Abstract
Background
Vascular endothelial growth factor is upregulated in hepatocellular carcinoma(HCC) and is further upregulated following transhepatic arterial chemoembolization. We conducted a phase II trial to evaluate safety and efficacy of bevacizumab combined with chemoembolization in patients with unresectable HCC.
Methods
Patients with Eastern Cooperative Oncology Group status 0–2, Child-Pugh score A–B and Barcelona Clinic Liver Cancer stage B–C were eligible. Treatment consisted of bevacizumab every two weeks and chemoembolization in week three, in a 6-week cycle, up to 3 cycles in 6 months. Primary endpoints were safety and efficacy.
Results
Twenty-five patients received chemoembolization and bevacizumab. The most common grade 3–4 events following cycle 1 were leukocytopenia(12%), fatigue(12%), hyponatremia(12%). Serious toxicities known to be associated with bevacizumab were observed in 4 patients. 30-day mortality was 0%. Median time-to-tumor progression of the targeted lesion(s) and overall survival were not reached and 10.8 months, respectively. The objective response rate was 60% using enhancement response evaluation criteria while the disease control rate was 100%.
Conclusions
Concurrent treatment with bevacizumab and chemoembolization is safe in carefully selected patients and shows antitumor activity in patients with unresectable HCC. These results support further development of bevacizumab combined with chemoembolization as a treatment for unresectable HCC.
Keywords: Transhepatic arterial chemoembolization, Hepatocellular Carcinoma, Bevacizumab
INTRODUCTION
Hepatocellular carcinoma (HCC) constitutes a major health issue, accounting for more than 598,000 deaths per year worldwide1, 2. The majority of patients with HCC presents at an advanced stage with a median survival less than 6 months3. Recently, sorafenib was shown in phase III clinical trials to prolong survival among patients with advanced HCC4, 5. Median survival, however, was still less than 1 year. For many patients with unresectable, intermediate HCC, transhepatic arterial chemoembolization is often recommended as the preferred treatment option6, 7. Several studies have shown the survival benefit of chemoembolization8. However, one of the main limitations of chemoembolization is the high incidence of recurrence. Even among patients with an initial response the 3-year cumulative recurrence rate can be as high as 65%9.
One possible reason for recurrence after chemoembolization is the stimulation of angiogenesis by chemoembolization -induced tumor hypoxia10. HCC is a highly vascular tumor in which angiogenesis mediated by vascular endothelial growth factor (VEGF) contributes to growth and metastatic spread. VEGF over-expression has been demonstrated to be a prognostic indicator of poor survival in patients with HCC11–14. VEGF is further up-regulated immediately following chemoembolization, and VEGF levels after treatment are an independent predictor of tumor response and survival15–17.
Bevacizumab, a humanized monoclonal antibody, prevents binding of VEGF to its receptors thereby inhibiting VEGF-mediated angiogenesis. Bevacizumab normalizes tumor vasculature, thereby improving tumor uptake of concomitantly administered therapeutic agents18. In addition, bevacizumab has been shown to modulate blood vessels and drug response in HCC in vitro19. Recently our group described our experience combining sorafenib with chemoembolization and showed that this approach was safe and potentially efficacious20. There is, however, a paucity of data on the use of chemoembolization combined with other biological agents such as bevacizumab.
We postulated that combined treatment with chemoembolization and bevacizumab might potentiate cytotoxic effects on HCC by preventing chemoembolization induced up-regulation of angiogenesis. In turn, combined chemoembolization and bevacizumab therapy might facilitate tumor uptake of the cytotoxic agents delivered through chemoembolization. To examine these hypotheses, we conducted a prospective two-center single-arm phase II trial to evaluate the safety and efficacy of bevacizumab combined with chemoembolization in patients with unresectable HCC.
MATERIALS AND METHODS
Study Population and Eligibility criteria
Patients (≥18 years) with a diagnosis of unresectable HCC based on either histology obtained by needle biopsy, or a hypervascular lesion >2 cm on cross-sectional imaging and an α-fetoprotein level of ≥200 ng/mL, were evaluated for this study21. Eligibility and exclusion criteria were similar to other phase II trials reported by our group20. The study was approved by our Institutional Review Boards, and conducted in accordance with the principles of the Declaration of Helsinki.
Study design
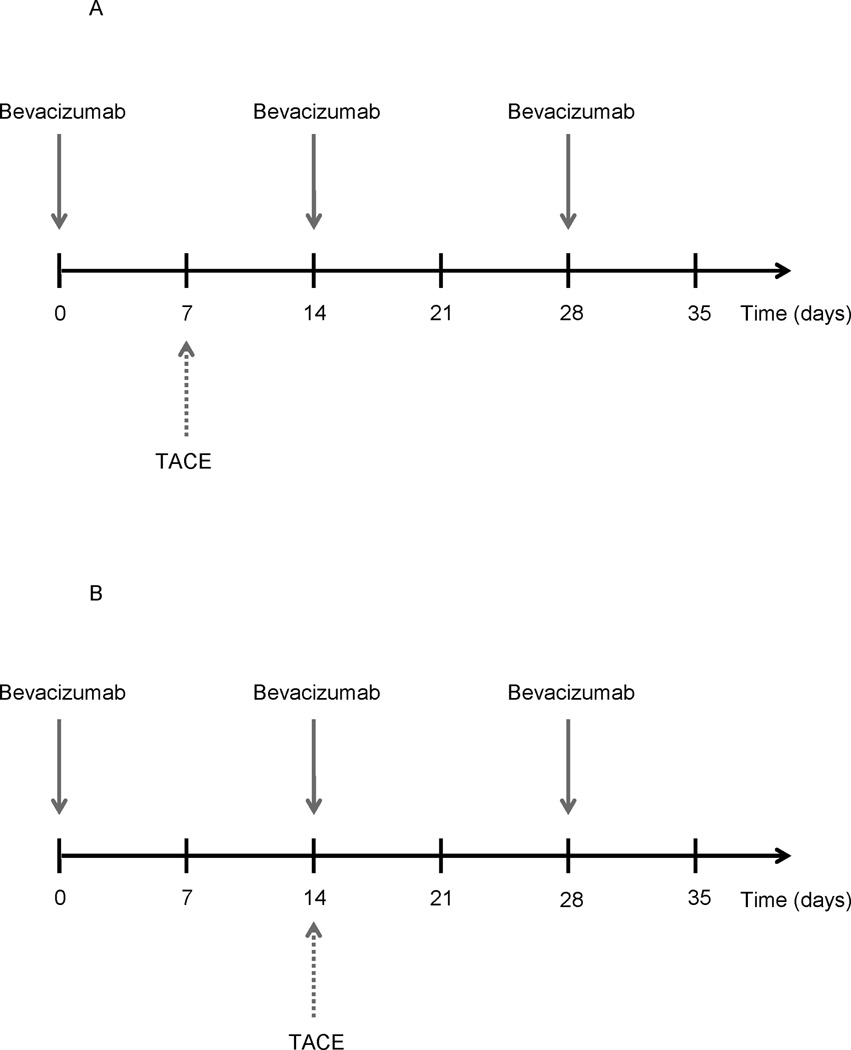
Patients were treated with intravenous bevacizumab (10mg/kg, Genentech, San Francisco, CA) and chemoembolization, up to 3 cycles in 6 months (Figure 1a). Five out of the first six patients, however, had not fully recovered from chemoembolization in week 3, which required withholding of the second dose of bevacizumab on week 4. Following this, the protocol was amended (Figure 1b). Bevacizumab and chemoembolization were given the same day (but not exactly at the same time). After completion of the last treatment cycle, follow-up included clinic visits and cross-sectional imaging every 8–12 weeks. After completion of the protocol, patients were allowed to receive other therapies. Chemoembolization procedures were performed as previously described22.
Figure 1.
Treatment protocol before (a) and after (b) protocol amendment.
Safety and Efficacy
Each study visit included a clinical assessment, laboratory evaluations, and toxicity assessments (according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0). In the event of a dose limiting toxicity (DLT), bevacizumab was held until resolution; no dose reductions were allowed. The interim safety assessment demonstrated no DLTs, therefore, enrollment was continued per protocol.
Tumor response was assessed using contrast-enhanced MR imaging at baseline, 3 weeks following each chemoembolization, and 4 weeks following the completion of the final treatment cycle. Images were centrally viewed by an independent diagnostic radiologist who was blinded to the fact that patients were part of a clinical trial. Response was evaluated and categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).23,24 Specifically, tumor response was assessed by standard RECIST criteria as well as by assessing tumor enhancement. Regarding assessment of tumor enhancement, a CR was defined as 100% tumor necrosis of the target lesion(s) upon completion of any of 3 cycles of chemoembolization therapy; PR was defined as patients demonstrating >50% tumor necrosis of the target lesion(s) upon completion of any of 3 cycles of chemoembolization therapy; SD was defined as patients not meeting criteria for CR or PR and not demonstrating evidence of disease progression. SD was measured from the start of treatment until the criteria for progression were met, taking as reference the smallest measurements of target lesion(s) recorded since treatment started. PD was defined as either reappearance or increased tumor enhancement >25% in the target lesion(s). Response was assessed considering the entire volume of the tumor(s) by an experienced radiologist (IK).
Statistical Considerations
The primary endpoint was time to tumor progression (TTP) of the targeted lesions. Our analysis followed the current recommendations for the design of HCC clinical trials, which recommends that deaths during follow-up without evidence of radiological progression should be censored25. Secondary endpoints included TTP of non-targeted lesions within the liver and overall TTP. TTP time points were measured from initiation of therapy until documentation of progression. Overall survival (OS) was measured both from the date of diagnosis of unresectable HCC and from the time of initiation of therapy until death.
Survival endpoints were estimated using the Kaplan Meier method. The objective response rate (ORR; the total of complete and partial responses of targeted lesions) and the disease control rate (DCR; the total of complete, partial, and stable responses of targeted lesions) were compared using using linear regression models fit with generalized estimating equations (GEE) to account for multiple tumors within the same patient. All analyses were conducted using statistical freeware R version 2.11.1 (www.rproject.org).
RESULTS
Patient Characteristics
Between September 2006 and April 2009, a total of 26 patients consented to the study and were enrolled. One patient left the study prior to receiving chemoembolization after receiving one administration of bevacizumab. This patient was included in the safety analysis, but excluded from the efficacy analysis. The characteristics of the study population are noted in Table 1.
Table 1.
Baseline Patient Characteristics (N=26)
| Characteristic | Number (N) |
|---|---|
| Institution Northwestern University Johns Hopkins University |
10 16 |
| Age (Years) Mean Range |
64 31–85 |
| Gender Male Female |
21 5 |
| Race Asian African American Caucasian Hispanic |
2 5 18 1 |
| ECOG 0 1 2 |
17 8 1 |
| Etiology HBV HCV Other |
4 12 10 |
| Prior Treatment None Transhepatic arterial chemoembolization Radioembolization Systemic chemotherapy Resection Transplantation |
15 4 3 1 4 1 |
| Liver Cirrhosis Yes No |
20 6 |
| Portal Vein Thrombosis Yes No |
5 21 |
| Child-Pugh Score A B C |
20 6 0 |
| BCLC Stage B C |
9 17 |
| AFP >200 ng/ml Number of patients Mean (ng/ml) Median (ng/ml) |
8 14,323 3,373 |
| Metastatic Disease Yes No |
6 20 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus,; BCLC, Barcelona Clinic Liver Cancer; AFP, Alpha-fetoprotein. All data are expressed as number of patients unless otherwise stated.
Safety and Treatment Toxicity
Overall, the 26 patients enrolled in the study were treated with a total of 43 cycles of therapy. The median number of treatment cycles per patient was 2 (range, 1–3). At the time of last follow-up, 6 patients were alive and were censored at this time point. The median number of chemoembolization treatments per patient was 2 (range, 1–3). The median number of weeks of bevacizumab therapy delivered per patient was 4 (range, 1–9). A total of 14 bevacizumab administrations were withheld due to adverse events (n=6 prior to protocol amendment).
During cycle 1, 100% of patients experienced some toxicity. The most common toxicities during weeks 1–2, cycle 1 (i.e. bevacizumab only) included hyperbilirubinemia (12%), elevated PT (8%), nausea (8%), and elevated AST (8%). In general, most toxicities during weeks 1–2, cycle 1 were not severe (G1–2, 83%; G3–4, 17%) (Table 2). Uncommon G3–G4 toxicities seen in weeks 1–2, cycle 1 were fatigue (4%), ascites (4%), hemorrhage of esophageal varices (4%), hyponatremia (4%), and encephalopathy (4%). Following week 3, cycle 1 (i.e. chemoembolization administration), each patient experienced at least one toxicity. The most common G1–G2 toxicities following chemoembolization consisted of hypoalbuminemia (44%), anorexia (40%), anemia (36%), fatigue (36%), lymphopenia (28%), elevated ALT (24%), elevated AST (24%) and hyperglycemia (24%). One patient had a G1 vascular event consisting of an arterial dissection involving the hepatic artery. The dissection was noted on MRI pre- chemoembolization and was again incidentally noted during the chemoembolization procedure but did not preclude successful completion of the procedure. This event and other lower grade events resolved without any sequelae. The most common G3–G4 toxicities following chemoembolization consisted of leukocytopenia (12%), fatigue (12%), and hyponatremia (12%).
Table 2.
Toxicities
| Toxicities | Cycle 1 (Pre-TACE, bevacizumab only) n=26 |
Cycle 1 (Post- TACE) n=25 |
Cycles 2–3 n=14 |
||||
|---|---|---|---|---|---|---|---|
| # events | # events | # events | |||||
| Grade | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 5 |
| *Blood/bone marrow | 0 | 0 | 22 | 5 | 20 | 2 | 0 |
| **Anemia | - | - | 9 | 1 | - | - | - |
| Leukocytopenia | - | - | 4 | 3 | 7 | - | - |
| Lymphopenia | - | - | 7 | 1 | 10 | 2 | - |
| Cardiovascular | 0 | 0 | 4 | 3 | 3 | 1 | 0 |
| Heart failure | - | - | - | - | - | 1 | - |
| Edema | - | - | 2 | 1 | 1 | 1 | - |
| Ischemia | - | - | - | 1 | - | - | - |
| Coagulation | 3 | 0 | 10 | 0 | 5 | 0 | 0 |
| Constitutional | 2 | 1 | 19 | 5 | 14 | 1 | 0 |
| Fatigue | 1 | 1 | 9 | 3 | 10 | 1 | - |
| Night sweats | - | - | 1 | 1 | - | - | - |
| Weight loss | 1 | - | 5 | 1 | 2 | - | - |
| Dermatologic | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Endocrine | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Gastrointestinal | 5 | 1 | 28 | 4 | 12 | 1 | 1 |
| Ascites | - | 1 | - | 1 | 2 | 1 | - |
| Anorexia | 1 | - | 10 | 1 | 2 | - | - |
| Duodenal perforation | - | - | - | - | - | - | 1 |
| Nausea | 2 | - | 3 | 1 | 1 | - | - |
| Vomiting | 1 | - | 5 | 1 | 2 | - | - |
| HEENT | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Hemorrhage/bleeding | 0 | 1 | 2 | 0 | 3 | 0 | 0 |
| Esoph varices | - | 1 | - | - | - | - | - |
| Hepatic function | 7 | 0 | 32 | 5 | 24 | 2 | 0 |
| Elevated ALT | 1 | - | 6 | 1 | 2 | - | - |
| Elevated AST | 2 | - | 6 | 1 | 5 | 1 | - |
| Hyperbilirubinemia | 3 | - | 4 | 2 | 3 | 1 | - |
| Liver failure | - | - | - | 1 | - | - | - |
| Infection | 0 | 0 | 2 | 2 | 1 | 1 | 0 |
| Cellulitis | - | - | - | 1 | - | - | - |
| C.difficile | - | - | - | - | - | 1 | - |
| Colangitis | - | - | - | 1 | - | - | - |
| Metabolic | 3 | 1 | 18 | 8 | 21 | 2 | 0 |
| Hyperglycemia | - | - | 6 | 2 | 10 | 1 | - |
| Hypocalcemia | - | - | 2 | 1 | 1 | 1 | - |
| Hypokalemia | - | - | - | 2 | 1 | - | - |
| Hyponatremia | 1 | 1 | 4 | 3 | 4 | - | - |
| Musculo/skeletal | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Fracture | - | - | - | - | - | 1 | - |
| Neurologic | 2 | 1 | 7 | 2 | 5 | 2 | 0 |
| Cord compression | - | - | - | - | - | 1 | - |
| Delerium | - | - | - | 1 | - | - | - |
| Encephalopathy | - | 1 | - | - | - | - | - |
| Psychosis | - | - | - | 1 | - | - | - |
| Stroke | - | - | - | - | - | 1 | - |
| Pain | 1 | 0 | 9 | 6 | 7 | 0 | 0 |
| Abdominal NOS | - | - | 5 | 1 | - | - | - |
| Epigastric | - | - | - | 1 | - | - | - |
| RUQ | 1 | - | 3 | - | 4 | - | - |
| Chest | - | - | - | 2 | - | - | - |
| Other | - | - | 1 | 2 | 3 | - | - |
| Pulmonary | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| Dyspnea | - | - | 1 | 1 | 1 | - | - |
| Renal | 1 | 0 | 2 | 2 | 2 | 0 | 0 |
| Acute renal failure | - | - | - | 1 | - | - | - |
| Proteinuria | 1 | - | 1 | 1 | 2 | - | - |
| Respiratory | 1 | 0 | 3 | 0 | 5 | 0 | 0 |
| TOTAL | 25 | 5 | 161 | 43 | 128 | 14 | 1 |
ABBREVIATIONS: TACE: transarterial chemoembolization. HEENT: head/eyes/ears/nose/throat. Esoph: esophageal. ALT: alanine aminotransferase. AST: aspartate aminotransferase. C.difficile: Clostridium difficile. NOS: not otherwise specified. RUQ: right upper quadrant
total number events per system listed in highlighted rows.
grade 3-4-5 events listed in non-highlighted rows
In total, during cycle 1, 25 out of 25 patients experienced at least one toxicity associated with bevacizumab plus chemoembolization. However, the overall toxicity profile for cycle 1 was good as only 21% of all reported toxicities were G3–G4, with most reported toxicities being G1–G2 (79%). Possible bevacizumab related toxicities included one patient who had chest pain one week following the third bevacizumab dose during cycle one.
Fourteen patients were treated with 2–3 cycles of therapy. In general, G1–G2 toxicities following 2–3 cycles was higher than the toxicity seen after cycle 1 alone: hypoalbuminemia (79%), lymphopenia (71%), fatigue (71%), hyperglycemia (71%), and leukocytopenia (50%). Similar to cycle 1, one patient had a G1 vascular event consisting of an arterial dissection involving the hepatic artery during cycle 2, which resolved without sequelae. Similar to cycle 1, most toxicities during cycle 2–3 were not severe (G1–2, 90%; G3–4, 10%). The most common G3–G4 toxicity after 2–3 cycles was lymphopenia (14%); other G3–G4 adverse events included edema, fatigue, ascites, elevated AST, hyperbilirubinemia, hyperglycemia, hypocalcemia, cord compression and fracture, all occurring in only one patient (7%). Possible bevacizumab related toxicities included one patient who was admitted for clostridium difficile infection after completing cycle two. During this admission the patient suffered from volume overload and a more detailed cardiac work-up atrial flutter and cardiomyopathy. A second patient, who had cerebral vascular disease diagnosed on follow-up imaging, experienced a cerebral infarct during cycle 2. A separate patient suffered a G5 toxicity due to a ruptured duodenal ulcer. This patient had completed the second cycle of therapy and was noted to have a perforated duododenal perforation, developed sepsis and subsequently died (Table 2).
Efficacy
23 patients were evaluable for tumor response by imaging. One patient withdrew from the study prior to follow-up imaging; a second patient exited from the study due to cardiac toxicity and a third patient was lost to follow-up. Treatment was associated with a decrease in median tumor size from 5.8 cm to 4.6 cm (P<0.0001); in addition, median tumor enhancement was noted to decrease from 100% to 25% (P<0.0001)(Table 3). The ORR was 35% by RECIST and 60% by tumor enhancement (Table 3). Using either tumor response criteria, the DCR was 100% while the patients underwent treatment. One patient had an objective response lasting more than two years and, as a result, was successfully bridged to transplantation. Of note, on pathological analysis of the explant liver, there was showed no evidence of residual viable HCC.
Table 3.
Efficacy (n=23 patients)
| Features | Baseline Median (Range) |
Post therapy Median (Range) |
Mean percent change from baseline, 95% CI |
p-value | |
|---|---|---|---|---|---|
| Tumor size (cm), n=36 lesions | 5.8 (1.4–20) |
4.6 (1.4–18.2) |
−9.9% [−15%,−4.8%] |
<0.0001 | |
| Contrast-enhancement (%), n=36 lesions | 100 (50–100) |
25 (0–100) |
−57% [−71%, −43%] |
<0.0001 | |
|
Tumor Enhancement |
RECIST | ||||
| No. | % | No. | % | ||
| Response of the targeted lesion Complete Response Partial Response Stable Disease Progressive Disease |
4 10 9 0 |
17 43 39 0 |
0 8 15 0 |
0 35 65 0 |
|
Abbreviations: Tumor Enhancement; RECIST, Response Evaluation Criteria in Solid Tumors.
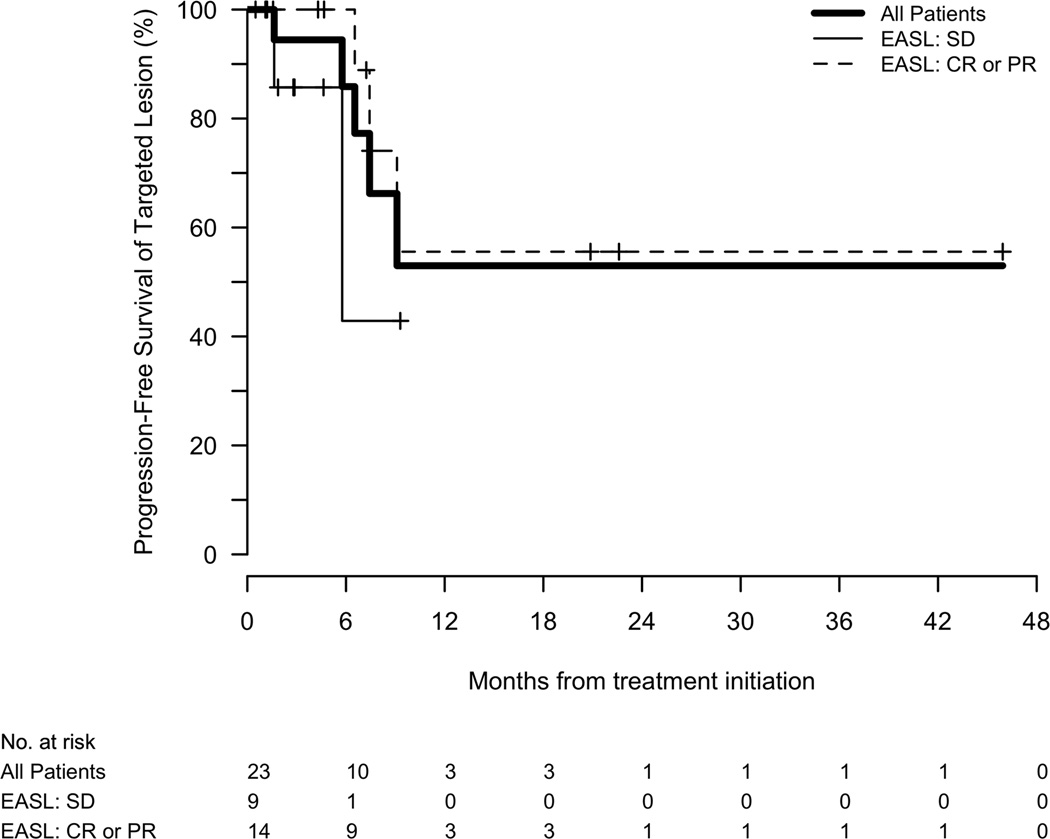
Among those lesions that were targeted, the majority had no evidence of progression at 6 months (86%) or 1 year (53%). The median TTP of the targeted lesion from time of therapy was not reached at last follow-up. In contrast, the median TTP in non-targeted lesions was 9.1 months (95%CI, 6.5–9.1). The median overall TTP from initiation of therapy was 7.2 months (95%CI, 5.8–9.1), with a corresponding 6 month and 1 year progression-free survival of 65% (95%CI, 47–90%) and 23% (95%CI, 9–60%), respectively. Responders tended to have a longer TTP (7.5 months) compared with patients who had stable disease (5.8 months)(P=0.09)(Figure 2a).
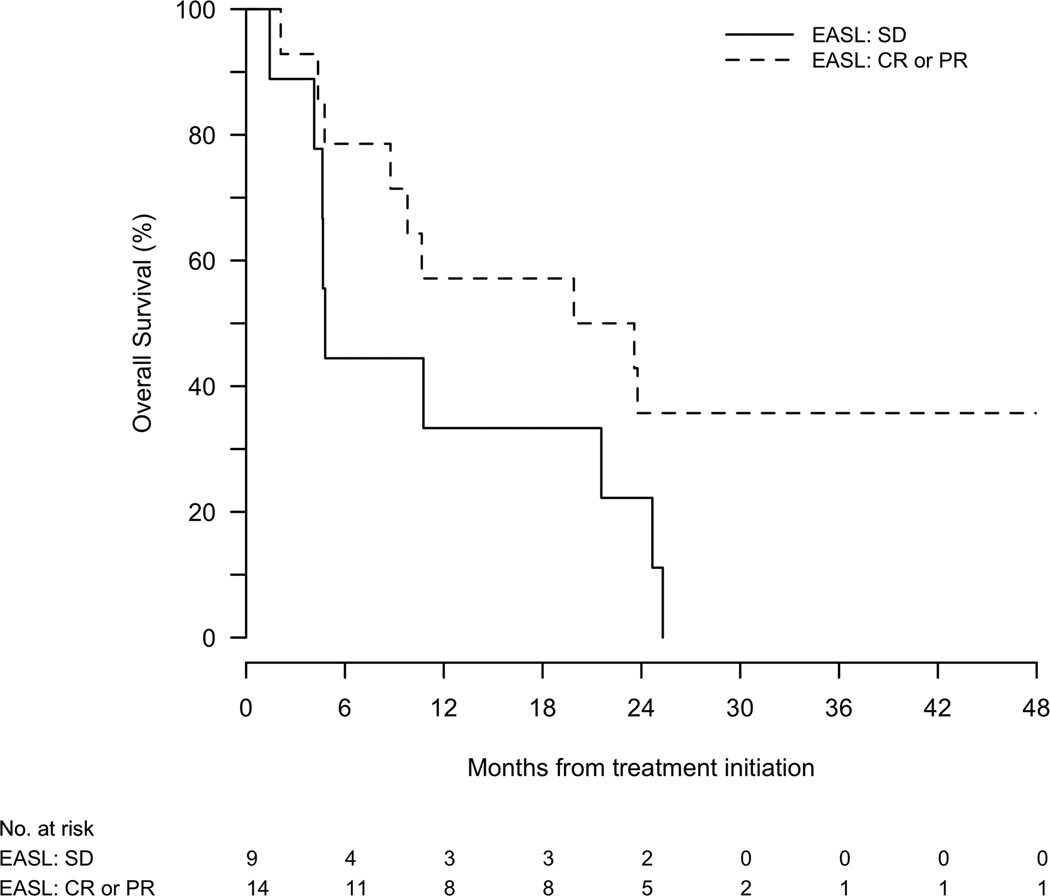
Figure 2.
(a) Time to tumor progression of the targeted lesion, stratified by imaging response. (b) Overall survival stratified by imaging response.
Median OS was 10.8 (95%CI, 4.8–24.7) and 23.6 (95%CI, 12.8–31.5) months from start of therapy and from diagnosis, respectively. Responders tended to have a longer median overall survival (21.7 months) compared with patients who only had stable disease (4.8 months)(P=0.09)(Figure 2b).
DISCUSSION
There has been increasing interest in combining systemic agents with intra-arterial therapy for advanced HCC. While intra-arterial therapy has been shown to effectively induce necrosis of targeted lesions, there is concern that liver-directed therapy may induce hypoxia-mediated factors that could stimulate neo-angiogenesis and new tumor growth10, 15–17. Our group has had an interest in intra-arterial therapy for HCC, as well as combination therapy using intra-arterial therapy with systemic agents20. The current paper is important because we demonstrate that the combination of chemoembolization and bevacizumab was safe in carefully selected patients. chemoembolization has previously been shown to cause systemic side effects, largely related to the post embolization syndrome, which typically include nausea, vomiting, abdominal pain, fever and loss of appetite22. The toxicities reported here with combined chemoembolization and bevacizumab therapy were expected and mostly related to chemoembolization, as the toxicity profile of combination therapy was similar to that reported after treatment with chemoembolization alone22. In addition to defining the safety and toxicity profile of combined chemoembolization and bevacizumab therapy, we noted that this combination had considerable antitumor effects when utilized in patients with unresectable HCC. Specifically, we noted no radiologic progression of targeted lesions during therapy. Collectively, these data suggest that combined therapy with chemoembolization and bevacizumab is safe and may have efficacy in treating patients with advanced HCC.
To date, the use of combined chemoembolization and bevacizumab in the advanced HCC population has not been previously reported in detail. In one unpublished pilot study, HCC patients – all of whom underwent chemoembolization – were then randomized to receive either concurrent continuous bevacizumab or observation26. The reported preliminary results in seven patients showed that the combination of chemoembolization and bevacizumab was relatively well-tolerated. However, Grade 3–4 toxicities were seen in four patients and included hypertension and a variceal bleed. A separate, randomized, placebo-controlled trial of chemoembolization and bevacizumab in patients with advanced HCC was recently reported27. In this trial, patients were treated with standard chemoembolization and either placebo or bevacizumab (5 mg/kg) every 2 weeks over a period of 48 weeks. This trial, however, was stopped early due to safety concerns related to bevacizumab toxicity. In the current study, we noted an overall toxicity profile for combined therapy with chemoembolization and bevacizumab. Specifically, while 100% of patients experienced some toxicity during cycle 1, the overwhelming toxicities were G1–2 (79%). The lower toxicity reported with cycle 1 of chemoembolization and bevacizumab in our study is most likely multi-factorial. We closely monitored blood pressure both pre-therapy and during therapy; in addition, all patients with large varices on imaging were required to undergo endoscopy and be treated with banding prior to therapy. Our study design also allowed for bevacizumab treatment breaks of several weeks between cycles, which may help explain the better toxicity profile. It is important to note, however, that toxicity with chemoembolization and bevacizumab did seem to increase with additional cycles of therapy. In fact, 7 out of 14 (50%) patients who were treated with 2–3 cycles of therapy experienced a G3–4 toxicity. Perhaps more importantly, several of these appeared to be related to bevacizumab and were similar to those reported in other phase I/II studies with bevacizumab28, 29. As such, while combined chemoembolization and bevacizumab appears safe, judicious patient screening and selection are needed. More investigation will be needed to define better whether more than one cycle of combined chemoembolization and bevacizumab is indeed more toxic.
Combined chemoembolization and bevacizumab therapy did demonstrate antitumor effects. While patients were on the protocol, combination therapy yielded a 100% disease control rate as assessed by radiographic criteria. Perhaps as expected, no evaluable patient had a complete response according to RECIST criteria. These findings are consistent with data from other studies evaluating chemoembolization or anti-angiogenic agents, which have noted that tumors that respond to these types of treatments do not necessary “shrink” or show a decrease in their cross-sectional diameter30, 31. Rather, tumor necrosis – rather than tumor size – appears to be a better indicator of therapeutic efficacy24. In the current study, we noted clear evidence of tumor necrosis of the lesions targeted by chemoembolization. There was a significant decrease in contrast enhancement of the targeted lesions following treatment with combined chemoembolization and bevacizumab after treatment. Based on these criteria, we noted an ORR response rate of 60% following treatment. This translated into a median overall TTP of 7.2 months. The response rates and time to progression that we noted for combined chemoembolization and bevacizumab were more favorable than the reported ORR and TTP reported in recent phase I/II studies with bevacizumab regimens that did not include chemoembolization for unresectable HCC28, 32.
Although not a primary aim of the current study, we did examine OS following combined therapy. The OS of 10.8 months reported following chemoembolization and bevacizumab was comparable with the previously reported OS of 9.6 months in HCC patients treated with a combination of bevacizumab and gemcitabine and oxaliplatin29. It was also comparable to the median survival noted in the SHARP trial 5. Other studies, however, have noted a slightly better median OS of 12.4 months and 15.7 months, respectively28, 32. The higher median OS in these studies cannot, however, be directly compared with the results of the current study. Patient selection, as well as administration of therapy, varied. For example, in the study by Siegel et al. the investigators utilized continuous dosing of bevacizumab and excluded all patients with metastatic disease28. Unlike the SHARP trial, some patients in the current study also had received prior treatments such as prior embolization, yttrium-90 or liver transplant. The heterogeneity in prior treatment exposures is one limitation of the current paper. In addition to not being treatment naïve, the slightly lower OS in the current study may have also been due to the fact that according to our protocol, patients who progressed at any time later than 6 months after initiation of therapy and those who had a bevacizumab break longer than 2 months, did not receive additional treatment cycles. A number of patients who did progress may have benefited from additional treatment cycles and the reported OS may therefore be an underestimate.
In conclusion, in this single arm, phase II study, we provide important data that begin to define the safety and efficacy of combined loco-regional and systemic treatment of patients with advanced HCC. We noted that combined chemoembolization and bevacizumab was safe in carefully selected patients and had antitumor activity. These results support further development and investigation of chemoembolization combined with bevacizumab as a treatment for advanced, unresectable HCC.
Acknowledgments
Partially funded by: Genentec; Biosphere Medical; NIH T32 grant 5T32EB006351-04 (Manon Buijs)
Footnotes
Financial disclosures: None
REFERENCES
- 1.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 3.Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100(9):1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, Bekaii-Saab T, Ben-Josef E, Blumgart L, Clary BM, Curley SA, et al. Hepatobiliary cancers. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(8):728–750. doi: 10.6004/jnccn.2006.0064. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 10.Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol. 2011;80(1):40–53. doi: 10.1016/j.critrevonc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115(21):4895–4906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- 12.Kaseb AO, Hassan MM, Lin E, Xiao L, Kumar V, Pathak P, et al. V-CLIP: Integrating plasma vascular endothelial growth factor into a new scoring system to stratify patients with advanced hepatocellular carcinoma for clinical trials. Cancer. doi: 10.1002/cncr.25791. Epub 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng CS, Lo HW, Chen PH, Chuang WL, Juan CC, Ker CG. Clinical significance of plasma D-dimer levels and serum VEGF levels in patients with hepatocellular carcinoma. Hepatogastroenterology. 2004;51(59):1454–1458. [PubMed] [Google Scholar]
- 14.Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4(2):220–226. [PubMed] [Google Scholar]
- 15.Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11(5):1077–1084. [PubMed] [Google Scholar]
- 16.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 17.Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15(36):4582–4586. doi: 10.3748/wjg.15.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong YQ, Sun HC, Zhu XD, Zhang W, Zhuang PY, Zhang JB, et al. Bevacizumab enhances chemosensitivity of hepatocellular carcinoma to adriamycin related to inhibition of survivin expression. J Cancer Res Clin Oncol. 2011;137(3):505–512. doi: 10.1007/s00432-010-0914-8. [DOI] [PubMed] [Google Scholar]
- 20.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29(30):3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buijs M, Vossen JA, Frangakis C, Hong K, Georgiades CS, Chen Y, et al. Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience. Radiology. 2008;249(1):346–354. doi: 10.1148/radiol.2483071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 26.Britten CDFR, Gomes AS, et al. A pilot study of IV bevacizumab in hepatocellular cancer patients undergoing chemoembolization. J Clin Oncol, 2005 ASCO Annual Meeting Proceedings. 2005;(23):4138. [Google Scholar]
- 27.Ulbrich GKC, Pinter M, et al. AVATACE-1 trial: Bevacizumab as inhibitor of collateral tumor-vessel-growth during transarterial chemoebolization (TACE) for hepatocellular carcinoma- a double-blind, randomized, placebo-controlled pilot-trial. Hepatology, The Liver Meeting. 2010;(52):1159–1160. [Google Scholar]
- 28.Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu AX, Blaszkowsky LS, Ryan DP, Clark JW, Muzikansky A, Horgan K, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(12):1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 30.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250(2):466–473. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuma RS. Sometimes size doesn't matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98(18):1272–1274. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27(6):843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]