Abstract
Neurotrophic factors and steroid hormones interact to regulate a variety of neuronal processes such as neurite outgrowth, differentiation, and neuroprotection. The coexpression of steroid hormone and neurotrophin receptor mRNAs and proteins, as well as their reciprocal regulation provides the necessary substrates for such interactions to occur. This review will focus on androgen-BDNF interactions in the spinal cord, describing androgen regulation of BDNF in neuromuscular systems following castration, androgen manipulation, and injury. Androgens interact with BDNF during development to regulate normally-occurring motoneuron death, and in adulthood, androgen-BDNF interactions are involved in the maintenance of several features of neuromuscular systems. Androgens regulate BDNF and trkB expression in spinal motoneurons. Androgens also regulate BDNF levels in the target musculature, and androgenic action at the muscle regulates BDNF levels in motoneurons. These interactions have important implications for the maintenance of motoneuron morphology. Finally, androgens interact with BDNF after injury, influencing soma size, dendritic morphology, and axon regeneration. Together, these findings provide further insight into the development and maintenance of neuromuscular systems and have implications for the neurotherapeutic/neuroprotective roles of androgens and trophic factors in the treatment of motoneuron disease and recovery from injury.
Keywords: testosterone, motoneuron, muscle, morphology, neurotrophic factors
Introduction
Neurotrophic factors and steroid hormones interact to regulate a variety of neuronal processes such as neurite outgrowth, differentiation and neuroprotection. The coexpression of steroid hormone and neurotrophin receptor mRNAs and proteins (Toran-Allerand et al., 1992; Toran-Allerand, 1996; Shugrue et al., 2000), as well as their reciprocal regulation (Sohrabji et al., 1994) provides the necessary substrates for such interactions to occur. Steroid hormone receptors and neurotrophin receptors and their ligands are coexpressed in several brain regions, including the hippocampus, cerebral cortex, cingulate cortex, olfactory bulb and basal ganglia (Miranda et al., 1993; Jezierski and Sohrabji, 2000). For example, estrogens support brain-derived neurotrophic factor (BDNF) mRNA and protein expression in hippocampal, basal forebrain, and pyriform cortical neurons (Gibbs, 1999; Jezierski and Sohrabji, 2001; Solum and Handa, 2002). In addition, cross-coupling of steroid hormone and neurotrophic factor intracellular signaling pathways underlies the activation of genes involved in neurite outgrowth and differentiation (Toran-Allerand et al., 1999). Furthermore, the gene encoding BDNF contains an estrogen response element (Sohrabji et al., 1995), which provides a direct mechanism for steroid hormone control of BDNF expression.
These steroid hormone-neurotrophic factor interactions not only occur in the brain but throughout the central and peripheral nervous system. This review will focus on androgen-BDNF interactions in the spinal cord, and in particular, on androgenic regulation of BDNF in neuromuscular systems following androgen manipulation, and injury. The effects of androgen and BDNF treatment on motoneuron morphology and BDNF, trkB, and androgen receptor expression, in sexually dimorphic and somatic motoneuron populations and their target muscles will be discussed.
BDNF is a member of the neurotrophin family of neurotrophic factors and binds with high affinity to the trkB receptor. BDNF is expressed throughout the body, including in the central (Yan et al., 1997) and peripheral nervous system (Matsuoka et al., 1991; Griesbeck et al., 1995) and skeletal muscles (Kust et al., 2002; Mousavi et al., 2004; Verhovshek et al., 2010). BDNF is produced in limited quantities in target tissues and retrogradely transported back to the CNS where it exerts a variety of effects (Thoenen, 1991). Indeed, BDNF is retrogradely transported from skeletal muscle to motoneurons (DiStefano et al., 1992; Koliatsos et al., 1993). Thus, levels of BDNF protein in spinal motoneuron somata can be altered through either changes in peripheral production or retrograde transport. However, BDNF is also produced centrally and released in an autocrine and/or paracrine manner within neuronal populations (Lom and Cohen-Cory, 1999; Lom et al., 2002; Horch, 2004), including spinal motoneurons (Buck et al., 2000). Thus, levels of BDNF protein in spinal motoneuron somata could also be altered through changes in BDNF message in the motoneurons themselves, local production of BDNF, or even differential trafficking into dendrites. The actions of BDNF in the central nervous system have traditionally been thought of as facilitative, promoting growth or survival of neurons (Snider, 1994; McAllister et al., 1999; Pitts et al., 2006). For example, BDNF supports neuron survival (Henderson et al., 1993; Rasika et al., 1999; Xu et al., 2001), somal area (Wissman and Brenowitz, 2009), axonal outgrowth (Cabelli et al., 1995; Cohen-Cory and Fraser, 1995; Lom and Cohen-Cory, 1999; Mamounas et al., 2000), and dendritic branching in vitro and in vivo (McAllister et al., 1995; McAllister et al., 1997; Horch and Katz, 2002; Finsterwald et al., 2009).
BDNF-androgen interactions: maintenance of structure
Sexually dimorphic neuromuscular systems
The lumbar spinal cord of male rats contains a sexually dimorphic motor nucleus, the spinal nucleus of the bulbocavernosus (SNB; Breedlove and Arnold, 1980) that consists of approximately 200 medially-located motoneurons (Arnold et al., 1988). SNB motoneurons innervate the bulbocavernosus and levator ani muscles of the perineum (McKenna and Nadelhaft, 1986), and control penile reflexes important for copulatory behavior (Sachs, 1982). Androgenic effects on SNB motoneurons were first identified over 30 years ago and since then almost every feature of the SNB neuromuscular system has been shown to be regulated by gonadal hormones in some way (Sengelaub and Forger, 2008). The SNB target musculature plays a critical role in mediating androgenic effects in the SNB neuromuscular system. Maintenance of SNB soma size is regulated by androgens and influenced by motoneuron contact with the SNB target muscles (Araki et al., 1991). Removal of the target musculature in early postnatal life downregulates androgen receptor expression, blocking androgen sensitivity in SNB motoneurons (Lubischer and Arnold, 1995). Furthermore, androgens are thought to control normally-occurring cell death in the SNB (Fishman and Breedlove, 1992) and regulate SNB dendritic morphology by acting at the SNB target musculature (Rand and Breedlove, 1995). It had been suggested that this peripheral regulation of SNB motoneuron morphology results from the actions of target-derived trophic substances (Breedlove, 1986) such as BDNF, and more recent work supports this hypothesis (see below).
Motoneurons
SNB motoneurons express BDNF mRNA and protein, and this expression is regulated by androgens (Ottem et al., 2007; Verhovshek et al., 2010). Castration of adult male rats reduced BDNF mRNA and protein in SNB somata and treating castrates with testosterone maintained BDNF message and protein at levels similar to those of gonadally intact males (Ottem et al., 2007). Interestingly, due to the unusual structure of the BDNF gene, BDNF mRNA can be regulated in various ways. The Bdnf gene contains multiple promoters that control transcription of a number of 5’ non-coding exons and a common 3’ exon coding for the BDNF protein. Through the use of multiple promoters and alternative splicing, a variety of BDNF transcripts are produced from the Bdnf gene, all coding for the BDNF protein, but containing different 5’ exons (Timmusk et al., 1993). To add to the diversity of BDNF transcripts produced, the Bdnf gene contains multiple polyadenylation sites that produce BDNF mRNA with short or long 3’ UTRs (An et al., 2008). The diversity of BDNF mRNA produced from a single gene allows for tissue-specific expression of BDNF mRNA and protein (An et al., 2008; Timmusk et al., 1993).
The androgenic regulation of BDNF mRNA is isoform-specific: 5’ non-coding exon VI was the only identified isoform that displayed androgen regulation, and its expression decreased after castration, but was restored to levels of those found in gonadally intact males in castrates treated with testosterone (Ottem et al., 2010). This exon-specific androgenic regulation of BDNF mRNA provides a mechanism for localization of BDNF within SNB motoneurons in response to androgen manipulation, as transcript-specific localization of BDNF mRNA to somata and dendrites has been previously demonstrated (An et al., 2008; Ottem et al., 2010; Pattabiraman et al., 2005). Additionally, BDNF protein is reduced in SNB motoneurons (Verhovshek et al., 2010) and proximal dendrites (Ottem et al., 2007) following castration, and testosterone treatment prevented these castration-induced decreases in SNB motoneurons. Together, these findings demonstrate that androgen depletion results in a concomitant decrease in BDNF that is restored by testosterone treatment. Further, this alteration of BDNF protein may reflect an androgenic regulation of BDNF mRNA expression.
Similarly, trkB, the high-affinity BDNF receptor, is present in SNB motoneurons, and its expression is sensitive to androgens (Osborne et al., 2007; Ottem et al., 2007). In adult male rats, castration decreased trkB mRNA (Ottem et al., 2007) and protein (Osborne et al., 2007) in SNB motoneurons and testosterone treatment can prevent or restore this castration-induced trkB downregulation (Osborne et al., 2007; Ottem et al., 2007).
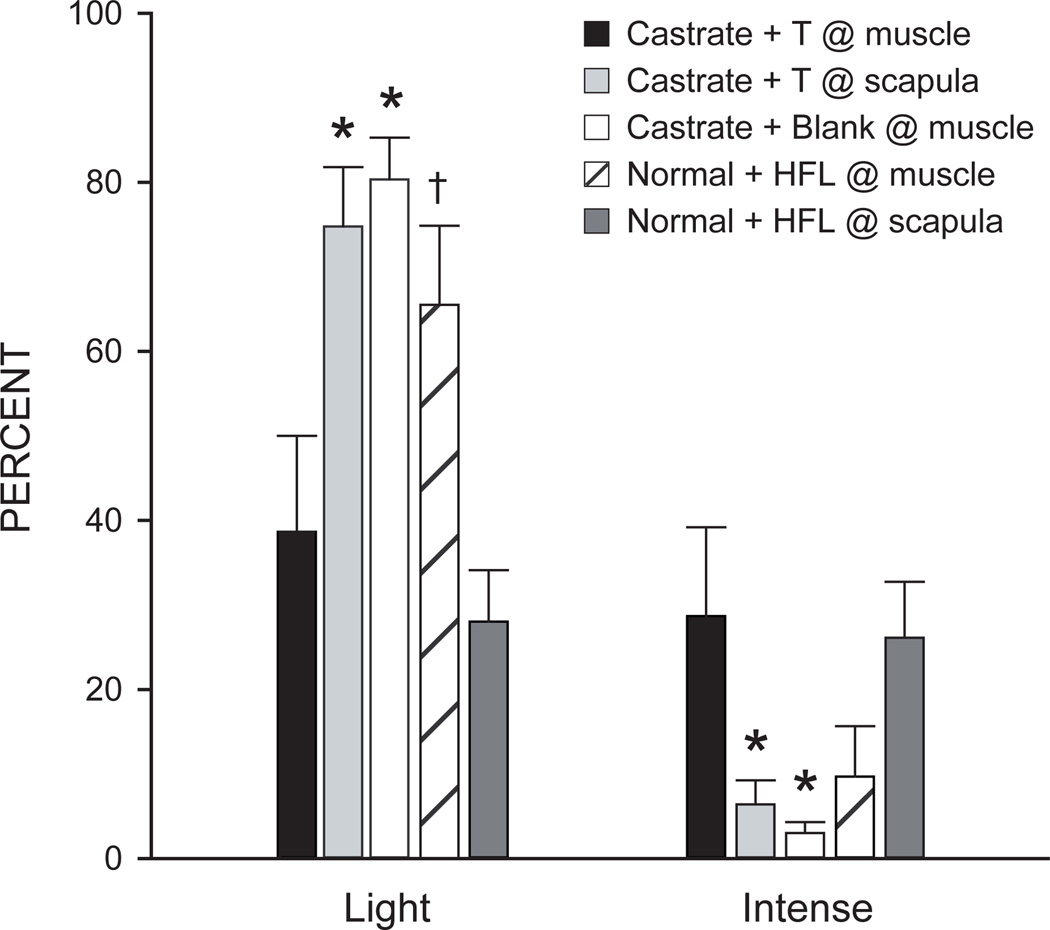
Although the castration-induced decreases in BDNF mRNA and protein in SNB motoneurons demonstrate that changes in systemic androgen levels regulate neurotrophin levels in the SNB, it does not elucidate the site of action for this androgenic regulation of BDNF in these motoneurons. Because SNB motoneurons express androgen receptors (Matsumoto et al., 1996) it is possible that androgens act directly at the motoneuron to regulate BDNF production. However, the SNB target muscles also express androgen receptors (Monks et al., 2004; Monks et al., 2006), implicating the muscle as a potential site for androgenic regulation of BDNF in SNB motoneurons. Indeed, the dendritic morphology of SNB motoneurons can be regulated by androgenic action at the SNB target musculature (Rand and Breedlove, 1995), demonstrating that the peripheral action of androgens can regulate motoneuron morphology. Furthermore, BDNF is expressed in the SNB target musculature (Verhovshek et al., 2010), can be retrogradely transported from skeletal muscle to spinal motoneurons (DiStefano et al., 1992; Koliatsos et al., 1993), and its role as a retrogradely transported, target-derived neurotrophic factor has been well established (Huang and Reichart, 2001), providing a likely mechanism for the peripheral androgenic regulation of BDNF levels in SNB motoneurons. Recent evidence suggests that BDNF can act in an autocrine fashion to increase local BDNF levels (Cheng et al., 2011), allowing for local changes in the amount of BDNF available for retrograde transport from the target muscle to motoneurons. Based on these data, it is possible that androgens act at the SNB target muscle to locally increase levels of BDNF, and excess BDNF could be retrogradely transported, resulting in an increase in BDNF protein in SNB motoneurons following androgen treatment in castrated male rats. In fact, restricting testosterone treatment in castrated males directly to the SNB target musculature via a microimplant affixed directly to the SNB target musculature was sufficient to maintain the staining intensity of BDNF-immunolabeled SNB somata at those of normal males (Verhovshek and Sengelaub, 2010a; Fig. 1). Placement of the same implant in the interscapular region had no effect on BDNF immunolabeling, demonstrating that a site of action for androgenic regulation of BDNF in the SNB is the target musculature. Furthermore, in gonadally intact adult male rats treated with microimplants at the target musculature containing the androgen receptor blocker hydroxyflutamide, the staining intensity of BDNF-immunolabeled SNB somata was decreased compared to gonadally intact animals that had the same microimplants placed interscapularly or when compared to castrated males with a testosterone microimplant at the target muscle. These results demonstrate that the SNB target musculature is a critical site of action for the androgenic regulation of BDNF protein in SNB motoneurons and that this androgenic regulation of BDNF occurs through the peripheral action of androgens via androgen receptors.
Figure 1.
Histogram of the number of lightly and intensely immunostained SNB motoneuron somata after immunolabeling for BDNF in castrated males with a testosterone (T) implant placed at the target muscle (black bars), castrated males with a T implant placed interscapularly (lightly shaded bars), castrated males with a blank implant placed at the target muscle (open bars), gonadally intact males with a hydroxyflutamide (HFL) implant placed interscapularly (hatched bars) and gonadally intact males with a hydroxyflutamide implant placed at the target muscle (darkly shaded bars). Bar heights represent means ± SEM. * Significantly different from castrated males with a T implant placed at the target muscle; † Significantly different from gonadally intact males with a hydroxyflutamide implant placed interscapularly. (Verhovshek et al., 2010a)
As previously mentioned, changes in the levels of BDNF in SNB motoneurons could occur through a variety of mechanisms. Treatment of castrates with androgen at the target muscle could increase the amount of peripherally-produced and/or retrogradely transported BDNF, resulting in maintenance of BDNF immunolabeling in SNB motoneurons. The retrograde transport of BDNF is activity-dependent (Watson et al., 1999), and castration decreases activity in the SNB neuromuscular system (Fargo et al., 2003; Holmes and Sachs, 1992). Thus, this castration-induced decrease in activity could potentially result in lower rates of the retrograde transport of target-derived BDNF, which could account for the accumulation of BDNF in the muscle and a concomitant decrease in SNB motoneuron BDNF protein following castration. Alternatively, androgen-dependent alterations in BDNF levels in SNB motoneurons could reflect changes in local transcription, translation, or trafficking of BDNF within SNB motoneurons.
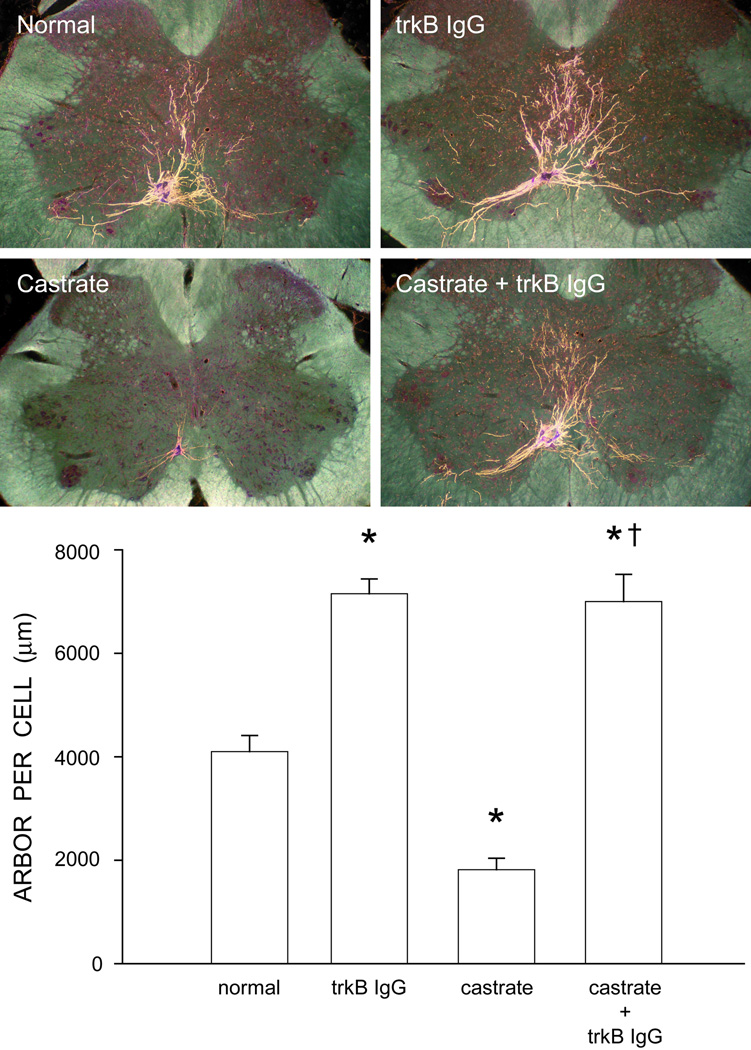
It is clear that androgens regulate BDNF and trkB expression in SNB motoneurons and this interaction has important implications for the maintenance of SNB motoneuron morphology. Castration decreases BDNF mRNA and protein in SNB motoneurons, and if BDNF signaling promotes the maintenance of SNB motoneuron morphology, these reductions could underlie the somal and dendritic regression seen after castration. Paradoxically, treating gonadally intact males with trkB IgG, a fusion protein that interrupts BDNF action, resulted in a hypertrophy of SNB dendritic arbors (Verhovshek and Sengelaub, 2010b; Fig. 2), suggesting that BDNF may be part of a signaling cascade exerting a tonic restraint on SNB motoneuron morphology. Interestingly, castration markedly elevated BDNF protein in the SNB target musculature and treatment with trkB IgG prevented the typical castration-induced dendritic atrophy; dendrite lengths in these animals were similar to those of gonadally intact males treated with trkB IgG. This was the first demonstration that the dendritic arbors of the highly androgen-sensitive SNB motoneurons could be maintained in the absence of androgens, and further suggested that the elevated BDNF levels in muscle were responsible for regressive changes in SNB morphology (Verhovshek and Sengelaub, 2010b). Together, these results suggest that BDNF exerts regulatory effects on SNB dendrites, because when the actions of BDNF are blocked, dendritic hypertrophy occurs, and castration-induced somal atrophy is prevented.
Figure 2.
(Top) Darkfield photomicrographs of transverse sections through the lumbar spinal cord of normal (top left), trkB IgG-treated (top right), castrated (lower left), and trkB IgG-treated castrated (lower right) males after unilateral BHRP injections into SNB target muscles. (Bottom) Dendritic length per labeled motoneuron in normal, trkB IgG-treated, castrated, and trkB IgGtreated castrated males. Bar heights represent means ± SEM. * Significantly different from normal males; † Significantly different from castrated males. (Adapted from Verhovshek and Sengelaub, 2010b)
This conclusion is consistent with other results that demonstrate BDNF can drive regressive processes that affect neuronal morphology. For example, BDNF inhibits dendritic growth in developing layer VI cortical neurons (McAllister et al., 1997), decreases dendritic complexity in retinal ganglion cells (Lom and Cohen-Cory, 1999), and can decrease dendritic length in neurons of the nucleus of the solitary tract (Martin et al., 2012). Although it is not known if direct application or overexpression of BDNF in SNB motoneurons would result in decreased dendritic lengths, our study demonstrates that blockade of BDNF signaling results in dendritic hypertrophy in SNB motoneurons, suggesting that BDNF has a regulatory role in the maintenance of SNB morphology. Future studies should assess the effects of BDNF treatment on SNB morphology, and determine the site of action for BDNF regulation of SNB dendrogenesis.
Alternatively, trkB IgG may not cross the blood-brain barrier, and thus could be acting only peripherally to block BDNF signaling in the target muscles. SNB motoneurons could respond to the loss of peripheral BDNF with a compensatory increase in BDNF expression centrally to promote SNB dendrite growth. However, such an increase in BDNF expression in SNB motoneurons would have to be through an androgen-independent pathway, as dendritic hypertrophy after treatment with trkB IgG was seen in both gonadally intact and castrated males (and castration decreases BDNF protein and message in SNB motoneurons; Osborne et al., 2007; Ottem et al., 2007). Examination of BDNF protein and message expression in SNB motoneurons and their target musculature in trkB IgG-treated animals could address this critical outstanding question.
Although androgenic regulation of BDNF and trkB in SNB motoneurons and the morphological effects of BDNF blockade in adult rats have been well-documented, there is little research that has examined androgen-BDNF interactions on SNB motoneurons during development. Androgens play a critical role in the perinatal development of SNB motoneuron number: testosterone action at the SNB target musculature spares these motoneurons from normally-occurring cell death (Breedlove and Arnold, 1983; Freeman et al., 1996; Nordeen et al., 1985; Sengelaub and Arnold, 1986). Exogenous testosterone treatment in female rats prevents motoneuron cell death, resulting in a masculine SNB neuromuscular system (Breedlove and Arnold, 1983; Nordeen et al., 1985), presumably through androgen regulation of target-derived neurotrophic factors such as BDNF from the SNB target musculature. Indeed, blocking BDNF action at the SNB target musculature prevents the androgenic sparing of SNB motoneurons in newborn female rats (Xu et al., 2001). It is believed that androgens act at the target musculature to prevent SNB motoneuron death perinatally, possibly through an androgenic upregulation of BDNF in the target muscle that rescues motoneurons from cell death. The BDNF-producing capabilities of the developing SNB target muscles are not known, but because other skeletal muscles and corresponding motoneurons produce BDNF during development (Koliatsos et al., 1993; Griesbeck et al., 1995), it is possible that androgen-regulated, peripherally produced BDNF is retrogradely transported to SNB motoneurons to promote survival during the perinatal period (Xu et al., 2001).
Target musculature
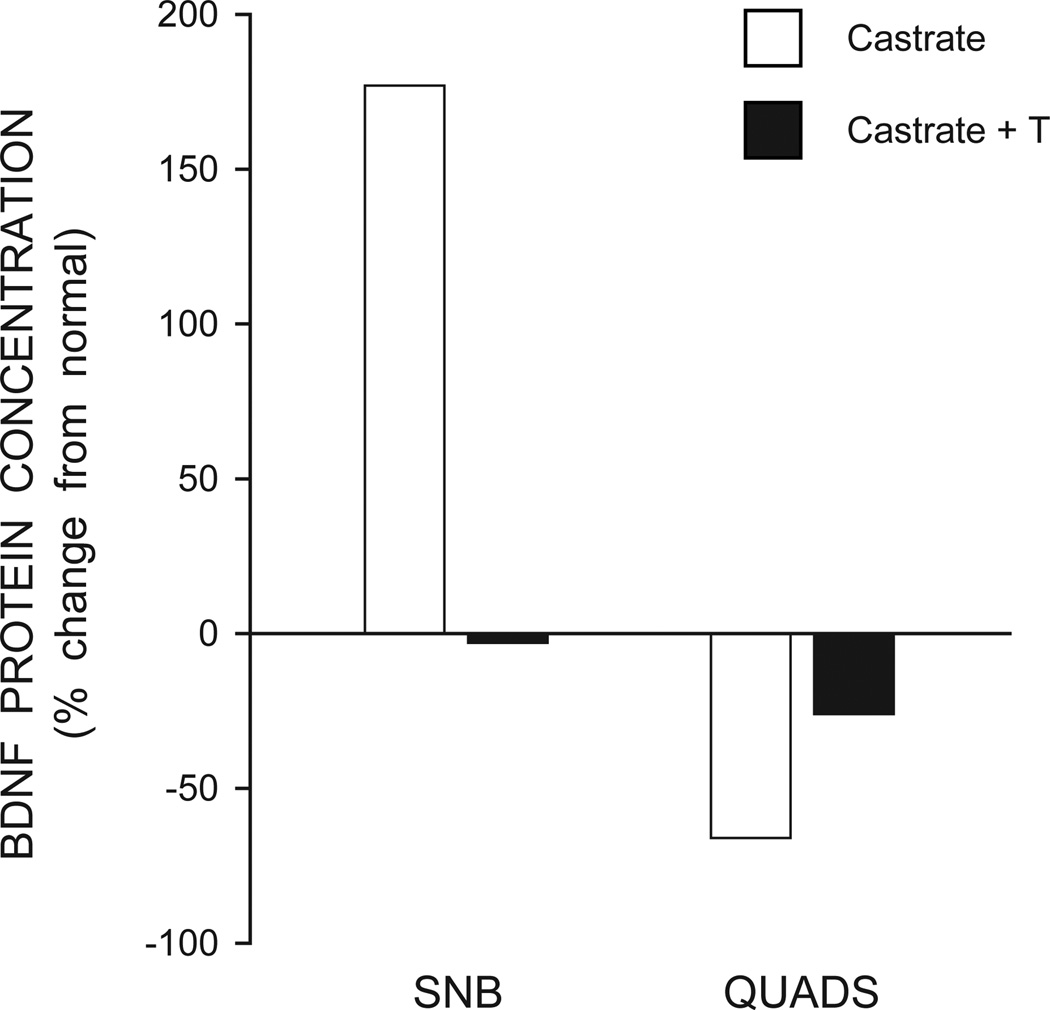
The SNB target musculature is critical for androgenic regulation of SNB morphology during development and in adulthood, and more recent work has explored BDNF and its androgen regulation in the SNB target muscles. BDNF mRNA and protein are present in skeletal muscle (Koliatsos et al., 1993; Kust et al., 2002; Mousavi et al., 2004) including the SNB target muscles (Verhovshek et al., 2010). BDNF levels in the SNB target musculature are regulated by androgens: in adult male rats, castration results in a significant increase in BDNF levels compared to gonadally intact animals, and treating castrates with testosterone reduces BDNF levels to those found in intact control males (Verhovshek et al., 2010; Fig. 3). Because both message and protein for BDNF are found in skeletal muscles, this suggests that the ability of androgens to regulate BDNF in skeletal muscle may reflect a change in the peripheral production of BDNF.
Figure 3.
BDNF protein concentrations in the target musculature of SNB (left) and quadriceps (QUADS, right) of castrated (open bars) and testosterone-treated (T) male rats (black bars). Concentrations are expressed as percent change from levels in gonadally intact males. (Adapted from Verhovshek et al., 2010)
In addition to the androgenic regulation of BDNF levels in muscle, BDNF and androgens interact to influence gross muscle morphology as well. Blockade of BDNF signaling using trkB IgG resulted in hypertrophy of the SNB target musculature in gonadally intact male rats, and muscle weights were significantly greater for trkB IgG-treated animals compared to controls (Verhovshek and Sengelaub, 2010b). Similarly, trkB IgG treatment in castrated males attenuated the castration-induced decrease in SNB target muscle weight (Verhovshek and Sengelaub, 2010b). However, trkB IgG treatment did not maintain muscle weights at that of gonadally intact males, suggesting that although BDNF contributes to the regulation of SNB target muscle weight, other potentially androgen-sensitive factors are likely involved. These results represent the first demonstration that interfering with BDNF signaling can have trophic effects on skeletal muscle. Although the exact mechanism for BDNF’s influence on skeletal muscle remains unclear, it has been suggested that BDNF plays an important role in the regulation of muscle homeostasis (Chevrel et al., 2006).
Somatic motor systems
Motoneurons
Androgenic regulation of BDNF and trkB in motoneurons is not restricted to androgen-sensitive neuromuscular systems. The non-dimorphic quadriceps motoneurons express BDNF and trkB, and the expression of this neurotrophic factor and its receptor are regulated by circulating hormones. In adult male rats, castration resulted in a significant decrease in both BDNF and trkB protein in quadriceps motoneurons (Osborne et al., 2007; Verhovshek et al., 2010). The castration-induced decrease in quadriceps motoneuron BDNF and trkB was restored to levels of those found in gonadally intact males by testosterone treatment (Osborne et al., 2007; Verhovshek et al., 2010).
Target musculature
In contrast to the SNB system, while BDNF levels in the quadriceps muscles are also regulated by androgens, castration reduces, rather than increases, BDNF protein in the muscle. BDNF protein levels are significantly lower in the quadriceps muscle of castrated adult male rats compared to gonadally intact animals, and treating castrates with testosterone restored BDNF levels to those of gonadally intact animals (Verhovshek et al., 2010; Fig. 3). In a potential naturally-occurring analog, BDNF mRNA in skeletal muscles is decreased in aged animals (Ming et al., 1999). Circulating androgen levels are also decreased in aged rats (Ghanadian et al., 1975; Kaler and Neaves, 1981), and thus, similar to castration effects in young adults, the decrease in BDNF mRNA in skeletal muscle could reflect age-related decreased androgen. Alternatively, BDNF levels are activity-dependent, for example, increased after exercise (Gomez-Pinilla et al., 2001). Therefore, an age-related decrease in activity could be responsible for lower BDNF mRNA levels in the skeletal muscle of aged rats.
Summary of results
The pattern of androgenic regulation of trkB and BDNF in the highly androgen-sensitive motoneurons of the SNB is identical to that observed in the more typical somatic motoneurons innervating the quadriceps (Osborne et al., 2007; Verhovshek et al., 2010). In both systems, castration resulted in a decrease in trkB and BDNF protein levels in motoneurons, and testosterone treatment restored trkB and BDNF levels of those found in gonadally intact animals (Osborne et al., 2007; Verhovshek et al., 2010). In the SNB neuromuscular system, a site of action for androgenic regulation of BDNF in motoneurons is the target musculature (Verhovshek and Sengelaub, 2010a), but it has not been determined where androgens act to regulate BDNF expression in quadriceps motoneurons. Furthermore, the effect of interrupting BDNF signaling on quadriceps motoneurons should be assessed in order to compare the effects of BDNF blockade on motoneuron morphology across spinal motoneuron populations. Although these findings from quadriceps motoneurons suggest a general ability of androgens to regulate BDNF in spinal motoneurons, other somatic motor populations apparently do not display androgenic regulation of BDNF and trkB. In the retrodorsolateral nucleus (RDLN), a non-sexually dimorphic somatic motor population innervating foot muscles, castration had no effect on BDNF mRNA, protein, or trkB mRNA, and treating castrates with testosterone did not alter BDNF protein in proximal RDLN dendrites (Ottem et al., 2007). These contrasting results could simply reflect a difference between the RDLN and quadriceps motor populations. Alternatively, BDNF protein levels were measured 14 days post-castration in RDLN motoneurons compared to 28 days after castration in quadriceps motoneurons. Therefore it is possible that castration-induced changes in BDNF protein occur later in quadriceps compared to RDLN motoneurons. Alternatively, BDNF was measured in dendrites in the RDLN motoneurons, but in the somata of SNB motoneurons, and there could be differences in castration-induced changes in BDNF expression across cellular structures.
Androgens also regulate BDNF levels in target muscles in both the SNB and quadriceps neuromuscular systems. Interestingly, castration increased BDNF in the SNB target muscle, but decreased BDNF levels in the quadriceps, and in both systems, testosterone treatment in castrates restored BDNF levels to those of gonadally intact animals. Opposite effects of castration on BDNF concentrations in peripheral tissues have been previously observed. Castration decreased BDNF in the vas deferens but increased BDNF in the vesicular and prostate glands of rats (Mirabella et al., 2006). Together, these results demonstrate that within a neural system, androgen manipulation differentially affects BDNF levels in a structure-dependent fashion. Future studies should explore the role of steroid hormone-neurotrophic factor interactions in both sexually dimorphic as well as non-dimorphic populations of motoneurons.
The weights of the SNB and quadriceps motoneuron target muscles are also differentially sensitive to castration. In the SNB neuromuscular system, castration results in a decrease in target muscle weight, whereas quadriceps muscle weight remains unaffected after gonadectomy (Verhovshek et al., 2010). It is interesting to speculate that these changes could be potentially mediated by changes in BDNF levels in muscle. The increased levels of BDNF protein in the SNB target musculature after castration could underlie the decreases in muscle weight, and in fact trkB IgG treatment in castrates attenuates this decrease (Verhovshek and Sengelaub, 2010b). BDNF levels do not increase in the quadriceps muscle after castration (in fact, they decrease; Verhovshek et al., 2010), potentially protecting the muscle from atrophy.
The differential androgen sensitivity of SNB target muscles and quadriceps muscles is likely mediated by differences in androgen receptor expression. Androgen receptors are present in substantially higher concentrations in the SNB target muscle compared to the quadriceps muscle (Dube et al., 1976). This difference in muscle androgen receptor density appears to confer androgen sensitivity on motoneuron morphology. Unlike SNB motoneurons, the morphology of quadriceps motoneurons is normally unaffected by androgen manipulation (Huguenard et al., 2011). As described above, BDNF and trkB protein in quadriceps motoneurons decrease after castration, and the lack of a concomitant decrease in dendritic length suggests that BDNF signaling does not promote dendritic growth in this system. However, androgen sensitivity can be induced in quadriceps motoneuron dendrites by increasing androgen receptor expression in the target musculature of transgenic rats (Huguenard et al., 2011). It would be interesting to examine if the pattern of changes in BDNF levels in response to androgen manipulations in the quadriceps system of androgen receptor overexpressing transgenic rats mimics that seen in the normally androgen-sensitive SNB neuromuscular system.
BDNF-androgen interactions in injury models
Androgens exhibit a wide array of neuroprotective and neurotherapeutic effects (Jones, 1993; Jones et al., 2001). For example, testosterone protects against cell death in cultured hippocampal neurons (Pike, 2001) and injury-induced dendritic atrophy in cortical pyramidal cells (Forgie and Kolb, 2003). In addition, neuroprotective and neurotherapeutic effects of androgens have also been demonstrated in motoneurons, including supporting cell survival, axonal regeneration, and dendritic maintenance (Fargo et al., 2009). The androgenic regulation of trophic factors has potentially important implications not only for adult maintenance of motoneuron morphology and function, but for neurotherapeutic or protective actions after motoneuron injury or disease (Fargo et al., 2009).
Axotomy
SNB motoneurons
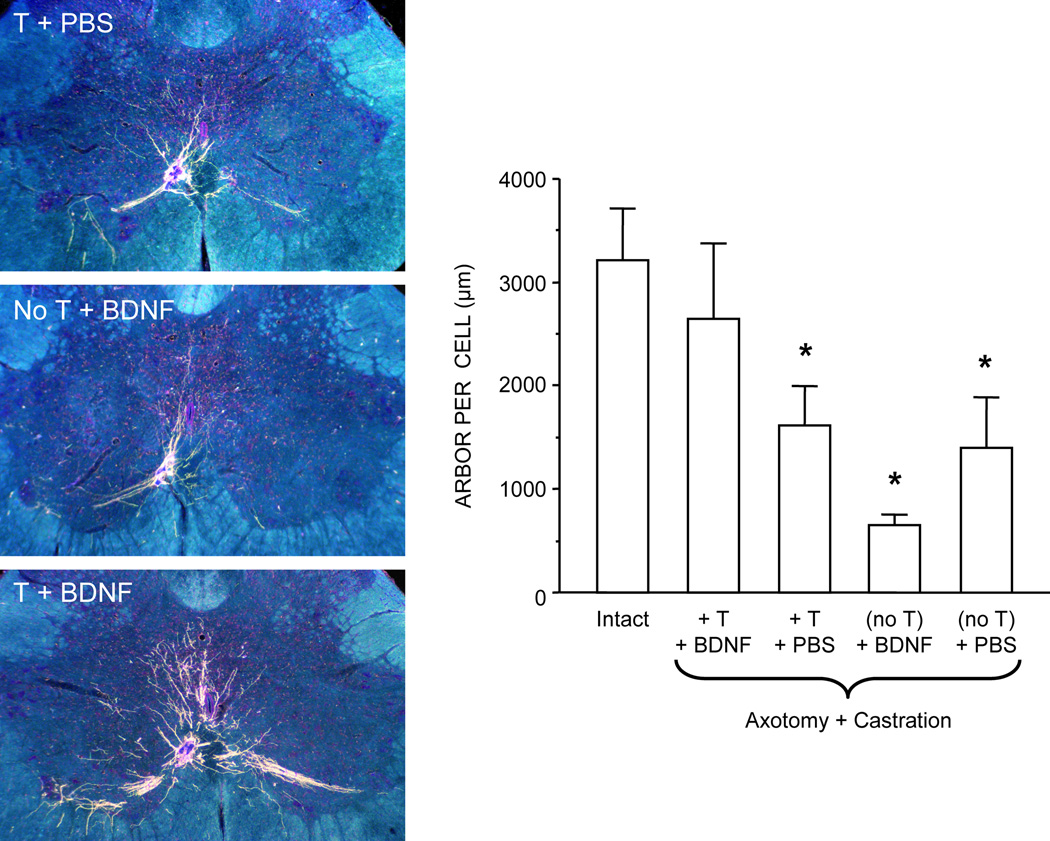
BDNF-like immunoreactivity in SNB motoneurons is decreased dramatically after axotomy, suggesting that BDNF produced by the target muscles is retrogradely transported to SNB motoneurons (Yang and Arnold, 1998). Along with its androgen sensitivity, SNB motoneuron morphology is also influenced by BDNF. For example, BDNF regulates SNB soma size after axotomy: treatment with BDNF alone reversed the axotomy-induced SNB somal atrophy in castrated and gonadally intact animals (Yang and Arnold, 2000). Additionally, testosterone and BDNF interact to regulate several features of adult spinal motoneurons. For example, post-axotomy treatment with BDNF enhanced nuclear androgen receptor expression in SNB motoneurons, but only in the presence of testosterone (Yang and Arnold, 2000). In the absence of testosterone, BDNF treatment was ineffective, and androgen receptor immunoreactivity was comparable to the reduced levels found in castrated animals (Yang and Arnold, 2000). Similarly, following axotomy, combinatorial treatment with testosterone and BDNF maintained SNB motoneuron dendritic length in adult male rats; treatment with either testosterone or BDNF alone was ineffective (Yang et al., 2004; Fig. 4). The combinatorial treatment effects of testosterone and BDNF on androgen receptor expression and dendritic morphology in SNB motoneurons indicate that the neuromuscular periphery is a critical source of BDNF, as BDNF was applied directly to the cut axons (Yang and Arnold, 2000; Yang et al., 2004).
Figure 4.
(Left) Dark-field digital images of transverse sections through the lumbar spinal cord showing BHRP labeling of SNB dendrites in axotomized and castrated males treated with testosterone (T) and phosphate buffered saline (PBS) (top), BDNF alone (middle), or T and BDNF (bottom). (Right) Dendritic length per labeled motoneuron in intact males and axotomized and castrated males with T and BDNF (T+BDNF), T alone (T+PBS), BDNF alone (no T+BDNF), and PBS alone (no T+PBS) applied to the cut SNB axons. Bar heights represent means ± SEM. * Significantly different from intact males. (Adapted from Yang et al., 2004)
These findings demonstrate that BDNF and testosterone interact to provide trophic effects on SNB motoneuron morphology following axotomy (Yang et al., 2004). These results stand in strong contrast with the previously described inhibitory effects of BDNF on SNB dendritic morphology that occur in motoneurons with intact axons. This apparent conflict most likely reflects the major differences in the manipulations used in the two studies. For example, axotomy completely severs motoneuron contact with the periphery, whereas castration has no such effect. Indeed, motoneuron contact with the periphery is critical for androgen receptor and neurotrophin levels in SNB motoneurons: if cut axons are allowed to re-innervate their targets following axotomy, the axotomy-induced decrease in androgen receptor expression in SNB motoneurons is fully restored (Al-Shamma and Arnold, 1995). Additionally, other peripherally-derived signaling molecules may interact dynamically to regulate SNB dendritic morphology and by axotomizing SNB motoneurons, all of the potential effects of these various target-derived signals are disrupted. Indeed, axotomy alters the trophic factor responsitivity of motoneurons (Funakoshi et al., 1993; Koliatsos et al., 1994). On the other hand, blocking BDNF signaling with trkB IgG treatment may only have disrupted the BDNF component of this dynamic target-motoneuron interaction. Thus, it is likely that a variety of signaling pathways are differentially affected in these experimental manipulations, accounting for the different effects.
Peripheral nerve regeneration
Axons in the peripheral nervous system are known for their regenerative capacity, but recovery is protracted and often suboptimal (Fargo et al., 2009). A variety of factors have been identified that can be utilized to enhance nerve regeneration after nerve crush, including testosterone (Kujawa et al., 1989) and BDNF (Zhang et al., 2000). Relevant to this review, a few studies have examined the potential interaction of androgens and BDNF in peripheral nerve regeneration.
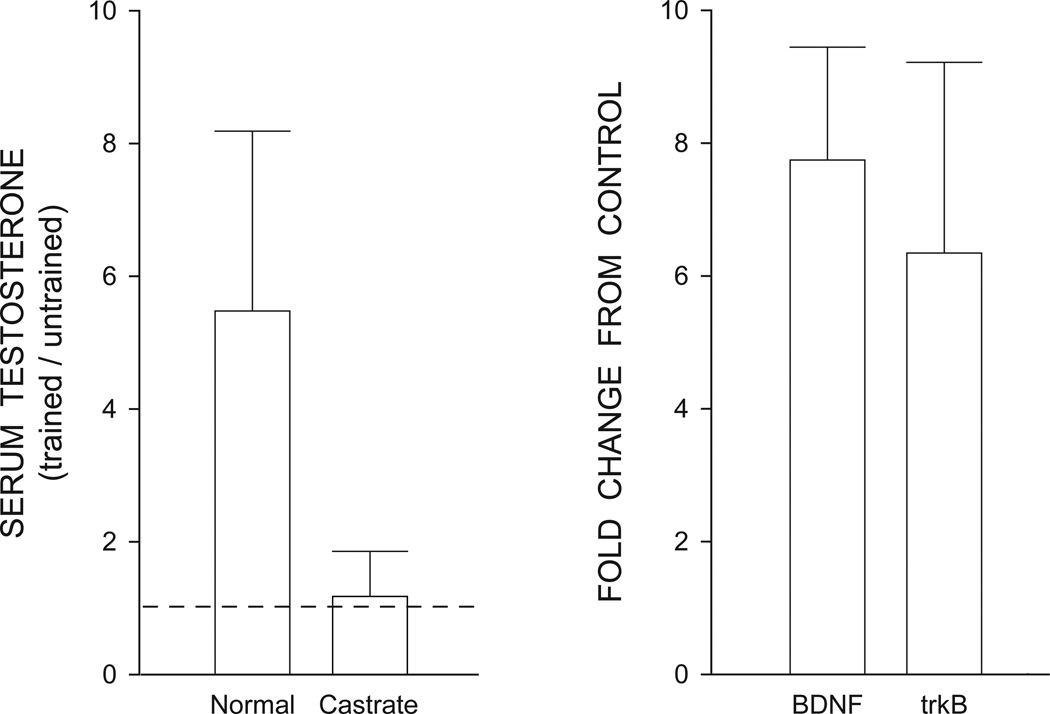
In thy-1-YFP-H mice, nerve grafts and exercise have been used as a treatment for peripheral nerve injury (Wood et al., 2011). After cutting a terminal branch of the sciatic nerve, male mice were subjected to treadmill training, and axon regeneration profiles were assessed at the end of the exercise treatment (Wood et al., 2011). Axon regeneration was enhanced by exercise, an effect likely mediated by an interaction of androgen and BDNF (Wood et al., 2011). Exercised mice had significantly greater levels of serum testosterone as well as increased BDNF and trkB mRNA in lumbar spinal cord compared to sedentary animals (Wood et al., 2011; Fig. 5). The effects of exercise on axon regeneration are dependent upon androgen receptor activation. For example, treatment with the androgen receptor blocker flutamide attenuated exercise effects on axon regeneration (Thompson et al., 2012). Similarly, exercise-induced enhancement of axon regeneration is also dependent on BDNF. Exercise had no effect on axon regeneration in BDNF-null mice (Wilhelm et al., 2012). These effects likely reflect an interaction of androgens and BDNF, as BDNF is increased immediately after peripheral nerve injury, and testosterone treatment further enhances and sustains BDNF mRNA levels, resulting in a long-term upregulation of BDNF in motoneurons (Sharma et al., 2010).
Figure 5.
(Left) Serum testosterone levels in treadmill trained normal and castrated male mice. Testosterone levels are expressed as the mean ratio of serum measurements in trained mice to untrained mice; dashed line indicates no change. (Right) BDNF and trkB expression in the lumbar spinal cords of gonadally intact treadmill trained male mice. Data are from real time PCR analysis expressed as mean fold change in BDNF mRNA relative to untrained mice. Bars in both histograms represent means ± 95% confidence intervals. (Adapted from Wood et al., 2012)
Mechanism of androgen regulation of BDNF
BDNF expression is regulated through a calcium-dependent signaling pathway, involving the phosphorylation of the cAMP response element (CRE) and its binding protein CREB (Shieh et al., 1998; Tao et al., 1998; Tao et al., 2002). Testosterone has been shown to activate both CRE and CREB (Aarnisalo et al., 1998; Auger et al., 2001) and thus, it is possible that the changes in BDNF immunolabeling in SNB motoneurons could involve an androgen-mediated regulation of the cAMP signaling pathway for BDNF. Alternatively, many of the actions of testosterone occur through its conversion to dihydrotestosterone or estrogenic metabolites (Hutchinson, 1997), and thus it is possible that the effects of castration and/or testosterone replacement on BDNF immunolabeling could be either androgenic or estrogenic in nature. Estrogenic regulation of BDNF has been reported previously in several brain regions (Singh et al., 1995; Sohrabji et al., 1995; Gibbs, 1999; Jezierski and Sohrabji, 2001; Liu et al., 2001; Blurton-Jones et al., 2004) and although there is no evidence for estrogen accumulation in SNB motoneurons (Breedlove and Arnold, 1980), the SNB target musculature binds estrogens as well as androgens (Dube et al., 1976). Subsequent studies with estrogens or nonaromatizable androgens can address this question.
Implications of the androgen-BDNF interactions
This review highlights how androgens influence BDNF expression and motoneuron morphology by acting at the target musculature to regulate target-derived neurotrophic signals. Additionally, we provide evidence that BDNF’s production, axonal transport, and androgenic regulation could be relevant for a variety of injury paradigms. Androgen-BDNF interactions may have relevance to therapeutic approaches in the treatment of neurodegenerative diseases or other human myopathies, as previous reports have suggested that abnormal expression of trophic factors and their receptors may play a role (Kust et al., 2002). It is important to note that in addition to its well-established trophic effects, BDNF can also have deleterious effects on neuron survival and morphology. For example, BDNF can render motoneurons vulnerable to excitotoxic insult in vitro (Fryer et al., 2000; Hu and Kalb, 2003; Mojsilovic-Petrovic et al., 2006) and has regressive effects on dendritic morphology in vitro (McAllister et al., 1997; Martin et al., 2012) and in vivo (Lom and Cohen-Cory, 1999).
In the case of neurodegenerative diseases, BDNF concentrations are elevated in human biceps muscles of patients with amyotrophic lateral sclerosis, a progressive motoneuron disease (Kust et al., 2002). Additionally, results of clinical tests using neurotrophic factors for the treatment of motoneuron disease are largely negative. Intrathecal treatment with recombinant methionyl human BDNF produced mild sensory symptoms, sleep disturbance, dry mouth, agitation and other behavioral effects requiring reductions in dosage (Ochs et al., 2000). Similarly, a Phase III multicenter clinical trial of ALS patients failed to show a benefit of BDNF treatment (The BDNF Study Group, 1999). Our recent evidence that blockade of excess BDNF production may be neuroprotective (Verhovshek and Sengelaub, 2010b) may be relevant for treating these types of diseases. Conversely, BDNF levels increase after testosterone treatment in multiple sclerosis patients, but this marked increase in BDNF occurs only in patients who have achieved full recovery, suggesting that heightened BDNF production is associated with positive outcomes for patients with certain diseases (Gold et al., 2008). Overall, it is possible that therapeutic strategies involving the regulation of trophic factors, rather than their simple administration, may be useful in treating degenerative neuromuscular diseases in which abnormal expression of trophic factors is a suspected cause.
Summary
This review has discussed studies demonstrating androgenic influences on BDNF signaling in neuromuscular systems. Further, we review evidence that suggests a mechanism for the trophic effects of androgens on spinal motoneurons via androgenic suppression of BDNF in the SNB target musculature. It is important to note that central upregulation of BDNF and trkB does not always indicate trophic effects on motoneuron morphology, and androgen-nregulated changes in BDNF protein and mRNA expression in neuromuscular systems should be directly evaluated. Also, we have compared androgen-BDNF interactions following peripheral nerve injury in rodents. Results indicate that BDNF and androgens promote regeneration following axonal injury, potentially by an androgen-enhanced upregulation of BDNF in spinal motoneurons. Taken together, these findings provide further insight into the maintenance of neuromuscular systems in adulthood and have implications for the neurotherapeutic/neuroprotective roles of androgens and trophic factors in the treatment of motoneuron disease and recovery from injury.
We review androgen-BDNF interactions in the spinal cord.
We describe these interactions following androgen manipulation and injury.
Androgens interact with BDNF to regulate motoneuron death in development.
In adulthood, androgens regulate BDNF and trkB expression in spinal motoneurons.
In adulthood, androgens regulate BDNF levels in the target musculature.
Androgen-BDNF interactions affect morphology, axon regrowth of injured motoneurons.
Acknowledgements
We would like to thank our anonymous reviewers for their extremely helpful and insightful comments on the previous draft.
Supported by NIH-NINDS NS047264 to D.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnisalo P, Palvimo J, Janne O. CREB-binding protein in androgen receptor-mediated signaling. PNAS USA. 1998;95:2122–2127. doi: 10.1073/pnas.95.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamma AH, Arnold AP. Importance of target innervation in recovery from axotomy-induced loss of androgen receptor in rat perineal motoneurons. J Neurobiol. 1995;28(3):341–353. doi: 10.1002/neu.480280307. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vaneski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki I, Harada Y, Kuno M. Target-dependent hormonal control of neuron size in the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1991;11:3025–3033. doi: 10.1523/JNEUROSCI.11-10-03025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Matsumoto A, Micevych PE. Neural plasticity in a hormone-sensitive spinal nucleus. Bull TMIN. 1988;16:41–46. [Google Scholar]
- Auger A, Hexter D, McCarthy M. Sex differences in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kuan P, Tuszynski M. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- Breedlove S. Cellular analyses of hormone influence on motoneuronal development and function. J Neurobiol. 1986;17:157–176. doi: 10.1002/neu.480170304. [DOI] [PubMed] [Google Scholar]
- Breedlove S, Arnold A. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove S, Arnold A. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormonal manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal-Control of a Developing Neuromuscular System .2. Sensitive Periods for the Androgen-Induced Masculinization of the Rat Spinal Nucleus of the Bulbocavernosus. J Neurosci. 1983;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C, Sebum K, Cope T. Neurotrophin expression by spinal motoneurons in adult and developing rats. J Comp Neurol. 2000;416:309–318. doi: 10.1002/(sici)1096-9861(20000117)416:3<309::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Cabelli R, Hohn A, Shatz C. Inhibition of ocular dominance column formation of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. PNAS. 2011;45:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrel G, Hohlfeld R, Sendtner M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve. 2006;33:462–476. doi: 10.1002/mus.20444. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser S. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- DiStefano P, Friedman C, Radziejewski C, Alexander C, Boland P, Schick C, Lindsay R, Wiegand S. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Dube J, Lesage R, Tremblay R. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Foster AM, Harty MW, Sengelaub DR. Estrogen alters the excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J Neurobiol. 2003;56:66–77. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Fargo K, Foecking E, Jones K, Sengelaub DR. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol. 2009;30:130–141. doi: 10.1016/j.yfrne.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C, Fiumelli H, Martin J. Role of trkB-mediated signaling pathways in the regulation of cortical dendritic development by BDNF. Society for Neuroscience Itinerary Planner. 2009:312.11. [Google Scholar]
- Forgie M, Kolb B. Manipulation of gonadal hormones in neonatal rats alters the morphological response of cortical neurons to brain injury in adulthood. Behav Neurosci. 2003;117:257–262. doi: 10.1037/0735-7044.117.2.257. [DOI] [PubMed] [Google Scholar]
- Freeman L, Watson N, Breedlove SM. Androgen Spares Androgen-Insensitive Motoneurons from Apoptosis in the Spinal Nucleus of the Bulbocavernosus in Rats. Horm Behav. 1996;30:424–433. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- Fryer H, Wolf D, Knox R, Strittmatter S, Pennica D, O'Leary R, Russell D, Kalb R. Brain-derived neurotrophic factor induces excitotoxic sensitivity in cultured embryonic rat spinal motor neurons through activation of the phosphatidylinositol 3-kinase pathway. J Neurochem. 2000;74:582–595. doi: 10.1046/j.1471-4159.2000.740582.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge V, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanadian R, Lewis J, Chisholm J. Serum testosterone and dihydrotestosterone changes with age in rat. Steroids. 1975;25:753–762. doi: 10.1016/0039-128x(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation. 2008;5:32. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian A, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- Henderson C, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon S, Armanini M, Berkemeier L, Phillips H, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Horch H. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Horch H, Katz L. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Sachs BD. Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Horm Behav. 1992;26:406–419. doi: 10.1016/0018-506x(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Hu P, Kalb R. BDNF heightens the sensitivity of motor neurons to excitotoxic insults through activation of trkB. J Neurochem. 2003;84:1421–1430. doi: 10.1046/j.1471-4159.2003.01599.x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard AL, Fernando SM, Monks DA, Sengelaub DR. Overexpression of androgen receptors in target musculature confers androgen sensitivity to motoneuron dendrites. Endocrinology. 2011;152(2):639–650. doi: 10.1210/en.2010-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J. Gender-specific steroid metabolism in neural differentiation. Cell Mol Neurobiol. 1997;176:603–626. doi: 10.1023/a:1022581902880. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jones KJ. Gonadal steroids and neuronal regeneration: a therapeutic role. In: Seil F, editor. Neural injury and regeneration, advances in neurology. vol. 59. New York: Raven Press; 1993. pp. 227–240. [PubMed] [Google Scholar]
- Jones K, Brown T, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Kaler L, Neaves W. The androgen status of aging male rats. Endocrinology. 1981;108:712–719. doi: 10.1210/endo-108-2-712. [DOI] [PubMed] [Google Scholar]
- Koliatsos V, Price D, Clatterbuck R. Motor neurons in Onuf's nucleus and its rat homologues express the p75 nerve growth factor receptor: sexual dimorphism and regulation by axotomy. J Comp Neurol. 1994;345:510–527. doi: 10.1002/cne.903450404. [DOI] [PubMed] [Google Scholar]
- Koliatsos V, Clatterbuck R, Winslow J, Cayouette M, Price D. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Kurz E, Sengelaub D, Arnold A. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Kust B, Copray J, Brouwer N, Troost D, Boddeke H. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol. 2002;177:419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler C, Young L, Yan Q, Insel T, Wang Z. Expression and estrogenic regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lom B, Cogen J, Lontok Sanchez A, Vu T, Cohen-Cory S. Local and target derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubischer J, Arnold A. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995;694:61–68. doi: 10.1016/0006-8993(95)00766-j. [DOI] [PubMed] [Google Scholar]
- Mamounas L, Altar C, Blue M, Kaplan D, Tessarollo L, Lyons W. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Brown AL, Balkoweic A. Glia determine the course of brain-derived neurotrophic factor-mediated dendritogenesis and provide a soluble inhibitory cue to dendritic growth in the brainstem. Neuroscience. 2012;207:333–346. doi: 10.1016/j.neuroscience.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Prins G. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol. 1996;8:553–559. doi: 10.1046/j.1365-2826.1996.04899.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka I, Meyer M, Hofer M, Thoenen H. Differential regulation of nerve growth factor and brain-derived neurotrophic factor expression in the peripheral nervous system. Ann N Y Acad Sci. 1991;633:550–552. doi: 10.1111/j.1749-6632.1991.tb15657.x. [DOI] [PubMed] [Google Scholar]
- McAllister A, Lo D, Katz L. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister A, Katz L, Lo D. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- McAllister A, Katz L, Lo D. Neurotrophins and synaptic plasticity. Ann Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McKenna K, Nadelhaft I. Organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Ming Y, Bergman E, Edstrom E, Ulfhake B. Reciprocal changes in the expression of neurotrophin mRNAs in target tissues and peripheral nerves of aged rats. Neurosci Lett. 1999;273:187–190. doi: 10.1016/s0304-3940(99)00655-2. [DOI] [PubMed] [Google Scholar]
- Mirabella N, Squillacioti C, Immacolata P, Ciarcia R, Russo M, Paino G. Effects of castration on the expression of brain-derived neurotrophic factor (BDNF) in the vas deferens and male accessory genital glands of the rat. Cell Tissue Res. 2006;323:513–522. doi: 10.1007/s00441-005-0084-1. [DOI] [PubMed] [Google Scholar]
- Miranda R, Sohrabji F, Toran-Allerand C. Presumptive estrogen target neurons express mRNAs for both neurotrophin receptors: A basis for the potential developmental interactions of estrogen with the neurotrophins. Mol Cell Neurosci. 1993;4:510–525. doi: 10.1006/mcne.1993.1063. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong G, Crocker A, Arneja A, David S, Russell D, Kalb R. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks D, O'Bryant E, Jordan C. Androgen receptor immunoreactivity in skeletal muscle: Enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks D, Kopachik W, Breedlove S, Jordan C. Anabolic responsiveness of skeletal muscle correlates with androgen receptor protein but not mRNA. Can J Physiol Pharmacol. 2006;84:272–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- Mousavi K, Parry D, Jasmin B. BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am J Physiol Cell Physiol. 2004;287:C22–C29. doi: 10.1152/ajpcell.00583.2003. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens Prevent Normally Occurring Cell-Death in a Sexually Dimorphic Spinal Nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(3):201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- Osborne M, Verhovshek T, Sengelaub D. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem E, Beck L, Jordan C, Breedlove S. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Poort JE, Wang H, Jordan CL, Breedlove SM. Differential expression and regulation of brain-derived neurotrophic factor (BDNF) mRNA isoforms in androgen-sensitive motoneurons of the rat lumbar spinal cord. Mol Cell Endo. 2010;328:40–46. doi: 10.1016/j.mce.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Pike C. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pitts E, Potluri S, Hess D, Balice-Gordan R. Neurotrophin and trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin. 2006;44:21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- Rand M, Breedlove S. Androgens alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Sachs B. Role of striated penile muscle in penile reflexes, copulation and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Arnold AP. Development and Loss of Early Projections in a Sexually Dimorphic Rat Spinal Nucleus. J Neurosci. 1986;6:1613–1620. doi: 10.1523/JNEUROSCI.06-06-01613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengelaub D, Forger N. The Spinal Nucleus of the Bulbocavernosus: Firsts in androgen-dependent neural se differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Shieh P, Hu S, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Shugrue P, Scrimo P, Merchenthaler I. Estrogen binding and estrogen receptor characterization in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer E, Simpkins J. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Snider W. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda R, Toran-Allerand C. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda R, Toran-Allerand C. Identification of a putative estrogen response element in the gene coding for BDNF. PNAS USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum D, Handa R. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold D, Shaywitz A, Greenberg M. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tao X, West A, Chen W, Corfas G, Greenberg M. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- The BDNF Study Group Phase III. A controlled trial of recombinant methionyl human BDNF in ALS. Neurology. 1999;52(7):1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Thompson N, Sengelaub DR, English AW. Enhancement of peripheral axon regeneration by exercise requires androgen receptor signaling in both male and female mice. 2012 Society for Neuroscience Abstracts Viewer/Itinerary Planner. 2012 [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. Mechanisms of estrogen action during neural development: mediation by interactions with the neurotrophins and their receptors. J Steroid Biochem Mol Biol. 1996;56:169–178. doi: 10.1016/0960-0760(95)00234-0. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C, Miranda R, Bentham W, Sohrabji F, Brown T, Hochberg R, MacLusky N. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. PNAS USA. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand C, Singh M, Setalo JR G. Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne M, Sengelaub D. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Sengelaub D. Local application of testosterone at the target musculature regulates brain-derived neurotrophic factor in spinal motoneurons. Society for Neuroscience Abstract Viewer and Itinerary Planner. 2010a;Vol 40 [Google Scholar]
- Verhovshek T, Sengelaub D. Trophic effects of bran-derived neurotrophic factor blockade in an androgen-sensitive neuromuscular system. Endocrinology. 2010b;151(11):5337–5348. doi: 10.1210/en.2010-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32(14):5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman A, Brenowitz E. The role of Neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci. 2009;29:6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol. 2011;72:688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gingras K, Bengston L, DiMarco A, Forger N. Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons. J Neurosci. 2001;21:4366–4372. doi: 10.1523/JNEUROSCI.21-12-04366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld R, CR, Hawkins N, Lopez O, Bennett L, Welcher A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yang L, Arnold A. Axotomy decreases BDNF expression in the SNB motoneurons of male rats. Society for Neuroscience Abstracts. 1998;24:1547. [Google Scholar]
- Yang L, Arnold A. BDNF regulation of androgen receptor expression in axotomized SNB motoneurons of adult male rats. Brain Res. 2000;852:127–139. doi: 10.1016/s0006-8993(99)02225-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Verhovshek T, Sengelaub D. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]