Abstract
In this study, we first assessed the effect of intragastric infection of pregnant mice with Listeria monocytogenes on relative expression of select genes associated with T cell subsets. Relative gene expression was moderately increased in placental tissues for IFNγ, IL-4, IL-17a, IL-22, CD3, and FoxP3. To assess the roles of IL-17a and IL-22 in resistance to listeriosis during pregnancy, we compared the severity of maternal and fetal infection in IL-17a(−/−), IL-22(−/−), and IL-17a(−/−)/IL-22(−/−) mice with that of wild type C57BL/6 mice. Intragastric infection with modest numbers of bacterial cells (105 CFU) caused reproducible maternal and fetal infection in all four mouse strains. We recovered greater numbers of CFU from the bloodstream of pregnant IL-22(−/−) mice than pregnant wild type mice. Otherwise we found no significant difference in bacterial load in maternal or fetal tissues (spleen, liver, fetoplacental units) from pregnant IL-17a(−/−), IL-22(−/−), or IL-17a(−/−)/IL-22(−/−) or wild type mice. Nor did we observe histopathologic differences in severity of inflammation in maternal or fetal tissues from the various groups of mice. Although IL-17a and IL-22 are up-regulated in placental tissue, our study suggests that antibacterial resistance and the host inflammatory response are not dependent on IL-17a or IL-22 during infection of mice with L. monocytogenes at 10-14 days of gestation.
Keywords: Listeria monocytogenes; mouse; pregnancy; IL-17a, IL-22
1. Introduction
Listeria monocytogenes is commonly linked to foodborne disease outbreaks, especially in industrialized nations. Listeriosis results in an estimated 1600 cases per year in the U.S.A [1]. Despite its relatively low incidence, L. monocytogenes is associated with a high mortality, causing an estimated 250 deaths annually in the United States [1]. Immunocompromised or pregnant individuals are at higher risk for clinical disease due to listeriosis [2, 3]. Pregnant women represent 16% of L. monocytogenes clinical disease in the USA and have 20 times greater risk for developing moderate to severe clinical disease than the average adult [4, 5]. The 3rd trimester is the most common time of L. monocytogenes infection during pregnancy [6]. Although most infections are mild or asymptomatic, 20% of L. monocytogenes infections during pregnancy result in stillbirth and spontaneous abortion and 68% of live births to infected mothers result in neonatal infection [6]. It remains unclear why pregnant women cannot protect their fetuses against listeriosis.
Increased susceptibility to listeriosis during pregnancy has been hypothesized to be due to loss of homeostasis between pro- and anti-inflammatory T cell populations. In a non-pregnant animal, Th1 cells are significantly more abundant than Th2 cells, especially in response to infection with intracellular pathogens [7]. Pregnancy is associated with diminished T helper (Th) 1 cells, which are critical for host defense against listeriosis [3, 4, 8]. The balance among Th cell populations is important for defense against infection with L. monocytogenes, because cytokines associated with the Th1 cell response (e.g. interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα)), are critical for protective cellular immunity to L monocytogenes [9]. However, recent findings of additional distinct types of T cells, such as Th17 cells and T regulatory cells (reviewed in [10]), complicate assessment of the roles of T cells in protecting the fetus against Listeria infection during pregnancy.
Th17 cells are the predominant source of the cytokines IL-17a and IL-22 [11-13]. These cytokines have pro- and anti-inflammatory properties that are critical to inflammation and immune surveillance in mucosal and endothelial tissues. IL-17a is a pro-inflammatory cytokine, produced primarily by Th17 cells, which influences expression of multiple proinflammatory chemokines, cytokines, peptides, and other proteins involved in the acute phase response (reviewed in [14]). IL-17a also stimulates neutrophil differentiation, migration, and activation [15]. IL-17a produced by TCR γδ T cells was isolated from the livers of non-pregnant mice inoculated i.p. with L. monocytogenes [16]. Orgun et al. showed that mice inoculated i.v. with L. monocytogenes produced a significant Th17 response when IFNγ, and the type 1 IFN receptor, were experimentally neutralized in non-pregnant mice [17]. Non-pregnant L. monocytogenes-infected IL-17a(−/−) mice had a significantly greater microbial load in the liver and spleen, than wild type mice, although the difference was less than 1 log10 CFU [18]. IL-17a has been detected in the human placenta during inflammation, and is associated with miscarriage and other pregnancy abnormalities [19]. IL-17a is found in the mouse placenta during normal pregnancy, but its presence in the placenta following intragastric L. monocytogenes infection has not been reported [20].
IL-22 is produced by several subsets of T cells, including Th17 cells, and has been implicated in host defense, inflammation, and tissue repair [21-23]. IL-22 regulates function of parenchymal cells, such as epithelial cells and hepatocytes [22, 24]. In some circumstances, IL-22 works synergistically with IL-17a and is reported to have multiple functions that are both pro- and anti-inflammatory (reviewed in [25]). IL-22 has been found in human placenta, and was shown to be significantly elevated in experimental L. monocytogenes infection [26, 27]. Several reports showed that bacterial clearance was not imapired in IL-22(−/−) mice infected i.v. with L. monocytogenes [28, 29]. However, the importance of IL-22 in the pathogenesis of oral infection with L. monocytogenes has not been reported.
Here we investigated whether L. monocytogenes infection during pregnancy alters the relative expression of inflammatory cytokines, including IL-17a and IL-22,. Using gene knockout mice, we go on to show that IL-17a and IL-22 are dispensable for resistance to listeriosis during pregnancy.
2. Results and Discussion
2.1 Expression of inflammatory cytokine mRNAs in maternal and fetal tissues from L. monocytogenes infected pregnant mice
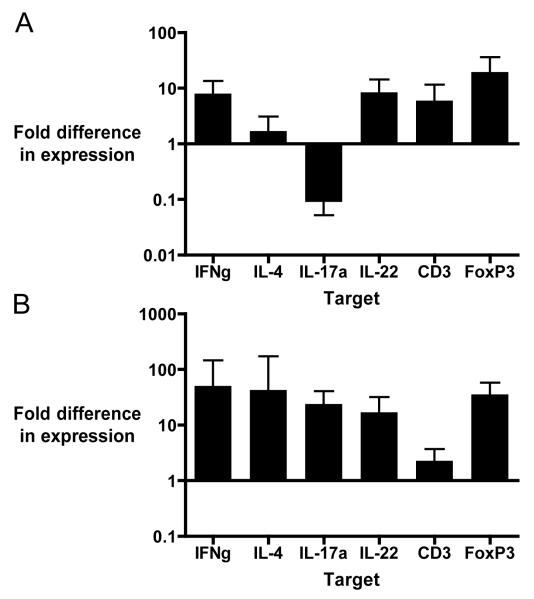
Quantitative PCR analysis showed minimal changes in expression of T cell associated genes in maternal liver from L. monocytogenes infected pregnant C57BL/6 mice, compared to uninfected pregnant mice. IL-4 and IL-17a expression did not differ and were slightly decreased, respectively, in infected pregnant mice compared to uninfected mice (Fig. 1A). Placental tissues from L. monocytogenes infected mice had increased expression of inflammatory cytokine mRNA as compared to uninfected pregnant mice (Fig. 1B). The relative expression difference (ΔΔCt) of all the cytokines of interest were increased in L. monocytogenes infected placental tissue compared to uninfected fetal tissue. ΔCt values for infected vs. uninfected placental tissues suggested a trend towards increased relative gene expression, but did not achieve statistical significance (data not shown).
Fig. 1. Changes in expression of T cell associated mRNAs in maternal liver and placental tissue following oral infection with L. monocytogenes.
Pregnant C57BL/6 mice (10-14 days gestation) were infected by intragastric lavage with 105 CFU LM2203 in 100μL of 1% saline. Tissues were harvested 72 hours post infection for RNA isolation and qPCR analysis. The Ct method was used to calculate relative expression, using 3 reference genes (ActB, GAPDH, and RPL8) between L. monocytogenes infected and uninfected mice. (A) mRNA expression in liver tissue from L. monocytogenes infected pregnant mice (n=8 from 3 separate experiments) is compared to uninfected control pregnant mice (n=10 from 3 separate experiments.) (B) mRNA expression in placental tissue from L. monocytogenes infected pregnant mice (n=15 from 3 separate experiments) is compared to uninfected pregnant control mice (n=9 from 3 separate experiments.) Data are expressed as the mean ± SD.
Cluster of differentiation (CD3) is a four-chain protein complex that is part of the T cell receptor, and a marker for activated T cells. CD3 has been reported to be up-regulated in L. monocytogenes infection [30]. Here we show that CD3 expression is up-regulated in placental tissue, which is consistent with our findings of increased relative expression of T cell associated genes. relative expression of IFNγ and IL-4 were similarly upregulated in L. monocytogenes infected placental tissue. This finding contradicts previously reported increased Th2:Th1 cytokine ratios in fetal tissues, (3,4,7,8) IL-17a is part of the IL-17 family, which influences the production of chemokines (CXCL1, CXCL2, CXCL5, CXCL8) and cytokines (IL-6, GM-CSF, and G-CSF) that are responsible for recruitment, differentiation, and activation of neutrophils [11, 31]. Listeriosis is typified by mixed infiltration of macrophages and neutrophils into tissues. One exception is the placenta, which experiences primarily neutrophilic inflammation [32, 33]. Our results show that IL-17a expression is up-regulated in murine placental tissue following intragastric infection with L. monocytogenes compared to uninfected control placental tissue. This finding is consistent with our initial hypothesis that IL-17a is involved in placental inflammation during L. monocytogenes infection.
IL-22, like IL-17a, is produced primarily by Th17 cells, which in turn are regulated by IL-23 during L. monocytogenes infection [34]. Because IL-22 has both pro and anti-inflammatory functions in epithelial tissues, and in certain circumstances works synergistically with IL-17a, we hypothesized that it would play a significant role in resistance to intragastric L. monocytogenes infection [22, 24, 25]. Previous publications reported that IL-22 deficient mice do not differ from wild type mice in clearance of L. monocytogenes, or tissue protection [28, 29]. However, both of these studies infected mice i.v. rather than via the gastrointestinal tract, which is the natural route of infection. We chose to examine the role of IL-22 in an intragastric infection model, which more closely follows the physiologic route of infection. We found relative expression of IL-22 is moderately increased in placental tissue following L. monocytogenes infection compared to placental tissue from uninfected control C57BL/6 mice. This finding supported our hypothesis that IL-22 is involved in the pathogenesis of listeriosis in the pregnant mouse.
Forkhead box p3 (FoxP3) is part of the forkhead box (FOX) gene family that regulates development of cells. FoxP3 regulates development and function of CD4+CD25+ Treg cells, which prevent autoimmunity and regulate maternal tolerance to the fetus [35, 36]. Previous studies have shown that FoxP3+ regulatory T cells are required to maintain pregnancy, but compromise host defense against prenatal bacterial pathogens [37]. However, regulatory T cell function is not always able to suppress the host immune response. For example the suppressive function of T reg cells was over-ridden by L. monocytogenes infection in a murine cardiac allograft model [38]. We found that relative FoxP3 expression was moderately elevated in murine placental tissue, as was fetal inflammation. These findings are consistent with the previous murine allograft model and suggests that L. monocytogenes infection triggers an inflammatory response the dampens regulatory T cell function.
The multiplex qPCR was performed at a single time point (72 hours) at which we observed consistent microbial load and histopathology in 10-14 day pregnant C56BL/6 mice [39]. It is possible that more pronounced changes in relative gene expression occur earlier or later in the course of the host immune response, but this was not investigated. We interpret with caution the ΔΔCt and ΔCt values because the Ct values were high (30-35 cycles), indicating relatively low gene expression. We attempted to quantify antigen-specific T cells expressing the cytokines of interest but did not recover sufficient numbers of cells from placental tissue to do so. We also attempted to quantify cytokine protein in tissues using a multiplex fluorescent bead array. However, we obtained variable results, probably due to interfering factors in placental tissue.
2.2 IL-17a and IL-22 are not required for placental resistance to L. monocytogenes infection in pregnant mice of 10-14 days gestation
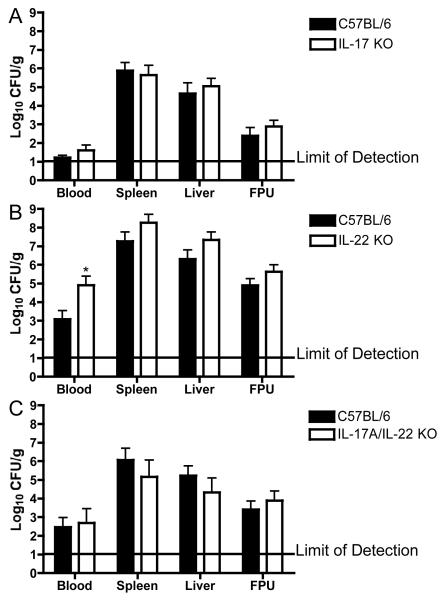
We utilized next mouse strains with gene deletions for IL-17a(−/−), IL-22(−/−), and IL-17a(−/−)/IL-22(−/−) to test the role of these cytokines in the maternal and fetal response to intragastric infection with L. monocytogenes. Overall, we observed similar levels of L. monocytogenes infection in pregnant IL-17a(−/−) (Fig. 2A), IL-22(−/−) (Fig. 2B), IL-17a(−/−)/IL-22(−/−) (Fig. 2C), and wild type C57BL/6 mice. We did, however, observe a significantly greater bacteremia IL-22(−/−) than wild type pregnant mice (Fig. 2B). Although there was some variability in CFU data among sets of experiments,, this is not surprising in light of the expected variability following intragastric infection [39]. In all experiments, both wild type and gene knockout mice displayed moderate signs of systemic illness (lack of grooming, movement, hunched posture) by the end of the 72-hour infection period. The tissues that make up the fetoplacental unit (fetus, placenta, amniotic membranes, and amniotic fluid) were homogenized as a single unit to determine microbial load in CFU/g. This was done because L. monocytogenes infection resulted in inflammation and necrosis of the fetal tissues that made it impossible to dissect these free of maternal tissues.
Fig. 2. Bacterial burden in maternal and fetal tissues is not dependent on IL-17a, or IL-22.
Mice were infected with L. monocytogenes as described in the Materials and Methods by intragastric lavage with 105 CFU LM2203 in 100μL of 1% saline. Mice were euthanized 72 hours later and blood, spleen, liver and fetoplacental units (FPUs) were removed (2-3 per mouse) and processed for bacterial culture. Data are presented as the mean ± SEM log10 CFU/g of tissue or log10 CFU/ml of blood. (A) Data from 3 separate experiments are combined for a total of 9 pregnant C57BL/6 (25 FPU) and 15 pregnant IL-17a(−/−) (33 FPU) mice. (B) Data from 2 separate experiments are combined for a total of 12 pregnant C57BL/6 (23 FPU) and 9 pregnant IL-22(−/−) (23 FPU) mice. (C) Data from 2 separate experiments are combined for a total of 10 pregnant C57BL/6 (22 FPU) and 9 pregnant IL-17a(−/−)/IL-22(−/−) (19 FPU) mice. The asterisk indicates p<0.05 compared to wild type mice.
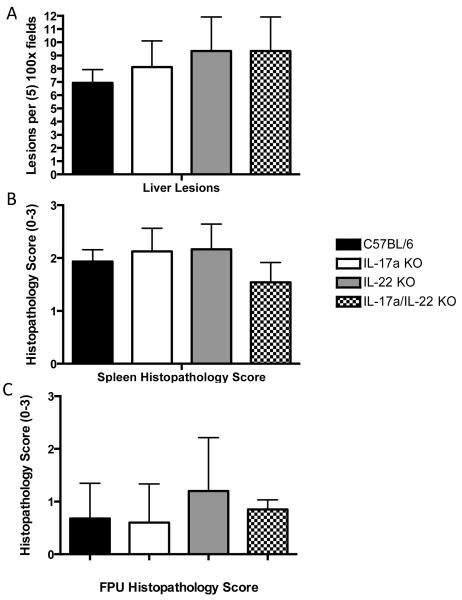
Portions of the tissues removed at necropsy were submitted for histopathological analysis. Focal inflammatory lesions with mixed leukocyte infiltrates(granulocytes and macrophages) were observed in the livers of infected mice. When we quantified liver lesions, there was no significant differences between control and gene knockout mice (p≥0.7 among all experiments) (Fig. 3A). We also quantified the severity of splenic inflammation using a scoring system described in the Materials and Methods, (Fig. 3B). Here too we observed no significant difference among the groups of mice (p≥0.8 in all experiments). Nor did we discern a significant difference in inflammation and necrosis in the FPUs of L. monocytogenes infected wild type and gene knockout mice (Fig. 3C) (p≥0.1). The histopathology scores for FPUs were variable within a uterine horn, similar to our previous report [39]. We did not allow fetuses to come to term and assess fetal viability, as all infected mice displayed signs of systemic illness and were euthanized 72 hours after inoculation.
Fig. 3. Severity of inflammation in maternal liver and spleen is not dependent on IL-17a or IL-22 during L. monocytogenes infection.
Mice were infected as described in the Materials and Methods. Portions of spleen and liver tissues were fixed in 10% buffered formalin and prepared for histopathological analysis. Liver and spleen sections were sectioned, stained and scored as described in the Materials and Methods. (A) Inflammatory lesions per five 100× fields of maternal liver. Tissues were from the same mice illustrated in Fig. 2. We observed no significant difference between L. monocytogenes infected pregnant C57BL/6 mice (n=24) and pregnant IL-17a(−/−) (n=8), IL-22(−/−) (n=6), and IL-17a(−/−)/IL-22(−/−) (n=12) mice. (B) Spleen tissue from the same mice were scored for severity of inflammation (0-3) as described in the Methods. We observed no significant difference in splenic inflammation between control C57BL/6 wild type mice and IL-17a or IL-22 gene knockout mice. Data represent the mean ± SEM and the asterisk indicates p<0.05.
Despite evidence for increased relative expression of IL-17a and IL-22 in placental tissue of L. monocytogenes infected pregnant mice, the severity of infection and inflammation of maternal and fetal tissues were dependent on neither IL-17a nor IL-22 in our experimental model. One exception was the bacteremia in IL-22(−/−) pregnant mice, which was significantly greater than in pregnant wild type mice. Perhaps this observation indicates that the presence of IL-22 on epithelial surfaces, especially gastrointestinal epithelial cells, affects the ability of L. monocytogenes to translocate across the gut mucosa and cause bacteremia [22-24]. However, the overall bacterial burden was particularly high in the IL-22(−/−) mice experiments, which could account in part for the increased bacteremia. We did not observe incresed bacteremia in the IL-17a(−/−)/IL-22(−/−) mice. It is possible that IL-17a and IL-22 have earlier effects in the host response, but this was not examined in our study.
Because IL-17a recruits and activates neutrophils, which are important in resistance to L. monocytogenes infection, we expected inflammation to be decreased in the placental tissue of IL-17a(−/−) mice [32, 33]. However, inflammation in maternal and fetal tissues were similar among IL-17a(−/−), IL-22(−/−), and wild type mice. Inflammation was of mixed leukocyte populations, as we did not differentiate among cell types in histopathology samples.
In conclusion, intragastric infection of pregnant (10-14 days of gestation) C57BL/6 mice with L. monocytogenes causes increased relative expression of IL-17a and IL-22 in placental tissues. However, neither bacterial burden nor inflammation in fetal tissues is dependent on IL-17a, or IL-22 in mice infected at 10-14 days gestation.
4. Materials and Methods
4.1 Preparation of L. monocytogenes
L. monocytogenes strain 2203 (serotype 4b) was generously donated by Dr. Sophia Kathariou (Raleigh, NC) [40]. L. monocytogenes cells were stored at −20°C on Cryobank™ Cryobeads (Copan Diagnostics, Inc., Corana, CA). For each experiment, a bead was placed into 5 ml of brain heat infusion (BHI) broth and incubated overnight with shaking at 37°C. Bacterial cells were harvested by centrifugation (3,500 × g for 5 minutes), washed three times in phosphate buffered saline and kept on ice prior to inoculating mice. The bacterial suspensions were diluted to the desired concentration, and numbers of viable L. monocytogenes confirmed by plating serial dilutions onto tryptic soy agar with 5% sheep blood (BD® Biosciences).
4.2 Strains of mice and inoculation
Female inbred C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) at 6 weeks of age and housed under microisolator caps at the School of Veterinary Medicine animal care facility. Breeding pairs of IL-17a(−/−) and IL-22(−/−) mice (C57BL/6 genetic background) were obtained from Dr. Iwakura (Univ. of Tokyo) and Genentech (San Francisco, CA), respectively. IL-17a(−/−) and IL-22(−/−) mice were housed and bred under microisolator caps under supervision of the Research and Animal Care Biotron facility. IL-17a(−/−)/IL-22(−/−) mice were derived breeding IL-17a(−/−) females with IL-22(−/−) males. The IL-17a(−/−)/IL-22(−/−) mice were bred to provide 6 week old female mice for experiments. All null mouse strains were genotyped (Table 1) to ensure their status using tail snip tissue submitted to an outside vendor (Transnetyx, Cordova, TN). Mice obtained from the breeding colony were acclimated for 1 week in the School of Veterinary Medicine animal care facility prior to being paired with a breeding male. Female mice were allowed to reach 7-10 days of gestation prior to use in an experiment. Mice received food and water ad libitum until 5 hours prior to an intragastric inoculation experiment, at which time food was removed from the cage. This was done to minimize the risk of delivery of the bacterial inoculum into stomachs that were engorged with mouse chow, which could lead to aspiration of the inoculum into the lungs. Mice were anesthetized by i.p. injection of sodium pentobarbital (40 mg/kg). When the mice were sedated, the listerial inoculum was introduced (as a total volume of 0.1 ml) via a 1.5 in.-long, 24 gauge, stainless steel oral esophageal tube attached to a 1-ml syringe.
Table 1.
Primers used to genotype IL-17a and IL-22 null mice.
| Primer specificity |
Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Product Size |
|---|---|---|---|
| IL-17a WT | ACTCTTCATCCACCTCACACGA | GCCATGATATAGACGTTGTGGC | 1300 bp |
| IL-17a(−/−) | ACTCTTCATCCACCTCACACGA | CAGCATCAGAGACTAGAAGGGA | 500 bp |
| IL-22 WT | CAGCTGGCGGCCAAAGTCCC | GATACAGGTGCAGCTAAGCGAG | 196 bp |
| IL-22(−/−) | CTCAGACCTCTACAGACAATCATC | GATACAGGTGCAGCTAAGCGAG | 374 bp |
4.3 Recovery of L. monocytogenes from tissues of infected mice
At the desired time points (10-14 days gestation), mice were humanely euthanized by asphyxiation with CO2 followed by exsanguination and cervical dislocation. Blood was collected into a syringe containing sodium citrate as an anticoagulant. The blood was then serially diluted in sterile saline, plated (0.1 ml) on trypticase soy agar with 5% sheep’s blood, and the plates incubated at 37°C. The abdominal cavity was then aseptically opened and portions of the spleen, liver, and fetoplacental units (FPU) (2-3 FPUs per pregnant mouse) were removed. The FPU consisted of the fetus, placenta, and amniotic fluid. The FPU was not dissected and CFU data is expressed per FPU in CFU/g of tissue. These tissues were weighed in sterile weigh boats and placed into separate sterile tissue tubes that contained 1 ml of cold, sterile saline. The tissues were homogenized with sterilized Zirconium Oxide beads using a Bullet Blender® (Next Advance, Averill Park NY). The homogenates were diluted in sterile saline, and plated on blood agar. The plates were allowed to dry and then incubated at 37°C for 48 hours. Colonies were counted and the data expressed as mean ± standard error of the mean (SEM) log10 CFU of L. monocytogenes per gram of tissue (wet weight).
4.4 Histopathology
At the time of necropsy, portions of the spleen, liver, and fetoplacental units were removed, placed in plastic cassettes, and fixed in 10% buffered formalin. Following fixation and embedding into paraffin, the tissues were serial sectioned, mounted on glass slides, and stained with hemotoxylin and eosin or a tissue gram stain. The sections were coded and evaluated by a veterinary pathologist who is board certified by the American College of Veterinary Pathologists (H.S.). Liver sections were scored based on number of focal inflammatory lesions per five −100× fields. Pathological changes in spleen and fetoplacental units (fetus, placenta, and amniotic fluid) were scored on a 0-3 scale with 0 defined as no lesions present, 1 mild to moderate inflammation, 2 moderate to severe inflammation, and 3 severe inflammation and necrosis.
4.5 Cytokine response to L. monocytogenes infection
At the time of necropsy, 80-100 mg pieces of maternal liver and entire placentas were removed, placed in cryogenic vials (Corning, Corning NY), snap-frozen in liquid nitrogen, and stored at −80°C. RNA was extracted from tissue samples using the Applied Biosystems RNAqeuous kit (Life Technologies, Carlsbad CA) following the manufacturer’s protocol. RNA concentration was determined using a spectrophotometer (NanoDrop ND-1000, NanoDrop Technologies, Wilmington, DE). RNA purity was assessed by OD260/280, all samples greater or less than 1.8-2.0 were discarded. RNA integrity was tested by running the sample on an agarose gel with ethidium bromide fluorescent staining [41]. One microgram of RNA was heated to 70°C for 10 minutes prior to transcription to cDNA, using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Life Technologies) with an Eppendorf Mastercycler EP Gradient S thermocycler (Eppendorf, Hamburg). Relative gene expressions of IFNγ, IL-4, IL-17a, IL-22, CD3, and FoxP3 between experimental and control groups of tissues, using beta actin (Actβ), 60S ribosomal protein L8 (RPL8), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reference genes for normalization, were measured by real-time PCR using the Applied Biosystems StepOne Plus RT PCR system and analyzed with StepOne Software v2.1 (Life Technologies). Samples were loaded in triplicate into a 96-well optical reaction plate (Applied Biosystems), and sealed with optical sealing tape (MicroAmp Optical Adhesive Film, Applied Biosystems). The Power SYBR Green master mix and AmpErase Uracil N-glycosylase were used (Applied Biosystems) with a 1:50 dilution of cDNA from the qPCR reaction. Each reaction was optimized according to the manufacturer’s protocol. Primer concentrations can be found in Table 2. The cytokine primer sequences were constructed using the National Center for Biotechnology Information (NCBI) Gene online software and manufactured by the University of Wisconsin Biotechnology Center (Madison, WI). All primer sets were tested on cDNA isolated from control murine liver and placental tissues using regular PCR and products were sequenced by the University of Wisconsin Biotechnology Center. Sequences were then analyzed using NCBI Gene online software ensure accurate amplification of the desired cytokines. The parameter for development of the primer sequences consisted of a primer length of 15-25 bp, an amplicon of 50-150 bp, a 30-80% GC content, and a melting point of about 60°C [41]. Primer sequences and amplicon size can be found in Table 2. Specific PCR amplification was confirmed by a dissociation curve, PCR products with a melting temperature lower than 81°C were not included in the relative quantification analysis [41]. Relative gene expression was then calculated using the ΔΔCt method[41].
Table 2.
Primers Used for real time PCR.
| Primer specificity |
Forward primer (5′→3′) |
Reverse primer (5′→3′) | cDNA amplicon size |
Concentration used (Liver/Placenta uM) |
|---|---|---|---|---|
| Actb* | TGTGATGGTGGGAAT GGGTCAGAA |
TGTGGTGCCAGATCTT CTCCATGT |
140 bp | 200/400 |
| GAPDH* | CTTTGTCAAGCTCAT TTCCTGG |
TCTTGCTCAGTGTCCT TGC |
133 bp | 400/400 |
| RPL8* | CTACGTGCTGTGGA CTTCG |
CGGCCAGGGTCATGA ATG |
77 bp | 600/200 |
| IFNγ | GGCTGTTACTGCCAC GGCACA |
CACCATCCTTTTGCCA GTTCCTCCA |
130 bp | 600/600 |
| IL-4 | CGAATGTACCAGGA GCCATATC |
TCTCTGTGGTGTTCTT CGTTG |
149 bp | 600/600 |
| IL-17a | TCCAGAATGTGAAGG TCAACC |
TATCAGGGTCTTCATT GCGG |
129 bp | 600/600 |
| IL-22 | AGCTTGAGGTGTCCA ACTTC |
GGTAGCACTCATCCTT AGCACTG |
150 bp | 600/600 |
| CD3 | TGGAGCAAGAATAG GAAGGC |
CATAGTCTGGGTTGGA ACAG |
115 bp | 600/600 |
| FoxP3 | AAGTACCACAATATA TGCGACCC |
TCTGAAGTAGGCGAAC ATGC |
132 bp | 600/400 |
Denotes reference gene
4.6 Statistical analysis
For comparison of bacterial burden and inflammation between groups of mice, non-parametric statistical analysis was done using a Kruskal-Wallis test of analysis of variance (ANOVA) followed with a Dunn’s Multiple Comparison test of groups (Instat, GraphPad). Statistical significance for all comparisons was set at P<0.05.
Acknowledgements
The authors would like to thank Dr. Sophia Kathariou (Raleigh, NC) for generously providing L. monocytogenes strain LM2203. We would also like to thank Dr. Bruce Klein (Madison, WI) and Genentech (SanFransico, CA) for providing the IL-17a null and IL-22 null mouse strains, respectively. This work was funded by the National Institutes of Health Ruth L. Kirschstein National Research Service Award Institutional Training Grant T32 RR023916 from the National Center for Research Resources, The Michael and Winona Foster Wisconsin Distinguished Fellowship Award, The Walter and Martha Renk Laboratory Endowed Laboratory for Food Safety, USDA Special Cooperative Agreement 58-1935-1-128, and the UW-Madison Food Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schlech WF., 3rd Listeria gastroenteritis--old syndrome, new pathogen. N Engl J Med. 1997;336:130–2. doi: 10.1056/NEJM199701093360211. [DOI] [PubMed] [Google Scholar]
- [3].Posfay-Barbe KM, Wald ER. Listeriosis. Semin Fetal Neonatal Med. 2009;14:228–33. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [4].Jacobson L. Listeriosis. Pediatr Rev. 2008;29:410–1. doi: 10.1542/pir.29-11-410. [DOI] [PubMed] [Google Scholar]
- [5].Jackson KA, Iwamoto M, Swerdlow D. Pregnancy-associated listeriosis. Epidemiol Infect. 2010:1–7. doi: 10.1017/S0950268810000294. [DOI] [PubMed] [Google Scholar]
- [6].Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–9. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- [7].Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- [8].Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- [9].Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–23. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- [10].Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [11].Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [12].Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pappu R, Ramirez-Carrozzi V, Ota N, Ouyang W, Hu Y. The IL-17 family cytokines in immunity and disease. J Clin Immunol. 2010;30:185–95. doi: 10.1007/s10875-010-9369-6. [DOI] [PubMed] [Google Scholar]
- [15].Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, et al. IL-17 A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–63. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12 p40 and type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180:4109–15. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J Immunol. 2010;185:5879–87. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- [19].Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, et al. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–11. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- [20].Ostojic S, Dubanchet S, Chaouat G, Abdelkarim M, Truyens C, Capron F. Demonstration of the presence of IL-16, IL-17 and IL-18 at the murine fetomaternal interface during murine pregnancy. Am J Reprod Immunol. 2003;49:101–12. doi: 10.1034/j.1600-0897.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- [21].Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- [22].Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [23].Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- [24].Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–84. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- [25].Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17 A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, et al. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol. 2008;38:1204–14. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A. 2001;98:9511–6. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PLoS One. 2011;6:e17171. doi: 10.1371/journal.pone.0017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feng H, Zhang D, Palliser D, Zhu P, Cai S, Schlesinger A, et al. Listeria-infected myeloid dendritic cells produce IFN-beta, priming T cell activation. J Immunol. 2005;175:421–32. doi: 10.4049/jimmunol.175.1.421. [DOI] [PubMed] [Google Scholar]
- [31].Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–73. doi: 10.1016/j.jaci.2004.10.019. quiz 74. [DOI] [PubMed] [Google Scholar]
- [32].Redline RW, Lu CY. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–55. [PubMed] [Google Scholar]
- [33].Takahashi K, Naito M, Katabuchi H, Higashi K. Development, differentiation, and maturation of macrophages in the chorionic villi of mouse placenta with special reference to the origin of Hofbauer cells. J Leukoc Biol. 1991;50:57–68. doi: 10.1002/jlb.50.1.57. [DOI] [PubMed] [Google Scholar]
- [34].Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–34. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- [35].Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- [36].Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- [37].Rowe JH, Ertelt JM, Way SS. Foxp3(+) Regulatory T cells, Immune Stimulation and Host Defense against Infection. Immunology. 2012;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–33. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Poulsen KP, Faith NG, Steinberg H, Czuprynski CJ. Pregnancy reduces the genetic resistance of C57BL/6 mice to Listeria monocytogenes infection by intragastric inoculation. Microb Pathog. 2011;50:360–6. doi: 10.1016/j.micpath.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].MacDonald PD, Whitwam RE, Boggs JD, MacCormack JN, Anderson KL, Reardon JW, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677–82. doi: 10.1086/427803. [DOI] [PubMed] [Google Scholar]
- [41].Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1–5. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]