Abstract
Oral tolerance is defined as the specific suppression of humoral and / or cellular immune responses to an antigen by administration of the same antigen through the oral route. Due to its absence of toxicity, easy administration, and antigen specificity, oral tolerance is a very attractive approach to prevent unwanted immune responses that cause a variety of diseases or that complicate treatment of a disease. Many researchers have induced oral tolerance to efficiently treat autoimmune and inflammatory diseases in different animal models. However, clinical trials yielded limited success. Thus, understanding the mechanisms of oral tolerance induction to therapeutic proteins is critical for paving the way for clinical development of oral tolerance protocols. This review will summarize progress on understanding the major underlying tolerance mechanisms and contributors, including antigen presenting cells, regulatory T cells, cytokines, and signaling pathways. Potential applications, examples for therapeutic proteins and disease targets, and recent developments in delivery methods are discussed.
Keywords: oral tolerance, dendritic cells, Treg, IL-10, TGF-β, Tr1, Th3, nanoparticles, transgenic plants, oral delivery, protein antigen
1. Introduction
The human mucosal surfaces of the gastrointestinal tract, with an area of around 300 m2, are constantly in contact with a large variety of antigens, including dietary proteins and constituents of commensal bacteria [1]. Approximately 30 kg of food proteins per year reach the gut, and 1012 bacteria per g of stool colonize the human intestinal mucosa [2, 3]. Under such a high antigen pressure, it is natural for the gut to develop a mechanism to abrogate potentially injurious inflammatory responses and favor a tolerogenic environment. However, most pathogens also enter the human body through GI tract [4]. The gut immune system has evolved a complicated and tightly regulated mechanism to suppress unwanted inflammatory responses while at the same time protecting the body from pathogenic organisms. Generally, there are at least three types of responses to oral antigen administration – a local secretory IgA antibody response which protects mucus layer by forming a barrier capable of neutralizing the pathogen before it get to the cells; local and systemic suppression of the activation of damaging immunological responses (this type of response is termed as oral tolerance); systemic immune responses including generating serum antibodies such as IgG and cell-mediated immunity such as by cytotoxic T lymphocytes (CTL) [5].
Particularly, oral tolerance is defined as the specific suppression of humoral and / or cellular immune responses, such as antibody formation of different isotypes, production of inflammatory cytokines, and cellular immune responses including lymphocyte proliferation, delayed type-hypersensitivity reactions, etc. to an antigen by prior administration of the same antigen through the oral route [6]. Tolerance should not be restricted to the local intestinal tissue but also include the systemic immune system [6]. It is an active adaptive immune response rather than passive unresponsiveness or blindness of the immune system to exogenous antigens. It is “any mechanism by which a potentially injurious immune response is prevented, suppressed, or shifted to a non-injurious class of immune responses [7].”
Currently, intravenous infusion of recombinant protein is a routine treatment for genetic deficiencies such as hemophilia and lysosomal storage diseases. However, immune responses develop in a certain percentage of patients receiving the therapeutic protein, which represents a big hurdle for therapy [8–10]. All these urgently call for an efficient immune tolerance protocol. Due to the absence of toxicity, easy administration, and targeting of a specific antigen, oral tolerance is a very attractive candidate. Indeed, people have successfully lowered antibody titers and cell mediated immunity with oral tolerance therapy in animal models of autoimmune diseases and inflammatory diseases, including experimental autoimmune encephalomyelitis (EAE), uveitis, thyroiditis, myasthenia gravis, arthritis, diabetes, experimental colitis, as well as graft-versus host disease, allergy, anti-phospholipid syndrome, asthma, stroke, and atherosclerosis [11]. Several clinical trials have also been conducted in multiple sclerosis, uveitis, thyroid disease, Crohn’s disease, rheumatoid arthritis, hepatitis, and diabetes [7, 12]. The results from human studies have yielded limited success, possibly due to inappropriate selection of dosage and delivery methods, indicating there is still a long way to go in understanding the mechanisms of oral tolerance. The present review will focus on the molecular and cellular mechanisms currently known to direct oral tolerance, emphasizing clinical applications of oral tolerance in protein therapy, and discussing several emerging and promising delivery methods for oral tolerance therapy.
2. Mechanisms
2.1 GALT
The intestine has the most abundant populations of immune cells, with every meter of human small intestine housing 1012 lymphoid cells [13]. These immune cells are located in three compartments: scattered throughout the epithelium; in the lamina propria of the mucosa; or residing in the gut-associated lymphoid tissue (GALT), which are organized lymphoid aggregates along the submucosa of the entire small intestine [14]. Consisting of three parts - Peyer’s patches, appendix, and isolated lymphoid follicles, the GALT contains approximately 5x1010 lymphocytes [4]. Its structure is similar to lymph nodes in that they both have follicular B cell zones, inter-follicular T cell zones, and antigen-presenting cells like dendritic cells (DCs) and macrophages [15]. However, the GALT is not encapsulated and contains no lymphatic vessels, acquiring antigens directly from the intestinal mucosal surface [15]. GALT and the mesenteric lymph node (MLN), the largest lymph node in the body, are considered the primary inductive sites of adaptive immune responses, whereas the lamina propria and epithelium of mucosa have effector and memory functions (Fig. 1). For example, B cell differentiate into plasma cells and generating antibodies in lamina propria with helper T cells getting signals from local antigen presenting DC [14]. Intraepithelial lymphocytes (IELs) regulate innate and adaptive immune responses. About one IEL can be found per 10 intestinal villous epithelial cells [16]. The majority of IELs are CD8+ T cells and express α β or γδ T cell receptors, which is required for oral tolerance. Depletion of γδ IEL impaired oral tolerance induction and maintenance [17].
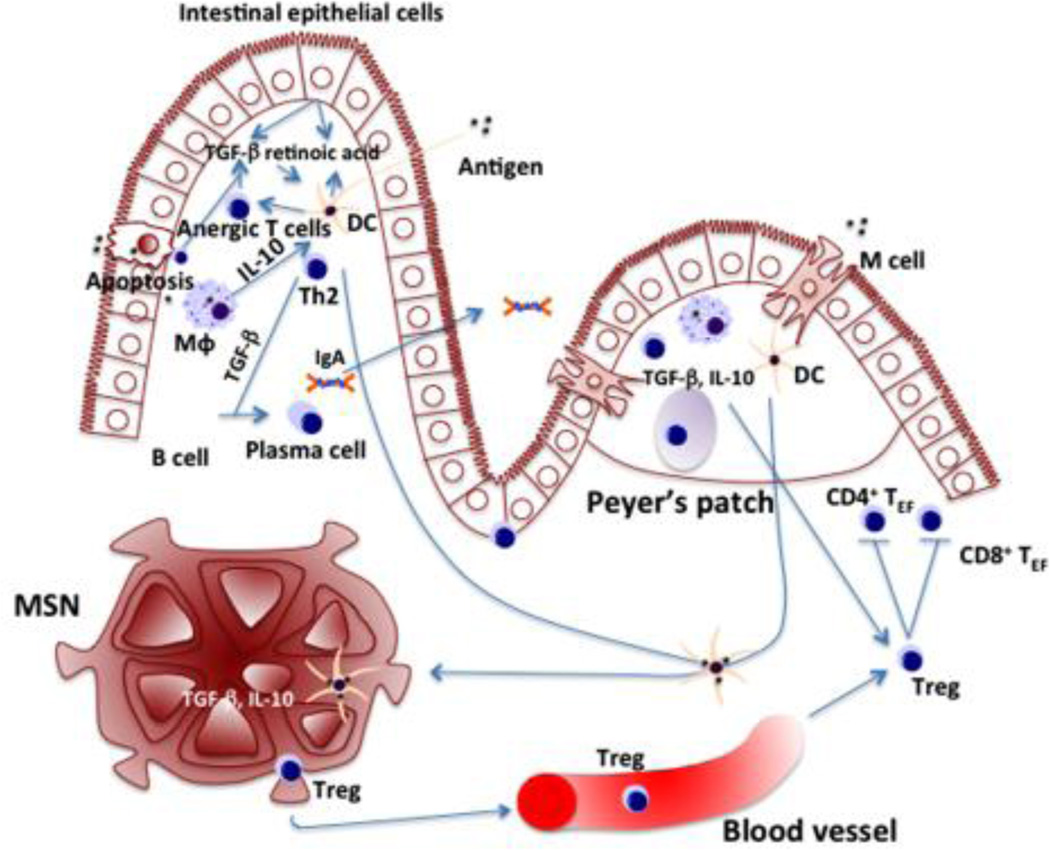
Fig. 1. Overview of mechanism of oral tolerance induction.
Oral antigens are sampled by different ways: M cells transfer antigen to DCs by transcytosis; DCs directly acquire antigen from gut lumen; antigens endotyosized by enterocytes and DCs or macrophages engulf enterocytes debris containing antigens; antigens directly cross the epithelial layer. Plasmacytoid or CD11c+ DCs, CD103+ DCs, and CD11b+ DCs are specialized in inducing oral tolerance. DCs get educated in local microenvironment through local factors and crosstalking with different cell types: intestinal epithelial cells releasing TGF-β and retinoic acid; lamina propria macrophages producing large amount of IL-10 and inhibit DCs induction of Th-17 cells; oral immunized memory T cells secrete IL-4 and IL-10 to educate DCs and induce naïve T cells to produce the same cytokines. DCs are critical in gut hemostasis: DCs from Peyer’s patches and MLN induced B cells to transform into plasma cells and produce IgA; DCs abrogate a large fraction of antigen-specific CD8+ T cells in liver and MLNs; activated DCs migrate to MLNs and induce Treg. Generally high dose of oral antigens induce T cell deletion / anergy and low dose antigens induce Treg. These two mechanisms occur simultaneously and overlap. Apoptotic T cells, macrophages and DCs that clean up the debris, express high level of TGF-β and suppress inflammatory cytokines production. Treg suppressed effector T cells responses including inhibition of T cell proliferation and blockade of inflammatory cytokines release. TGF-b and IL-10 are high in gut microenvironment, which is essential for Treg function and maintenance.
The development of the GALT requires stimulation from commensal microbiota and food antigens. Newborns develop germinal centers, and IgA positive plasmablasts after 1 month [18]. Germ free mice have underdeveloped lymphoid follicles and low IgA production, which normalized after 1-month exposure to conventional gut microbiata [19]. Similarly, mice on a balanced protein free diet showed immature GALT with reduced IgA [20]. Microbiota play a role in tolerance induction, as numbers of regulatory T cells (Treg), one of the key cell types in tolerance, were lower in germ-free mice than in normal microbiota mice [21]. Colonization of germ-free mice studies demonstrated that Clostridium species, but not B. Fragilis, Lactobacillus, segmented filamentous bacteria (SFB), or Bacteroides, specifically promoted intestinal epithelial cells to produce transforming growth factor-β (TGF-β), which leads to Treg differentiation and survival [22].
Interestingly, MLN but not GALT was demonstrated to be essential and sufficient for oral tolerance (Fig. 1). Clostridium can induce Treg accumulation in mice with deficient Peyer’s patches and lymphoid follicles [22]. Oral tolerance can be induced in the absence of Peyer’s patches and M cells [23], though oral tolerance can be induced in Peyer’s patches as well [2]. Functional MLN are essential for oral tolerance induction as lymphotoxin α deficient mice, which is a model for Peyer’s patches and lymph node deficiency, lost oral tolerance induction; whereas reconstitution of MLN with anti-lymphotoxin β receptor antibody was able to restore oral tolerance [24]. This is additionally supported by a study where surgical ablation of the MLN resulted in decreased oral tolerance [25]. Similarly, surgical removal of nose-draining lymph nodes abolished nasal tolerance, which can be restored by transplantation of nosedraining lymph nodes but not peripheral lymph nodes [26].
2.2 APC
Orally administrated antigens are sampled by different mechanisms and cell types. Microfold cells (M cells), DCs, and enterocytes were all reported to actively take up antigens [27]. In addition, some protein antigens can directly cross the epithelial layer and get into circulation. The route and mechanism of antigen uptake may be dependent on the natural characteristics of that antigen [28]. Peyer’s patches might be involved more with bacteria antigens [29], whereas some haptens might reach the liver via portal vein, which is believed to play a prominent role in oral tolerance induction [30]. Antigen presenting cells (APC) in the gut are particularly prone to induce antigen-specific Treg, which migrate and suppress damaging immune responses and secret antigen non specific cytokines such as TGF-β, contributing to bystander suppression (Fig. 1) [27].
2.2.1 M cells
M cells, with broad microfolds instead of microvilli on their apical surface, are mainly located in the follicle associated epithelium that lines Peyer’s patches and isolated lymphoid follicles in the small intestine (Table 1). M cells take up antigens in gut lumen by endocytosis and effectively transport them to professional antigen presenting cells residing in the subepithelial dome region of the follicles. Antigen loaded APCs further present the antigens to naïve T cells in the GALT or migrate to gut-draining MLNs [29]. Targeting M cells using a recombinant reovirus protein sigma 1(pσ1) conjugated with ovalbumin (OVA), enhanced oral tolerance as antibody titers and CD4+ T cell responses against OVA were greatly suppressed compared with controls. CD4+CD25+ forkhead box protein 3 (Foxp3+) T cells from the spleen, MLNs, and Peyer’s patches showed dramatically increased expression of TGF-β and IL-10 (Fig. 1) [31]. In one example, fed antigen predominantly localized to the interphase between M cells and CD11c+ DC in Peyer’s patches, consistent with the model that M cells take up antigen and deliver to DC by transcytosis. Two to five hours after oral gavage, antigen can be found in the liver and plasma (Fig. 2) [32]. This transcellular-M pathway was also identified as one of the mechanisms in nanoparticle oral delivery system by fluorescent image studies [33]. Particles with a diameter of less than 5 µm were able to be taken up by M cells, with a diameter around 100nm having higher efficiency compared with bigger nanoparticle sizes [34, 35].
Table 1.
Key cells, cytokines, and pathways involved in oral tolerance induction
| Characteristics | Function | Findings in oral tolerance | Reference | ||
|---|---|---|---|---|---|
| Cells | M cells | Broad microfolds. Located in Peyer’s patches and lympho follicles in small intestine | Take up antigens and effectively transport to antigen presenting cells | Targeting M cells using pσ1 conjugation with antigen enhanced oral tolerance. | [29,31,32] |
| Dendritic cells | CD11c+ DCs, CD103+ DCs, and CD11b+ DCs specialize in inducing oral tolerance. Gut DCs are mainly located in Lamina propria, Peyer’s patches, MLN. | Antigen presenting to T cells. Gut DCs may induce Treg, increase T cells expression of gut homing receptors, and induce B cells production of IgA. | In vivo expansion of DCs enhances oral tolerance whereas depletion of DCs diminished oral tolerance. Adoptive transfer of MLN pDCs transferred tolerance. | [36,40,41,44,45] | |
| Tr1 | CD4+ T cells with low proliferative capacity, high levels of IL-10, and low levels of IL-2, no IL-4. | Suppress T cells responses through IL-10. Induce Foxp3+ Treg. | Involved in low dose oral tolerance | [74,80,110] | |
| Th3 | CD4+ T cells producing TGF-β with various amounts of IL-4 and IL-10. Dependent on IL-4 rather than IL-2 for growth. First isolated from MLN of orally tolerized mice. | Suppress T cells responses. Induce Foxp3+ Treg. | Population increased in orally tolerized mice and peripheral blood of humans fed MBP. | [6,79,99] | |
| Th17 | Secrete high amounts of IL-17 and IL-22. Induced by IL-23 or IL-6. | Pro-inflammation. Important in autoimmune diseases. Decrease Treg. | Oral tolerance decreased Th17 cells | [49,195] | |
| Foxp3+ Treg | CD4+ CD25+ Foxp3+ regulatory T cells | Suppressing an effector T cell response such as T cell proliferation and inflammatory cytokine productions. | Oral antigen administration induced Foxp3+ Treg, which transferred tolerance to naïve recipients. | [76,77] | |
| LAP+ Treg | CD4+ LAP+ regulatory T cells in both CD25+ and CD25− | Suppressing an effector T cell response | Population increased in oral tolerance. Anti-TGF-β antibody reversed the suppressive effects. | [81–83] | |
| Cytokines | TGF-β | Abundant in the gut microenvironment. Secreted by CD4+ CD8+ T cells, macrophages, enterocytes, and epithelial cells | Pivotal in epithelial cell differentiation, IgA class switching, and strong immunosuppressive effects on lymphocytes. Induce Treg. | Up-regulated in oral tolerance. Neutralized TGF-β impaired oral tolerance. TGF-β knockout mice exhibited no tolerance in low dose feeding and partial tolerance in high dose feeding. | [90,91,97,98,100,101,103] |
| IL-10 | Highly expressed in the intestine. Secreted by certain CD4+ T cells (Th2 cells, Tr1 cells, Th3 cells, Foxp3+ Treg), epithelial cells, macrophages, NK cells, B cells, DCs and some CD8+ T cells. | Broad anti-inflammatory cytokine. Inhibits Th1 responses, down-regulates MHC class II and costimulatory molecules. Important for Treg induction and function. | Antigen feeding induced IL-10 production and Tr1 cells. IL-10 deficient mice failed in oral tolerance induction. However, some reported oral tolerance could be induced with anti-IL-10 antibody administration. | [80,109,113,115–118] | |
| IFN-Υ | Th1 type cytokine | Regulates the expression of adhesion molecules essential for Treg migration. | Controversial. Some reported that oral tolerance cannot be induced or adoptive transferred in IFN-Υ deficient mice. Others found it did not affect oral tolerance induction or maintenance. | [121–124] | |
| Pathways | Cox-2 | Inducible enzyme in arachidonic acid metabolism | Induces Foxp3 expression | Selective Cox-2 inhibition led to loss of oral tolerance | [126,127] |
| Retinoic acid | Metabolite of vitamin A. Produced by mucosal DCs, epithelial cells, MLN stromal cells, and macrophages. | Critical for mucosal DCs function. Regulates Th17 cell responses. Boost Treg induction. | CD103+ DCs in MLN expressed high level of Aldhla2, which made their own RA. | [51–53,135–139] | |
| Foxp3 | Mainly expressed in CD4+ T cells. Marker of Treg. | Transcriptional factor. Pivotal for Treg functions. | Mutation caused severe autoimmune disease. Retroviral gene transfer turned naïve T cells to Treg-like phenotype and function. | [142–148,150,151] |
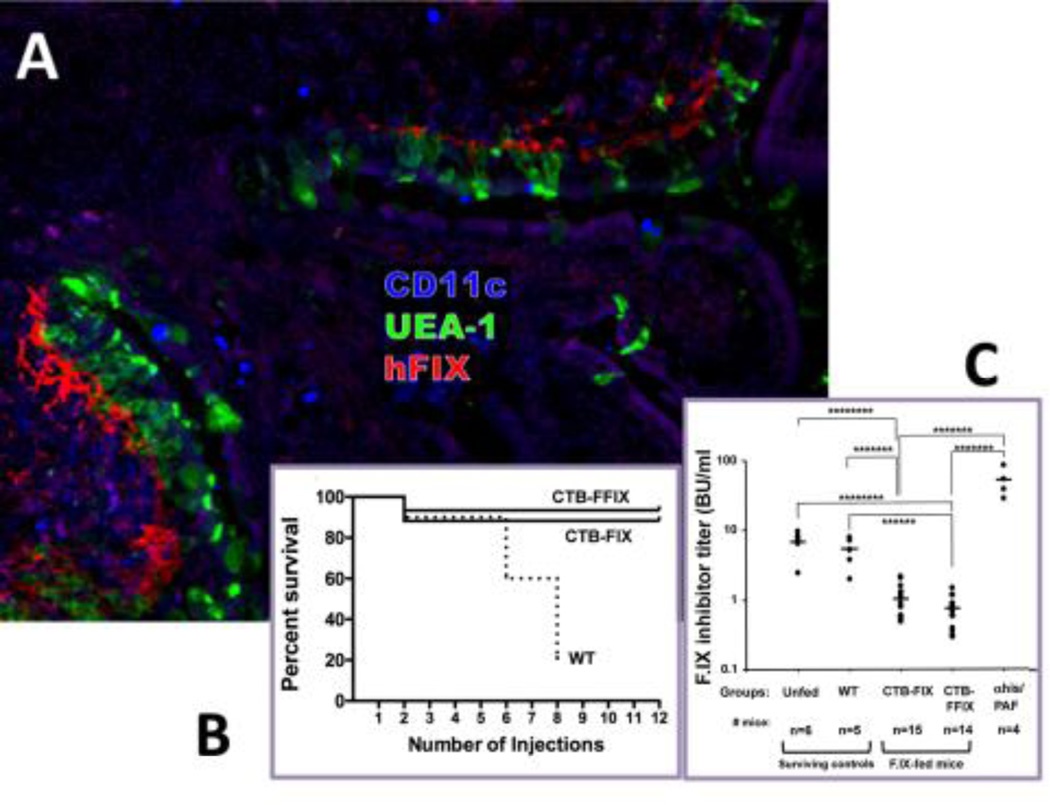
Fig. 2. Example of prevention of a systemic immune response by oral tolerance induction.
Prevention of inhibitory antibody formation and of anaphylactic reactions against intravenous human F.IX (hF.IX) by oral administration of CTB-hF.IX chloroplast transgenic plant material in hemophilia B mice. A. Delivery of hF.IX antigen to the GALT. Shown are Peyer’s patch and villi of ileum of a fed mouse stained for hF.IX (red), M cells (UEA-1, green), and CD11c (blue). B. Survival of mice fed with wild-type (WT, n= 10 mice at the onset of protein therapy), CTB-FIX (n=17), or CTB-FFIX (n=15) plant material as a function of the number of intravenous injections of hF.IX protein (CTB-FFIX contained a furin cleavage site between the CTB and hF.IX portions of the fusion protein). C. Inhibitor titers (in BU/ml) at 3-month time point mice (i.e. after 8 weekly IV injections of hF.IX) in unfed, WT fed, CTB-FIX fed, and CTB-FFIX fed hemophilia B mice. αhis/PAF: titers in unfed mice that received anti-histamine/anti-PAF prior to a 6th injection of hF.IX to prevent anaphylaxis. Modified from Proc Natl Acad Sci USA 107(15): 7101-6, 2010; © 2010 by The National Academy of Sciences of the USA.
2.2.2 DC
Intestinal DCs, the major APCs in the gut, are located in the lamina propria, Peyer’s patches, and MLN [36]. DCs constitutively engulf apoptotic enterocytes, which sampled intestine luminal antigens. Additionally, DCs under the epithelial of lamina propria are able to open the tight junctions between adjacent epithelial cells and extend their dendrites to reach luminal content to acquire antigen directly from intestine lumen (Fig. 1) [37, 38].
It was suggested that resident DCs in the gut have an intrinsic non-inflammatory characteristics (Table 1). Compared to splenic DCs, DCs from Peyer’s patches induced tolerance rather than primed T cells to an effector phenotype [39]. DCs isolated from MLN and Peyer’s patches induced B cells production of IgA, and T cells expression of gut-homing receptors CCR9 and α4β7 [40, 41], which are critically required for oral tolerance [11]. In vivo expansion of DCs using flt3 ligand enhanced oral tolerance [36]. Although intestinal antigens were shown to reach the circulation and peripheral lymph nodes [42], evidence indicated that it is not sufficient to induce significant responses and oral tolerance relies on the gut DCs uptake of antigens and subsequent migration to MLN on a CCR7-dependent manner [25].
Besides inducing Treg, gut’s DCs are able to initiate effector T cells in response to invasive pathogens, depending on subsets of DCs involved and surface receptors engaged [43]. Plasmacytoid or conventional CD11c+ DCs, CD103+ DCs, and CD11b+ DCs may be specialized for inducing oral tolerance. In an asthma model, transferring splenic CD11c+ plasmacytoid DCs (pDCs) isolated from OVA-fed mice transferred tolerance [44]. In a delayed type hypersensitivity model, in vivo depletion of pDCs diminished oral tolerance [45]. Adoptively transferring pDCs from liver and MLN but not spleen of antigen fed animals transferred tolerance. A study showed pDCs that contributed to around 70–80% anergy/deletion of antigen-specific cells [45]. In a contact dermatitis model, pDCs first abrogated a large fraction of antigen-specific CD8+ T cells in liver and MLNs, followed by induction of suppressive Treg [30]. CD103+ DCs produced retinoic acid, which is important in boosting Foxp3 Treg [46]. CD103+ DCs also expressed indoleamine-2,3-dioxygenase (IDO), required for the induction of Foxp3 Treg and development of oral tolerance. TGF-β, as well as prostaglandin E2, was involved in inducing IDO expression in CD103+ DCs [47]. CD11b+ DCs, which produced IL-10 and IL-27, increased dramatically during oral tolerance. IL-27 enhanced IL-10 production by Treg [48]. CD11b deficient animals were unable to induce oral tolerance [49].
Gut mucosal environment is important in educating DCs (Fig. 1). As they migrate from the lamina propria to the MLNs, DCs are influenced by local factors and crosstalking with different cell types. Intestinal epithelial cells are in close contact with lamina propria DCs and have been shown to condition DCs into “tolerogenic cells” by releasing TGF-β, retinoic acid, and thymic stromal lymphopoietin [50, 51]. Other innate cells may play roles, such as lamina propria macrophages producing large amount of IL-10 and inhibit DCs induction of TH-17 cells [52]. MLN stromal cells are essential for DC induced CCR9 expression of T cells and express high level of retinoic acid-producing enzymes [53]. Orally immunized memory CD4+ T cells secrete IL-4 and IL-10 to educate DCs, which further induce naïve T cells to produce the same cytokines as the immunized T cells producing [54].
2.3 T cell responses
T cell responses play a central role in immunity. Oral tolerance is orchestrated by distinct mechanisms (Fig. 3). Generally, when given a high dose oral antigen, it induces T cell deletion/anergy with IgA production; when adding certain adjuvants, the response can be converted to systemic activation such as CTLs and IgG. When given low doses, it results in active suppression with IgA secretion and induction of Treg producing IL-4, IL-10 and TGF-β [6]. These two mechanisms may occur simultaneously and overlap instead of being mutually exclusive, as they shared some of the same characteristics such as similar cytokines production and anergic T cells perform regulatory function identical to Treg [55], which will be discussed further below. Suppression of specific effector T cell responses by oral antigen administration has a different susceptibility. For example, Th1 type inflammation can be easily suppressed long-term with lower doses of antigen [56]. Th2 responses need higher amounts of antigen or increased feeding frequency [57], except for IL-4 dependent IgE response, which is highly susceptible to oral tolerance induction [58].
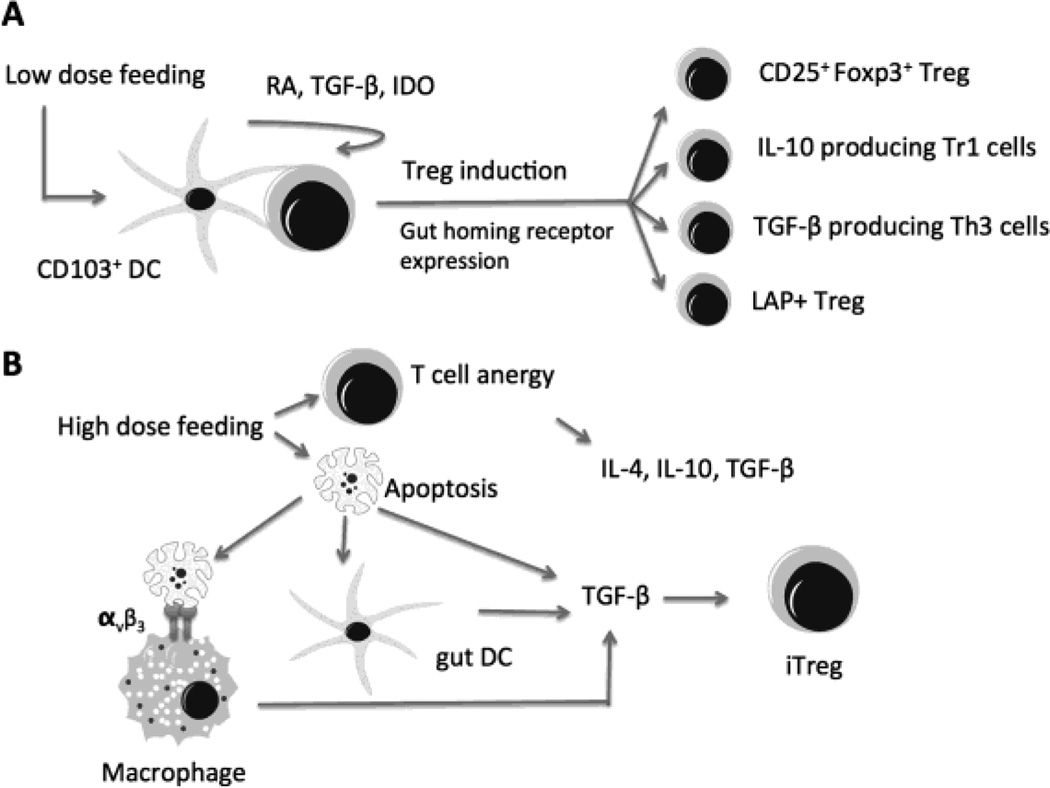
Fig. 3. Mechanisms of Treg induction in oral tolerance.
A. Low dose antigen feeding results in active induction of Treg, which involves cross talk between different cell types. For example, CD103+ gut DCs are specialized in inducing Treg via production of retinoic acid (RA), TGF-β, and expression of indoleamine-2,3-dioxygenase (IDO). B. High dose oral antigen induces T cell anergy. Anergic T cells produce cytokines including IL-4, IL-10, TGF-β, and act as suppressor cells to evoke tolerance. High dose antigen feeding also increases susceptibility to apoptosis. Macrophage and DC clean up the apoptotic cells and exhibit up-regulation of TGF-β and down regulation of inflammatory cytokines. Apoptotic cells can also secrete TGF-β, which is critical for inducing and maintaining Treg. These two mechanisms may occur simultaneously and overlap as they shared some of the same characteristics such as cytokines production profiles and generation of induced Treg (iTreg).
The concept of suppression mediated by T cells was first described in the 1970’s [59]. More than 1000 scientific papers were published on that topic, mainly referring to CD8+ (Lyt- 2) T cells [59, 60]. However, due to lack of specific markers and poor characterization, suppressor T cells were nearly abandoned by the end of 1980’s [61]. In the mid-1990s, a new subset of suppressive CD4+ T cells was characterized as regulatory T cells (or “Treg”). Besides high level of IL-2 receptor (IL-2Rα or CD25) expression, other markers were also reported such as transcriptional factor Foxp3, cytotoxic T lymphocyte antigen 4 (CTLA-4), and glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) [59]. Commonly, CD4+ T cells now are divided into two distinct lineages, conventional T helper (Th) cells and Treg [59]. Conventional Th cells regulate the adaptive immunity by activating other effector cells including CD8+ CTLs, B cells, and macrophages in an antigen dependent manner. Treg are defined as T cells diminishing potentially harmful activity of Th cells [62]. Treg exhibit regulatory functions to suppress an effector T cell response including inhibition of T cell proliferation and blockade of inflammatory cytokines release. Treg can transfer tolerance to naïve recipients [59]. They either secret high levels of IL-10 and TGF-β, or require TGF-β for the development [62].
It is well accepted that oral antigen administration activates or induces Treg, though the mechanisms are not completely clear, most likely involves cross talking between macrophages, DCs and T cells (Fig. 3). As mentioned above, gut DCs had the intrinsic characteristic to induce tolerogenic T cells response rather than prime effector T cells. An interesting link between apoptosis and generation of Treg has been described in high dose feeding. Feeding high doses antigen in mice seems to increase the susceptibility of lymphocytes to apoptosis [63]. Similarly, human lamina propria T cells show increased susceptibility to Fas-mediated apoptosis upon CD2 pathway stimulation [64]. Macrophages, which clean up the apoptotic cells through αvβ3 integrin, exhibit up-regulation of TGF-β and down-regulation of pro-inflammatory cytokines production [65]. In addition, a subset of gut DCs were discovered to take up apoptotic enterocytes, and have relatively low stimulatory activity for T cells [66]. Apoptotic T cells also secrete TGF-β, as a result from existing cytokine redistribution following loss of mitochondrial membrane potential [67]. TGF-β increased from macrophages, DCs and apoptotic T cells, is a critical cytokine in the differentiation and survival of Treg. Another explanation for high dose oral tolerance is anergy of specific T cells. Anergy is the basis of peripheral tolerance to self-antigens. It also plays role in oral tolerance, first indicated that T cell tolerance can be reversed by exogenous IL-2 in vitro [68]. Anergic T cells are not just passive in the tolerance they evoke. They produce cytokines including IL-4, IL-10, TGF-β and act as suppressor cells in vivo and in vitro [69, 70]. Anergic CD4+ T cells from mice tolerized by feeding casein can mediate active suppression when transferred to severe combined immunodeficiency (SCID) mice [71].
Various subsets of Treg in oral tolerance have been reported, such as thymus derived Treg or natural Treg, induced FoxP3+ Treg, Tr1 cells, and Th3 cells (Table 1). Thymus derived CD4+CD25+Foxp3+ Treg or natural Treg play critical role in abrogation of auto-immunity and maintain self-tolerance [72]. In addition, they involve in suppressing immune responses toward commensal bacteria in the gut [73]. They are reported to be unregulated in oral/mucosal tolerance [11]. However, in a mice model containing a monoclonal population of CD4+ T cells specific for OVA crossed on a RAG-1 deficient background, it was demonstrated that oral tolerance to OVA was effectively induced without thymus-derived Treg [74].
Peripherally induced or adaptive Treg are essential for oral tolerance [75]. They develop outside the thymus within specific microenvironment. For example, CD4+CD25+Foxp3+ Treg were markedly induced in Peyer’s patches of OVA TCR transgenic mice fed high doses of OVA. They have suppressive properties in vitro and mediate tolerance transfer to naive animals [76]. CD4+CD25+CTLA4+ Treg were upregualted as long as 4 weeks after OVA feeding. They expressed high level of TGF-β and IL-10 and mediate adoptive tolerance transfer in BALB/c mice [77]. In a high dose OVA feeding model, induced CD4+CD25+ Treg expressed TGF-β and were anergic T cells. Their oral tolerance was IL-18 dependent [78]. One subset of CD4+ T cells producing TGF-β is termed Th3 cells, which was initially isolated and cloned from the lymphoid tissues of mice tolerated by low dose antigen feeding (Table 1) [79]. They appear to be dependent on IL-4 rather than IL-2 for growth and may produce variable amounts of Th2 cytokines like IL-4 and IL-10 [6]. Another subset of IL-10 producing type 1 regulatory T cells, named Tr1 cells, mediate suppression through IL-10 and induce Foxp3+ Treg, are reported to be involved in lose dose oral tolerance (Table 1) [74, 80]. A membrane-bound form of TGF-β containing latency-associated peptide (LAP) has been identified [81]. LAP+ Treg occurs in both CD25+ or CD25− cells (Table 1) [82]. All these subsets are not mutually exclusive as they produce similar cytokines. For instance, Foxp3+ Treg, Th3 cells, and Tr1 cells, all secrete IL-10. As a result of different experimental settings and lack of exclusive markers, one can hardly determine what percentage a certain cell type contribute in oral tolerance. Moreover, various subsets might act in synergy and connected to each other in performing their functions. As such, Th3 and Tr1 cells induce Foxp3+ Treg through TGF-β and IL-10 [83].
Additionally, a subpopulation of CD8+ lymphocytes might be also involved in tolerance induction. Intestinal epithelial cells (IEC) activated CD8+ T cells with a regulatory function [84]. Feeding a MHC class I immunodominant peptide of OVA induced CD8+ T cells with regulatory function. These cells inhibited Th1/Th17 responses but not Th2 responses [85]. Defects in CD8+ T cells were observed in inflammatory bowel disease patients and correlated with a failure to induce oral tolerance in these patients [86]. However, anti-CD8 antibody studies demonstrated that CD8+ cells are not essential for oral tolerance induction and maintenance of systemic tolerance [87, 88].
2.4 Cytokines
2.4.1 Transforming growth factor-β (TGF-β)
TGF-β a multifunctional polypeptide involved in various cellular processes, such as extracellular matrix production, angiogenesis, differentiation, proliferation, apoptosis, and immunomodulation [89]. Three isoforms exist in mammals including TGF- β1, TGF-β2, and TGF-β3. TGF-β1 is the foremost in leukocyte populations [89]. TGF-β has emerged as a key regulator of both innate and adaptive immune responses, in a context dependent manner [89]. Abundant in the gut microenvironment, TGF- β is pivotal in epithelial cell differentiation, IgA class switching, and has strong immunosuppressive effects on lymphocytes [90]. TGF-β mediates immune tolerance via induction and maintenance of Foxp3+ Treg. In vitro studies revealed that TGF-β activates naïve T cells into Foxp3+ expressing T cells with suppressive capacity [91]. Neutralization of TGF-β during induction of oral tolerance diminished Foxp3 expression in MLN and spleen [74]. TGF-β inhibits T cell proliferation with no exogenous IL-2, and decreases the differentiation of naïve T cells into effector Th1 and Th2 cells via the downregulation of T-bet and GATA3 [92–94]. TGF-β is secreted as an inactivated form. Latent TGF-β can be activated by gut DCs and IECs through integrins αvβ8 and α4β6 [95, 96]. Mucosal DCs expressed more αvβ8 than splenic DCs and release more bioactive TGF-β, which might in part explain why mucosal DC inducing more Foxp3+ Treg [95].
Involvement of TGF-β in oral tolerance was extensively documented (Fig. 4). TGF-β production was unregulated in Peyer’s patches, lamina propria and draining lymphoid tissures after induction of oral tolerance [97, 98]. TGF- β producing Th3, other CD4+ cells, and CD8+ T cells were isolated and cloned from the Peyer’s path and MLN of orally tolerized mice, and peripheral blood of humans fed MBP [6, 99]. In the experimental models of EAE and IBD, oral antigen feeding reduced diseases dependent on TGF-β productions, as neutralized TGF-β with antibodies impaired the development of oral tolerance [100, 101]. TGF-β deficient mice die soon after birth from wide spread inflammation [102]. Using an anti-LFA-1 antibody to inhibit inflammation in TGF-β deficient mice, it was shown that low doses and high dose feeding of OVA lymphocytes had reduced proliferation [103]. However, some mice in low dose feeding exhibited no tolerance or even priming systemic proliferation responses; and the degree of tolerance was only partial for all systemic responses compared with wild type mice [103]. Thus, TGF-β plays a critical role in oral tolerance, although it may not be the exclusive mechanism involved.
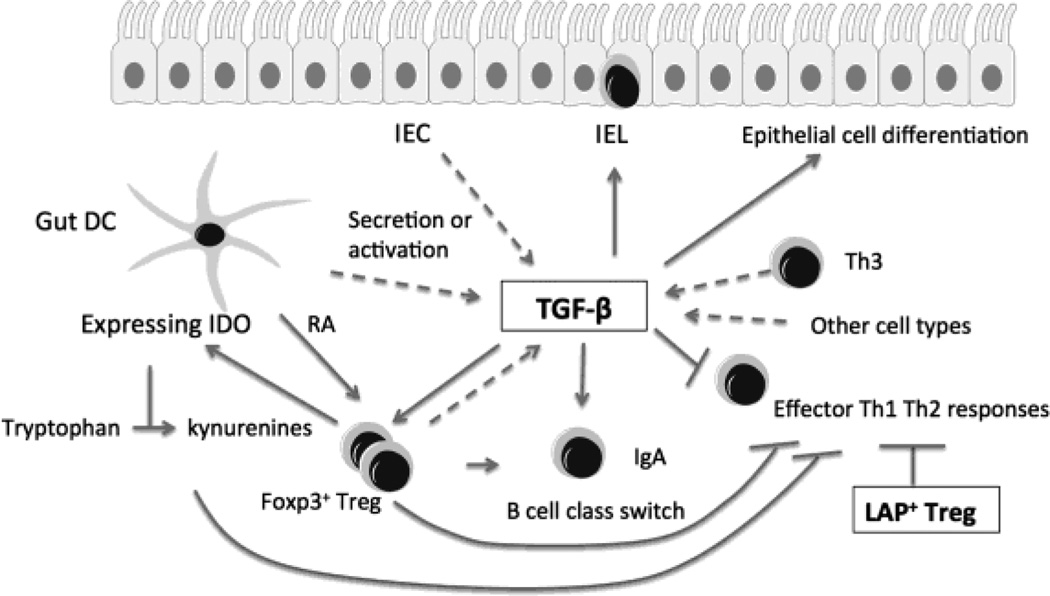
Fig. 4. Induction and multifunction of TGF-β in the gut.
TGF-β is a key cytokine for oral tolerance. Abundant in the gut microenvironment, TGF-β has multiple functions, including epithelial cell differentiation, IgA class switching, and induction and maintenance of Foxp3+ Treg. Additionally, TGF-β decreases the differentiation of naïve T cells into effector Th1 and Th2 cells, drives the development of TCRαβ+CD8αα+ intestinal intraepithelial lymphocytes (IEL). TGF-β is secreted by a variety of cells including CD4+, CD8+ T cells, Th3 cells, macrophages, enterocytes, antigen-pulsed intestinal epithelial cells (IEC), and gut DCs. Gut DCs produce retinoic acid (RA), which enhance Foxp3+ Treg induction by TGF-β. In MLN DCs, interaction with Foxp3+ Treg increases expression of indoleamine-2,3-dioxygenase (IDO), which depletes tryptophan into kynurenines and suppresses effector T cell responses. Besides the secreted form, an active membrane-bound form (Latency-associated peptide, LAP) exists. Some Treg express LAP and TGF-β on their cell surface, which is associated with the suppressive activity of Treg. LAP+ Treg are expanded in oral tolerance.
The source of TGF-β in gut is not only from CD4+ or CD8+ T cells. Many other cell types such as macrophages and enterocytes can also produce this cytokine [104]. One study even indicated that antigen-pulsed epithelial cells might inhibit T cell activation through secretion of TGF-β [105].
In addition to the secreted form of TGF-β, an active membrane-bound form was also described. Latency-associated peptide (LAP) is the amino-terminal domain of the TGF- β precursor peptide. After cleavage, LAP forms the latent complex with TGF- β via noncovalent association [82]. Certain Treg express LAP and TGF- β on their cell surface, which is associated with the suppressive activity of Treg [81, 82]. LAP+ Treg are expanded in oral tolerance, both CD4+CD25+ and CD4+CD25- [82]. Anti-TGF- β antibody can reverse the suppressive effects of LAP+ Treg [83].l
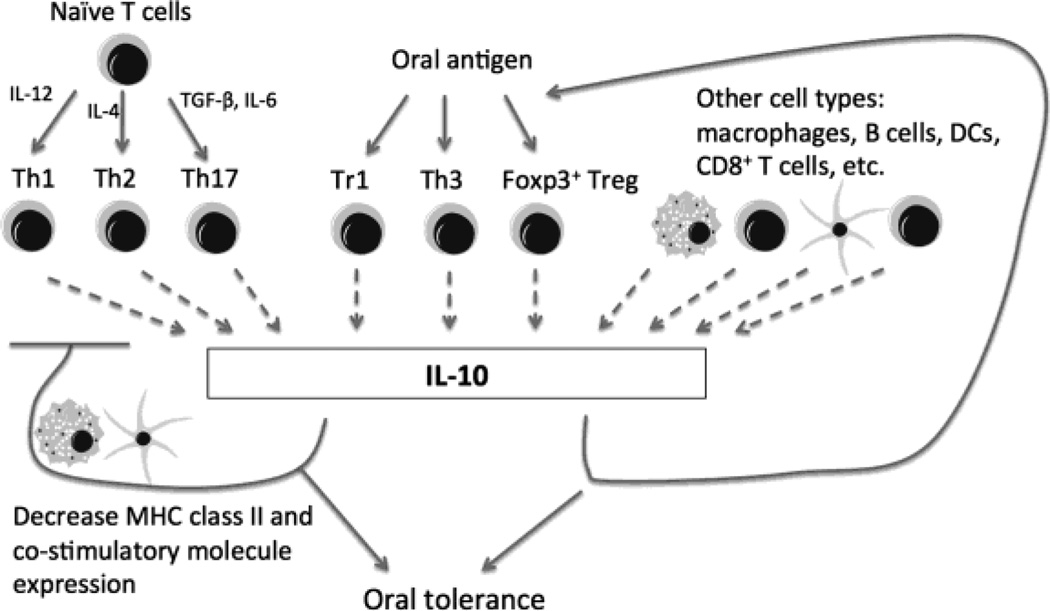
2.4.2 Interleukin-10 (IL-10)
IL-10 initially has been described as a Th2 type cytokine to suppress Th1 type responses, including cytokines IL-2 and IFN-γ production and cell proliferation in oral tolerance, which is still widely used in measurement of oral tolerance induction by many researchers [54, 106]. Low dose antigen feeding induced IL-10 and IL-4 in tolerated mice [107]. However, oral tolerance also inhibits Th2 type responses although the susceptibility is different [57]. The role of Th2 cells in mediating oral tolerance is further questioned since neutralizing IL-4 with antibody or IL-4 deficient mice, which is a model for Th2 cell deficiency, still develop oral tolerance [108].
IL-10 is now regarded as a broad anti-inflammatory cytokine (Fig. 5). It not only inhibits Th1 responses, but also down-regulate MHC class II and costimulatory molecules expression [109]. Besides Th2 cells, IL-10 can be secreted from a variety of cells. Specifically, one regulatory subset of CD4+ T cells mainly producing IL-10, are named Tr1 cells [110]. Other populations of CD4+ T cells are capable of secreting IL-10, such as Th3 cells, Th17 cells, Foxp3+ Treg [109]. Epithelial cells, macrophages, NK cells, B cells, DCs and CD8+ T cells, all have been reported to produce IL-10 [109]. An interesting linking has been revealed between IL-10 producing mucosal APC and IL-10 producing T cells, as plasmacytoid DCs from MLN, CD11b+ DCs from PP, and CD11b+F4/80+ lamina propria macrophages can induce IL-10 producing T cells through their own secretion of IL-10 [52].
Fig. 5. IL-10 expression and function in the gut.
IL-10 is a cytokine with broad anti-inflammatory properties and constitutes another key component in oral tolerance. Highly expressed in the intestine, IL-10 producing cells increased even more after antigen feeding, especially in Peyer’s patch (PP), lamina propria, and MLN. Specifically, one regulatory subset of CD4+ T cells mainly producing IL-10, named Tr1 cells, is induced in oral tolerance. Other regulatory T cells such as Th3. Foxp3+ Treg may also secrete IL-10 and are induced by antigen feeding. Macrophages, B cells, DCs and CD8+ T cells, epithelial cells, NK cells all have been reported to produce IL-10. IL-10 can be produced by Th2, Th17, and Th1 cells in certain situations, which serves as a negative feedback loop to limit effector T cell responses, possibly via regulation of antigen presenting DCs and macrophages. IL-10 is critical for induction of certain subsets of regulatory T cells and/or their function, which provides a positive regulatory loop for establishment of oral tolerance.
Highly expressed in the intestine, IL-10 is critical in establishing tolerance to intestinal commensal bacteria. IL-10 deficient mice develop spontaneous colitis associated with abnormal T cell activation [111]. Exogenous IL-10 was able to restore tolerance in the colitis mice [109]. It has been reported that all IL-10 producing T cells in the colonic lamina propria express Foxp3, but not in the small intestine [112]. Selective deletion of IL-10 in Foxp3+ T cells in the colon lead to spontaneous colitis, indicating IL-10 is important in Foxp3+ Treg suppressive function in the colon [113]. In addition, IL-10 producing macrophages in lamina propria are crucial in supporting the Foxp3+ Treg function as well [114].
The role of IL-10 in oral tolerance is controversial. Lots of IL-10 producing T cells exist in the gut microenvironment [115]. Antigen feeding increases IL-10 production even more, especially in PP, MLN, also in spleen and serum [80]. Tr1 cells are induced in oral tolerance [116]. However, oral tolerance can still be induced in mice treated with anti-IL-10 antibodies and anti-IL-10 antibodies do not abrogate established tolerance in vivo [117]. But in one study with IL-10 deficient mice treated recombinant IL-2, multiple feeding of antigen failed to induce tolerance [80]. Another IL-4 and IL-10 knockout mouse study demonstrated that both cytokines are required for low dose oral tolerance [118].
Cytokines are essential for oral tolerance induction. Cross-talking between different cytokine producing cells occurs in oral tolerance. TGF- β may induce the differentiation of IL-10 producing cells [112, 119]. IL-10 may be involved in TGF-β production as anti-IL-10 treatment decreased TGF-β levels [120].
Besides the two major suppressive cytokines TGF-β and IL-10, other cytokines may also be involved. IFN-γ regulates the expression level of adhesion molecules essential for Treg migration [121]. It is reported that oral tolerance cannot be induced in IFN-γ deficient mice or adoptive transfer of TCR transgenic T cells with an IFN-γ deficient background [121, 122]. However, other reports described that deficiency of IFN-γ does not affect oral tolerance induction or maintenance [123, 124]. Some anti-tolerance cytokines have also been described. IL-6 and IL-17 treatment can decrease the Treg cell population and impair tolerance [125]. Oral tolerance suppresses Th17 cells, which secrete high amounts of IL-17 and IL-22 [49].
2.5 Other immune regulatory pathways
Many signal pathways are important in regulating immune responses for oral tolerance (Table 1). Cyclooxyenase-2 (COX-2) is the inducible enzyme in arachidonic acid metabolism. PGE2, the product of COX-2 was shown to directly induce Foxp3 expression in T cells [126, 127]. Selective COX-2 inhibition led to loss of tolerance to oral antigen administration [126]. NF-κB and MAPK pathways are essential for Treg development and Akt has a negative effect on Treg [128]. Retinoic acid and Foxp3 are two pathways which were intensively studied, and will be discussed further below.
2.5.1 Retinoic acid (RA)
Retinoic acid (RA) is the metabolite of vitamin A by alcohol dehydrogenase (ADH) and retinal dehydrogenase (RALDH) [129]. It is critical for proper function of mucosal DCs in oral tolerance. Mucosal DCs induction of gut homing receptors including αEβ7 and CCR9 in T and B cells is dependent on the ability of DCs to convert vitamin A into RA [130, 131]. RA regulates Th17 cell responses, contingent on the local cytokine environment [132, 133]. TGF-β mediated Foxp3+ Treg induction can be boosted by RA [134, 135]. It is well known that mucosal DCs have a greater capacity to induce Foxp3 expression in T cells than non-mucosal DCs, which is associated with the DCs ability to convert vitamin A into RA [134]. CD103+ DCs in MLN express high level of Aldhla2, the gene encoding RALDH2 [46]. So that CD103+ DCs but not CD103− DCs can induce Foxp3+ Treg in the absence of exogenous RA and TGF-β. CD103− DCs have weaker induction of Foxp3+ Treg compared with CD103+ DCs even in the present of exogenous TGF-β [46].
Multiple pathways might mediate the mechanisms of RA enhancement of Foxp3+ Treg induction by TGF-β. First, RA inhibits the production of some cytokines in effector and memory T cells, which suppress naïve T cell differentiation into Foxp3+ Treg [136, 137]. Furthermore, RA suppresses the effects of pro-inflammatory cytokines on naïve T cells, such as down-regulation of IL-6 receptors levels [138]. Vitamin A deficiency results in increased inflammation [129]. In addition, RA increases TGF-β signaling by raising the expression of Smad3 [138]. RA up-regulates Foxp3 expression directly via binding to the promoter region with RA receptor α [139]. Moreover, RA attenuates the co-stimulation from interfering Foxp3+ Treg differentiation [135]. However, there is a report that RA’s effects can be independent of inhibitory cytokines and RA induction of Foxp3 expression is largely Smad3 independent [140]. Thus, the effects of RA are most likely integrative instead of just relying on a single pathway.
Besides mucosal DCs, other cell types in the gut are able to convert vitamin A into RA, such as intestinal epithelial cells, MLN stromal cells, and lamina propria macrophages [51–53]. The epithelial cells and stromal cells generated RA might be transferred to DC as a mechanism of local education of DCs. DCs were reported to take RA from outside, store it, and acquire tolerogenic characteristics [141]. Macrophages might function similar way with DCs in that macrophages induce Foxp3+ Treg dependent on RA and TGF-β [52]. The difference is macrophage require IL-10 in inducing Treg besides RA and TGF-β [52].
2.5.2 Treg expressing transcription factor FoxP3
Foxp3 belongs to the forkhead / winged-helix family of transcriptional factors and is highly conserved in humans [142, 143]. Acting as the activator or suppressor, Foxp3 binds to the promoter region of 700–1100 genes, most of the genes associated with TCR signaling [142, 143]. The expression of Foxp3 is largely in T cells, mainly in CD4+ T cells, some CD8+ T cells also express it, very low or undetectable in B cells, natural killer cells, macrophages and DCs [142, 143]. To date, Foxp3 is considered one of the most reliable Treg markers, although some reports found Foxp3 also exist in non-Treg [144]. There is an overlap of expression between Foxp3 and CD25, the traditional Treg marker. In lymph nodes and spleen, most CD4+CD25+ cells express Foxp3 [144, 145]. Although Foxp3 can be positive in CD4+CD25+ and CD4+CD25− cells, it is more abundant in CD4+CD25+ cells in mice [145].
Foxp3 is a pivotal factor for Treg function. Foxp3 mutations cause X-linked autoimmunity allergic dysregulation syndrome and immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, which are severe autoimmune diseases in humans [146–148]. Scurfy mice, the mice orthologue of Foxp3 deficiency, develop lymphoproliferative disorder and die one month after birth [149]. T cell targeted deficiency of Foxp3 lead to lymphoproliferative disease [144]. Retroviral-mediated Foxp3 expression in naïve T cells resulted in those T cells acquiring Treg-like phenotype and functions [150, 151]. Foxp3 transgenic mice show markedly increased number of CD4+CD25+ Treg, and CD8+ T cells expressing Foxp3 also demonstrate immunosuppressive activity [152]. Continuous Foxp3 expression is associated with maintenance of the Treg in the periphery [153]. Expression of Foxp3 is stabilized by demethylation, and this epigenetic modification is essential for the development of a permanent Treg cell lineage [154].
Foxp3+ Treg can convert to conventional T cells. In vitro TGF- β induced Treg with partial promoter demethylation lost Foxp3 expression and suppressive activity upon restimulation in the absence of TGF-β [154]. A large fraction of labeled Foxp3+ Treg became Foxp3− after 4 weeks transfer into T cell deficient mice. Some of them converted to Th1 cells, Th2 cells, and Th17 cells in lymph nodes and spleen [155, 156]. Some converted to Tfh cells that function in assisting IgA production by B cells in Peyer’s patches [157]. The mechanism of this conversion is not clear, possibly involving inflammatory cytokines. IL-6 can decrease Foxp3 expression and reprogram Treg into Th17 cells [158]. In the presence of inflammatory signals, Treg differentiated into Th17 cells in vivo [159].
Glucocorticoid-Induced Tumor necrosis factor Receptor family-related protein (GITR; also called TNFRSF18 or CD357) is constitutively expressed on Treg cells and is inducible upon activation on naïve CD4+ T cells [160–162]. Although the number of FoxP3+ Treg is normal under steady state, it is significantly induced in wild-type mice after administration of soluble Fc-GITR-L fusion protein, which has been shown to transiently induced tolerance to the coagulation factor IX expression in a murine model of hemophilia B [163]. Transgenic hCD19-GITR-L mice also showed increased numbers of FoxP3+ Treg and were more resistant to the induction of experimental autoimmune encephalitis (EAE) [164]. In addition to regulating the balance between Teff and Treg cells, the GITR-L / GITR interaction also controls the development of DC, macrophages and/or monocytes. Therefore, the numbers of tolerogenic CD103+ DCs and pDCs are reduced in GITR deficient mice, thereby causing the expansion of Th1 cells and inhibiting the development of FoxP3+ Treg after their exposure to the large amount of food antigens as well as to commensal bacteria [165]. GITR-L / GITR engagement may also potentially influence the establishment of oral tolerance by a reverse signaling through GITR-L in pDC. For example, GITR-L engagement by GITR::Fc fusion protein induced expression of IDO in pDC, a pathway that may also aid in inducing oral tolerance [166].
3. Therapeutic proteins & disease targets
Hemophilia A and B are X-linked bleeding diseases with a deficiency of coagulation factor VIII (F.VIII) or factor IX (F.IX), respectively. Current clinical treatment is based on periodical intravenous administration of the deficient coagulation factor. The most problematic complication associated with therapeutic protein replacement is the development of neutralizing antibodies (inhibitors) that occurs in treatment of 25–30% of hemophilia A and of 2–4% of hemophilia B patients. Some hemophilia B patients with inhibitors are also at risk for severe allergic / anaphylactic reactions to F.IX, contributing to a higher morbidity and mortality rate. Inhibitors develop due to the induction of adaptive immune responses against the therapeutic proteins, which are perceived as non-self antigens. High-level inhibitors decrease the therapeutic effects of coagulation factors and require either bypass therapy or immune tolerance induction (ITI). However, several disadvantages limited these applications: high cost; inability to induce tolerance in some patients; and requirement for frequent monitoring [167, 168].
Pompe disease, which is an autosomal recessive lysosomal storage disorder with a deficiency of acid α-glucosidase (GAA), causes death early in childhood due to hypertrophic cardiomyopathy and respiratory failure. Similarly, enzyme replacement therapy (ERT) based on intravenous infusion of recombinant human GAA, is the standard therapy, which prolongs the life of affected infants. However, anti-GAA antibodies responses develop and compromise the beneficial effects. Only recently, immune tolerance protocols to prevent anti-GAA antibody formation in patients with infantile onset disease are being developed to maintain the efficacy of ERT [10]. However, these heavily rely on immune suppression. One study addressed oral tolerance as an alternative (Table 2). Oral recombinant human GAA administration successfully lowed anti-GAA antibody titers in C57BL/6 and Balb/c mice. GAA specific IgE titers in post-immune serum were lowered too [169].
Table 2.
Studies on oral administration of therapeutic protein to induce tolerance in treatment of disease
| Therape utic protein | Disease | Species | Feeding regimen | Results | Reference |
|---|---|---|---|---|---|
| F.VIII | Hemophi lia A | Human | 1250 IU F.VIII with sodium bicarbonate once daily for 3 months | 2 of 3 patients had decreased inhibitor titer lasted for 6 months; the other patients had decreased in vivo proliferation rate of mononucleated cells and reduced inflammatory cytokines productions | [170] |
| F.VIII | Hemophi lia A | Human | 20 to 70 U/kg F.VIII oral administrated at intervals from 1 day to 1 week | Diminished inhibitors titers from 42 BU/ml to 11 BU/ml in one patient; Inhibitors come back when high dose of prothrombin complex concentrates was used. | [171] |
| F.VIII C2 | Hemophi lia A | Mice | Oral or nasal 1–50 µg F.VIII C2 for 5 consecutive days | Decreased the titer of anti-F.VIII C2 inhibitors and antibodies; tolerance was associated with increased IL-10 and can be adoptively transferred. | [172] |
| F.VIII | Hemophi lia A | Mice | 1 to 2 µg F.VIII oral gavage neonatal mice every 2 to 3 days for 9 total feedings | No protection against inhibitors formation developed after F.VIII iv injection. | [196] |
| F.IX | Hemophi lia B | Mice | Tobacco leaves contain CTB-F.IX or CTB-FF.IX twice per week for 2 months; equivalent to 5–80 µg/kg recombinant F.IX | Eliminated fatal anaphylactic reactions; blocked formation of inhibitory antibodies undetectable or up to 100-fold less than controls; controlled inhibitor formation and anaphylaxis longterm, up to 7 months, about 40% life span of this mouse strain | [32] |
| GAA | Pompe disease | Mice | 16 mg recombinant human GAA 5 times every other day for 6 days | Significantly lower anti-GAA antibody titers; reduced specific IgE against GAA titers. Not effective with 1 mg and 10 mg oral GAA 3 times every other day. | [169] |
| Insulin | Diabetes | Human | 2.5 or 7.5 mg oral insulin or placebo daily for 1 year in autoantibody positive patients | No differences were seen in the time course or titers of antibodies to insulin, glutamic acid decarboxylase or islet antigen 2. Similar insulin requirements, haemoglobin A1c concentrations, and C peptide concentrations. | [197] |
| Insulin | Diabetes | Human | 1 or 10 mg insulin tolerance trial in newly diagnosed diabetic patients with cytoplasmic islet cell autoantibodies | Improved plasma C-peptide responses in patients diagnosed at ages greater than 20 years, best seen at low (1 mg/daily) over high (10 mg/day) dose. There were no adverse effects. | [198] |
| Insulin | Diabetes | Human | Oral insulin or placebo treatment of type 1 diabetes for 1 year | In oral insulin group, significantly higher TGF-β;markedly reduced IFN-γ; similar levels of IL-4 and IL-5 in challenged lymphocytes; significantly lower circulating levels of IgG1 and IgG3 against insulin. | [199] |
| Insulin | Diabetes | Mice | Tobacco or lettuce leaves contain CTB-proinsulin (about 14 µg of fusion protein) or control once a week for 7 weeks | Decreased infiltration of cells characteristics of lymphocytes; insulin producing β cells were preserved; increased expression of immunosuppressive cytokines | [173] |
| Insulin | Diabetes | Mice | Silkworm produced GFP-CTBinsulin (about 50 µg of fusion protein) every other day for 5 weeks | Induced special tolerance; delayed the development of diabetic symptoms; increased the numbers of CD4+CD25+Foxp3+ Treg. | [200] |
| Insulin | Diabetes | Mice | Potato expressed CTB-insulin, CTB, or insulin (20 –30 µg) once per week for 5 weeks | Only CTB-insulin group showed a reduction in insulitis and a delay in the diabetes progression, but not insulin or CTB alone group. | [201] |
| Insulin | Diabetes | Mice | Glutamic acid decarboxylase expressing transgenic plant as a dietary supplement | Inhibits the development of diabetes in the non-obese diabetic mouse | [202] |
| Insulin | Diabetes | Mice | 1 mg porcine insulin orally twice a week for 5 weeks, then weekly until 1 year. | Reduced lymphocytic infiltration of pancreatic islets; splenic T cells from orally treated animal adoptively transfer protection against diabetes | [178] |
| Insulin | Diabetes | Mice | 0.8 mg human insulin in PBS 3 times a week for 2 – 4 weeks | Induced insulin B-chain reactive regulatory T cells to block cytokine secretion and migration of diabetogenic effector T cells | [203] |
| Insulin | Diabetes | Mice | 600 µg insulin B chain or 250 µg peptide 5 consecutive days, once a week thereafter | Slowed diabetes development in a co-transfer model of diabetes in NOD mouse; a decrease in Th1 cytokine and increase in Th2 cytokines. | [204] |
| Insulin | Diabetes | Mice | 1 mg equine insulin or ovalbumin twice a week for 5 weeks | Considerably less insulitis in insulin fed NOD mice; residual mononuclear cells expressing IL-4, IL-10, PGE, TGF- β, and an absence of IL-2, IFN-γ or TNF-α | [205] |
| Insulin | Diabetes | Rat | 0.5 or 1 mg insulin 3 times weekly for 90 days | No protection effect was observed in Diabetes prone or Diabetes resistant BB rats | [206] |
| Egg white | Egg allergy | Human | 2 g egg white powder per day for 10 months; 10 g egg white power till 22 months | 55% in oral-immunotherapy group passed the oral food challenge after 10 months therapy; 75% of children were desensitized in the oral-immunotherapy group at 24 months. | [207] |
Oral tolerance for inherited protein deficiencies offers many advantages in suppressing adaptive immune responses: low costs; low adverse effects; easy delivery; and higher efficacy in achieving antigen specific peripheral tolerance. Several studies have been performed in hemophilia (Table 2). F.VIII orally administrated with sodium bicarbonate at a dose of 1250 IU once daily for 3 months successfully decreased F.VIII inhibitors titers in two of three patients, lasted for 6 months after termination of the treatment. The other patient showed no decrease of inhibitors but still had a decreased in vivo proliferation rate of mononucleated cells and reduced cytokine production, including IL-6, IL-1β and TNF-α[170]. Another study reported that orally administered F.VIII at intervals ranging from 1 day to 1 week, dosage increasing form 20 to 70 units/kg, markedly diminished the inhibitors from 42 BU/ml to 11 BU/ml in one patient. However, the inhibitors relapsed when high dosage of prothrombin complex concentrates (contains F.VIII light chain fragment) were administrated due to bleeding control [171]. Mouse studies showed that F.VIII-C2 oral and nasal applications significantly suppressed antibody and inhibitor formation to this domain but not to the entire F.VIII protein. Tolerance was associated with increased IL-10 production and could be adoptively transferred. However, tolerance was not sustained after additional F.VIII-C2 injections [172]. Oral delivery of human F.IX bioencapsulated in chloroplast transgenic plant cells was successful in controlling inhibitor formation to factor replacement therapy in hemophilia B mice with F9 gene deletion long-term [32]. Moreover, this treatment also prevented IgE formation and the associated life-threatening anaphylactic reactions that normally occur in response to repeated intravenous administration of human F.IX in these mice (Fig. 2).
In contrast to the still limited data in genetic disorders caused by single gene deficiencies, oral tolerance has initially and most intensively been studied in autoimmune disease. These investigations include well-established animal models and clinical trial trials, particularly in multiple sclerosis (MS), rheumatoid arthritis (RA), and diabetes (Table 2). For example, oral insulin has been used to slow or prevent diabetes in the non-obese diabetic (NOD) and in a viral induced diabetic mouse models, accompanied by decreased IFN-γ and increased IL-4, IL- 10 and TGF-β responses to the insulin antigen. In other work, oral antigens tolerized mice showed markedly reduced disease severity in collagen-induced arthritis model, adjuvant arthritis model, pristine-induced arthritis model, and silicone-induced arthritis [11, 12]. Studies from different groups demonstrated that oral myelin basic protein (MBP) significantly suppressed experimental autoimmune encephalitis (EAE), which is a murine model for MS [6]. Human trials have also been performed in autoimmune diseases, MS, RA, and diabetes[11, 12]. Although clinical efficacy has been limited, and approval of an oral tolerance drug is still a distance away from reality, these trials continue to yield valuable information. For example, no systemic toxicity and exacerbation of diseases have been observed. Several small studies showed positive effects but also indicate a need for further optimization of dosage, scheduling of the regimen, and delivery methodologies [11, 12].
4. Delivery methods
The majority of the animal experiments and human trials were conducted using protein concentrates or solutions. Although the suppressions of immune responses from animal studies were dramatic and sufficient to protect from diseases, clinical trials in patients were generally of limited success. One possible explanation is that the doses of antigens applied to humans were low compared with that of mice, due to the large absorptive intestinal mucosal area [7, 173]. Thus one critical question to address in oral tolerance clinical applications is to enhance the efficacy or to obtain sufficient amounts of antigen for repeated oral administration.
Transgenic plants are an attractive system for the production of oral antigens. Plants are easy to produce for large scale-up at low cost with no need of expensive culture media. They are highly amenable to oral application without extensive protein purification. They are stable and easy to store. The protein and peptide synthesized in plants can be structurally and functionally identical to their native counterparts, and are protected by bioencapsulation. Indeed, many publications have reported the production of pharmaceutical proteins such as antibodies, cytokines, enzymes, vaccine antigens, hormones etc in plant cells. Some of them have entered clinical development stage. Transgenic plants including tobacco, rice, potato, lettuce, and tomato have all been created, either as a stable transgenic like nuclear system or transient expression using plant virus vectors [174]. Several interesting studies have tried plant material in oral tolerance therapy in animal models of allergies, rheumatoid arthritis, diabetes and hemophilia B. One study, using transgenic rice with mite allergen encapsulated in endoplasmic reticulum derived protein bodies in mice, markedly suppressed allergen-specific IgE and IgG production, allergen-induced CD4+ T cell proliferation and production of Th2 cytokines, infiltration of eosinophils, neutrophils, and mononuclear cells into the airways [175]. Another group generated transgenic rice with T cell epitopes of cedar pollen fused with cholera toxin B (CTB), suppressed allergen specific IgE responses and pollen induced clinical asthma symptoms [176]. Oral application of CTB-fused proinsulin fusion protein expressed in lettuce and tobacoo chloroplasts, significantly inhibited insulitis in NOD mice. Insulin producing β cells were greatly preserved; blood and urine glucose levels were reduced; and IL-4 and IL-10 were increased [173]. Feeding DBA/1 mice, which is a model of rheumatoid arthritis, with transgenic rice seeds of collagen type II peptides, lowered serum specific IgG2a responses against subsequent and repeated intraperitoneal injection of type II collagen [177]. Our studies utilized oral delivery of chloroplast transgenic (“transplastomic)” leaf material containing CTB-fused F.IX to control inhibitor formation and anaphylaxis to human F.IX in hemophilia B mice in a highly effective manner for at least 7 months, about 40% of the life span for the mouse strain used [32]. From these early plant studies, it appears that the effective dosage required for plant delivery system is considerably lower than for conventional protein or peptides concentrates, especially when the plant expressed protein or peptide were fused with a mucosal carrier like CTB. For example, 1 mg of porcine insulin twice a week for 5 weeks, and then weekly for 1 year was used to effectively suppress diabetes in NOD mice [178]; whereas only about 14 µg of the CTB-proinsulin fusion protein expressed in tobacco once a week for 7 weeks markedly suppressed insulitis in NOD mice [173]. In the hemophilia A mouse studies, 500 µg of F.VIII C2 peptide protein concentrations was used for oral administration to induce partial immune tolerance in hemophilia A mice model; whereas we used only 0.14 µg to 2 µg F.IX with CTB fusion expressed in tobacco induced complete oral tolerance in the hemophilia B mouse model [32, 172]. 100 µg collagen type II (250–270) synthetic peptide every other day for 20 days (total 1 mg) vs 25 µg same peptide expressed in rice seeds per day for 2 weeks (total 350 µg) successfully suppressed immune responses against type II collagen in DBA/1 mice [177, 179].
CTB, the nontoxic cholera toxin B subunit, is a potent antigen-adjuvant. It binds to GM1 ganglioside expressed on the live intestinal epithelial cells, and facilitates uptake via an actinand ATP-dependent processes [173, 180]. The binding of CTB does not alter the organization of the plasma membrane and has minimal effect on the diffusion of other molecules [180]. CTB-coupled antigens have been shown to suppress DC activation and promote induction of FoxP3+ Treg [181–183].
One of the reasons for requirement of lower dosage of protein bioencapsulated in plant cells is protection of antigens from degradation in the stomach by acids and enzymes [184]. Also, transplastomic plants are capable of very high levels of expression of therapeutic proteins, up to 70% of the total leaf protein [185]. In addition, multi-gene engineering to express several antigens in a single transformation event is a major advantage [185–187]. Plant cells can be lyophilized and stored at room temperature for several months or years without degradation of antigens or autoantigens [188]. Most importantly, harvest of leaves before emergence of reproductive structures and maternal inheritance of transformed chloroplast genomes containing genes coding for therapeutic proteins offer several layers of biological containment of transgenes [189]; such containment addresses one of the major environmental concerns in using genetically modified plants. Therefore, plant delivery system has several unique advantages in delivering autoantigens to confer oral tolerance.
Nanometer-sized particles like proteins and viruses are actively involved in diverse cellular activities. Recently, synthetic nanoparticles were developed to transport drugs and proteins of interest into target cells. Oral formulation of nanoparticles encapsulates drugs or proteins, protects and releases them in a temporally or spatially controlled manner. Particle size, surface charge, and surface chemistry all influence delivery efficacy. The particle surface can be modified or coated to enhancing specific targeting. For example, coating insulin-nanoparticles with pH sensitive material or conjugating with vitamin B-12 or derivatives can greatly enhance insulin absorption and bioavailability. To date, the well recognized oral nanoparticle formulation is complex, multilayered, mucoadhesive, biodegradable, biocompatible, and acid-protected [190]. A few oral tolerance studies have used nanoparticle as a delivery method. Polylactic-co-glycolic acid (PLGA) nanopartilces delivered collagen type II protected experimental arthritis in mice [191]. Another study used poly-ethylene glycol (PEP) conjugated collagen peptides suppressed collagen-induced arthritis [192]. Besides protein, peptide or drugs, cDNA can be effectively delivered using chitosan, which is a cationic polymer interacts with cDNA via electorstatic interactions, protects entrapped cDNA from digestion and facilitates gut delivery. Using chitosan-based nanoparticles, F.VIII DNA was successfully delivered into hemophilia A knockout mice with functional F.VIII protein detected in plasma at a peak level of 2–4% F.VIII activity. 13 out of 30 mice showed a phenotypic correction in bleeding challenge [193]. Another study, using canine F.VIII cDNA formulated in chitosan, provided transient F.VIII activity that was however sustainable upon re-administration. No neutralizing F.VIII antibody was detected, suggesting oral tolerance involvement [194].
In conclusion, elucidation of the mechanisms of oral tolerance will further facilitate the success of oral tolerance as a means of controlling or preventing immune responses to therapeutic proteins. Further investigations are needed in several areas such as the characterization of therapeutic antigens, modes of antigen processing and presentation, costimulatory requirements, induction of cytokine responses, and others. In this context, murine studies may be supplemented with translational studies in large animal models of disease. As we understand more, we will get closer to effective clinical development of oral tolerance in treatment of human diseases. For example, targeting pivotal antigen presentation cells, adding specific adjuvants, co-administration of cytokines such as IL-10 or TGF-β, optimization of oral antigen delivery, its dosage and feeding frequency, all seem promising aspects that would greatly improve efficacy of oral tolerance in human disease therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xiaomei Wang, Email: wangxiaomei@ufl.edu.
Alexandra Sherman, Email: alex2309@ufl.edu.
Gongxian Liao, Email: gliao@bidmc.harvard.edu.
Kam W. Leong, Email: kam.leong@duke.edu.
Henry Daniell, Email: Henry.Daniell@ucf.edu.
Cox Terhorst, Email: cterhors@bidmc.harvard.edu.
Roland W Herzog, Email: rherzog@ufl.edu.
References
- 1.Moog F. The lining of the small intestine. Sci Am. 1981;245:154–158. doi: 10.1038/scientificamerican1181-154. 160, 162 et passiom. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–S18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl. 1997;222:3–9. doi: 10.1080/00365521.1997.11720708. [DOI] [PubMed] [Google Scholar]
- 4.du Pre MF, Samsom JN. Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy. 2011;66:478–490. doi: 10.1111/j.1398-9995.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 5.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 6.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci. 2004;1029:211–224. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- 8.Dimichele D, Rivard G, Hay C, Antunes S. Inhibitors in haemophilia: clinical aspects. Haemophilia. 2004;10(Suppl 4):140–145. doi: 10.1111/j.1365-2516.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 9.DiMichele DM. Inhibitors in haemophilia: a primer. Haemophilia. 2000;6(Suppl 1):38–40. doi: 10.1046/j.1365-2516.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS. Pompe disease gene therapy. Hum Mol Genet. 2011;20:R61–R68. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner HL, Mayer LF, Strober W. Oral tolerance : new insights and prospects for clinical application. New York Academy of Sciences, New York N.Y. 2004 [Google Scholar]
- 13.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 14.Brandtzaeg P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol. 2010;7:380–400. doi: 10.1038/nrgastro.2010.80. [DOI] [PubMed] [Google Scholar]
- 15.Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–577. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Sheridan BS, Lefrancois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep. 2010;12:513–521. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618. [PubMed] [Google Scholar]
- 18.Nahmias A, Stoll B, Hale E, Ibegbu C, Keyserling H, Innis-Whitehouse W, Holmes R, Spira T, Czerkinsky C, Lee F. IgA-secreting cells in the blood of premature and term infants: normal development and effect of intrauterine infections. Adv Exp Med Biol. 1991;310:59–69. doi: 10.1007/978-1-4615-3838-7_6. [DOI] [PubMed] [Google Scholar]
- 19.Crabbe PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970;22:448–457. [PubMed] [Google Scholar]
- 20.Menezes JS, Mucida DS, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, de Faria AM. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2003;15:447–455. doi: 10.1093/intimm/dxg043. [DOI] [PubMed] [Google Scholar]
- 21.Barnes MJ, Powrie F. Immunology. The gut's Clostridium cocktail. Science. 2011;331:289–290. doi: 10.1126/science.1201291. [DOI] [PubMed] [Google Scholar]
- 22.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Ivanov S Hori, II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32:1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolvers DA, Coenen-de Roo CJ, Mebius RE, van der Cammen MJ, Tirion F, Miltenburg AM, Kraal G. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol. 1999;162:1994–1998. [PubMed] [Google Scholar]
- 27.Peron JP, de Oliveira AP, Rizzo LV. It takes guts for tolerance: the phenomenon of oral tolerance and the regulation of autoimmune response. Autoimmun Rev. 2009;9:1–4. doi: 10.1016/j.autrev.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13:485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 30.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–1028. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 31.La Terza A, Miceli C, Luporini P. Differential amplification of pheromone genes of the ciliate Euplotes raikovi. Dev Genet. 1995;17:272–279. doi: 10.1002/dvg.1020170312. [DOI] [PubMed] [Google Scholar]
- 32.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci U S A. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdinc B, Neufeld RJ. Protein micro and nanoencapsulation within glycolchitosan/Ca(2)+/alginate matrix by spray drying. Drug development and industrial pharmacy. 2011;37:619–627. doi: 10.3109/03639045.2010.533681. [DOI] [PubMed] [Google Scholar]
- 34.Awaad A, Nakamura M, Ishimura K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer's patches using fluorescent organosilica particles. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:627–636. doi: 10.1016/j.nano.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharmaceutical research. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 36.Mowat AM. Dendritic cells and immune responses to orally administered antigens. Vaccine. 2005;23:1797–1799. doi: 10.1016/j.vaccine.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 38.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 39.Everson MP, Lemak DG, McGhee JR, Beagley KW. FACS-sorted spleen and Peyer's patch dendritic cells induce different responses in Th0 clones. Adv Exp Med Biol. 1997;417:357–362. doi: 10.1007/978-1-4757-9966-8_58. [DOI] [PubMed] [Google Scholar]
- 40.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 41.Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 42.Smith KM, Davidson JM, Garside P. T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology. 2002;106:144–158. doi: 10.1046/j.1365-2567.2002.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 44.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol. 2006;176:1481–1489. doi: 10.4049/jimmunol.176.3.1481. [DOI] [PubMed] [Google Scholar]
- 45.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med. 2007;204:1519–1524. doi: 10.1084/jem.20062292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 51.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 52.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17- producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 53.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P. 'Educated' dendritic cells act as messengers from memory to naive T helper cells. Nat Immunol. 2004;5:615–622. doi: 10.1038/ni1077. [DOI] [PubMed] [Google Scholar]
- 55.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 56.Kay RA, Ferguson A. The immunological consequences of feeding cholera toxin. I. Feeding cholera toxin suppresses the induction of systemic delayed-type hypersensitivity but not humoral immunity. Immunology. 1989;66:410–415. [PMC free article] [PubMed] [Google Scholar]
- 57.Mowat AM, Steel M, Worthey EA, Kewin PJ, Garside P. Inactivation of Th1 and Th2 cells by feeding ovalbumin. Ann N Y Acad Sci. 1996;778:122–132. doi: 10.1111/j.1749-6632.1996.tb21121.x. [DOI] [PubMed] [Google Scholar]
- 58.Hanson DG, Miller SD. Inhibition of specific immune responses by feeding protein antigens. V. Induction of the tolerant state in the absence of specific suppressor T cells. J Immunol. 1982;128:2378–2381. [PubMed] [Google Scholar]
- 59.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 60.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 61.Moller G. Do suppressor T cells exist? Scand J Immunol. 1988;27:247–250. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 62.Stockinger B, Barthlott T, Kassiotis G. T cell regulation: a special job or everyone's responsibility? Nat Immunol. 2001;2:757–758. doi: 10.1038/ni0901-757. [DOI] [PubMed] [Google Scholar]
- 63.Garside P, Steel M, Worthey EA, Kewin PJ, Howie SE, Harrison DJ, Bishop D, Mowat AM. Lymphocytes from orally tolerized mice display enhanced susceptibility to death by apoptosis when cultured in the absence of antigen in vitro. Am J Pathol. 1996;149:1971–1979. [PMC free article] [PubMed] [Google Scholar]
- 64.Boirivant M, Pica R, DeMaria R, Testi R, Pallone F, Strober W. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J Clin Invest. 1996;98:2616–2622. doi: 10.1172/JCI119082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 66.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, Wahl SM. Requirement for transforming growth factor beta1 in controlling T cell apoptosis. J Exp Med. 2001;194:439–453. doi: 10.1084/jem.194.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 69.Taams LS, van Rensen AJ, Poelen MC, van Els CA, Besseling AC, Wagenaar JP, van Eden W, Wauben MH. Anergic T cells actively suppress T cell responses via the antigen-presenting cell. Eur J Immunol. 1998;28:2902–2912. doi: 10.1002/(SICI)1521-4141(199809)28:09<2902::AID-IMMU2902>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Cauley LS, Cauley KA, Shub F, Huston G, Swain SL. Transferable anergy: superantigen treatment induces CD4+ T cell tolerance that is reversible and requires CD4-CD8- cells and interferon gamma. J Exp Med. 1997;186:71–81. doi: 10.1084/jem.186.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirahara K, Hisatsune T, Nishijima K, Kato H, Shiho O, Kaminogawa S. CD4+ T cells anergized by high dose feeding establish oral tolerance to antibody responses when transferred in SCID and nude mice. J Immunol. 1995;154:6238–6245. [PubMed] [Google Scholar]
- 72.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 73.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 74.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Treg. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauet-Broere F, Unger WW, Garssen J, Hoijer MA, Kraal G, Samsom JN. Functional CD25- and CD25+ mucosal regulatory T cells are induced in gut-draining lymphoid tissue within 48 h after oral antigen application. Eur J Immunol. 2003;33:2801–2810. doi: 10.1002/eji.200324115. [DOI] [PubMed] [Google Scholar]
- 76.Nagatani K, Sagawa K, Komagata Y, Yamamoto K. Peyer's patch dendritic cells capturing oral antigen interact with antigen-specific T cells and induce gut-homing CD4(+)CD25(+) regulatory T cells in Peyer's patches. Ann N Y Acad Sci. 2004;1029:366–370. doi: 10.1196/annals.1309.020. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 78.Tsuji NM, Nowak B. IL-18 and antigen-specific CD4(+) regulatory T cells in Peyer's patches. Ann N Y Acad Sci. 2004;1029:413–415. doi: 10.1196/annals.1309.049. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 80.Tsuji NM, Mizumachi K, Kurisaki J. Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology. 2001;103:458–464. doi: 10.1046/j.1365-2567.2001.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Reddy J, Ochi H, Frenkel D, Kuchroo VK, Weiner HL. Recovery from experimental allergic encephalomyelitis is TGF-beta dependent and associated with increases in CD4+LAP+ and CD4+CD25+ T cells. Int Immunol. 2006;18:495–503. doi: 10.1093/intimm/dxh390. [DOI] [PubMed] [Google Scholar]
- 84.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–1526. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 85.Arnaboldi PM, Roth-Walter F, Mayer L. Suppression of Th1 and Th17, but not Th2, responses in a CD8(+) T cell-mediated model of oral tolerance. Mucosal Immunol. 2009;2:427–438. doi: 10.1038/mi.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 87.Barone KS, Jain SL, Michael JG. Effect of in vivo depletion of CD4+ and CD8+ cells on the induction and maintenance of oral tolerance. Cell Immunol. 1995;163:19–29. doi: 10.1006/cimm.1995.1094. [DOI] [PubMed] [Google Scholar]
- 88.Garside P, Steel M, Liew FY, Mowat AM. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995;7:501–504. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- 89.Wahl SM, Wen J, Moutsopoulos N. TGF-beta: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 90.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 91.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 93.Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 94.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lundin BS, Karlsson MR, Svensson LA, Hanson LA, Dahlgren UI, Telemo E. Active suppression in orally tolerized rats coincides with in situ transforming growth factor-beta (TGF-beta) expression in the draining lymph nodes. Clin Exp Immunol. 1999;116:181–187. doi: 10.1046/j.1365-2249.1999.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barone KS, Tolarova DD, Ormsby I, Doetschman T, Michael JG. Induction of oral tolerance in TGF-beta 1 null mice. J Immunol. 1998;161:154–160. [PubMed] [Google Scholar]
- 104.Barnard JA, Warwick GJ, Gold LI. Localization of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 105.Galliaerde V, Desvignes C, Peyron E, Kaiserlian D. Oral tolerance to haptens: intestinal epithelial cells from 2,4-dinitrochlorobenzene-fed mice inhibit hapten-specific T cell activation in vitro. Eur J Immunol. 1995;25:1385–1390. doi: 10.1002/eji.1830250537. [DOI] [PubMed] [Google Scholar]
- 106.Weiner HL, Gonnella PA, Slavin A, Maron R. Oral tolerance: cytokine milieu in the gut and modulation of tolerance by cytokines. Res Immunol. 1997;148:528–533. doi: 10.1016/s0923-2494(98)80146-6. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CA, Jr., Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci U S A. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolvers DA, van der Cammen MJ, Kraal G. Mucosal tolerance is associated with, but independent of, up-regulation Th2 responses. Immunology. 1997;92:328–333. doi: 10.1046/j.1365-2567.1997.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 111.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 112.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 113.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 114.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Battaglia M, Gianfrani C, Gregori S, Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann N Y Acad Sci. 2004;1029:142–153. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 117.Fowler S, Powrie F. CTLA-4 expression on antigen-specific cells but not IL-10 secretion is required for oral tolerance. Eur J Immunol. 2002;32:2997–3006. doi: 10.1002/1521-4141(2002010)32:10<2997::AID-IMMU2997>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 118.Rizzo LV, Morawetz RA, Miller-Rivero NE, Choi R, Wiggert B, Chan CC, Morse HC, 3rd, Nussenblatt RB, Caspi RR. IL-4 and IL-10 are both required for the induction of oral tolerance. J Immunol. 1999;162:2613–2622. [PubMed] [Google Scholar]
- 119.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGFbeta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 121.Lee HO, Miller SD, Hurst SD, Tan LJ, Cooper CJ, Barrett TA. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology. 2000;119:129–138. doi: 10.1053/gast.2000.8542. [DOI] [PubMed] [Google Scholar]
- 122.Kweon MN, Fujihashi K, VanCott JL, Higuchi K, Yamamoto M, McGhee JR, Kiyono H. Lack of orally induced systemic unresponsiveness in IFN-gamma knockout mice. J Immunol. 1998;160:1687–1693. [PubMed] [Google Scholar]
- 123.Kjerrulf M, Grdic D, Ekman L, Schon K, Vajdy M, Lycke NY. Interferon-gamma receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology. 1997;92:60–68. doi: 10.1046/j.1365-2567.1997.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mowat AM, Steel M, Leishman AJ, Garside P. Normal induction of oral tolerance in the absence of a functional IL-12-dependent IFN-gamma signaling pathway. J Immunol. 1999;163:4728–4736. [PubMed] [Google Scholar]
- 125.Wang GY, Sun B, Kong QF, Zhang Y, Li R, Wang JH, Wang DD, Lv GX, Li HL. IL-17 eliminates the therapeutic effects of myelin basic protein-induced nasal tolerance in experimental autoimmune encephalomyelitis by activating IL-6. Scand J Immunol. 2008;68:589–597. doi: 10.1111/j.1365-3083.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 126.Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- 127.Broere F, du Pre MF, van Berkel LA, Garssen J, Schmidt-Weber CB, Lambrecht BN, Hendriks RW, Nieuwenhuis EE, Kraal G, Samsom JN. Cyclooxygenase-2 in mucosal DC mediates induction of regulatory T cells in the intestine through suppression of IL-4. Mucosal Immunol. 2009;2:254–264. doi: 10.1038/mi.2009.2. [DOI] [PubMed] [Google Scholar]
- 128.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 129.Chang SY, Cha HR, Chang JH, Ko HJ, Yang H, Malissen B, Iwata M, Kweon MN. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology. 2010;138:1468–1478. doi: 10.1053/j.gastro.2009.11.006. 1478 e1461-1466. [DOI] [PubMed] [Google Scholar]
- 130.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 131.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 132.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 133.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 134.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cantorna MT, Nashold FE, Chun TY, Hayes CE. Vitamin A down-regulation of IFN-gamma synthesis in cloned mouse Th1 lymphocytes depends on the CD28 costimulatory pathway. J Immunol. 1996;156:2674–2679. [PubMed] [Google Scholar]
- 138.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 142.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 144.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 145.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 148.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 149.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 150.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 151.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 152.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 153.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 154.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 157.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 158.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou L, Lopes JE, Ivanov MM Chong, II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 161.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 162.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 163.Liao G, Nayak S, Regueiro JR, Berger SB, Detre C, Romero X, de Waal Malefyt R, Chatila TA, Herzog RW, Terhorst C. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int Immunol. 2010;22:259–270. doi: 10.1093/intimm/dxq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Olffen RW, Koning N, van Gisbergen KP, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RA, Nolte MA. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J Immunol. 2009;182:7490–7500. doi: 10.4049/jimmunol.0802751. [DOI] [PubMed] [Google Scholar]
- 165.Gongxian Liao CD, Berger Scott B, Engel Pablo, Malefyt Rene de Waal, Herzog Roland W, Bhan Atul K, Terhorst Cox. Glucocorticoid-Induced Tumor Necrosis Factor Receptor Family-Related Protein Regulates CD4(+)T Cell-Mediated Colitis in Mice. Gastroenterology. 2012 Mar;142(3):582–591. doi: 10.1053/j.gastro.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 167.Herzog RW, Dobrzynski E. Immune implications of gene therapy for hemophilia. Semin Thromb Hemost. 2004;30:215–226. doi: 10.1055/s-2004-825635. [DOI] [PubMed] [Google Scholar]
- 168.Herzog RW, High KA. Problems and prospects in gene therapy for hemophilia. Curr Opin Hematol. 1998;5:321–326. doi: 10.1097/00062752-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 169.Ohashi T, Iizuka S, Shimada Y, Eto Y, Ida H, Hachimura S, Kobayashi H. Oral administration of recombinant human acid alpha-glucosidase reduces specific antibody formation against enzyme in mouse. Mol Genet Metab. 2011;103:98–100. doi: 10.1016/j.ymgme.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 170.Lindgren A, Wadenvik H, Tarkowski A, Tengborn L. Does peroral administration of factor VIII induce oral tolerance in patients with acquired haemophilia A? Thromb Haemost. 2000;83:632–633. [PubMed] [Google Scholar]
- 171.Terada K, Yagi Y, Niizuma T, Kataoka N. Is oral tolerance therapy possible for haemophilia with inhibitors? Vox Sang. 2001;80:61–62. doi: 10.1046/j.1423-0410.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 172.Rawle FE, Pratt KP, Labelle A, Weiner HL, Hough C, Lillicrap D. Induction of partial immune tolerance to factor VIII through prior mucosal exposure to the factor VIII C2 domain. J Thromb Haemost. 2006;4:2172–2179. doi: 10.1111/j.1538-7836.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 173.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts--oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lico C, Santi L, Twyman RM, Pezzotti M, Avesani L. The use of plants for the production of therapeutic human peptides. Plant Cell Rep. 2012;31:439–451. doi: 10.1007/s00299-011-1215-7. [DOI] [PubMed] [Google Scholar]
- 175.Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, Ohtomo T, Ohmachi Y, Noda Y, Hirose S, Okumura K, Ogawa H, Takada K, Hirasawa M, Hiroi T, Takaiwa F. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol J. 2011;9:982–990. doi: 10.1111/j.1467-7652.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- 176.Takagi H, Hiroi T, Yang L, Takamura K, Ishimitsu R, Kawauchi H, Takaiwa F. Efficient induction of oral tolerance by fusing cholera toxin B subunit with allergen-specific T-cell epitopes accumulated in rice seed. Vaccine. 2008;26:6027–6030. doi: 10.1016/j.vaccine.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 177.Hashizume F, Hino S, Kakehashi M, Okajima T, Nadano D, Aoki N, Matsuda T. Development and evaluation of transgenic rice seeds accumulating a type II-collagen tolerogenic peptide. Transgenic Res. 2008;17:1117–1129. doi: 10.1007/s11248-008-9187-2. [DOI] [PubMed] [Google Scholar]
- 178.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991;88:10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Khare SD, Krco CJ, Griffiths MM, Luthra HS, David CS. Oral administration of an immunodominant human collagen peptide modulates collagen-induced arthritis. J Immunol. 1995;155:3653–3659. [PubMed] [Google Scholar]
- 180.Day CA, Kenworthy AK. Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes. PLoS One. 2012;7:e34923. doi: 10.1371/journal.pone.0034923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Odumosu O, Nicholas D, Payne K, Langridge W. Cholera toxin B subunit linked to glutamic acid decarboxylase suppresses dendritic cell maturation and function. Vaccine. 2011;29:8451–8458. doi: 10.1016/j.vaccine.2011.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Odumosu O, Payne K, Baez I, Jutzy J, Wall N, Langridge W. Suppression of dendritic cell activation by diabetes autoantigens linked to the cholera toxin B subunit. Immunobiology. 2011;216:447–456. doi: 10.1016/j.imbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun JB, Czerkinsky C, Holmgren J. B lymphocytes treated in vitro with antigen coupled to cholera toxin B subunit induce antigen-specific Foxp3(+) regulatory T cells and protect against experimental autoimmune encephalomyelitis. J Immunol. 2012;188:1686–1697. doi: 10.4049/jimmunol.1101771. [DOI] [PubMed] [Google Scholar]
- 184.Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–961. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kanagaraj AP, Verma D, Daniell H. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol. 2011;76:323–333. doi: 10.1007/s11103-011-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2012 doi: 10.1111/pbi.12008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc Natl Acad Sci U S A. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim WU, Lee WK, Ryoo JW, Kim SH, Kim J, Youn J, Min SY, Bae EY, Hwang SY, Park SH, Cho CS, Park JS, Kim HY. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109–1120. doi: 10.1002/art.10198. [DOI] [PubMed] [Google Scholar]
- 192.Lee WK, Park JY, Jung S, Yang CW, Kim WU, Kim HY, Park JH, Park JS. Preparation and characterization of biodegradable nanoparticles entrapping immunodominant peptide conjugated with PEG for oral tolerance induction. J Control Release. 2005;105:77–88. doi: 10.1016/j.jconrel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 193.Bowman K, Sarkar R, Raut S, Leong KW. Gene transfer to hemophilia A mice via oral delivery of FVIII-chitosan nanoparticles. J Control Release. 2008;132:252–259. doi: 10.1016/j.jconrel.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dhadwar SS, Kiernan J, Wen J, Hortelano G. Repeated oral administration of chitosan/DNA nanoparticles delivers functional FVIII with the absence of antibodies in hemophilia A mice. J Thromb Haemost. 2010;8:2743–2750. doi: 10.1111/j.1538-7836.2010.04116.x. [DOI] [PubMed] [Google Scholar]
- 195.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 196.Kaplan J, Genyea C, Secord E. Factor VIII inhibitors. Potential for prevention of inhibitor formation by immune tolerance. Semin Thromb Hemost. 2000;26:173–178. doi: 10.1055/s-2000-9820. [DOI] [PubMed] [Google Scholar]
- 197.Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 198.Ergun-Longmire B, Marker J, Zeidler A, Rapaport R, Raskin P, Bode B, Schatz D, Vargas A, Rogers D, Schwartz S, Malone J, Krischer J, Maclaren NK. Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci. 2004;1029:260–277. doi: 10.1196/annals.1309.057. [DOI] [PubMed] [Google Scholar]
- 199.Monetini L, Cavallo MG, Sarugeri E, Sentinelli F, Stefanini L, Bosi E, Thorpe R, Pozzilli P. Cytokine profile and insulin antibody IgG subclasses in patients with recent onset type 1 diabetes treated with oral insulin. Diabetologia. 2004;47:1795–1802. doi: 10.1007/s00125-004-1521-5. [DOI] [PubMed] [Google Scholar]
- 200.Meng Q, Wang W, Shi X, Jin Y, Zhang Y. Protection against autoimmune diabetes by silkworm-produced GFP-tagged CTB-insulin fusion protein. Clin Dev Immunol. 2011;2011:831704. doi: 10.1155/2011/831704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 202.Ma SW, Zhao DL, Yin ZQ, Mukherjee R, Singh B, Qin HY, Stiller CR, Jevnikar AM. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat Med. 1997;3:793–796. doi: 10.1038/nm0797-793. [DOI] [PubMed] [Google Scholar]
- 203.Bergerot I, Arreaza GA, Cameron MJ, Burdick MD, Strieter RM, Chensue SW, Chakrabarti S, Delovitch TL. Insulin B-chain reactive CD4+ regulatory T-cells induced by oral insulin treatment protect from type 1 diabetes by blocking the cytokine secretion and pancreatic infiltration of diabetogenic effector T-cells. Diabetes. 1999;48:1720–1729. doi: 10.2337/diabetes.48.9.1720. [DOI] [PubMed] [Google Scholar]
- 204.Polanski M, Melican NS, Zhang J, Weiner HL. Oral administration of the immunodominant B-chain of insulin reduces diabetes in a co-transfer model of diabetes in the NOD mouse and is associated with a switch from Th1 to Th2 cytokines. J Autoimmun. 1997;10:339–346. doi: 10.1006/jaut.1997.0148. [DOI] [PubMed] [Google Scholar]
- 205.Hancock WW, Polanski M, Zhang J, Blogg N, Weiner HL. Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration is associated with selective expression of interleukin-4 and -10, transforming growth factor-beta, and prostaglandin-E. Am J Pathol. 1995;147:1193–1199. [PMC free article] [PubMed] [Google Scholar]
- 206.Mordes JP, Schirf B, Roipko D, Greiner DL, Weiner H, Nelson P, Rossini AA. Oral insulin does not prevent insulin-dependent diabetes mellitus in BB rats. Ann N Y Acad Sci. 1996;778:418–421. doi: 10.1111/j.1749-6632.1996.tb21161.x. [DOI] [PubMed] [Google Scholar]
- 207.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, Scurlock AM, Shreffler WG, Plaut M, Sampson HA. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]