Abstract
Context
A report by the National Cancer Institute identified that an important gap in symptom research is the investigation of multiple symptoms of cancer that might identify common biological mechanisms among cancer-related symptoms.
Objectives
We applied novel statistical methods to assess whether variants of 37 inflammation genes may serve as biologic markers of risk for severe pain, depressed mood, and fatigue in non-Hispanic white patients with non-small cell lung cancer.
Methods
Pain, fatigue, and depressed mood were assessed prior to cancer treatment. We used a generalized, multivariate, classification tree approach to explore the influence of single nucleotide polymorphisms in the inflammation genes in pain, depressed mood, and fatigue in lung cancer patients.
Results
Among patients with advanced-stage disease, IL-8-T251A was the most relevant genetic factor for pain (odds ratio [OR]=2.18, 95% confidence interval [CI]=1.34,3.55; P=0.001), depressed mood (OR=0.37, 95% CI=0.14,1.0), and fatigue (OR=2.07, 95% CI=1.16,3.70). Among those with early-stage NSCLC, variants in the IL-10 receptor were relevant for fatigue among women. Specifically, women with genotype Lys_Glu or Glu_Glu in the IL-10 gene had a 0.49 times lower risk of severe fatigue compared with those with genotype Lys_Lys (OR=0.49, 95% CI=0.25, 0.92; P=0.027). Among men with early-stage lung cancer, a marginal significance was observed for IL-1A C-889T, C/T or T/T genotype: these men had a lower risk of severe fatigue compared with those with genotype C/C (OR=0.38, 95% CI=0.13, 1.06).
Conclusion
The interaction of multiple inflammation genes, along with non-genetic factors, underlies the occurrence of symptoms. IL-8 and IL-10 may serve as potential targets for treating multiple symptoms of cancer.
Keywords: Pain, depression, fatigue, cytokines, symptoms, genes
Introduction
Lung cancer is the most common fatal cancer in the United States (1), with an estimated 222,520 new cases and 157,300 deaths in 2010 alone (2). Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. Most patients with NSCLC present with advanced-stage disease, with only 16% of cases diagnosed at the earliest and most curable stage. Patients with NSCLC suffer from severe and debilitating symptoms associated with cancer and its treatment; pain, depression, and fatigue are prevalent among these patients (3).
Symptoms in cancer patients have been hypothesized to result from a complex interaction between the tumor and host characteristics, including the immune status of the host, comorbidities, and the type of antineoplastic treatments being given. Recent pre-clinical and clinical studies suggest the importance of inflammation in cancer (4-7) and cancer-related symptoms (8-12). Circulating cytokines and inflammatory proteins are associated with pain, cognitive impairment, depression, fatigue, cachexia, and sleep disorders (9-11).
Cytokine genes are highly polymorphic. Polymorphisms found in the regulatory regions, including promoters and untranslated regions (UTRs), can, in many cases, affect in vitro expression of the gene product. Polymorphisms in inflammation genes have been shown to influence pain, depression, and fatigue (13-15). In a study of lung cancer patients, Reyes-Gibby and colleagues (16) genotyped functional single-nucleotide polymorphisms (SNPs) in tumor necrosis factor-alpha (TNF-α)-308G/A, interleukin (IL)-6-174G/C, and IL-8-251T/A and determined their associations with pain severity. They found that after controlling for epidemiologic (age and sex), clinical (stage of disease, comorbidities), and symptom (depressed mood and fatigue) variables known to influence pain severity, variant alleles in IL-8-251T/A were a significant factor for severe pain for white patients. In a follow-up study (13), they assessed if the same genetic variants would influence analgesic intake in lung cancer patients who were referred for pain control. They found that variant alleles in TNF-α-308G/A remained significantly associated with pain severity (b = 0.226; P = 0.036) and carriers of the IL-6 -174C/C genotypes required a 4.7 times higher dose of opioids for pain relief relative to G/G and G/C genotypes. A study of pancreatic cancer patients (14) further confirmed that select polymorphisms in IL-8 were associated with pain severity scores. Other studies have likewise shown the influence of genetic polymorphisms in inflammation genes on the severity of cancer-related symptoms (17, 18). A significant limitation of these studies, however, is that although symptoms are never expressed in isolation, these studies examined symptoms as mutually exclusive entities. In particular, these studies used univariate analytical approaches, where each symptom was analyzed separately. Although the univariate approach is simple and straightforward, it ignores the joint information among co-occurring and potentially correlated symptoms, which may have led to incorrect inferences (19). Advances in statistical methods have now enabled the assessment of putative genetic mechanisms underlying correlated phenotypes (e.g., pain, depression, and fatigue). Multivariate approaches that account for the correlation among multiple outcomes of pain, depression and fatigue could improve the predictive accuracy and can outperform individual univariate approaches (19, 20).
Therefore, in this study, we used a nonparametric, tree-based, multivariate approach to assess the influence of 59 genetic variants in 37 inflammation genes on the severity of pain, fatigue, and depressed mood in 599 newly diagnosed patients with NSCLC, accounting for the correlation among these three symptoms. Our exploratory hypothesis is that polymorphisms of inflammation genes will help predict pain, depressed mood, and fatigue in lung cancer patients.
To our knowledge, this study is among the first to explore the relation of SNPs in the inflammation pathway to pain, depressed mood, and fatigue simultaneously in lung cancer patients. Identifying host genetic polymorphisms of inflammation genes, as well as the potential interactive effect between these genetic components and environmental factors, will improve our knowledge of symptom burden in lung cancer patients and may provide clues for potential drug targets. Because host genetic polymorphisms are stable markers, it will enable early identification of cancer patients at high risk for severe symptoms, and will help facilitate early symptom management.
Materials and Methods
Study Subjects
The study sample was drawn from a case-control study of lung cancer (21), with an overall participation/response rate of 80%. We focused our analyses on case patients with newly diagnosed, histologically confirmed NSCLC who were recruited at the time of initial registration at The University of Texas M. D. Anderson Cancer Center, prior to the initiation of any cancer treatment. There were no restrictions with regard to age, sex, ethnicity, or disease stage. Only U.S. residents were recruited. This study was approved by the Institutional Review Board at M. D. Anderson Cancer Center.
Collection of Demographic, Clinical, and Symptom Data
Demographic, clinical, and symptom data were collected prior to the initiation of any cancer treatment. Using self-administered questionnaires, subjects rated their pain on a 0 to 10 rating scale (0 = no pain and 10 = pain as bad as you can imagine), a standard method for assessing pain. Fatigue and depressed mood were assessed using two items from the 12-Item Short-Form Health Survey (SF-12®) (22): “During the past 4 weeks, have you had a lot of energy?” and “During the past 4 weeks, have you been feeling downhearted and blue?,” respectively. Response options were: 1 = none of the time, 2 = little of the time, 3 = some of the time, 4 = good bit of time, 5 = most of the time, and 6 = all of the time. Clinical data abstracted from patients’ charts were stage of disease and history of comorbid conditions (heart disease, stroke, diabetes, etc.).
Blood Sample Collection, DNA Extraction, and Genotyping
Blood samples were collected at presentation to the cancer center (4). All SNPs were genotyped using the SNPlex™ Genotyping System (Life Technologies, Grand Island, NY), which enables simultaneous genotyping of up to 48 SNPs in a single tube using an oligonucleotide ligation assay.
SNP and Gene Selection
We selected for genotyping SNPs in immune-response genes that met at least two of three criteria: 1) minor allele frequency of at least 5%; 2) location in the promoter, UTR, or coding region of the gene; and 3) previous report of an association with cancer and symptom severity. The final list comprised SNPs in the following genes: pro-inflammatory cytokines and related molecules (IL-1A, IL-1B, IL-2, IL-6, IL-8, IL-12, IL-16, TNF-α, TNF-b, GM-CSF, MCP, MIF, INFg); anti-inflammatory cytokines and related molecules (IL-1RA, IL-4, IL-4R, IL-10, IL-10RA, IL-10RB, IL-13); prostaglandin and nitric oxide (PTGS2, ENOS, INOS), and intracellular signaling molecules (IKB, PPARA, PPARD, PPARG).
Statistical Analyses
Descriptive statistics were used to summarize the subject characteristics. The Kolmogorov-Smirnov Z test was used to assess the normality distribution of pain, fatigue, and depressed mood. Since normality was not met, we dichotomized the values of pain, fatigue and depressed mood. Therefore, in this study, we used the widely accepted and previously applied cut-off points (23-25) to dichotomize the responses to severe versus non-severe symptoms. We used the National Comprehensive Cancer Network (NCCN) cut-off score of > 7 for severe pain (26). Based on previous studies (13, 14, 27, 28), we also combined responses to the SF-12 questionnaire to categorize severe levels of depressed mood and fatigue. For the question, “During the past 4 weeks, have you been feeling downhearted and blue?,” responses of “most of the time” and “all of the time” were combined to indicate severe levels of depressed mood, and “none of the time,” “little of the time,” “some of the time,” and “good bit of the time” were combined to indicate non-severe levels of depressed mood. For the fatigue question (“During the past 4 weeks, have you had a lot of energy?”), we combined the responses “none of the time” and “little of the time” to indicate non-severe levels of fatigue and the responses “most of the time,” “all of the time,” “some of the time,” and “good bit of the time” to indicate severe levels of fatigue.
Bivariate Analysis
We assessed the association between the non-genetic variables (age, sex, comorbid conditions, and stage of disease) and the variables pain, fatigue, and depressed mood using Chi-square statistics. The extent of correlation between pain, depressed mood, and fatigue was assessed using Pearson correlation coefficients.
Tree-Based Multivariate Analysis
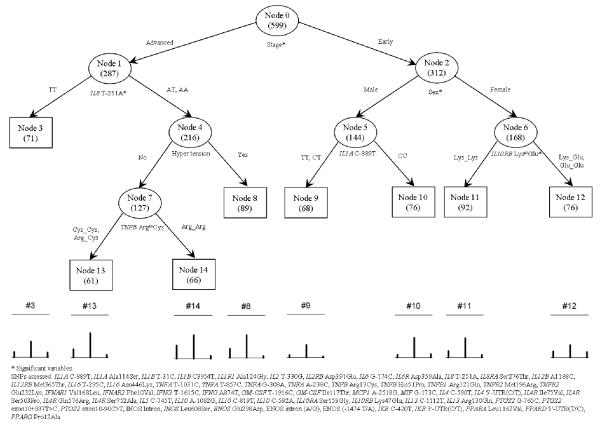
Because the symptoms of pain, fatigue, and depressed mood are highly correlated with each other, we used a generalized classification tree model (29) to analyze the association between the multiple outcomes (pain, fatigue, depressed mood) and the non-genetic (age, sex, co-morbid conditions, stage of disease) and genetic variables (59 SNPs in 37 inflammation genes) (Fig. 1).
Figure 1.
Classification and Regression Tree Analyses for Pain, Depressed Mood, and Fatigue
Briefly, a tree-based model is a stepwise, nonparametric procedure that uses exhaustive search and sort technique to classify subjects into several homogeneous subgroups and produce a tree-structured output (30). Therefore, the classification of variables is assessed relative to a split or cut-point. The single best predictor (the one whose optimal cut-point maximizes the number of correct classifications) is selected as the starting variable at the top of a hierarchical tree. Individuals with values less than the cut-point are moved to one category (the first box), and those with values greater than the cut-point are moved into second category (the second box) of the hierarchical tree. Cut-points are then assessed in a step-wise manner for the remaining predictors. A classification tree is generated that grows until maximal classification is achieved.
The classification/regression tree for a univariate discrete response was later extended (29) to handle multiple correlated binary outcomes. With the new method, multiple binary responses can be analyzed using generalized entropy criterion (homogeneity), which is the maximum likelihood of the joint (i.e., multivariate) distribution of the multiple binary responses (29, 31, 32). In building the tree (or as the tree grows) for multiple binary responses, a parent node always splits into two child nodes, and the procedure is repeated for each subsequent child node. Each node splits only on one covariate, and each splitting produces mutually exclusive subgroups. For the analysis of our data, the initial tree had 14 nodes, and then a sequence of seven nested optimal subtrees was derived. We used a 10-fold cross-validation and repeated 10 times to estimate the cost-complexity of the subtrees. We found that the cost increases as the subtree becomes simpler. Therefore, we selected the initially grown tree, which would be the optimal tree selected according to the data used.
Calculation of Odds Ratio
Given the exploratory nature of tree-based models, we calculated the odds ratios (ORs) and P-values for each split (including one parent and two child nodes) in order to assess the risk associated with each variable. The ORs and P-values were adjusted for the variables (genetic and non-genetic) significantly associated with the outcome variables, respectively, in the corresponding subsets of individuals.
Results
Characteristics of the Study Sample
A total of 599 non-Hispanic white patients with previously untreated and histologically confirmed NSCLC comprised our sample. Mean age was 61 years (SD=12). There was an almost equal distribution of the sample between early-stage (stage I-IIIA; n=285) and advanced-stage (stage IIIB-IV; n=287) disease. There were more men (n=316) than women (n=283). A majority (69.7%) of the sample reported comorbid conditions, with hypertension being the most prevalent (n=188). Table 1 shows the characteristics of the patient sample by severe levels of pain (Panel A), fatigue (Panel B), and depressed mood (Panel C). In the bivariate analyses, we found that severe pain varied by age (P<0.007) and stage of disease (P<0.001) and severe fatigue only varied by stage of disease (P<0.01).
Table 1.
Characteristics of the Lung Cancer Cases (N=599)
| Variable | Pain | Fatigue | Depressed Mood | |||
|---|---|---|---|---|---|---|
| Severe /non-severe |
P- value |
Severe/ non-severe |
P-value | Severe /non- severe |
P-value | |
| Age (yrs) | ||||||
| >50 | 65/400 | 195/270 | 31/434 | |||
| <=50 | 32/102 | 0.007 | 65/69 | 0.18 | 13/121 | 0.24 |
| Sex | ||||||
| Male | 43/273 | 132/184 | 18/298 | |||
| Female | 54/229 | 0.07 | 128/155 | 0.39 | 26/257 | 0.11 |
| Heart Disease | ||||||
| Yes | 24/104 | 62/66 | 12/116 | |||
| No | 59/321 | 0.39 | 165/215 | 0.32 | 26/354 | 0.35 |
| Diabetes | ||||||
| Yes | 5/32 | 17/20 | 1/36 | |||
| No | 78/393 | 0.63 | 210/261 | 0.87 | 37/434 | 0.28 |
| Hypertension | ||||||
| Yes | 29/159 | 78/110 | 13/175 | |||
| No | 54/266 | 0.67 | 149/171 | 0.27 | 25/295 | 0.71 |
| Stroke | ||||||
| Yes | 4/19 | 12/11 | 4/19 | |||
| No | 79/406 | 0.89 | 215/270 | 0.46 | 34/451 | 0.08 |
| Stage of Disease | ||||||
| Advanced stage | 62/225 | 140/147 | 19/268 | |||
| Early stage | 32/253 | 0.001 | 109/176 | 0.01 | 23/262 | 0.51 |
Pearson Correlation Analyses
We found significant correlations (all P<0.0001) between pain and depressed mood (r=0.294), pain and fatigue (r=0.385) and depressed mood and fatigue (r=0.495). Thirty-three percent (n=198) of the sample did not report any severe symptoms. The most prevalent symptom was fatigue (n= 176; 29%), followed by pain (n=97; 16%) and depressed mood (n=44; 7%). Two percent of the sample (n=14) reported all three symptoms of severe intensity; 9% (n=54) reported severe pain and fatigue; 3% (n=16) reported severe fatigue and depressed mood; 0.5% (n=3) reported pain and depressed mood. As many as 29% (n=176) reported severe fatigue only, followed by pain only (n=26; 4%), and lastly, depressed mood (n=11; 2%).
Tree-Based Analysis
We also classified subjects into several homogeneous subgroups using a tree-based analysis. Figure 1 shows the final optimized tree structure. The number of subjects within each node of the tree is given in parentheses. The splitting variables are given under the nodes. The terminal nodes are represented by squares. The diagram under the tree structure shows the percentages of the three symptoms (severe pain, severe fatigue, and severe depression, from left to right) in the terminal nodes. The top and bottom bars in each diagram define the unit of one. Table 2 reports the numbers of subjects with different symptoms in each node.
Table 2.
Risk Estimates for Symptoms of Pain, Depressed Mood, and Fatigue
| Severe Symptoms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Node Number |
Node Size |
Node Description |
Pain |
Fatigue |
Depressed Mood |
||||||
| Yes (%) | OR (95% CI) |
P | Yes (%) | OR (95% CI) |
P | Yes (%) | OR (95% CI) |
P | |||
| 0 | 599 | All individuals | 97 (16.19) | 260 (43.41) | 44 (7.35) | ||||||
| 1 | 287 | Advanced stage | 62 (21.60) | 2.18 (1.34,3.55) |
0.002 | 140 (48.78) | 1.93 (1.32,2.82) |
<0.001 | 19 (6.62) | 1.04 (0.51,2.13) |
0.912 |
| 2 | 312 | Early stagea | 32 (11.23) | 109 (38.25) | 23 (8.07) | ||||||
| 3 | 71 | IL-8 T-251A={TT}a | 9 (12.68) | 2.47 (1.10,5.55) |
0.028 | 26 (36.62) | 2.07 (1.16,3.70) |
0.014 | 8 (11.27) | 0.37 (0.14,1.00) |
0.049 |
| 4 | 216 | IL-8 T-251A={AT,AA} | 50 (24.15) | 109 (52.66) | 10 (4.83) | ||||||
| 5 | 144 | Malea | 10 (6.94) | 2.9 (1.18,7.11) |
0.020 | 56 (38.89) | 1.32 (0.78,2.25) |
0.307 | 9 (6.25) | 1.86 (0.77,4.51) |
0.170 |
| 6 | 168 | Female | 25 (14.88) | 64 (38.10) | 16 (9.52) | ||||||
| 7 | 127 | No hypertensiona | 33 (25.98) | 0.78 (0.38,1.59) |
0.498 | 71 (55.91) | 0.98 (0.52,1.85) |
0.962 | 8 (6.30) | 0.8 (0.16,3.89) |
0.778 |
| 8 | 89 | Hypertension | 14 (21.54) | 35 (53.85) | 3 (4.62) | ||||||
| 9 | 68 | IL-1A C-889T={TT,CT} | 4 (5.88) | 0.43 (0.08,2.33) |
0.326 | 20 (29.41) | 0.38 (0.13,1.06) |
0.065 | 2 (2.94) | 0.3 (0.06,1.57) |
0.153 |
| 10 | 76 | IL-1A C-889T={CC}a | 5 (6.67) | 35 (46.67) | 6 (8.00) | ||||||
| 11 | 92 | IL-10RB Lys47Glu= {Lys_Lys}a |
14 (15.22) | 0.85 (0.35,2.05) |
0.715 | 42 (45.65) | 0.49 (0.25,0.92) |
0.028 | 6 (6.52) | 2.58 (0.65,10.26) |
0.177 |
| 12 | 76 | IL-10RB Lys47Glu= {Lys_Glu,Glu_Glu} |
11 (14.47) | 22 (28.95) | 10 (13.16) | ||||||
| 13 | 61 | TNFB Arg13Cys= {Cys_Cys,Arg_Cys} |
12 (19.67) | 0.52 (0.22,1.21) |
0.132 | 35 (57.38) | 1.37 (0.64,2.97) |
0.420 | 4 (6.56) | 1.61 (0.35,7.40) |
0.541 |
| 14 | 66 | TNFB Arg13Cys= {Arg_Arg}a |
19 (30.16) | 34 (53.97) | 4 (6.35) | ||||||
Reference for evaluating ORs and P-values.
We observed that of the candidate variables (i.e., age, sex, stage of disease, comorbidity, and the 59 genetic variants), the initial split was on the variable “stage of disease,” suggesting that this is the most important factor for pain, depressed mood and fatigue. Described below are the genetic and non-genetic markers by stage of disease.
Advanced Stage of Disease
Among those with advanced stage of disease (Stage IIIB and IV), the genetic variant IL-8-T251A was the most strongly associated with pain, fatigue, and depressed mood, indicating that there is a joint effect between IL-8-T251A and advanced stage of lung cancer. Patients with IL-8-T251A T/T genotypes were less likely to have severe pain (12.68% vs. 24.15%) or severe fatigue (36.62% vs. 52.66%) but more likely to have severe depression (11.27% vs. 4.83%) relative to those with A/T and A/A genotypes (Table 2). Further split was selected on the variable hypertension, among the subset of individuals with advanced stage of lung cancer and genotype A/T or A/A for the genetic variant IL-8-T251A. However, we did not observe significant increase/decrease for the risk of severe pain, fatigue and depression - according to the ORs and P-values. Moreover, for the subgroup of patients with: 1) advanced stage of lung cancer; 2) genotype A/T or A/A for genetic variant IL-8-T251A; and 3) without hypertension, another split was selected on the genetic variable TNF-b Arg13Cys. But no significant increase/decrease for any of the symptoms was observed.
Early Stage of Disease
Among patients with early-stage disease, the variables sex, IL-1A C-889T in males, and IL-10RB Lys47Glu in females were factors that further selected subsets in this study population. More women experienced severe pain than men (14.88% vs. 6.94%). More women with the Lys_Lys genotype of IL-10RB Lys47Glu experienced severe fatigue than women with Lys_Glu or Glu_Glu genotypes (45.65% vs. 28.95%). More men with the C/C genotype of IL-1A C-889T experienced severe fatigue than men with C/T or T/T genotypes (46.67% vs. 29.41%). This observation indicates that there is a joint effect of sex and genetic polymorphisms on symptoms in patients with early-stage disease (Table 2).
Assessment of Risks
We report the corresponding ORs and P-values for each node in the tree model. Relative to those with early-stage lung cancer, patients with advanced disease had a 2.18 times higher risk of severe pain (P=0.0017; OR=2.18, 95% confidence interval [CI]=1.34, 3.55) and a 1.93 times higher risk of severe fatigue (P=0.0007; OR=1.93, 95% CI=1.32, 2.82). Among those with advanced disease, patients with the IL-8-T251A A/T or A/A genotype had a 2.47 times higher risk of severe pain (P=0.0278; OR=2.47, 95% CI=1.10, 5.55) and a 2.07 times higher risk of severe fatigue (P=0.0135; OR=2.07, 95% CI=1.16, 3.70) compared with those with the T/T genotype. Interestingly, we observed that carriers of genotype A/T or A/A had a 0.37 times lower risk of severe depression compared to those with genotype T/T (P=0.049; OR=0.37, 95% CI=0.14, 1.00).
Among those with early-stage NSCLC, women had a 2.90 times higher risk of severe pain (P=0.020; OR=2.90, 95% CI=1.18, 7.11). Furthermore, women with early-stage disease and with genotype Lys_Glu or Glu_Glu in the IL-10 gene had a 0.49 times lower risk of severe fatigue compared to those with genotype Lys_Lys (P=0.0275; OR=0.49, 95% CI=0.25, 0.92).
Among men with early-stage lung cancer, the split was selected on the genetic variable IL-1A C-889T. A marginal significance was observed for the carriers of genotype C/T or T/T, who had a 0.38 times lower risk of severe fatigue compared with those with genotype C/C (P-=0.0654; OR=0.38, 95% CI=0.13, 1.06).
Discussion
In this study, we assessed the role of host genetic susceptibility for multiple symptoms by applying a tree-based multivariate approach for exploring 59 variants in 37 inflammation genes as potential biologic markers of risk for severe pain, depressed mood, and fatigue in 599 non-Hispanic white patients with NSCLC. We identified the associated risks from both genetic and non-genetic factors and, in particular, found important associations between severe symptoms and SNPs of IL-8. To our knowledge, this study is among the first to simultaneously and systematically examine multiple genes in the inflammation pathway as biomarkers of risk for the multiple symptom outcomes of pain, depressed mood, and fatigue in lung cancer patients. Of the 59 SNPs assessed, the T251A polymorphism in IL-8 is most significant among patients with advanced stage of disease, with the A/T or A/A genotype conferring about twice the risk of severe pain and severe fatigue compared with the T/T genotype.
Among the important findings are the joint effects of IL-8 genotypes and advanced stage of disease. Polymorphisms in the IL-8 gene result in variable IL-8 levels. The A-allele of the -251T/A polymorphism, for example, results in higher production of IL-8 (33). IL-8 is a chemokine and a major mediator of inflammatory response. Elevated IL-8 levels have been observed among patients with chronic back pain (34, 35), fibromyalgia,(36) and chronic fatigue syndrome (37). It is also hypothesized that IL-8 is directly involved in nociceptive signal transduction (38). IL-8 also was shown to evoke dose-dependent hyperalgesia, which could be blocked by anti-IL-8 serum (39).
Our study is among the first to show that IL-8-251T/A SNP is an important factor for fatigue in lung cancer patients. Most studies of fatigue in cancer patients focus on only a few cytokines--with studies showing significance for the IL-1 receptor antagonist (40) (IL-1RA), TNF-α receptor II (41), and IL-6, failing to assess a more comprehensive number of genes. Interestingly, IL-8 has been shown to induce the production of other cytokines, including IL-6 and TNF-α (42). Consistent with our findings, the influence of IL-8 on fatigue also has been shown in studies of chronic fatigue syndrome (CFS). Natelson and colleagues (37) compared spinal fluid samples from patients who met the 1994 case definition for CFS and from healthy controls. Of the 11 cytokines detectable in spinal fluid, they found that levels of IL-8 were higher in patients with sudden, influenza-like onset of CFS than in patients with gradual onset or in controls. They concluded that immune dysregulation within the central nervous system may be involved in CFS.
Consistent with other studies, we found that sex was an important grouping among patients with early-stage NSCLC and that variants in the IL-10 receptor were relevant for severe symptoms among women. Located in chromosome 1, IL-10 is known as the human cytokine synthesis inhibitory factor. IL-10 has been shown to suppress the production and function of all pro-inflammatory cytokines (43), and ongoing investigations focus on IL-10’s potential use for pain therapy. IL-10 activity is mediated by its specific cell surface receptor complex, which is expressed on a variety of cells. The IL-10 receptor complex consists of at least two separate receptor chains, a ligand-binding IL-10R_ chain (44) and an IL-10 chain (45), which are essential for signal transduction. Polymorphisms in genes encoding the IL-10 receptor could interrupt IL-10-mediated immune regulation. IL-10RB rs2834167 codes for a nonsynonymous substitution and is considered to be functional. Associations between this IL-10RB polymorphism and several diseases have been reported (46-48). Our findings provide preliminary evidence of the important role of IL-10 for severe fatigue, particularly in women.
We also observed the potential significance of IL-1, a pro-inflammatory cytokine, particularly among men. Studies have shown the role of IL-1 in pain response and postoperative morphine consumption (49, 50), depression (51), and fatigue (52). The IL-1 family contains two agonists (IL-1α and IL-1β), an inhibitor (IL-1 receptor antagonist [IL-1RA]), and two receptors (biologically active IL-1R and inactive type II IL-1R). IL-1 coordinates systemic host defense responses to injury and within the central nervous system (53). Both IL-1α and IL-1β induce the expression of other pro-inflammatory genes. IL-1 leads to the expression of cyclooxygenase-2 (Cox-2) and inducible nitric oxide syntheses (iNOS). Cox-2 (54, 55) and NOS (56-66) inhibitors are therapeutic targets for pain, depression, and fatigue.
This paper is one of the first to apply the generalized classification tree approach to genetic and epidemiologic data to better understand symptoms in cancer patients. This tree-based approach allows for the analysis of multiple correlated outcomes (e.g., pain, fatigue, and depressed mood) simultaneously. Thus, the correlation of these symptoms could be incorporated into the model by defining the joint probability distribution. Compared with multiple univariate regressions (which analyze one symptom at a time), this approach would be more informative to identify the common genetic mechanism underlying correlated symptoms of pain, depressed mood, and fatigue.
Although, in general, tree-based approaches are subject to over-fitting (increase in number of models but with no significant improvement in model accuracy) (67, 68), among the strengths of our current approach is that it does not require a minimum sample size for the number of variables analyzed in the model (69). Furthermore, after the initial tree was grown, we used the two-step approach, evaluating the cost-complexity of a nested sequence of subtrees and performing cross-validation to select an optimal subtree (69) to avoid over-fitting. We also used a nonparametric multivariate approach. Compared to parametric approaches, nonparametric approaches do not assume a specific distribution for the outcome variables (70) and can be more suitable to deal with a relatively large number of variables in the study (69).
There are limitations to this study. One could argue that although clinical practice guidelines suggest the use of a one-item score to initiate clinical intervention (26), using a single item to measure symptoms is inadequate in assessing pain, depressed mood and fatigue. However, studies have found that single-item questions correlate well with multi-item symptom assessment tools (71-74). We also acknowledge that although we assessed several SNPs in several genes, there is more genetic variation for each gene, and inclusion of multiple SNPs per gene would have allowed examination of haplotypes, which may have yielded different outcomes. Furthermore, the tested SNP may not necessarily be the functional SNP, but may be in linkage disequilibrium with the true functional SNP. It also is known that there is considerable interaction between cytokines that initiate gene activation and suppression, and thus, more genes with functional significance should be assessed. It also could be argued that dichotomization of symptom data may have led to losing considerable data variability. However, clinical studies (23) have adapted this approach to simplify the analysis and for better interpretation of the results (24, 25). In some situations, the dichotomization of data even results in higher power for statistical analysis (23, 75, 76) .
In conclusion, the occurrence of severe symptoms is a significant concern for lung cancer patients. Using novel statistical methodologies, we have shown, in a preliminary fashion, that inflammation, particularly from IL-8 and IL-10, may be a common biological mechanism that underlies the occurrence of pain, depressed mood and fatigue and that these genes may serve as potential targets for treating multiple symptoms of cancer. In addition, future studies that dissect the independent contributions of the disease evolution and host factors involved in symptom outcomes will have high clinical significance. In the future, we anticipate that genetic profiling could become an integral component of an individualized treatment program for symptoms in patients with lung cancer.
Acknowledgments
This study was funded by grants CA109043, CA128069, CA127219-02, and DE022891 from the National Institutes of Health and is supported in part by the NIH/NCI through M. D. Anderson’s Cancer Center Support Grant CA016672 and Faculty fellowship (Wang) from The University of Texas M. D. Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors declare no conflicts of interest.
References
- (1).Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- (2).American Cancer Society Cancer facts & figures 2010. 2011 Available from http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010.
- (3).Sarna L, Cooley ME, Brown JK, et al. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17(5):455–467. [PMC free article] [PubMed] [Google Scholar]
- (4).Engels EA, Wu X, Gu J, et al. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67(13):6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- (5).Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–2036. doi: 10.1097/jto.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- (7).Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6(4):824–833. doi: 10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- (8).Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- (9).Reyes-Gibby CC, Wu X, Spitz M, et al. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9(8):777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- (11).Lundstrom S, Furst CJ. Symptoms in advanced cancer: relationship to endogenous cortisol levels. Palliat Med. 2003;17(6):503–508. doi: 10.1191/0269216303pm780oa. [DOI] [PubMed] [Google Scholar]
- (12).Mantovani G, Maccio A, Lai P, et al. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog. 1998;9(2):99–106. doi: 10.1615/critrevoncog.v9.i2.10. [DOI] [PubMed] [Google Scholar]
- (13).Reyes-Gibby CC, El Osta B, Spitz MR, et al. The influence of tumor necrosis factor-alpha - 308 G/A and IL-6 -174 G/C on pain and analgesia response in lung cancer patients receiving supportive care. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3262–3267. doi: 10.1158/1055-9965.EPI-08-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Reyes-Gibby CC, Shete S, Yennurajalingam S, et al. Genetic and nongenetic covariates of pain severity in patients with adenocarcinoma of the pancreas: assessing the influence of cytokine genes. J Pain Symptom Manage. 2009;38(6):894–902. doi: 10.1016/j.jpainsymman.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rausch SM, Gonzalez BD, Clark MM, et al. SNPs in PTGS2 and LTA predict pain and quality of life in long term lung cancer survivors. Lung Cancer. 2012;77(1):217–223. doi: 10.1016/j.lungcan.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Reyes-Gibby CC, Spitz M, Wu X, et al. Cytokine genes and pain severity in lung cancer: exploring the influence of TNF-alpha-308 G/A IL6-174G/C and IL8-251T/A. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2745–2751. doi: 10.1158/1055-9965.EPI-07-0651. [DOI] [PubMed] [Google Scholar]
- (17).Rausch SM, Clark MM, Patten C, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116(17):4103–4113. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav Immun. 2008;22(8):1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lee W, Liu Y. Simultaneous multiple response regressionandinverse covariance matrix estimation via penalized Gaussian maximum likelihood. J Multivar Anal. 2012;111:241–255. doi: 10.1016/j.jmva.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Breiman L, Friedman JH. Predicting multivariate responses in multiple linear regression. J Roy Statist Soc Series B. 1997;59:3–54. [Google Scholar]
- (21).Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- (22).Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- (23).Yue S, Minge X. A note on dichotomization of continuous response variable in the presence of contamination and model misspecification. Stat Med. 2010;29(21):2200–2214. doi: 10.1002/sim.3966. [DOI] [PubMed] [Google Scholar]
- (24).MacCallum RC, Zhang S, Preacher KJ, Rucker D. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- (25).Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- (26).National Comprehensive Cancer Network [Accessed September 12, 2007];WHAT IS THE TITLE OF THE ONLINE ARTICLE / GUIDELINE? [updated 2007 July 07]. Available from http://www.nccn.org/professionals/physician_gls/PDF/pain.pdf. URL DIDN’T WORK.
- (27).Reyes-Gibby CC, Spitz M, Wu X, et al. Cytokine genes and pain severity in lung cancer: exploring the influence of TNF-alpha-308 G/A IL6-174G/C and IL8-251T/A. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2745–2751. doi: 10.1158/1055-9965.EPI-07-0651. [DOI] [PubMed] [Google Scholar]
- (28).Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain. 2009;10(11):1146–1150. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhang HP. Classification trees for multiple binary responses. J Am Stat Assoc. 1998;93(441):180–193. [Google Scholar]
- (30).Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Wadsworth; Pacific Grove CA: 1984. [Google Scholar]
- (31).Cox DR. Analysis of multivariate binary data. J Roy Statist Soc Series C-Applied Statistics. 1972;21(2):113–120. [Google Scholar]
- (32).Zhao LP, Prentice RL. Correlated binary regression using a quadratic exponential model. Biometrika. 1990;77(3):642–648. [Google Scholar]
- (33).Puthothu B, Krueger M, Heinze J, Forster J, Heinzmann A. Impact of IL8 and IL8-receptor alpha polymorphisms on the genetics of bronchial asthma and severe RSV infections. Clin Mol Allergy. 2006;4:2. doi: 10.1186/1476-7961-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976 ) 2002;27(9):911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- (35).Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- (36).Ang DC, Moore MN, Hilligoss J, Tabbey R. MCP-1 and IL-8 as Pain biomarkers in fibromyalgia: a pilot study. Pain Med. 2011;12(8):1154–1161. doi: 10.1111/j.1526-4637.2011.01179.x. [DOI] [PubMed] [Google Scholar]
- (37).Natelson BH, Weaver SA, Tseng CL, Ottenweller JE. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin Diagn Lab Immunol. 2005;12(1):52–55. doi: 10.1128/CDLI.12.1.52-55.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Oh SB, Tran PB, Gillard SE, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21(14):5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ribeiro RA, Vale ML, Thomazzi SM, et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387(1):111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- (40).Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (Abeta 1-42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005;159(1-2):66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- (43).Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- (44).Ho AS, Liu Y, Khan TA, et al. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci U S A. 1993;90(23):11267–11271. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kotenko SV, Krause CD, Izotova LS, et al. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16(19):5894–5903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Gong QM, Kong XF, Yang ZT, et al. Association study of IFNAR2 and IL10RB genes with the susceptibility and interferon response in HBV infection. J Viral Hepat. 2009;16(9):674–680. doi: 10.1111/j.1365-2893.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- (47).Sivula J, Turpeinen H, Volin L, Partanen J. Association of IL-10 and IL-10Rbeta gene polymorphisms with graft-versus-host disease after haematopoietic stem cell transplantation from an HLA-identical sibling donor. BMC Immunol. 2009;10:24. doi: 10.1186/1471-2172-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hikami K, Ehara Y, Hasegawa M, et al. Association of IL-10 receptor 2 (IL10RB) SNP with systemic sclerosis. Biochem Biophys Res Commun. 2008;373(3):403–407. doi: 10.1016/j.bbrc.2008.06.054. [DOI] [PubMed] [Google Scholar]
- (49).Neeb L, Hellen P, Boehnke C, et al. IL-1beta stimulates COX-2 dependent PGE synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011;6(3):e17360. doi: 10.1371/journal.pone.0017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Martucci C, Trovato AE, Costa B, et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137(1):81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- (51).Baune BT, Dannlowski U, Domschke K, et al. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry. 2010;67(6):543–549. doi: 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- (52).Von Ah DM, Kang DH, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31(2):134–144. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- (53).Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78(2):151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- (54).Muller N, Riedel M, Schwarz MJ. Psychotropic effects of COX-2 inhibitors--a possible new approach for the treatment of psychiatric disorders. Pharmacopsychiatry. 2004;37(6):266–269. doi: 10.1055/s-2004-832682. [DOI] [PubMed] [Google Scholar]
- (55).Muller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- (56).Ramachandran R, Ploug KB, Hay-Schmidt A, et al. Nitric oxide synthase (NOS) in the trigeminal vascular system and other brain structures related to pain in rats. Neurosci Lett. 2010;484(3):192–196. doi: 10.1016/j.neulet.2010.08.050. [DOI] [PubMed] [Google Scholar]
- (57).Chen Y, Boettger MK, Reif A, et al. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. 2010;6:13. doi: 10.1186/1744-8069-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Presciuttini S, Curcio M, Chillemi R, et al. Promoter polymorphisms of the NOS3 gene are associated with hypnotizability-dependent vascular response to nociceptive stimulation. Neurosci Lett. 2009;467(3):252–255. doi: 10.1016/j.neulet.2009.10.056. [DOI] [PubMed] [Google Scholar]
- (59).Dolan S, Field LC, Nolan AM. The role of nitric oxide and prostaglandin signaling pathways in spinal nociceptive processing in chronic inflammation. Pain. 2000;86(3):311–320. doi: 10.1016/S0304-3959(00)00262-1. [DOI] [PubMed] [Google Scholar]
- (60).Handy RL, Wallace P, Moore PK. Inhibition of nitric oxide synthase by isothioureas: cardiovascular and antinociceptive effects. Pharmacol Biochem Behav. 1996;55(2):179–184. doi: 10.1016/s0091-3057(96)00051-2. [DOI] [PubMed] [Google Scholar]
- (61).Joubert J, Malan SF. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21(4):537–560. doi: 10.1517/13543776.2011.556619. [DOI] [PubMed] [Google Scholar]
- (62).Kumar A, Garg R, Gaur V, Kumar P. Nitric oxide modulation in protective role of antidepressants against chronic fatigue syndrome in mice. Indian J Pharmacol. 2011;43(3):324–329. doi: 10.4103/0253-7613.81506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Kumar A, Garg R, Kumar P. Nitric oxide modulation mediates the protective effect of trazodone in a mouse model of chronic fatigue syndrome. Pharmacol Rep. 2008;60(5):664–672. [PubMed] [Google Scholar]
- (64).Sendur OF, Turan Y, Tastaban E, Yenisey C, Serter M. Serum antioxidants and nitric oxide levels in fibromyalgia: a controlled study. Rheumatol Int. 2009;29(6):629–633. doi: 10.1007/s00296-008-0738-x. [DOI] [PubMed] [Google Scholar]
- (65).Maes M, Mihaylova I, Kubera M, Bosmans E. Not in the mind but in the cell: increased production of cyclo-oxygenase-2 and inducible NO synthase in chronic fatigue syndrome. Neuro Endocrinol Lett. 2007;28(4):463–469. [PubMed] [Google Scholar]
- (66).Nijs J, Van de Velde B, De Meirleir K. Pain in patients with chronic fatigue syndrome: does nitric oxide trigger central sensitisation? Med Hypotheses. 2005;64(3):558–562. doi: 10.1016/j.mehy.2004.07.037. [DOI] [PubMed] [Google Scholar]
- (67).Oates T, Jensen D. The effects of training set size on decision tree complexity. Proceedings of the Fourteenth International Conference on Machine Learning; San Francisco: Morgan Kaufmann; 1997. pp. 254–262. [Google Scholar]
- (68).Morgan J, Daugherty R, Hilchie A, Carey B. Sample size and modeling accuracy of decision tree based data mining tools. Academy of Information and Management Sciences Journal. 2003;6(2):71–99. [Google Scholar]
- (69).Zhang HP. Classification trees for multiple binary responses. J Am Stat Assoc. 1998;93:180–193. [Google Scholar]
- (70).Tan P-N, Steinbach M, Kumar V. Introduction to data mining. Addison Wesley; Boston: 2006. [Google Scholar]
- (71).Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55(2):195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- (72).Jensen MP, Schnitzer TJ, Wang H, et al. Sensitivity of single-domain versus multiple-domain outcome measures to identify responders in chronic low-back pain: pooled analysis of 2 placebo-controlled trials of etoricoxib. Clin J Pain. 2012;28(1):1–7. doi: 10.1097/AJP.0b013e3182236209. [DOI] [PubMed] [Google Scholar]
- (73).Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35(1):20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- (74).Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34(6):628–638. doi: 10.1016/j.jpainsymman.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Risch N, Zhang H. Extreme discordant sib pairs for mapping quantitative trait loci in humans. Science. 1995;268(5217):1584–1589. doi: 10.1126/science.7777857. [DOI] [PubMed] [Google Scholar]
- (76).Abecasis GR, Cookson WO, Cardon LR. The power to detect linkage disequilibrium with quantitative traits in selected samples. Am J Hum Genet. 2001;68(6):1463–1474. doi: 10.1086/320590. [DOI] [PMC free article] [PubMed] [Google Scholar]