Abstract
Leishmaniasis causes significant morbidity and mortality worldwide and there are no vaccines available against this disease. Previously, we had shown that the amastigote specific protein p27 (Ldp27) is a component of an active cytochrome c oxidase complex in L. donovani and upon deletion of its gene the parasite had reduced virulence in vivo. In this study, we have shown that Ldp27−/− parasites do not survive beyond 20 weeks in BALB/c mice, hence are safe as an immunogen. Upon virulent challenge, 12 weeks post-immunized mice showed significantly lower parasite burden in liver and spleen. When mice were challenged 20 weeks post immunization, there was still a significant reduction in parasite burden suggesting long term protection by Ldp27−/− immunization. Immunization with Ldp27−/− induced both pro- and anti- inflammatory cytokine responses and activated splenocytes for enhanced leishmaniacidal activity in association with NO production. Protection in both short and long term immunized mice after challenge with the wild type parasite correlated with the stimulation of multifunctional Th1 type CD4 and CD8 T cells. Adoptive transfer of T cells from long term immunized mice conferred protection against virulent challenge in naïve recipient mice suggesting involvement of memory T cell response in the protection against Leishmania infection. Immunization of mice with Ldp27−/− also demonstrated cross-protection against the Leishmania major and Leishmania braziliensis infection. Our data show that genetically modified live attenuated Ldp27−/− parasites are safe, induce protective immunity even in the absence of parasites and can provide protection against homologous and heterologous Leishmania species.
Introduction
Leishmaniasis is an insect vector borne disease caused by the protozoan parasite Leishmania. Approximately 350 million people worldwide living in tropical and subtropical regions are under the threat of such infections. There are 12 million infections worldwide with ~2 million new clinical cases reported annually and an estimated death toll of ~50,000 persons/year (1). The clinical manifestation of leishmaniasis depends mostly on the infecting Leishmania species as well as host factors that range from mild cutaneous leishmaniasis (CL) to potentially fatal visceral leishmaniasis (VL) (2–4). VL is one of the major health problems on the Indian subcontinent, East Africa and most of the Latin American countries (5–7). Available drugs are toxic, and moreover emergence of drug resistant parasites as well as co-infection with HIV make the drug treatment regime even more complex (8). Currently there are no vaccines against Leishmania infections in human. In leishmaniasis, the host defense against intracellular Leishmania is cell mediated, which involves Th1 responses due to T-cells primed mostly by dendritic and macrophage cells producing IL-12 (9–12). Production of IL-12 by antigen presenting cells and IFNγ by T cells are crucial for controlling the parasite growth by enhancing the nitric oxide generation (13). The Th2 cytokines, mainly IL-10, suppress host immunity and help parasite survival (13–14), however IL-10 also protects the host from tissue damage by excessive inflammatory cytokines (15). Unlike cutaneous leishmaniasis, the Th1/Th2 dichotomy is not as clear in visceral infection of mice and even less so in human VL (16). The immune response and pathology of visceral leishmaniasis is complex involving a number of genetic and cellular factors in the process of susceptibility or resistance to parasites (17).
In past years, several approaches have been tested for the Leishmania vaccine development such as DNA vaccination, subunit vaccination and heat killed parasite vaccination with and without adjuvant (8, 18–19). Some of these worked in animal models however none have been successful so far in humans. Leishmanization, a process in which deliberate infections with Leishmania major cause a controlled skin lesion with very low number of parasites has been shown to provide protection against reinfection (20–21). Immunity can also be acquired by pre-exposure to infection as was demonstrated in individuals who migrated from an L. major endemic region were reactive to Leishmania antigen, and had a lower risk of developing VL (22–23). Therefore, these studies suggest that for an effective vaccine against leishmaniasis a controlled parasitic infection that can provide the complete array of antigens of a wild type parasite might be necessary for developing a protective immune response. Past experience with other pathogens has suggested that live attenuated pathogens can fulfill such a requirement (24–26)
Attempts to develop a live attenuated Leishmania vaccine, including chemical mutagenesis, long-term serial in vitro cultures, irradiation, temperature sensitivity and targeted gene deletions of both alleles have been made in the past (18, 27–33). However, most of the mutated parasite cell lines were developed in species causing cutaneous leishmaniasis, such as L. major or L. mexicana. Attempts were also made using partial targeted gene deleted parasites for the A2-A2rel gene cluster in L. donovani (34) and SIR2 gene in L. infantum (35) to test as immunogens. However, such mutants developed in VL causing parasites cannot be used as vaccine candidates because they still carry single alleles of the wild type gene that could revert to the wild genotype and regain virulence. Therefore it is critical to develop attenuated parasites through complete gene knockouts in which all the alleles of a virulence gene are non-functional and hence not capable of reversion to cause the disease. L. donovani biopterin transporter (BT1) null mutant parasites with both alleles disrupted were tested in mice showing reduced infectivity and induced protection against infection with wild type parasites (36). However this study did not address the issue of safety and correlates of immune protection for genetically modified live attenuated L. donovani parasites. To address this question, we previously developed an amastigote specific replication deficient Centrin gene deleted L. donovani parasite cell line (LdCen−/−) that was tested in a rodent model, and found to have limited persistence and induce a protective cellular immune response in immunized animals (37). Recently, we developed another L. donovani cell line devoid of the p27 gene, encoding an amastigote specific cytochrome c oxidase component (38) and demonstrated that Ldp27−/− parasites persist longer (> 12 weeks) compared to LdCen−/− (5 weeks) in mice (37–38). We therefore evaluated whether longer persistence of Leishmania antigens can produce better protection. In our current study we showed that the Ldp27−/− parasite cell line, which can persist for an extended period of time without causing pathogenesis, can elicit an effective cell mediated effector protective immune response against homologous and heterologous Leishmania species.
Materials and Methods
Animals and parasites
Five to six week old female BALB/c mice from the National Cancer Institute, Bethesda, MD were used in the experiments. Procedures used were reviewed and approved by the Animal Care and Use Committee, Center for Biologics Evaluation and Research, Food and Drug Administration. Among parasites, the wild type (Wt) L. donovani (Ld1S) maintained in Golden Syrian hamsters and p27 gene deleted (Ldp27−/−) of L. donovani (Ld1S2D) (38) were used. The parasites were cultured according to the procedure previously described (39–40). Leishmania major (Friedlin) and L. braziliensis promastigotes were grown at 26°C in medium 199 supplemented (M199/S) with 20% fetal calf serum.
Immunizations and challenge studies
The mice were immunized via tail vein with 3 × 106 stationary phase Ldp27−/− parasites. In each study 4 to 5 mice were used per group. Immunized mice were challenged after different time periods of immunization via tail vein with 105 stationary phase Wt L. donovani parasites. Age matched naïve mice as controls were also similarly challenged with 105 virulent stationary phase Wt L. donovani parasites. In separate experiments, mice immunized (with Ldp27−/−) or not (naïve with saline) were also challenged by injecting s. c./ i. d. on the left hind footpad with 105 stationary phase L. major or 106 stationary phase L. braziliensis parasites. After various periods post challenge, parasite load was measured from spleens and livers from the L. donovani challenged mice and from footpads and lymph nodes from the L. major or L. braziliensis challenged mice by limiting dilutions as previously described (41). As an additional confirmation of the presence of parasites in tissues, total DNA obtained from spleens of mice from certain groups as specified in the results was also used as template in real-time PCR. The real-time PCR was based on the target from the kinetoplast minicircle DNA using primers and methods as described (42).
For the immune-suppression study, mice were infected with either Wt or Ldp27−/− parasites, and 20 weeks or 25 weeks post-infection 2 mg/kg Dexamethasone sodium phosphate (Sigma Aldrich) in PBS was administered subcutaneously 3 times per week (43). At the end of this treatment mice were sacrificed and evaluated for parasite burden from visceral organs.
Multiplex Cytokine ELISA
Single cell suspensions were prepared from splenocytes after lysis of red blood cells by using Ammonium-Chloride-Potassium (ACK) lysing buffer (Lonza, MD, USA). Cells were washed with medium and plated in 24 well plates and stimulated with either freeze-thaw L. donovani antigen (FTAg) or without Ag in complete RPMI 1640 medium at 37°C in 5% CO2 with 95% humidity incubator. After 72 hours of culture cell supernatants were collected and stored in −80°C until analyzed using multiplex kits, Milliplex Mouse Cytokine/ Chemokine Magnetic Panel from Millipore, USA and the plate was read in a Luminex -100™ (Luminex, Austin, USA) system using Bioplex manager software 5.0. The cytokine analysis procedure has been performed according to manufacturer’s instructions and the level of cytokine concentration determined by using a standard curve of each specific cytokine.
Intracellular staining and flow cytometry
Splenocytes were plated in 24-well plates in complete RPMI medium at 37°C and stimulated with or without FTAg (80μg/ml) (44). After 48 hrs at 37°C Protein Transport Inhibitor (BD Golgi Stop, BD Pharmingen) was added to the wells. Six hours after cells were blocked at 4°C with Rat anti mouse CD16/32 (5μg/ml) from BD Pharmingen for 20 minutes. Cells were surface stained with anti mouse CD3 APC-eFluor@780, anti mouse CD4 eFluor@450, anti mouse CD8a eFluor@605NC, anti mouse CD44 FITC, and anti mouse CCR7 PE-Cy5 antibodies (eBioscience, USA) for 30 min (each with 1:200 dilution; 4°C). The cells were then stained with Live/Dead Fixable Aqua (Invitrogen, Molecular Probes) to stain dead cells. Cells were washed with wash buffer and fixed with the Cytofix/ Cytoperm kit (BD Bioscience) for 20 min (room temperature). Intracellular staining was done with anti mouse IFNγ PE-Cy7, anti mouse TNFα PerCp-eFluor@710, anti mouse IL-2 APC and anti mouse IL-10 PE (eBioscience, USA) for 30 min (each with 1:300 dilution; 4°C). Cells were acquired on LSRII (BD Biosciences, USA) equipped with 405, 488, 532, and 638 laser lines using DIVA 6.1.2 software. Data were analyzed with the FlowJo software version 9.1.5 (Treestar, San Carlos, CA). For analysis, first doublets were removed using width parameter; dead cells were excluded based on staining with the Live/Dead Aqua dye. Lymphocytes were identified according to their light-scattering properties. CD4 and CD8 T-cells were identified as CD3+ lymphocytes uniquely expressing either CD4 or CD8. Upon further gating intracellular cytokines were measured in CD44Hi CCR7Low cells. FMO controls were used for proper gating of positive events for designated cytokines.
Antibody responses
Specific antibody responses were measured by conventional enzyme-linked immunoadsorbent assay (ELISA). Briefly, ELISA plates were coated overnight at room temperature with FTAg (15μg/ml). A serial dilution of the sera was carried out to determine the titer, which is defined as the inverse of the highest serum dilution factor giving an absorbance of >0.2. The titers for the antibodies were determined using the following horseradish peroxidase-conjugated secondary antibodies: Rabbit anti-mouse IgG (H+L)-HRP; Rabbit anti-mouse IgG1-HRP, Rabbit anti-mouse IgG2a-HRP; Southern Biotech, Birmingham, AL; all with 1:1000 dilutions). SureBlue™ (KPL, Gaithersburg, MD) was used as a peroxidase substrate. After 15 min, the reaction was stopped by the addition of 100 μl of 1M H2SO4, and the absorbance was read at 450 nm.
Histological staining
Mouse livers were fixed in fixative solutions (10% buffered formalin phosphate solution), and sent to Histoserv, Gaithersburg, MD USA for sectioning and H&E staining. Stained sections were analyzed under the microscope (Nikon Eclipse TE2000-U).
NO quantification
Splenocytes or macrophages obtained from peritoneal fluid (45) were cultured in complete RPMI medium in the presence or absence of FTAg for 24 h at 37°C. NO (nitrite/nitrate) production was determined from the supernatants of the cultures by the Griess reaction kit (Sigma-Aldrich Corporation, St-Louis, MO) (37).
CFSE proliferation assay
Proliferative capacity of T cells was assessed by a CFSE dilution assay in Ldp27−/− immunized mice before and after challenge with wild type parasites (46). Age matched naïve mice served as negative controls for Ag-specific proliferation. Splenocytes from different groups of mice were isolated, incubated in 5μM CFSE (Molecular Probes/Invitrogen) for 10 min in RPMI 1640 without fetal calf serum (FCS) followed by 5 min of quenching in ice-cold RPMI1640 plus 10% FCS and subsequently washed thoroughly before plating in 96 well tissue culture plates at 2 × 105 cells/well. Cells were cultured for 5 days at 37°C with 5% CO2 under stimulation with or without of FTAg (50μg/ml). Cells were harvested, washed and were blocked with anti-CD16/32 (5μg/ml) for 20 min (4°C), cell surface stained with anti mouse CD3 APC-eFluor@780, anti mouse CD4 eFluor@450, anti mouse CD8a eFluor@605NC (eBioscience, USA) for 30 min (each with 1:200 dilution; 4°C). Cells were acquired on LSRII (BD Biosciences, USA) equipped with 405, 488, 532, and 638 laser lines using DIVA 6.1.2 software. Data were analyzed with the FlowJo software version 9.1.5.
Adoptive cell transfer
Total T cells, or CD4 or CD8 T cells from mice 16 weeks post-immunization with Ldp27−/− or non-immunized naïve mice were isolated and transferred into naïve mice. Purification of T cells was performed with the Midi-MACS system (Miltenyi Biotec, USA) using Pan-T cells, CD4 T cells or CD8 T cells isolation kits, respectively as recommended by the manufacturer. 5 × 106 total T cells or CD4 or CD8 T cells were transferred to recipient mice and 24 hrs later all groups of mice were infected with L. donovani Wt parasites. The purity of the isolated T cells population was > 95% as observed by flow cytometry.
Statistical analysis
Statistical analysis of differences between means of groups was determined by unpaired two tailed ‘Student t test’ using Graph Pad Prism 5.0 software. A p value less than 0.05 was considered as significant and a p value less than 0.01 was considered as highly significant.
Results
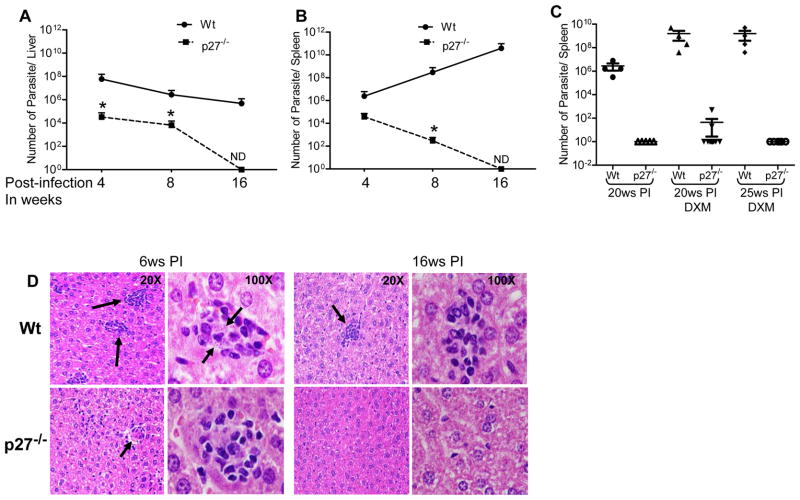
Live attenuated Ldp27−/− parasites disseminate into the visceral organs with limited persistence and are non pathogenic
We previously demonstrated that the Ldp27−/− mutant is unable to sustain beyond 13 weeks post-infection and the defect is overcome by episomal expression of p27 in the Ldp27−/− cell line (38). To further dissect the organ specific survival of Ldp27−/− parasites, mice were injected intravenously with stationary phase wild type (Wt) or Ldp27−/− promastigotes and the parasite burden was measured in the spleen and liver after 4, 8 and 16 weeks post-infection (Figure 1A & B). The parasite burden is significantly high in the spleen and the liver of Wt infected mice up to the 16th week reflecting the normal course of infection. However, Ldp27−/− mutants showed a progressive decline from 4 weeks post-infection in both spleen and liver. In both time points, 4 and 8 weeks post-infection, Ldp27−/− infected mice had significantly (p< 0.05) less parasite burden compared to Wt infected mice in both the organs (Figure 1A & B). Interestingly, in Ldp27−/− infected mice by the 16th week, the parasites were cleared from both the organs, as we could not recover any live parasites from serial dilutions of cultured organ cell suspension of these mice. Similar results were obtained in a confirmatory real-time PCR study using DNA from spleens of mice infected either with Wt or Ldp27−/− parasites (supplementary Table 1). Detection of the parasite specific minicircle DNA target indicated the presence of a substantial number of parasites in Wt-infected (12 weeks) and Ldp27−/− infected (12 weeks) mice. However, 20 weeks after infection with Ldp27−/−, the mice no longer had detectable parasites whereas there was significant parasite burden in Wt-infected spleens, suggesting the absence of any residual attenuated parasite persistence. Taken together these experiments suggested that deletion of the Ldp27 gene does not affect the visceralizing capacity of this cell line, however the parasite growth is severely impaired in host organs leading to clearance by the host.
Figure 1.
Avirulent properties of p27 knockout L. donovani (Ldp27−/−) parasite in BALB/c mice. Survival of wild type (Wt) or Ldp27−/− parasites in Liver (A) and Spleen (B) of BALB/c mice. Mice were infected with Wt or Ldp27−/− parasites, and at 4, 8 and 16 weeks (ws) post-infection (PI) parasite load in infected mice was measured. (C) Effect of immunosuppressive drug Dexamethasone (DXM) on infected mice. Parasite load was measured in spleen of mice infected with Wt or p27−/− parasite for different weeks of post-infection and treated with DXM. (D) Histopathology of liver from Wt or Ldp27−/− infected mice after 6 weeks and 16 weeks post-infection. Black arrows pointing infection foci or intra-cellular parasites. The data presented are representative of three independent experiments with similar results and 4 mice in each group. Mean and SEM of each group are shown. *, p < 0.05.
To rule out the survival of any undetectable Ldp27−/− mutant parasites in visceral organs beyond 20 weeks post-infection, we treated Wt or Ldp27−/− parasite infected mice with Dexamethasone (DXM), a known immune-suppressor to allow proliferation of any residual parasites. In the Wt-infected group, treatment with DXM enhances the parasite growth in the spleen (Figure 1C) and liver (data not shown) at 20 weeks and 25 weeks after infection. In Ldp27−/− infected mice at 20 weeks post-infection, only 2 out of 12 (16%) mice showed a very low number of parasites only in the spleen not in the liver. However, at 25 weeks post-infection, with or without DXM treatment we did not observe any Ldp27−/− parasites in the spleen, liver or bone marrow. These observations confirm that Ldp27−/− is safe as an immunogen and does not persist for the long term.
To ensure that Ldp27−/− do not cause any pathogenesis we analyzed histopathology of the liver. Stained liver sections (Figure 1D) showed that initially (6 weeks post infection) Ldp27−/− parasites generated an inflammatory response resulting in development of inflammatory foci. Unlike the Wt-infected mouse liver, all the inflammatory foci were devoid of parasites in Ldp27−/− infected mice. Moreover, at a later stage of infection (16 weeks post-infection) Ldp27−/− infected mouse liver was completely free of any inflammatory foci and the liver cells showed normal morphology. On the contrary in the wild type L. donovani infected mouse liver, there were still some inflammatory foci, although most of these foci were free of parasites. These observations confirm that Ldp27−/− causes initial mild pathogenesis in the host liver, however eventually the host clears the parasite and restores the normal architecture of liver tissue.
Immunization with Ldp27−/− protects against challenge with virulent L. donovani
To determine the protective efficacy of Ldp27−/− immunization, naïve BALB/c mice were immunized with the Ldp27−/− parasites or saline by intravenous injection. Twelve weeks post-immunization mice were challenged with wild type L. donovani parasites and assessment of parasite burden in spleens and livers was done at three time points post-challenge, measured by limiting dilution. The results showed immunization with Ldp27−/− significantly reduces the liver or spleen parasite burden 4, 8 or 12 weeks post-challenge compared with the non-immunized mice group (Figure 2A and B). However 12 weeks post-challenge, immunized mice showed the most significant protection having 2.5 log and 4 log fold reduction in parasite number in liver and spleen respectively compared to non-immunized mice (Figure 2A & B). Non-immunized infected mice showed significant splenomegally compared to immunized infected mice (data not shown). To evaluate the ability of Ldp27−/− immunization to confer long-term protection, mice were challenged with virulent L. donovani parasites 20 weeks following Ldp27−/− immunization, and the parasite load was evaluated 12 weeks post-challenge (Fig 2C). There was significant reduction in parasite burden in spleen and liver of mice challenged 20 weeks post-immunization compared to naïve challenged mice (Fig. 2C). However the reduction in parasite burden was less in the 20 weeks post-immunization group (2 log fold in spleen and 1.5 log fold in liver) than it was in the 12 week immunized group (4 log fold in spleen and 2.5 log fold in liver, compare data in Figure 2A &B with Figure 2C). Overall these data suggest that Ldp27−/− immunization confers significant sustained protection even at 20 weeks post-immunization.
Figure 2.
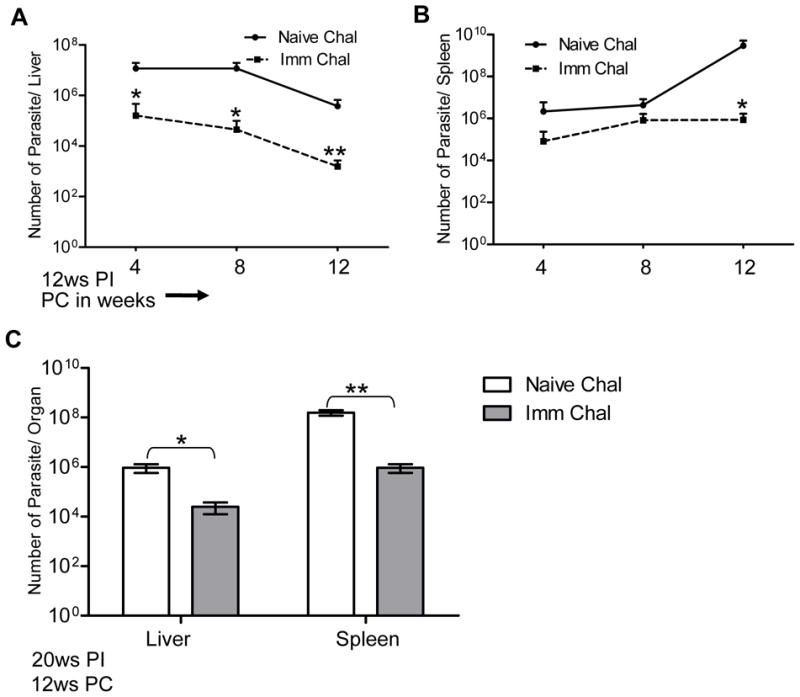
Protection by Ldp27−/− parasite immunization against L. donovani infection. Mice were either immunized with Ldp27−/− parasites or with PBS, 12 weeks post-immunized mice were challenged with Wt parasites. Parasite number in Liver (A) and Spleen (B) was measured 4 weeks, 8 weeks and 12 weeks post- infection. Mean and SEM of four mice in each group are shown. (C) Long term effect of immunization. 20 weeks post-immunized mice were challenged with Wt parasites and after 12 weeks of infection parasite burden was determined. The data are a representation of two independent experiments with similar results and 4- 5 mice in each group. Mean and SEM of each group are shown. PI, post-immunization; PC, post-challenge *, p < 0.05 and **, p < 0.01.
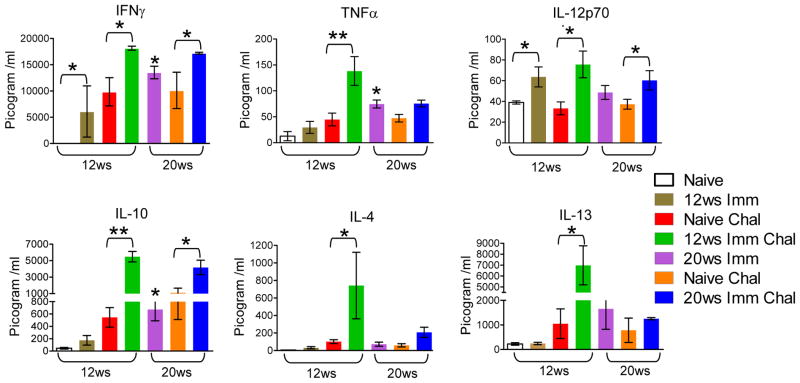
Ldp27−/− immunization induces a mixed pro-inflammatory and anti-inflammatory cytokine response upon virulent challenge
To characterize the immune response induced by the live attenuated Ldp27−/− parasites, we analyzed antigen-specific cytokine secretion by splenocytes from naïve, immunized, non-immunized challenged and immunized challenged mice. There was a significant induction of Leishmania antigen specific IFNγ and IL-12p70 secretion in the splenocyte culture supernatants of 12 weeks post-immunized mice (Figure 3). IFNγ and TNFα were also induced in 20 weeks post-immunized mice (Figure 3). Interestingly, following 3 weeks of challenge with wild type L. donovani parasites, the 12 weeks post-immunized mice showed significantly enhanced IFNγ, TNFα and IL-12 secretion whereas 20 weeks post-immunized mice showed significantly enhanced IFNγ and IL-12, but not TNFα secretion compared to non-immunized challenged mice. In 12 weeks post-immunized and challenged mice there was significant induction of anti inflammatory cytokines like IL-10, IL-4 and IL-13 compared to naïve challenged. Although there was significant induction of IL-10 secretion in 20 weeks post-immunized mice and immunized challenged mice compared to non-immunized challenged mice; there was no difference in the levels of IL-4 and IL-13 in 20 week immunized challenged mice. Overall these results suggest that Ldp27−/− parasite immunization primes a selective long-term mixed pro and anti-inflammatory immune response against wild type parasites.
Figure 3.
Leishmania antigen stimulated cytokine profiles in splenocyte culture supernatants from naïve, Ldp27−/− immunized (Imm), naïve challenged (Naïve Chal) and Ldp27−/− immunized challenged (Imm Chal) mice. 12 or 20 weeks post immunized mice were challenged with wild type parasite and 3 weeks post challenge mice were euthanized (shown below each bar diagram) and spleens were collected. Concentration of cytokines in culture supernatants were measured using the multiplex mouse cytokine kit as described in Materials & Methods section. The data presented are representative of two independent experiments with similar results. Mean and SEM of each group are shown. *, p < 0.05 and **, p < 0.01.
Resistance to infection induced by Ldp27−/− immunization is associated with induction of Nitric Oxide in splenocytes
Most of the pro-inflammatory cytokines activate macrophages and induce NO production. NO is one of the macrophage derived effector molecules and crucial for the control of intracellular Leishmania infections (47). A significant amount of Leishmania antigen specific nitrite production was observed in both 12 weeks as well as 20 weeks post-immunized mice compared to non-immunized mice splenocytes (Figure 4). However a much greater amount of NO was observed in Ldp27−/− immunized mice upon wild type L. donovani challenge compared to naïve challenged both in 12 and 20 weeks post-immunized mice (Figure 4). Interestingly the induction of nitrite concentration was similar in challenged animals immunized for 12 or 20 weeks. Overall, the sustained nitrite secretion level by splenocytes indicates a pro-inflammatory cytokine dominating milieu in immunized mouse spleens which in turn favors the host in clearing parasites.
Figure 4.
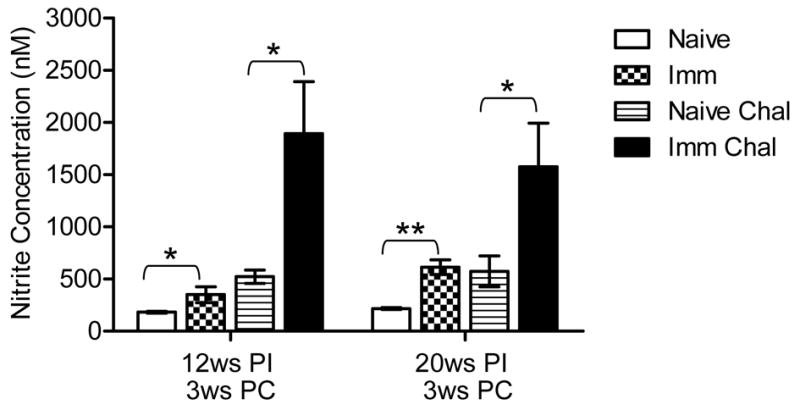
Immunization with Ldp27−/− induces Nitric Oxide (NO) production by splenocyte. The activity of NOS2 is indicated by the amount of released nitrite in the antigen stimulated splenocytes supernatants (48 hrs) and was measured by the Griess reaction. The data presented are representative of two experiments with similar results and 4–5 mice in each group. Mean and SEM of three or more mice in each group are shown. PI, post-immunization; PC, post-challenge * p< 0.05; ** p < 0.01
Immunization with Ldp27−/− induces a sustained level of Ag-specific T cell proliferation response
Impairment of the cell mediated immune response is a hallmark of active VL in humans or in animals (48). Restoration of antigen specific immune response is crucial for effective vaccine induced immunity. To investigate the proliferative potential upon reencounter with a Leishmania antigen, spleen cells were isolated from naïve; naïve challenged, 20 weeks post-immunized and immunized challenged mice. Splenocytes were CFSE labeled and stimulated in vitro with FTAg or medium alone. After 5 days of Ag stimulation, the cells were stained with anti-CD3, anti-CD4 and anti CD8 antibodies, and their CFSE profile was analyzed with the flow-cytometer (Figure 5A). FlowJo analysis showed that all CD3+ T cells including CD4 and CD8 isolated from mice immunized with Ldp27−/− have significantly higher proliferative capacity in response to Leishmania FTAg antigen compared to T cells isolated from naïve mice (Figure 5B). Further, T cells isolated from mice immunized and challenged with virulent L. donovani parasites have significantly (p< 0.05) higher proliferative capacity than the naïve challenged group (Figure 5B). The significant proliferative capacity of T cells even in 20 weeks post-immunized mice, suggests that some of them may have derived from a memory response.
Figure 5.
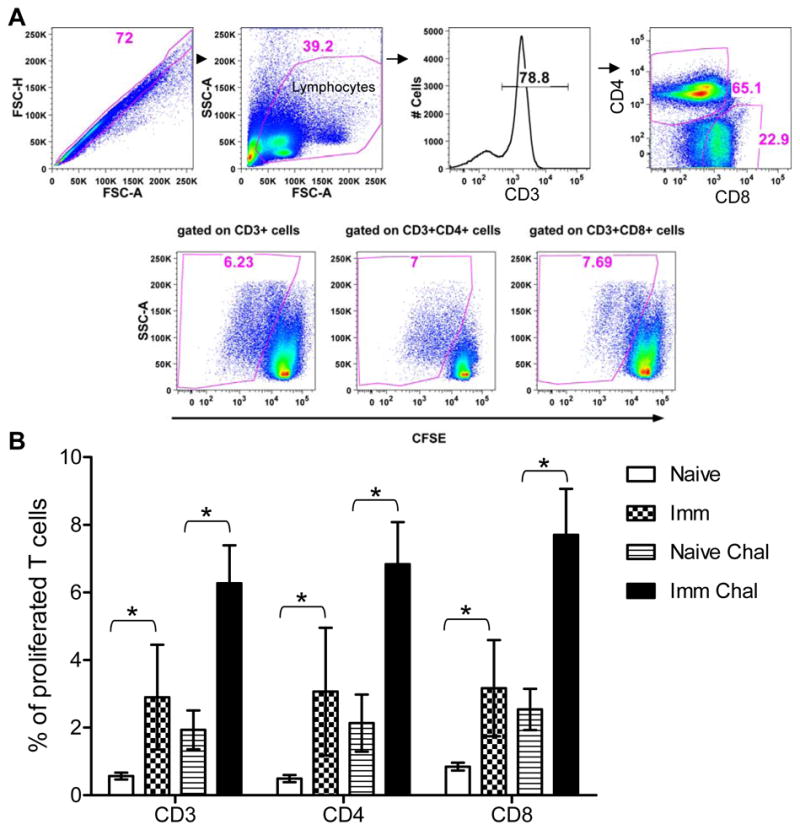
Proliferative capacity of Ag-specific T cells after 20 weeks post-immunization with Ldp27−/−. Splenocytes were isolated from each naïve, 20 weeks post-immunized (Imm), naïve and 3 weeks post-challenged (Naïve Chal) or 20 weeks immunized and 3 weeks post-challenge (Imm Chal) mice, stained with CFSE, and stimulated in vitro with FTAg for 5 days. (A) Cells were analyzed by flow cytometry, where CFSE dilution on particular gated cells was used as readout for Ag-specific proliferation. (B) Percentage of gated CD3, CD4 and CD8 proliferated T cells were calculated. The data presented are representative of two independent experiments with similar results and 4–5 mice in each group. Mean and SEM of each group were shown. *, p< 0.05.
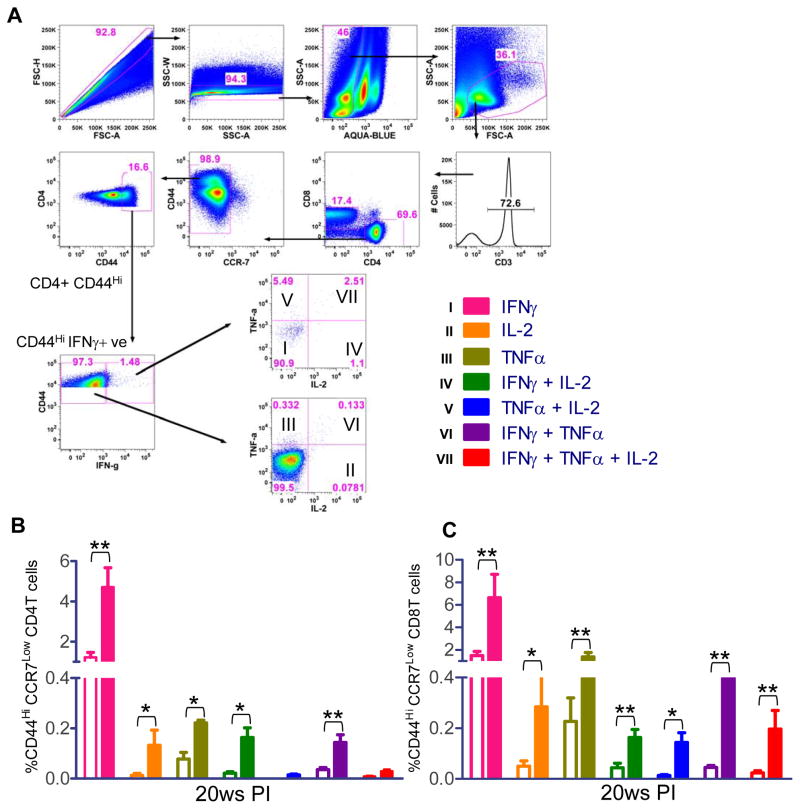
Induction of multifunctional antigen experienced Th1 effector cells correlates with Ldp27−/− induced immunity
Having shown that the immunization with the Ldp27−/− parasite establishes stable, long-lived immunological protection against the wild type Leishmania parasite, we subsequently characterized the phenotype and cytokine production of the antigen experienced effector memory T cells CD44HI/CCR7Low in 20 weeks post-immunized animals (49–50). Using intracellular FACS staining, antigen experienced CD4 and CD8 T cells were gated based on their surface expression of CD44 and CCR7 (Figure 6A). The CD44HI/CCR7Low cells were separated into seven distinct subpopulations based on their production of IFNγ, TNFα or IL-2 in any combination within the pool of effector memory T cells (Figure 6A). The results showed 20 weeks post-immunization, in CD4 T cells, IFNγ single cytokine secreting cells are dominating and there are a substantial number of single IL-2 and TNFα+ producing as well as double IFNγ+ IL-2+ and IFNγ+ TNFα+ producing cells (Figure 6B). In CD8 T cells, although IFNγ+ cells are dominating, there are significant number of IL-2 and TNFα+ single producing cells as well as double cytokine producing IFNγ+IL-2+, IFNγ+TNFα+, TNFα+IL-2+ or triple cytokines producing IFNγ+TNFα+IL-2+ cells (Figure 6C). Most importantly the CD8 T cell compartment had higher percentage of double and triple cytokine producing cells than in CD4 T cells compartment (Figure 6C vs Figure 6B).
Figure 6.
Multiparameter flow cytometry based analysis for single, double or triple cytokine secreting CD44HI/CCR7Low CD4 or CD8 T cells. (A) The common gating steps shown in this study. (B) Spleen cells of 20 weeks post-immunized mice were stimulated with FTAg for 48 hrs and stained with various antibodies as described in Materials & Methods. Antigen experienced effector cells were gated and divided into seven distinct subpopulations and the frequencies of the various subpopulations were calculated. Open bars represent naïve mice and solid color bars represent immunized mice. The data presented are representative of two experiments with similar results and 4–5 mice in each group. Mean and SEM of four mice in each group are shown. *, p< 0.05; **, p< 0.01
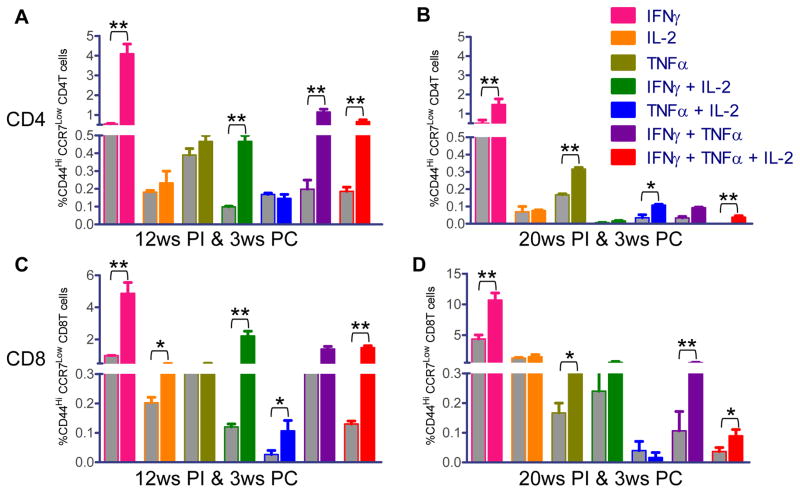
Having shown that Ldp27−/− immunization induces a long-term immune response characterized by a high level of multifunctional CD4 and CD8 T cells, we characterized the nature of the immune response after challenge with the wild type parasite. In CD4 and CD8 T cells from spleens of both 12 weeks and 20 weeks post-immunized and challenged mice, mostly IFNγ cytokine secreting cells were significantly higher than the non-immunized challenged mice (Figure 7A–D). In addition, a significantly higher percentage of double or triple cytokine producing CD4 (Figure 7A & B) and CD8 (Figure 7C & D) T cells were observed for many cytokines in both 12 weeks and 20 weeks post-immunized and challenged mice compared to non-immunized challenged mice. Particularly, in CD4 T cells IFNγ+ IL-2+, IFNγ+ TNFα+ and IFNγ+ IL-2+ TNFα+ producing cells were significantly higher in 12 weeks post-immunized and challenged mice. Similarly, in the 20 weeks immunized-challenged mice TNFα+ IL-2+ and IFNγ+ IL-2+ TNFα+ cells were higher compared to non-immunized challenged mice. Albeit the percentage of such cells was smaller compared to 12 weeks post-immunized and challenged mice. Interestingly, in 20 week immunized mice, the percentage of both double and triple cytokine producing CD8 T cells was higher compared to CD4 T cells similar to the results observed in immunized mice before challenge. Overall these results indicate that Ldp27−/− immunized mice induce a strong antigen experienced effector memory T cell mediated immune response after 20 weeks post-immunization at a time point when majority of mice have cleared the Ldp27−/− parasite.
Figure 7.
Antigen specific intra-cellular cytokine secretion analysis of CD4 and CD8 T cells from Ldp27−/− immunized and non-immunized mice after virulent challenge. 12 weeks or 20 weeks post-immunized or non-immunized mice were challenged for 3ws with wild type parasite. Intracellular cytokine analysis was done as shown in Fig 6A and divided into seven distinct subpopulations. Cytokine analysis of CD4 T Cells from 12 weeks PI & 3 weeks PC mice (A), from 20 weeks PI & 3 weeks PC mice (B). Cytokine analysis of CD8 T cells analysis of 12 weeks PI & 3 weeks PC (C) and 20 weeks PI & 3 weeks PC (D). Grey bars represent non-immunized and challenged mice and colored bars represent immunized and challenged mice. The data presented are representative of two experiments with similar results. Mean and SEM of 4 mice in each group are shown. *, p< 0.05; **, p< 0.01 PI, post-immunization; PC, post-challenged
We also quantified antigen experienced CD4 and CD8 T cells that produce IL-10, a crucial anti-inflammatory cytokine in the pathogenesis of VL (16, 37, 51). In the antigen stimulated CD4 and CD8 T cells from the spleen, the IFNγ/ IL-10 ratio was significantly higher in the 12ws immunized mice after challenge compared to non-immunized challenged mice (Figure 8A). These results indicate that increased IFNγ secretion and simultaneously decreased IL-10 production by antigen experienced CD4 and CD8 T cells results in a strong Th1 response that could translate into protective immunity. In addition IL-10 has been shown to be a critical immune-regulatory molecule and is necessary to shape the amplitude of immune response as well as to prevent infection associated lesions (15, 52). Particularly, IFNγ and IL-10 coproducing cells play an important role to prevent collateral tissue damage due to an extensive amount of inflammatory cytokines (52). We analyzed the percentage of IFNγ and IL-10 coproducing antigen experienced CD4 and CD8 T cells. In mice that were challenged 12ws post-immunization there were significantly higher percentage of both the CD4 and CD8 T cells that were positive for both IFNγ and IL-10 compared to naïve challenged mice (Figure 8B). Interestingly, the percentage of IFNγ/IL-10 coproducing CD8 T cells was significantly higher than the coproducing CD4 T cells. Overall these data clearly indicate that Ldp27−/− immunization induces host protective cell mediated controlled proinflammatory immune response against challenge with wild type infection.
Figure 8.
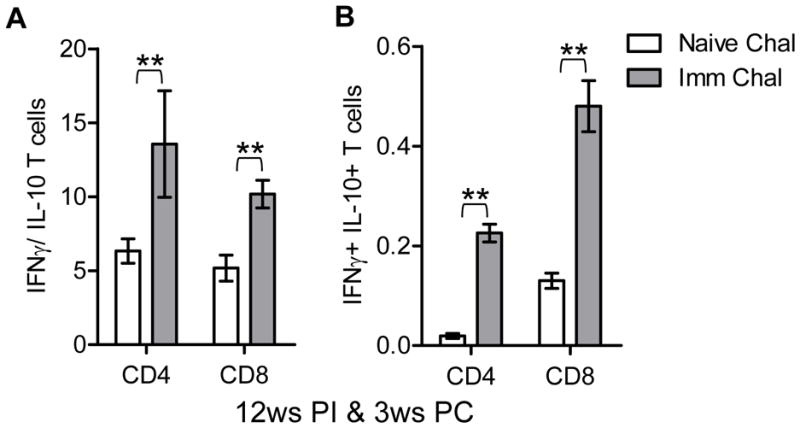
IFNγ and IL-10 producing CD4 and CD8 T cells. Splenocytes were cultured from either non-immunized challenged or 12 weeks immunized challenged mice. (A) Ratio of IFNγ and IL-10 producing CD4 and CD8 T cells form spleens of either non-immunized and 3 weeks challenged or 12 weeks Ldp27−/− immunized mice and 3 weeks challenged. (B) CD4 or CD8 T cells co-producing IFNγ and IL-10 from splenocytes of 12 weeks immunized and 3ws challenged mice. Mean and SEM of 4 mice in each group are shown. *, p< 0.05; **, p< 0.01
Adoptive transfer of isolated T cells from Ldp27−/− immunized mice confers protection to recipient mice
To confirm that T cell mediated immunity is sufficient to protect against wild type parasite infection, we performed adoptive transfer of T cells. The recipient mice were infected with wild type parasites for 10 weeks and parasite load was measured. Mice that received T cells from immunized mice showed significant reduction in parasite burden compared to recipients of T cells from non-immunized mice. There was 4 log fold, 2 log fold and 3.5 log fold reduction of parasite burden in spleens of recipients of Ldp27−/− immunized total T cells, CD4 T cells or CD8 T cells respectively compared to mice receiving non-immunized T cells (Figure 9). These results clearly indicate that attenuated parasite induced immunity is T cell mediated immunity and both CD4 and CD8 T cells are major effector T-cell populations that are important in controlling the parasite burden in immunized mice.
Figure 9.
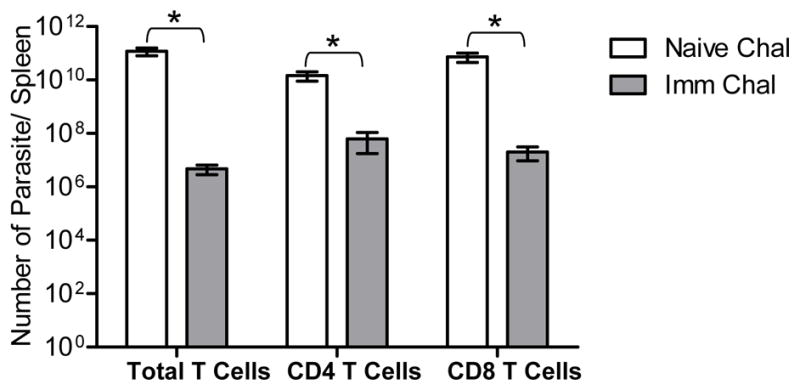
Adoptive transfer of T cells from Ldp27−/− immunized mice protects naïve recipient mice against Wt infection. Purified total, CD4 or CD8 T cells derived from immunized mouse spleens were transferred into naïve recipient mice and after 24hrs these mice were challenged with wild type parasites. Bar diagram shows parasite burden in spleens of naïve challenged and immunized challenged mice. Naïve challenge indicates the group of mice that received T cells from naïve mice. Imm Chal indicates mice the group of mice that received T cells from immunized mice. Each group of recipient mice included 4–5 animals. Mean and SEM of four mice in each group are shown. *, p< 0.05; **, p< 0.01.
Ldp27−/− immunization cross protects mice against challenge with other Leishmania species
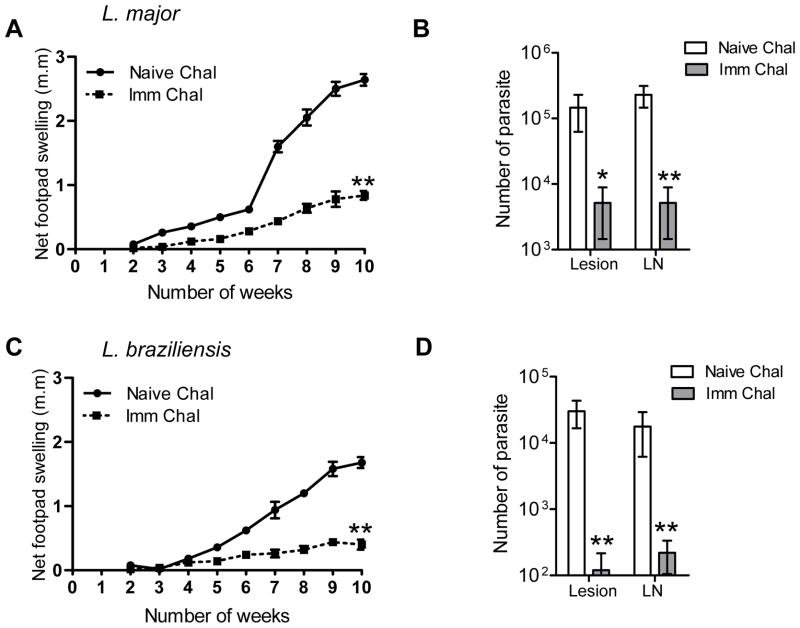
We further investigated whether immunization with Ldp27−/− could provide protection against heterologous species of Leishmania. Mice immunized (i. v.) with Ldp27−/− parasites for 12 weeks were infected (in footpad) with either L. major or L. braziliensis. The naïve mice infected with L. major started developing progressive lesions from 3 weeks post- challenge and by ten weeks post challenge they developed a large swollen footpad with lesions. On the contrary, Ldp27−/− immunized mice developed significantly smaller lesions compared to naïve mice that did not continue to swell (Figure 10A). The immune-challenged mice also showed significantly lower parasite burden both in the footpads and lymph nodes compared to the naïve challenged mice (Figure 10B). Similarly we found a significantly reduced footpad swelling compared to non-immunized mice in Ldp27−/− immunized mice after infection with L. braziliensis, (Figure 10C). The parasite burden in the footpad and draining lymph nodes was also significantly reduced in immunized mice compared to non-immunized mice (Fig. 10D). These results suggest immunization with live attenuated L. donovani parasites can control parasitemia from non visceralizing Leishmania infections.
Figure 10.
Cross-protection of BALB/c mice immunized with Ldp27−/− against heterologous challenge with L. major or L. braziliensis. 12 weeks post-immunized mice (PI) were infected with stationary phase parasites of L. major or L. braziliensis in the left hind footpad of each mouse. Graph shows footpad swelling due to infection in the injected footpad, calculated by measuring the difference in the footpad size between the two hind footpads (A & C). Parasite numbers per infected footpad or draining lymph nodes from 10ws post-challenge with either L. major or L. braziliensis are shown in (B & D). The data presented are representative of two independent experiments with similar results and 5 animals in each group. Mean and SEM of each group are shown. LN, lymph node; *, p < 0.05 and **, p < 0.01.
Induction of humoral response in the immunized mice
Although there is no specific evidence of a role of Leishmania specific antibodies in determining the outcome of VL (53), from the dominance of immunoglobulin subtype one can predict the outcome of the immune response in vivo. Mouse sera were assessed for L. donovani specific IgG1 and IgG2 isotypes, surrogate markers of Th2 and Th1 CD4 T cell differentiation respectively. In Ldp27−/− immunized mice the levels of IgG2a and IgG1 were not significantly different at 8 weeks post infection. However, the IgG2a levels were progressively higher in 12 and 16 weeks post-immunization groups of mice (Figure S1A), which correlated with the clearance of Ldp27−/− parasites from the visceral organs (Fig. 1AB). On the contrary in mice infected with Wt parasites, the IgG2a/ IgG1 ratio was lower, suggesting a Th2 type immune response which correlates with the disease progression as is evident by the increased parasite burden (Figure 2AB). Interestingly, in 20 weeks post-immunized and challenged mice the IgG2a/ IgG1 ratio is significantly higher than the non-immunized and challenged mice, suggesting a host protective Th1 type immune response (Figure S1B). The increased serum IgG2a levels observed in immunized and challenged mice correlate with the increased Th1 response.
Discussion
In Leishmania infection, individuals who recover from natural infection are protected from reinfection and develop life-long protection, suggesting that infection may be a prerequisite for immunological memory. The genetically altered live-attenuated parasites with controlled infectivity could achieve such immunological memory (37). In addition, live attenuated parasites can provide a broad spectrum of parasite antigens to generate a diverse antigen specific memory immune response that is important for protection against infection. Live attenuated vaccination is an old and widely accepted method against a broad spectrum of diseases like bacille Calmette-Guerin (BCG), polio vaccine, small pox, (24–25). In this study we evaluated safety and potency of Leishmania live attenuated vaccine candidate, Ldp27−/− parasite against homologous and heterologous Leishmania species. We observed that the host protection correlates with the induction of a Leishmania antigen specific cell mediated immune response.
Safety of live attenuated parasites as a vaccine is essential because such parasites should not regain virulence when used as human immunogens. Therefore we first tested Ldp27−/− parasites for virulence in mice. Ldp27−/− parasites had no reduced growth during the promastigote stage however, their growth was attenuated during the intracellular amastigote stage. These parasites do visceralize to spleen and liver in mice but are cleared after 20 weeks of infection as is demonstrated by the absence of parasite in immune-suppressed mice. Six weeks after immunization with the Ldp27−/− parasite mice recruit infiltrating mononuclear cells and form foci in the liver similar to wild type infection. However, these foci lack parasites unlike wild type infection induced foci. Furthermore, 16 weeks post-immunization the liver cell morphology returns to normal similar to the uninfected condition. Thereby suggesting that the Ldp27−/− parasites are safe and illicit a host response without causing pathogenesis.
The efficacy of the Ldp27−/− parasites as immunogens was confirmed by significant parasite control in immunized mice after infection both at early and late stages of immunization. It is well established in the literature that in VL control of parasitemia is correlated with the induction of both pro and anti-inflammatory cytokine response (54–55). Such a correlation was observed using Ldp27−/− parasites as immunogens as indicated by significant enhanced secretion of pro-inflammatory cytokines like IFNγ, TNFα and IL-12 as well as significant induction of anti-inflammatory cytokines like IL-10, IL-4 and IL-13. However, there has to be a balance between the pro-inflammatory and anti-inflammatory cytokines to avoid tissue injury. Recent studies suggested that an exacerbated response to infections may result in deleterious lesion and extensive tissue damage, and IL-10 prevents the development of immuno-pathological tissue damage (15). Therefore, lack of liver tissue damage and the absence of splenomegaly in live attenuated parasite immunized mice could be the outcome of induction of IL-10 secretion by Ldp27−/− parasite balancing the effect of the extensive amount of pro-inflammatory cytokines such as IFNγ. In addition, IL-4 and IL-13 have been shown to be crucial for the clearance of L. donovani parasite from liver and spleen (56–58). Therefore, enhanced IL-4 and IL-13 levels observed during the early stage of Ldp27−/− parasite immunization in our study may be important for the development of hepatic granuloma maturation with leishmaniacidal activity resulting in enhanced NO production which in turn results in significant reduction of parasite in visceral organs. Taken together these results clearly suggest Ldp27−/− parasites induce a mixed Th1 and Th2 response without causing tissue damage and control the parasite burden by enhancing the leishmaniacidal activity.
In mice, vaccine induced T cell response has been shown to correlate with protection against Leishmaniasis (37, 59–61). Therefore, we analyzed CD4 and CD8 T cells that are CD44HI/CCR7Low populations and represent antigen experienced effector memory T cells (61–62) to determine correlates of immune protection for Ldp27−/− parasites. In the current study, the Ldp27−/− immunization induced a significantly high frequency of antigen experienced CD4 and CD8 T cells that could be detected even after 20 weeks of post-immunization and conferred a significant level of protection. In addition, there was a significant level of Ag specific T cell recall response as indicated by T cell proliferation even after 20 weeks post-immunization in the absence of parasite persistence suggesting generation of Ag-specific memory cells. IFN-γ and TNF-α are the two major cytokines involved in the clearance of intracellular pathogens like Leishmania, and multifunctional cytokine producing cells are much more effective than the single cytokine secretory cells (37, 46, 61, 63). In the current study immunization with Ldp27−/− increased the percent of Leishmania specific CD4 and CD8 cells expressing Th1 cytokines (IFNγ, TNFα and IL-2) either single or in multiple combinations in response to wild type parasite challenge. Importantly IFNγ+ TNFα+ is one of the major double cytokine producing cells in Ldp27−/− immunized and challenged mice as was observed with protection against L. major infections (60–61). From the previous studies it was well established that CD8 T cells play a potential role in cure of leishmaniasis particularly in VL (5, 37, 64–66). In this study we also observed that there were significant numbers of single or multiple cytokine producing CD8 T cells in immunized and challenged mice confirming that immunization with live attenuated Ldp27−/− parasites, CD8 T cells play an essential role in host protection. Furthermore, significantly more Leishmania antigen responsive CD8 T cells are present in immunized mice than in naïve mice. The protective function of these cells may derive from their cytokine secretion as well as cytotoxic activity. However, at this time we have not analyzed the cytotoxic function of these cells and will be the focus of future studies.
We also found significantly increased IFNγ/ IL-10 ratio both in CD4 and CD8 T cells in immunized and infected mice compared to non-immunized infected mice, suggesting polarization towards the Th1 type cell development as was observed in L. infantum infected mice after immunization with SIR2+/− (35). Further, co-production of IFNγ and IL-10 by both antigen experienced CD4 and CD8 T cells in immunized and infected mice suggests a desirable balance in the immune response allowing brief parasite persistence that facilitates protection without causing host damage (37). Therefore both production of multiple cytokine producing effector memory T cells and T cells co-producing IFN-γ and IL10 upon immunization with Ldp27−/− parasites suggests that these could be used as markers of immune correlates of protection for Ldp27−/− vaccine candidate.
It has been debated whether persistence of parasites or a pool of central memory T cells is essential for protective immunity against reinfection with Leishmania (67–69). In CL it has been reported that, anti-Leishmania memory cells develop after infection and are maintained in the absence of parasites, however the quality of memory cells maintained in the presence and absence of live parasites are different (68, 70). Our studies showed that there was strong recall of cellular immune response both during the persistence of attenuated parasites and also in the absence of attenuated parasites. However, the observation that short term immunized mice show a higher level of protection than long term immunized mice suggests that with persistence there is a pool of effector cells that rapidly respond to infection where as in the absence of parasite persistence, which serves as a source of antigen, there is a small pool of central memory cells which respond slowly to infection by transitioning into effector memory cells that eventually help in controlling the parasite burden. Therefore persistence of parasite antigen and antigen specific effector T cells are probably more effective than central memory cells alone. Further adoptive transfer experiments confirm the generation of antigen specific both CD4 and CD8 T cells in immunized mice which confer protection during re-infection with wild type parasites. Future studies are needed to analyze the type of T cells (potentially memory T cells) and their specific role in live attenuated parasite immunization.
Various Leishmania species have different tissue tropism resulting in different phenotypes. An ideal Leishmania vaccine would be the one which will control most Leishmania infections. There are several reports that have shown cross protection against L. major infections using either whole parasite lysates or defined proteins from L. donovani (71–72). Additionally, L. donovani DNA vaccines and L. infantum sterol 24-c-methyl-transferase partial knock-out parasites induced a strong protection against L. mexicana and L. major infections respectively (60, 73). Similarly, in our previous studies we showed LdCen−/− parasite immunized mice are cross-protected against L. braziliensis infection (37). In this study we found that Ldp27−/− immunized mice were cross-protected against L. major (CL) as well as L. braziliensis (MCL) infections. All these studies suggest that the efficacy of a vaccine candidate will depend on the quality of cellular immunity and the antigen conservation among species. Therefore, it is possible to develop Leishmania vaccines especially the live attenuated parasites which can provide an opportunity for broad based antigen repertoire for the immune system to respond and hence cross protect from infections from other Leishmania species.
Overall this study established that the Ldp27−/− live attenuated parasite could be a potential vaccine candidate against VL, CL and MCL. However, there are significant differences in host immunity depending on the mode of primary infections like needle inoculums vs sand fly mediated infections. Particularly in CL, it has been reported that vaccine induced immunity confers protection against needle challenge but fails to protect mice against sand fly mediated infection (69, 74). These studies strongly suggest testing the efficacy of all Leishmania vaccines against sand fly mediated infections. Recently we have developed a rodent VL model with sand fly vector mediated transmission (75). Vector-transmission generated a slower progression of VL that resembled the chronicity of the disease following natural transmission in the field. The slower progression of disease may be relevant to the evolution of immunity to infection and to pathogenesis. Therefore, the slow progression of vector-initiated VL may be more appropriate for studies of early immune events, parasite establishment, and the screening of drugs and vaccines. Such a model will allow us to test the live attenuated vaccine candidates against sand fly mediated L. donovani infections.
In summary, our results demonstrate that immunization with Ldp27−/− parasites provides a significant protection against infection with homologous as well as heterologous species of Leishmania parasites. The immunization induced antigen specific strong multifunctional CD4 and CD8 T cells as correlates of immune protection. Ldp27−/− parasites elicit memory response both in the presence and absence of parasite persistence and the antigen specific cell mediated immunity correlated with robust NO generation and humoral response. Further Ldp27−/− parasites are safe since they do not persist for a long time hence reducing the chance of reversion to wild type and do not cause pathogenesis. Taken together, these studies strongly support that the Ldp27−/− mutant parasite is a safe and effective immunogen and has a potential to be a vaccine against a broad spectrum of leishmaniasis.
Supplementary Material
Acknowledgments
We want to thank Drs. Alain Debrabant, Sanjai Kumar and Sreenivas Gannavaram for their critical review of the manuscript.
Source of grants: The funding for these studies was provided by the intramural funds and Critical Path initiative of Center for Biologics Evaluation and Research, Food and Drug Administration, USA.
Footnotes
Findings of this study are an informal communication and represent authors own best judgment. These comments do not bind or obligate FDA.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Human leishmaniases: epidemiology and public health aspects. World Health Stat Q. 1992;45:267–275. [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Banuls AL, Bastien P, Pomares C, Arevalo J, Fisa R, Hide M. Clinical pleiomorphism in human leishmaniases, with special mention of asymptomatic infection. Clin Microbiol Infect. 2011;17:1451–1461. doi: 10.1111/j.1469-0691.2011.03640.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–1470. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 7.Harhay MO, Olliaro PL, Costa DL, Costa CH. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 2011;27:403–409. doi: 10.1016/j.pt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Selvapandiyan A, Dey R, Gannavaram S, Lakhal-Naouar I, Duncan R, Salotra P, Nakhasi HL. Immunity to visceral leishmaniasis using genetically defined live-attenuated parasites. J Trop Med. 2012;2012:631460. doi: 10.1155/2012/631460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacellar O, Brodskyn C, Guerreiro J, Barral-Netto M, Costa CH, Coffman RL, Johnson WD, Carvalho EM. Interleukin-12 restores interferon-gamma production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996;173:1515–1518. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 10.Bacellar O, D’Oliveira A, Jr, Jeronimo S, Carvalho EM. IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine. 2000;12:1228–1231. doi: 10.1006/cyto.2000.0694. [DOI] [PubMed] [Google Scholar]
- 11.Engwerda CR, Murphy ML, Cotterell SE, Smelt SC, Kaye PM. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Ghalib HW, Whittle JA, Kubin M, Hashim FA, Mel-Hassan A, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–4629. [PubMed] [Google Scholar]
- 13.Murray HW, Hariprashad J, Coffman RL. Behavior of visceral Leishmania donovani in an experimentally induced T helper cell 2 (Th2)-associated response model. J Exp Med. 1997;185:867–874. doi: 10.1084/jem.185.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–569. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 16.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Cummings HE, Tuladhar R, Satoskar AR. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol. 2010;2010:294389. doi: 10.1155/2010/294389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvestre R, Cordeiro-da-Silva A, Ouaissi A. Live attenuated Leishmania vaccines: a potential strategic alternative. Arch Immunol Ther Exp (Warsz) 2008;56:123–126. doi: 10.1007/s00005-008-0010-9. [DOI] [PubMed] [Google Scholar]
- 19.Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133(Suppl):S87–112. doi: 10.1017/S0031182006001831. [DOI] [PubMed] [Google Scholar]
- 20.Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89(Suppl 1):83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 21.Haldar JP, Ghose S, Saha KC, Ghose AC. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983;42:702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamil AA, Khalil EA, Musa AM, Modabber F, Mukhtar MM, Ibrahim ME, Zijlstra EE, Sacks D, Smith PG, Zicker F, El-Hassan AM. Alum-precipitated autoclaved Leishmania major plus bacille Calmette-Guerrin, a candidate vaccine for visceral leishmaniasis: safety, skin-delayed type hypersensitivity response and dose finding in healthy volunteers. Trans R Soc Trop Med Hyg. 2003;97:365–368. doi: 10.1016/s0035-9203(03)90171-4. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 24.Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol. 2010;28:573–579. doi: 10.1038/nbt.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svenson S, Kallenius G, Pawlowski A, Hamasur B. Towards new tuberculosis vaccines. Hum Vaccin. 2010;6:309–317. doi: 10.4161/hv.6.4.10711. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L, Wei H, Letvin NL, Frothingham R, Haynes BF, Chen ZW, Jacobs WR., Jr Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander J, Coombs GH, Mottram JC. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- 28.Gorczynski RM. Immunization of susceptible BALB/c mice against Leishmania braziliensis. I. Resistance induced using as immunogen adherent or nonadherent cells from infected mice. Cell Immunol. 1985;94:1–10. doi: 10.1016/0008-8749(85)90080-2. [DOI] [PubMed] [Google Scholar]
- 29.Daneshvar H, Coombs GH, Hagan P, Phillips RS. Leishmania mexicana and Leishmania major: attenuation of wild-type parasites and vaccination with the attenuated lines. J Infect Dis. 2003;187:1662–1668. doi: 10.1086/374783. [DOI] [PubMed] [Google Scholar]
- 30.Kimsey PB, Theodos CM, Mitchen TK, Turco SJ, Titus RG. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect Immun. 1993;61:5205–5213. doi: 10.1128/iai.61.12.5205-5213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell GF, Handman E, Spithill TW. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust J Exp Biol Med Sci. 1984;62(Pt 2):145–153. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- 32.Rivier D, Shah R, Bovay P, Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 1993;15:75–84. doi: 10.1111/j.1365-3024.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 33.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 34.Zhang WW, Matlashewski G. Characterization of the A2-A2rel gene cluster in Leishmania donovani: involvement of A2 in visceralization during infection. Mol Microbiol. 2001;39:935–948. doi: 10.1046/j.1365-2958.2001.02286.x. [DOI] [PubMed] [Google Scholar]
- 35.Silvestre R, Cordeiro-Da-Silva A, Santarem N, Vergnes B, Sereno D, Ouaissi A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J Immunol. 2007;179:3161–3170. doi: 10.4049/jimmunol.179.5.3161. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulou B, Roy G, Breton M, Kundig C, Dumas C, Fillion I, Singh AK, Olivier M, Ouellette M. Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect Immun. 2002;70:62–68. doi: 10.1128/IAI.70.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, Nakhasi HL. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol. 2009;183:1813–1820. doi: 10.4049/jimmunol.0900276. [DOI] [PubMed] [Google Scholar]
- 38.Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, Duncan R. Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol Microbiol. 2010;77:399–414. doi: 10.1111/j.1365-2958.2010.07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvapandiyan A, Duncan R, Debrabant A, Bertholet S, Sreenivas G, Negi NS, Salotra P, Nakhasi HL. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J Biol Chem. 2001;276:43253–43261. doi: 10.1074/jbc.M106806200. [DOI] [PubMed] [Google Scholar]
- 40.Debrabant A, Joshi MB, Pimenta PFP, Dwyer DM. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Intl J Parasitology. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 42.Selvapandiyan A, Duncan R, Mendez J, Kumar R, Salotra P, Cardo LJ, Nakhasi HL. A Leishmania minicircle DNA footprint assay for sensitive detection and rapid speciation of clinical isolates. Transfusion. 2008;48:1787–1798. doi: 10.1111/j.1537-2995.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 43.Rousseau D, Suffia I, Ferrua B, Philip P, Le Fichoux Y, Kubar JL. Prolonged administration of dexamethasone induces limited reactivation of visceral leishmaniasis in chronically infected BALB/c mice. Eur Cytokine Netw. 1998;9:655–661. [PubMed] [Google Scholar]
- 44.Buxbaum LU, Denise H, Coombs GH, Alexander J, Mottram JC, Scott P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol. 2003;171:3711–3717. doi: 10.4049/jimmunol.171.7.3711. [DOI] [PubMed] [Google Scholar]
- 45.Dey R, Sarkar A, Majumder N, Bhattacharyya Majumdar S, Roychoudhury K, Bhattacharyya S, Roy S, Majumdar S. Regulation of impaired protein kinase C signaling by chemokines in murine macrophages during visceral leishmaniasis. Infect Immun. 2005;73:8334–8344. doi: 10.1128/IAI.73.12.8334-8344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 47.Wei XQ, I, Charles G, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho EM, Teixeira RS, Johnson WD., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981;33:498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2010;17:618–625. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 51.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores-Garcia Y, Rosales-Encina JL, Satoskar AR, Talamas-Rohana P. IL-10-IFN-gamma double producers CD4+ T cells are induced by immunization with an amastigote stage specific derived recombinant protein of Trypanosoma cruzi. Int J Biol Sci. 2011;7:1093–1100. doi: 10.7150/ijbs.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhowmick S, Mazumdar T, Ali N. Vaccination route that induces transforming growth factor beta production fails to elicit protective immunity against Leishmania donovani infection. Infect Immun. 2009;77:1514–1523. doi: 10.1128/IAI.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol. 2000;30:2935–2943. doi: 10.1002/1521-4141(200010)30:10<2935::AID-IMMU2935>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Bruhn KW, Birnbaum R, Haskell J, Vanchinathan V, Greger S, Narayan R, Chang PL, Tran TA, Hickerson SM, Beverley SM, Wilson ME, Craft N. Killed but metabolically active Leishmania infantum as a novel whole-cell vaccine for visceral leishmaniasis. Clin Vaccine Immunol. 2012;19:490–498. doi: 10.1128/CVI.05660-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McFarlane E, Carter KC, McKenzie AN, Kaye PM, Brombacher F, Alexander J. Endogenous IL-13 plays a crucial role in liver granuloma maturation during Leishmania donovani infection, independent of IL-4Ralpha-responsive macrophages and neutrophils. J Infect Dis. 2011;204:36–43. doi: 10.1093/infdis/jir080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stager S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun. 2003;71:4804–4807. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray HW, Tsai CW, Liu J, Ma X. Visceral Leishmania donovani infection in interleukin-13−/− mice. Infect Immun. 2006;74:2487–2490. doi: 10.1128/IAI.74.4.2487-2490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto Y, Bhatia A, Raman VS, Vidal SE, Bertholet S, Coler RN, Howard RF, Reed SG. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27:2884–2890. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 62.Kaveh DA, V, Bachy S, Hewinson RG, Hogarth PJ. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PLoS One. 2011;6:e21566. doi: 10.1371/journal.pone.0021566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogdan C, Moll H, Solbach W, Rollinghoff M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990;20:1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 64.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, Udey MC, Sacks D. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol. 2002;168:3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- 66.Stager S, Rafati S. CD8(+) T cells in leishmania infections: friends or foes? Front Immunol. 2012;3:5. doi: 10.3389/fimmu.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seder RA, Sacks DL. Memory may not need reminding. Nat Med. 2004;10:1045–1047. doi: 10.1038/nm1004-1045. [DOI] [PubMed] [Google Scholar]
- 68.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 69.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathog. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res. 2008;41:123–136. doi: 10.1007/s12026-008-8016-2. [DOI] [PubMed] [Google Scholar]
- 71.Rachamim N, Jaffe CL. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol. 1993;150:2322–2331. [PubMed] [Google Scholar]
- 72.Tonui WK, Titus RG. Cross-protection against Leishmania donovani but not L. Braziliensis caused by vaccination with L. Major soluble promastigote exogenous antigens in BALB/c mice. Am J Trop Med Hyg. 2007;76:579–584. [PubMed] [Google Scholar]
- 73.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, Garcia-Miss R, del M, Palatnik de Sousa CB, Dumonteil E. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73:812–819. doi: 10.1128/IAI.73.2.812-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers ME, Sizova OV, Ferguson MA, Nikolaev AV, Bates PA. Synthetic glycovaccine protects against the bite of leishmania-infected sand flies. J Infect Dis. 2006;194:512–518. doi: 10.1086/505584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aslan H, Dey R, Meneses C, Castrovinci P, Jeronimo S, Oliva G, Fischer L, Duncan R, Nakhasi HL, Valenzuela J, Kamhawi S. A New Model of Progressive Visceral Leishmaniasis in Hamsters by Natural Transmission via Bites of Vector Sand Flies. J Infect Dis. 2012 doi: 10.1093/infdis/jis932. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.