 phosphorylation
HIMEC monolayers were exposed to optimal stimulatory doses of TNF-α or TLR2/6, TLR4, NOD1 or NOD2 ligands for 48 h, harvested, lysed, and protein extracted for immunoblotting using the respective antibody and GAPDH antibody. Lower panel shows densitometric analysis of the ratio of p-VEGF-R2/VEGF-R2 under each condition. Figure is representative of 3 control and 5 IBD HIMEC.
C) TLR and NLR activation-induced FAK phosphorylation
HIMEC monolayers were exposed to optimal stimulatory doses of VEGF or TLR2/6, TLR4, NOD1 or NOD2 ligands for 48 h, harvested, lysed, and protein extracted for immunoblotting using the respective antibody and GAPDH antibody. Lower panel shows densitometric analysis of the ratio of p-FAK/total FAK under each condition. Figure is representative of 3 control and 5 IBD HIMEC.
We finally investigated additional molecules critically involved in events required for angiogenesis: these included VEGF-R2, the main receptor mediating the angiogenic activity of VEGF, and FAK, which is broadly involved in cell spreading and migration37,38. Compared to unstimulated cells, all ligands upregulated the expression of VEGF-R2 to a degree comparable to that induced by TNF-α, and induced its phosphorylation to an even higher degree (Fig. 7B); the same ligands induced phosphorylation of FAK similar to or greater than that induced by VEGF, while levels of total FAK remained unchanged (Fig. 7C). In all experiments investigating RIP2, TRAF6, MAPK and NF-kB, VEGF-R2 and FAK no differences were observed between control and IBD HIMEC.
DISCUSSION
This study demonstrates that microbial products can directly activate mucosal endothelial cells and induce proliferation, migration, transmigration, tube formation and vessel sprouting. These responses are mediated through various TLRs and NLRs and promote angiogenesis. Additionally, microbial products contribute indirectly to angiogenesis by inducing the TLR- and NLR-dependent production of pro-angiogenic factors by mucosal mesenchymal cells. Together, these observations suggest that the intestinal microbiota contributes to angiogenesis during intestinal inflammation18,19.
The type and degree of TLR2/6-, TLR4-, NOD1- and NOD2-mediated responses depended primarily on the effect induced without significant differences between control and IBD HIMEC. Activation of TLR4 and NOD1 was particularly effective in inducing transmigration, tube formation and ex vivo and in vivo vessel sprouting, while TLR2/6 and NOD2 activation had the strongest effect on migration. NOD2 ligation was relatively less effective, probably due to lower expression or affinity in HIMEC, as suggested by lower mRNA expression compared to NOD1 mRNA (unpublished). Overall responses were comparable to those induced by bFGF or VEGF, two of the most powerful pro-angiogenic factors. Therefore, it appears that TLRs and NLRs better promote endothelial cell movement than growth. Additional to these in vitro observations, the collagen gel assays confirmed that single ligands specific for TLR4 and NOD1 are sufficient to induce angiogenesis in vivo. Crude LPS, which contains other bacterial products29, was more potent that ultrapure LPS, suggesting additive or synergistic effects in angiogenesis induction, a response likely to reflect the actual in vivo situation.
We also tested the pro-angiogenic activity of TNF-α, a prototypical inflammatory cytokine abundantly produced in IBD39. The role of TNF-α as a pro-angiogenic mediator is controversial because few studies claim that TNF-α exerts pro-angiogenic activity in vivo, whereas the majority of reports fail to find such activity in vitro40. TNF-α or TNF-α-conditioned medium failed to induce HIMEC migration, transmigration or proliferation, and did not promote tube formation. Conversely, TNF-α was at least as effective as TLR and NLR ligands in inducing IL-8 production by HIMEC and HIF and upregulating VEGF-R2 expression on HIMEC. This suggests that microbial stimuli may be more effective than inflammatory cytokines in the direct induction of mucosal angiogenesis; inflammatory cytokines, like TNF-α, may complement this effect indirectly by priming endothelial cells, stimulating mesenchymal cells to secrete angiogenic factors, and upregulating their receptor on the nearby microvasculature.
TLR and NLR activation robustly stimulated HIMEC movement, and this was mediated by a time-dependent phosphorylation of signaling molecules of the MAPK and NF-κB pathways, including p38, ERK and IKBa. In addition, TLR and NLR activation led to phosphorylation of FAK, a tyrosine kinase essential to integrin-mediated cell adhesion41. FAK phosphorylation is indispensable to cell spreading, migration, survival and cycle progression and it has been recently shown to also promote angiogenesis38. TLR and NLR activation also upregulated VEGF-R2, the main receptor through which VEGF exerts pro-angiogenic activity37. Together, the activation of the MAPK and NF-kB pathways, the induction of p-FAK, and of VEG-R2 mimic the action of classical pro-angiogenic factors, supporting the notion that microbial products are important mediators of intestinal angiogenesis acting through selective TLRs and NLRs.
Next we investigated the role molecules traditionally involved in TLR and NLR signaling, including the adaptor RIP2 for NLRs and TRAF6 for TLRs and NLRs33,34. RIP2 knockdown significantly inhibited NOD1- and NOD2-induced transmigration and tube formation. Unexpectedly, RIP2 knockdown inhibited tube formation of HIMEC seeded in medium alone, a response not due to the inadvertent presence of NOD ligands in medium or Matrigel™. Thus, RIP2 exerts functions not exclusively related to NLR signaling, as indicated by reports showing that RIP2 may be involved in cell death and survival in Schwann cells, T cell receptor-mediated NF-κB activation, and myogenic responses42–44. The involvement of TRAF6 was also evidenced when its knockdown inhibited transmigration and tube formation. So, TLR/NLR signaling pathways acting in immune and epithelial cells are also functional in mucosal microvascular endothelial cells with a cell-type specific outcome, i.e., promotion of intestinal angiogenesis.
Leaking of microbial products in the inflamed intestine allows interaction with mucosal cells bearing their specific receptors45, including endothelial and mesenchymal cells14,15,21. That endothelial cells, including HIMEC, produce their own pro-angiogenic factors acting in an autocrine fashion and gut mucosal extracts contain pro-angiogenic factors is known18,19. However, the ability of mucosal mesenchymal cell-derived factors to act in a paracrine pro-angiogenic fashion was unknown. Medium conditioned by HIF alone was able to induce HIMEC proliferation, which increased further when HIF were pre-stimulated with LPS. Indeed, HIF produced vast amounts of IL-8 in response to TLR2/6 activation, and increased amounts in response to TLR4 and NOD1 activation. HIF also produced IGF-1, IL-6, PDGF-BB, MCP-1 and MIP-1α, all of which likely act in a complementary fashion to promote mucosal angiogenesis. Blocking some of these pro-angiogenic factors in HIF-conditioned medium inhibited TLR/NLR-induced HIMEC proliferation and migration, demonstrating a crucial role of fibroblasts in inflammation-induced angiogenesis comparable to that seen in neoplasia-induced angiogenesis31.
In summary, this study shows that different microbiota-derived products induce mucosal endothelial and mesenchymal cells to promote intestinal angiogenesis through the selective induction of specific pro-angiogenic pathways. Angiogenesis fosters recruitment of immune cells and sustains inflammation, and therapeutic manipulation of the gut microbiome may prevent or attenuate chronic intestinal inflammation.
Acknowledgments
Grant support: This work was supported by grants from the National Institutes of Health (DK50984 and DK069854 to CF and DK082437 to CM), the Deutsche Forschungsgemeinschaft (RI1735/2-1) and the Crohn's & Colitis Foundation of America (to FR), the Charité, Berlin, Germany, and the Nancy & Gerald Goldberg AmTrust Charitable Foundation (to AS), and the International Society for Advancement of Cytometry (to KA).
Abbreviations
- bFGF
basic fibroblast growth factor
- FAK
focal adhesion kinase
- HIF
human intestinal fibroblast
- HIMEC
human intestinal microvascular endothelial cell
- NLR
Nod-like receptor
- TLR
Toll-like receptor
- TRAF6
TNF receptor-associated factor 6
- RIP2
receptor interacting protein-2
- VEGF
vascular endothelial growth factor
- VEGF-R2
vascular endothelial growth factor receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None by all authors
Author contribution Anja Schirbel: study concept and design; acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; analysis and interpretation of data; statistical analysis.
Sean Kessler: study design; acquisition of data; critical revision of the manuscript for important intellectual content; drafting of the manuscript; technical support.
Florian Rieder: acquisition of data; critical revision of the manuscript for important intellectual content; technical and material support.
Gail West: acquisition of data; analysis and interpretation of data; administrative and technical support.
Nancy Rebert: acquisition of data; analysis and interpretation of data; administrative and technical support.
Kewal Asosingh: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Christine McDonald: study design; acquisition of data; obtained funding; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Claudio Fiocchi: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; administrative, technical and material support; study supervision.
References
- 1.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 5.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartor RB. Microbial influences in inflammatory bowel disease. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Danese S. Nonimmune cells in inflammatory bowel disease: from victim to villain. Trends Immunol. 2008;29:555–564. doi: 10.1016/j.it.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Sydora BC, MacFarlane SM, Walker JW, et al. Epithelial barrier disruption allows nondisease-causing bacteria to initiate and sustain IND in the IL-10 gene-deficient mouse. Inflamm Bowel Dis. 2007;13:947–954. doi: 10.1002/ibd.20155. [DOI] [PubMed] [Google Scholar]
- 10.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 11.Fritz JH, Ferrero RL, Philpott DJ, et al. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 12.Erridge C. The roles of Toll-like receptors in atherosclerosis. J Innate Immun. 2009;1:340–9. doi: 10.1159/000191413. [DOI] [PubMed] [Google Scholar]
- 13.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: an update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 14.Faure E, Thomas L, Xu H, et al. Bacterial lipopolysaccharde and IFN-γ induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 15.Davey MP, Martin TM, Planck SR, et al. Human endothelial cells express NOD2/CARD15 and increase IL-6 in response to muramyl dipeptide. Microvasc Res. 2006;71:103–107. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Saber T, Veale DJ, Balogh E, et al. Toll-like receptor 2 induced angiogenesis and invasion is mediated through the Tie2 signalling pathway in rheumatoid arthritis. PLoS One. 2011;6:e23540. doi: 10.1371/journal.pone.0023540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danese S, Sans M, delaMotte C, et al. Angiogenesis as a novel components of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 19.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–95. e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 20.Rieder F, Fiocchi C. Intestinal fibrosis in IBD - a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 21.Uehara A, Takada H. Functional TLRs and NODs in human gingival fibroblasts. J Dental Res. 2007;86:249–154. doi: 10.1177/154405910708600310. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, You Y, Case SM, et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:830–5. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 23.Inohara N, Koseki T, Lin J, et al. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–31. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- 24.Masson VV, Devy L, Grignet-Debrus C, et al. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 27.Auerbach R, Lewis R, Shinners B, et al. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 28.Aplin AC, Fogel E, Zorzi P, et al. The aortic ring model of angiogenesis. Methods Enzymol. 2008;443:119–36. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 29.Morath S, Geyer A, Spreitzer I, et al. Structural decomposition and heterogeneity of commercial lipoteichoic Acid preparations. Infect Immun. 2002;70:938–44. doi: 10.1128/iai.70.2.938-944.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Noma K, Smalley KS, Lioni M, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–93. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enzerink A, Vaheri A. Fibroblast activation in vascular inflammation. J Thromb Haemost. 2011;9:619–26. doi: 10.1111/j.1538-7836.2011.04209.x. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Kim YG, McDonald C, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–8. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 36.Fritz JH, Ferrero RL, Philpott DJ, et al. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–7. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 37.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 38.Peng X, Ueda H, Zhou H, et al. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc Res. 2004;64:421–30. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor a-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 40.Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–43. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 42.Khursigara G, Bertin J, Yano H, et al. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. J Neurosci. 2001;21:5854–63. doi: 10.1523/JNEUROSCI.21-16-05854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruefli-Brasse AA, Lee WP, Hurst S, et al. Rip2 participates in Bcl10 signaling and T-cell receptor-mediated NF-kappaB activation. J Biol Chem. 2004;279:1570–4. doi: 10.1074/jbc.C300460200. [DOI] [PubMed] [Google Scholar]
- 44.Munz B, Hildt E, Springer ML, et al. RIP2, a checkpoint in myogenic differentiation. Mol Cell Biol. 2002;22:5879–86. doi: 10.1128/MCB.22.16.5879-5886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clavel T, Haller D. Bacteria- and host-derived mechanisms to control intestinal epithelial cell homeostasis: implications for chronic inflammation. Inflamm Bowel Dis. 2007;13:1153–1164. doi: 10.1002/ibd.20174. [DOI] [PubMed] [Google Scholar]