Abstract
Posttraumatic stress disorder (PTSD) is difficult to treat and current PTSD treatments are not effective for all people. Despite limited evidence for its efficacy, some clinicians have implemented biofeedback for PTSD treatment. As a first step in constructing an effective biofeedback treatment program, we assessed respiration, electroencephalography (EEG) and heart rate variability (HRV) as potential biofeedback parameters for a future clinical trial. This cross-sectional study included 86 veterans; 59 with and 27 without PTSD. Data were collected on EEG measures, HRV, and respiration rate during an attentive resting state. Measures were analyzed to assess sensitivity to PTSD status and the relationship to PTSD symptoms. Peak alpha frequency was higher in the PTSD group (F(1,84) = 6.14, p = 0.01). Peak high-frequency HRV was lower in the PTSD group (F(2,78) = 26.5, p<0.00005) when adjusting for respiration rate. All other EEG and HRV measures and respiration were not different between groups. Peak high-frequency HRV and peak alpha frequency are sensitive to PTSD status and may be potential biofeedback parameters for future PTSD clinical trials.
Keywords: Posttraumatic stress disorder, Combat veterans, Heart rate variability, Peak alpha frequency, Biofeedback
Introduction
Posttraumatic stress disorder (PTSD) is a serious and growing health issue with high personal and societal costs. PTSD affects approximately 7.7 million American adults (3.5 %) with veterans being especially vulnerable to acquiring it (Kessler et al. 2005). Approximately 30.9 % of Vietnam veterans and 5–20 % of Iraq (OIF) and Afghanistan (OEF) veterans report PTSD symptoms in their lifetime. As many as 50 % recent veterans screen positive for PTSD symptoms when returning from deployment (Ramchand et al. 2010). People with PTSD not only experience its debilitating symptoms, but also increased psychiatric and physical co-morbid conditions (Seal et al. 2011; Stecker et al. 2010). Additionally, people with PTSD have higher suicidal ideation and attempt suicide more often (Jakupcak et al. 2009). This is especially true of OIF/OEF veterans whose suicide rates rose significantly from 2005 to 2007 (Bruce 2010). Overall, the quality of life of Vietnam and OIF/OEF veterans with PTSD is severely impacted resulting in poor functioning, lower objective living conditions, and life satisfaction (Schnurr et al. 2009). The chronicity of symptoms, increased co-morbidities, marked functional impairment, and economic costs render PTSD a serious public health concern (Brunello et al. 2001; Solomon and Davidson 1997).
PTSD may occur when a person has been exposed to a traumatic event that involves actual or threatened death or serious injury or threat to the physical integrity of self or others; and the person’s response involves intense fear, helplessness, or horror. People who acquire PTSD after a traumatic event then experience a constellation of symptoms in three categories: hyper arousal, re-experiencing, and avoidance. People with PTSD experience symptoms of increased arousal and sympathetic nervous system hyperactivity that were not present before the trauma (Buckley et al. 2004; Pole 2007). These symptoms include hyper-vigilance, difficulty concentrating, difficulty falling or staying asleep, irritability or outbursts of anger, or exaggerated startle response. People with PTSD also show physiological evidence of increased sympathetic dominance such as decreased heart rate variability (HRV) (Cohen et al. 1998; Lakusic et al. 2007; Mellman et al. 2004), increased heart rate (Blanchard et al. 1996; Muraoka et al. 1998; Pole 2007), and increased blood pressure (Pole 2007). People with PTSD also persistently re-experience their trauma through recurrent and intrusive distressing recollections of the event or distress when exposed to cues that symbolize or resemble an aspect of the trauma (American Psychiatric Association 1994; Diagnostic and Statistical Manual of Mental Disorders: DSM-IV). These re-experiencing symptoms may be a result of dysfunction in the prefrontal cortex which regulates fear extinction. This concept is supported by multiple neuro-imaging studies demonstrating prominent hypo-activations in the prefrontal cortex, anterior cingulate cortex, and thalamus in people with PTSD compared to controls (Etkin and Wager 2007). These regions are associated with the experience or regulation of emotion. Prefrontal cortex activation is also inversely correlated with PTSD symptom severity (Shin et al. 2006). Finally, PTSD is characterized by pervasive avoidance symptoms such as avoiding thoughts, feelings, conversations, people, places, or activities that remind them of their trauma. Avoidance behaviors are theorized to maintain PTSD symptoms in that they interfere with processing of traumatic memories and habituation/relearning to conditioned stimuli. Thus, PTSD diagnosis currently consists of hyper-arousal in response to intrusive symptoms and trauma-related cues, intense re-experiencing symptoms, and avoidance of emotions, thoughts, and situations that could evoke these experiences. More recently a four-factor rather than three factor diagnostic model has received strong evidence for its use and has been proposed for the DSM-V (Friedman et al. 2011).
Unfortunately because of PTSD’s complex psychopathology and concomitant co-morbid conditions, it is difficult to treat, and current therapies are not effective for all people. Trauma-focused psychotherapy and pharmaceuticals have the most evidence for PTSD treatment (Berg et al. 2007; Defense 2010). However, many people with PTSD refuse or drop out of these interventions (Grunert et al. 2007; Lockwood et al. 2009). Thus, additional treatments are needed for people with PTSD.
Biofeedback may be a potential treatment for PTSD considering its pathology in multiple physiological systems. However, there is no consensus on the most appropriate biofeedback measures for PTSD as of yet. Current evidence in the literature includes studies for electroencephalography (EEG), HRV, respiration, temperature, and electromyography (EMG).
EEG
EEG neurofeedback for PTSD was initiated with an alpha/ theta neurofeedback protocol for 29 combat veterans with PTSD and reported improvements in personality scores and decreases in psychotropic medicine use (Peniston and Kulkosky 1991). Two reviews have since examined neurofeedback for anxiety disorders with inconclusive results (Hammond 2005; Moore 2000). Additionally, non-clinical cross-sectional studies report multiple alterations in EEG parameters in people with PTSD compared to controls: increased theta over central regions (Begic et al. 2001); increased beta activity over frontal, central cortex (Begic et al. 2001; Jokic-Begic and Begic 2003); decreased alpha power (Jokic-Begic and Begic 2003; Veltmeyer et al. 2006); increased theta/alpha ratio (Veltmeyer et al. 2006); and increased alpha power in the left hemisphere compared to the right (Jokic-Begic and Begic 2003; Metzger et al. 2004; Rabe et al. 2006).
HRV
HRV feedback for PTSD was tested in one uncontrolled study and three controlled studies. The uncontrolled study administered HRV feedback weekly for 4 weeks to 10 combat veterans, 5 with and 5 without PTSD, and found increased cardiac coherence [defined as peak power/(total power—peak power)2] in all participants (Ginsberg et al. 2010). A controlled study found reduced depression symptoms and improvements in HRV indices for the HRV biofeedback group compared to a progressive muscle relaxation control while both groups significantly reduced PTSD and insomnia symptoms (Zucker et al. 2009). Another controlled study (n = 39) used four sessions of HRV feedback compared to treatment-as-usual and reported no group differences on PTSD and depression outcomes (Lande et al. 2010). Conversely, a final controlled study using eight sessions of HRV biofeedback compared to treatment-as-usual found significant improvements in HRV indices in the biofeedback group compared to controls (Tan et al. 2011). While HRV is most often reported lower in people with PTSD and HRV biofeedback studies appear to improve HRV indices, there is no evidence to date that it can actually improve PTSD or PTSD-related symptoms.
Respiration
Only one study on respiration biofeedback has been conducted. It compared paced breathing to paced counting in thirty-six alcohol-dependent inpatients scoring high in trait anxiety. The paced breathing group had reduced self-rated tension, state anxiety, and skin conductance levels compared to the control group (Clark and Hirschman 1990).
Temperature
A mixed mind–body intervention used temperature feedback as one of its methods in 82 adolescents with PTSD compared to a wait list control and found decreased PTSD symptoms in the mind–body group (Gordon et al. 2008).
EMG
Two studies have examined EMG biofeedback’s effect on PTSD symptoms. One uncontrolled study of six veterans with PTSD reported slight to marked improvement in the State-Trait Anxiety Inventory Scale and the Beck Depression Inventory, a decrease in overall Minnesota Multiphasic Personality Inventory scores, and lowered EMG and subjective tension ratings for all participants (Hickling et al. 1986). Another controlled study of 14 post-traumatic headache patients found improvements in the EMG group compared to controls regarding headache-free days but not on other measures (Tatrow et al. 2003).
EEG and HRV have been most widely studied in cross-sectional studies and as biofeedback parameters; respiration, temperature, and EMG have limited cross-sectional and biofeedback studies. Unfortunately, many of the conducted studies are small, report contradictory findings, and/ or have not been replicated. Not knowing the potentially viable biofeedback parameters to use in clinical practice is an obstacle in the effective biofeedback treatment of PTSD. Also, in biofeedback therapy there are many potential parameters to choose from and the therapy is time consuming. Thus, additional evidence for the most appropriate biofeedback measure would be helpful in guiding future clinical practice and research studies.
In order to collect additional evidence, we conducted a cross-sectional study to assess group differences in veterans with and without PTSD on respiration, EEG and HRV measures. The rationale for this study was to use rigorous methods to provide more evidence for physiological changes in people with PTSD and ascertain potentially sensitive biofeedback measures for future clinical practice and research studies. This cross-sectional study’s purpose was to evaluate respiration and a number of EEG and HRV outcomes examined in previous cross-sectional studies or already being used in clinical biofeedback protocols in veterans with and without PTSD. Because PTSD status as determined by the current diagnostic criteria may not capture the full relationship between physiological measures and PTSD symptoms, a secondary analysis was incorporated to evaluate these relationships. The EEG measures included delta, theta, alpha, and beta amplitude, frontal alpha asymmetry, and peak alpha frequency. The HRV measures included heart rate and frequency domain measures. The study hypothesis was that the PTSD group, or those with greater PTSD symptoms, would have decreased theta and alpha EEG amplitude and HRV, and increased frontal alpha asymmetry, peak alpha frequency, heart rate, and respiration rate compared to the no-PTSD group based on previous cross-sectional and treatment studies as described above.
Methods
Participants
Potential participants were recruited through flyers at the Portland Veterans Administration Medical Center, Portland Veterans Center, and other veterans groups throughout the Portland Metropolitan area. Veterans were excluded for the following reasons: if they were over 65 years old, had a current significant chronic medical illness, bipolar, schizoaffective, or psychotic disorders; any DSM-IV cognitive disorder; substance dependence disorder within 3 months of the study or current substance use other than two drinks or less of alcohol per day; or sexual assault as primary PTSD events. Participants were asked about traumatic brain injury or blast injury during the telephone screening and Structured Clinical Interview for DSM Disorders when assessing for cognitive impairment. Participants with diagnosed traumatic brain injury were excluded. Participants with asymptomatic blast exposure without loss of consciousness were not excluded. The primary traumatic event needed to be combat exposure to avoid variability from the traumatic exposure type that can elicit PTSD symptoms (i.e. sexual assault, combat, motor vehicle accident) and thus, reduce heterogeneity. Although veterans are mostly male, women were included because 10.26 % of the 22.8 million Veterans Administration users are women. Also, women are more likely to develop PTSD when exposed to similar types of trauma (36 % women compared to 6 % men exposed to interpersonal violence developed PTSD) (Breslau et al. 1998). Participants were required to be in good general health and on stable doses of medications for at least 4 weeks prior to study onset. Medications were recorded and classified according to their effect on EEG or electrocardiography (ECG). The study was approved by the institutional review boards of Portland Veterans Administration Medical Center and Oregon Health & Science University. Written informed consent was obtained from all subjects.
Study Procedures
Participants underwent a telephone screening, a screening visit, and a laboratory visit.
Screening Visit
Study procedures, inclusion/exclusion criteria, and the risks and benefits of participating were explained during a telephone screening. The screening visit included informed consent, completion of questionnaires (Combat Exposure Scale, Beck Depression Inventory, PTSD Checklist Scale) and clinician screening interviews. The Clinician-Administered PTSD Scale for DSM-IV (CAPS) (Blake et al. 1995) established a PTSD diagnosis. Participants in the PTSD group met DSM-IV Criteria A, Criteria B (Re-experiencing, at least 1 item), Criteria C (Avoiding/Numbing, at least 3 items), Criteria D (Hyper-arousal, at least 2 items), Criteria E (Duration of the disturbance is more than 1 month), and Criteria F (The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning). Criteria B, C, and D were met when the frequency plus intensity score were ≥4. Participants in the non-PTSD groups did not meet full syndrome criteria as described above although they may have positively endorsed some items without reaching full DSM-IV PTSD diagnosis. The Structured Clinical Interview for DSM-IV-Patient Edition was performed to screen for excluded DSM-IV disorders and concomitant mental health disorders (First et al. 2002). Combat exposure was determined with the self-report Combat Exposure Scale (CES) (Keane et al. 1989). The Posttraumatic Stress Disorder Checklist Scale (PCL) (Blanchard et al. 1996) and the Beck Depression Inventory (BDI) (Beck et al. 1996) assessed self-report PTSD and depression symptoms. The PCL includes re-experiencing, numbing and avoiding, and hyper-arousal sub-scores as well as a total score.
Laboratory Visit
The laboratory visit occurred in a light and sound attenuated room. Data were collected between 10:30 am and 2:30 pm. Subjects were seated in the laboratory room for 30 min prior to data recording. EEG, ECG, and respiration data were then collected during a 5 min eyes-closed baseline. The baseline condition was designed to keep the participant alert and awake by intermittently responding to auditory tones as previously described (Salinsky et al. 2003). In brief, participants held a trigger button in each hand and clicked the right button after hearing a high tone (2,000 Hz) and the left button after hearing a low tone (1,000 Hz). The tones were presented prior to task initiation to familiarize participants with the tones and check their ability to distinguish between them. The tones were randomly presented for one second with a random and variable between-tone interval (4–14 s). The task was created and presented in EPrime 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA).
Physiological Data Collection and Analysis
EEG
EEG data were recorded at 1,024 Hz through 32 active electrode channels placed in an electrode cap at standardized electrode positions (20 international 10–20 system locations and 12 intermediate “10–10” locations) (Active 2, Biosemi, Amsterdam, Netherlands). Active electrode offsets (rather than impedance as used with standard electrodes) were kept below manufacturer guidelines. Electro-oculogram was measured with electrodes placed inferior to the external canthi on the right eye and superior to the external canthi on the left eye.
All EEG data were processed offline using Brain Vision Analyzer Version 2.0 (Brain Products GmbH, Inc., Gilching, Germany). Data were digitally filtered with 1 Hz high pass, 70 Hz low pass and 60 Hz notch filters, visually inspected, and gross artifacts removed. Independent Component Analysis was performed, and components containing eye blink artifacts were identified and removed (Jung et al. 2000). Two seconds from the beginning of tone onset were removed to exclude tone-related evoked potentials. Channels were referenced to a local average reference (Nunez and Pilgreen 1991). Remaining data were segmented into 2 s epochs. Any segment containing gradient voltage step greater than 125 µV/50 ms, and amplitudes greater than ±75 µV were flagged with semi-automatic artifact rejection and checked manually for removal. Fast fourier transform was done with a 40 % Hanning window and 0.5 Hz resolution over delta (0.5–3.99 Hz), theta (4–7.99 Hz), alpha (8–12.99 Hz), and beta (13–31.99 Hz).
Delta, theta, alpha, and beta amplitude, frontal alpha asymmetry, and peak alpha frequency measures were used because of their clinical use as neurofeedback parameters and/or they were included in previous research studies. Data from EEG channels F3, F4, C3, C4, P3, P4, PO3, PO4, Fz, Cz, Pz, Oz were exported and epochs averaged. Peak alpha frequency was defined as the frequency bin with the largest magnitude between 5.0 and 14.99 Hz. A global EEG variable was created by averaging the F3, F4, C3, C4, P3, P4, PO3, PO4, Fz, Cz, Pz, Oz channels. If the EEG global variable was significant between groups, post hoc variables of: Right- F4, C4, P4, PO4; Left- F3, C3, P3, PO3; Anterior- F3, F4, Fz, C3, C4, Cz; and Posterior- P3, P4, Pz, PO3, PO4, Oz were analyzed. Frontal alpha asymmetry index was calculated as the natural logarithm of spectral power (ln F3–ln F4) (Davidson et al. 2003). Muscle movement was assessed with activity in the 50–70 Hz frequency range (EMG). Participants on psychotropic medications (e.g., benzodiazepines, antidepressants, and sedatives) were noted and this variable used as a covariate because psychotropics affect the EEG (Oken et al. 1995).
ECG
ECG data were collected at 1,024 Hz with active electrodes placed bilaterally just inferior to the clavicles. R waves were detected automatically and beat-to-beat intervals extracted using Brain Vision Analyzer 2.0. Artifacts and RR intervals with erroneous detections or aberrant beats (e.g., premature contractions) were eliminated by visual inspection off-line. RR intervals were imported into Kubios HRV v 2.0 (University of Kuopio, Kuopio, Finland) for heart rate and frequency domain measures. Frequency domain measures include low (LF: 0.04–0.15 Hz) and high (HF: 0.15–0.4 Hz) frequency peak, absolute and normalized amplitude, and LF/HF ratio. HRV analysis parameters included a 100 s window width, 50 % window overlap; autoregressive spectrum model order = 16 with no factorization, and interpolation rate = 4 Hz. The average data length was 275 ± 46 s. Based on the guidelines for heart rate variability standards of measurement, physiological interpretation, and clinical use by the Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology, a minimum of 5 min of data is need to calculate very low-frequency and time domain measures other than heart rate and so these parameters were not calculated (Malik 1996). Kubios also computes nonlinear HRV parameters such as Poincaré plots, approximate entropy, sample entropy, detrended fluctuation analysis, correlation dimension, and recurrence plot analysis; however, the nonlinear parameters were not included in this analysis because they do not have high reliability (Mukherjee et al. 2011), are not widely used and their clinical meaning is not clearly defined, which would limit inferences about their results and/or comparing our findings to other studies. Participants on medications that affected ECG (e.g., beta blockers and calcium channel blockers) were noted and this variable use as a covariate because these medications affect the ECG (Goodnick et al. 2002; Olgin and Zipes 2007).
Respiration
Respiration was measured with a light elastic piezoelectric belt (Ambu-Sleepmate, Maryland) around the participant’s chest near the diaphragm. It was recorded using BioSemi, and the data imported to Matlab r2007a. Peaks were identified, counted, and average breaths per minute calculated in Matlab.
Statistical Analyses
The overall statistical plan included four steps: (1) evaluate the normality of variables, (2) assess any group differences on demographic and lifestyle characteristics by PTSD status, (3) evaluate any group differences by PTSD status on EEG, ECG, and respiration measures with analysis of covariance (primary analyses), and (4) assess the relationship between EEG, ECG, and respiration measure on continuous variables of PTSD symptoms (secondary analyses).
Means, medians, and standard deviations were calculated for each variable and values examined for outliers and normality of distribution. EEG data were log transformed to normalize distributions for statistical analyses. Some group demographic data were analyzed with non-parametric tests (as noted in Table 1) because they were non-normally distributed.
Table 1.
Control and PTSD Veteran groups are similar
| Characteristic | PTSD n = 57 |
No PTSD n = 29 |
Statistics |
|---|---|---|---|
| Gender | 100 % male | 100 % male | |
| Age | 54.4 ± 11.5 | 53.1 ± 11.3 | W (z) = −1.1; p = 0.28 |
| Race (% Caucasian) | 83 % | 93 % | X2 = 9.3; p = 0.10 |
| Education (% > 14 years) | 45 % | 37 % | X2 = 3.1; p = 0.38 |
| Marital status (% married) | 53 % | 22 % | X2 = 9.0; p = 0.01 |
| Era (% Vietnam) | 67 % | 52 % | X2 = 8.0; p = 0.02 |
| (% OEF/OIF) | 21 % | 10 % | |
| Years in service | 6.6 ± 7.0 | 3.2 ± 1.3 | F(1,85) = 6.9; p = 0.01 |
| CAPS | 66.3 ± 19.6 | 11.7 ± 12.8 | W(z) = −7.2; p<0.0005 |
| PTSD checklist | 55.5 ± 12.0 | 30.5 ± 11.8 | W(z) = −6.3; p<0.0005 |
| Combat exposure scale | 25.8 ± 10.0 | 10.0 ± 11.3 | W(z) = −5.3; p<0.0005 |
| Beck depression inventory | 21.3 ± 11.8 | 11.4 ± 8.9 | F(1,85) = 14.6; p = 0.0003 |
The two groups show no significant group differences on important demographic variables (p < 0.004 considered significant with multiple comparison correction). Depression is different between the two groups and is tested as a confounding covariate in subsequent analyses of significant results. Values are reported as means and standard deviation. OEF/OIF-Operation Enduring Freedom/Operation Iraqi Freedom; W- two-sample Wilcoxon rank-sum (Mann–Whitney) test for non-normal variables
Group characteristics differences were assessed with the Chi square test for discrete variables, analysis of variance for normally distributed variables, and the two-sample Wilcoxon rank-sum (Mann–Whitney) test for non-normally distributed variables.
The primary analysis consisted of assessing group differences in EEG, ECG, and respiration measures. EEG measures (EEG frequency amplitudes, peak alpha frequency, and frontal alpha asymmetry) were analyzed with analysis of variance (ANOVA) and/or covariance (ANCOVA) because of the two group cross-sectional design with potential covariates. First, a simple ANOVA was conducted for each measure by PTSD status. If any measure was significant with the ANOVA analysis, then the analysis was repeated with covariates to test if the significant group difference was actually due to important EEG covariates. Age (Gaal et al. 2010), depression (BDI score) (Knott et al. 2001), medications (Blume 2006), and EMG activity (Whitham et al. 2007) were the covariates used in EEG analyses because they affect EEG. Of note, depression is a common co-morbid condition in PTSD and was not excluded in this study (Hofmann et al. 2003; Marciniak et al. 2005). Non-significant covariates were then removed from the model. Any significant global EEG measure was further tested with anterior, posterior, right and left channel designations in post hoc analyses. ECG measures were analyzed using ANCOVA with respiration as a covariate to account for respiratory sinus arrhythmia (Aysin and Aysin 2006). Significant results were further tested with ECG medication status as a covariate to ensure medication status was not confounding the results. Respiration was analyzed using ANOVA.
The secondary post hoc analysis consisted of assessing the relationships between EEG, ECG, and respiration measures with the continuous PTSD symptom variable (CAPS/PCL). This was done because some participants in the No-PTSD group endorsed PTSD symptoms although they did not technically have a PTSD diagnosis according to the DSM-IV. In order to assess PTSD symptoms regardless of diagnosis status, Spearman’s rank correlations were conducted.
Missing data, due to poor signal or technological malfunctions, were handled by excluding participants from specific analyses where they had missing data and including them in analyses where data were present. Thus, some analyses will have a different n. A Holm-Bonferroni correction for multiple comparisons was used for each category resulting in the following p values as cut-offs for each measure to be considered significant [Group characteristics (11 listed in Table 1; p = 0.004), EEG frequency amplitudes and peak alpha frequency (5 listed in Table 2; p = 0.01), frontal alpha asymmetry (p = 0.05), and ECG (7 listed in Table 3; p = 0.006)] (Holm 1979). Post hoc analyses were considered significant at p = 0.05. Statistical analyses were performed with SPSS 19.0 (IBM, USA) and STATA 10.0 (Statacorp, LP, Texas, USA).
Table 2.
EEG parameters by group listed as means plus or minus the standard deviation
| EEG measure | PTSD n = 57 | No PTSD n = 29 |
Statistics |
|---|---|---|---|
| Delta magnitude global (uV) | 4.4 ± 3.2 | 5.1 ± 3.8 | F(1,84) = 0.99; p = 0.32a |
| Theta magnitude global (uV) | 3.3 ± 1.1 | 3.6 ± 2.8 | F(1,84) = 0.75; p= 0.39a |
| Alpha magnitude global (uV) | 9.9 ± 11.1 | 11.0 ± 10.9 | F1,84) = 0.16; p = 0.69a |
| Beta magnitude global (uV) | 3.7 ± 2.5 | 4.3 ± 3.0 | F(1,84) = 0.87; p = 0.35a |
| Peak alpha global (Hz) | 9.7 ± 0.99 | 9.2 ± 0.51 | F(1,84) = 6.14; p = 0.01a |
| Anterior | 9.7 ± 1.1 | 9.2 ± 0.73 | F(1,84) = 6.91; p = 0.01b |
| Posterior | 9.8 ± 1.1 | 9.3 ± 0.61 | F(1,84) = 4.2; p = 0.04b |
| Right | 9.9 ± 1.1 | 9.2 ± 0.65 | F(1,84) = 8.8; p = 0.004b |
| Left | 9.6 ± 1.1 | 9.2 ± 0.56 | F(1,84) = 4.1; p = 0.04b |
| Frontal alpha asymmetry (n = 79 due to missing data) | −0.14 ± 0.15 | −0.17 ± 0.13 | F(1,77) = 0.61; p = 0.44c |
There were no group differences in delta, theta, alpha, or beta EEG amplitudes. Peak alpha global was different between groups with the PTSD group having a higher peak alpha frequency. Post-hoc analyses of anterior, posterior, right and left peak alpha frequency showed significantly higher values in all areas. Channel allocations were as follows: Global- F3, F4, C3, C4, P3, P4, PO3, PO4, Fz, Cz, Pz, Oz; Right- F4, C4, P4, PO4; Left- F3, C3, P3, PO3; Anterior- F3, F4, Fz, C3, C4, Cz; and Posterior- P3, P4, Pz, PO3, PO4, Oz
With the Holm-Bonferroni multiple comparison correction for the EEG parameters, each measure needed to have a p value lower than 0.01 to be considered significant
Post-hoc analysis significance at p = 0.05
Frontal alpha symmetry significant at p = 0.05
Table 3.
Relationship between PTSD symptoms and peak alpha frequency
| Re-experiencing | Numbing avoiding | Hyper-arousal | PTSD Checklist total | CAPS | |
|---|---|---|---|---|---|
| Peak alpha global | |||||
| Spearman’s rho | 0.27 | 0.26 | 0.33 | 0.32 | 0.22 |
| p | 0.01* | 0.01* | 0.002* | 0.003* | 0.04* |
Peak alpha frequency was correlated to total PTSD and sub-category symptom scores using Spearman’s rho correlation in secondary analysis. p = 0.05 considered significant in this secondary analysis. (n = 86). CAPS-Clinician Administered PTSD Structured Interview
Results
The participants were 86 veterans; 59 with PTSD and 27 without PTSD (Fig. 1). There were no group differences on important demographic variables such as age, race, education, exercise, sleep, marital status, alcohol use, smoking, military era, and years in service (all p’s>0.01) (Table 1). Combat exposure differed between groups as expected, as did the CAPS, PCL, and BDI.
Fig. 1.
Recruitment chart. 228 potential volunteers underwent the telephone screening. 142 were excluded or declined to participate in the study. 86 participants were consented and completed the study
Peak Alpha Frequency Higher in PTSD Group
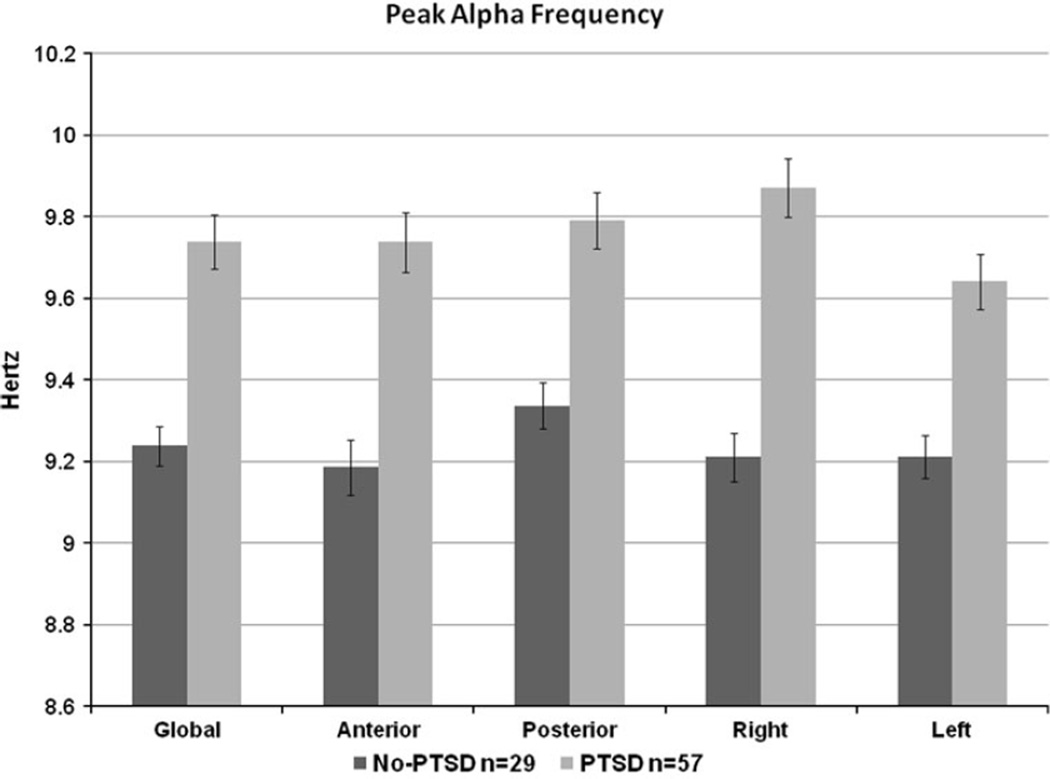
The PTSD and No-PTSD groups had similar delta, theta, alpha, and beta frequency amplitudes (all p’s>0.10) and frontal alpha asymmetry (p = 0.44). The PTSD group had higher peak alpha frequency (F(1,84) = 6.14; p = 0.01) (Table 2; Fig. 2). Post-hoc analyses revealed that anterior, posterior, right and left peak alpha frequency were all higher in the PTSD group. No covariates were significant and both groups had the same EMG activity in all areas (PTSD 1.1 ± 1.2; No-PTSD 1.2 ± 1.0; F(1,84) = 0.16; p = 0.69). In post hoc paired t tests, the PTSD group had a higher right than left peak alpha frequency (Right 9.9 ± 1.1, Left 9.6 ± 1.1; t = 2.9; df = 57; p = 0.005), while the no-PTSD group had similar right and left peak alpha frequency.
Fig. 2.
Peak Alpha Frequency higher in PTSD group. Mean values for peak alpha frequency are represented in this bar graph. Error bars are standard error. Peak alpha frequency was higher in the PTSD group globally, and also in post hoc anterior, posterior, right and left side designations. The right and left side were significantly different within the PTSD group but not within the no-PTSD group
Peak Alpha Frequency Correlated to PTSD Symptoms
Global peak alpha frequency was correlated with total PTSD symptoms (CAPS and PCL) and PCL sub-scores of re-experiencing, numbing-avoiding, and hyper-arousal (Table 3). There were no relationships between delta, theta, alpha, beta, or frontal alpha asymmetry and PTSD symptoms (CAPS or PCL total or sub-scores, all p’s>0.30).
Peak High-Frequency HRV Lower in PTSD Group
Five participants were missing ECG data. In the remaining 81 participants, the PTSD group had lower HF peak frequency (F(2,78) = 26.53; p<0.00005) when including respiration as a covariate (Table 4). This group difference remained when medication status was also included as a covariate to ensure medication status was not influencing the results (F(3,77) = 17.7, p<0.00005). When respiration was not included as a covariate the peak HF HRV frequency was not significant (F(1,79) = 1.20; p = 0.28). The two groups had similar heart rates (PTSD- 72.5 ± 9.9; No-PTSD-70.1 ± 10.4; p>0.05). They also had similar LF/HF ratio, LF peak, absolute, and normalized power, and HF absolute and normalized power (all p’s>0.05) (Table 4). The ECG measures were not correlated with PTSD symptoms as continuous variables (CAPS, PCL total and sub-scores) (all p’s>0.05).
Table 4.
Power spectral HRV in combat veterans with and without PTSD
| HRV parameter | PTSD n = 52 | No-PTSD n = 29 | Statistics |
|---|---|---|---|
| LF peak frequency (Hz) | 0.09 ± 0.02 | 0.08 ± 0.02 | F(2,78) = 2.85; p = 0.06a |
| LF absolute power (ms2) | 407.9 ± 533.7 | 620.6 ± 1,123.9 | F(2,78) = 4.69; p = 0.01a |
| LF power (normalized units) | 63.3 ± 17.7 | 68.1 ± 16.0 | F(2,78) = 3.87; p = 0.03a |
| HF peak frequency (Hz) | 0.21 ± 0.07 | 0.23 ± 0.06 | F(2,78) = 26.5; p<0.00005a |
| HF absolute power (ms2) | 237.9 ± 438.6 | 236.9 ± 395.9 | F(2,78) = 1.21; p = 0.30a |
| HF power (normalized units) | 36.7 ± 17.7 | 31.8 ± 16.0 | F(2,78) = 3.87; p = 0.03a |
| LF/HF ratio | 2.4 ± 2.0 | 2.9 ± 1.9 | F(2,78) = 1.02; p = 0.32a |
| Respiration | 15 ± 0.6 | 15 ± 0.8 | F(1,84) = 0.68, p = 0.41 |
The PTSD group had lower high-frequency HRV. No other HRV parameter was different between groups. Means and standard deviations are shown for each group. Five participants ECG data did not allow for analysis. (n = 81)
With the Holm-Bonferoni multiple comparison correction for the HRV parameters, each measure needed to have a p value lower than 0.006 to be considered significant
No difference in Respiration Rate
Respiration rate was the same in both groups (PTSD 15 ± 0.6; no-PTSD 15 ± 0.8; F(1,84) = 0.68, p = 0.41).
Discussion
In summary, this cross-sectional study evaluated respiration rate, and EEG and HRV measures in veterans with and without PTSD as a first step in assessing biofeedback therapy for PTSD. The two veteran groups were well-matched on important demographic and lifestyle factors and, as expected, the PTSD group had more combat exposure and depression symptoms. Women were recruited for this study, but none were eligible because military sexual rather than combat trauma was their primary trauma. Although excluding non-combat primary trauma was necessary to reduce heterogeneity because of our sample size, military sexual trauma is clearly an important clinical and research area as more women serve in the military (Kimerling et al. 2007; Suris and Lind 2008). Yet, this aspect of PTSD was not addressed by this study.
Peak alpha frequency was sensitive to PTSD status and symptoms, with an increased peak alpha frequency in the PTSD group. Anterior, posterior, right and left peak alpha frequency were all increased in the PTSD group in post hoc analyses. This is the first study to examine peak alpha frequency in people with PTSD. Although peak alpha frequency will vary based on reference, filters, age, and also state (i.e. the type of task the person is doing) (Osaka 1984), it may be a better EEG measure than amplitude to assess cross-sectional differences because it is a more stable EEG feature with greater test re-test reliability than other EEG measures (Salinsky et al. 1991). Peak alpha frequency status may also have health implications. Peak alpha frequency has been associated with higher intelligence and improved cognitive performance (Klimesch 1999) and relaxation (Cahn and Polich 2006). Extensive meditation practice decreases peak alpha frequency in various meditation traditions compared to controls (Cahn and Polich 2006). Meditation is associated with greater relaxation, reduced stress, and improved quality of life among other positive benefits (Grossman et al. 2004; Schoormans and Nyklicek 2011). Peak alpha frequency feedback has been used in other studies to improve cognitive performance in the elderly (Angelakis et al. 2007) and students (Hanslmayr et al. 2005). Peak alpha frequency can be lower in people with traumatic brain injury, however, we excluded those with this condition and so it is unlikely that the lower peak alpha in the control group is due to brain injury (Angelakis et al. 2004). Physiologically, slower peak alpha frequencies are associated with relaxation and the slower peak alpha frequency in the no-PTSD group may reflect a more relaxed state (Nunez et al. 2001) while the higher peak alpha frequency in the PTSD group reflects greater anxiety. It may also be that a lower peak alpha does not relate to relaxation so much as a higher peak alpha relates to cognitive preparedness (Angelakis et al. 2004). The higher peak alpha frequency may reflect the PTSD group’s increased vigilance to their surroundings. Peak alpha frequency also correlated with continuous PTSD symptom variables. The re-experiencing, numbing-avoiding, and hyper-arousal sub-symptoms were similar in association magnitude (r = 0.26–0.33) and so peak alpha frequency may not explain specific PTSD symptom subsets but be associated with PTSD symptoms in general.
Peak HF HRV was lower in the PTSD group compared to controls lending support to the rationale of using HF HRV as a biofeedback parameter. The HF peak of the HRV power spectrum is known to be caused by a mechanism activated by respiration and is often called the respiratory sinus arrhythmia. In paced breathing experiments, the peak HF frequency directly relates to the respiration rate (Yildiz and Ider 2006). The administration of atropine and surgical selective sinoatrial nodal parasympathectomy abolish the HF HRV peak (Houle and Billman 1999; Pomeranz et al. 1985; Randall et al. 1991). Both groups had similar respiration rates and we also included respiration as a covariate in the analysis. However, respiration rate was highly correlated to HF HFV peak frequency (r = 0.61; p < 0.00005) and so despite the apparent group similarities in respiration rate, respiration still may have been the driving factor for the differences in peak HF HRV. Also, when respiration was not included as a covariate there was no significant difference between groups. Further research is needed to establish the relationship between shifts in peak HF HRV frequency, respiration rate, and other HRV parameters.
Regardless, HF HRV peak frequency amplitude is still considered a reliable indicator of parasympathetic efferent activity (Pomeranz et al. 1985; Randall et al. 1991). During sympathetic activation in normal humans, there is a predominance in the LF frequency of HRV, blood pressure and sympathetic nerve activity. During sympathetic inhibition or parasympathetic activation, the HF component of cardiovascular variability predominates (Pagani et al. 1997). Thus, parasympathetic vagal nerve activity is a major although not exclusive contributor to the HF component of HRV (Malik 1996). Lower HF HRV and thus, parasympathetic activity in people with PTSD aligns with our understanding of PTSD hyper-arousal and increased sympathetic activity (Zucker et al. 2009). Sympathetic dominance is a characteristic symptom pattern in people with PTSD as observed with the hyper-arousal symptoms. There was no relationship between PTSD symptoms as continuous variables with any HRV measure. This may reflect the organic physiological changes as a result of a chronic PTSD diagnosis rather than self-report symptom acknowledgement of particular PTSD symptoms without a diagnosis. Other small and variable studies have found decreased HRV in people with PTSD compared to controls and also increased HRV and improved symptoms after training (Ginsberg et al. 2010; Tan et al. 2011; Zucker et al. 2009). These studies either did not test directly for or did not observe any changes in PTSD symptoms. Additional HRV feedback studies should be conducted with large subject numbers, an active control, consistent treatment protocols with greater than 4 sessions (the four session study had negative results whereas the eight session study had positive results), and clear PTSD outcomes to assess efficacy of HRV feedback for PTSD symptoms.
Unlike other EEG studies in PTSD, we found no amplitude differences in any EEG frequency band. Other EEG studies report decreased alpha power (Jokic-Begic and Begic 2003; Veltmeyer et al. 2005) and increased beta power (Begic et al. 2001; Ehlers et al. 2006; Jokic-Begic and Begic 2003) in PTSD compared to controls. Studies examining theta report contradictory results (Begic et al. 2001; Veltmeyer et al. 2005).
Multiple factors may contribute to these variable findings in EEG frequency analysis: frequency band, reference choice, data length, EMG, statistical methods, medications, and population. Most of these studies including ours used the standard and static definitions of EEG frequency ranges. Klimesch has argued that because alpha frequency varies with age, neurological disease, memory performance, brain volume, and task demands using a static alpha frequency range may be problematic and suggests instead using an “individual alpha frequency” range to remedy this problem. The “individual alpha frequency” is defined as the frequency range 2 Hz above and below the individual dominant EEG frequency (above the lower delta range) that desynchronizes during task demands (Klimesch 1999). Thus, the static definitions of EEG frequencies may preclude finding consistent and replicable results because of the individual variation of peak alpha, especially in cross-sectional studies and in the alpha band.
Each study used a different EEG reference montage. We chose a local average reference because of its ability to have surface data more accurately represent local underlying brain sources (Nunez and Pilgreen 1991). The studies also used very different data lengths ranging from 10 s to 15 min. Both reference choice and data length can affect EEG amplitude results (Salinsky et al. 1991). EMG can also influence EEG power, especially in the beta range (McMenamin et al. 2010; Whitham et al. 2007). We found no EMG group differences. As far as we know, no other study has examined EMG differences in people with and without PTSD as a covariate or otherwise. The statistical methods were also variable in their missing data handling, multiple comparison corrections, normalizing and transformation of data, and actual type of statistical analysis used (simple analysis of variance, covariate inclusion, repeated measures models). We included only people with complete datasets, adjusted for multiple comparisons, normalized data or used non-parametric tests, and included important covariates in statistical models to test for confounding. Most studies made an attempt to adjust for participants on medications in the analysis, as did we. Finally, the participants in the prior studies were heterogeneous (e.g. veteran versus non-veteran). Thus, to resolve discrepant observations, additional studies that specifically replicate methods are needed. Also, EEG may be more state dependent and insensitive to PTSD at rest. Comparing EEG during a stressor may reveal more consistent cross-sectional differences in people with and without PTSD.
Although the peak alpha frequency was different across hemispheres within the PTSD group, frontal alpha frequency amplitude was not different between groups. Frontal alpha asymmetry is well-documented in mood disorders and is used as a biofeedback parameter for mood disorders treatment (Baehr et al. 1999). However, frontal alpha asymmetry studies in PTSD are not consistent (Gordon et al. 2010). A meta-analysis of eight studies demonstrated that anxious symptoms correlated with relative right-sided frontal alpha asymmetry (r = 0.25). However, only one study in this review included people with PTSD (Thibodeau et al. 2006). Similarly, five other studies examining frontal alpha asymmetry in PTSD found no differences compared to controls (Gordon et al. 2010; Kemp et al. 2010; Metzger et al. 2004; Rabe et al. 2006; Shankman et al. 2008). Our study lends additional support that while frontal alpha symmetry may be sensitive to mood disorders; it is not sensitive to PTSD. Even though we did not see any group difference in frontal alpha amplitude asymmetry, we did see a difference in right and left peak alpha frequency. The no-PTSD group had similar peak alpha frequencies on contra-lateral channels while the PTSD group had a higher peak alpha frequency on the right than the left.
Heart rate and the other HRV frequency domain measures were not sensitive to PTSD status or symptoms in our study. Similarly, a meta-analysis found significant effect sizes for heart rate overall during traumatic laboratory conditions, but no differences in resting measures (Pole 2007). Heart rate differences may only appear under stressful conditions. Future studies would include a stressful condition along with a baseline condition to test this hypothesis. Our study carefully used respiration rate as a covariate to account for its known affect on high-frequency HRV (Ritz and Dahme 2006) and tested if ECG affecting medications confounded the results.
The two groups had similar respiration rates at rest. As far as we know, no study has formally evaluated respiration rate in people with PTSD compared to controls. Again, future studies assessing respiration rate during a stressor may elucidate cross-sectional differences.
There are limitations in this study. It was a cross-sectional study and did not allow for causal inference. Only male veterans participated in the study, although the study did not exclude females. This precludes generalizability of the results to women veterans and people with non combat-related PTSD. Future studies should focus recruitment methods to enroll female combat veterans and expand the participant pool to non-veterans to confirm findings. The short ECG data length precluded calculating very low-frequency HRV, time domain measures other than heart rate, and the need for result generalizability precluded the use of non-linear measures. Because medications affect EEG and ECG, more efforts to recruit medication free participants could be attempted. This however, may be very challenging as psychotropic medications are often a first line treatment for PTSD (Berg et al. 2007; Defense 2010). Also, depression symptoms may have influenced the alpha asymmetry score as depression may affect alpha asymmetry in a different way than PTSD does (Kemp et al. 2010). However, it would have been unfeasible to recruit as many participants in the time frame due to the high co-morbidity of PTSD and depression (Kinder et al. 2008).
Conclusions
Our study results of higher peak alpha frequency and lower peak frequency HF HRV in veterans with PTSD can inform clinical practice. Currently, no peak alpha frequency neurofeedback study has been conducted for PTSD. While other EEG feedback parameters for PTSD or general anxiety show some preliminary support, most studies are small, and/or uncontrolled (Hammond 2005; Michael et al. 2005; Moore 2000). Considering our lack of EEG amplitude findings, the limited evidence of amplitude biofeedback, and our positive findings in peak alpha frequency which is a more stable EEG measure, a study on peak alpha frequency neurofeedback training for people with PTSD may be worthwhile. Ideally, the study would be controlled, double-blinded, and include measures at session intervals (e.g. 10 sessions with PTSD measure collection every 2–3 sessions). A posteriorly placed electrode for training would be suggested as alpha rhythms originate in the occipital and parieto-occipital regions (Silva 1991). Additionally, continued studies on HF HRV biofeedback are warranted especially ones that specifically use PTSD measures as outcomes and incorporate at least eight training sessions. While no PTSD studies specifically focused on respiratory biofeedback have been conducted, slowed respiration rate training may help people with PTSD increase their HF HRV (Ginsberg et al. 2010; Tan et al. 2011; Zucker et al. 2009) and reduce anxiety in general (Clark and Hirschman 1990; Morarend et al. 2011).
In summary, people with PTSD have a higher peak alpha frequency compared to controls. Peak alpha frequency is a more stable measure of EEG and has not yet been tested clinically or in research studies as a potential biofeedback parameter. The PTSD group also had lower peak frequency HF HRV supporting previous studies with similar results and continued research in HF HRV biofeedback parameter for PTSD. Large, randomized controlled trials are needed for both these biofeedback parameters to test their utility as a treatment for PTSD.
Acknowledgments
This work was supported in part by National Institute ofHealth grants T32AT002688, K01AT004951, U19AT002656, UL1RR024140, K24AT005121, and a Tartar Trust Grant. Special thanks to Roger Ellingson, Irina Fonareva, Jennifer Bishop and Elena Goodrich for their assistance with this study.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Publishing Inc.; 1994. [Google Scholar]
- Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: An electroencephalographic measure of cognitive preparedness. Clinical Neurophysiology. 2004;115(4):887–897. doi: 10.1016/j.clinph.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Stathopoulou S, Frymiare JL, Green DL, Lubar JF, Kounios J. EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin Neuropsychol. 2007;21(1):110–129. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1776–1779. doi: 10.1109/IEMBS.2006.260773. [DOI] [PubMed] [Google Scholar]
- Baehr E, Rosenfeld JP, Baehr R, Earnest C. Clinical use of an alpha asymmetry neurofeedback in the treatment of mood disorders. In: Abarbanel A, Evans JR, editors. Introduction to quantitative EEG and neurofeedback. Massachusetts: Academic Press; 1999. pp. 181–201. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory -Second Edition Manual. San Antonio: Harcourt Brace & Company: 1996. [Google Scholar]
- Begic D, Hotujac L, Jokic-Begic N. Electroencephalographic comparison of veterans with combat-related post-traumatic stress disorder and healthy subjects. International Journal of Psychophysiology. 2001;40(2):167–172. doi: 10.1016/s0167-8760(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Berg AO, Breslau N, Goodman SN, Lezak MD, Matchar DB, Mellman TA, et al. Treatment of PTSD: An assessment of the evidence. Washington, DC: National Academies Press; 2007. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behaviour Research and Therapy. 1996;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Blume WT. Drug effects on EEG. Journal of Clinical Neurophysiology. 2006;23(4):306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Bruce ML. Suicide risk and prevention in veteran populations. Annals of the New York Academy of Sciences. 2010;1208:98–103. doi: 10.1111/j.1749-6632.2010.05697.x. [DOI] [PubMed] [Google Scholar]
- Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, et al. Posttraumatic stress disorder: Diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43(3):150–162. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Holohan D, Greif JL, Bedard M, Suvak M. Twenty-four-hour ambulatory assessment of heart rate and blood pressure in chronic PTSD and non-PTSD veterans. Journal of Traumatic Stress. 2004;17(2):163–171. doi: 10.1023/B:JOTS.0000022623.01190.f0. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Clark ME, Hirschman R. Effects of paced respiration on anxiety reduction in a clinical population. Biofeedback and Self Regulation. 1990;15(3):273–284. doi: 10.1007/BF01011109. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, et al. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a traumarelated reminder. Biological Psychiatry. 1998;44(10):1054–1059. doi: 10.1016/s0006-3223(97)00475-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Department of Veteran Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress. Washington, DC: Veterans Health Administration, Department of Veteran Affairs, Department of Defense; 2010. [Google Scholar]
- Ehlers CL, Hurst S, Phillips E, Gilder DA, Dixon M, Gross A, et al. Electrophysiological responses to affective stimuli in American Indians experiencing trauma with and without PTSD. Ann NY Acad Sci. 2006;1071:125–136. doi: 10.1196/annals.1364.011. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis 1 disorders-patient edition (SCID-I/P, 11/2002 revision) New York, New York: Biometrics Research Department; 2002. [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, Brewin CR. Considering PTSD for DSM-5. Depression & Anxiety. 2011;28(9):750–769. doi: 10.1002/da.20767. [DOI] [PubMed] [Google Scholar]
- Gaal ZA, Boha R, Stam CJ, Molnar M. Agedependent features of EEG-reactivity-Spectral, complexity, and network characteristics. Neuroscience Letters. 2010 doi: 10.1016/j.neulet.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Berry ME, Powell DA. Cardiac coherence and posttraumatic stress disorder in combat veterans. Alternative Therapies in Health and Medicine. 2010;16(4):52–60. [PubMed] [Google Scholar]
- Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: Focus on the QTc interval. Expert Opinion on Pharmacotherapy. 2002;3(5):479–498. doi: 10.1517/14656566.3.5.479. [DOI] [PubMed] [Google Scholar]
- Gordon E, Palmer DM, Cooper N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHDand conduct disorder. Clin EEG Neurosci. 2010;41(4):178–183. doi: 10.1177/155005941004100404. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Staples JK, Blyta A, Bytyqi M, Wilson AT. Treatment of posttraumatic stress disorder in postwar Kosovar adolescents using mind-body skills groups: A randomized controlled trial. Journal of Clinical Psychiatry. 2008;69(9):1469–1476. doi: 10.4088/jcp.v69n0915. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A metaanalysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Grunert BK, Weis JM, Smucker MR, Christianson HF. Imagery rescripting and reprocessing therapy after failed prolonged exposure for post-traumatic stress disorder following industrial injury. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(4):317–328. doi: 10.1016/j.jbtep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Hammond DC. Neurofeedback with anxiety and affective disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14(1):105–123, vii. 1. doi: 10.1016/j.chc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Association for Applied Psychophysiology and Biofeedback. 2005;30(1):1–10. doi: 10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- Hickling EJ, Sison GF, Jr, Vanderploeg RD. Treatment of posttraumatic stress disorder with relaxation and biofeedback training. Biofeedback and Self Regulation. 1986;11(2):125–134. doi: 10.1007/BF00999980. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Litz BT, Weathers FW. Social anxiety, depression, and PTSD in Vietnam veterans. Journal of Anxiety Disorders. 2003;17(5):573–582. doi: 10.1016/s0887-6185(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Houle MS, Billman GE. Low-frequency component of the heart rate variability spectrum: A poor marker of sympathetic activity. American Journal of Physiology. 1999;276(1 Pt 2):H215–H223. doi: 10.1152/ajpheart.1999.276.1.H215. [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Cook J, Imel Z, Fontana A, Rosenheck R, McFall M. Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan War veterans. Journal of Traumatic Stress. 2009;22(4):303–306. doi: 10.1002/jts.20423. [DOI] [PubMed] [Google Scholar]
- Jokic-Begic N, Begic D. Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD) Nordic Journal of Psychiatry. 2003;57(5):351–355. doi: 10.1080/08039480310002688. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111(10):1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Keane T, Fairbank JA, Caddell J, Zimering R, Taylor K, Mora C. Clinical evaluation of a measure to assess combat exposure. A Journal of Consulting and Clinical Psychology. 1989;1(1):53–55. [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, et al. Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology. 2010;85(2):350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimerling R, Gima K, Smith MW, Street A, Frayne S. The Veterans health administration and military sexual trauma. American Journal of Public Health. 2007;97(12):2160–2166. doi: 10.2105/AJPH.2006.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder LS, Bradley KA, Katon WJ, Ludman E, McDonell MB, Bryson CL. Depression, posttraumatic stress disorder, and mortality. Psychosomatic Medicine. 2008;70(1):20–26. doi: 10.1097/PSY.0b013e31815aac93. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research. Brain Research Reviews. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knott V, Mahoney C, Kennedy S, Evans K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Research. 2001;106(2):123–140. doi: 10.1016/s0925-4927(00)00080-9. [DOI] [PubMed] [Google Scholar]
- Lakusic N, Fuckar K, Mahovic D, Cerovec D, Majsec M, Stancin N. Characteristics of heart rate variability in war veterans with post-traumatic stress disorder after myocardial infarction. Military Medicine. 2007;172(11):1190–1193. doi: 10.7205/milmed.172.11.1190. [DOI] [PubMed] [Google Scholar]
- Lande RG, Williams LB, Francis JL, Gragnani C, Morin ML. Efficacy of biofeedback for post-traumatic stress disorder. Complementary Therapies in Medicine. 2010;18(6):256–259. doi: 10.1016/j.ctim.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Annals of Pharmacotherapy. 2009;43(7):1227–1232. doi: 10.1345/aph.1M017. [DOI] [PubMed] [Google Scholar]
- Malik M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, et al. The cost of treating anxiety: The medical and demographic correlates that impact total medical costs. Depression and Anxiety. 2005;21(4):178–184. doi: 10.1002/da.20074. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Greischar LL, Davidson RJ. Electromyogenic artifacts and electroencephalographic inferences revisited. Neuroimage. 2010;54(1):4–9. doi: 10.1016/j.neuroimage.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, et al. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. Journal of Abnormal Psychology. 2004;113(2):324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Michael AJ, Krishnaswamy S, Mohamed J. An open label study of the use of EEG biofeedback using beta training to reduce anxiety for patients with cardiac events. Neuropsychiatric Disease and Treatment. 2005;1(4):357–363. [PMC free article] [PubMed] [Google Scholar]
- Moore NC. A review of EEG biofeedback treatment of anxiety disorders. Clinical Electroencephalography. 2000;31(1):1–6. doi: 10.1177/155005940003100105. [DOI] [PubMed] [Google Scholar]
- Morarend QA, Spector ML, Dawson DV, Clark SH, Holmes DC. The use of a respiratory RATE biofeedback device to reduce dental anxiety: An exploratory investigation. Association for Applied Psychophysiology and Biofeedback. 2011;36(2):63–70. doi: 10.1007/s10484-011-9148-z. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Yadav R, Yung I, Zajdel DP, Oken BS. Sensitivity to mental effort and test-retest reliability of heart rate variability measures in healthy seniors. Clinical Neurophysiology. 2011;122(10):2059–2066. doi: 10.1016/j.clinph.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka MY, Carlson JG, Chemtob CM. Twentyfour- hour ambulatory blood pressure and heart rate monitoring in combat-related posttraumatic stress disorder. Journal of Traumatic Stress. 1998;11(3):473–484. doi: 10.1023/A:1024400628342. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: A method to improve EEG spatial resolution. Journal of Clinical Neurophysiology. 1991;8(4):397–413. [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatialtemporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Human Brain Mapping. 2001;13(3):125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Kishiyama S, Salinsky MC. Pharmacologically induced changes in arousal: Effects on behavioral and electrophysiologic measures of alertness and attention. Electroencephalography and Clinical Neurophysiology. 1995;95(1995):359–371. doi: 10.1016/0013-4694(95)00124-h. [DOI] [PubMed] [Google Scholar]
- Olgin J, Zipes D. Specific arrhythmias: Diagnosis and treatment. In: Libby P, Bonow R, Mann D, Zipes D, editors. Braunwald’s heart disease: A textbook of cardiovascular medicine. St. Louis, Mo: WB Saunders; 2007. [Google Scholar]
- Osaka M. Peak alpha frequency of EEG during a mental task: Task difficulty and hemispheric differences. Psychophysiology. 1984;21(1):101–105. doi: 10.1111/j.1469-8986.1984.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, et al. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95(6):1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave neuro-feedback therapy for Vietnam veterans with combatrelated post-traumatic stress disorder. Medical Psychotherapy. 1991;4:47–60. [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248(1 Pt 2):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Rabe S, Beauducel A, Zollner T, Maercker A, Karl A. Regional brain electrical activity in posttraumatic stress disorder after motor vehicle accident. Journal of Abnormal Psychology. 2006;115(4):687–698. doi: 10.1037/0021-843X.115.4.687. [DOI] [PubMed] [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, Caldarone LB. Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: Possible explanations. Journal of Traumatic Stress. 2010;23(1):59–68. doi: 10.1002/jts.20486. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Raisch RM, Yingling JD, Randall WC. SA nodal parasympathectomy delineates autonomic control of heart rate power spectrum. American Journal of Physiology. 1991;260(3 Pt 2):H985–H988. doi: 10.1152/ajpheart.1991.260.3.H985. [DOI] [PubMed] [Google Scholar]
- Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: Practice against better evidence? Psychosomatic Medicine. 2006;68(4):617–627. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis. Electroencephalography and Clinical Neurophysiology. 1991;79(5):382–392. doi: 10.1016/0013-4694(91)90203-g. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Oken BS, Storzbach D, Dodrill CB. Assessment of CNS effects of antiepileptic drugs by using quantitative EEG measures. Epilepsia. 2003;44(8):1042–1050. doi: 10.1046/j.1528-1157.2003.60602.x. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: Extension of findings to veterans of the wars in Iraq and Afghanistan. Clinical Psychology Review. 2009;29(8):727–735. doi: 10.1016/j.cpr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Schoormans D, Nyklicek I. Mindfulness and psychologic well-being: Are they related to type of meditation technique practiced? Journal of Alternative and Complementary Medicine. 2011;17(7):629–634. doi: 10.1089/acm.2010.0332. [DOI] [PubMed] [Google Scholar]
- Seal KH, Cohen G, Waldrop A, Cohen B, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2010.11.027. 2011 Jan 28 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shankman SA, Silverstein SM, Williams LM, Hopkinson PJ, Kemp AH, Felmingham KL, et al. Resting electroencephalogram asymmetry and posttraumatic stress disorder. Journal of Traumatic Stress. 2008;21(2):190–198. doi: 10.1002/jts.20319. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Silva F. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalography and Clinical Neurophysiology. 1991;79(1991):81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Davidson JR. Trauma: Prevalence, impairment, service use, cost. Journal of Clinical Psychiatry. 1997;58(Suppl 9):5–11. [PubMed] [Google Scholar]
- Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-occurring medical, psychiatric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51(6):503–507. doi: 10.1176/appi.psy.51.6.503. [DOI] [PubMed] [Google Scholar]
- Suris A, Lind L. Military sexual trauma: A review of prevalence and associated health consequences in veterans. Trauma Violence Abuse. 2008;9(4):250–269. doi: 10.1177/1524838008324419. [DOI] [PubMed] [Google Scholar]
- Tan G, Dao TK, Farmer L, Sutherland RJ, Gevirtz R. Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Association for Applied Psychophysiology and Biofeedback. 2011;36(1):27–35. doi: 10.1007/s10484-010-9141-y. [DOI] [PubMed] [Google Scholar]
- Tatrow K, Blanchard EB, Silverman DJ. Posttraumatic headache: An exploratory treatment study. Association for Applied Psychophysiology and Biofeedback. 2003;28(4):267–278. doi: 10.1023/a:1027326808356. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Veltmeyer MD, Clark CR, McFarlane AC, Felmingham KL, Bryant RA, Gordon E. Integrative assessment of brain and cognitive function in post-traumatic stress disorder. Journal of Integrative Neuroscience. 2005;4(1):145–159. doi: 10.1142/s0219635205000719. [DOI] [PubMed] [Google Scholar]
- Veltmeyer MD, McFarlane AC, Bryant RA, Mayo T, Gordon E, Clark CR. Integrative assessment of brain function in PTSD: Brain stability and working memory. Journal of Integrative Neuroscience. 2006;5(1):123–138. doi: 10.1142/s0219635206001057. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, et al. Scalp electrical recording during paralysis: Quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118(8):1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Yildiz M, Ider YZ. Model based and experimental investigation of respiratory effect on the HRV power spectrum. Physiological Measurement. 2006;27(10):973–988. doi: 10.1088/0967-3334/27/10/004. [DOI] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Association for Applies Psychophysiology and Biofeedback. 2009;34(2):135–143. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]