Abstract
The anti-tumor effects of paclitaxel are generally attributed to the suppression of microtubule dynamics resulting in defects in cell division. New data demonstrated that in ultra-low non-cytotoxic concentrations, paclitaxel modulated in immune cells in vitro the activity of small Rho GTPases, the key regulators of intracellular actin dynamics. However, the immunomodulatory properties of paclitaxel in vivo have not been evaluated. Using here the ret transgenic murine melanoma model, which mimics human cutaneous melanoma, we tested effects of ultra-low non-cytotoxic dose paclitaxel on functions of myeloid-derived suppressor cells (MDSCs), chronic inflammatory mediators, and T cell activities in the tumor microenvironment in vivo. Administration of paclitaxel significantly decreased accumulation and immunosuppressive activities of tumor-infiltrating MDSCs without alterations of the bone marrow hematopoiesis. This was associated with the inhibition of p38 MAPK activity, TNF-α and production and S100A9 expression in MDSCs. The production of mediators of chronic inflammation in the tumor milieu was also diminished. Importantly, reduced tumor burden and increased animal survival upon paclitaxel application was mediated by the restoration of CD8 T cell effector functions. We suggest that the ability of paclitaxel in non-cytotoxic dose to block the immunosuppressive potential of MDSCs in vivo represents a new therapeutic strategy to down-regulate immunosuppression and chronic inflammation in the tumor microenvironment for enhancing the efficacy of concomitant anti-cancer therapies.
Keywords: melanoma, paclitaxel, myeloid-derived suppressor cells, chronic inflammation, immunosuppression, tumor microenvironment
Introduction
Conventional chemotherapy based on maximum tolerated doses represents one of the major cancer treatments despite its severe toxicity, development of drug resistance and strong immunosuppression. However, a growing body of evidence indicate that lowering the dose of anti-tumor cytotoxic agents and combining chemotherapy with other modalities may not only decrease the toxicity of chemotherapy but also up-regulate the efficacy of different anticancer therapies by altering differentiation and activity of immune regulatory and effector cells (1). Although the signaling pathways targeted by chemotherapeutic agents at low doses in immune cells are still unknown, new data suggest that the immunomodulating activity of these agents in non-cytotoxic, non-cytostatic concentrations is mediated by drug-specific activation of signal transduction pathways. For instance, paclitaxel can modulate the activity of small Rho GTPase family members that regulate the assembly and organization of actin-based structures in cells (1, 2).
Furthermore, certain drugs (e.g., paclitaxel or 5-fluorouracil) at ultra-low non-cytotoxic doses were reported to display the immunomodulating effects including the stimulation of maturation and functions of human and murine dendritic cells (DCs) in vitro (3, 4) and augmentation of the anti-tumor efficiency of DC vaccination in the transplantable mouse model of lung cancer (5). It has been recently demonstrated that low-dose paclitaxel blocked tumor-induced polarization of conventional DCs into immunosuppressive regulatory DCs (6), which was prevented by a small Rho GTPase inhibitor (Zhong et al., submitted).
However, it is still unknown whether paclitaxel can modulate homing and function of myeloid-derived suppressor cells (MDSCs), a key cell subset responsible for maintaining the immunosuppressive and tolerogenic tumor microenvironment in many cancers (7–9). This heterogeneous population of immature myeloid cells was reported to inhibit the anti-tumor immune cell responses via different mechanisms and markedly restrict the efficiency of anti-tumor immunotherapies (10–12). Malignant melanoma is characterized by a strong immunosuppression driven by chronic inflammation that induces the MDSC recruitment and activation (13–17). However, no clinically feasible strategies are developed so far to down-regulate the emergence and function of MDSCs in the melanoma microenvironment.
Here we have tested how paclitaxel changes MDSC accumulation and activity in the ret transgenic mouse model of spontaneous melanoma that closely resembles human melanoma regarding histopathology and clinical development (18, 19). We also determine the signaling pathways in MDSCs that are involved in their inhibition and verified the involvement of chronic inflammation in the anti-tumor action of paclitaxel. Our results revealed that paclitaxel at non-cytotoxic dose reduced the number of tumor-infiltrating MDSCs and abrogated nitric oxide (NO) production by MDSCs in the metastatic lymph nodes (LN) and bone marrow (BM) of melanoma-bearing mice without affecting hematopoietic stem cells. Tumor-derived MDSCs from paclitaxel-treated animals showed lower immunosuppressive activity associated with decreased expression of p38 MAPK and S100A9. The production of chronic inflammatory mediators such as TGF-β, GM-CSF, IL-1β, IL-10, TNF-α and IFN-γ was reduced in primary tumors. The anti-tumor effect of paclitaxel was associated with the restoration of CD8 T cell activity and significantly increased survival of tumor-bearing mice. These results suggest that the reversal of immunosuppression in the tumor microenvironment induced by ultra-low non-cytotoxic doses of paclitaxel represents an efficient therapeutic approach and can be combined with immunotherapies for increasing their anti-tumor efficiency.
Materials and Methods
Mice
C57BL/6 mice expressing human ret transgene in melanocytes under the control of mouse metallothionein-I promoter-enhancer (18) were provided by Dr. I. Nakashima (Chubu University, Aichi, Japan). Animals were crossed and kept under specific pathogen-free conditions in the animal facility of German Cancer Research Center (Heidelberg, Germany). Experiments were performed in accordance with government and institutional guidelines and regulations.
Reagents and antibodies
Paclitaxel was purchased from Hexal. Rat anti-mouse directly conjugated mAbs (CD3-PerCP-Cy5.5, CD4-FITC, CD8-APC-Cy7, CD25-APC, CD45.2-PerCP-Cy5.5, CD11b-PE, Gr1-PE-Cy7, CD11c-APC), purified rat anti-mouse CD16/CD32 (Fc-block), mouse anti-mouse p-p38 MAPK (pT180/pY182)-Alexa Fluor 647, mouse anti-mouse pStat3 (pY705) Alexa Fluor 488, rat anti-mouse TNF-α-Alexa Fluor 488, mouse anti-human Ki67-FITC, purified mouse anti-human arginase-1 (ARG-1) (both cross reacting with respective mouse markers), and rat anti-mouse IgG-FITC were purchased from BD Biosciences. FoxP3 fixation/permeabilization kit and rat anti-mouse Foxp3-PE mAbs were from eBioscience. Rat anti-mouse F4/80-PE (Biolegend), purified rat anti-mouse S100A9 and PE-conjugated mouse anti-mouse TCR ζ-chain mAbs (Abcam) were also used. Mouse RPE-conjugated dextramers containing H-2 Kb and the TRP-2-derived peptide SVYDFFVWL were from Immudex. Intracellular NO was detected using the staining with diaminofluoresciein-2 diacetate (DAF-2DA, Cell Technology) according to the manufacturer's instructions. Rat anti-mouse CD8 depleting mAbs were from Serotec and IgG from rat serum was from Sigma.
Paclitaxel treatment
Ret transgenic tumor-bearing mice and non-transgenic littermates were weekly injected intraperitoneally with 1 mg/kg paclitaxel in 0.2 ml PBS three times. Control group of mice with tumors of similar size received 0.2 ml PBS. Both groups were monitored daily for tumor progression. Some paclitaxel-treated and untreated mice were injected i.p. with CD8+ T cell depleting mAbs or control IgG from rat serum (both 100 μg/mouse) at days 0, 2, 14 and 28 after the initiation of the experiment. The quality of depletion was determined at day 10.
Preparation of single cell suspensions
Fresh BM, spleen, LN and tumor samples were mechanically dissociated in ice-cold PBS and filtered through the cell strainers (BD Falcon). Tumor, BM and spleen cell samples were depleted of erythrocytes by ammonium chloride lysis, washed twice and stored on ice.
Flow cytometry
Single cell suspensions were treated with Fc-block and mAbs for 30 min at 4°C. For intracellular staining, samples were pre-incubated with the corresponding fixation/permeabilization buffers for phosphoprotein staining (BD Biosciences, according to recommendations of manufacturer for specific phosphoproteins) or FoxP3 fixation/permeabilization kit for FoxP3, TCR-ζ chain and ARG-1 stainings. Stainings for p-p38 MAPK and TNF-α were made according to BD Phosflow™ protocol for p-p38. Staining for pSTAT3 was performed as recommended by BD Phosflow™ protocol for pSTAT3. Acquisition was done by five- or six-color flow cytometry using FACSCanto II with FACSDiva software (both from BD Biosciences) with dead cell exclusion based on scatter profile or propidium iodide inclusion. FlowJo software (Tree Star) was used to analyze at least 100,000 events. Data were expressed as dot plots.
Bio-plex assay
Snap frozen primary tumor samples were mechanically disrupted and treated with lysis solution (Bio-Rad). After sonication, samples were centrifuged at 4,500 g for 10 min at 4°C. Protein concentration in lysates was determined using Pierce BCA protein assay kit (Thermo Scientific) and adjusted to 1000 µg/ml using serum diluent (Bio-Rad). Concentrations of inflammatory factors in tissue lysates were measured by multiplex technology (Bio-Rad).
In vitro proliferation assay
CD11b+ cells were isolated from tumors of transgenic mice using CD11b+ MicroBeads isolation kit (Miltenyi Biotec) according to the manufacturer’s protocol. The purity of Gr1+CD11b+ MDSCs in isolated cell population was around 80%. Normal splenocytes were labeled with the cell proliferation dye (CPD, eBioscience), stimulated with soluble anti-CD3 and anti-CD28 mAbs (0.5 µg/ml each) and co-cultured with MDSCs in triplicates (at the splenocytes:MDSC ratio 1:1) for 96 h. T cell proliferation was evaluated by the reduction of the CPD expression using flow cytometry.
Immunohistochemistry
Melanoma-bearing mice were treated with a single i.p. injection of paclitaxel at ultra-low (1 mg/kg) or conventional (15 mg/kg) doses. Upon 48 h, tumor samples were fixed in 4% paraformaldehyde overnight followed by paraffin embedding. Consecutive sections were used for the TdT-mediated dUTP nick end labeling (TUNEL) assay and the staining with anti-Ki-67 mAbs. Apoptotic cells were visualized by using TdT In Situ Apoptosis Detection Kit (Trevigen, Inc). Proliferating Ki67+ cells were stained using the IHC Kit (Eton Bio). All staining procedures were carried out according to the manufacture’s protocols.
Statistical analysis
Statistical analyses were performed by GraphPad Prism software using non-parametric Mann-Whitney U test and ANOVA after evaluation for normality. A value of p < 0.05 was considered statistically significant.
Results
Paclitaxel at ultra-low non-toxic doses reduces MDSC numbers and activity without alteration of hematopoiesis in vivo
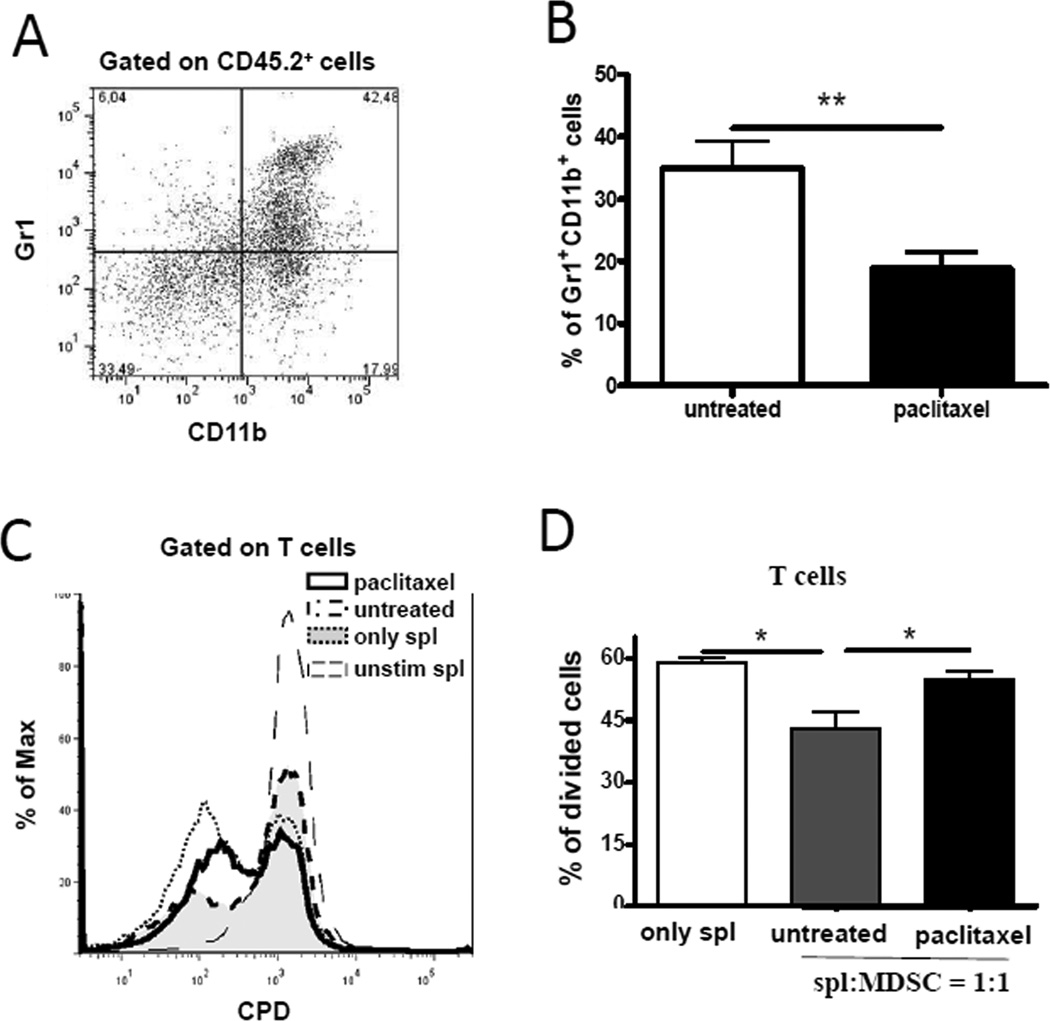
MDSCs were shown to be important regulators of chronic inflammation, tumorigenesis and tumor progression (7, 10). Therefore, we tested the hypothesis that non-toxic ultra-low dose of paclitaxel (1 mg/kg, weekly × 3) affected MDSCs in primary skin tumors and lymphoid organs (Fig. 1A, B; Supplemental Fig. 1A). Administration of taxol caused a significant reduction (1.9-fold; p < 0.01) in frequencies of tumor-infiltrating MDSCs as compared to PBS-treated group (Fig. 1B). MDSC frequency in the metastatic LN, spleen and BM remained unchanged (Supplemental Fig. 1A). In contrast to MDSCs, the frequency of tumor-infiltrating DCs and macrophages was not significantly altered upon the paclitaxel treatment (Supplemental Fig. 1B).
FIGURE 1.
Paclitaxel down-regulates the number and function of tumor-infiltrating MDSCs in vivo. Tumor-bearing ret transgenic mice were treated with ultra-low dose paclitaxel (1 mg/kg). CD11b+Gr1+ MDSCs were assessed by flow cytometry. (A) A representative dot plot of primary tumor is shown. (B) Cumulative data from six independent experiments assessing MDSCs in primary tumors expressed as the percentage within live leukocytes (mean ± SD; 28 mice per group). (C–D) MDSCs were isolated from tumors followed by the co-culture with normal splenocytes labeled with CPD eFluor® 670 and stimulated with anti-CD3 and anti-CD28 mAbs. (C) A representative histogram from one experiment out of three with similar results. (D) Data (mean ± SD; 9 mice per group) for the inhibition of T cell proliferation by MDSCs are presented as the percentage of divided T cells. Splenocytes (spl):MDSC ratio was 1:1. * p < 0.05; ** p < 0.01.
The production of NO and elevated ARG-1 activity are the key factors of immunosuppression mediated by MDSCs (8, 10). To test, whether these pathways are involved in the effect of paclitaxel on MDSCs, we used an intracellular NO staining with DAF-2DA and found a significant reduction of NO-producing Gr1+CD11b+ MDSCs in metastatic LN from paclitaxel-treated melanoma-bearing mice as compared to PBS-treated control group (p < 0.05); however, the influence of paclitaxel on these cells in the BM was less pronounced (Supplemental Fig. 2). Levels of NO-producing MDSCs in primary skin tumors and the spleen of treated animals were not significantly different from those in PBS-treated mice (data not shown). In addition, we did not see significant inhibition of ARG-1 expression in MDSCs after paclitaxel administration (data not shown), suggesting that the effect of paclitaxel on MDSCs in vivo was tissue- and signaling-specific.
Next, to determine the immunosuppressive potential of MDSCs, we verified a direct impact of tumor-infiltrating MDSCs from melanoma-bearing mice on T cell proliferative activity. MDSCs isolated from primary tumors were co-cultured with splenocytes from untreated syngeneic mice stimulated by anti-CD3 and anti-CD28 mAbs. Our results demonstrated a significant reduction (up to 1.4-fold; p < 0.05) in the capacity of tumor-infiltrating MDSCs from paclitaxel-treated mice to suppress T cell proliferation as compared to MDSCs from PBS-treated group (Fig. 1C, D). This suggests that paclitaxel inhibited the immunosuppressive activity of MDSCs in vivo.
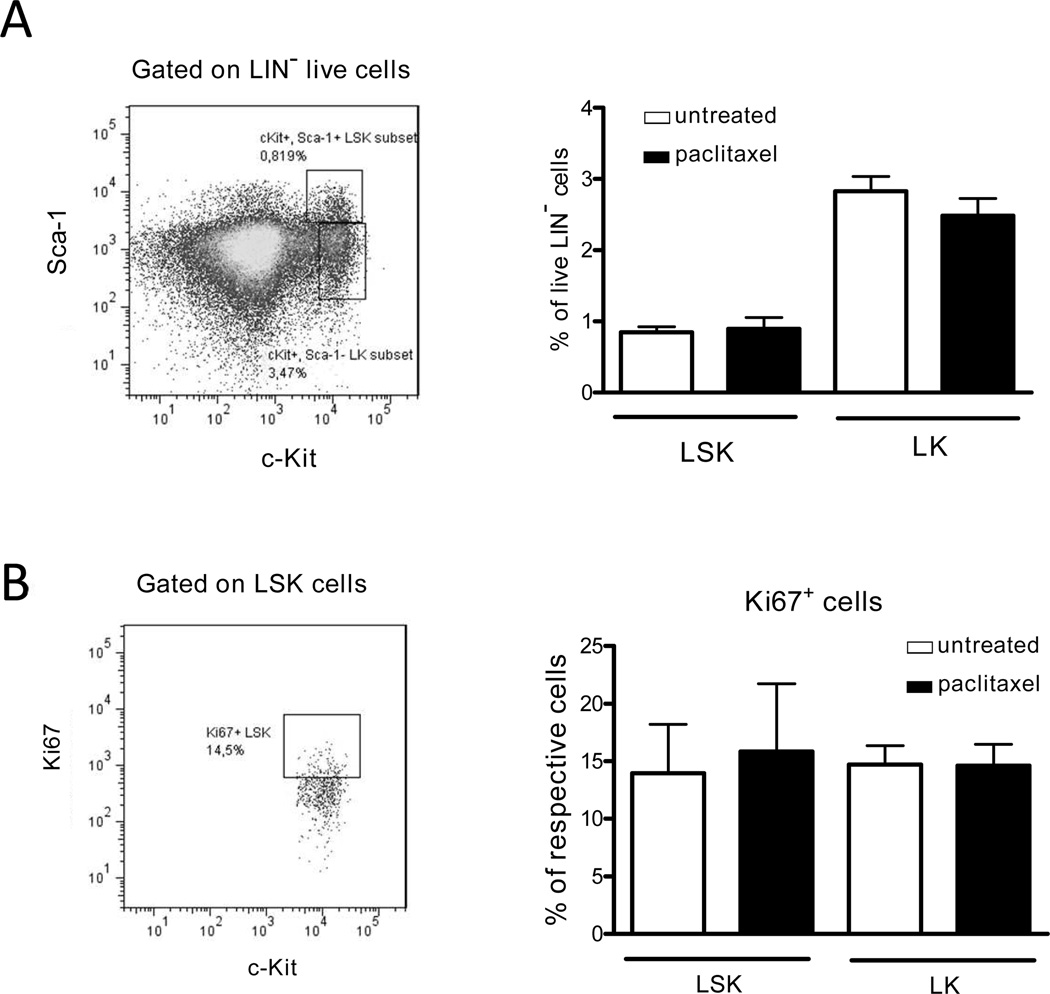
In addition, analyzing how paclitaxel affected the BM hematopoiesis, we found no alterations in the levels of LSK (Lin−Sca-1+c-Kit+) and LK (Lin−Sca-1−c-Kit+) cells or in their proliferative capacity measured by the expression of Ki67 (Fig. 2), suggesting a selective effect of paclitaxel administration on MDSCs.
FIGURE 2.
Administration of ultra-low dose paclitaxel is not toxic for bone marrow hematopoietic cells. C57BL/6 mice were treated with ultra-low doses of paclitaxel as described in Material and Methods. Hematopoietic early progenitor cells, Lin−Sca-1+c-Kit+ (LSK) and Lin−Sca-1−c-Kit+ (LK) were analyzed by flow cytometry. (A) Results are presented as the percentage of LSK or LK cells among Lin− cells (8 mice per group). (B) Proliferation of hematopoietic cells is shown as the percentage of Ki67+ cells within respective cell subset (mean ± SD; 8 mice per group). Data of two independent experiments are depicted.
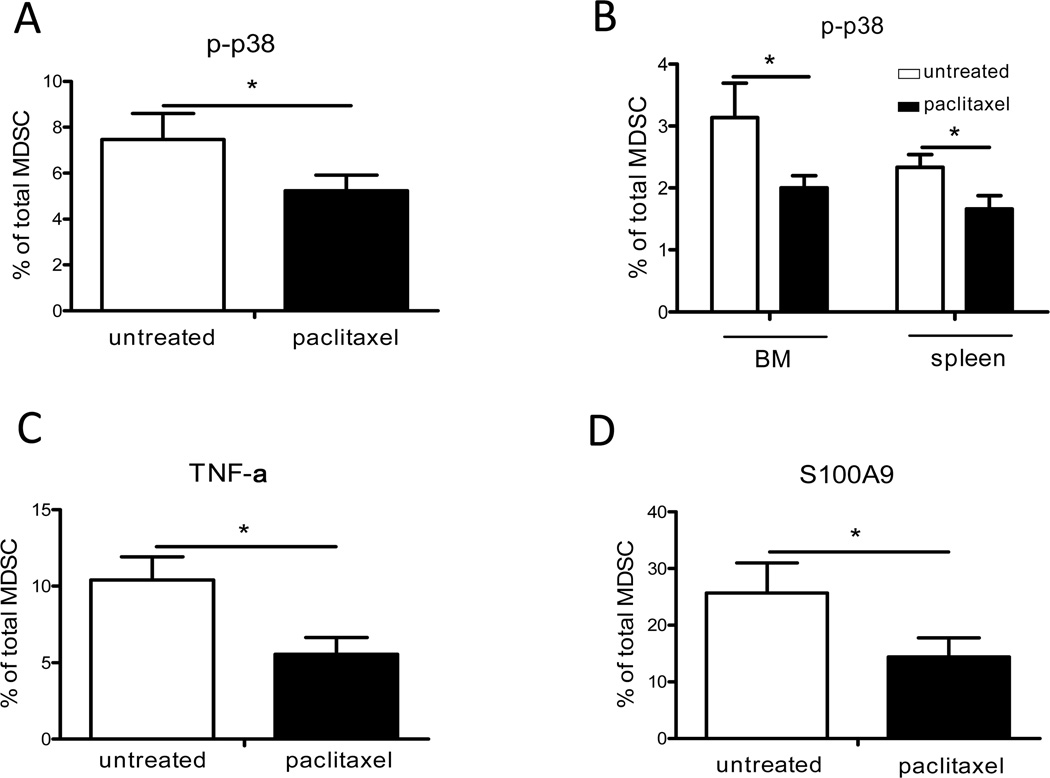
Paclitaxel modulates p38 MAPK, S100A8/A9 and STAT-3 signaling pathways in MDSCs
Signaling pathways involving p38 MAPK, S100A8/A9 and STAT-3 play an important role in the myeloid cell activation in chronic inflammatory tumor microenvironment (8). To determine which signaling pathways in MDSCs are affected by paclitaxel in vivo, we assessed the activity of p38 MAPK by measuring phosphorylation of threonine 180 and tyrosine 705 (pT180/pY182). The results revealed a significant down-regulation of p38 activation (up to 1.5-fold; p < 0.05) in tumor-infiltrating MDSCs upon the paclitaxel treatment (Fig. 3A). Moreover, p38 phosphorylation was significantly reduced (p < 0.05) in MDSCs from the BM and spleen in paclitaxel-treated tumor-bearing mice (Fig. 3B). In contrast, no differences in the level of MDSCs expressing activated p38 MAPK were demonstrated in the metastatic LN (data not shown). Since p38 MAPK can regulate the TNF-α production (20), we measured its intracellular expression in tumor-infiltrating MDSCs. As expected, primary skin tumors contained significantly less amount of MDSCs producing TNF-α upon the paclitaxel treatment as compared to control group (p < 0.05; Fig. 3C). Since p38 MAPK is also known to be activated by secreted S100A8/A9 complex (21, 22), we evaluated the intracellular expression of S100A9 and detected a strong reduction in the number of tumor-infiltrating MDSCs expressing S100A9 upon the treatment with paclitaxel as compared to untreated animals (26 ± 5 % versus 14 ± 3 % cells, respectively; p < 0.05; Fig. 3D). In contrast, the amount of S100A9-positive MDSCs in the metastatic LNs was not significantly altered (data not shown).
FIGURE 3.
Paclitaxel modulates p38 MAPK and S100A9 signaling in tumor-associated MDSCs in vivo. Tumor-bearing mice were treated with ultra-low doses of paclitaxel. Expression of phosphorylated p38 MAPK (p-p38 MAPK), S100A9 and TNF-α were detected in CD11b+Gr1+ MDSCs from skin tumors and lymphoid organs by flow cytometry. Data of three independent experiments are presented as the percentage of p-p38 MAPK expressing cells within total MDSCs in the tumor (A) and lymphatic organs (B) (mean ± SD; 13–17 mice per group). TNF-α producing (C) and S100A9 expressing (D) tumor-infiltrating MDSCs were shown as the percentage of respective cells among total MDSCs (mean ± SD; 14 mice per group). * p < 0.05.
Since the STAT3 pathway is responsible for the regulation of MDSC-mediated immunosuppression (8, 10), we next analyzed the phosphorylation of tyrosine 705 in the STAT3 molecule (pY705). In the metastatic LN, a significant reduction (up to 2.3-fold; p < 0.05) of p-STAT3-positive MDSCs was detected upon the ultra-low dose paclitaxel treatment (Supplemental Fig. 3). However, STAT3 phosphorylation in tumor-infiltrating MDSCs was not profoundly altered, suggesting a tissue-specific effect of paclitaxel on MDSC signaling pathways in vivo (data not shown).
Paclitaxel diminishes chronic inflammation and inhibits tumor development
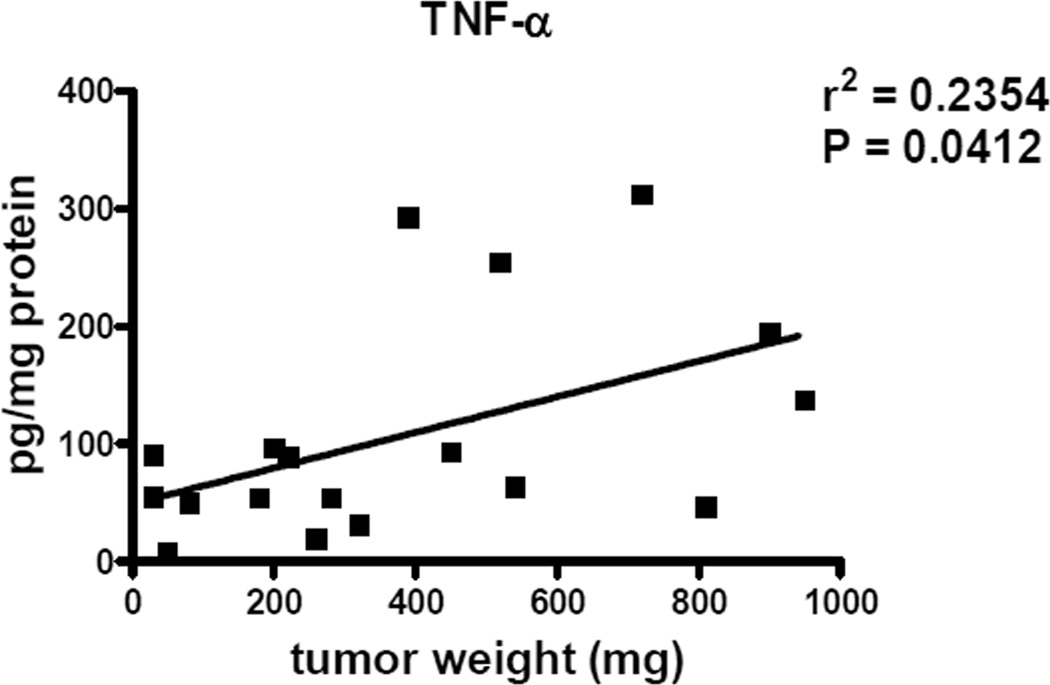
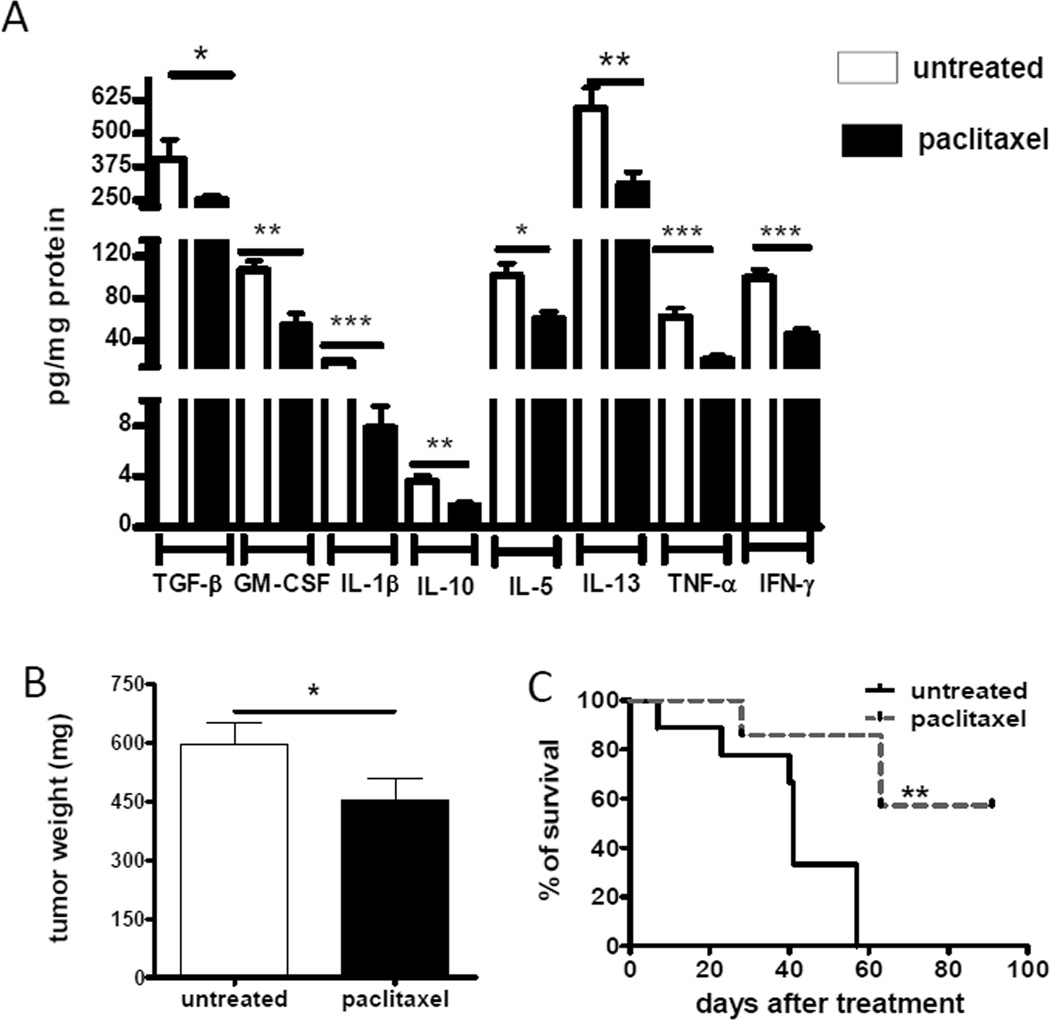
Recent reports by us and others have demonstrated that numerous chronic inflammatory mediators can attract and activate MDSCs in the tumor microenvironment (8, 10, 13, 23). In addition, we demonstrated here a positive correlation between intratumoral concentrations of TNF-α and tumor progression (Fig. 4). Since these inflammatory factors could be produced by myeloid cells upon the activation of p38 MAPK signaling (20–22, 24–26), we defined whether their levels were influenced by the ultra-low dose paclitaxel treatment in vivo. The results revealed a remarkable decrease in the levels of TGF-β, GM-CSF, IL-1β, IL-10, IL-5, IL-13, TNF-α and IFN-γ in the primary skin tumor microenvironment (Fig. 5A). For instance, the concentration of IL-1β was 4.2 times lower upon paclitaxel administration (p < 0.001) These data are also in agreement with the above mentioned down-regulation of TNF-α production by MDSCs infiltrating skin tumors (Fig. 2C). Moreover, the reduction of chronic inflammation was associated with a significantly decreased tumor burden estimated at day 21 after the beginning of the paclitaxel administration as compared to untreated group (from 593 ± 60 mg to 453 ± 57 mg of tumor weight, respectively; p < 0.05; Fig. 5B). In addition, we revealed a profound prolongation of the survival of treated tumor-bearing mice (p < 0.05; Fig. 5C). Importantly, using the proliferation marker Ki-67 and the TUNEL assay, we observed no direct cytostatic or cytotoxic effects of paclitaxel on tumor cells at day 2 after its administration in ultra-low dose (Supplemental Fig. 4). In contrast, paclitaxel at conventional dose (15 mg/kg) induced both effects on melanoma cells under the same conditions. These results suggest that paclitaxel in ultra-low dose (in contrast to conventional therapeutic dose) is not able to directly suppress tumor cell proliferation or induce their apoptosis in vivo but affects immune regulators in the tumor milieu.
FIGURE 4.
TNF-α production in tumors of ret transgenic mice. TNF-α concentration was measured in primary tumors in two independent experiments (18 mice) by bio-plex and plotted against the tumor weight. The correlation between two variables was calculated using a linear regression analysis.
FIGURE 5.
Paclitaxel inhibits chronic inflammation and tumor development in vivo. (A) Proinflammatory mediators were measured in skin tumors by bio-plex assay as described in Materials and Methods. Data from three independent experiments are expressed as pg/mg protein (mean ± SD; 11 mice per group). (B) The weight of tumors from treated and untreated mice was measured at day 21 after initiation of the treatment and expressed in mg (mean ± SD; 40 mice per group). (C) Survival of mice (8 animals per group) is shown as a Kaplan-Meier curve. * p < 0.05; ** p < 0.01, *** p < 0.001.
Restoration of the anti-tumor activity of CD8+ T cells upon the treatment with paclitaxel
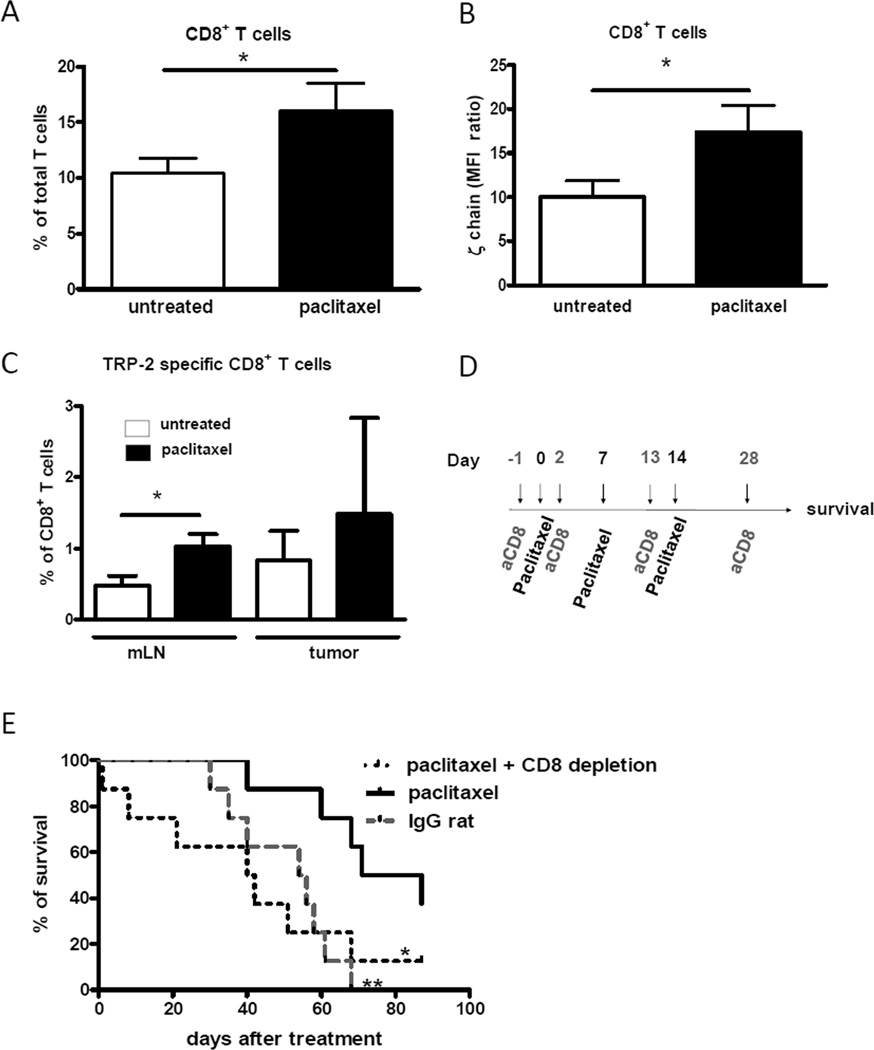
Since the development of the anti-tumor immune response is associated with the accumulation and activation of tumor infiltrating CD8+ T cells (TILs) in the tumor milieu (27) and MDSCs are known to suppress TILs activity (8), we next tested whether reduced chronic inflammation as well as MDSC amounts and suppressive functions could increase TIL frequencies and restore their functions in vivo. We detected a significant accumulation of CD8+ TILs in skin melanoma lesions from paclitaxel-treated mice as compared to the control group (16 ± 3 % versus 10 ± 1 % among total TILs, respectively; p < 0.05; Fig. 6A). Since TCR ζ-chain expression is important for T cell functions (23), we estimated the ability of CD8+ TILs to accomplish their functions in situ by assessing ζ-chain levels in these cells. The results revealed a significant elevation of its expression in CD8+ TILs from paclitaxel-treated mice as compared to TILs from untreated animals (p < 0.05; Fig. 6B).
FIGURE 6.
Paclitaxel induces restoration of CD8+ T cells in vivo. CD8+ T cells from melanoma lesions were analyzed by flow cytometry. (A) Results of six independent experiments (mean ± SD; 20–24 mice per group) are shown as the percentage of CD8+ T cells among all T cells. (B) ζ-chain level in CD8+ T cells is expressed as the mean fluorescence intensity (MFI) ratio (MFI of experimental samples/MFI of respective negative controls). Data of three independent experiments are shown (mean ± SD; 9–15 mice per group). * p < 0.05. (C) Cells from metastatic LN (mLN) and primary tumors were stained with mAbs for CD3 and CD8 and with tetramers containing Kb and the TRP-2 derived peptide SVYDFFVWL followed by flow cytometry. Frequencies of TRP-2-specific (tetramer-positive) CD8+ T cells among total CD8+ T cells are shown (mean ± SD; 8 mice per group). (D) Paclitaxel-treated mice were injected with rat anti-mouse mAbs depleting CD8+ T cells. IgG from rat serum were inoculated in untreated mice. (E) Survival (8 mice per group) is shown as a Kaplan-Meier curve. * p < 0.05, paclitaxel only versus paclitaxel and CD8+ T cell depletion; ** p < 0.01, paclitaxel only versus untreated group.
To determine the effect of paclitaxel on tumor-specific CD8+ TILs, we next measured the amount of CD8+ T cells specific for the melanoma-associated antigen tyrosinase related protein-2 (TRP-2) that infiltrated melanoma lesions. Under the treatment conditions, we demonstrated a significant increase in the frequency of TRP-2-specific CD8+ T cells in metastatic LN of paclitaxel-treated mice as compared to untreated animals (1.02 ± 0.17 % versus 0.48 ± 0.14 % among total CD8+ T cells; p < 0.05; Fig. 6C). In addition, we detected a strong tendency to the increase in the frequency of TRP-2-specific CD8+ TILs within total CD8+ TILs, despite a high variability in individual animals (p = 0.0765; Fig. 6C). These findings suggest that paclitaxel was able to increase not only the frequency of total CD8+ TILs but also that of TRP-2-specific CD8+ T cell subset in the tumor milieu in vivo.
To directly prove the role of effector T cells in the anti-tumor activity mediated by the paclitaxel administration, we depleted CD8+ T cells during paclitaxel treatment (Fig. 6D, E). The depletion efficiency was checked at day 10 in the spleen, peripheral blood and LN, showing more than 95% decrease in CD8+ T cell numbers. The results showed that the removal of CD8+ T cells in paclitaxel-treated mice resulted in the complete abrogation of the beneficial anti-melanoma effect of ultra-low dose paclitaxel documented by the absence of differences in survival with the control group of tumor-bearing mice (Fig. 6E).
Taken together, paclitaxel applied at ultra-low, non-toxic doses demonstrated a remarkable anti-tumor effect in vivo, indicated by a prolonged survival of melanoma-bearing mice and a reduced tumor burden. This effect was shown to be linked to the elevated CD8+ TIL numbers and activity in melanoma lesions associated with the down-regulation of chronic inflammation, leading to diminished amounts and immunosuppressive functions of MDSCs in the tumor microenvironment.
Discussion
We have recently reported a novel immunomodulatory approach based on the application of various chemotherapeutic drugs (including paclitaxel) at ultra-low non-cytotoxic doses called chemoimmunomodulation (1, 2). In particular, the administration of ultra-low dose paclitaxel has been shown to enhance the maturation and immunostimulatory functions of mouse DCs in vitro and in the transplantable tumor model in vivo (1, 2, 5). In the current study we highlight the complex effect of paclitaxel on the major myeloid cell subset with immunosuppressive functions, MDSCs, as well as on chronic inflammation in the tumor microenvironment in vivo.
MDSCs have been previously demonstrated by us and others to be recruited to the tumor site and activated both in cancer patients and tumor-bearing mice (8, 10–12, 13, 16, 17). Moreover, it has been reported that a constant activation of inflammatory pathways in myeloid cells led to a systemic enrichment of MDSCs, development of chronic inflammation and tumorigenesis (7). Using ret transgenic mice spontaneously developing skin melanoma with metastases in LN, liver, lungs and the BM, we detected here a significant reduction of MDSC frequencies in melanoma lesions upon the paclitaxel treatment. Interestingly, a similar decrease of CD11b+Gr1+ immature myeloid cells in spleens and LN was earlier observed in tumor-free C57BL/6 mice treated with the same non-toxic doses of paclitaxel (28). Furthermore, although MDSC numbers were not altered in the metastatic LN and BM of treated tumor-bearing mice, we revealed a reduction in frequencies of MDSCs producing NO that is involved in MDSC-mediated immunosuppression in these organs (10). Importantly, tumor-infiltrating MDSCs also displayed a significantly lower ability to suppress the proliferation of stimulated normal T cells indicating their decreased immunosuppressive function after paclitaxel administration. The mechanisms of observed differences in the paclitaxel-mediated effects on MDSCs in skin tumors and metastatic LN are currently under investigation.
Next we addressed a question about signaling pathways in MDSCs affected by the paclitaxel-based chemoimmunomodulation. We demonstrated that the phosphorylation of p38 MAPK in MDSCs from skin tumors, the BM and spleen was strongly down-regulated upon the paclitaxel treatment as compared to the untreated group. Signaling through p38 MAPK in myeloid cells was previously reported to induce a reduction of DC immunostimulatory capacities in ret transgenic melanoma-bearing mice (29) and to stimulate the production of chronic inflammatory mediators TNF-α, IL-1β, IL-10, TGF-β and IL-6 (24–26, 30, 31). Indeed, we have found here that a down-regulation of the p38 MAPK signaling in MDSCs was strongly associated with reduction of TNF-α production by these cells and decreased levels of the abovementioned factors in the chronic inflammatory tumor microenvironment. Furthermore, an autocrine regulation of p38 MAPK activation is possible since TNF-α and IL-1β were reported to activate p38 MAPK signaling (32, 33), stimulating thereby a production of IL-6 (34), an activation of inducible NO synthase (34, 35) and the accumulation of MDSCs (36). In agreement with these publications, our findings demonstrated that paclitaxel-mediated abrogation of p38 MAPK activation in MDSCs resulted in the decrease in their numbers in tumors as well as in their capacity to produce NO and suppress T cell functions. Decreased amounts of tumor-infiltrating MDSCs could be also due to an enhanced MDSC differentiation. Indeed, our recent in vitro studies demonstrated that paclitaxel in ultra-low concentrations can promote the MDSC differentiation into functional conventional DCs (37). Since p38 MAPK is also involved in signaling pathways activated by secreted S100A8/A9 in autocrine manner (21, 22, 38), we evaluated the intracellular expression of S100A9 in MDSCs. A significant reduction in the number of tumor-infiltrating MDSCs expressing S100A9 was found after administration of paclitaxel, additionally highlighting the importance of the stimulation of p38 MAPK-S100A8/A9 pathways in MDSC-mediated immunosuppression.
Taking into account a critical role of MDSCs and chronic inflammatory mediators in supporting tumor progression, we also assessed the anti-tumor potential of non-toxic application of paclitaxel and revealed a significant delay in tumor development indicated by a prolonged survival of treated animals. Investigating the mechanism of this effect, we found an increased numbers of CD8+ TILs and TCR ζ-chain expression in these cells upon the treatment. Importantly, in the metastatic LN, frequencies of TRP-2 specific CD8+ T cells were also significantly higher, although the total numbers of CD8+ T cells were not changed. Along this line, we have previously reported that administration of ultra-low dose paclitaxel in healthy C57BL/6 mice strongly increased the frequencies of TRP-2 specific spleen T cells upon the peptide vaccination (28). These results are in agreement with data reported by us and others that the reduction of MDSC numbers and/or immunosuppressive functions resulted in the restoration of effector functions of tumor-specific CD8+ T cells (13, 39). A selective depletion of CD8+ T cells led to the complete abrogation of the beneficial anti-tumor activity of paclitaxel, underlying a critical role of CD8+ T cells in this effect. In addition, a lack of direct toxic effect of paclitaxel in ultra low-dose (in contrast to conventional doses) on melanoma cells in vivo further emphasizes its immunomodulating potential.
In conclusion, we demonstrated here for the first time that administration of ultra-low non-cytotoxic dose of paclitaxel caused a significant reduction of tumor-associated immunosuppression due to the decrease in MDSC numbers and functions in the tumor lesions. These effects were strongly related to the down-regulation of p38 MAPK signaling in MDSCs associated with a decreased production of chronic inflammatory mediators in the tumor microenvironment. Abrogation of MDSC-driven immunosuppression strongly affected melanoma progression indicated by the prolonged survival of tumor-bearing animals through the CD8+ T cell-dependent mechanism. Together with our previous data on the stimulation of anti-tumor activities of DCs and modulation of intratumoral cytokine network (3–5), our findings here demonstrated that the paclitaxel-based chemoimmunomodulation could efficiently condition the tumor microenvironment, decreasing its immunosuppressive potential. This suggests that administration of paclitaxel in non-cytotoxic doses can be considered as a novel therapeutic approach for decreasing the protumorigenic potential of the tumor microenvironment and increasing the efficacy of associated anti-cancer therapies.
Supplementary Material
Acknowledgements
We thank I. Nakashima for initially providing ret transgenic mice, and K. Frank for excellent technical assistance.
Financial Support: This work was supported by the Dr. Mildred Scheel Foundation for Cancer Research (grant 108992, to V. Umansky), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer (to V. Umansky) and NIH NCI RO1 CA154369 (M.R. Shurin).
Abbreviations used in this article
- ARG-1
arginase-1
- BM
bone marrow
- DAF-2DA
diaminofluoresciein-2 diacetate
- DC
dendritic cells
- LK
Lin−Sca-1−c-Kit+ cells
- LN
lymph nodes
- LSK
Lin−Sca-1+c-Kit+ cells
- MDSC
myeloid-derived suppressor cells
- TIL
tumor-infiltrating lymphocytes
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Shurin MR, Naiditch H, Gutkin DW, Umansky V, Shurin GV. ChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agents. Curr. Med. Chem. 2012;19:1792–1803. doi: 10.2174/092986712800099785. [DOI] [PubMed] [Google Scholar]
- 2.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J. Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J. Transl. Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12- dependent mechanism. J. Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin. Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Shurin GV, Gutkin DW, Shurin MR. Tumor-associated regulatory dendritic cells. Semin. Cancer Biol. 2012;22:298–306. doi: 10.1016/j.semcancer.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Yan C, Czader M, Foreman O, Blum JS, Kapur R, Du H. Inhibition of PPARγ in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood. 2012;119:115–126. doi: 10.1182/blood-2011-06-363093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer. Immunol. Immunother. 2012;61:255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J. Immunother. 2012;35:107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 13.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. U S A. 2011;108:17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol. Immunother. 2012;61:827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 18.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 19.Umansky V, Abschuetz O, Osen W, Ramacher M, Zhao F, Kato M, Schadendorf D. Melanoma-specific memory T cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res. 2008;68:9451–9458. doi: 10.1158/0008-5472.CAN-08-1464. [DOI] [PubMed] [Google Scholar]
- 20.Campbell J, Ciesielski CJ, Hunt AE, Horwood NJ, Beech JT, Hayes LA, Denys A, Feldmann M, Brennan FM, Foxwell BM. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J. Immunol. 2004;173:6928–6937. doi: 10.4049/jimmunol.173.11.6928. [DOI] [PubMed] [Google Scholar]
- 21.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp. Cell Res. 2006;312:184–197. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin. Cancer Biol. 2006;16:80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL, Muñoz-Cánoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell. Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat. Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho FM, Lai CC, Huang LJ, Kuo TC, Chao CM, Lin WW. The antiinflammatory carbazole, LCY-2-CHO, inhibits lipopolysaccharide-induced inflammatory mediator expression through inhibition of the p38 mitogen-activated protein kinase signaling pathway in macrophages. Br. J. Pharmacol. 2004;141:1037–1047. doi: 10.1038/sj.bjp.0705700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer. Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 28.Sevko A, Kremer V, Falk C, Umansky L, Shurin MR, Shurin GV, Umansky V. Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J. Immunotoxicol. 2012;9:275–381. doi: 10.3109/1547691X.2012.655343. [DOI] [PubMed] [Google Scholar]
- 29.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin. Cancer Res. 2009;15:4382–4390. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 30.Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J. Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- 31.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D, Su B, Wang HY, Wang RF. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity. 2012;36:43–54. doi: 10.1016/j.immuni.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou FH, Foster BK, Zhou XF, Cowin AJ, Xian CJ. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J. Bone Miner. Res. 2006;21:1075–1088. doi: 10.1359/jbmr.060410. [DOI] [PubMed] [Google Scholar]
- 33.Gao D, Bing C. Macrophage-induced expression and release of matrix metalloproteinase 1 and 3 by human preadipocytes is mediated by IL-1β via activation of MAPK signaling. J. Cell. Physiol. 2011;226:2869–2880. doi: 10.1002/jcp.22630. [DOI] [PubMed] [Google Scholar]
- 34.Chae HJ, Kim SC, Chae SW, An NH, Kim HH, Lee ZH, Kim HR. Blockade of the p38 mitogen-activated protein kinase pathway inhibits inducible nitric oxide synthase and interleukin-6 expression in MC3T3E-1 osteoblasts. Pharmacol. Res. 2001;43:275–283. doi: 10.1006/phrs.2000.0778. [DOI] [PubMed] [Google Scholar]
- 35.Chen BC, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–129. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 2005;78:210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- 37.Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J. Immunotoxicol. 2012;9:292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006;72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Apetoh L, Végran F, Ladoire S, Ghiringhelli F. Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr. Mol. Med. 2011;11:365–372. doi: 10.2174/156652411795976574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.