Abstract
Background
We describe the incidence, risk factors, and outcomes of invasive candidiasis in infants >1500 g birth weight.
Methods
We conducted a retrospective cohort study of infants >1500 g birth weight discharged from 305 NICUs in the Pediatrix Medical Group from 2001–2010. Using multivariable logistic regression, we identified risk factors for invasive candidiasis.
Results
Invasive candidiasis occurred in 330/530,162 (0.06%) infants. These were documented from positive cultures from ≥1 of these sources: blood (n=323), cerebrospinal fluid (n=6), or urine from catheterization (n=19). Risk factors included day of life >7 (OR 25.2; 95% CI 14.6–43.3), vaginal birth (OR 1.6 [1.2–2.3]), exposure to broad-spectrum antibiotics (OR 1.6 [1.1–2.4]), central venous line (OR 1.8 [1.3–2.6]), and platelet count <50,000/mm3 (OR 3.7 [2.1–6.7]). All risk factors had poor sensitivities, low positive likelihood ratios, and low positive predictive values. The combination of broad-spectrum antibiotics and low platelet count had the highest positive likelihood ratio (46.2), but the sensitivity of this combination was only 4%. Infants with invasive candidiasis had increased mortality (OR 2.2 [1.3–3.6]).
Conclusions
Invasive candidiasis is uncommon in infants >1500 g birth weight. Infants at greatest risk are those exposed to broad-spectrum antibiotics and with platelet counts of <50,000/mm3.
Keywords: candidiasis, candidemia, neonates, neonatal intensive care unit
Invasive candidiasis is an important cause of late-onset sepsis in the neonatal intensive care unit (NICU) and is associated with up to 40% mortality and increased risk of neurodevelopmental impairment among survivors.1–6 Extremely low birth weight (ELBW, <1000 g birth weight) infants are at highest risk, with a cumulative incidence of 7%.7–9 Although risk factors for invasive candidiasis in infants <1500 g birth weight have been well described,3,10,11 there are only limited data on risk factors in larger infants. Previous multicenter studies examining risk factors for invasive candidiasis included larger infants together with ELBW infants; studies that focused on larger infants alone have been predominantly single-center.12–14
The high morbidity associated with invasive candidiasis has led to the evaluation of prophylaxis and empirical antifungal treatment in high-risk infants in the NICU. Two clinical trials involving preterm infants revealed benefits of antifungal prophylaxis,8,9 and an earlier study showed that empirical antifungal therapy was associated with improved mortality and morbidity of ELBW infants with invasive candidiasis.7 We sought to describe the epidemiology and risk factors for invasive candidiasis in infants >1500 g birth weight using a large, multicenter database to inform the need and approach for antifungal prophylaxis or empirical antifungal therapy in this population.
METHODS
Population
We identified all infants >1500 g birth weight discharged from Pediatrix Medical Group NICUs between 2001 and 2010. During our study period, the Pediatrix Medical Group included 305 centers representing both academic and community hospitals in the United States. We included all blood, urine (obtained by in-and-out catheterization) and cerebrospinal fluid (CSF) cultures obtained between day of life (DOL) 1 and 120. The data were obtained from an administrative database that prospectively captures information from daily progress notes using a computer-assisted tool. Information that was collected included maternal history, infant demographics, medications, laboratory results, culture results, diagnoses, and respiratory support. Evaluations for infection were performed using standard-of-care practices at each NICU. Decisions with regard to obtaining cultures and initiating therapies were site- and physician-dependent.
Definitions
We defined invasive candidiasis as a positive culture with Candida spp. from blood, urine, or CSF. We considered all cultures with Candida spp. within a 21-day period as a single episode of invasive candidiasis and retained the first positive cultures in the dataset for analysis. We selected pertinent baseline underlying conditions based on prior published research in neonatal candidiasis from single-center studies in term infants and multicenter studies in preterm infants: necrotizing enterocolitis (NEC), gastrochisis, omphalocele, intestinal atresia, Hirschprung’s disease, intestinal perforation, congenital diaphragmatic hernia (CDH), and congenital heart disease.12,13 We only included NEC treated medically or surgically and excluded suspected NEC. For congenital heart disease, we included atrioventricular septal defects, valvular pathologies, obstructive lesions, cyanotic heart conditions, and single ventricular lesions; we excluded patent ductus arteriosus, atrial septal defects/patent foramen ovale, and ventricular septal defects from this category. We defined broad-spectrum antibiotic exposure as treatment with one of the following antibiotics within seven days prior to the culture: third-generation cephalosporins, carbapenems, ticarcillin, or piperacillin.11,15 We defined antifungal prophylaxis as an infant with all of the following: exposure to an antifungal in the first six days of life, antifungal exposure prior to a positive culture for Candida spp., and antifungal therapy continued for a minimum of eight days. We defined antacid exposure as exposure to any of the following medications within seven days prior to the culture: cimetidine, famotidine, ranitidine, omeprazole, or pantoprazole. We defined mechanical ventilation (conventional or high-frequency) and inotropic support as being present on the day of the culture. We identified the maximum fraction of inspired oxygen (FiO2) recorded. We defined central line use as the presence of an umbilical catheter, central venous line, and/or peripherally inserted central line on the day of culture, and categorized this risk factor into three groups: no central line, central line duration ≤7 days, and central line duration >7 days. We defined platelet counts as the highest platelet count obtained on the day of culture. If no platelet count was obtained on the day of culture, we used, in order of preference, the platelet count from the previous day or the platelet count from the following day. Death was defined as death prior to hospital discharge. For length of stay, we limited our analysis to survivors transferred out or discharged from the NICU.
Statistical Analysis
We compared continuous and categorical variables using the Student’s t-test and chi-square test, respectively. The unit of observation for risk factor analysis was the culture. We performed univariable logistic regression of demographic and known risk factors for neonatal candidiasis. All risk factors were included as candidate variables in a multivariable model. Because each patient had multiple culture observations, we clustered our multivariable model by patient. We used stepwise variable selection with an entry P-value <0.1 and a P-value <0.15 to remain in the model. We reported sensitivity, specificity, and likelihood ratios of demographics and postnatal age, as well as known risk factors for neonatal candidiasis alone and in combination. We then compared mortality and length of stay between infants with and without invasive candidiasis, adjusting for gestational age and underlying baseline conditions to account for these important covariates. Statistical significance was defined as P <0.05 for all tests. We analyzed the data using STATA 12 (College Station, TX). This analysis was approved by the Duke University Institutional Review Board without the need for written informed consent because the data were collected without identifiers.
RESULTS
There were 530,162 infants >1500 g birth weight. The mean birth weight was 2751 g (5th, 95th percentile: 1660, 4020), and the mean gestational age was 36 weeks (32, 40). Of these, 411,866 (78%) infants had ≥1 blood, urine, or CSF culture, and 330 infants (0.06%) developed invasive candidiasis (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B371 which shows demographics). These episodes were documented from positive growth of Candida spp. from 323 blood cultures, six CSF cultures, and 19 urine cultures. Among infants with cultures performed, the mean birth weights of infants with and without invasive candidiasis were 2377 g (1554, 3776) and 2749 g (1656, 4005), respectively (P <0.001). The mean gestational ages of infants with and without invasive candidiasis were 35 weeks (30, 40) and 36 weeks (32, 40), respectively (P <0.001). The mean age of the first episode of invasive candidiasis was 30 days (1, 90). Among the infants with invasive candidiasis, 213 (65%) infants had a positive Candida spp. culture within the first 28 days of life. Antifungal prophylaxis was used in 156 infants (0.03%); 118 infants (76%) were given fluconazole prophylaxis.
While only 4% (19,379/530,162) of infants >1500 g birth weight had pertinent baseline underlying conditions (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B371), these infants represented 42% (140/330) of the cases of invasive candidiasis. Among the 122 centers with >2000 total discharges, the incidence of invasive candidiasis ranged from 0.0 to 4.1 per 1000 infants (Figure 1).
Figure 1.
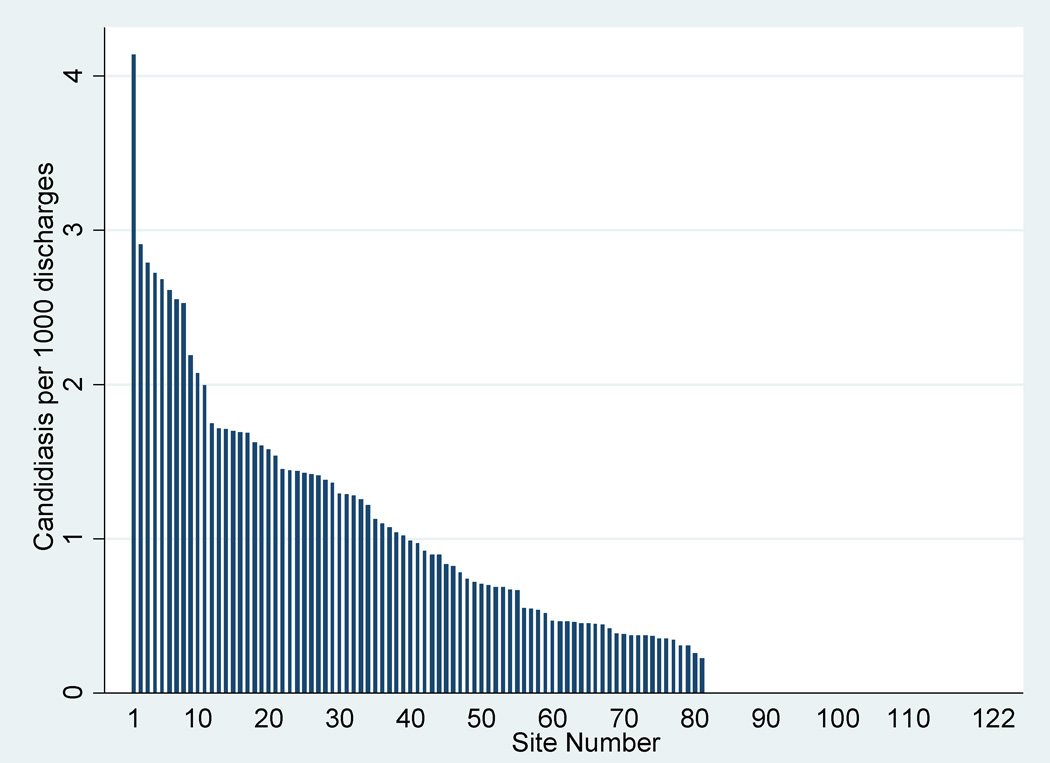
Invasive candidiasis by site (sites with >2000 total discharges). Site numbers 80–122 did not have any episodes of invasive candidiasis.
Risk Factors
Risk factors for invasive candidiasis identified on unadjusted analysis were gestational age, lower birth weight, DOL >7, baseline underlying conditions, mechanical ventilation, central lines, broad-spectrum antibiotics, antacids, antifungal prophylaxis, and platelet count <50,000/mm3 (Table 1). Risk factors on adjusted analysis included vaginal birth, DOL >7, presence of central lines, broad-spectrum antibiotics, antifungal prophylaxis, and platelet count <50,000/mm3 (Table 1).
TABLE 1.
Risk Factors for Invasive Candidiasis
| Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|
| Gestational age (weeks) | |||
| <29 | 15.3 (6.2–37.9) | ||
| 29–33 | 2.6 (2.0–3.5) | ||
| 34–36 | 1.4 (1.0–1.8) | ||
| ≥ 37 | Reference | ||
| Birth weight (g) | |||
| 1501–1999 | 3.5 (2.6–4.8) | ||
| 2000–2999 | 1.8 (1.3–2.4) | ||
| ≥3000 | Reference | ||
| Vaginal delivery | 1.3 (1.0–1.6) | 1.6 (1.2–2.3) | |
| Day of life | |||
| 0–7 | Reference | Reference | |
| 8–30 | 53.0 (38.1–73.6) | 24.0 (13.9–41.6) | |
| 31–60 | 91.1 (62.9–131.7) | 35.3 (18.7–66.9) | |
| >60 | 79.2 (50.6–123.6) | 18.1 (7.4–44.5) | |
| Broad-spectrum antibiotics | 14.6 (11.6–18.5) | 1.6 (1.1–2.4) | |
| Antacids | 14.3 (11.0–18.5) | 1.5 (1.0–2.3) | |
| Inotropes | 1.0 (0.5–2.0) | 0.4 (0.2–0.9) | |
| Antifungal prophylaxis | 22.4 (10.5–48.2) | 6.0 (1.6–22.8) | |
| Mechanical ventilation | 1.5 (1.2–2.1) | ||
| FiO2 (%) | |||
| 21 | Reference | ||
| 22–30 | 1.0 (0.7–1.6) | ||
| 31–50 | 0.7 (0.5–0.9) | ||
| >50 | 0.8 (0.6–1.2) | ||
| Central line duration (days) | |||
| 0 | Reference | Reference | |
| ≤7 | 0.7 (0.5–1.0) | 2.3 (1.5–3.5) | |
| >7 | 21.3 (16.6–27.4) | 2.3 (1.7–3.1) | |
| Platelets <50,000/mm3 | 9.3 (5.7–14.9) | 3.7 (2.1–6.7) | |
| Baseline underlying conditions | 9.2 (7.4–11.4) | ||
The combination of broad-spectrum antibiotics and low platelet count had the highest positive likelihood ratio (46.2) (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B372 which shows estimates of sensitivity, specificity, positive likelihood ratios, and positive predictive values for predictors of invasive candidiasis). Exposure to broad-spectrum antibiotics, presence of central lines, and platelet count <50,000/mm3 had sensitivities of 30%, 36%, and 7%, respectively (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B372). Likelihood ratios of individual risk factors were low. Almost all individual risk factor had positive predictive values (PPV) <1%. Using combinations of risk factors produced higher PPVs (1–3%), but sensitivities for these combinations were also low (≤8%).
Outcomes Following Invasive Candidiasis
Of the 278 infants with invasive candidiasis and known mortality outcomes, 18 (7%) died. This was significantly higher than mortality of infants without invasive candidiasis (2907/369,892 [0.8%], P <0.001). On multivariable analysis, infants with invasive candidiasis were more likely to die (odds ratio [OR] = 2.2; 95% confidence interval: 1.3, 3.6) than infants without invasive candidiasis. After excluding infants who died, the mean length of stay for infants with and without invasive candidiasis were 61 days (7, 166) and 12 days (2, 35), respectively (P <0.001).
DISCUSSION
This report of infants >1500 g birth weight with invasive candidiasis found that infants with invasive candidiasis had higher odds of the following risk factors: vaginal delivery, DOL >7 days, broad-spectrum antibiotics, antifungal prophylaxis, presence of indwelling central line, and platelet counts <50,000/mm3. In very low birth weight (<1500 g birth weight) infants, risk factors for invasive candidiasis include lower gestational age, lower birth weight, presence of central venous catheter, broad-spectrum antibiotics, duration of mechanical ventilation, and thrombocytopenia.3,10,15–17 In our study, vaginal birth, DOL >7, exposure to broad-spectrum antibiotics, presence of a central line, and thrombocytopenia were significant predictors of invasive candidiasis. Our adjusted ORs for thrombocytopenia and broad-spectrum antibiotics were similar to those previously described for ELBW infants: the adjusted OR for thrombocytopenia and broad-spectrum antibiotics in infants ≤1250 g birth weight was 3.6 (2.8–4.7) and 1.8 (1.3–2.3), respectively.11 Our adjusted OR for presence of a central line was lower (2.3) compared with infants of all birth weights (OR = 3.9 [1.5 – 12.3]).16 In this previous study, central line days were calculated as all line days in the two weeks prior to the episode of candidemia. Alternatively, we considered a central line as a risk factor only if it was present when the culture was drawn, and we analyzed this risk factor by considering the number of days that the central venous catheter had been in place.
Small case series have identified mechanical ventilation and need for inotropes as risk factors for invasive candidiasis in term infants.14,16 In our cohort, mechanical ventilation and use of inotropes were not associated with invasive candidiasis. This is analogous to findings in premature infant studies: earlier single-center studies reported ventilation and inotropes as risk factors for premature infants and invasive candidiasis, but subsequent multicenter studies did not report such a strong association.3,11,18 These differences may be due to differences in definitions used in our study compared with previous studies. We defined mechanical ventilation as support on the day that cultures were drawn, and we did not include intubation and mechanical ventilation events prior to the drawing of blood cultures. We considered inotrope use if present only on days when cultures were drawn. Previous studies defined these risk factors as being present if infants were ever intubated or required inotropic support.16 The definition used in the present study was more representative of the severity of illness of the infant at the time of the culture.
A comorbidity identified by previous studies as a strong risk factor for invasive candidiasis in infants is gastrointestinal pathology.12,13,19 Gastrointestinal pathologies (NEC, gastrochisis, omphalocele, intestinal atresia, Hirschprung’s disease, intestinal perforation, CDH) comprised 32% (104/333) of infants with invasive candidiasis in our cohort. On multivariable analysis, gastrointestinal diagnoses by themselves were associated with neonatal candidiasis (OR: 1.5 [1.1–2.2]). The lack of association between cardiovascular diagnoses and invasive candidiasis was consistent with findings in a previous study where investigators found no difference in the proportion of infants ≥1000 g birth weight with cardiovascular diagnoses (22/86 vs. 75/258, P=0.53) and invasive candidiasis.20
Antifungal prophylaxis decreases candidiasis in ELBW infants who are hospitalized at institutions with a high incidence of invasive candidiasis.8,9 We attempted to identify risk factors that would place larger infants at higher risk for invasive candidiasis to better target antifungal prophylaxis. However, sensitivity and PPV of all risk factors were low. Combining risk factors worsened sensitivities while making only slight improvements in PPV. Increasing the PPV substantially reduces the absolute number of cultures that meet criteria for prophylaxis. For example, in the instance where PPV was the highest (3.3% for the combination of broad-spectrum antibiotics and thrombocytopenia), the proportion of all cultures that would be included in this category was <1/1000 (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B372). Designing a prospective trial of antifungal prophylaxis in infants >1500 g birth weight will involve recruiting infants at the highest risk, such as those identified by the combination of risk factors found in our study. Given the rarity of this combination of risk factors and the low incidence of invasive candidiasis, a trial of this nature will require a prohibitively large number of NICUs.
Invasive candidiasis increases mortality, length of stay, and medical costs in hospitalized adults, children, and infants.2,21,22 ELBW infants with invasive candidiasis were twice as likely to die compared with ELBW infants without invasive candidiasis (OR = 2.2 [1.4–3.5]); furthermore, between 29% and 57% of infants who survived had significant neurodevelopmental delays at two years of age.3,20,23 We showed that invasive candidiasis was associated with 40-day increased length of stay, and infants with invasive candidiasis were more likely to die (OR = 2.2 [1.3–3.6]). Although the adjusted OR for death was similar to studies in premature infants, the mortality of infants with invasive candidiasis in our study (7%) was lower than previously reported in more premature infants (20–54%).14,19,23,24
Bacterial infections in infants are also associated with increased mortality and morbidity.4,25 Although the incidence of early-onset bacterial sepsis in infants is <1%,26,27 empirical antibiotics are widely used because of the poor clinical outcomes resulting from the delay in treatment.28 Similarly, Candida spp. infections are associated with increased mortality and morbidity.3 Delay in treatment of invasive candidiasis results in higher odds of death or neurodevelopmental impairment.7 Given that the overall incidence of candidiasis was 0.6 per 1000 infants in our study, infants >1500 g birth weight should not be routinely given empirical antifungal therapy except when other risk factors are present (e.g., exposure to broad-spectrum antibiotics and thrombocytopenia).
Our study design allowed us to capture a relatively large number of infants with invasive candidiasis from a multicenter NICU cohort, but it has several limitations. Compared with infants >1500 g birth weight in all levels of care, those who were admitted to the NICU were more likely to have pulmonary disease, congenital anomalies, and require invasive procedures.29,30 Our estimate of the incidence of invasive candidiasis would be much lower if we included all infants >1500 g birth weight regardless of level of care. Our dataset did not have documentation of perinatal Candida spp. colonization or use of total parenteral nutrition. We thus were not able to examine these previously reported risk factors.16,31 We did not have records of ophthalmological assessment for fungal retinitis and solid organ (e.g., liver) involvement. Records of strains of Candida spp. isolated were not available in our database, and this has limited our availability to investigate the association between strains and clinical outcomes. Because we do not have data on indication for drug prescription, we acknowledge that, within our chosen definition of antifungal prophylaxis, some of this exposure may have been empirical antifungal therapy rather than antifungal prophylaxis.
The small number of infants with invasive candidiasis limited our ability to provide a better estimate of our clinical outcome measures or factors associated with these outcomes (e.g., center). As part of a sensitivity analysis, we repeated the adjusted risk factor analysis accounting for center differences by using a random effect model but did not find significant differences in the odds ratios. Because the Pediatrix Medical Group manages a large number of infants, the database is considered to be representative of neonatal care in the United States and has been queried for information by federal agencies such as the Food and Drug Administration and the National Institute of Child Health and Human Development. However, the potential generalizability of our findings to NICUs outside of North America may be limited given the marked variation in incidence of candidiasis in NICUs worldwide.
Invasive candidiasis is rare in infants >1500 g birth weight. Although we identified several risk factors for invasive candidiasis, these risk factors are not sufficiently robust to identify populations that will be feasible for the conduct of definitive trials. Given the low incidence of invasive candidiasis, we did not identify a population in which prophylaxis would be indicated. The decision to start empirical antifungal therapy should occur after examining risk factors in the infant in the context of the baseline incidence of invasive candidiasis for that particular NICU.
Acknowledgments
sources of funding: Dr. Benjamin receives support from the U.S. government for his work in pediatric neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01) and is principal investigator of the Pediatric Trials Network (government contract HHSN275201000002I); from the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org); and from industry for neonatal pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives support from NICHD-1K23HD060040-01, DHHS-1R18AE000028-01, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Cohen-Wolkowiez receives support from the U.S. government for his work in pediatric clinical pharmacology (government contract HHSN267200700051C, PI: Benjamin); from the National Institute of Child Health and Human Development (1K23HD064814-01); and from the non-profit organization Thrasher Research Foundation. He is also a consultant for Pfizer and Janssen Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The remaining authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Downey LC, Smith PB, Benjamin DK., Jr Risk factors and prevention of late-onset sepsis in premature infants. Early Hum Dev. 2010;86(Suppl 1):7–12. doi: 10.1016/j.earlhumdev.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith PB, Morgan J, Benjamin JD, et al. Excess costs of hospital care associated with neonatal candidemia. Pediatr Infect Dis J. 2007;26:197–200. doi: 10.1097/01.inf.0000253973.89097.c0. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 5.Wynn JL, Benjamin DK, Jr, Benjamin DK, Cohen-Wolkowiez M, Clark RH, Smith PB. Very late onset infections in the neonatal intensive care unit. Early Hum Dev. 2012;88:217–225. doi: 10.1016/j.earlhumdev.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin DK, Jr, Ross K, McKinney RE, Jr, Benjamin DK, Auten R, Fisher RG. When to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics. 2000;106:712–718. doi: 10.1542/peds.106.4.712. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg RG, Benjamin DK, Jr, Gantz MG, et al. Empiric antifungal therapy and outcomes in extremely low birth weight infants with invasive candidiasis. J Pediatr. 2012 Mar 15; doi: 10.1016/j.jpeds.2012.01.053. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 9.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 10.Cotten CM, McDonald S, Stoll B, et al. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118:717–722. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 12.Rabalais GP, Samiec TD, Bryant KK, Lewis JJ. Invasive candidiasis in infants weighing more than 2500 grams at birth admitted to a neonatal intensive care unit. Pediatr Infect Dis J. 1996;15:348–352. doi: 10.1097/00006454-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Feja KN, Wu F, Roberts K, et al. Risk factors for candidemia in critically ill infants: a matched case-control study. J Pediatr. 2005;147:156–161. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badran EF, Al Baramki JH, Al Shamyleh A, Shehabi A, Khuri-Bulos N. Epidemiology and clinical outcome of candidaemia among Jordanian newborns over a 10-year period. Scand J Infect Dis. 2008;40:139–144. doi: 10.1080/00365540701477550. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin DK, Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–e873. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Saiman L, Ludington E, Dawson JD, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Linder N, Levit O, Klinger G, et al. Risk factors associated with candidaemia in the neonatal intensive care unit: a case-control study. J Hosp Infect. 2004;57:321–324. doi: 10.1016/j.jhin.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Shetty SS, Harrison LH, Hajjeh RA, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. Pediatr Infect Dis J. 2005;24:601–604. doi: 10.1097/01.inf.0000168751.11375.d6. [DOI] [PubMed] [Google Scholar]
- 20.Zaoutis TE, Heydon K, Localio R, Walsh TJ, Feudtner C. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–1193. doi: 10.1086/513196. [DOI] [PubMed] [Google Scholar]
- 21.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 22.Shorr AF, Gupta V, Sun X, Johannes RS, Spalding J, Tabak YP. Burden of early-onset candidemia: analysis of culture-positive bloodstream infections from a large U.S. database. Crit Care Med. 2009;37:2519–2526. doi: 10.1097/CCM.0b013e3181a0f95d. quiz 2535. [DOI] [PubMed] [Google Scholar]
- 23.Lee BE, Cheung PY, Robinson JL, Evanochko C, Robertson CM. Comparative study of mortality and morbidity in premature infants (birth weight, < 1,250 g) with candidemia or candidal meningitis. Clin Infect Dis. 1998;27:559–565. doi: 10.1086/514712. [DOI] [PubMed] [Google Scholar]
- 24.Blyth CC, Chen SC, Slavin MA, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 26.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lannering B, Larsson LE, Rojas J, Stahlman MT. Early onset group B streptococcal disease. Seven year experience and clinical scoring system. Acta Paediatr Scand. 1983;72:597–602. doi: 10.1111/j.1651-2227.1983.tb09777.x. [DOI] [PubMed] [Google Scholar]
- 29.Philips JB, 3rd, Dickman HM, Resnick MB, Nelson RM, Jr, Eitzman DV. Characteristics, mortality, and outcome of higher-birth weight infants who require intensive care. Am J Obstet Gynecol. 1984;149:875–879. doi: 10.1016/0002-9378(84)90607-0. [DOI] [PubMed] [Google Scholar]
- 30.Lian WB, Yeo CL, Ho LY. Two-year outcome of normal-birth-weight infants admitted to a Singapore neonatal intensive care unit. Ann Acad Med Singapore. 2002;31:199–205. [PubMed] [Google Scholar]
- 31.Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. 2010;11:240–245. doi: 10.1097/PCC.0b013e3181b808fb. [DOI] [PubMed] [Google Scholar]


