Abstract
Wolbachia are obligate intracellular bacteria that cause cytoplasmic incompatibility in mosquitoes. In an incompatible cross, eggs of uninfected females fail to hatch when fertilized by sperm from infected males. We used polyacrylamide gel electrophoresis and tandem mass spectrometry to identify Wolbachia proteins in infected mosquito gonads. These included surface proteins with masses of 25 and 18 kDa and the DNA binding protein, HU beta. Using reverse transcriptase polymerase chain reaction, we showed that the HU gene is transcribed in Wolbachia-infected Culex pipiens and Aedes albopictus mosquitoes. We sequenced HU genes from four Wolbachia strains and compared deduced protein sequences with additional homologs from the databases. Among the Rickettsiales, Wolbachia HU has distinct N- and C-terminal basic/acidic amino acid motifs as well as a pair of conserved, cysteine residues.
1. INTRODUCTION
Hertig (1936) first described Wolbachia as pleomorphic rods and coccoid bodies in stained gonad smears of Culex pipiens mosquitoes. These Gram-negative, alpha proteobacteria, now known as Wolbachia pipientis, are classified in the family Anaplasmataceae, order Rickettsiales, and infect many orders of insects. Wolbachia manipulate and distort insect reproduction, causing cytoplasmic incompatibility (CI), parthenogenesis, feminization, and male-killing (Serbus et al., 2008). These reproductive distortions skew offspring ratios in a way that provides a reproductive advantage to females infected by Wolbachia, facilitating the organism’s spread through naïve insect populations. Wolbachia-mediated CI represents a unique tool for manipulating mosquito populations, with potential applications such as vector life shortening (McMeniman et al., 2009), gene drive and population replacement (Rasgon et al., 2006) and reduction of vector competence (Frentiu et al., 2010). An understanding of the molecular basis for CI would represent an important advance towards these goals.
In mosquitoes, CI is a form of conditional sterility that occurs when sperm from Wolbachia-infected males fertilize eggs from uninfected females. CI is thought to result from a Wolbachia-mediated modification on developing spermatocytes/spermatids (Clark et al., 2003) that can be rescued when the male pronucleus matures in the cytoplasm of eggs from infected females. In eggs from uninfected females, sperm modification in the absence of the rescue factor disrupts cell cycle synchrony between male and female pronuclei (Serbus et al., 2008; Callaini et al., 1997); in diploid insects such as mosquitoes, eggs from a CI cross fail to hatch.
Although molecules that mediate CI have not been discovered, several lines of evidence are consistent with the hypothesis that Wolbachia secrete one or more effector protein(s) that associates with sperm DNA and interferes with male pronuclear chromatin architecture. Presgraves (2000) showed that the CI effect in Drosophila originates from a modification on paternal chromatin. Landmann et al. (2009) then showed that CI in Drosophila is associated with impaired ability to deposit maternal histones on male pronuclear chromatin. High CI expression correlates with high Wolbachia load in testes (Clark et al., 2003; Clark et al., 2002), which would conceivably raise the concentration of an effector protein, making CI more potent. Increased copulation lowers CI rates, implying depletion of an effector molecule as new sperm develop (Karr et al., 1998). Wolbachia genomes encode all of the components of the bacterial type IV secretion system (T4SS), which mediates extracellular export of proteins and DNA (Rances et al., 2005). Moreover, in sperm and ovarian tissues Wolbachia often localize around the nucleus and directly contact the nuclear envelope, consistent with the possibility that they secrete molecules into the nucleus (Ferree et al., 2005; Clark et al., 2002; 2003).
The link between CI potency and Wolbachia density argues against the secretion or activation of a signaling molecule or transcription factor needed at low concentrations. More likely would be secretion of a protein whose global concentration directly causes CI; such a protein might be expressed at high enough levels to be detected by mass spectrometry, so long as one could acquire enough tissue with a high Wolbachia infection. An argument against a secreted effector protein is the observation that in Nasonia vitripennis, Wolbachia do not need to be present in the germline to induce CI (Clark et al., 2008). However, this point is countered by the fact that in such cases, Wolbachia heavily infect the somatic cyst cells surrounding the developing sperm. In Drosophila, these cyst cells are linked directly to the developing sperm by gap junctions that potentially mediate passage of chemical effector molecules (Kiger et al., 2000; Tazuke et al., 2002).
Despite the possibility of a proteomic basis for CI, mass spectrometry-based approaches to study of Wolbachia infections have been underutilized. Using SDS PAGE and tandem mass spectrometry we identified protein bands with masses of approximately 25 and 18 kDa that were present in gonads of Culex pipiens mosquitoes infected with Wolbachia pipientis, wPip, but absent in uninfected tissues. Among smaller protein bands, we detected a Wolbachia protein (gi|190571020) homologous to the DNA-binding protein, HU beta, in Escherichia coli. Wolbachia HU beta is transcribed in both Culex pipiens and Aedes albopictus infected mosquitoes, further showing that the protein is present in-vivo and that it is expressed by both wPip and wAlbA/B strains of Wolbachia.
2. EXPERIMENTAL PROCEDURES
2.1 Mosquitoes and other insects
Colonies of Culex pipiens pipiens (Buckeye strain) mosquitoes were maintained at 25°C as described previously (Beckmann and Fallon, 2012). Larvae were fed pulverized rat chow and yeast. Adults were allowed to feed on 10% sucrose in water. Cx. pipiens mosquitoes are naturally infected with wPip. A cured colony of mosquitoes was established by tetracycline treatment. Infection status was verified by PCR as detailed previously (Beckmann and Fallon, 2012). Aedes albopictus mosquitoes (Houston strain, doubly infected with wAlbA and wAlbB) were generously provided by Dr. S. L. Dobson (University of Kentucky). Bedbugs (Cimex lectularius) were provided by Dr. S. Kells (Department of Entomology, University of Minnesota) and their Wolbachia infection is designated wLec. Wolbachia from the planthopper Laodelphax striatellus (wStr) originated from an infected Ae. albopictus AeAl2 cell line (Noda, 2002).
2.2 Protein extraction
Testes (150) or ovaries (30) were dissected in 100% ethanol and collected in a 1.5 ml tube filled with 100% ethanol, which prevented tissues from sticking to the metal dissecting tools. Pooled tissues were sonicated at 40 mA for 10 seconds in a Kontes GE 70.1 ultrasonic processor, and trichloroacetic acid (TCA) was added to a final concentration of 10% (v/v). After centrifugation at 13,000 rpm in a microcentrifuge, the resulting pellets were washed with acetone:water (9:1), dried, and stored at −20°C.
2.3 SDS PAGE and mass spectrometry
Protein samples were reconstituted in SDS sample buffer and boiled prior to electrophoresis, which was usually conducted on 8–18% gradient polyacrylamide gels. Protein gels were submitted to the University of Minnesota’s Center for Mass Spectrometry and Proteomics for gel staining with Deep Purple (GE Healthcare), imaging, and in-gel trypsin digestion as described by Anderson et al. (2010). Tryptic peptides were rehydrated in water/acetonitrile (ACN)/formic acid (FA) 98:2:0.1 and loaded using a Paradigm AS1 autosampler system (Michrom Bioresources, Inc., Auburn, CA). Each sample was subjected to Paradigm Platinum Peptide Nanotrap (Michrom Bioresources, Inc.) pre-column (0.15×50 mm, 400-μl volume) followed by an analytical capillary column (100 μm×12 cm) packed with C18 resin (5 μm, 200 Å MagicC18AG, Michrom Bioresources, Inc.) at a flow rate of 250 nl/min. Peptides were fractionated on a 60 min (10– 40% ACN) gradient on a MS4 flow splitter (Michrom Bioresources, Inc.).
Mass spectrometry (MS) was performed on an LTQ (Thermo Electron Corp., San Jose, CA). Ionized peptides eluting from the capillary column were subjected to an ionizing voltage (2.0 kV) and selected for MS/MS using a data-dependent procedure alternating between an MS scan followed by five MS/MS scans for the five most abundant precursor ions. Tandem mass spectra were extracted by Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 27, rev. 12. Charge state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 27, rev. 12). Sequest was set up to search an rs_wolbachia_aedes_v200808_cRAP_flavivirusREV database (containing protein entries from sequenced Wolbachia genomes, the Aedes aegypti genome, and flavivirus genomes available as of July, 2011, 74570 entries) assuming the digestion enzyme trypsin and specifying two missed trypsin cleavage sites and one non-tryptic terminus. Sequest was searched with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 1.00 Da. Iodoacetamide derivative of cysteine was specified in Sequest as a fixed modification, and oxidation of methionine was specified as a variable modification. Scaffold (version Scaffold_3.6.0, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at greater than 95.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Peptide results were only reported under the condition that they met the following criteria: 1) molecular weight matches the apparent migration on gels, 2) the protein should contain at least 3 tryptic peptides identified by mass spectrometry from the database, 3) the tandem spectra should contain at least three consecutive ions, ie., b5,b6,b7, for each reported peptide, and 4) all spectral peaks above background are assigned to the peptide. As a negative control a decoy-database was searched with all samples and no decoy protein hits were detected with greater than 20% protein probability.
2.4 DNA extraction and Polymerase Chain Reaction (PCR)
DNA was extracted from decapitated mosquitoes (Beckmann and Fallon, 2012) as described by Livak (1984). Infection status was monitored by PCR amplification with primers S12F and S7R as detailed previously (Beckmann and Fallon, 2012); hupB sequences were produced with primers HuCloneF: 5′ TGGGAATTCGAACAATATTAAGGTAATTTATGAG (near wPip rpsI gene at 664557-664578 of the Culex quinquefasciatus Pel wPip genome) and HuCloneR: 5′ TGGGAATTCGAACGAGGCTATATTTCATGGC (in wPip pdxJ at 664923-664941 of the Culex quinquefasciatus Pel wPip genome; underlined bases correspond to a BstBI restriction enzyme site added for cloning purposes). After an initial denaturation at 94 °C for 5 min, DNA was denatured at 94°C for 1 min, annealed at 52°C for 1 min, and extended at 72°C for 1 min for 35 cycles with a final extension at 72°C for 3 min. PCR products were electrophoresed on 2% agarose gels and visualized by ethidium bromide staining. PCR products were sequenced at the University of Minnesota Biomedical Genomics Center.
2.5 RNA extraction, DNase treatment and Reverse Transcriptase (RT) PCR
Cultured cells were pelleted by centrifugation at 800 rpm for 10 minutes, washed in phosphate-buffered saline, pelleted again by centrifugation at 800 rpm for 10 minutes and resuspended in ice cold lysis buffer (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, 0.5% (v/v) Nonidet P-40). Pools of 15 decapitated mosquitoes were frozen in liquid nitrogen and ground to powder. The powder was resuspended in 375 μl ice cold lysis buffer and held on ice for 5 minutes. Particulate material was pelleted by centrifugation at 4°C, 13,000 rpm and the supernatant was placed into a new tube. SDS (4 μl of a 20% stock) was added (a final concentration of 0.2%) and immediately mixed into the extract. Proteinase K (2.5 μl of 20 mg/ml stock) was added (a final concentration of 120 μg/ml) and incubated at 37°C for 15 minutes. RNA was then extracted twice with 400 μl phenol/chloroform/isoamyl alcohol (25:24:1) and once with chloroform/isoamyl (24:1). The aqueous phase was recovered, and 40 μl of 3 M sodium acetate, pH 5.2 was added, followed by 2 volumes of 100% ethanol. A precipitate was allowed to form overnight at −20°C. Pellets were washed in 70% ethanol, dried, and resuspended in water. Immediately prior to RT-PCR, samples were treated with Promega’s RQ1 RNase-Free DNase (catalog # M610A), according to the manufacturer’s instructions (Promega Corporation Madison, WI.) RT-PCR was carried out as described in the Applied Biosystems GeneAmp RNA PCR kit (catalog # N808-0017) with slight modifications. The initial annealing step for the reverse transcriptase reactions was done at 50°C for 5 min, the extension step was at 42°C for 1 h, and the reaction was terminated by heating at 99°C for 5 min. The primers used in the reverse transcriptase reaction to make cDNA were HuMosR and HuR (see below). PCR reactions had an initial denaturation at 94°C for 5 min, then 35 cycles of 94°C denaturing for 1 min, 50°C, 56°C, or 65°C annealing for 1 min, and extension at 72°C for 1 min. Primer pairs used in the PCR reactions were the wPip specific HU primers, HuF: 5′ AGGATCAGCTAAGTCGCAAAGGCG and HuR: 5′ ACCCTTGTCTTTTCAGGAACGGTC (56°C annealing), the Wolbachia HU primers designed to be conserved within Wolbachia that infect mosquitoes, HuMosF: 5′ ATGAGTAAAGAAGATATARTAAAC and HuMosR: 5′ TCATAATCTCACCATTTTGAG, (50°C annealing) and the mosquito ribosomal protein S3 (RpS3) primers 40S3F: 5′ ATGCCGAGAAGGTCGCCAC and 40S3R: 5′ GCACGGATCTCCGGAATGG (65°C annealing).
2.6 Sequence Alignments
Experimentally obtained DNA sequences were translated using the ExPASy translate tool, http://web.expasy.org/translate/, from the SIB Swiss Institute of Bioinformatics. Amino acid sequence alignments were constructed by using MUSCLE in the MEGA 5.05 software program. Alignments were performed under default settings with Gap penalties: Gap open −2.9, Gap Extend 0, Hydrophobicity Multiplier 1.2, and Memory/Iterations: Max Memory in MB 1686, Max Iteration 8 (Edgar, 2004). Sequence data from the Aedes albopictus sample potentially containing both wAlbB and wAlbA DNA was identical to the NCBI translated protein Reference Sequence: ZP_09542731.1 from wAlbB. Sequence data from wPip was identical to the published sequence, GenBank: AM999887.1; sequences from wStr and wLec are deposited under GenBank accession numbers JX984572 and JX984573, respectively.
3. RESULTS
3.1 Wolbachia membrane proteins in infected gonads
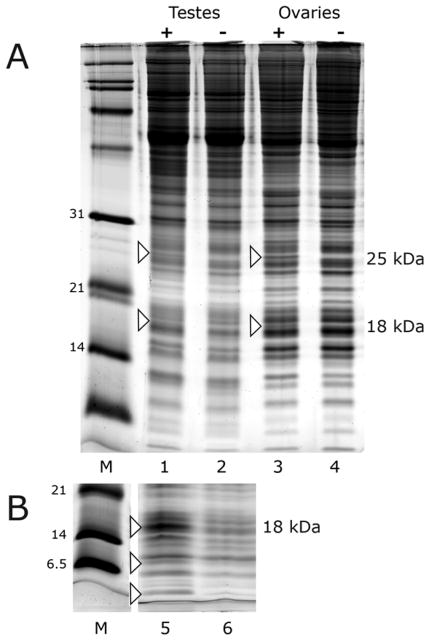
SDS polyacrylamide gradient gels were used to compare proteins in testes and ovaries from infected and tetracycline-cured Cx. pipiens mosquitoes (Fig. 1). The similar pattern of stained proteins in infected and cured tissues indicated that in mosquito reproductive tissues, Wolbachia infection is accompanied by little overall change in proteins detectable by visual inspection of stained polyacrylamide gels. Despite the overall similarity, bands at 25 and 18 kDa, unique to infected testes and ovaries, were observed in 8 biological replicates with independent pools of dissected tissues (Fig. 1A). Additional bands with masses below 14 kDa were also typical of infected testes (Fig. 1B) and ovaries (not shown). We began analysis by excising the 25 kDa and 18 kDa bands from gels, subjecting tryptic peptides to mass spectrometry, and assessing the presence of Wolbachia encoded products.
Figure 1.
Electrophoretic analysis of proteins extracted from Cx. pipiens gonads. Panel A. Lanes 1 and 2 are 150 individual testes from Cx. pipiens; lanes 3 and 4 are 30 individual ovaries. Lanes 1 and 3 (+) are from Wolbachia-infected mosquitoes; lanes 2 and 4 are from a tetracycline-cured sister colony. Panel B represents an independent biological replicate from testes of infected (lane 5) and cured (lane 6) Cx. pipiens. Protein bands at 25 kDa and 18 kDa were visible in 8 biological replicates. Lane 5 shows a better replicate of the 18 kDa band as well as smaller bands that were examined by mass spectrometry.
The most abundant protein in the 25 kDa band was the Wolbachia surface protein, WSP. The best replicate revealed a total of 44 peptides covering 48% of the total protein (Table 1 and Fig. 2A). Detection was repeated in five separate isolates. Not surprisingly, a smaller number of peptides from host proteins were also recovered from the 25 kDa band, including the ~ 25 kDa proteasome subunits and ubiquitin, previously found to be up-regulated when cultured cells were newly infected with wAlbB (Fallon and Witthuhn, 2009). The 18 kDa band contained 26 peptides covering 36% of a “putative Wolbachia membrane protein” in the best replicate (Figs. 1A and 2B). Detection of this membrane protein was repeated in two biological samples. In aggregate, these data reveal that abundantly expressed Wolbachia surface proteins can be identified by gel electrophoresis and mass spectrometry against a background of host proteins.
Table 1.
Wolbachia protein identities obtained from MS/MS analysis of the best individual replicate, Scaffold v3.6.
| Band | Extract source | Protein | kDa | (p) | TS | UP | %C | R | Accession |
|---|---|---|---|---|---|---|---|---|---|
| 25 kDa | testes, ovaries | Wolbachia surface protein | 25 | 100% | 44 | 16 | 48% | 5 | gi|190571332 |
| 18 kDa | testes, ovaries | Wolbachia putativemembrane protein | 17 | 100% | 26 | 9 | 36% | 2 | gi|190570988 |
| <14 kDa | testes, ovaries | Wolbachia DNA binding HU | 12 | 100% | 11 | 7 | 67% | 5 | gi|190571020 |
Abbreviations are as follows: (p), identity probability; TS, total spectra matches; UP, unique peptides; %C, % coverage; R, replicates.
Figure 2.
Total peptide coverage detected for the three Wolbachia proteins. A. Wolbachia surface protein, WSP. Coverage varied from 39% – 50% among 5 replicates. B. Wolbachia putative membrane protein. Coverage varied from 14% – 36% among 2 replicates C. Wolbachia DNA binding protein HU beta. Coverage varied from 36% – 67% among 5 replicates. Shaded boxes indicate mass spectrometry identified peptides.
3.2 A Wolbachia DNA binding protein
Infected tissues differentially expressed bands with masses less than 14 kDa (Fig. 1B) in some samples, but these bands were not consistently replicated in every sample. Likewise, in studies with Wolbachia-infected cell lines, we noted that extracts sometimes included differentially expressed, radiolabeled bands with masses below 14 kDa (Fallon et al, submitted). Protein from this region was extracted and subjected to tryptic digestion and mass spectrometry analysis. A high proportion of peptides (7 peptides; 50% coverage) corresponded to the Wolbachia DNA binding protein HU (gi|190571020). In subsequent experiments, we identified HU peptides in five biological replicates, including both testes and ovaries, with high confidence and protein coverage ranging from 36% – 67% among replicates (Fig. 2C and Table 1).
3.3 Sequence and BLAST analysis of HU
BLAST analysis indicated that the Wolbachia HU protein was the ortholog of the 90 amino acid protein, HU beta, encoded by hupB in Escherichia coli, with 23 amino acid identities spanning the length of the protein, and an E value of 1.00e-09 (Fig. 3A). The most striking differences between HU beta from wPip and E. coli were a five amino acid insertion (KQDCV) near the N terminus of the Wolbachia protein within alpha helical region α1, and eight residues at the C-terminus extending beyond helical region α3 in the E. coli homolog. Alpha helix α1 is the dimerization site that mediates an interaction between HU alpha and HU beta proteins in E. coli, but Wolbachia genomes lack the hupA ortholog, suggesting that in Wolbachia, HU beta forms a homodimer, interacts with a unique partner, or functions as a monomer. HU beta from wPip also maintains a conserved intercalating proline residue essential for DNA binding in E. coli HU beta (identified by the solid downward pointing arrow in Fig. 3A), and a second proline (identified in Fig. 3B with an open arrow) was conserved among Wolbachia homologs. Just upstream of the conserved proline was the second of a pair of cysteine residues (shaded grey), which potentially forms a disulfide bond with the unique cysteine in the N-terminal KQDCV insertion in Wolbachia HU beta.
Figure 3.
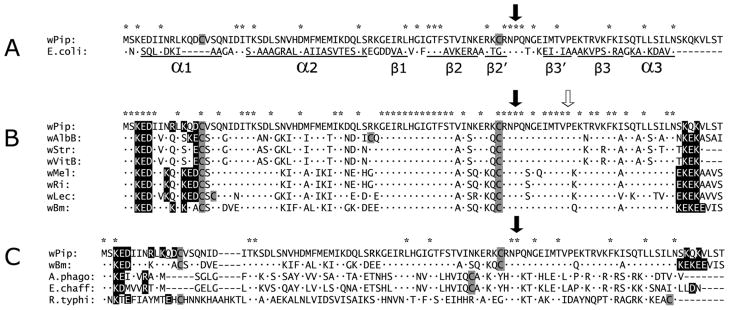
Comparisons among HU beta proteins. A. Amino acid sequence alignment of wPip HU beta and its E. coli orthologp. Asterisks indicate amino acid identities, and the downward-pointing arrow indicates a proline residue important in DNA binding. Under the E. coli sequence, residues that participate in alpha helix (α) and beta sheet structure (β) as defined by Swinger and Rice (2004) are underlined. B. Alignment of HU beta amino acid sequences. Wolbachia genes from strains wPip, wAlbB, wLec, and wStr were experimentally determined in this study; others were from Wolbachia genomes available in the database. C. Amino acid sequence alignment of Wolbachia HU beta from (wPip and wBm) compared with representative orthologs from Anaplasma phagocytophilum, Ehrlichia chaffeensis, and Rickettsia typhi. N and C-terminal charged motifs composed of lysine, arginine, glutamic acid, and aspartic acid in HU beta are shown in black boxes. Cysteine residues are shown in grey. For other residues, dots indicate identities.
To determine whether the unique aspects of the N and C termini and the cysteine residues were conserved among Wolbachia HU beta homologs, we sequenced the hupB genes from the Wolbachia strains available in our lab and aligned them with annotated sequences from the databases. Internal primers based on wPip hupB nucleotide sequences did not reliably produce hupB PCR products in other Wolbachia strains, suggesting variability among hupB homologs. To address this possibility, primers in better conserved regions, including the 135 nt intergenic region downstream of the gene encoding ribosomal protein S9 (rpsI; HuCloneF) and within the gene encoding pyridoxine 5′-phosphate synthase (pdxJ; HuCloneR, Fig. 4) were used to obtain PCR products encoding hupB from wPip, wStr (from the planthopper, Laodelphax striatellus), wAlbB (from the mosquito Aedes albopictus), and wLec (from the bedbug, Cimex lectularius). During our sequence analysis the protein sequences for the wAlbB HU beta homolog (ZP_09542731.1) became available. Our translated wAlbB protein sequence and our wPip hupB DNA sequence (Gene ID: 6385678) matched published sequences perfectly. Sequences for wStr hupB and wLec hupB were deposited in the GenBank database under accession numbers JX984572 and JX984573.
Figure 4.
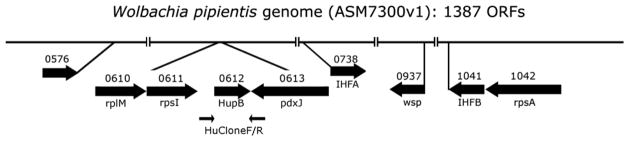
Partial linearized genome of Wolbachia pipientis. Genes described in this study are mapped by solid arrows showing the direction of transcription. Flanking the hupB (0612) are primers HUCloneF/R (indicated by small black arrows), which map immediately downstream of rpsI and within pdxJ, respectively, used to clone HU sequences from other Wolbachia strains described in Fig. 3. Amino acid sequences of HU, WSP (0937) and the putative membrane protein (0576) are described in Fig. 2. Genes encoding IHF alpha (0738) and IHF beta (1041), transcribed in opposite directions, are also shown. Locations of ribosomal protein genes potentially transcribed as operons that include hupB (rplM, rpsI) and ihfB (rpsA) are also represented.
Alignment of Wolbachia HU beta proteins showed 52 amino acid identities common to all available homologs (see the asterisks at the top of the alignment in Fig. 3B), including the N-terminal KED motif, the two internal cysteines, two conserved prolines, and KEK motifs at the C-terminus. Strains wAlbB and wLec have an additional third cysteine, but their positions are not conserved. The C-terminal charged motifs appear to be a unique characteristic of Wolbachia HU beta proteins, relative to homologs in the other genera in the Rickettsiales: Anaplasma, Ehrlichia, and Rickettsia (Fig 3C). At least one cysteine residue occurred in each of the HU beta representatives of the order Rickettsiales aligned in Fig. 3C.
3.4 Expression of Wolbachia hupB
We verified that hupB is transcribed in two species of infected mosquitoes by reverse-transcriptase PCR (RT-PCR). The hupB transcript was detected in RNA prepared from Wolbachia-infected male and female Cx. pipiens and Ae. albopictus mosquitoes (Fig. 5, lanes 3, 5, 7, 9), but not in uninfected cell cultures (Fig. 5, lane 1), indicating that this gene is transcribed from the wPip and wAlbA/B genomes, in vivo, at levels detectable in total RNA prepared from whole mosquitoes. RT-PCR bands were excised and sequenced to confirm that the PCR product encoded the hupB transcript. Positive controls for RNA quality were performed by amplifying a PCR product from mosquito ribosomal protein RpS3 gene transcripts (Fig. 5, lanes 11–15). Negative controls were uninfected mosquito cells (lane 1) and RNA assayed without reverse transcriptase (lanes 2, 4, 6, 8, 10).
Figure 5.
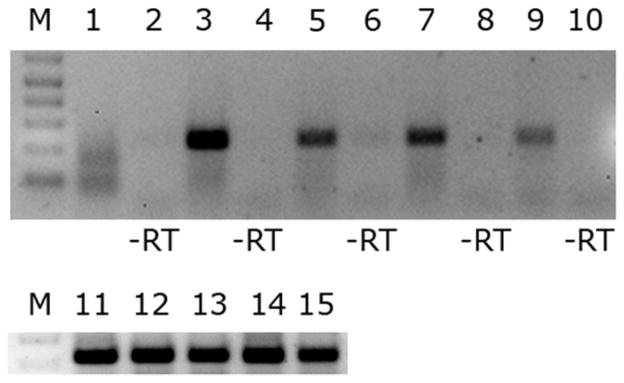
RT PCR analysis of the hupB transcript in pooled decapitated mosquitoes and uninfected mosquito cells. M is a DNA marker. Lanes 1 and 11 are RNA extracted from uninfected C7–10 mosquito cells. Lanes 3, 4, and 12 are total RNA extracted from Culex pipiens females. Lanes 5, 6, and 13 are RNA extracted from Culex pipiens males. Lanes 7, 8, and 14 are RNA extracted from Aedes albopictus females. Lanes 9, 10, and 15 are RNA extracted from Aedes albopictus males. Lanes 2, 4, 6, 8, and 10 were assayed without reverse transcriptase as a control for DNA contamination of RNA preparations. Lanes 11–15 are positive controls for RNA quality using primers for the mosquito ribosomal protein RpS3.
3.5 In silico analysis of HU partners
In E. coli, HU beta is a member of a group of four nucleoid proteins: two HU proteins, HU alpha and HU beta and two IHF (integration host factor) proteins, IHF alpha and IHF beta, each of which can form homo- and hetero- dimers (α/α, β/β, α/β), but HU proteins do not dimerize with IHF proteins. As described above, all Wolbachia genomes lack a gene corresponding to E. coli hupA. However, Wolbachia genomes encode three HU proteins: gi|190571020, which aligns most closely with E. coli HU beta (Fig 3); gi|190571140, which most closely resembles E coli IHF alpha, and gi|190571428, which is the homolog of E. coli IHF beta. Relative to E. coli, wPip IHF alpha had 34% identity over 99 residues, uniformly distributed over the length of the protein (Fig. 6A), and among strains of Wolbachia, IHFA was highly conserved (Fig. 6B). Relative to E. coli, wPip IHF beta had 28% identity over 92 residues (Fig. 6C), with strong identity among Wolbachia strains (Fig. 6D). We note that overall, among Wolbachia homologs, IHF alpha appears to be evolving more slowly than IHF beta and both IHF proteins are evolving more slowly than HU beta.
Figure 6.
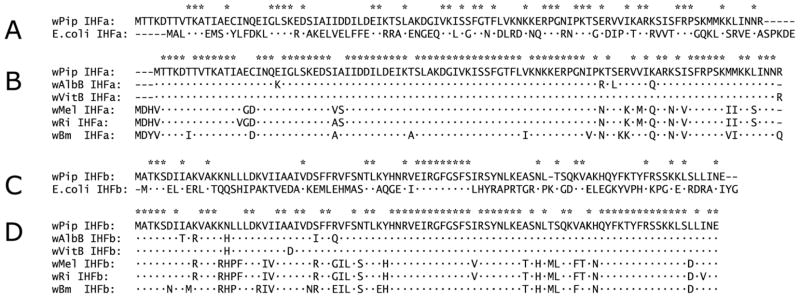
IHF amino acid sequence alignments. A. Sequence alignment of wPip IHF alpha with its E. coli ortholog. B. Sequence alignment of known Wolbachia IHF alpha homologs. C. Sequence alignment of wPip IHF beta with its E. coli ortholog. D. Sequence alignment of known Wolbachia IHF beta homologs. Asterisks indicate amino acid identities. Identities relative to the wPip proteins are indicated by dots.
Finally, to evaluate possibilities for co-expression and/or potential expression of these genes as part of an operon(s), we examined the relative locations of the Wolbachia genes encoding the proteins that we have detected in this study (Fig. 4). The membrane protein encoding genes, hypothetical protein (wpa 0576) and wsp (wpa 0937) are encoded by distant genes, and transcribed in opposite directions. Interestingly, both hupB and ihfB might be included in separate operons, each of which includes upstream ribosomal protein genes; strong constitutive expression would be expected if they were co-transcribed with components of the translational machinery. hupB is flanked on the 5′ end by rplM, encoding 50S ribosomal protein L13, and rpsI, encoding 30S ribosomal protein S9; ihfB is immediately downstream of rpsA, encoding the 30S ribosomal protein S1. Conservation of the relative order of these genes in E. coli is consistent with possible inclusion of hupB and ihfB proteins in operons that encode key components of the translational machinery.
4. DISCUSSION
4.1 Detection of Wolbachia proteins
Although CI was successfully used for mosquito population replacement more than 40 years ago (Laven, 1967), the molecular basis for CI remains elusive. We have initiated proteomic studies addressing the hypothesis that in infected mosquito testes, Wolbachia secrete effector molecules that interact with sperm DNA. Here we use mass spectrometry to identify three proteins abundantly expressed in Wolbachia-infected tissues: two membrane proteins and a DNA binding protein. In addition to WSP, previously identified in Drosophila reproductive tissues (Sasaki et al., 1998; Braig et al., 1998) we also detected an 18 kDa band on SDS gels that contained peptides from a second Wolbachia membrane protein of unknown function. Although detection of abundant membrane proteins was not surprising, it remained to be seen whether mass spectrometry was capable of detecting less abundant Wolbachia proteins. To optimize collection of sufficient tissue, we dissected mosquitoes in 100% ethanol, rather than buffered saline, to reduce tissue adherence to dissecting instruments. Improved recovery of protein allowed us to detect bands that migrated at masses of less than 14 kDa. Wolbachia peptides recovered from this region of the gel corresponded to those of a DNA binding protein homologous to HU beta in E. coli.
4.2 Structure and function of HU
In E. coli, HU proteins are a constitutive component of the bacterial nucleoid with roles in replication, transposition, gene inversion, and expression (Nash and Robertson, 1981; Ryan et al., 2002; and Azam et al., 1999). HU binds bacterial DNA and wraps it into a nucleosome-like particle providing compaction and protection. The three dimensional structure of E. coli HU beta is composed of three alpha helices, with five beta sheets between the second and third helix. Alpha 1 helix acts as the dimerization site, and DNA binding involves the five beta sheets and a conserved intercalating proline. In dimer form, two beta sheet arms interact with the minor groove of DNA and force it to bend 180 degrees around the base of the protein (Swinger and Rice, 2004). Note that Wolbachia and E. coli HU beta proteins share the strongest concentration of identities in this key DNA binding region, including the intercalating proline present in all Wolbachia homologs (Fig. 3).
The most striking differences in primary structure of the Wolbachia homologs, relative to HU beta in E. coli, are the acidic/basic motifs near the N and C termini, precisely where charged protein signals often mediate nuclear localization (Assier et al., 1999), and are thought to be an important signal for Wolbachia’s type IV secretion system (Vergunst et al., 2005). Moreover, because these motifs interrupt and flank amino acids that constitute the E. coli helices α1 and α3, they might affect higher order structure and protein function. These changes are particularly intriguing because all known Wolbachia genomes have lost the partner gene hupA, and therefore can form only homodimers. Interestingly, other researchers have reported unusual histone1-like PAAK and KAAK additions on the C-terminal domain of a mycobacterial HU protein which lowers its binding constant and makes its interaction with DNA more specific (Kumar et al., 2010). Moreover, the pair of cysteines in Wolbachia HU beta potentially form intra or intermolecular disulfide bonds that would potentially decrease the distance between acidic/basic “KEKE-like” motifs, which are thought to be involved in assembly of protein complexes by a putative “charged zipper” mechanism (Realini et al., 1994).
In Chlamydia and E. coli, HU proteins have been shown to regulate transitions in growth cycle (Wagar and Stephens, 1988; Azam et al., 1999) and to regulate transposition and recombination (Friedman, 1988), which could have profound impacts on genetic manipulation of Wolbachia. The relative importance of HU in Wolbachia biology is underscored by the fact that of the seven major bacterial nucleoid proteins listed for E. coli (Dorman 2009), the Wolbachia pipientis genome encodes only three; HU beta and the two IHF proteins described above. Because Wolbachia lacks most of the nucleoid proteins known from E. coli (Azam et al., 1999; Dorman, 2009) HU beta is likely to play a key role in the Wolbachia cell cycle. HU has limited homology to chromosome partitioning protein, MUKB, reviewed in (Nasmyth and Haering, 2005), a 170 kDa prokaryotic member of the SMC (structural maintenance of chromatin) protein family involved in condensation and segregation of chromosomes. In E. coli, HU and MUKB cooperate in chromosome segregation. Double hupA/hupB mutants are defective in chromosome partitioning and show the same phenotype as mukB mutants (Jaffe et al., 1997); that is they develop a population of anucleate cells. Interestingly, hupA/mukB double mutants are lethal, but having one gene seems to compensate for the loss of the other. The absence of mukB and hupA homologs in Wolbachia genomes further underscores the uniqueness of Wolbachia HU beta, which presumably accomplishes chromosome segregation in the absence of MUKB.
Of the three nucleoid poteins encoded by Wolbachia genomes, we note that both HU beta and IHF beta genes lie immediately downstream of ribosomal protein genes, raising the possibility that they could be co-transcribed with highly expressed components of the translational machinery. However, if HU beta and IHF beta are co-expressed, it is curious that that we did not detect peptides corresponding to Wolbachia IHF proteins, which would be expected to migrate with masses similar to that of HU beta. Recovery of several peptides from HU beta, relative to the absence of peptides from IHF beta, leads us to speculate that HU beta may be expressed at higher levels than the IHF proteins, or alternatively, may be more stable, compatible with a possible role for HU beta in mediating CI. We also note that during preparation of this manuscript Darby et al. (2012) published a global study of transcription in Wolbachia from the nematode, Onchocerca ochengi, and noted up-regulation of an HU transcript in germ tissue as compared to somatic tissues.
4.3 HU as a candidate CI effector molecule
We hypothesize that if the acidic/basic motifs at the N terminus of Wolbachia HU beta are recognized by the T4SS, HU beta might be accumulated in the host cell nucleus and bind to sperm DNA, at the time when histones are being replaced by protamines. In Drosophila it is claimed that any small positively charged/basic protein can assume the protamine packaging function (Hennig, 2003). All Wolbachia HU beta proteins have cysteines that do not occur in E coli HU proteins; cysteine residues are known to be essential for protamine-mediated DNA compaction in upper eukaryotes (Ballhorn, 2007). The possibility that HU beta interacts with DNA in mosquito sperm is further supported by structural and functional similarity between bacterial HU proteins and eukaryotic chromatin architectural HMG (High Mobility Group) box proteins (Oberto et al., 1994; Bianchi, 1994) known to be important for nuclear condensation and chromatin structure in early Drosophila embryos (Ner and Travers, 1994). In particular, HMG box proteins specifically favor cruciform DNA. Likewise, In E. coli, HU beta homodimers (the only form possible in Wolbachia) bind preferentially to cruciform DNA, while heterodimers associate with both linear and cruciform DNA (Pinson et al., 1999). An enrichment of Wolbachia-derived homodimeric HU beta in the paternal pronucleus conceivably disrupts chromatin changes associated with fertilization by an antagonistic/competitive mechanism with maternal histones and HMG Box proteins, causing the improper chromatin condensation (Landmann et al., 2009; Breeuwer and Werren, 1990) and aberrant synchrony between male and female pronuclei that have been observed in incompatible crosses in Drosophila simulans (Callaini et al. 1997). Future research on HU will focus on identifying additional proteins with which it may interact, further characterization of its role in the Wolbachia replication cycle, and evaluation of its possible role in cytoplasmic incompatibility.
HIGHLIGHTS.
W olbachia express a DNA binding protein, HU, in mosquito testes and ovaries.
H U abundance is comparable to that of Wolbachia surface proteins.
W olbachia HU has unique amino acid motifs absent in E. coli homologs.
H U is the first Wolbachia DNA binding protein detected by mass spectrometry.
Acknowledgments
This work was supported by NIH grant AI081322 and by the University of Minnesota Agricultural Experiment Station, St. Paul, MN. Mass spectrometry analysis was performed at the University of Minnesota Center for Mass Spectrometry and Proteomics (supporting agencies are listed at http://www.cbs.umn.edu/msp/about). We thank Gerry Baldridge and Grace Li for helpful discussion and Cassandra Kurtz for help maintaining the mosquito colonies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JD, Boylan KL, Xue FS, Anderson LB, Witthuhn BA, Markowski TW, Higgins L, Skubitz AP. Identification of candidate biomarkers in ovarian cancer serum by depletion of highly abundant proteins and differential in-gel electrophoresis. Electrophoresis. 2010;31:599–610. doi: 10.1002/elps.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assier E, Bouzinba-Segard H, Stolzenberg M, Stephens R, Bardos J, Freemont P, Charron D, Trowsdale J, Rich T. Isolation, sequencing, and expression of RED, a novel human gene encoding an acidic-basic dipeptide repeat. Gene. 1999;230:145–154. doi: 10.1016/s0378-1119(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Azam TA, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballhorn R. The protamine family of sperm nuclear proteins. Genome Biol. 2007;8:227. doi: 10.1186/gb-2007-8-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JF, Fallon AM. Decapitation improves detection of Wolbachia pipientis (Rickettsiales: Anaplasmataceae) in Culex pipiens (Diptera: Culicidae) mosquitoes by the polymerase chain reaction. J Med Entomol. 2012;49:1103–1108. doi: 10.1603/me12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. Prokaryotic HU and eukaryotic HMB1: a kinked relationship. Mol Microbiol. 1994;14:1–5. doi: 10.1111/j.1365-2958.1994.tb01261.x. [DOI] [PubMed] [Google Scholar]
- Braig HR, Zhou W, Dobson SL, O’Neill SL. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer JAJ, Werren JH. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature. 1990;346:558–560. doi: 10.1038/346558a0. [DOI] [PubMed] [Google Scholar]
- Callaini G, Dallai R, Giovanna Riparbelli M. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J Cell Sci. 1997;110:271–280. doi: 10.1242/jcs.110.2.271. [DOI] [PubMed] [Google Scholar]
- Clark ME, Bailey-Jourdain C, Ferree PM, England SJ, Sullivan W, Windsor DM, Werren JH. Wolbachia modification of sperm does not always require residence within developing sperm. Heredity. 2008;101:420–428. doi: 10.1038/hdy.2008.71. [DOI] [PubMed] [Google Scholar]
- Clark ME, Veneti Z, Bourtzis K, Karr T. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech of Dev. 2002;111:3–15. doi: 10.1016/s0925-4773(01)00594-9. [DOI] [PubMed] [Google Scholar]
- Clark ME, Veneti Z, Bourtzis K, Karr T. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech of Dev. 2003;120:185–198. doi: 10.1016/s0925-4773(02)00424-0. [DOI] [PubMed] [Google Scholar]
- Darby AC, et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012 doi: 10.1101/gr.138420.112. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman CJ. Nucleoid-associated proteins and bacterial physiology. Adv Appl Microbiol. 2009;67:47–64. doi: 10.1016/S0065-2164(08)01002-2. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Baldridge GD, Higgins LA, Witthuhn BA. Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell Dev Biol – Animal. 2012 doi: 10.1007/s11626-012-9571-3. submitted. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Witthuhn BA. Proteasome activity in a naïve mosquito cell line infected with Wolbachia pipientis wAlbB. In Vitro Cell Dev Biol – Animal. 2009;45:460–466. doi: 10.1007/s11626-009-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree PM, Frydman HM, Li JM, Cao J, Wieschaus E, Sullivan W. Wolbachia utilizes host microtubules and dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 2005;1:e14. doi: 10.1371/journal.ppat.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu FD, Robinson J, Young PR, McGraw EA, O’Neill SL. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PloS One. 2010;5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DI. Integration host factor: a protein for all reasons. Cell. 1988;55:545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Hennig W. Chromosomal proteins in the spermatogenesis of Drosophila. Chromosoma. 2003;111:489–494. doi: 10.1007/s00412-003-0236-6. [DOI] [PubMed] [Google Scholar]
- Hertig M. The rickettsia, Wolbachia pipientis (Gen. et sp n.) and associated inclusions of the mosquito, Culex pipiens. Parasitology. 1936;28:453–486. [Google Scholar]
- Jaffe A, Vinella D, D’Ari R. The Escherichia coli histone-like protein HU affects DNA initiation, chromosome partitioning via MukB, and cell division via MinCDE. J Bacteriol. 1997;179:3494–3499. doi: 10.1128/jb.179.11.3494-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr TL, Yang W, Feder ME. Overcoming cytoplasmic incompatibility in Drosophila. Proc R Soc Lond B. 1998;265:391–395. doi: 10.1098/rspb.1998.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sardesai AA, Basu D, Muniyappa K, Hasnain SE. DNA clasping by mycobacterial HU: the C-terminal region of hupB mediates increased specificity of DNA binding. PLoS One. 2010;5:e12551. doi: 10.1371/journal.pone.0012551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F, Orsi GA, Loppin B, Sullivan W. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PloS Pathog. 2009;5:e1000343. doi: 10.1371/journal.ppat.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang Y, O’Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- Nash HA, Robertson CA. Purification and properties of the Escherichia coli protein factor required for λ integrative recombination. J Biol Chem. 1981;256:9246–9253. [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- Ner SS, Travers AA. HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. EMBO J. 1994;13:1817–1822. doi: 10.1002/j.1460-2075.1994.tb06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Noda H, Miyoshi T, Koizumi Y. In vitro cultivation of Wolbachia in insect and mammalian cell lines. In Vitro Cell Dev Biol – Animal. 2002;38:423–427. doi: 10.1290/1071-2690(2002)038<0423:IVCOWI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Oberto J, Drlica K, Rouviere-Yaniv Histones, HMG, HU, IHF: Meme combat. Biochimie. 1994;76:901–908. doi: 10.1016/0300-9084(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Pinson V, Takahashi M, Rouviere-Yaniv J. Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J Mol Biol. 1999;287:485–497. doi: 10.1006/jmbi.1999.2631. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. A genetic test of the mechanism of Wolbachia-induced cytoplasmic incompatibility in Drosophila. Genetics. 2000;154:771–776. doi: 10.1093/genetics/154.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rances E, Voronin D, Tran-Van V, Mavingui P. Genetic and functional characterization of the type IV secretion system in Wolbachia. J Bacteriol. 2008;190:5020–5030. doi: 10.1128/JB.00377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Ren X, Petridis M. Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl and Environ Microb. 2006;72:7718–7722. doi: 10.1128/AEM.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini C, Rogers SW, Rechsteiner M. Keke motifs, proposed roles in protein-protein association and presentation of peptides by MHC class I receptors. FEBS Letters. 1994;348:109–113. doi: 10.1016/0014-5793(94)00569-9. [DOI] [PubMed] [Google Scholar]
- Ryan VT, Grimwade JE, Nievera CJ, Leonard AC. IHF and HU stimulate assembly of pre-replication complexes at Escherichia coli oriC by two different mechanisms. Mol Microbiol. 2002;46:113–124. doi: 10.1046/j.1365-2958.2002.03129.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Braig HR, O’Neill SL. Analysis of Wolbachia protein synthesis in Drosophila in vivo. Insect Mol Biol. 1998;7:101–105. doi: 10.1046/j.1365-2583.1998.72057.x. [DOI] [PubMed] [Google Scholar]
- Serbus LR, Casper-Lindly C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- Swinger KK, Rice PA. IHF and HU: flexible architects of bent DNA. Curr Opin Struc Biol. 2004;14:28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, Ephrussi A, Wood CG, Lehmann R, Fuller MT. A germline specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–2539. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- Vergunst AC, van Lier MCM, Dulk-Ras AD, Grosse Stuve TA, Ouwehand A, Hooykaas PJJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci USA. 2005;102:832–837. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar EA, Stephens RS. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988;56:1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]